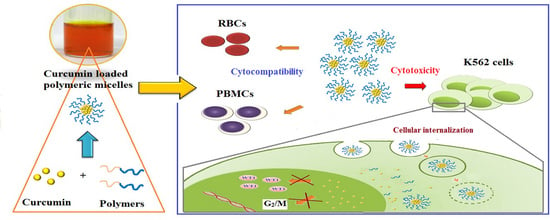
Modification of Polyethylene Glycol-Hydroxypropyl Methacrylate Polymeric Micelles Loaded with Curcumin for Cellular Internalization and Cytotoxicity to Wilms Tumor 1-Expressing Myeloblastic Leukemia K562 Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
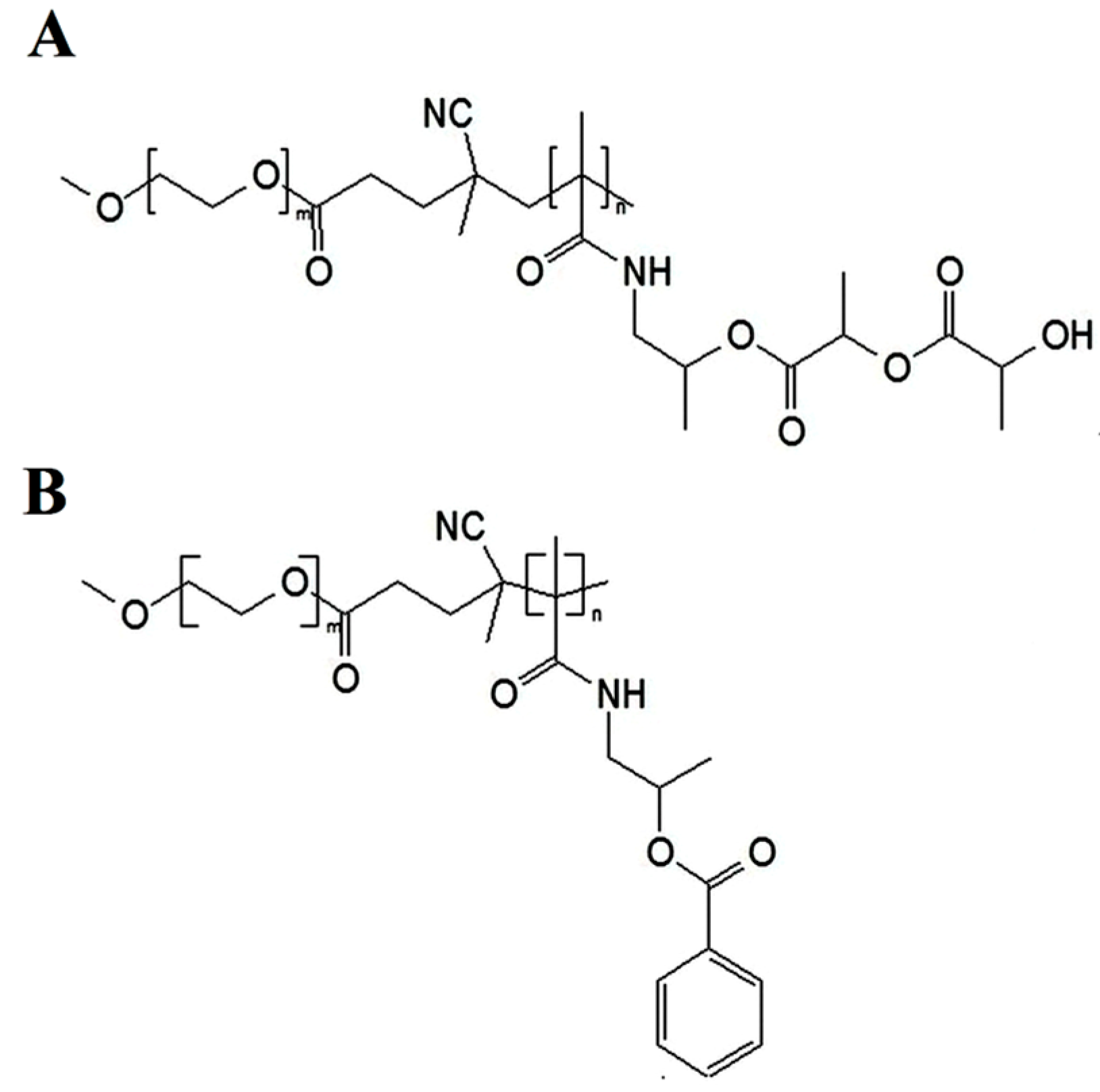
2.2. Preparation of Polymeric Micelles
2.3. Cell Preparation
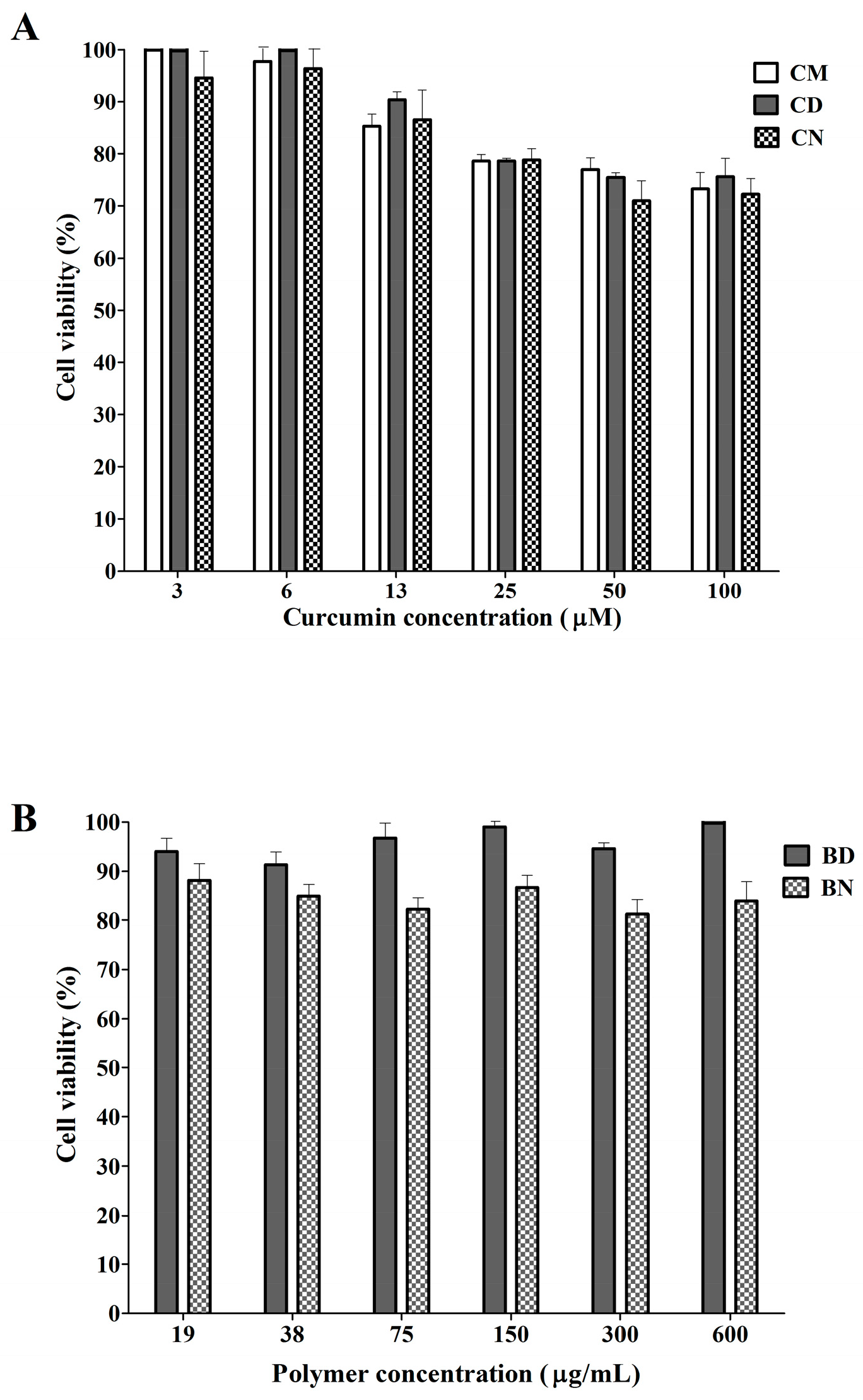
2.4. Effect of CD and CN on Normal Cells
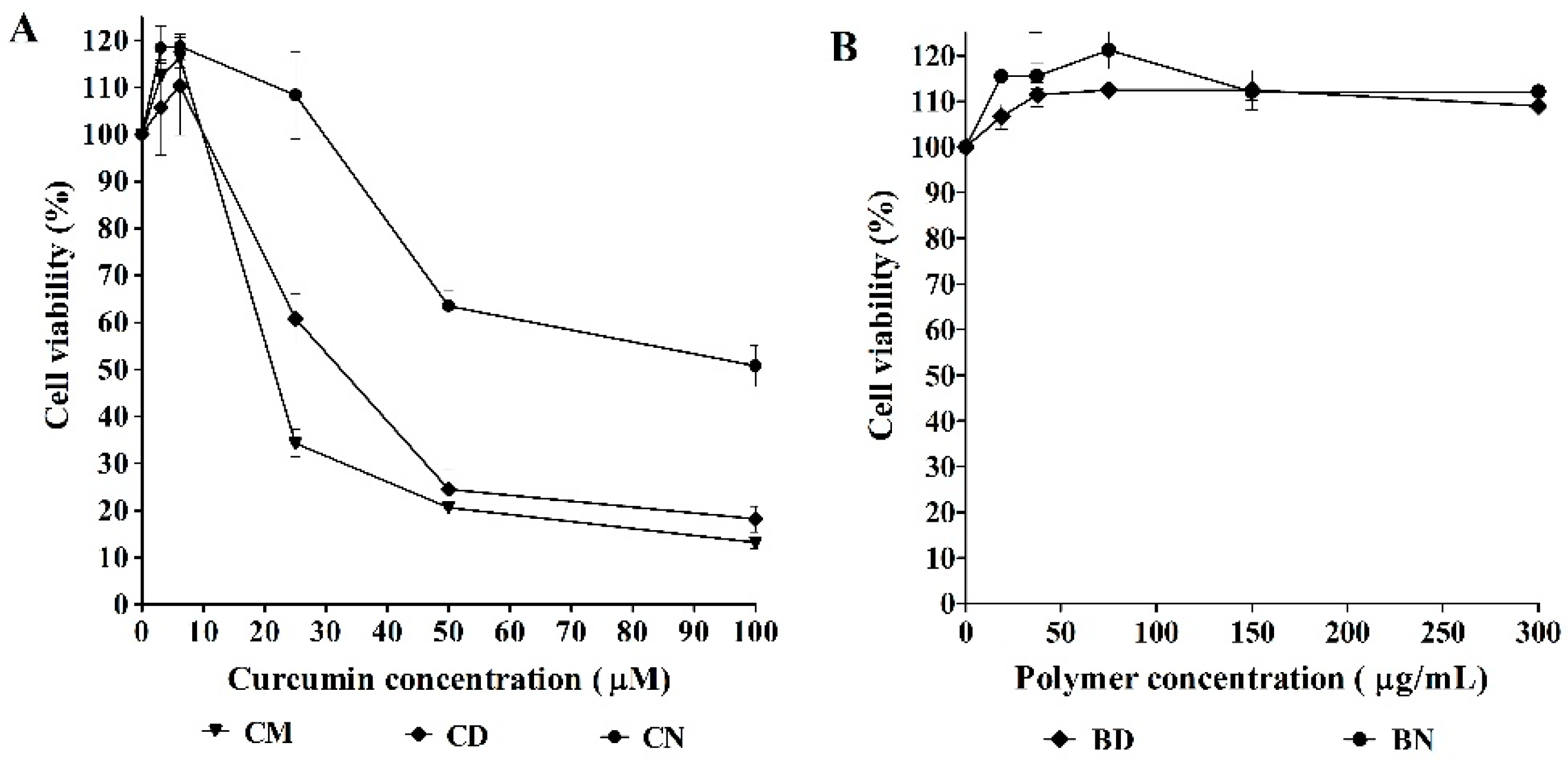
2.5. Effect of CD and CN on Leukemic Cells
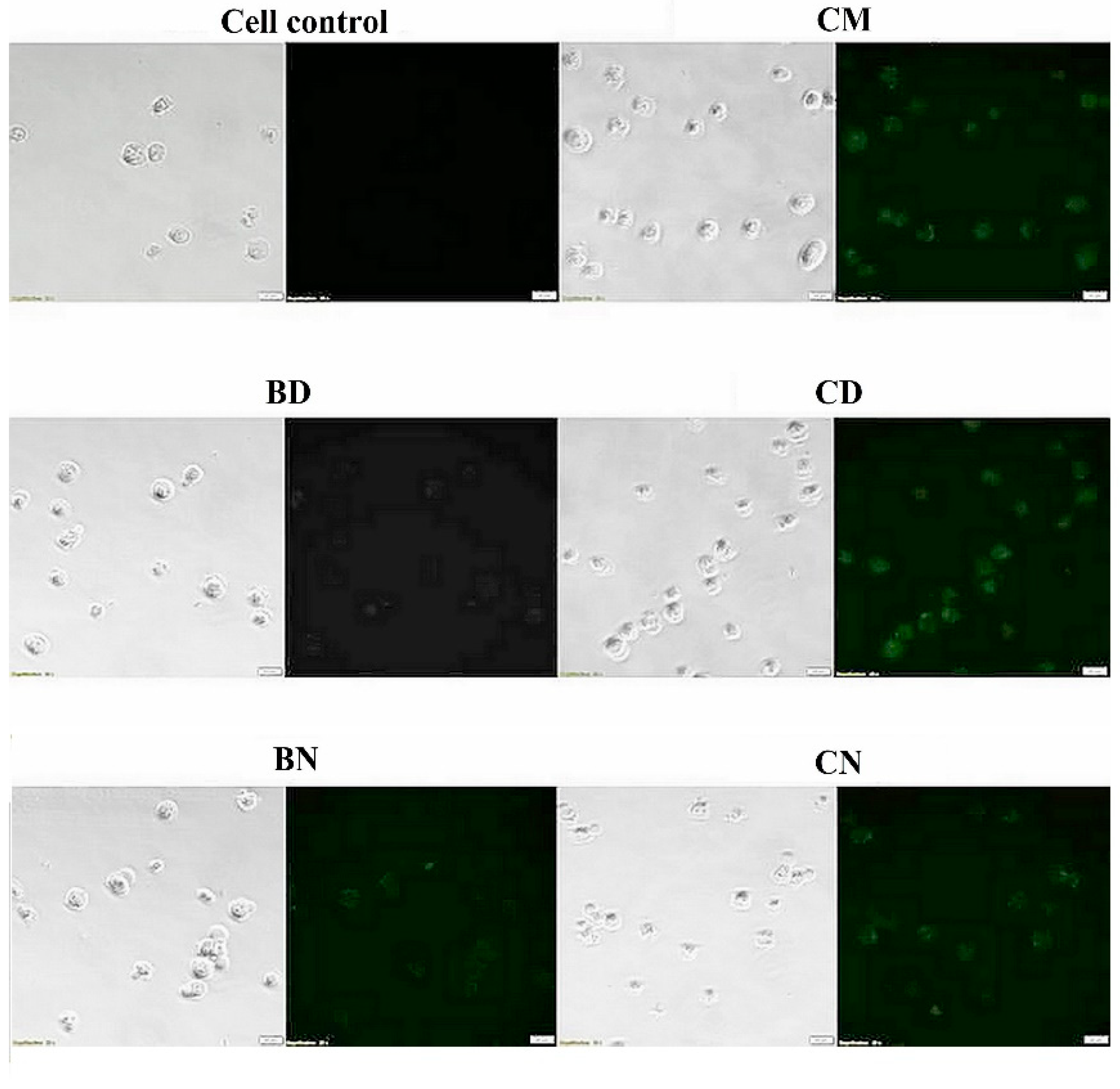
2.6. Cellular Uptake of CD and CN to Leukemic Cells
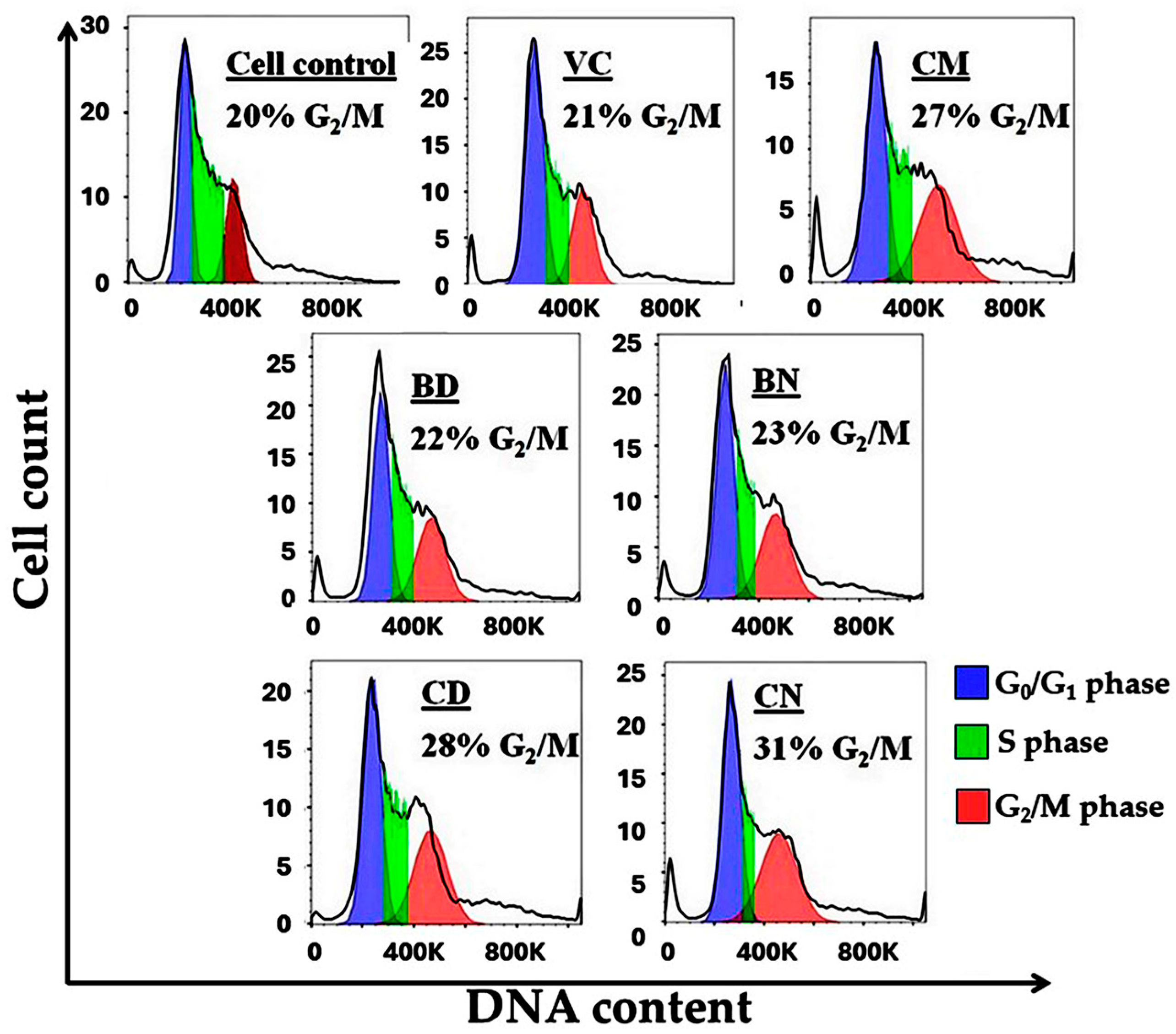
2.7. Cell-Cycle Analysis
2.8. Effect of CD and CN on WT1 Protein Suppression
2.9. Statistical Analysis
3. Results and Discussion
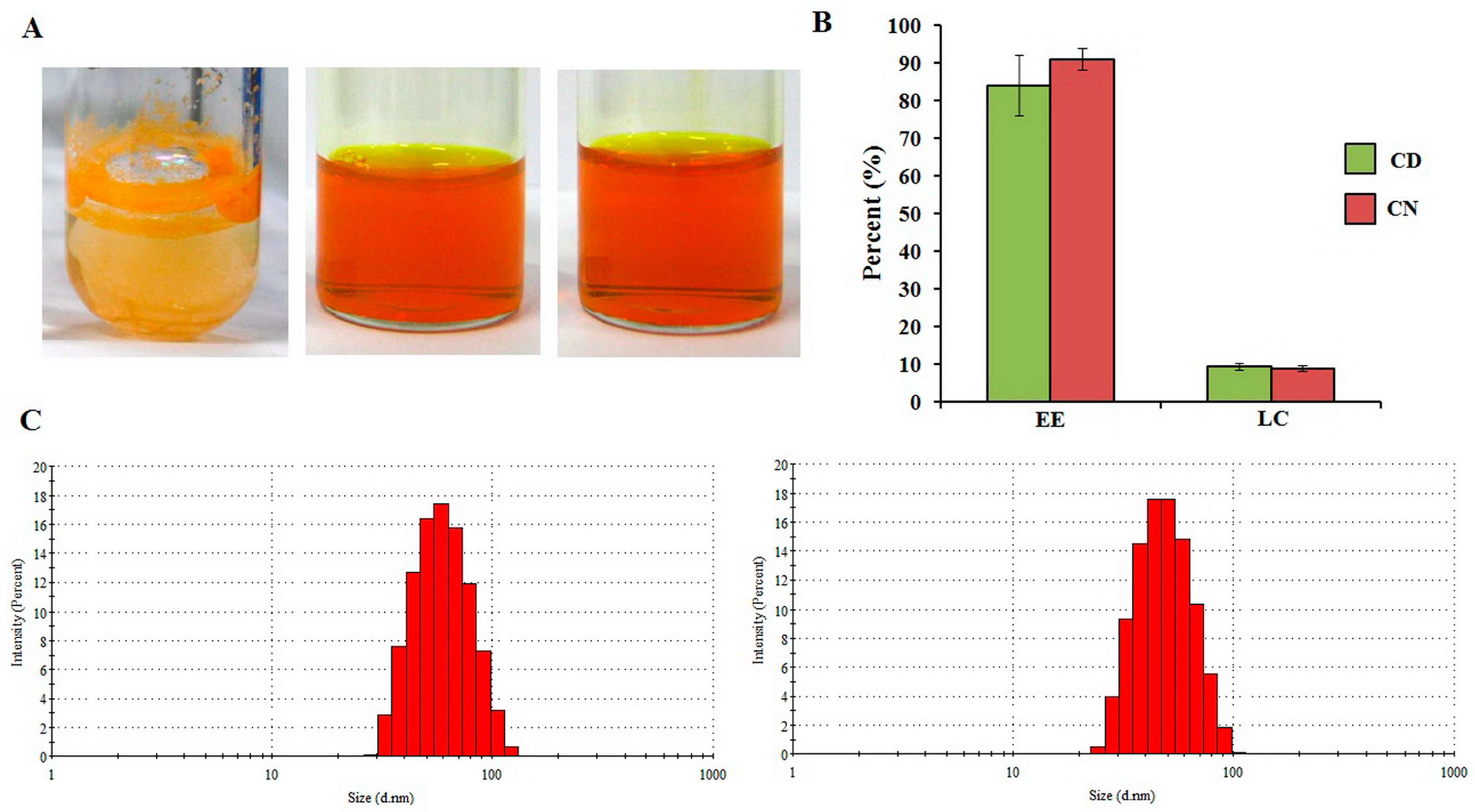
3.1. Preparation of Polymeric Micelles
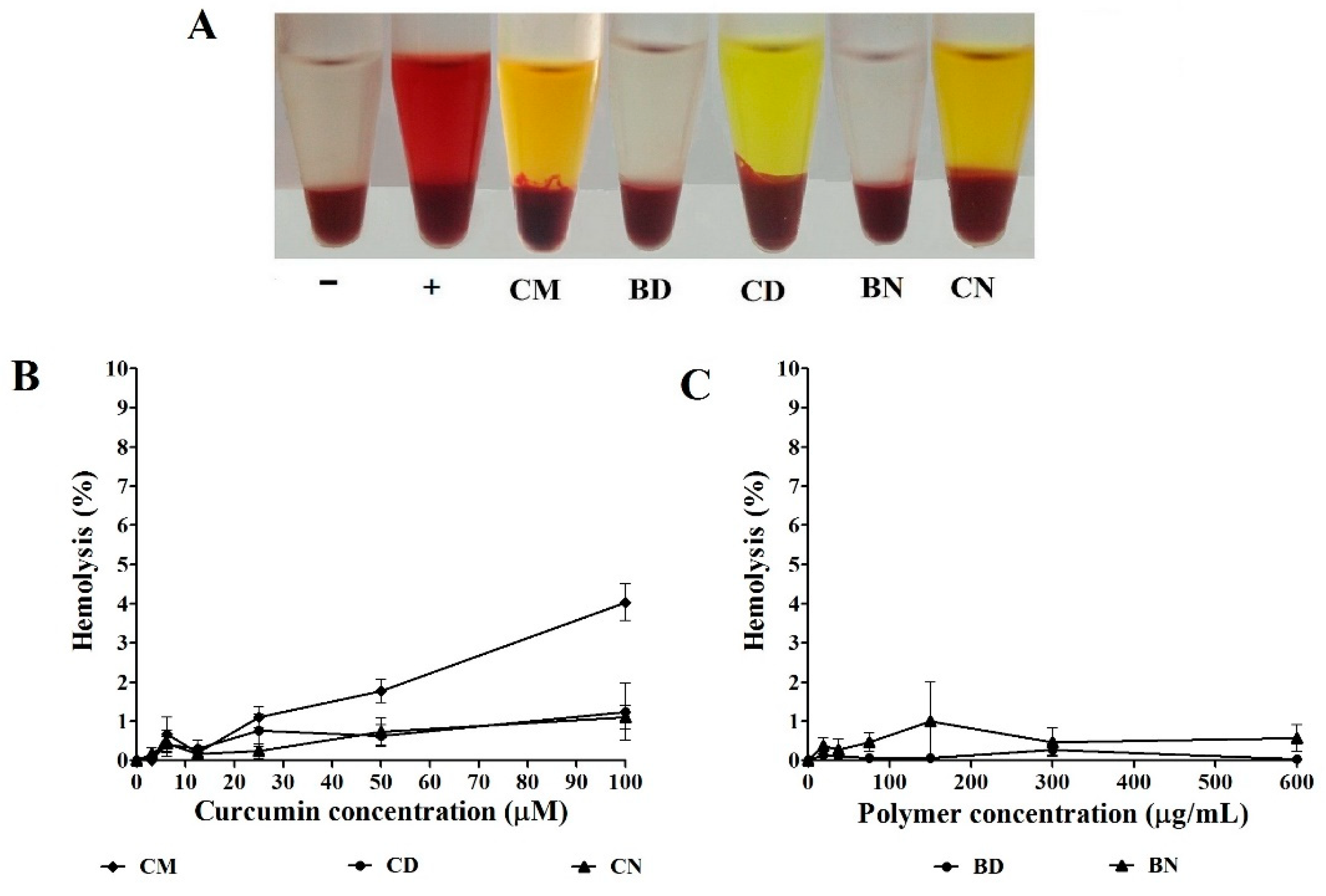
3.2. Effect of CD and CN on Normal Cells
3.3. Effect of CD and CN on Leukemic Cells
3.4. Cellular Uptake of CD and CN to Leukemic Cells
3.5. Cell-Cycle Analysis
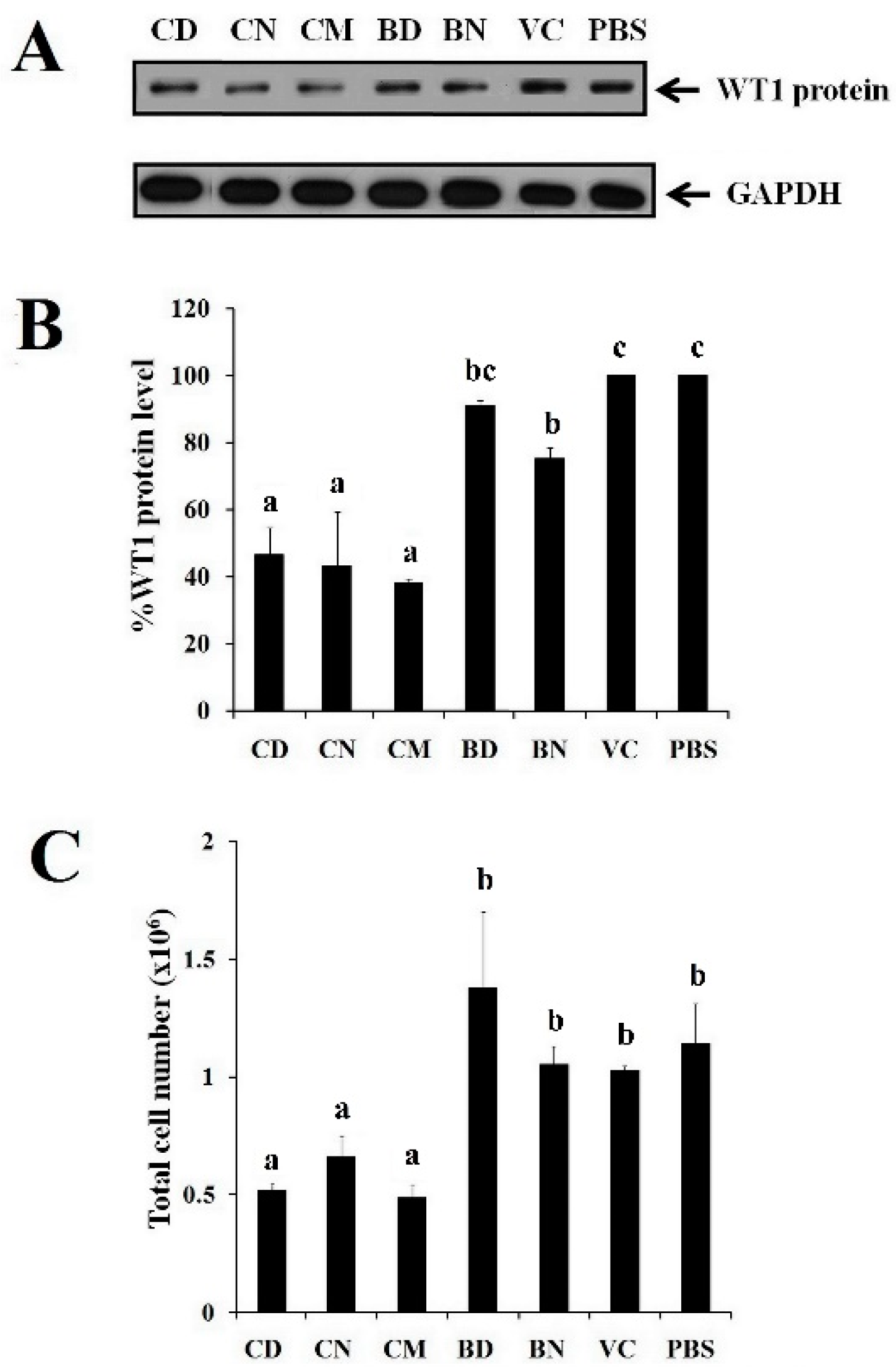
3.6. Effect of CD and CN on WT1 Protein Suppression
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 2016, 66, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Lv, X.; Hua, F.; Lin, H.; Sun, W.; Cao, W.; Fu, X.; Xie, J.; Yu, J.; Li, Z. Targeting acute myeloid leukemia with a proapoptotic peptide conjugated to a toll-like receptor 2-mediated cell-penetrating peptide. Int. J. Cancer 2014, 134, 692–702. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Oo Khor, T.; Shu, L.; Su, Z.Y.; Fuentes, F.; Lee, J.H.; Tony Kong, A.N. Plants vs. cancer: A review on natural phytochemicals in preventing and treating cancers and their druggability. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem. Agents) 2012, 12, 1281–1305. [Google Scholar] [CrossRef] [PubMed]
