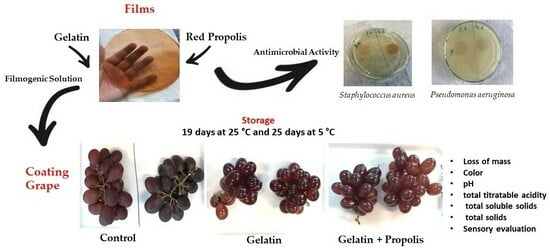
Effect of Adding Red Propolis to Edible Biodegradable Protein Films for Coating Grapes: Shelf Life and Sensory Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization of Propolis
2.2.1. Determination of Bioactive Compounds
2.2.2. Antioxidant Capacity
2.3. Production of Films for Antimicrobial Activity (“Halo Test”)
2.4. Production of Film Solutions to Cover the Grapes
2.5. Preparation of Grapes and Application of Film Solutions
2.6. Characterization of the Grapes
2.6.1. Visual Appearance and Color Parameters
2.6.2. Loss of Mass
2.6.3. Determining pH
2.6.4. Total Titratable Acidity (TTA)
2.6.5. Total Soluble Solids (TSS)
2.6.6. Total Solids
2.6.7. Sensory Evaluation
2.7. Statistical Analysis
3. Results and Discussion
3.1. Characterization of the Bioactive Compounds and Antioxidant Activity of Red Propolis Extract
3.2. Antimicrobial Activity of the Films (“Halo Test”)
3.3. Characterization of the Film Added with Red Propolis Extract
Visual Assessment
3.4. Characterization of the Grapes Covered with the Film Solution
3.4.1. Visual Appearance and Color Parameters
3.4.2. Loss of Mass
3.4.3. Determination of pH, Total Titratable Acidity, Total Soluble Solids and Total Solids
3.4.4. Sensory Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nogueira, G.F.; Oliveira, R.A.D.; Velasco, J.I.; Fakhouri, F.M. Methods of Incorporating Plant-Derived Bioactive Compounds into Films Made with Agro-Based Polymers for Application as Food Packaging: A Brief Review. Polymers 2020, 12, 2518. [Google Scholar] [CrossRef] [PubMed]
- Fakhouri, F.M.; Maria Martelli, S.; Canhadas Bertan, L.; Yamashita, F.; Innocentini Mei, L.H.; Collares Queiroz, F.P. Edible Films Made from Blends of Manioc Starch and Gelatin—Influence of Different Types of Plasticizer and Different Levels of Macromolecules on Their Properties. LWT 2012, 49, 149–154. [Google Scholar] [CrossRef]
- Zhang, Y.-L.; Cui, Q.-L.; Wang, Y.; Shi, F.; Liu, Y.-P.; Liu, J.-L.; Nie, G.-W. Effect of Carboxymethyl Chitosan-Gelatin-Based Edible Coatings on the Quality and Antioxidant Properties of Sweet Cherry during Postharvest Storage. Sci. Hortic. 2021, 289, 110462. [Google Scholar] [CrossRef]
- Pellá, M.C.G.; Silva, O.A.; Pellá, M.G.; Beneton, A.G.; Caetano, J.; Simões, M.R.; Dragunski, D.C. Effect of Gelatin and Casein Additions on Starch Edible Biodegradable Films for Fruit Surface Coating. Food Chem. 2020, 309, 125764. [Google Scholar] [CrossRef] [PubMed]
- Mannucci, A.; Serra, A.; Remorini, D.; Castagna, A.; Mele, M.; Scartazza, A.; Ranieri, A. Aroma Profile of Fuji Apples Treated with Gelatin Edible Coating during Their Storage. LWT Food Sci. Technol. 2017, 85, 28–36. [Google Scholar] [CrossRef]
- Lv, Y.; Deng, Y.; Wang, M.; Li, C.; Xie, P.; Sun, B.; Yang, X.; Lang, Y. Effect of Chitosan-Gelatine Edible Coating Containing Nano-Encapsulated Clove Ethanol Extract on Cold Storage of Chilled Pork. Meat Sci. 2023, 204, 109288. [Google Scholar] [CrossRef]
- Da Silva, A.C.P.; Barbosa, J.R.; Da Silva Araújo, C.; Sousa Batista, J.T.; Xavier Neves, E.M.P.; Pereira Cardoso, D.N.; Peixoto Joele, M.R.S.; De Fátima Henriques Lourenço, L. A New Edible Coating of Fish Gelatin Incorporated into Açaí Oil to Increase the Post-Harvest Shelf Life of Tomatoes. Food Chem. 2024, 438, 138047. [Google Scholar] [CrossRef]
- Sekarina, A.S.; Supriyadi; Munawaroh, H.S.H.; Susanto, E.; Show, P.L.; Ningrum, A. Effects of Edible Coatings of Chitosan—Fish Skin Gelatine Containing Black Tea Extract on Quality of Minimally Processed Papaya during Refrigerated Storage. Carbohydr. Polym. Technol. Appl. 2023, 5, 100287. [Google Scholar] [CrossRef]
- Schapoval, E.E.S. Controle Biológico de Qualidade de Produtos Farmacêuticos, Correlatos e Cosméticos. Rev. Bras. Cienc. Farm. 2005, 41, 279–280. [Google Scholar] [CrossRef]
- De Morais Sampaio, G.A.; Lacerda-Santos, R.; Cavalcanti, Y.W.; Vieira, G.H.A.; Nonaka, C.F.W.; Alves, P.M. Antimicrobial Properties, Mechanics, and Fluoride Release of Ionomeric Cements Modified by Red Propolis. Angle Orthod. 2021, 91, 522–527. [Google Scholar] [CrossRef] [PubMed]
