Advancing Food Preservation: Sustainable Green-AgNPs Bionanocomposites in Paper-Starch Flexible Packaging for Prolonged Shelf Life
Abstract
1. Introduction
2. Results and Discussion
2.1. Product Characterisation
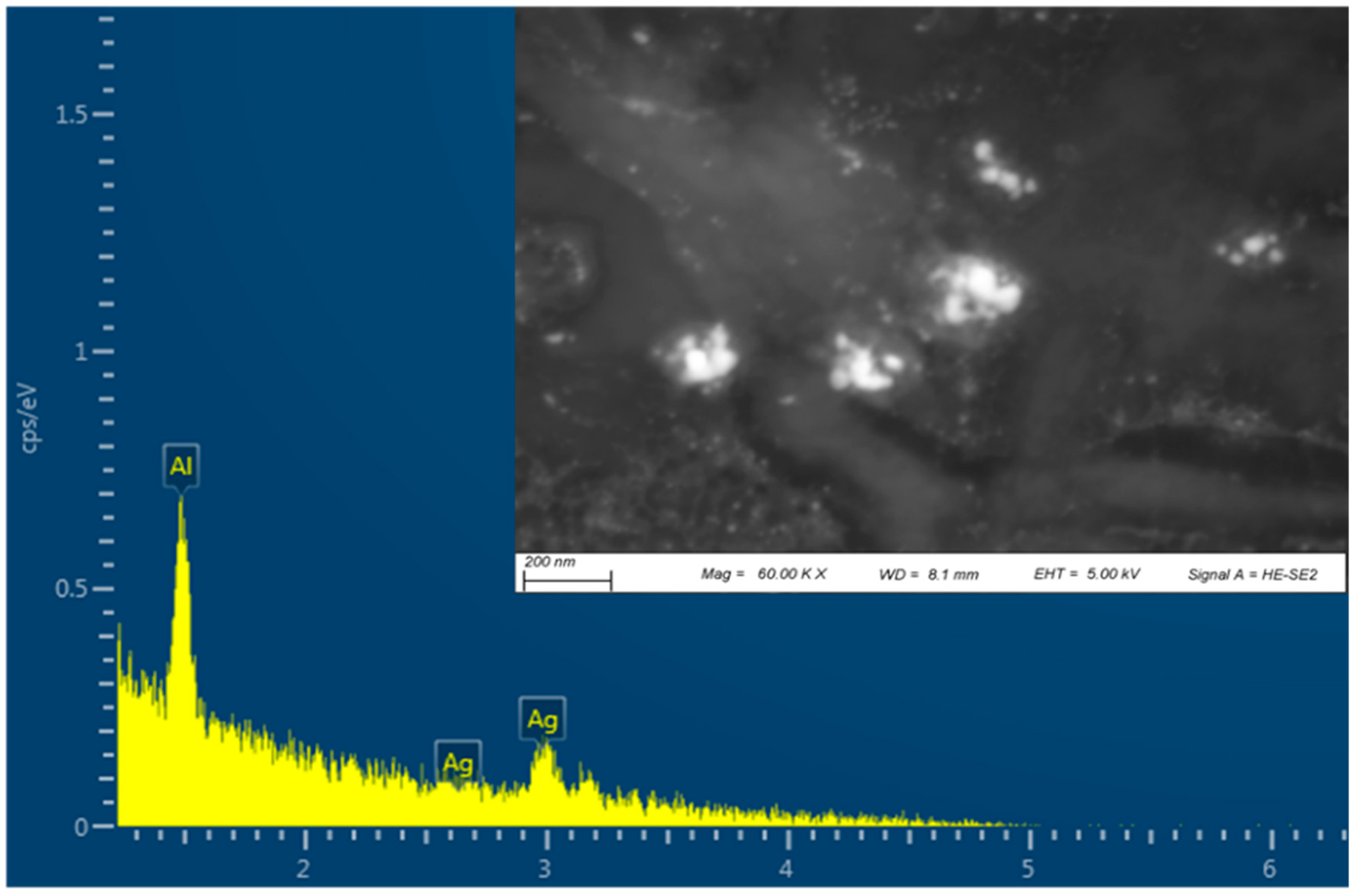
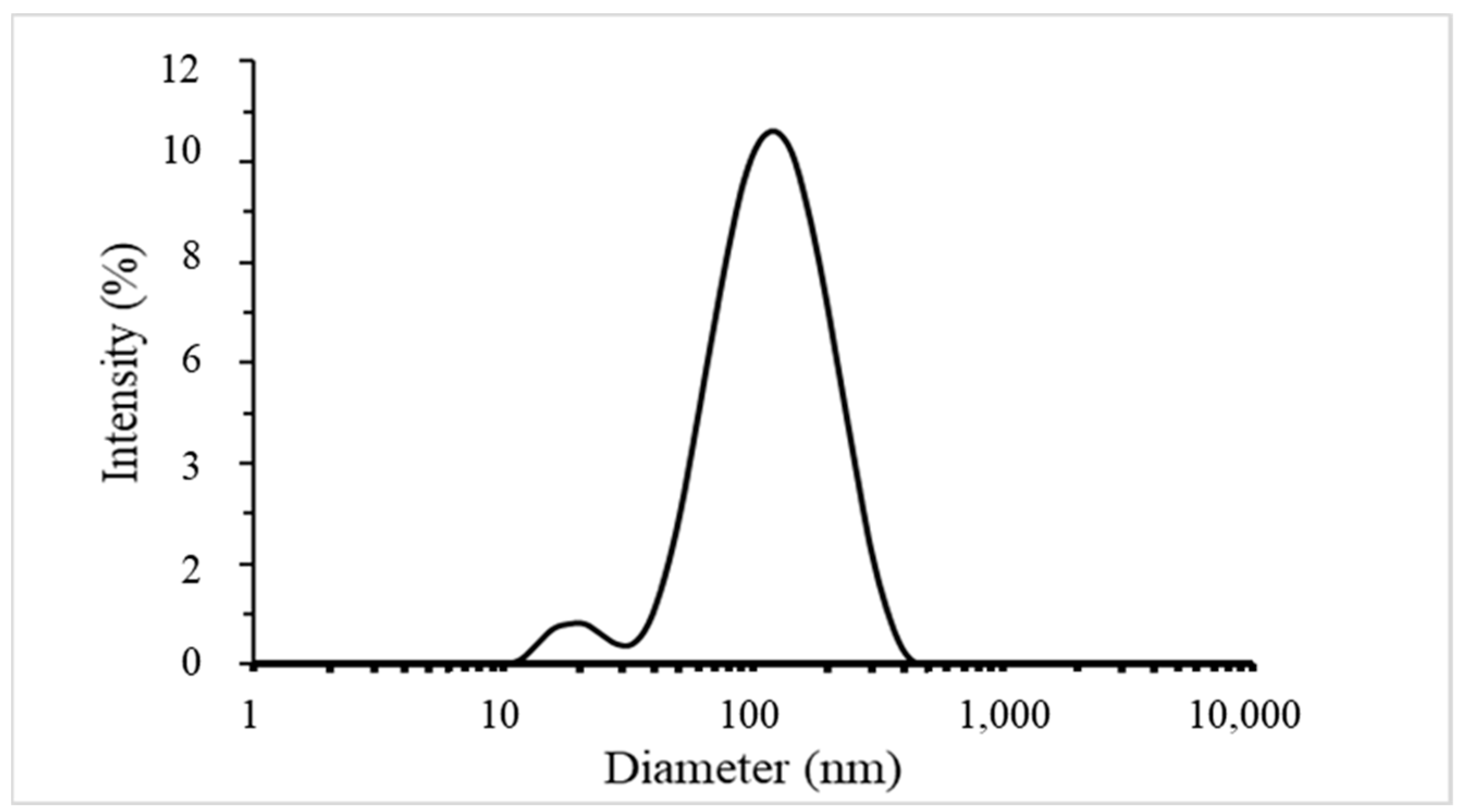
Silver Nanoparticles Characterisation
2.2. Paper-Based Packaging Characterisation
2.2.1. Starch-Based Coating FTIR Analysis
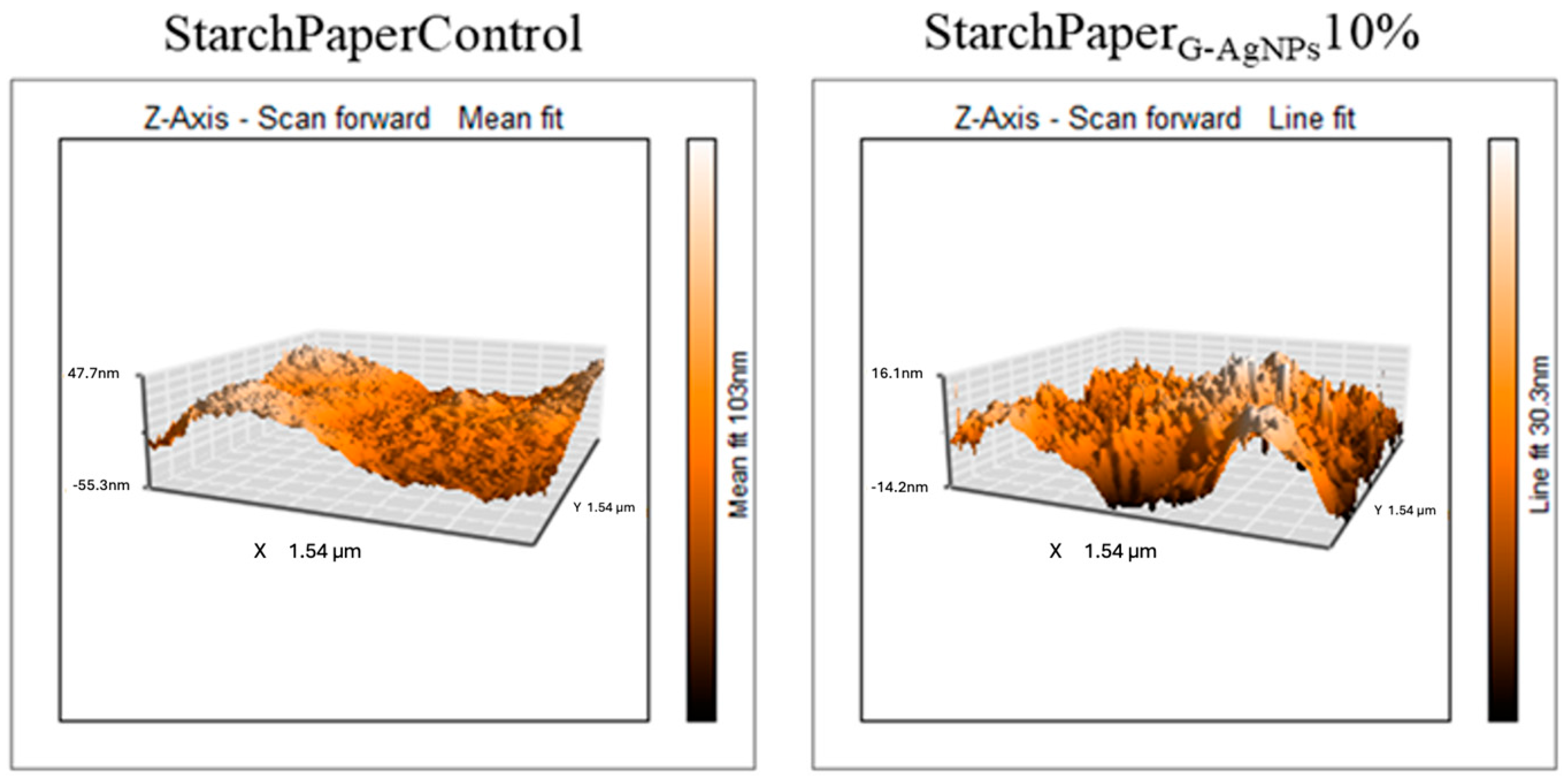
2.2.2. Morphological Properties of Paper-Based Packaging
2.2.3. Mechanical Properties of Paper-Based Packaging
| Sample | EaB% | UTS (Mpa) | EaB% Data Literature [44,45] |
|---|---|---|---|
| PaperControl | 5.05 ± 1.85 | 48.48 ± 5.3 | |
| StarchPaper | 3.19 ± 0.61 | 110.54 ± 18.01 | 7.6 ± 2.5 * |
| StarchPaperG-AgNPs5% | 6.97 ± 2.30 | 68.38 ± 35.68 | 6.8 ± 2.0/10.2 ± 2.5 ** |
| StarchPaperG-AgNPs10% | 7.14 ± 1.35 | 45.25 ± 8.17 | |
| StarchPaperG-AgNPs20% | 7.62 ± 2.46 | 67.01 ± 26.33 | |
| StarchPaperG-AgNPs30% | 8.34 ± 1.66 | 87.63 ± 24.23 |
2.2.4. Water Vapour Permeability (WVP) and Water Vapour Transmission Rate (WVTR)
2.2.5. Hydrophobicity
2.2.6. Thermal Analysis
2.2.7. G-AgNP Migration
2.3. Paper-Based Packaging Antimicrobial Activities
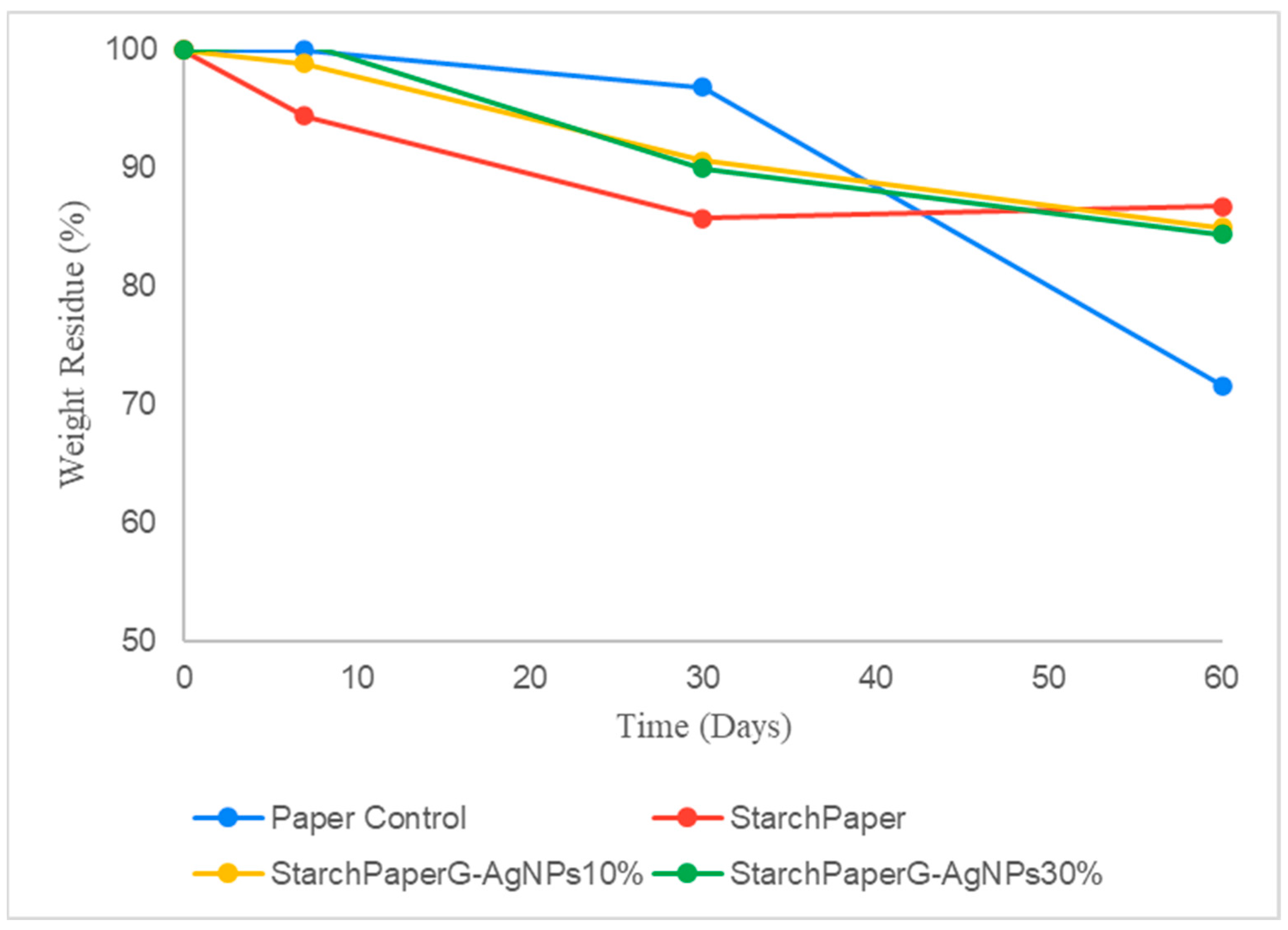
2.4. Bio-Disintegration of Starch Coated Papers
2.4.1. Soil Bio-Disintegration
2.4.2. Simulated Seawater Bio-Disintegration
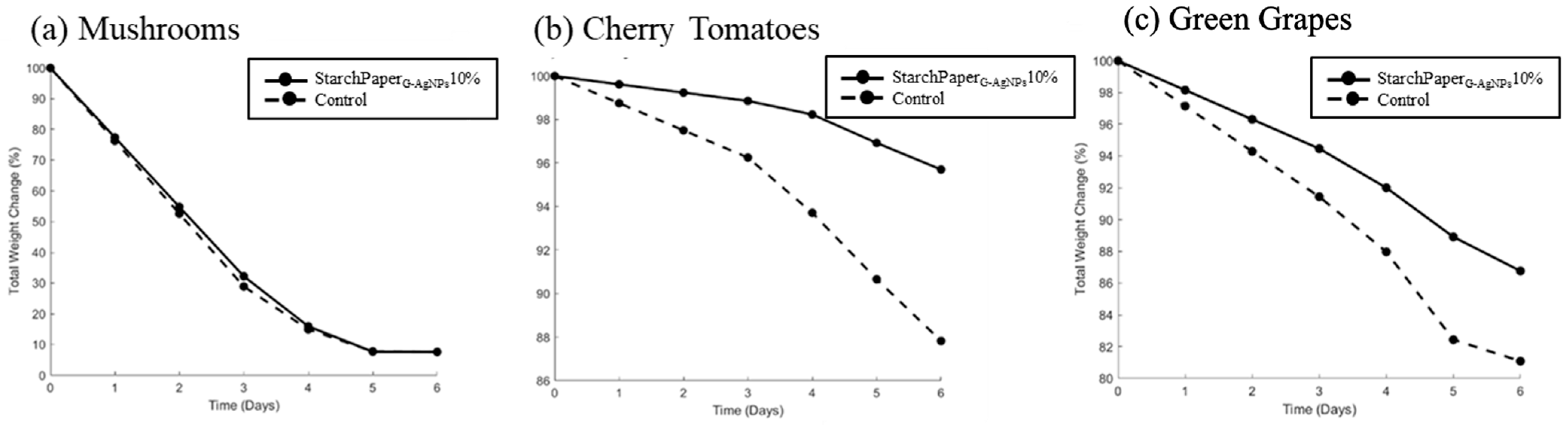
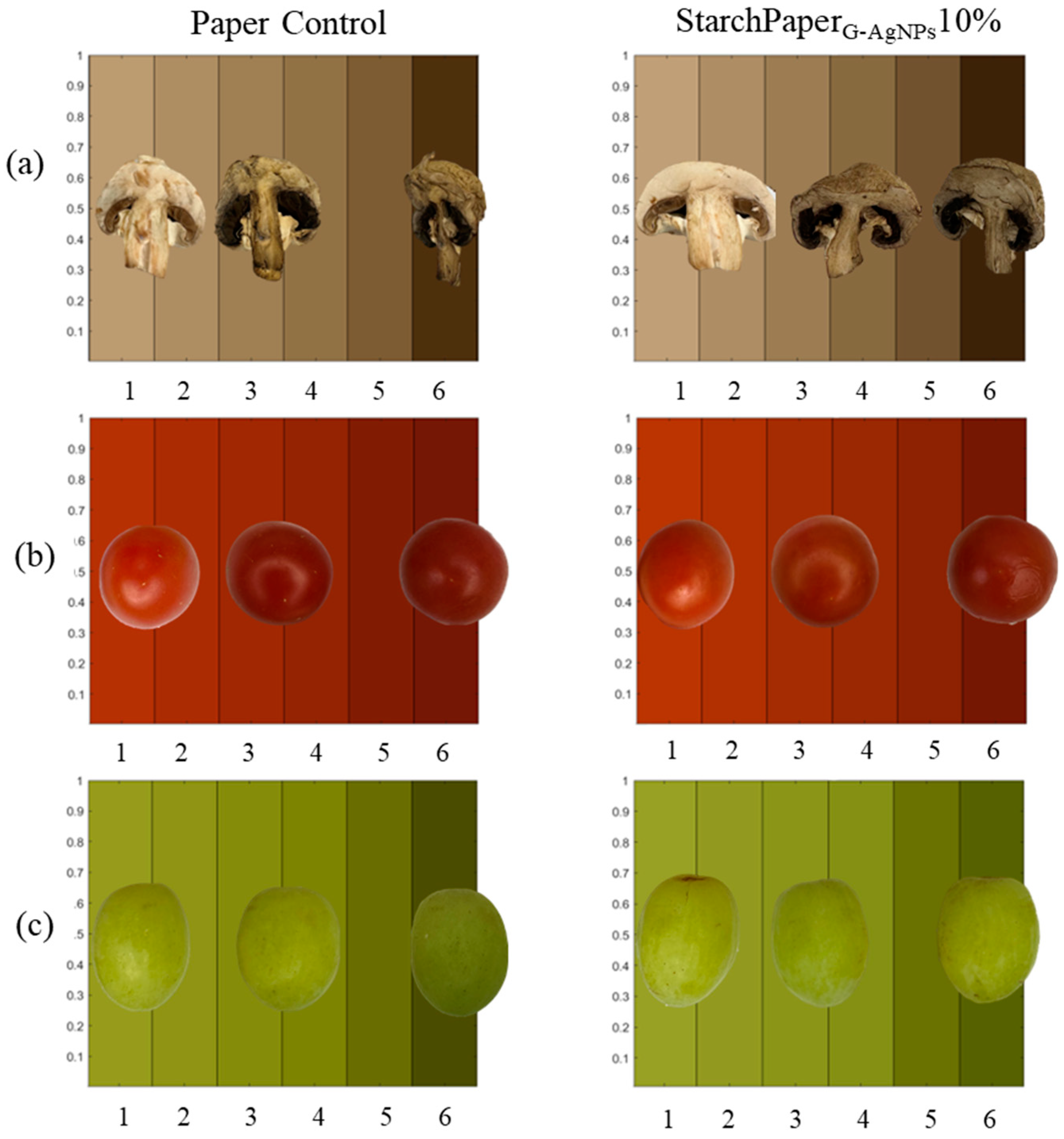
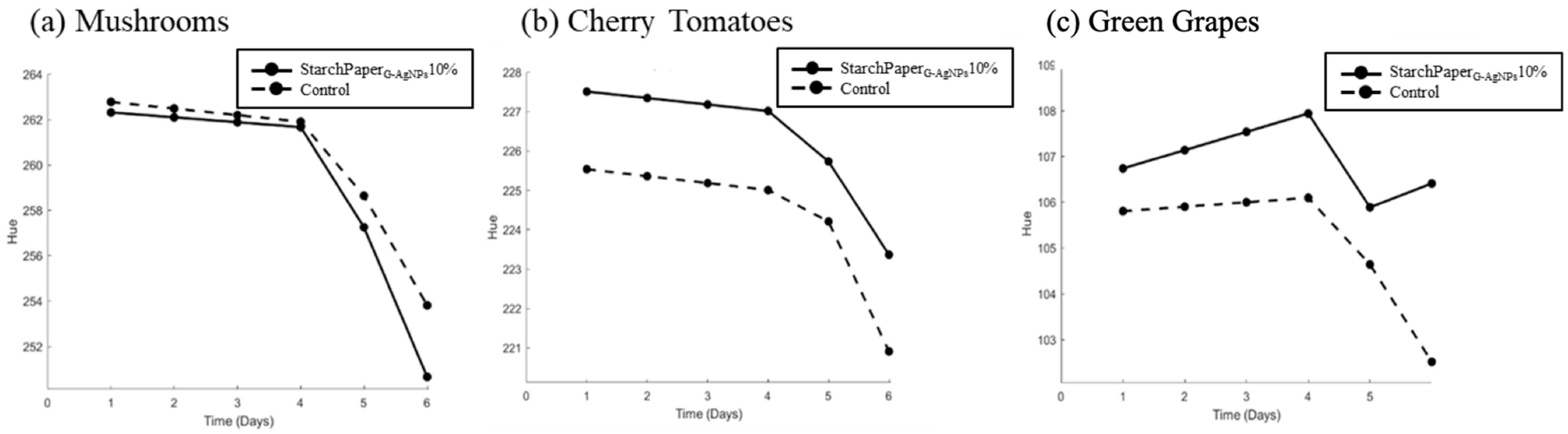
2.5. Food Trials
2.5.1. Weight Loss
2.5.2. Hue Test
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. AgNPs Characterisation
4.2.1. AgNPs UV–Vis Analysis
4.2.2. Scanning Electron Microscopy (SEM) with Energy Dispersive Spectrometry (EDS)
4.2.3. Particles Size Distribution
4.3. Preparation of Starch/Green Silver Nanoparticles (G-AgNPs) Paper-Based Packaging
4.3.1. Starch/G-AgNPs, Nanocomposite
