Integrated Heat Recovery System Based on Mixed Ionic–Electronic Conducting Membrane for Improved Solid Oxide Co-Electrolyzer Performance
Abstract
:1. Introduction
2. Theoretical Background and Model Selection
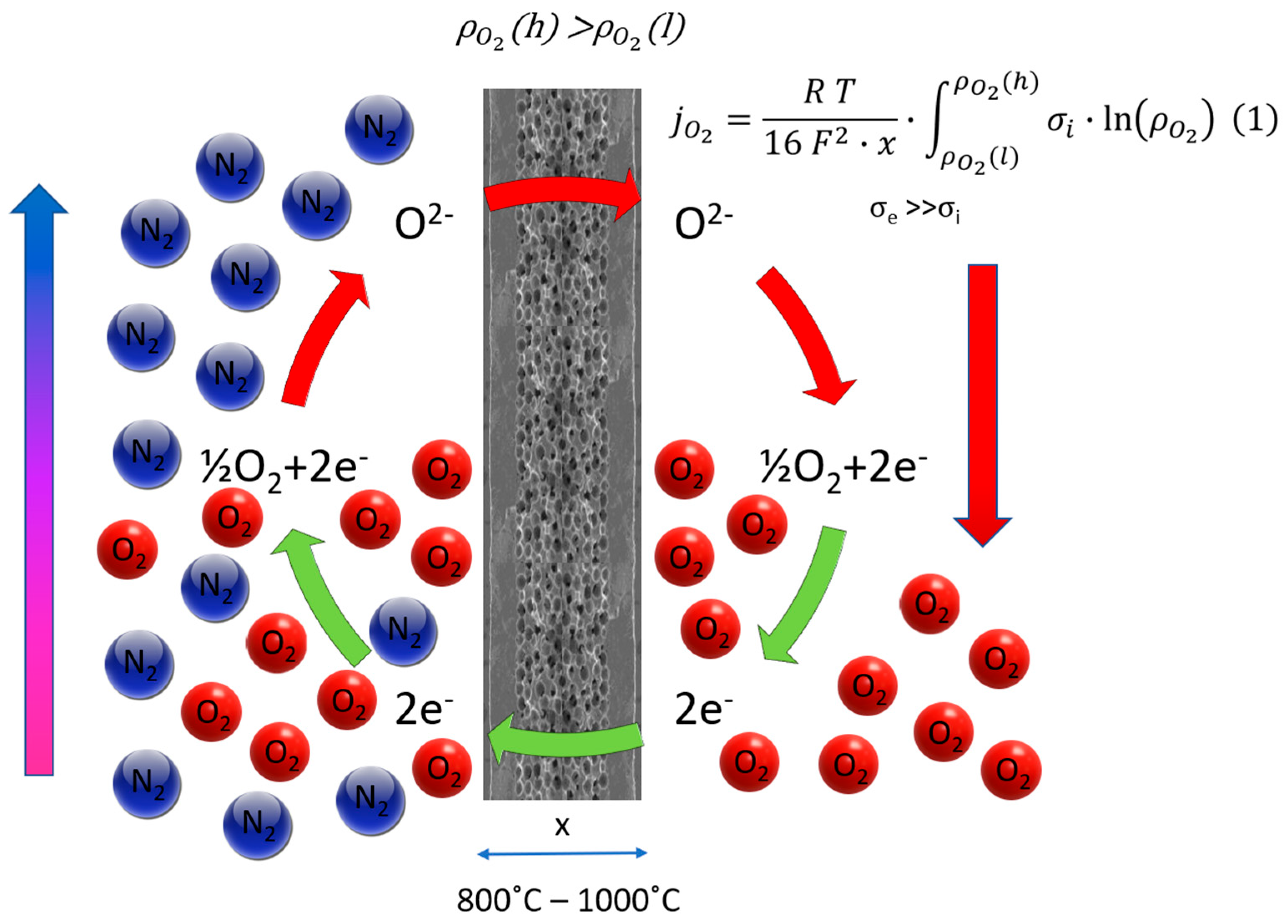
2.1. Modeling MIEC Membranes for Oxygen Separation
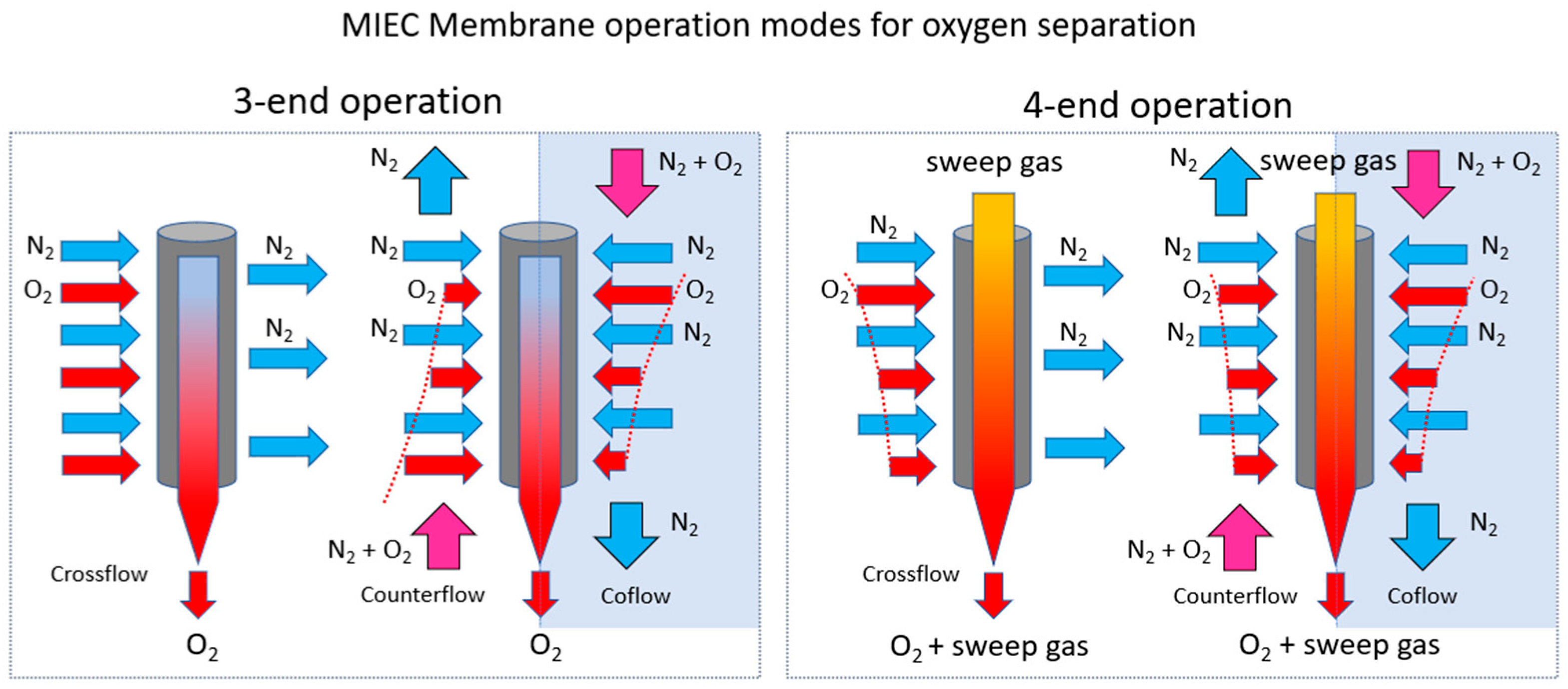
2.2. Oxygen Separation System Based on MIEC Membranes
- Reduced overpotential: The presence of N2 as a sweep gas helps in maintaining optimal operating conditions, minimizing overpotential at the anode. This, in turn, improves the kinetics of the electrochemical processes, leading to enhanced overall efficiency.
- Enhanced reaction rates: The removal of water vapor and oxygen through the sweep gas prevents side reactions and corrosion, contributing to more controlled and efficient electrochemical reactions at the anode. This results in improved reaction rates and electrolyzer performance.
- Extended cell lifespan: By preventing the accumulation of detrimental by-products, such as carbon deposition and oxide formation, the utilization of N2 as a sweep gas can contribute to an extended lifespan of the SOEC. This is crucial for the long-term durability and reliability of the co-electrolyzer.
- Improved gas purity: The presence of a sweep gas helps in maintaining a cleaner anode environment by carrying away impurities. This ensures the production of high-purity hydrogen or other desired products at the cathode without contamination from unwanted gases.
- Optimized gas distribution: The controlled flow of N2 as a sweep gas aids in achieving a well-distributed gas gradient within the anode compartment. This is essential for uniform electrochemical reactions across the electrode surface, preventing localized issues and ensuring consistent performance.