- Do Nascimento, T.G.; De Almeida, C.P.; Da Conceição, M.M.; Dos Santos Silva, A.; De Almeida, L.M.; De Freitas, J.M.D.; Grillo, L.A.M.; Dornelas, C.B.; Ribeiro, A.S.; Da Silva, J.F.; et al. Caseinates Loaded with Brazilian Red Propolis Extract: Preparation, Protein-Flavonoids Interaction, Antioxidant and Antibacterial Activities. J. Therm. Anal. Calorim. 2022, 147, 1329–1343. [Google Scholar] [CrossRef]
- Reis, J.H.D.O.; Barreto, G.D.A.; Cerqueira, J.C.; Anjos, J.P.D.; Andrade, L.N.; Padilha, F.F.; Druzian, J.I.; Machado, B.A.S. Evaluation of the Antioxidant Profile and Cytotoxic Activity of Red Propolis Extracts from Different Regions of Northeastern Brazil Obtained by Conventional and Ultrasound-Assisted Extraction. PLoS ONE 2019, 14, e0219063. [Google Scholar] [CrossRef] [PubMed]
- Dantas Silva, R.P.; Machado, B.A.S.; Barreto, G.D.A.; Costa, S.S.; Andrade, L.N.; Amaral, R.G.; Carvalho, A.A.; Padilha, F.F.; Barbosa, J.D.V.; Umsza-Guez, M.A. Antioxidant, Antimicrobial, Antiparasitic, and Cytotoxic Properties of Various Brazilian Propolis Extracts. PLoS ONE 2017, 12, e0172585. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.P.; Silva, T.M.; Mengarda, A.C.; Salvadori, M.C.; Teixeira, F.S.; Alencar, S.M.; Luz Filho, G.C.; Bueno-Silva, B.; De Moraes, J. Brazilian Red Propolis Exhibits Antiparasitic Properties in Vitro and Reduces Worm Burden and Egg Production in an Mouse Model Harboring Either Early or Chronic Schistosoma mansoni Infection. J. Ethnopharmacol. 2021, 264, 113387. [Google Scholar] [CrossRef] [PubMed]
- Botteon, C.E.A.; Silva, L.B.; Ccana-Ccapatinta, G.V.; Silva, T.S.; Ambrosio, S.R.; Veneziani, R.C.S.; Bastos, J.K.; Marcato, P.D. Biosynthesis and Characterization of Gold Nanoparticles Using Brazilian Red Propolis and Evaluation of Its Antimicrobial and Anticancer Activities. Sci. Rep. 2021, 11, 1974. [Google Scholar] [CrossRef]
- Siqueira, A.L.; Dantas, C.G.; Gomes, M.Z.; Padilha, F.F.; Albuquerque Junior, R.L.C.D.; Cardoso, J.C. Estudo Da Ação Antibacteriana Do Extrato Hidroalcoólico de Própolis Vermelha Sobre Enterococcus Faecalis. Rev. Odontol. UNESP 2014, 43, 359–366. [Google Scholar] [CrossRef]
- Bankova, V. Recent Trends and Important Developments in Propolis Research. Evid. Based Complement Alternat. Med. 2005, 2, 29–32. [Google Scholar] [CrossRef]
- Marcucci, M.C. Propolis: Chemical Composition, Biological Properties and Therapeutic Activity. Apidologie 1995, 26, 83–99. [Google Scholar] [CrossRef]
- Burdock, G.A. Review of the Biological Properties and Toxicity of Bee Propolis (Propolis). Food Chem. Toxicol. 1998, 36, 347–363. [Google Scholar] [CrossRef]
- Crupi, P.; Palattella, D.; Corbo, F.; Clodoveo, M.L.; Masi, G.; Caputo, A.R.; Battista, F.; Tarricone, L. Effect of Pre-Harvest Inactivated Yeast Treatment on the Anthocyanin Content and Quality of Table Grapes. Food Chem. 2021, 337, 128006. [Google Scholar] [CrossRef]
- Song, H.; Asghari, M.; Zahedipour-Sheshglani, P.; Diao, E.; Xiang, X.; Liang, X.; Abdollahi Mandoulakani, B.; Qian, S. Investigation of Pectolytic and PR Genes Expression, Quality and Phytochemical Contents in Organic and Non-Organic Table Grapes at Harvest and during Storage. Food Res. Int. 2023, 167, 112717. [Google Scholar] [CrossRef]
- Shahkoomahally, S.; Sarkhosh, A.; Richmond-Cosie, L.M.; Brecht, J.K. Physiological Responses and Quality Attributes of Muscadine Grape (Vitis Rotundifolia Michx) to CO2-Enriched Atmosphere Storage. Postharvest Biol. Technol. 2021, 173, 111428. [Google Scholar] [CrossRef]
- Paul, V.; Pandey, R. Role of Internal Atmosphere on Fruit Ripening and Storability—A Review. J. Food Sci. Technol. 2014, 51, 1223–1250. [Google Scholar] [CrossRef] [PubMed]
- Gustafsson, J.; Cederberg, C.; Sonesson, U.; Emanuelsson, A. The Methodology of the FAO Study: “Global Food Losses and Food Waste-Extent, Causes and Prevention”—FAO, 2011; SIK—The Swedish Institute for Food and Biotechnology: Göteborg, Sweden, 2013. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.-E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Fakhouri, F.M.; Fontes, L.C.; Innocentini-Mei, L.H.; Collares-Queiroz, F.P. Effect of Fatty Acid Addition on the Properties of Biopolymer Films Based on Lipophilic Maize Starch and Gelatin. Starch-Stärke 2009, 61, 528–536. [Google Scholar] [CrossRef]
- M02-A12; NCCLS Performance Standards for Antimicrobial Disk Susceptibility Tests, Approved Standard. 8th ed. NCCLS: Wayne, PA, USA, 2003; Document M2-A8.
