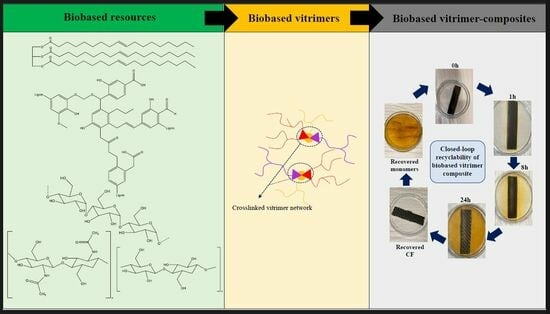
Recyclable and Biobased Vitrimers for Carbon Fibre-Reinforced Composites—A Review
Abstract
:1. Introduction
2. Potential Feedstocks for Bio-Based Vitrimer Synthesis
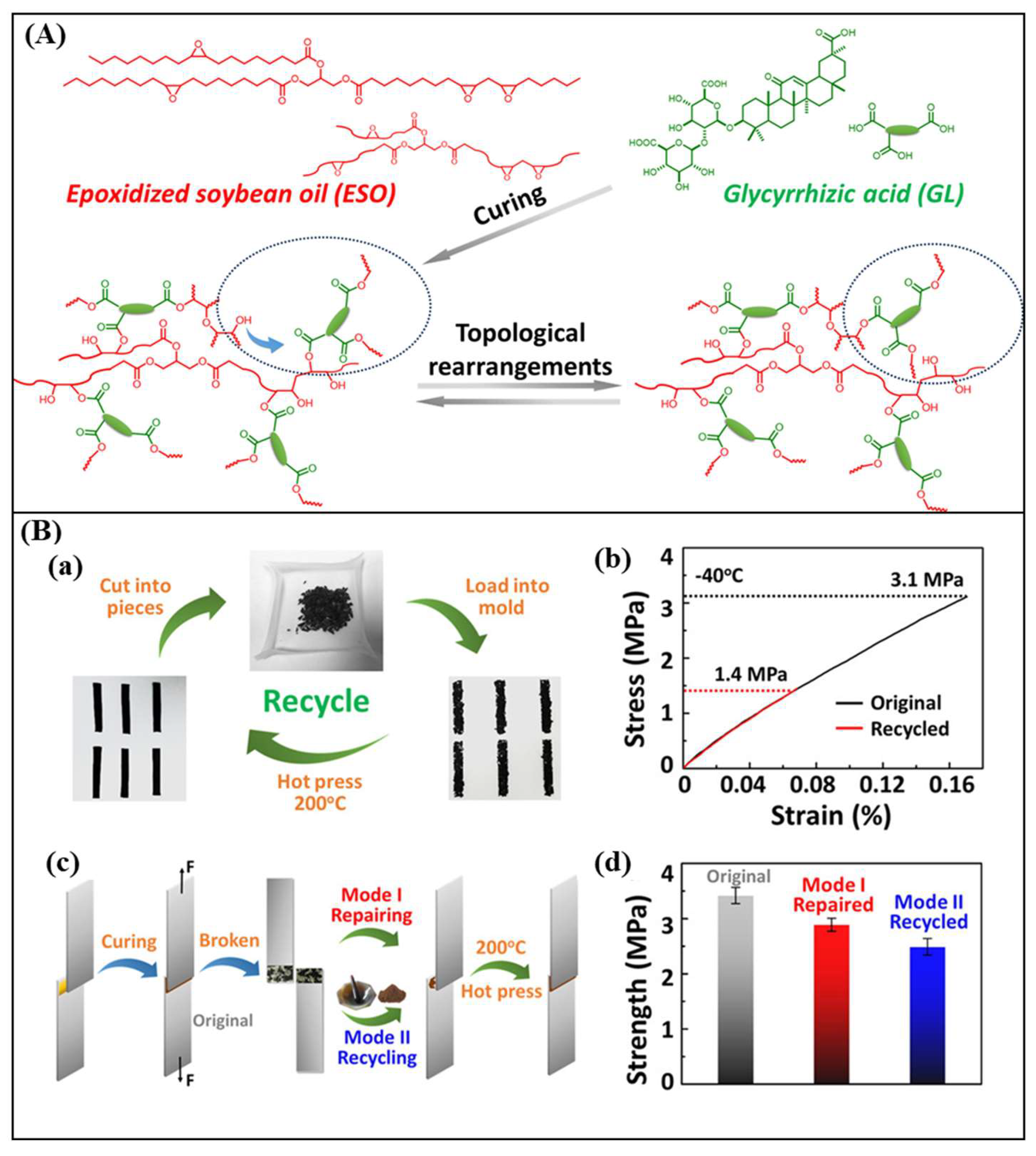
2.1. Vegetable Oils/Epoxidized Vegetable Oils
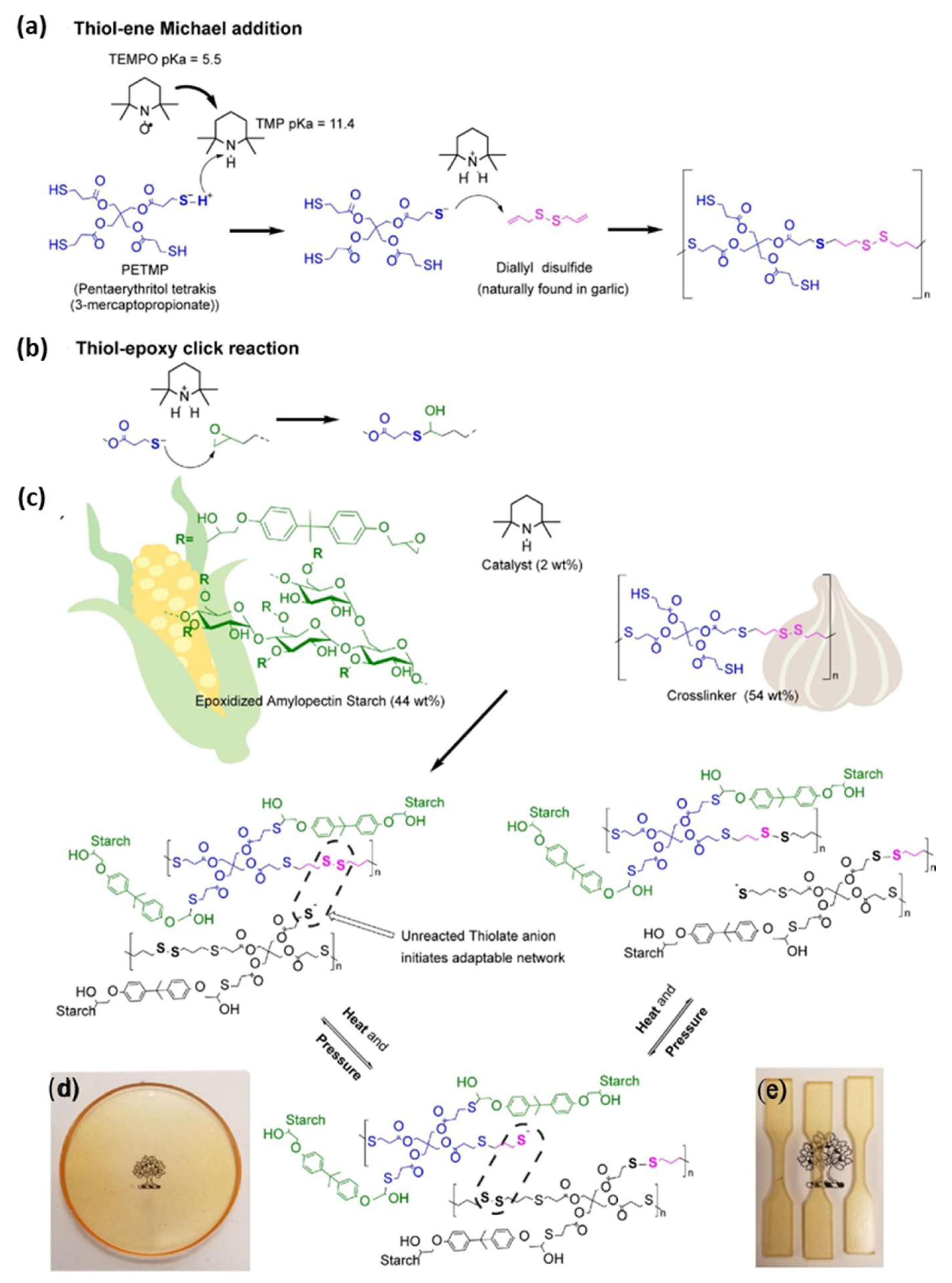