3. Methodology Modeling
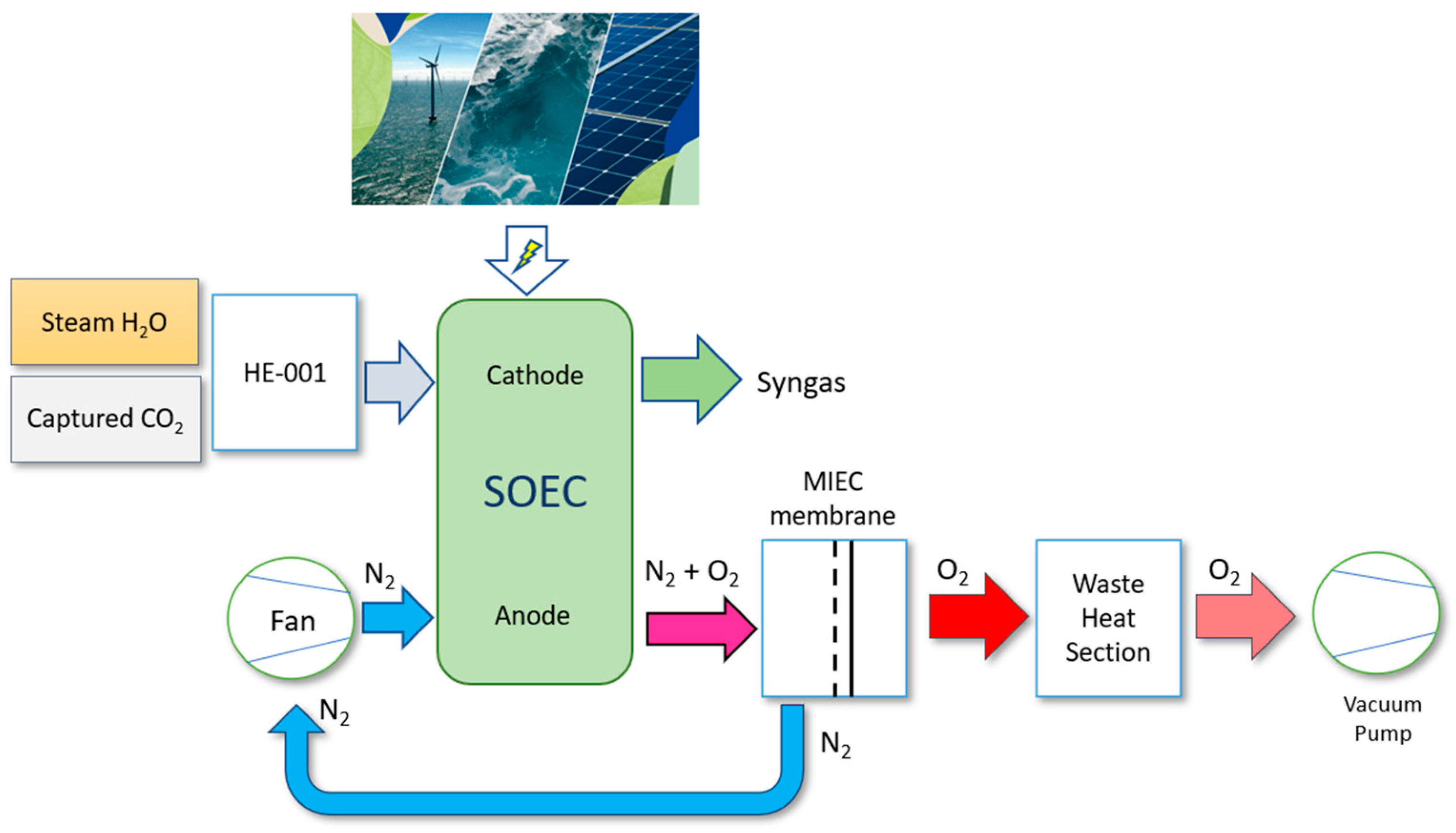
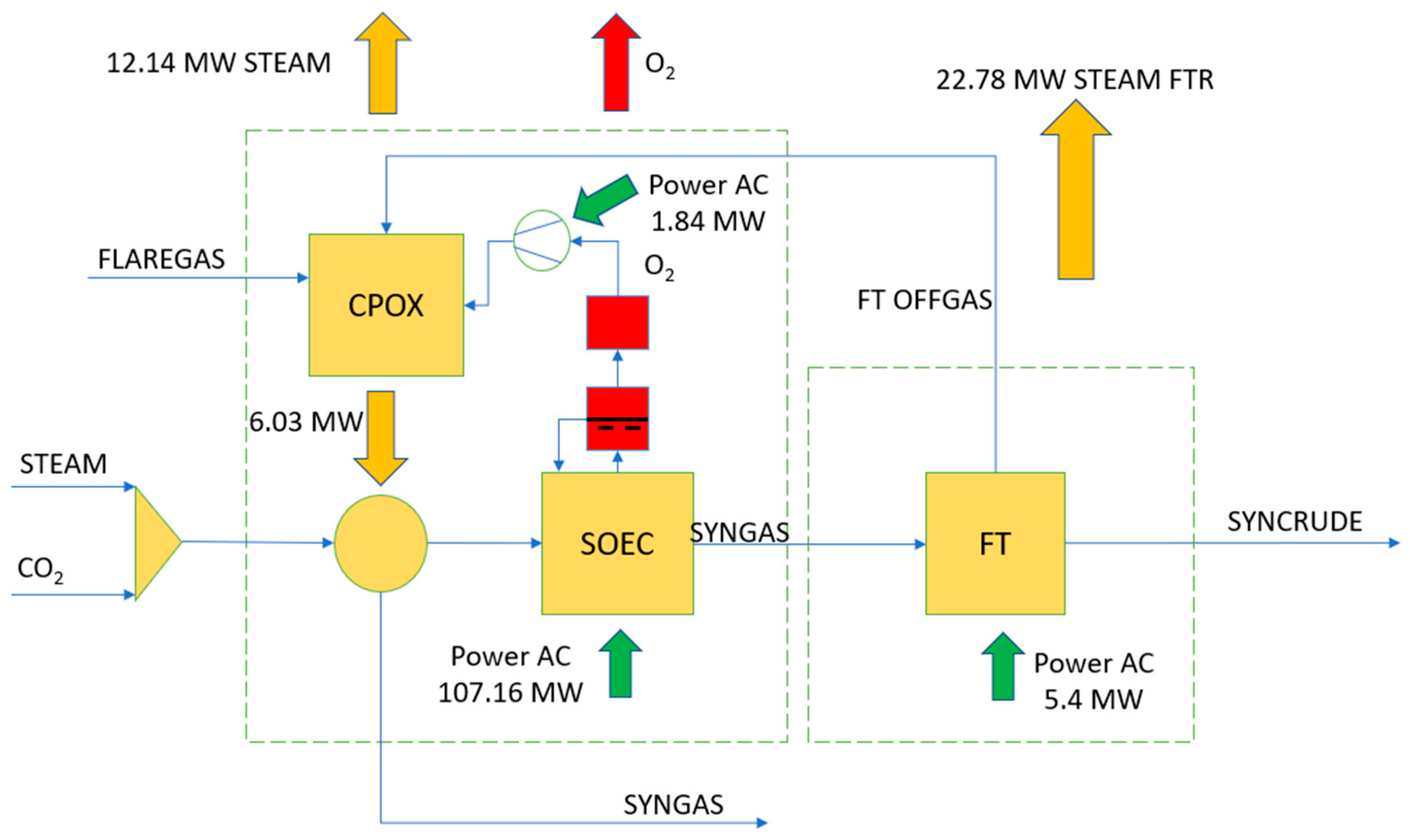
3.1. Process Model General Description
- Valorization of the extracted oxygen through vacuum in other industrial applications and processes.