- Cunniff, P.; Association of Official Analytical Chemists. Official Methods of Analysis of AOAC International; Association of Official Analytical Chemists: Washington, DC, USA, 1995; ISBN 978-0-935584-54-7. [Google Scholar]
- IAL—Instituto Adolfo Lutz. Normas Analíticas do Instituto Adolfo Lutz: Métodos Químicos e Físicos para Análises de Alimentos, 3rd ed.; IMESP: São Paulo, Brazil, 1985. [Google Scholar]
- Dutcosky, S.D. Análise Sensorial de Alimentos, 4th ed.; Exatas; rev. e ampl.; Champagnat: Curitiba, Brazil, 2013; ISBN 978-85-7292-303-3. [Google Scholar]
- Macfie, H.J.; Bratchell, N.; Greenhoff, K.; Vallis, L.V. Designs to balance the effect of order of presentation and first-order carry-over effects in hall tests. J. Sens. Stud. 1989, 4, 129–148. [Google Scholar] [CrossRef]
- Aldana-Mejía, J.A.; Ccana-Ccapatinta, G.V.; Ribeiro, V.P.; Arruda, C.; Veneziani, R.C.S.; Ambrósio, S.R.; Bastos, J.K. A Validated HPLC-UV Method for the Analysis of Phenolic Compounds in Brazilian Red Propolis and Dalbergia ecastaphyllum. J. Pharm. Biomed. Anal. 2021, 198, 114029. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, M.A.E.; Amarante, M.K.; Conti, B.J.; Sforcin, J.M. Cytotoxic Constituents of Propolis Inducing Anticancer Effects: A Review. J. Pharm. Pharmacol. 2011, 63, 1378–1386. [Google Scholar] [CrossRef] [PubMed]
- Amankwaah, F.; Addotey, J.N.; Orman, E.; Adosraku, R.; Amponsah, I.K. A Comparative Study of Ghanaian Propolis Extracts: Chemometric Analysis of the Chromatographic Profile, Antioxidant, and Hypoglycemic Potential and Identification of Active Constituents. Sci. Afr. 2023, 22, e01956. [Google Scholar] [CrossRef]
- Gomes Sá, S.H.; Chalella Mazzocato, M.; Saliba, A.S.M.C.; Alencar, S.M.; Sílvia Favaro-Trindade, C. Evaluation of the Release, Stability and Antioxidant Activity of Brazilian Red Propolis Extract Encapsulated by Spray-Drying, Spray-Chilling and Using the Combination of Both Techniques. Food Res. Int. 2023, 164, 112423. [Google Scholar] [CrossRef]
- Guzelmeric, E.; Özdemir, D.; Sen, N.B.; Celik, C.; Yesilada, E. Quantitative Determination of Phenolic Compounds in Propolis Samples from the Black Sea Region (Türkiye) Based on HPTLC Images Using Partial Least Squares and Genetic Inverse Least Squares Methods. J. Pharm. Biomed. Anal. 2023, 229, 115338. [Google Scholar] [CrossRef]
- Mello, B.C.B.S.; Hubinger, M.D. Antioxidant Activity and Polyphenol Contents in B Razilian Green Propolis Extracts Prepared with the Use of Ethanol and Water as Solvents in Different pH Values. Int. J. Food Sci. Technol. 2012, 47, 2510–2518. [Google Scholar] [CrossRef]
- Lima, A.B.S.D.; Batista, A.S.; Santos, M.R.C.; Rocha, R.D.S.D.; Silva, M.V.D.; Ferrão, S.P.B.; Almeida, V.V.S.D.; Santos, L.S. Spectroscopy NIR and MIR toward Predicting Simultaneous Phenolic Contents and Antioxidant in Red Propolis by Multivariate Analysis. Food Chem. 2022, 367, 130744. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.-Z.; Sun, M.; Xing, J.; Luo, Q.; Corke, H. Structure–Radical Scavenging Activity Relationships of Phenolic Compounds from Traditional Chinese Medicinal Plants. Life Sci. 2006, 78, 2872–2888. [Google Scholar] [CrossRef]
- Ponce, A.G.; Fritz, R.; Del Valle, C.; Roura, S.I. Antimicrobial Activity of Essential Oils on the Native Microflora of Organic Swiss Chard. LWT Food Sci. Technol. 2003, 36, 679–684. [Google Scholar] [CrossRef]