- Karimi, A.; Naeini, F.; Niazkar, H.; Tutunchi, H.; Musazadeh, V.; Mahmoodpoor, A.; Asghariazar, V.; Mobasseri, M.; Tarighat-Esfanjani, A.A. Nano-curcumin supplementation in critically ill patients with sepsis: A randomized clinical trial investigating the inflammatory biomarkers, oxidative stress indices, endothelial function, clinical outcomes and nutritional status. Food Funct. 2022, 13, 6596–6612. [Google Scholar] [CrossRef] [PubMed]
- Marton, L.T.; Pescinini-e-Salzedas, L.M.; Camargo, M.E.C.; Barbalho, S.M.; Haber, J.F.; Do, S.; Sinatora, R.V.; Detregiachi, C.R.P.; Girio, R.J.S.; Buchaim, D.V.; et al. The Effects of Curcumin on Diabetes Mellitus: A Systematic Review. Front. Endocrinol. 2021, 12, 669448. [Google Scholar] [CrossRef] [PubMed]
- Pathomwichaiwat, T.; Jinatongthai, P.; Prommasut, N.; Ampornwong, K.; Rattanavipanon, W.; Nathisuwan, S.; Thakkinstian, A. Effects of turmeric (Curcuma longa) supplementation on glucose metabolism in diabetes mellitus and metabolic syndrome: An umbrella review and updated meta-analysis. PLoS ONE 2023, 18, e0288997. [Google Scholar] [CrossRef] [PubMed]
- Pivari, F.; Mingione, A.; Brasacchio, C.; Soldati, L. Curcumin and type 2 diabetes mellitus: Prevention and treatment. Nutrients 2019, 11, 1837. [Google Scholar] [CrossRef]
- Ravindran, J.; Prasad, S.; Aggarwal, B.B. Curcumin and cancer cells: How many ways can curry kill tumor cells selectively? AAPS J. 2009, 11, 495–510. [Google Scholar] [CrossRef]