- Recovery of the available heat in the oxygen stream.
- Energy savings at the co-electrolyzer system level by being able to recirculate the sweep gas stream, avoiding the need to provide the heat required to reach the temperature of the thermoneutral mode from ambient temperature.
3.2. Integrated Heat Recovery System
4. Results and Discussion
4.1. Case Study
4.2. Plant-Level Efficiency Improvement Results
4.3. Membrane Area
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Commission. The European Green Deal. Available online: https://ec.europa.eu/info/strategy/priorities-2019-2024/european-green-deal_en (accessed on 6 February 2024).
- European Commission. A Hydrogen Strategy for a Climate-Neutral Europe. 2020. Available online: https://ec.europa.eu/energy/sites/ener/files/hydrogen_strategy.pdf (accessed on 6 February 2024).
- Kaldellis, J.K.; Kapsali, M.; Kondili, E. Advancing the energy policy and the use of renewable energy sources in the European Union. Renew. Sustain. Energy Rev. 2014, 36, 9–22. [Google Scholar] [CrossRef]
- International Energy Agency. Net Zero by 2050: A Roadmap for the Global Energy Sector. 2021. Available online: https://www.iea.org/reports/net-zero-by-2050 (accessed on 6 February 2024).
- Aicart, J. Modeling and Experimental Validation of High Temperature Steam and Carbon Dioxide Co-Electrolysis. Ph.D. Thesis, Université de Grenoble, Saint-Martin-d’Hères, France, 2014. (In English). [Google Scholar]
- Richter, H.; Kriegel, R.; Klefenz, H.; Voigt, I. Assessment of the Energy Demand of a Prototype Stand-Alone Oxygen Generator Using MIEC Membranes. In Proceedings of the 13th International Conference on Inorganic Membranes (ICIM), Brisbane, Australia, 6–9 July 2014. [Google Scholar]
- Kleiminger, L.; Li, T.; Li, K.; Kelsall, G.H. Syngas (CO-H2) production using high temperature micro-tubular solid oxide electrolysers. Electrochim. Acta 2015, 179, 565–577. [Google Scholar] [CrossRef]
- Molina-Moreno, V.; Caamaño-Martín, E.; Alcántara-Payán, J.M. Power-to-Liquid (PtL) technology as a tool for energy transition in the EU: A techno-economic analysis. Energy Policy 2021, 153, 112293. [Google Scholar] [CrossRef]
- van Bavel, S.; Verma, S.; Negro, E.; Bracht, M. Integrating CO2 Electrolysis into the Gas-to-Liquids–Power-to-Liquids Process. ACS Energy Lett. 2020, 5, 2597–2601. [Google Scholar] [CrossRef]
- Becker, W.; Braun, R.; Penev, M.; Melaina, M. Production of Fischer-Tropsch Liquid Fuels from High Temperature Solid Oxide Co-Electrolysis Units. Energy 2012, 47, 99–115. [Google Scholar] [CrossRef]
- Chen, G.; Feldhoff, A.; Weidenkaff, A.; Li, C.; Liu, S.; Zhu, X.; Sunarso, J.; Huang, K.; Wu, X.; Ghoniem, A.F.; et al. Roadmap for Sustainable Mixed Ionic-Electronic Conducting Membranes. Adv. Funct. Mater. 2022, 32, 2105702. [Google Scholar] [CrossRef]
- Liu, S.; Tan, X.; Li, K.; Hughes, R. Methane coupling using catalytic membrane reactors. Catal. Rev. 2001, 43, 147–198. [Google Scholar] [CrossRef]
- Hänggi, S.; Elbert, P.; Bütler, T.; Cabalzar, U.; Teske, S.; Bach, C.; Onder, C. A review of synthetic fuels for passenger vehicles. Energy Rep. 2019, 5, 555–569. [Google Scholar] [CrossRef]
- Singh, S.; Jain, S.; Venkateswaran, P.S.; Tiwari, A.K.; Nouni, M.R.; Pandey, J.K.; Goel, S. Hydrogen: A sustainable fuel for future of the transport sector. Renew. Sustain. Energy Rev. 2015, 51, 623–633. [Google Scholar] [CrossRef]
- Wilhelm, D.J.; Simbeck, D.; Karp, A.; Dickenson, R. Syngas production for gas-to-liquids applications: Technologies, issues and outlook. Fuel Process. Technol. 2001, 71, 139–148. [Google Scholar] [CrossRef]
- Wang, L.; Pérez-Fortes, M.; Madi, H.; Diethelm, S.; Van Herle, J.; Maréchal, F. Optimal design of solid-oxide electrolyzer based power-to-methane systems: A comprehensive comparison between steam electrolysis and co-electrolysis. Appl. Energy 2018, 211, 1060–1079. [Google Scholar] [CrossRef]
- dos Santos, R.G.; Alencar, A.C. Biomass-derived syngas production via gasification process and its catalytic conversion into fuels by Fischer Tropsch synthesis: A review. Int. J. Hydrogen Energy 2020, 45, 18114–18132. [Google Scholar] [CrossRef]