- Palmeira, J.D.; Ferreira, S.B.; de Souza, J.H.; de Almeida, J.M.; Figueiredo, M.C.; Pequeno, A.S.; Arruda, T.A.; Antunes, R.M.P.; Catão, R.M.R. Avaliação da atividade antimicrobiana in vitro e determinação da concentração inibitória mínima (CIM) de extratos hidroalcoólico de angico sobre cepas de Staphylococcus aureus. Rev. Bras. Anal. Clin. 2010, 42, 33–37. [Google Scholar]
- Takaisi-Kikuni, N.; Schilcher, H. Electron Microscopic and Microcalorimetric Investigations of the Possible Mechanism of the Antibacterial Action of a Defined Propolis Provenance. Planta Med. 1994, 60, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Grange, J.M.; Davey, R.W. Antibacterial Properties of Propolis (Bee Glue). J. R. Soc. Med. 1990, 83, 159–160. [Google Scholar] [CrossRef] [PubMed]
- Kujumgiev, A.; Tsvetkova, I.; Serkedjieva, Y.; Bankova, V.; Christov, R.; Popov, S. Antibacterial, Antifungal and Antiviral Activity of Propolis of Different Geographic Origin. J. Ethnopharmacol. 1999, 64, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Jamróz, E.; Juszczak, L.; Kucharek, M. Investigation of the Physical Properties, Antioxidant and Antimicrobial Activity of Ternary Potato Starch-Furcellaran-Gelatin Films Incorporated with Lavender Essential Oil. Int. J. Biol. Macromol. 2018, 114, 1094–1101. [Google Scholar] [CrossRef]
- Madigan, M.T.; Martinko, J.M.; Bender, K.S.; Buckley, D.H.; Stahl, D. A Microbiologia de Brock, 14th ed.; Artmed: Porto Alegre, Brazil, 2016; p. 960. [Google Scholar]
- Tongnuanchan, P.; Benjakul, S. Essential Oils: Extraction, Bioactivities, and Their Uses for Food Preservation. J. Food Sci. 2014, 79, R1231–R1249. [Google Scholar] [CrossRef]
- Freitas, S.F.; Shinohara, L.; Sforcin, J.M.; Guimarães, S. In Vitro Effects of Propolis on Giardia duodenalis Trophozoites. Phytomedicine 2006, 13, 170–175. [Google Scholar] [CrossRef]
- Gekker, G.; Hu, S.; Spivak, M.; Lokensgard, J.R.; Peterson, P.K. Anti-HIV-1 Activity of Propolis in CD4+ Lymphocyte and Microglial Cell Cultures. J. Ethnopharmacol. 2005, 102, 158–163. [Google Scholar] [CrossRef]
- Torlak, E.; Sert, D. Antibacterial Effectiveness of Chitosan–Propolis Coated Polypropylene Films against Foodborne Pathogens. Int. J. Biol. Macromol. 2013, 60, 52–55. [Google Scholar] [CrossRef] [PubMed]
- Tosi, E.A.; Ré, E.; Ortega, M.E.; Cazzoli, A.F. Food Preservative Based on Propolis: Bacteriostatic Activity of Propolis Polyphenols and Flavonoids upon Escherichia Coli. Food Chem. 2007, 104, 1025–1029. [Google Scholar] [CrossRef]
- Packer, J.F.; Luz, M.M.S.D. Método Para Avaliação e Pesquisa Da Atividade Antimicrobiana de Produtos de Origem Natural. Rev. Bras. Farmacogn. 2007, 17, 102–107. [Google Scholar] [CrossRef]
- Nakai, S.A.; Siebert, K.J. Organic Acid Inhibition Models for Listeria innocua, Listeria ivanovii, Pseudomonas aeruginosa and Oenococcus oeni. Food Microbiol. 2004, 21, 67–72. [Google Scholar] [CrossRef]
- Alves, V.D.; Mali, S.; Beléia, A.; Grossmann, M.V.E. Effect of Glycerol and Amylose Enrichment on Cassava Starch Film Properties. J. Food Eng. 2007, 78, 941–946. [Google Scholar] [CrossRef]
- Gheribi, R.; Puchot, L.; Verge, P.; Jaoued-Grayaa, N.; Mezni, M.; Habibi, Y.; Khwaldia, K. Development of Plasticized Edible Films from Opuntia Ficus-Indica Mucilage: A Comparative Study of Various Polyol Plasticizers. Carbohydr. Polym. 2018, 190, 204–211. [Google Scholar] [CrossRef]