4.3.2. Starch/G-AgNPs Deposition on Paper-Based Packaging
4.4. Paper-Based Packaging Characterisation
4.4.1. Morphological Characterisation—Atomic Force Microscopy
4.4.2. Mechanical Testing
4.4.3. Water Vapour Permeability (WVP)
4.4.4. Antimicrobial Activity
4.4.5. Hydrophobicity
4.4.6. Migration of G-AgNPs
4.4.7. Thermal Analysis
4.4.8. Fourier Transform Infrared Spectrometer (FTIR)
4.5. Bio-Disintegration
4.6. Food Trials
4.6.1. Packaging Food Samples
4.6.2. Weight Loss
4.6.3. Colour Measurement—Hue Test
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| Abbreviation | Full Description |
| G-AgNPs | Green Silver Nanoparticles obtained through Metalchemy’s green synthesis (environmentally friendly and nontoxic manufacturing method) |
| AgNPs | Silver Nanoparticles |
| SEM | Scanning Electron Microscope |
| EDS | Energy Dispersive Spectrometry |
| UTS | Ultimate Tensile Strength |
| EaB | Elongation at Break |
| WCA | Water Contact Angle |
| ICP-MS | Inductively Coupled Plasma Mass Spectrometry |
| SPR | Surface Plasmon Resonance |
| EFSA | European Food Safety Authority |
References
- Food Waste in the UK—House of Lords Library. Available online: https://lordslibrary.parliament.uk/food-waste-in-the-uk/ (accessed on 23 March 2023).
- Reducing Household Food Waste and Plastic Packaging (No Date) WRAP. Available online: https://wrap.org.uk/resources/report/reducing-household-food-waste-and-plastic-packaging (accessed on 14 March 2024).
- Eliminating Problem Plastics WRAP. Available online: https://wrap.org.uk/resources/report/eliminating-problem-plastics (accessed on 14 March 2024).
- Mujtaba, M.; Lipponen, J.; Ojanen, M.; Puttonen, S.; Vaittinen, H. Trends and challenges in the development of bio-based barrier coating materials for paper/cardboard food packaging; a review. Sci. Total. Environ. 2022, 851, 158328. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, P.; Salem, K.S.; Hubbe, M.A.; Pal, L. Advances in barrier coatings and film technologies for achieving sustainable packaging of food products—A review. Trends Food Sci. Technol. 2021, 115, 461–485. [Google Scholar] [CrossRef]