- Liu, Z.; Masel, R.I.; Chen, Q.; Kutz, R.; Yang, H.; Lewinski, K.; Kaplun, M.; Luopa, S.; Lutz, D.R. Electrochemical generation of syngas from water and carbon dioxide at industrially important rates. J. CO2 Util. 2016, 15, 50–56. [Google Scholar] [CrossRef]
- Yang, Z.; Wu, Y.; Zhang, Z.; Li, H.; Li, X.; Egorov, R.I.; Strizhak, P.A.; Gao, X. Recent advances in co-thermochemical conversions of biomass with fossil fuels focusing on the synergistic effects. Renew. Sustain. Energy Rev. 2019, 103, 384–398. [Google Scholar] [CrossRef]
- Dobladez, J.A.D.; Maté, V.I.Á.; Torrellas, S.Á.; Larriba, M.; Brea, P. Efficient recovery of syngas from dry methane reforming product by a dual pressure swing adsorption process. Int. J. Hydrogen Energy 2021, 46, 17522–17533. [Google Scholar] [CrossRef]
- Gupta, P.K.; Kumar, V.; Maity, S. Renewable fuels from different carbonaceous feedstocks: A sustainable route through Fischer–Tropsch synthesis. J. Chem. Technol. Biotechnol. 2021, 96, 853–868. [Google Scholar] [CrossRef]
- Herz, G.; Rix, C.; Jacobasch, E.; Müller, N.; Reichelt, E.; Jahn, M.; Michaelis, A. Economic assessment of Power-to-Liquid processes—Influence of electrolysis technology and operating conditions. Appl. Energy 2021, 292, 116655. [Google Scholar] [CrossRef]
- Tremel, A.; Wasserscheid, P.; Baldauf, M.; Hammer, T. Techno-economic analysis for the synthesis of liquid and gaseous fuels based on hydrogen production via electrolysis. Int. J. Hydrogen Energy 2015, 40, 11457–11464. [Google Scholar] [CrossRef]
- König, D.H.; Baucks, N.; Dietrich, R.-U.; Wörner, A. Simulation and evaluation of a process concept for the generation of synthetic fuel from CO2 and H2. Energy 2015, 91, 833–841. [Google Scholar] [CrossRef]
- Wang, L.; Chen, M.; Küngas, R.; Lin, T.-E.; Diethelm, S.; Maréchal, F.; Van Herle, J. Power-to-fuels via solid-oxide electrolyzer: Operating window and techno-economics. Renew. Sustain. Energy Rev. 2019, 110, 174–187. [Google Scholar] [CrossRef]
- Luo, Y.; Shi, Y.; Li, W.; Cai, N. Comprehensive modeling of tubular solid oxide electrolysis cell for co-electrolysis of steam and carbon dioxide. Energy 2014, 70, 420–434. [Google Scholar] [CrossRef]
- Schiestel, T.; Kilgus, M.; Peter, S.; Caspary, K.; Wang, H.; Caro, J. Hollow fibre perovskite membranes for oxygen separation. J. Membr. Sci. 2005, 258, 1–4. [Google Scholar] [CrossRef]
- Li, C.; Chew, J.J.; Mahmoud, A.; Liu, S.; Sunarso, J. Modelling of oxygen transport through mixed ionic-electronic conducting (MIEC) ceramic-based membranes: An overview. J. Membr. Sci. 2018, 567, 228–260. [Google Scholar] [CrossRef]
- Sunarso, J.; Baumann, S.; Serra, J.M.; Meulenberg, W.A.; Liu, S.; Lin, Y.S.; da Costa, J.C.D. Mixed ionic–electronic conducting (MIEC) ceramic-based membranes for oxygen separation. J. Membr. Sci. 2008, 320, 13–41. [Google Scholar] [CrossRef]
- Jin, Y.; Rui, Z.; Tian, Y.; Lin, Y.; Li, Y. Sequential simulation of dense oxygen permeation membrane reactor for hydrogen production from oxidative steam reforming of ethanol with ASPEN PLUS. Int. J. Hydrogen Energy 2010, 35, 6691–6698. [Google Scholar] [CrossRef]
- Kriegel, R. MIEC Membrane Plants—Competitive to commercial O2 Production. Presentation 2015. [Google Scholar] [CrossRef]
- Catalán-Martínez, D.; Santafé-Moros, A.; Gozálvez-Zafrilla, J.; García-Fayos, J.; Serra, J. Characterization of oxygen transport phenomena on BSCF membranes assisted by fluid dynamic simulations including surface exchange. Chem. Eng. J. 2020, 387, 124069. [Google Scholar] [CrossRef]
- Khajryan, K.; Storch, T.; Grab, T.; Kircheisen, R.; Ravkina, O.; Kriegel, R.; Fieback, T. O2 Separation Using MIEC Membranes and Steam Circulation Process. Chem. Ing. Tech. 2022, 95, 785–791. [Google Scholar] [CrossRef]
- Plazaola, A.A.; Labella, A.C.; Liu, Y.; Porras, N.B.; Tanaka, D.A.P.; Annaland, M.V.S.; Gallucci, F. Mixed Ionic-Electronic Conducting Membranes (MIEC) for Their Application in Membrane Reactors: A Review. Processes 2019, 7, 128. [Google Scholar] [CrossRef]