- Tima, S.; Ichikawa, H.; Ampasavate, C.; Okonogi, S.; Anuchapreeda, S. Inhibitory effect of turmeric curcuminoids on FLT3 expression and cell cycle arrest in the FLT3-overexpressing EoL-1 leukemic cell line. J. Nat. Prod. 2014, 77, 948–954. [Google Scholar] [CrossRef]
- Kurien, B.T.; Singh, A.; Matsumoto, H.; Scofield, R.H. Improving the solubility and pharmacological efficacy of curcumin by heat treatment. Assay Drug Dev. Technol. 2007, 5, 567–576. [Google Scholar] [CrossRef]
- Yang, K.Y.; Lin, L.C.; Tseng, T.Y.; Wang, S.C.; Tsai, T.H. Oral bioavailability of curcumin in rat and the herbal analysis from Curcuma longa by LC–MS/MS. J. Chromatogr. B 2007, 853, 183–189. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.L.; Hsu, C.H.; Lin, J.K.; Hsu, M.M.; Ho, Y.F.; She, T.S.; Ko, J.Y.; Lin, J.T.; Lin, B.R.; Wu, M.S.; et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001, 21, e2900. [Google Scholar]
- Khonkarn, R.; Mankhetkorn, S.; Hennink, W.E.; Okonogi, S. PEG-OCL micelles for quercetin solubilization and inhibition of cancer cell growth. Eur. J. Pharm. Biopharm. 2011, 79, 268–275. [Google Scholar] [CrossRef] [PubMed]
- Vauthey, S.; Santoso, S.; Gong, H.; Watson, N.; Zhang, S. Molecular self-assembly of surfactant-like peptides to form nanotubes and nanovesicles. Proc. Natl. Acad. Sci. USA 2002, 99, 5355–5360. [Google Scholar] [CrossRef] [PubMed]
- Motornov, M.; Roiter, Y.; Tokarev, I.; Minko, S. Stimuli-responsive nanoparticles, nanogels and capsules for integrated multifunctional intelligent systems. Prog. Polym. Sci. 2010, 35, 174–211. [Google Scholar] [CrossRef]
- Zheng, Y.; Oz, Y.; Gu, Y.; Ahamad, N.; Shariati, K.; Chevalier, J.; Kapur, D.; Annabi, N. Rational design of polymeric micelles for targeted therapeutic delivery. Nano Today 2024, 55, 102147. [Google Scholar] [CrossRef]