- Raheem, D. Application of plastics and paper as food packaging materials? An overview. Emir. J. Food Agric. 2013, 25, 177. [Google Scholar] [CrossRef]
- Allahvaisi, S. Polypropylene in the Industry of Food Packaging; IntechOpen Limited: London, UK, 2012; pp. 953–978. [Google Scholar]
- Grujić, R.; Vujadinović, D.; Savanović, D. Biopolymers as food packaging materials. In Advances in Applications of Industrial Biomaterials; Springer: Berlin/Heidelberg, Germany, 2017; pp. 139–160. [Google Scholar]
- Adeyeye, O.A.; Sadiku, E.R.; Babu Reddy, A.; Ndamase, A.S.; Makgatho, G.; Sellamuthu, P.S.; Perumal, A.B.; Nambiar, R.B.; Fasiku, V.O.; Ibrahim, I.D.; et al. The use of biopolymers in food packaging. In Green Biopolymers and Their Nanocomposites; Springer: Berlin/Heidelberg, Germany, 2019; pp. 137–158. [Google Scholar]
- Kumar, S.; Basumatary, I.B.; Mukherjee, A.; Dutta, J. An overview of natural biopolymers in food packaging. In Biopolymer-Based Food Packaging: Innovations and Technology Applications; Wiley: Hoboken, NJ, USA, 2022; pp. 1–28. [Google Scholar]
- Trotta, F.; Da Silva, S.; Massironi, A.; Mirpoor, S.F.; Lignou, S.; Ghawi, S.K.; Charalampopoulos, D. Silver bionanocomposites as active food packaging: Recent advances & future trends tackling the food waste crisis. Polymers 2023, 15, 4243. [Google Scholar] [CrossRef] [PubMed]
- Deshwal, G.K.; Panjagari, N.R.; Alam, T. An overview of paper and paper based food packaging materials: Health safety and environmental concerns. J. Food Sci. Technol. 2019, 56, 4391–4403. [Google Scholar] [CrossRef] [PubMed]