- Bertuzzi, M.A.; Castro Vidaurre, E.F.; Armada, M.; Gottifredi, J.C. Water Vapor Permeability of Edible Starch Based Films. J. Food Eng. 2007, 80, 972–978. [Google Scholar] [CrossRef]
- Villalobos, R.; Chanona, J.; Hernández, P.; Gutiérrez, G.; Chiralt, A. Gloss and Transparency of Hydroxypropyl Methylcellulose Films Containing Surfactants as Affected by Their Microstructure. Food Hydrocoll. 2005, 19, 53–61. [Google Scholar] [CrossRef]
- Silva, G.G.D.; Sobral, P.J.A.; Carvalho, R.A.; Bergo, P.V.A.; Mendieta-Taboada, O.; Habitante, A.M.Q.B. Biodegradable Films Based on Blends of Gelatin and Poly (Vinyl Alcohol): Effect of PVA Type or Concentration on Some Physical Properties of Films. J. Polym. Environ. 2008, 16, 276–285. [Google Scholar] [CrossRef]
- Chen, K.; Jiang, J.; Tian, R.; Kuang, Y.; Wu, K.; Xiao, M.; Liu, Y.; Qian, H.; Jiang, F. Properties of Konjac Glucomannan/Curdlan-Based Emulsion Films Incorporating Camellia Oil and the Preservation Effect as Coatings on ‘Kyoho’ Grapes. Int. J. Biol. Macromol. 2024, 258, 128836. [Google Scholar] [CrossRef]
- Duan, J.; Wu, R.; Strik, B.C.; Zhao, Y. Effect of Edible Coatings on the Quality of Fresh Blueberries (Duke and Elliott) under Commercial Storage Conditions. Postharvest Biol. Technol. 2011, 59, 71–79. [Google Scholar] [CrossRef]
- Salehi, F. Edible Coating of Fruits and Vegetables Using Natural Gums: A Review. Int. J. Fruit Sci. 2020, 20, S570–S589. [Google Scholar] [CrossRef]
- Fakhouri, F.M.; Martelli, S.M.; Caon, T.; Velasco, J.I.; Mei, L.H.I. Edible Films and Coatings Based on Starch/Gelatin: Film Properties and Effect of Coatings on Quality of Refrigerated Red Crimson Grapes. Postharvest Biol. Technol. 2015, 109, 57–64. [Google Scholar] [CrossRef]
- De Souza, W.F.C.; De Lucena, F.A.; Da Silva, K.G.; Martins, L.P.; De Castro, R.J.S.; Sato, H.H. Influence of Edible Coatings Composed of Alginate, Galactomannans, Cashew Gum, and Gelatin on the Shelf- Life of Grape Cultivar ‘Italia’: Physicochemical and Bioactive Properties. LWT 2021, 152, 112315. [Google Scholar] [CrossRef]
- Pastor, C.; Sánchez-González, L.; Marcilla, A.; Chiralt, A.; Cháfer, M.; González-Martínez, C. Quality and Safety of Table Grapes Coated with Hydroxypropylmethylcellulose Edible Coatings Containing Propolis Extract. Postharvest Biol. Technol. 2011, 60, 64–70. [Google Scholar] [CrossRef]
- Chitarra, M.I.F.; Chitarra, A.B. Pós-Colheita de Frutas e Hortaliças: Fisiologia e Manuseio, 2nd ed.; Editora UFLA: Lavras, Brazil, 2005. [Google Scholar]
- Baxter, C.J.; Carrari, F.; Bauke, A.; Overy, S.; Hill, S.A.; Quick, P.W.; Fernie, A.R.; Sweetlove, L.J. Fruit Carbohydrate Metabolism in an Introgression Line of Tomato with Increased Fruit Soluble Solids. Plant Cell Physiol. 2005, 46, 425–437. [Google Scholar] [CrossRef]
- Rodrigues, D.P.; Mitterer-Daltoé, M.L.; Lima, V.A.D.; Barreto-Rodrigues, M.; Pereira, E.A. Simultaneous Determination of Organic Acids and Sugars in Fruit Juices by High Performance Liquid Chromatography: Characterization and Differentiation of Commercial Juices by Principal Component Analysis. Cienc. Rural 2021, 51, e20200629. [Google Scholar] [CrossRef]
- Ribeiro, T.P.; Lima, M.A.C.D.; Alves, R.E.; Gonçalves, A.L.D.S.; Souza, A.P.C. Chemical Characterization of Winemaking Byproducts from Grape Varieties Cultivated in Vale Do São Francisco, Brazil. Food Sci. Technol. 2018, 38, 577–583. [Google Scholar] [CrossRef]