- Cui, C.; Yu, P.; Wu, M.; Zhang, Y.; Liu, L.; Wu, B.; Wang, C.X.; Zhuo, R.X.; Huang, S.W. Reduction-sensitive micelles with sheddable PEG shells self-assembled from a Y-shaped amphiphilic polymer for intracellular doxorubicine release. Colloids Surf. B Biointerfaces 2015, 129, 137–145. [Google Scholar] [CrossRef]
- Carstens, M.G.; de Jong, P.H.; van Nostrum, C.F.; Kemmink, J.; Verrijk, R.; De Leede, L.G.J.; Crommelin, D.J.A.; Hennink, W.E. The effect of core composition in biodegradable oligomeric micelles as taxane formulations. Eur. J. Pharm. Biopharm. 2008, 68, 596–606. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Liu, Y.; Feng, L.; Liu, F.; Zhang, L.; Zhang, N. Polymeric complex micelles with double drug-loading strategies for folate-mediated paclitaxel delivery. Colloids Surf. B Biointerfaces 2015, 131, 191–201. [Google Scholar] [CrossRef]
- Cabral, H.; Kataoka, K. Progress of drug-loaded polymeric micelles into clinical studies. J. Control Release 2014, 190, 465–476. [Google Scholar] [CrossRef]
- Matsumura, Y.; Kataoka, K. Preclinical and clinical studies of anticancer agent-incorporating polymer micelles. Cancer Sci. 2009, 100, 572–579. [Google Scholar] [CrossRef]
- Oerlemans, C.; Bult, W.; Bos, M.; Storm, G.; Nijsen, J.F.W.; Hennink, W.E. Polymeric micelles in anticancer therapy: Targeting, imaging and triggered release. Pharm. Res. 2010, 27, 2569–2589. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wu, Y.; Shang, H.; Sun, X.; An, K.; Zhang, Q.; Qiao, N. Preparation of pH/Light dual-responsive biocompatible polymer micelles: Application to curcumin delivery. J. Drug Deliv. Sci. Technol. 2023, 86, 104652. [Google Scholar] [CrossRef]