- Khan, B.; Bilal Khan Niazi, M.; Samin, G.; Jahan, Z. Thermoplastic starch: A possible biodegradable food packaging material—A review. J. Food Process Eng. 2017, 40, e12447. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Rhim, J.-W. Chitosan-based biodegradable functional films for food packaging applications. Innov. Food Sci. Emerg. Technol. 2020, 62, 102346. [Google Scholar] [CrossRef]
- Roy, S.; Priyadarshi, R.; Łopusiewicz, Ł.; Biswas, D.; Chandel, V.; Rhim, J.-W. Recent progress in pectin extraction, characterization, and pectin-based films for active food packaging applications: A review. Int. J. Biol. Macromol. 2023, 239, 124248. [Google Scholar] [CrossRef] [PubMed]
- Petersen, K.; Nielsen, P.V.; Bertelsen, G.; Lawther, M.; Olsen, M.B.; Nilsson, N.H.; Mortensen, G. Potential of biobased materials for food packaging. Trends Food Sci. Technol. 1999, 10, 52–68. [Google Scholar] [CrossRef]
- Robertson, G. State-of-the-art biobased food packaging materials. In Environmentally Compatible Food Packaging; Woodhead Publishing: Sawston, UK, 2008; pp. 3–28. [Google Scholar]
- Bobu, E.; Nicu, R.; Obrocea, P.; Ardelean, E.; Dunca, S.; Balaes, T. Antimicrobial properties of coatings based on chitosan derivatives for applications in sustainable paper conservation. Cellul. Chem. Technol. 2016, 50, 689–699. [Google Scholar]
- Nechita, P. Active-antimicrobial coatings based on silver nanoparticles and natural polymers for paper packaging functionalization. Nord. Pulp Pap. Res. J. 2017, 32, 452–458. [Google Scholar] [CrossRef]
- García-Guzmán, L.; Cabrera-Barjas, G.; Soria-Hernández, C.G.; Castaño, J.; Guadarrama-Lezama, A.Y.; Llamazares, S.R. Progress in Starch-Based Materials for Food Packaging Applications. Polysaccharides 2022, 3, 136–177. [Google Scholar] [CrossRef]
- Niranjana Prabhu, T.; Prashantha, K. A review on present status and future challenges of starch based polymer films and their composites in food packaging applications. Polym. Compos. 2018, 39, 2499–2522. [Google Scholar] [CrossRef]