- Engels, S.; Beggel, F.; Modigell, M.; Stadler, H. Simulation of a membrane unit for oxyfuel power plants under consideration of realistic BSCF membrane properties. J. Membr. Sci. 2010, 359, 93–101. [Google Scholar] [CrossRef]
- Spallina, V.; Melchiori, T.; Gallucci, F.; Annaland, M.V.S. Auto-thermal reforming using mixed ion-electronic conducting ceramic membranes for a small-scale H2 production plant. Molecules 2015, 20, 4998–5023. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.J.; Thomson, W.J. Oxygen permeation rates through ion-conducting perovskite membranes. Chem. Eng. Sci. 1999, 54, 3839–3850. [Google Scholar] [CrossRef]
- Fischer, C.D.; Iribarren, O.A. Oxygen integration between a gasification process and oxygen production using a mass exchange heuristic. Int. J. Hydrogen Energy 2016, 41, 2399–2410. [Google Scholar] [CrossRef]
- Turi, D.M.; Chiesa, P.; Macchi, E.; Ghoniem, A.F. High fidelity model of the oxygen flux across ion transport membrane reactor: Mechanism characterization using experimental data. Energy 2016, 96, 127–141. [Google Scholar] [CrossRef]
- van Hassel, B.A. Oxygen transfer across composite oxygen transport membranes. Solid State Ion. 2004, 174, 253–260. [Google Scholar] [CrossRef]
- Qiu, L.; Lee, T.; Liu, L.-M.; Yang, Y.; Jacobson, A. Oxygen permeation studies of SrCo0.8Fe0.2O3−δ. Solid State Ion. 1995, 76, 321–329. [Google Scholar] [CrossRef]
- Shao, Z.; Yang, W.; Cong, Y.; Dong, H.; Tong, J.; Xiong, G. Investigation of the permeation behavior and stability of a Ba0.5Sr0.5Co0.8Fe0.2O3−δ oxygen membrane. J. Membr. Sci. 2000, 172, 177–188. [Google Scholar] [CrossRef]
- Serra, J.M.; Meulenberg, W.A. Thin-film proton conducting BaZr0.85Y0.15O3 electrolytes: Towards an intermediate-temperature solid oxide fuel cell alternative. J. Am. Ceram. Soc. 2007, 90, 2082. [Google Scholar] [CrossRef]
- Kriegel, R. Use of ceramic BSCF membranes in a portable oxygen generator. In DKG Handbuch Technische Keramische Werkstoffe; Kriegesmann, J., Ed.; HvB-Verlag: Ellerau, Germany, 2010; Volume 119. (In German) [Google Scholar]
- Voigt, R.; Kiesel, I.; Kircheisen, L.; Kriegel, R. Efficient Oxygen Separation from Air with MIEC-Membranes. In Proceedings of the 14th International Conference on Inorganic Embranes ICIM, Atlanta, GA, USA, 10–13 July 2016. [Google Scholar]
- Sánchez-Luján, J.; Molina-García, A.; López-Cascales, J.J. Optimal integration modeling of Co—Electrolysis in a power-to-liquid industrial process. Int. J. Hydrogen Energy 2024, 52, 1202–1219. [Google Scholar] [CrossRef]
- Niehoff, P.; Baumann, S.; Schulze-Küppers, F.; Bradley, R.; Shapiro, I.; Meulenberg, W.; Withers, P.; Vaßen, R. Oxygen transport through supported Ba0.5Sr0.5Co0.8Fe0.2O3−δ membranes. Sep. Purif. Technol. 2014, 121, 60–67. [Google Scholar] [CrossRef]
- Bouwmeester, H.J.M.; Kruidhof, H.; Burggraaf, A.J. Importance of the surface exchange kinetics as rate limiting step in oxygen permeation through mixed-conducting oxides. Solid State Ion. 1994, 72, 185–194. [Google Scholar] [CrossRef]
- Tan, X.; Li, K. Modeling of Air Separation in a LSCF Hollow-Fiber Membrane Module. AIChE J. 2002, 48, 1469–1477. [Google Scholar] [CrossRef]
- Ghadimi, A.; Alaee, M.A.; Behrouzifar, A.; Asadi, A.A.; Mohammadi, T. Oxygen permeation of BaxSr1−xCo0.8Fe0.2O3−δ perovskite-type membrane: Experimental and modeling. Desalination 2011, 270, 64–75. [Google Scholar] [CrossRef]
- Zhu, Y.; Li, W.; Liu, Y.; Zhu, X.; Yang, W. Selection of oxygen permeation models for different mixed ionic-electronic conducting membranes. AIChE J. 2017, 63, 4043–4053. [Google Scholar] [CrossRef]
- Kircheisen, R.; Bernhardt, R.; Kriegel, M. Performance of oxygen generators based on tubular BSCF membranes. In Proceedings of the 15th International Conference on Inorganic Membranes ICIM, Dresden, Germany, 18–22 June 2018. [Google Scholar]
- Kriegel, R.; Kircheisen, R. Largest Membrane Plant Worldwide for High-Purity Oxygen Production. Available online: https://www.ikts.fraunhofer.de/en/departments/environmental_process_engineering/high_temperature_separation/high-temperature_membranes_storage_materials/cr_largest_membrane_plant_worldwide_for_high-purity_oxygen_production.html (accessed on 6 February 2024).