- Rezaeisadat, M.; Bordbar, A.; Omidyan, R. Molecular dynamics simulation study of curcumin interaction with nano-micelle of PNIPAAm-b-PEG co-polymer as a smart efficient drug delivery system. J. Mol. Liq. 2021, 332, 115862. [Google Scholar] [CrossRef]
- Farhoudi, L.; Kesharwani, P.; Majeed, M.; Johnston, T.; Sahebkar, A. Polymeric nanomicelles of curcumin: Potential applications in cancer. Int. J. Pharm. 2022, 617, 121622. [Google Scholar] [CrossRef] [PubMed]
- Patil, S.; Choudhary, B.; Rathore, A.; Roy, K.; Mahadik, K. Enhanced oral bioavailability and anticancer activity of novel curcumin loaded mixed micelles in human lung cancer cells. Phytomedicine 2015, 22, 1103–1111. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Kopeček, J. Design of smart HPMA copolymer-based nanomedicines. J. Control Release 2016, 240, 9–23. [Google Scholar] [CrossRef] [PubMed]
- Han, M.; Kim, J.; Kim, J.; Ko, J.; Magda, J.; Han, I. Temperature-dependent transparency of poly(HPMA-co-DMA) hydrogels: Effect of synthesis parameters. Polymer 2003, 44, 4541–4546. [Google Scholar] [CrossRef]
- Patel, T.; Itoo, A.; Paul, M.; Kondapaneni, L.; Ghosh, B.; Biswas, S. Block HPMA-based pH-sensitive gemcitabine pro-drug nanoaggregates for cancer treatment. Eur. Polym. J. 2023, 186, 111843. [Google Scholar] [CrossRef]
- Soga, O.; van Nostrum, C.F.; Fens, M.; Rijcken, C.J.F.; Schiffelers, R.M.; Storm, G.; Hennink, W.E. Thermosensitive and biodegradable polymeric micelles for paclitaxel delivery. J. Control Release 2005, 103, 341–353. [Google Scholar] [CrossRef]