- Heydari, A.; Alemzadeh, I.; Vossoughi, M. Functional properties of biodegradable corn starch nanocomposites for food packaging applications. Mater. Des. 2013, 50, 954–961. [Google Scholar] [CrossRef]
- Emamhadi, M.A.; Sarafraz, M.; Akbari, M.; Thai, V.N.; Fakhri, Y.; Linh, N.T.T.; Khaneghah, A.M. Nanomaterials for food packaging applications: A systematic review. Food Chem. Toxicol. 2020, 146, 111825. [Google Scholar] [CrossRef] [PubMed]
- Bumbudsanpharoke, N.; Choi, J.; Ko, S. Applications of Nanomaterials in Food Packaging. J. Nanosci. Nanotechnol. 2015, 15, 6357–6372. [Google Scholar] [CrossRef]
- Barage, S.; Lakkakula, J.; Sharma, A.; Roy, A.; Alghamdi, S.; Almehmadi, M.; Hossain, J.; Allahyani, M.; Abdulaziz, O. Nanomaterial in Food Packaging: A Comprehensive Review. J. Nanomater. 2022, 2022, 6053922. [Google Scholar] [CrossRef]
- Chaudhary, P.; Fatima, F.; Kumar, A. Relevance of nanomaterials in food packaging and its advanced future prospects. J. Inorg. Organomet. Polym. Mater. 2020, 30, 5180–5192. [Google Scholar] [CrossRef]
- Chadha, U.; Bhardwaj, P.; Selvaraj, S.K.; Arasu, K.; Praveena, S.; Pavan, A.; Khanna, M.; Singh, P.; Singh, S.; Chakravorty, A.; et al. Current Trends and Future Perspectives of Nanomaterials in Food Packaging Application. J. Nanomater. 2022, 2022, 2745416. [Google Scholar] [CrossRef]
- Trotta, F.; Dony, A.; Mok, M.; Grillo, A.; Whitehead-Clarke, T.; Homer-Vanniasinkam, S.; Kureshi, A. In vitro characterization of bionanocomposites with green silver nanoparticles: A step towards sustainable wound healing materials. Nano Sel. 2023, 5, 2300087. [Google Scholar] [CrossRef]
- Mensah, R.A.; Trotta, F.; Briggs, E.; Sharifulden, N.S.; Silva, L.V.B.; Keskin-Erdogan, Z.; Diop, S.; Kureshi, A.K.; Chau, D.Y.S. A Sustainable, Green-Processed, Ag-Nanoparticle-Incorporated Eggshell-Derived Biomaterial for Wound-Healing Applications. J. Funct. Biomater. 2023, 14, 450. [Google Scholar] [CrossRef]