- Qi, C.-Y.; Chi, Z.; Liu, G.-L.; Wang, P.; Chi, Z.-M. A New High Molecular Weight Polymalate Coating Film on Grape. Ind. Crops Prod. 2023, 202, 116994. [Google Scholar] [CrossRef]
- Teixeira, E.; Meinert, E.M.; Barbetta, P.A. Análise Sensorial de Alimentos; UFSC: Florianópolis, Brazil, 1987. [Google Scholar]






| Peak | R. Time | Area | Area (%) | Name |
|---|---|---|---|---|
| 1 | 25.854 | 5369065 | 7.23 | Copaene |
| 2 | 27.529 | 1129858 | 1.52 | Bicyclo[3.1.1]hept-2-ene, 2,6-dimethyl-6-(4-methyl-3-pentenyl)- |
| 3 | 31.356 | 867710 | 1.17 | .beta.-Bisabolene |
| 4 | 31.900 | 1126380 | 1.52 | Naphthalene, 1,2,3,5,6,8a-hexahydro-4,7-dimethyl-1-(1-methylethyl)-, (1S-cis)- |
| 5 | 42.392 | 1261623 | 1.70 | Squalene |
| 6 | 48.530 | 1007511 | 1.36 | Hexadecanoic acid, ethyl ester |
| 7 | 64.373 | 1637516 | 2.20 | Medicarpin |
| 8 | 67.982 | 2073647 | 1.67 | Phenol, 2-(3,4-dihydro-7-methoxy-2H-1-benzopyran-3-yl)-5-methoxy- |
| 9 | 69.216 | 1456579 | 2.79 | 2H-1-Benzopyran-7-ol, 3,4-dihydro-3-(2-hydroxy-4-methoxyphenyl)- |
| 10 | 70.029 | 6964543 | 1.96 | Octadecanoic acid, ethyl ester |
| 11 | 72.875 | 6964543 | 9.38 | 2,6,10,14,18-Pentamethyl-2,6,10,14,18-eicosapentaene |
| 12 | 73.095 | 4616562 | 6.21 | 2,6,10,14,18-Pentamethyl-2,6,10,14,18-eicosapentaene |
| 13 | 74.671 | 4507181 | 6.07 | 2,6,10,14,18-Pentamethyl-2,6,10,14,18-eicoosapentaene |
| 14 | 81.393 | 2096977 | 2.82 | 4,4,6a,6b,8a,11,11,14b-Octamethyl-1,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,14,14a,14b-octadecahydro-2H-picen-3-one |
| 15 | 82.102 | 12326129 | 16.59 | 4,4,6a,6b,8a,11,11,14b-Octamethyl-1,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,14,14a,14b-octadecahydro-2H-picen-3-one |
| 16 | 83.411 | 13730291 | 18.48 | Lupeol |
| 17 | 83.667 | 4208244 | 5.66 | D:B-Friedo-B’:A’-neogammacer-5-en-3-ol, |
| 18 | 85.278 | 2164620 | 2.91 | 4,4,6a,6b,8a,11,11,14b-Octamethyl-1,4,4a,5,6,6a,6b,7,8,8a,9,10,11,12,12a,14,14a,14b-octadecahydro-2H-picen-3-one |
| 19 | 86.695 | 3058562 | 4.12 | Urs-12-en-3-ol, acetate, (3.beta.)- |
| 20 | 86.871 | 3440076 | 4.63 | Lup-20(29)-en-3-ol, acetate, (3.beta.)- |
| Parameters | Control (25 °C) | Gelatine (25 °C) | Gelatine + Propolis (25 °C) | Control (5 °C) | Gelatine = (5 °C) | Gelatine + Propolis (5 °C) |
|---|---|---|---|---|---|---|
| Day 0 | ||||||
| L* | 28.89 ± 2.5 Ba* | 28.89 ± 2.5 Aa | 28.89 ± 2.5 Aa | 28.89 ± 2.5 Ba | 28.89 ± 2.5 Ba | 28.89 ± 2.5 Ba |
| a* | 6.36 ± 1.8 Aa | 6.36 ± 1.8 Aa | 6.36 ± 1.8 Aa | 6.36 ± 1.8 Aa | 6.36 ± 1.8 ABa | 6.36 ± 1.8 Aa |
| b* | 2.52 ± 1.7 Aa | 2.52 ± 1.7 Aa | 2.52 ± 1.7 Aa | 2.52 ± 1.7 Aa | 2.52 ± 1.7 Aa | 2.52 ± 1.7 Aa |
| Day 01 | ||||||
| L* | 27.27 ± 1.3 ABbc | 27.92 ± 3.5 Ac | 27.27 ± 3.1 Abc | 22.59 ± 1.4 Aab | 21.95 ± 2.0 Aa | 23.38 ± 5.6 Aabc |
| a* | 4.83 ± 2.0 Aa | 5.93 ± 1.6 Aab | 5.40 ± 1.2 Aa | 8.10 ± 1.9 Ab | 6.81 ± 1.1 Bab | 5.28 ± 1.3 Aa |
| b* | 3.68 ± 2.2 Aa | 2.00 ± 08 Aa | 2.21 ± 1.1 Aa | 3.40 ± 1.1 Aa | 2.77 ± 0.7 Aa | 2.95 ± 0.9 Aa |
| Day 04 | ||||||
| L* | 25.53 ± 5.4 Aba | 27.87 ± 4.6 Aa | 27.35 ± 2.7 Aa | 28.47 ± 1.0 Ba | 28.27 ± 2.6 Ba | 27.96 ± 1.8 ABa |
| a* | 6.19 ± 2.1 Aab | 5.83 ± 1.6 Aab | 5.00 ± 0.76 Aab | 6.46 ± 1.0 Ab | 5.42 ± 0.7 ABab | 4.39 ± 0.7 Aa |
| b* | 2.08 ± 0.7 Aa | 2.11 ± 1.0 Aa | 2.31 ± 0.6 Aa | 2.58 ± 0.7 a | 1.96 ± 0.7 Aa | 1.74 ± 0.5 Aa |
| Day 10 | ||||||
| L* | 26.10 ± 2.9 ABab | 27.69 ± 1.6 Abc | 29.71 ± 1.3 Ac | 23.69 ± 1.1 Aa | 24.94 ± 2.2 Aab | 25.76 ± 1.8 ABab |