- Rijcken, C.J.F.; Hofman, J.-W.; van Zeeland, F.; Hennink, W.E.; van Nostrum, C.F. Photosensitiser-loaded biodegradable polymeric micelles: Preparation, characterisation and in vitro PDT efficacy. J. Control Release 2007, 124, 144–153. [Google Scholar] [CrossRef] [PubMed]
- Talelli, M.; Rijcken, C.J.F.; Lammers, T.; Seevinck, P.R.; Storm, G.; van Nostrum, C.F.; Hennink, W.E. Superparamagnetic iron oxide nanoparticles encapsulated in biodegradable thermosensitive polymeric micelles: Toward a targeted nanomedicine suitable for image-guided drug delivery. Langmuir 2009, 25, 2060–2067. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; van Steenbergen, M.J.; Teunissen, E.A.; Novo, L.; Gradmann, S.; Baldus, M.; van Nostrum, C.F.; Hennink, W.E. Π–Π stacking increases the stability and loading capacity of thermosensitive polymeric micelles for chemotherapeutic drugs. Biomacromolecules 2013, 14, 1826–1837. [Google Scholar] [CrossRef] [PubMed]
- Naksuriya, O.; Shi, Y.; Van Nostrum, C.F.; Anuchapreeda, S.; Hennink, W.E.; Okonogi, S. HPMA-based polymeric micelles for curcumin solubilization and inhibition of cancer cell growth. Eur. J. Pharm. Biopharm. 2015, 94, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, H. WT1 (Wilms’ tumor gene 1): Biology and cancer immunotherapy. Jpn. J. Clin. Oncol. 2010, 40, 377–387. [Google Scholar] [CrossRef] [PubMed]
- Sugiyama, H. Wilms Tumor Gene WT1 as a: Tumor Marker for Leukemic Blast Cells and Its Role in Leukemogenesis. In Molecular Analysis of Cancer; Humana Press: Totowa, NJ, USA, 2002. [Google Scholar]
- Sassa-deepaeng, T.; Pikulkaew, S.; Okonogi, S.; Pikulkaew, S.; Okonogi, S. Development of chrysin loaded poloxamer micelles and toxicity evaluation in fish embryos. Drug Discov. Ther. 2016, 10, 150–155. [Google Scholar] [CrossRef] [PubMed]