- Massironi, A.; Morelli, A.; Grassi, L.; Puppi, D.; Braccini, S.; Maisetta, G.; Esin, S.; Batoni, G.; Della Pina, C.; Chiellini, F. Ulvan as novel reducing and stabilizing agent from renewable algal biomass: Application to green synthesis of silver nanoparticles. Carbohydr. Polym. 2019, 203, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Massironi, A.; Franco, A.R.; Babo, P.S.; Puppi, D.; Chiellini, F.; Reis, R.L.; Gomes, M.E. Development and characterization of highly stable silver nanoparticles as novel potential antimicrobial agents for wound healing hydrogels. Int. J. Mol. Sci. 2022, 23, 2161. [Google Scholar] [CrossRef] [PubMed]
- Reznickova, A.; Novotna, Z.; Kolska, Z.; Svorcik, V. Immobilization of silver nanoparticles on polyethylene terephthalate. Nanoscale Res. Lett. 2014, 9, 305. [Google Scholar] [CrossRef] [PubMed]
- Bumbudsanpharoke, N.; Lee, W.; Ko, S. A comprehensive feasibility study on the properties of LDPE-Ag nanocomposites for food packaging applications. Polym. Compos. 2018, 39, 3178–3186. [Google Scholar] [CrossRef]
- He, Y.; Li, H.; Fei, X.; Peng, L. Carboxymethyl cellulose/cellulose nanocrystals immobilized silver nanoparticles as an effective coating to improve barrier and antibacterial properties of paper for food packaging applications. Carbohydr. Polym. 2021, 252, 117156. [Google Scholar] [CrossRef]
- Ghaseminezhad, S.M.; Hamedi, S.; Shojaosadati, S.A. Green synthesis of silver nanoparticles by a novel method: Comparative study of their properties. Carbohydr. Polym. 2012, 89, 467–472. [Google Scholar] [CrossRef]
- de Souza, T.A.J.; Souza, L.R.R.; Franchi, L.P. Silver nanoparticles: An integrated view of green synthesis methods, transformation in the environment, and toxicity. Ecotoxicol. Environ. Saf. 2019, 171, 691–700. [Google Scholar] [CrossRef]
- ASTM E96; Methods for Water Vapor Transmission of Materials. ASTM International: West Conshohocken, PA, USA, 2013.