| a* | 4.87 ± 1.2 Aa | 5.77 ± 1.3 Aa | 5.79 ± 1.3 Aa | 7.10 ± 2.0 Aa | 5.47 ± 1.3 ABa | 4.86 ± 1.6 Aa |
| b* | 1.94 ± 0.8 Aa | 2.10 ± 0.8 Aa | 2.43 ± 0.8 Aa | 3.13 ± 1.3 Aa | 2.09 ± 0.6 Aa | 2.38 ± 1.2 Aa |
| Day 15 | ||||||
| L* | 22.26 ± 4.0 Aa | 28.07 ± 1.4 Ab | 27.85 ± 3.8 Ab | 22.06 ± 1.2 Aa | 24.20 ± 1.7 Aab | 26.31 ± 1.3 ABb |
| a* | 5.07 ± 2.3 Aa | 5.99 ± 1.4 Aa | 5.85 ± 0.9 Aa | 6.86 ± 1.2 Aa | 4.77 ± 0.7 Aa | 4.95 ± 1.8 Aa |
| b* | 2.40 ± 1.8 Aa | 2.35 ± 0.6 Aa | 2.82 ± 0.7 Aa | 3.15 ± 1.0 Aa | 1.90 ± 0.3 Aa | 2.97 ± 2.3 Aa |
| Day 19 | ||||||
| L* | 24.80 ± 3.9 ABab | 27.46 ± 3.0 Ab | 25.75 ± 2.9 Aab | 21.80 ± 1.9 Aa | 22.49 ± 1.7 Aa | 24.27 ± 1.6 ABab |
| a* | 5.42 ± 1.6 Aab | 5.90 ± 1.6 Aab | 5.45 ± 1.1 Aab | 7.11 ± 1.6 Ab | 5.28 ± 1.4 ABab | 4.58 ± 1.6 Aa |
| b* | 2.34 ± 1.0 Aa | 2.40 ± 0.7 Aa | 2.53 ± 0.7 Aa | 3.40 ± 1.2 Aa | 2.28 ± 0.9 Aa | 2.11 ± 1.0 Aa |
| Day 25 | ||||||
| L* | - | - | - | 23.09 ± 1.1 Aa | 24.17 ± 2.1 Aa | 23.03 ± 4.7 Aa |
| a* | - | - | - | 6.97 ± 1.5 Aa | 4.90 ± 1.4 ABa | 5.27 ± 1.7 Aa |
| b* | - | - | - | 2.97 ± 1.5 Aa | 2.27 ± 0.5 Aa | 3.50 ± 2.5 Aa |
| Formulations | pH (Decimal) | Titratable Total Acidity in Citric Acid (g 100 mL−1) | Total Soluble Solids (°Bx) | Total Solids (%) |
|---|---|---|---|---|
| Day 0 | ||||
| Control (25 °C) | 3.38 ± 0.0 Ba* | 0.66 ± 0.0 Aa | 19.00 ± 0.0 Aa | 19.72 ± 0.1 Aa |
| Gelatine (25 °C) | 3.38 ± 0.0 Aa | 0.66 ± 0.0 ABa | 19.00 ± 0.0 Ba | 19.72 ± 0.1 Ba |
| Gelatine + Propolis (25 °C) | 3.38 ± 0.0 Aa | 0.66 ± 0.0 ABa | 19.00 ± 0.0 Ca | 19.72 ± 0.1 Ba |
| Control (5 °C) | 3.38 ± 0.0 Ba | 0.66 ± 0.0 Aa | 19.00 ± 0.0 ABa | 19.72 ± 0.1 ABa |
| Gelatine (5 °C) | 3.38 ± 0.0 ABa | 0.66 ± 0.0 Aa | 19.00 ± 0.0 Aa | 19.72 ± 0.1 Aa |
| Gelatine + Propolis (5 °C) | 3.38 ± 0.0 Aa | 0.66 ± 0.0 Aa | 19.00 ± 0.0 Ba | 19.72 ± 0.1 Ba |
| Day 01 | ||||
| Control (25 °C) | 3.63 ± 0.0 DEab | 0.69 ± 0.0 Abc | 18.95 ± 0.0 Ab | 19.55 ± 0.2 Aab |
| Gelatine (25 °C) | 3.71 ± 0.0 Cbc | 0.75 ± 0.0 Bd | 19.00 ± 0.0 Bb | 19.43 ± 0.3 Bab |
| Gelatine + Propolis (25 °C) | 3.76 ± 0.1 Ec | 0.67 ± 0.0 ABCab | 18.20 ± 0.0 Ba | 18.79 ± 0.3 Aba |
| Control (5 °C) | 3.55 ± 0.0 Da | 0.70 ± 0.0 ABc | 19.00 ± 0.6 ABb | 19.96 ± 0.3 BCbc |
| Gelatine (5 °C) | 3.60 ± 0.0 Ea | 0.69 ± 0.0 Aabc | 19.50 ± 0.0 Bc | 21.27 ± 0.6 Ad |
| Gelatine + Propolis (5 °C) | 3.58 ± 0.0 Ca | 0.66 ± 0.0 Aa | 19.50 ± 0.0 Cc | 20.67 ± 0.1 Bcd |
| Day 04 | ||||
| Control (25 °C) | 3.50 ± 0.0 Cbc | 0.66 ± 0.0 Aa | 21.50 ± 0.0 Ca | 19.71 ± 0.5 Ab |
| Gelatine (25 °C) | 3.37 ± 0.0 Aa | 0.74 ± 0.0 Bb | 19.00 ± 0.0 Bb | 17.04 ± 0.5 Aa |
| Gelatine + Propolis (25 °C) | 3.50 ± 0.0 BCbc | 0.65 ± 0.0 AB | 19.95 ± 0.0 Dc | 17.89 ± 0.5 Aab |
| Control (5 °C) | 3.51 ± 0.0 Dc | 0.65 ± 0.0 Aa | 20.00 ± 0.0 BCc | 18.22 ± 0.3 Aab |
| Gelatine (5 °C) | 3.47 ± 0.0 CDb | 0.67 ± 0.0 Aa | 19.00 ± 0.0 Ab | 17.00 ± 0.8 Aa |
| Gelatine + Propolis (5 °C) | 3.40 ± 0.0 Aa | 0.67 ± 0.0 Aa | 18.00 ± 0.0 Aa | 16.29 ± 1.2 Aa |
| Day 10 | ||||
| Control (25 °C) | 3.55 ± 0.0 CDb | 0.68 ± 0.1 Aab | 21.00 ± 0.0 Bd | 22.24 ± 0.4 Ba |
| Gelatine (25 °C) | 3.55 ± 0.0 Bb | 0.63 ± 0.1 Aa | 21.50 ± 0.0 Ce | 22.73 ± 0.1 Da |
| Gelatine + Propolis (25 °C) | 3.53 ± 0.0 Cb | 0.61 ± 0.0 Aa | 21.00 ± 0.0 Ed | 22.13 ± 0.0 Ca |
| Control (5 °C) | 3.51 ± 0.0 Dab | 0.86 ± 0.1 Cc | 18.00 ± 0.0 Aa | 19.04 ± 0.1 Aba |
| Gelatine (5 °C) | 3.46 ± 0.0 BCDa | 0.80 ± 0.0 Bbc | 19.50 ± 0.0 Bb | 25.40 ± 9.0 Aa |