- Bussy, C.; Methven, L.; Kostarelos, K. Hemotoxicity of carbon nanotubes. Adv. Drug Deliv. Rev. 2013, 65, 2127–2134. [Google Scholar] [CrossRef]
- Rekha, M.R.; Sharma, C.P. Hemocompatible pullulan–polyethyleneimine conjugates for liver cell gene delivery: In vitro evaluation of cellular uptake, intracellular trafficking and transfection efficiency. Acta Biomater. 2011, 7, 370–379. [Google Scholar] [CrossRef]
- Rejinold, N.S.; Muthunarayanan, M.; Divyarani, V.V.; Sreerekha, P.R.; Chennazhi, K.P.; Nair, S.V.; Tamura, H.; Jayakumar, R. Curcumin-loaded biocompatible thermoresponsive polymeric nanoparticles for cancer drug delivery. J. Colloid Interface Sci. 2011, 360, 39–51. [Google Scholar] [CrossRef]
- Nichols, J.W.; Bae, Y.H. Odyssey of a cancer nanoparticle: From injection site to site of action. Nano Today 2012, 7, 606–618. [Google Scholar] [CrossRef]
- Sahay, G.; Alakhova, D.Y.; Kabanov, A.V. Endocytosis of nanomedicines. J. Control Release 2010, 145, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.; Yan, J.; Xu, J.; Zhou, S.; Hu, X. Novel electroactive proton-doped conducting poly(aromatic ethers) with good fluorescence properties via electropolymerization. Macromolecules 2010, 43, 4599–4608. [Google Scholar] [CrossRef]
- Wang, J.; Ma, W.; Tu, P. The mechanism of self-assembled mixed micelles in improving curcumin oral absorption: In vitro and in vivo. Colloids Surf. B Biointerfaces 2015, 133, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Al-Hussain, T.; Ali, A.; Akhtar, M. Wilms tumor: An update. Adv. Anat. Pathol 2014, 21, 166–173. [Google Scholar] [CrossRef] [PubMed]