- Abdullah, A.H.D.; Chalimah, S.; Primadona, I.; Hanantyo, M.H.G. Physical and chemical properties of corn, cassava, and potato starchs. In Proceedings of the 2nd International Symposium on Green Technology for Value Chains 2017 (GreenVC 2017), Jakarta, Indonesia, 23–24 October 2017; IOP Publishing: Bristol, UK, 2018; Volume 160, p. 012003. [Google Scholar]
- Jiang, C.; Wang, X.; Gunawidjaja, R.; Lin, Y.; Gupta, M.K.; Kaplan, D.L.; Naik, R.R.; Tsukruk, V.V. Mechanical Properties of Robust Ultrathin Silk Fibroin Films. Adv. Funct. Mater. 2007, 17, 2229–2237. [Google Scholar] [CrossRef]
- Hu, X.; Kang, H.; Li, Y.; Geng, Y.; Wang, R.; Zhang, L. Preparation, morphology and superior performances of biobased thermoplastic elastomer by in situ dynamical vulcanization for 3D-printed materials. Polymer 2017, 108, 11–20. [Google Scholar] [CrossRef]
- Perera, S.; Bhushan, B.; Bandara, R.; Rajapakse, G.; Rajapakse, S.; Bandara, C. Morphological, antimicrobial, durability, and physical properties of untreated and treated textiles using silver-nanoparticles. Colloids Surf. A Physicochem. Eng. Asp. 2013, 436, 975–989. [Google Scholar] [CrossRef]
- Soltanzadeh, M.; Peighambardoust, S.H.; Ghanbarzadeh, B.; Amjadi, S.; Mohammadi, M.; Lorenzo, J.M.; Hamishehkar, H. Active gelatin/cress seed gum-based films reinforced with chitosan nanoparticles encapsulating pomegranate peel extract: Preparation and characterization. Food Hydrocoll. 2022, 129, 107620. [Google Scholar] [CrossRef]
- Taghinejad, F.; Tabibiazar, M.; Bagheri, V.; Pezeshki, A.; Mahmoudzadeh, M. Characterization of Curcumin-Thymol Loaded Partially Hydrolyzed Zein-Ethyl Celloluse Active Packaging Film. SSRN Electron. J. 2022. [Google Scholar] [CrossRef]
- Ortega, F.; Sobral, P.; Jios, J.L.; Arce, V.B.; García, M.A. Starch Nanocomposite Films: Migration Studies of Nanoparticles to Food Simulants and Bio-Disintegration in Soil. Polymers 2022, 14, 1636. [Google Scholar] [CrossRef] [PubMed]
- Taha, I.M.; Zaghlool, A.; Nasr, A.; Nagib, A.; El Azab, I.H.; Mersal, G.A.M.; Ibrahim, M.M.; Fahmy, A. Impact of Starch Coating Embedded with Silver Nanoparticles on Strawberry Storage Time. Polymers 2022, 14, 1439. [Google Scholar] [CrossRef] [PubMed]
- Versino, F.; Lopez, O.V.; Garcia, M.A.; Zaritzky, N.E. Starch-based films and food coatings: An overview. Starch-Stärke 2016, 68, 1026–1037. [Google Scholar] [CrossRef]
- Zia, J.; Paul, U.C.; Heredia-Guerrero, J.A.; Athanassiou, A.; Fragouli, D. Low-density polyethylene/curcumin melt extruded composites with enhanced water vapor barrier and antioxidant properties for active food packaging. Polymer 2019, 175, 137–145. [Google Scholar] [CrossRef]
- Romainor, A.N.; Chin, S.F.; Lihan, S. Antimicrobial Starch-Based Film for Food Packaging Application. Starch-Stärke 2022, 74, 2100207. [Google Scholar] [CrossRef]
- Díaz-Montes, E.; Castro-Muñoz, R. Edible films and coatings as food-quality preservers: An overview. Foods 2021, 10, 249. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.W. Starch/agar-based functional films integrated with enoki mushroom-mediated silver nanoparticles for active packaging applications. Food Biosci. 2022, 49, 101–867. [Google Scholar] [CrossRef]
- Rozilah, A.; Jaafar, C.A.; Sapuan, S.M.; Zainol, I.; Ilyas, R.A. The effects of silver nanoparticles compositions on the mechanical, physiochemical, antibacterial, and morphology properties of sugar palm starch biocomposites for antibacterial coating. Polymers 2020, 12, 2605. [Google Scholar] [CrossRef] [PubMed]
- Kumar, K.K.; Ravi, M.; Pavani, Y.; Bhavani, S.; Sharma, A.K.; Rao, V.N. Investigations on PEO/PVP/NaBr complexed polymer blend electrolytes for electrochemical cell applications. J. Membr. Sci. 2014, 454, 200–211. [Google Scholar] [CrossRef]
- Grasso, A.; Ferrante, M.; Arena, G.; Salemi, R.; Zuccarello, P.; Fiore, M.; Copat, C. Chemical Characterization and Quantification of Silver Nanoparticles (Ag-NPs) and Dissolved Ag in Seafood by Single Particle ICP-MS: Assessment of Dietary Exposure. Int. J. Environ. Res. Public Health 2021, 18, 4076. [Google Scholar] [CrossRef]
- Hong, S.-I.; Cho, Y.; Rhim, J.-W. Effect of Agar/AgNP Composite Film Packaging on Refrigerated Beef Loin Quality. Membranes 2021, 11, 750. [Google Scholar] [CrossRef] [PubMed]
- Jo, Y.; Garcia, C.V.; Ko, S.; Lee, W.; Shin, G.H.; Choi, J.C.; Park, S.-J.; Kim, J.T. Characterization and antibacterial properties of nanosilver-applied polyethylene and polypropylene composite films for food packaging applications. Food Biosci. 2018, 23, 83–90. [Google Scholar] [CrossRef]
- Gram, L.; Ravn, L.; Rasch, M.; Bruhn, J.B.; Christensen, A.B.; Givskov, M. Food spoilage—Interactions between food spoilage bacteria. Int. J. Food Microbiol. 2002, 78, 79–97. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.T.; Min, B.; Kim, K.W. General Characteristics of Packaging Materials for Food System. In Innovations in Food Packaging; Han, J.V., Ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 13–35. [Google Scholar]
- Leistner, L. Basic aspects of food preservation by hurdle technology. Int. J. Food Microbiol. 2000, 55, 181–186. [Google Scholar] [CrossRef]
- ASTM D412; Standard Test Methods for Vulcanized Rubber and Thermoplastic Elastomers—Tension. ASTM International: West Conshohocken, PA, USA, 2021.