| Gelatine + Propolis (5 °C) | 3.52 ± 0.0 BCab | 0.63 ± 0.0 Aa | 20.50 ± 0.0 Ec | 20.56 ± 0.3 Ba |
| Day 15 | ||||
| Control (25 °C) | 3.70 ± 0.0 Eb | 0.67 ± 0.0 Aaab | 21.00 ± 0.0 Bb | 19.46 ± 0.2 Aa |
| Gelatine (25 °C) | 3.73 ± 0.0 Cb | 0.60 ± 0.0 Aa | 22.00 ± 0.0 Dc | 20.88 ± 0.4 Ca |
| Gelatine + Propolis (25 °C) | 3.65 ± 0.0 Db | 0.73 ± 0.0 BCb | 21.00 ± 0.0 Eb | 19.70 ± 1.0 Ba |
| Control (5 °C) | 3.45 ± 0.0 Ca | 0.69 ± 0.0 Aab | 20.00 ± 0.0 BCa | 20.28 ± 0.2 BCa |
| Gelatine (5 °C) | 3.50 ± 0.0 Da | 0.72 ± 0.0 Ab | 20.00 ± 0.0 Ca | 19.44 ± 0.3 Aa |
| Gelatine + Propolis (5 °C) | 3.50 ± 0.0 Ba | 0.68 ± 0.0 Aab | 20.00 ± 0.0 Da | 19.68 ± 0.2 Ba |
| Day 19 | ||||
| Control (25 °C) | 3.26 ± 0.0 Aa | 0.94 ± 0.0 Bc | 19.00 ± 0.0 Ac | 25.55 ± 0.6 Ce |
| Gelatine (25 °C) | 3.55 ± 0.0 Bd | 0.86 ± 0.0 Cbc | 18.00 ± 0.0 Ab | 23.84 ± 0.3 Ed |
| Gelatine + Propolis (25 °C) | 3.44 ± 0.0 ABc | 0.75 ± 0.0 Cab | 17.50 ± 0.0 Aa | 22.92 ± 0.4 Ccd |
| Control (5 °C) | 3.28 ± 0.0 Aa | 0.84 ± 0.0 Cabc | 21.00 ± 0.0 Cd | 21.39 ± 0.8 Cbc |
| Gelatine (5 °C) | 3.36 ± 0.0 Ab | 0.72 ± 0.0 ABa | 19.00 ± 0.0 Ac | 19.17 ± 0.3 Aa |
| Gelatine + Propolis (5 °C) | 3.36 ± 0.0 Ab | 0.81 ± 0.1 Bab | 19.00 ± 0.0 Bc | 20.52 ± 0.1 Bab |
| Day 25 | ||||
| Control (25 °C) | - | - | - | - |
| Gelatine (25 °C) | - | - | - | - |
| Gelatine + Propolis (25 °C) | - | - | - | - |
| Control (5 °C) | 3.29 ± 0.0 Aa | 0.83 ± 0.0 BCb | 22.50 ± 0.7 Da | 20.49 ± 0.8 BCa |
| Gelatine (5 °C) | 3.41 ± 0.0 ABCb | 0.67 ± 0.0 Aa | 22.50 ± 0.0 Da | 20.51 ± 0.3 Aa |
| Gelatine + Propolis (5 °C) | 3.40 ± 0.0 Ab | 0.71 ± 0. ABa | 22.00 ± 0.0 Fa | 21.92 ± 1.8 Ba |
| Sample | Global Appearance | Color | Glow | |||
|---|---|---|---|---|---|---|
| Grid Average | Acceptance (%) | Grid Average | Acceptance (%) | Grid Average | Acceptance (%) | |
| Control (5 °C) | 2.56 ± 1.5 | 42.73 | 3.22 ± 1.8 | 40.23 | 2.33 ± 1.3 | 38.79 |
| Gelatine (5 °C) | 7.07 ± 1.4 | 78.59 | 7.36 ± 1.4 | 81.52 | 7.24 ± 1.4 | 80.4 |
| Gelatine + Propolis (5 °C) | 7.45 ± 1.2 | 82.83 | 7.49 ± 1.2 | 83.23 | 7.84 ± 1.0 | 87.07 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Filgueiras, C.T.; Fakhouri, F.M.; Garcia, V.A.d.S.; Velasco, J.I.; Nogueira, G.F.; Ramos da Silva, L.; Oliveira, R.A.d. Effect of Adding Red Propolis to Edible Biodegradable Protein Films for Coating Grapes: Shelf Life and Sensory Analysis. Polymers 2024, 16, 888. https://doi.org/10.3390/polym16070888
Filgueiras CT, Fakhouri FM, Garcia VAdS, Velasco JI, Nogueira GF, Ramos da Silva L, Oliveira RAd. Effect of Adding Red Propolis to Edible Biodegradable Protein Films for Coating Grapes: Shelf Life and Sensory Analysis. Polymers. 2024; 16(7):888. https://doi.org/10.3390/polym16070888
Chicago/Turabian StyleFilgueiras, Cristina Tostes, Farayde Matta Fakhouri, Vitor Augusto dos Santos Garcia, José Ignacio Velasco, Gislaine Ferreira Nogueira, Luan Ramos da Silva, and Rafael Augustus de Oliveira. 2024. "Effect of Adding Red Propolis to Edible Biodegradable Protein Films for Coating Grapes: Shelf Life and Sensory Analysis" Polymers 16, no. 7: 888. https://doi.org/10.3390/polym16070888
APA StyleFilgueiras, C. T., Fakhouri, F. M., Garcia, V. A. d. S., Velasco, J. I., Nogueira, G. F., Ramos da Silva, L., & Oliveira, R. A. d. (2024). Effect of Adding Red Propolis to Edible Biodegradable Protein Films for Coating Grapes: Shelf Life and Sensory Analysis. Polymers, 16(7), 888. https://doi.org/10.3390/polym16070888