- Kriegel, R.; Voigt, I.; Burckhardt, W. Proceedings of the 10th International Conference of the European Ceramic Society (ECerS), Berlin, Germany, 17–21 June 2007; Heinrich, J.G., Aneziris, C., Eds.; Göller Verlag: Berlin, Germany, 2007; pp. 2161–2168. ISBN 3-87264-022-4. [Google Scholar]
- Lu, H.; Tong, J.; Cong, Y.; Yang, W. Partial oxidation of methane in Ba0.5Sr0.5Co0.8Fe0.2O3−δ membrane reactor at high pressures. Catal. Today 2005, 104, 154. [Google Scholar] [CrossRef]
- Kriegel, E.; Burckhardt, R.; Voigt, W.; Schulz, I.; Sommer, M. Benefits of the vacuum process for oxygen production from air using Ba0.5Sr0.5Co0.8Fe0.2O3-δ membranes. In Proceedings of the 10th International Conference on Inorganic Membranes ICIM, Tokyo, Japan, 18–22 August 2008; Poster Session l P8. ISBN 978-4904353-01-1. [Google Scholar]
- Liang, F.; Jiang, H.; Luo, H.; Kriegel, R.; Caro, J. High-purity oxygen production by a dead-end Ba0.5Sr0.5Co0.8Fe0.2O3−δ tube membrane. Catal. Today 2012, 193, 95–100. [Google Scholar] [CrossRef]
- Rautenbach, R.; Knauf, R.; Struck, A.; Vier, J. Simulation and design of membrane plants with AspenPlus. Chem. Eng. Technol. 1996, 19, 391–397. [Google Scholar] [CrossRef]
- Aspen Technology, Aspen Plus—Process Simulation for Chemicals. Available online: https://www.aspentech.com/products/engineering/aspen-plus (accessed on 6 February 2024).
- Tan, X.; Liu, Y.; Li, K. Mixed conducting ceramic hollow-fiber membranes for air separation. AIChE J. 2005, 51, 1991–2000. [Google Scholar] [CrossRef]
- Furfari, S.; Clerici, A. Green hydrogen: The crucial performance of electrolysers fed by variable and intermittent renewable electricity. Eur. Phys. J. Plus 2021, 136, 1–29. [Google Scholar] [CrossRef]
- Skov, I.R.; Schneider, N. Incentive structures for power-to-X and e-fuel pathways for transport in EU and member states. Energy Policy 2022, 168, 113121. [Google Scholar] [CrossRef]
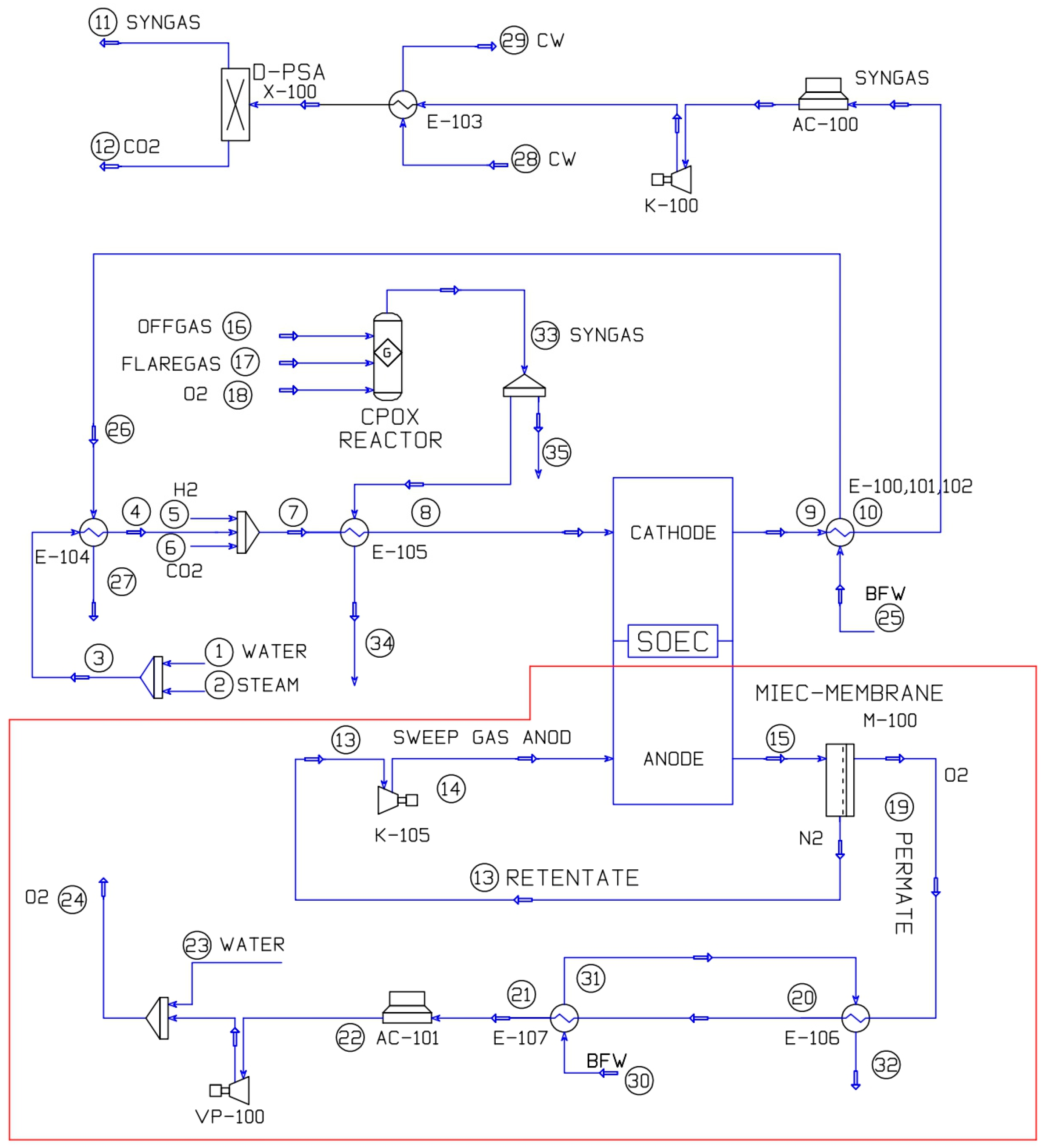