- Anuchapreeda, S.; Rungrojsakul, M.; Tima, S.; Chiampanichayakul, S.; Krig, S. Co-activation of WT1 and AP-1 proteins on WT1 gene promoter to induce WT1 gene expression in K562 cells. Cell. Signal 2019, 53, 339–347. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Okonogi, S.; Chittasupho, C.; Sassa-deepaeng, T.; Khumpirapang, N.; Anuchpreeda, S. Modification of Polyethylene Glycol-Hydroxypropyl Methacrylate Polymeric Micelles Loaded with Curcumin for Cellular Internalization and Cytotoxicity to Wilms Tumor 1-Expressing Myeloblastic Leukemia K562 Cells. Polymers 2024, 16, 917. https://doi.org/10.3390/polym16070917
Okonogi S, Chittasupho C, Sassa-deepaeng T, Khumpirapang N, Anuchpreeda S. Modification of Polyethylene Glycol-Hydroxypropyl Methacrylate Polymeric Micelles Loaded with Curcumin for Cellular Internalization and Cytotoxicity to Wilms Tumor 1-Expressing Myeloblastic Leukemia K562 Cells. Polymers. 2024; 16(7):917. https://doi.org/10.3390/polym16070917
Chicago/Turabian StyleOkonogi, Siriporn, Chuda Chittasupho, Tanongsak Sassa-deepaeng, Nattakanwadee Khumpirapang, and Songyot Anuchpreeda. 2024. "Modification of Polyethylene Glycol-Hydroxypropyl Methacrylate Polymeric Micelles Loaded with Curcumin for Cellular Internalization and Cytotoxicity to Wilms Tumor 1-Expressing Myeloblastic Leukemia K562 Cells" Polymers 16, no. 7: 917. https://doi.org/10.3390/polym16070917
APA StyleOkonogi, S., Chittasupho, C., Sassa-deepaeng, T., Khumpirapang, N., & Anuchpreeda, S. (2024). Modification of Polyethylene Glycol-Hydroxypropyl Methacrylate Polymeric Micelles Loaded with Curcumin for Cellular Internalization and Cytotoxicity to Wilms Tumor 1-Expressing Myeloblastic Leukemia K562 Cells. Polymers, 16(7), 917. https://doi.org/10.3390/polym16070917