- Gidik, H.; Vololonirina, O.; Ghantous, R.M.; Ankou, A. Impact of test parameters on the water vapor permeability of textiles. Int. J. Cloth. Sci. Technol. 2019, 31, 350–361. [Google Scholar] [CrossRef]
- ISO 22196; Measurement of Antibacterial Activity on Plastics and Other Non-Porous Surfaces. ASTM International: West Conshohocken, PA, USA, 2011.





| Samples | WVTR (g/m2/24 h) | WVP (×10−6 g/s·m·Pa) | WVP (×10−6 g/s·m·Pa) Data Literature [44,45] |
|---|---|---|---|
| PaperControl | 135.3 ± 7.2 | 2.22 ± 0.12 | |
| StarchPaper | 127.9 ± 8.2 | 2.17 ± 0.08 | 2.60 * |
| StarchPaperG-AgNPs5% | 122.6 ± 6.4 | 2.12 ± 0.03 | 2.45 ** |
| StarchPaperG-AgNPs10% | 123.8 ± 5.5 | 2.15 ± 0.05 | |
| StarchPaperG-AgNPs20% | 128.4 ± 6.8 | 2.16 ± 0.10 | |
| StarchPaperG-AgNPs30% | 117.9 ± 4.8 | 2.03 ± 0.06 |
| Samples | WCA | Water Drop Aspect |
|---|---|---|
| PaperControl | 82.3° ± 5.3° | 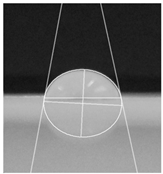 |
| StarchPaper | 86.9° ± 2.5° |  |
| StarchPaperG-AgNPs5% | 97.1° ± 5.0° | 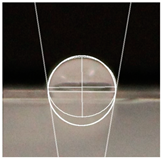 |
| StarchPaperG-AgNPs10% | 103.2° ± 5.1° |  |
| StarchPaperG-AgNPs20% | 103.9° ± 2.4° |  |
| StarchPaperG-AgNPs30% | 110.0° ± 3.5° | 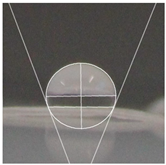 |
| Sample | T Peak Max (°C) | Weight Residue (%) |
|---|---|---|
| PaperControl | 338.4 ± 2.1 | 12.5 ± 0.9 |
| StarchPaper | 339.6 ± 1.6 | 13.4 ± 1.0 |
| StarchPaperG-AgNPs10% | 300.2 ± 1.1 | 15.4 ± 0.7 |
| StarchPaperG-AgNPs30% | 296.14 ± 1.0 | 18.0 ± 1.0 |
| Sample | Melting Temperature (°C) |
|---|---|
| PaperControl | 103.4 ± 2.8 |
| StarchPaperG-AgNPs10% | 89 ± 1.3 |
| Sample | Zone of Inhibition after 24 h (cm) | Zone of Inhibition after 48 h (cm) | Picture after 24 h |
|---|---|---|---|
| StarchPaper | No inhibition | No inhibition |  |
| StarchPaperG-AgNPs5% | No inhibition | 0.105 ± 0.012 |  |
| StarchPaperG-AgNPs10% | 0.114 ± 0.031 | 0.171 ± 0.032 |  |
| StarchPaperG-AgNPs20% | 0.141 ± 0.018 | 0.152 ± 0.022 |  |
| StarchPaperG-AgNPs30% | 0.158 ± 0.033 | 0.207 ± 0.044 |  |
| Sample | 7 Days | 30 Days | 60 Days |
|---|---|---|---|
| Paper Control |  |  |  |
| StarchPaper |  |  |  |
| StarchPaperG-AgNPs10% |  |  |  |
| StarchPaperG-AgNPs30% |  |  |  |
| Sample | G-AgNPs Added Volume (mL) | G-AgNPs Solution Concentration (ppm) | G-AgNPs/Starch wt/wt (%) |
|---|---|---|---|
| StarchPaperControl | 0 | / | / |
| StarchPaperG-AgNPs5% | 2.5 | 2.12 | 0.005 |
| StarchPaperG-AgNPs10% | 5 | 4.25 | 0.011 |
| StarchPaperG-AgNPs20% | 10 | 8.5 | 0.021 |
| StarchPaperG-AgNPs30% | 15 | 12.75 | 0.032 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trotta, F.; Da Silva, S.; Massironi, A.; Mirpoor, S.F.; Lignou, S.; Ghawi, S.K.; Charalampopoulos, D. Advancing Food Preservation: Sustainable Green-AgNPs Bionanocomposites in Paper-Starch Flexible Packaging for Prolonged Shelf Life. Polymers 2024, 16, 941. https://doi.org/10.3390/polym16070941
Trotta F, Da Silva S, Massironi A, Mirpoor SF, Lignou S, Ghawi SK, Charalampopoulos D. Advancing Food Preservation: Sustainable Green-AgNPs Bionanocomposites in Paper-Starch Flexible Packaging for Prolonged Shelf Life. Polymers. 2024; 16(7):941. https://doi.org/10.3390/polym16070941
Chicago/Turabian StyleTrotta, Federico, Sidonio Da Silva, Alessio Massironi, Seyedeh Fatemeh Mirpoor, Stella Lignou, Sameer Khalil Ghawi, and Dimitris Charalampopoulos. 2024. "Advancing Food Preservation: Sustainable Green-AgNPs Bionanocomposites in Paper-Starch Flexible Packaging for Prolonged Shelf Life" Polymers 16, no. 7: 941. https://doi.org/10.3390/polym16070941
APA StyleTrotta, F., Da Silva, S., Massironi, A., Mirpoor, S. F., Lignou, S., Ghawi, S. K., & Charalampopoulos, D. (2024). Advancing Food Preservation: Sustainable Green-AgNPs Bionanocomposites in Paper-Starch Flexible Packaging for Prolonged Shelf Life. Polymers, 16(7), 941. https://doi.org/10.3390/polym16070941