| No. | Stream | Temperature (°C) | Pressure (kPa) | No. | Stream | Temperature (°C) | Pressure (kPa) |
|---|---|---|---|---|---|---|---|
| 1 | Feed Water | 30 | 180 | 18 | O2 Feed | 40 | 1000 |
| 2 | Feed Steam | 250 | 180 | 19 | Permeate | 850 | 50 |
| 3 | Steam Mix | 142 | 180 | 20 | O2 | 450 | 30 |
| 4 | Steam | 550 | 175 | 21 | O2 | 120 | 10 |
| 5 | Hydrogen | 350 | 170 | 23 | Feed Water | 30 | 1000 |
| 6 | CO2 | 375 | 170 | 24 | O2 + Steam | 40 | 1000 |
| 7 | Feed Mix | 467 | 170 | 25 | BFW | 40 | 4000 |
| 8 | Feed Mix | 850 | 160 | 26 | Steam | 763.8 | 3980 |
| 9 | Syngas | 850 | 150 | 27 | Steam | 250.9 | 3975 |
| 10 | Syngas | 100 | 125 | 28 | Cooling Water | 25 | 2000 |
| 11 | Syngas | 50 | 990 | 29 | Cooling Water | 40 | 1995 |
| 12 | CO2 | 50 | 220 | 30 | BFW | 20 | 5005 |
| 13 | Retentate | 850 | 100 | 31 | Steam | 263.4 | 5000 |
| 14 | Sweep Gas Anode | 850 | 185 | 32 | Steam | 270 | 4995 |
| 15 | Sweep Gas Anode | 850 | 175 | 33 | Syngas | 1066 | 1000 |
| 16 | Off-gas Feed | 40 | 1000 | 34 | Syngas | 549.2 | 995 |
| 17 | Flare-gas Feed | 40 | 1000 | 35 | Syngas | 1066 | 1000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Luján, J.; Molina-García, Á.; López-Cascales, J.J. Integrated Heat Recovery System Based on Mixed Ionic–Electronic Conducting Membrane for Improved Solid Oxide Co-Electrolyzer Performance. Polymers 2024, 16, 932. https://doi.org/10.3390/polym16070932
Sánchez-Luján J, Molina-García Á, López-Cascales JJ. Integrated Heat Recovery System Based on Mixed Ionic–Electronic Conducting Membrane for Improved Solid Oxide Co-Electrolyzer Performance. Polymers. 2024; 16(7):932. https://doi.org/10.3390/polym16070932
Chicago/Turabian StyleSánchez-Luján, José, Ángel Molina-García, and José Javier López-Cascales. 2024. "Integrated Heat Recovery System Based on Mixed Ionic–Electronic Conducting Membrane for Improved Solid Oxide Co-Electrolyzer Performance" Polymers 16, no. 7: 932. https://doi.org/10.3390/polym16070932