2.2. Carbohydrates
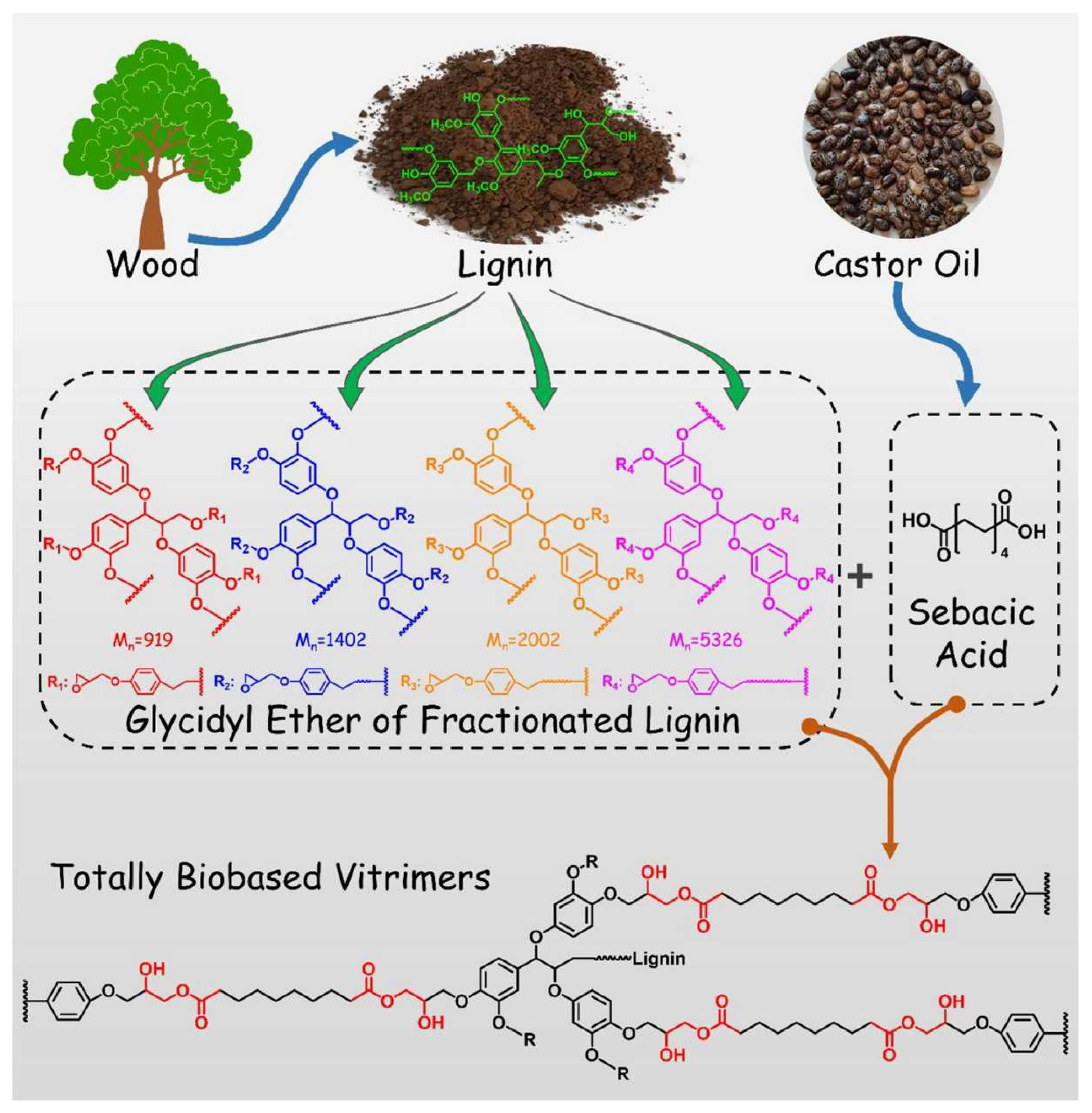
2.3. Lignin and Derivatives
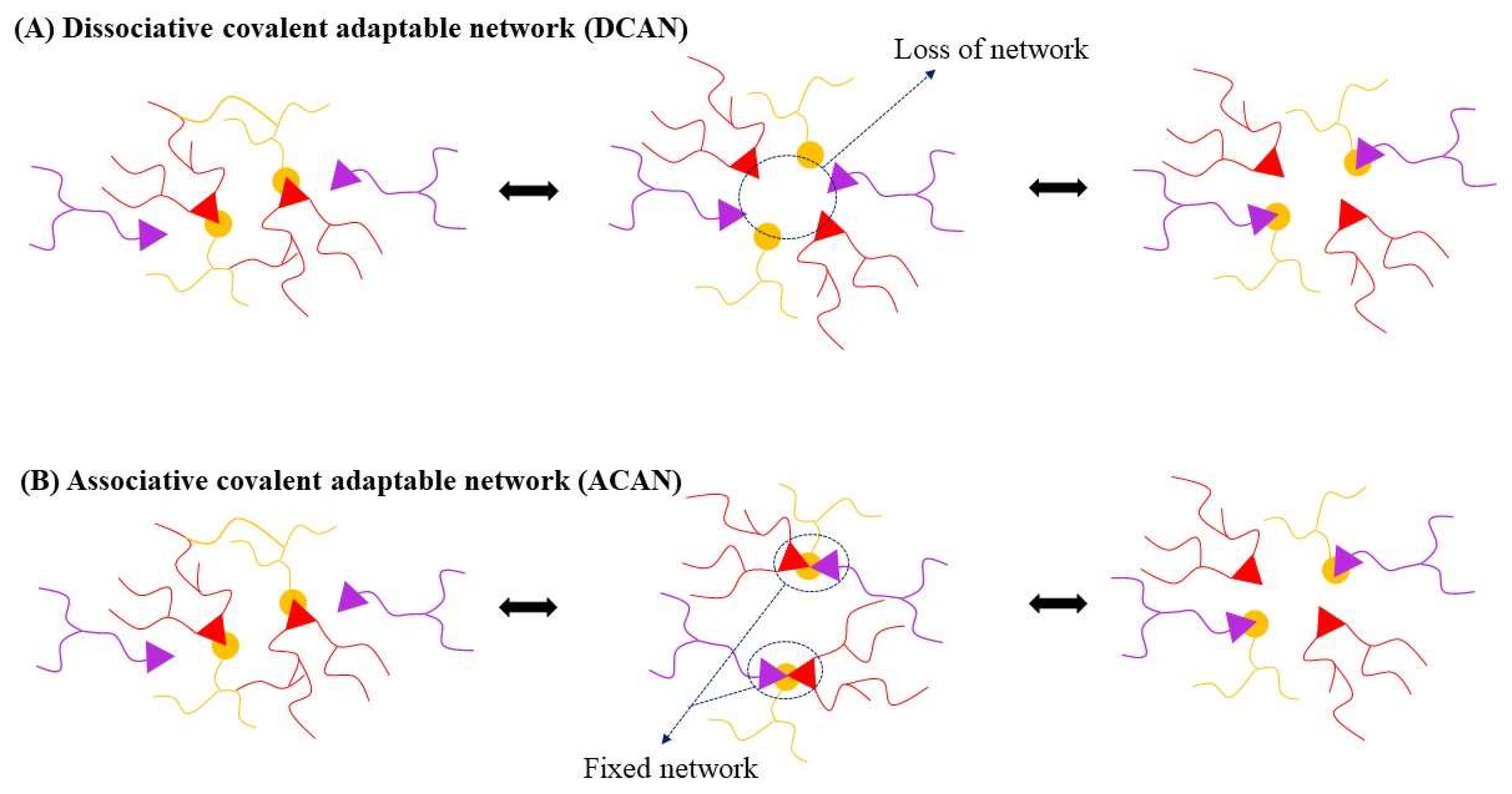
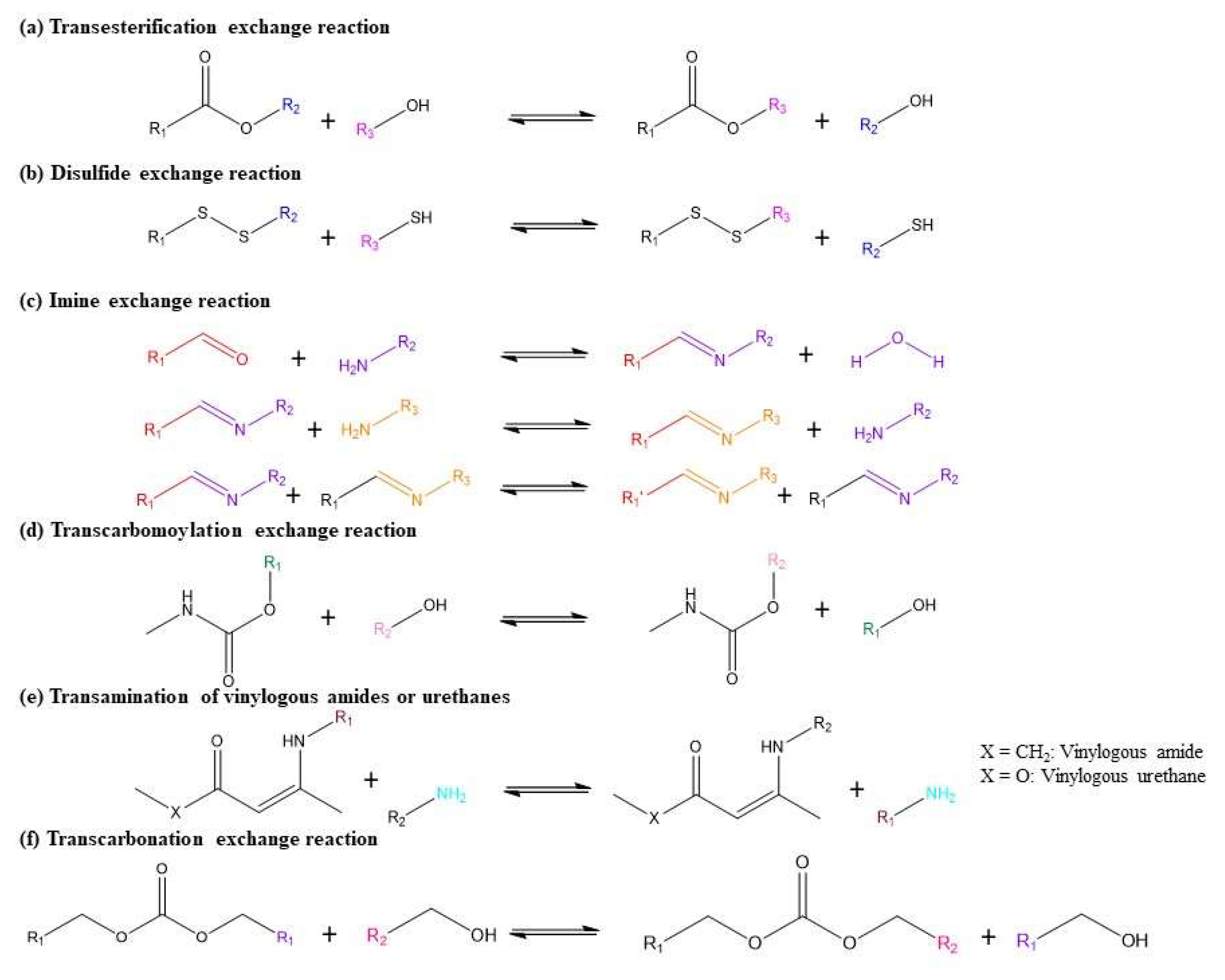
3. Types of Vitrimers
3.1. Transesterification Vitrimers
3.2. Disulfide Vitrimer
3.3. Polyimine Vitrimer
3.4. Other Dynamic Chemistries
| Materials | Mechanism | Properties | References | Cost Estimation a | |||
|---|---|---|---|---|---|---|---|
| Glass Transition Temperature (Tg) | Tensile Strength (MPa) | Young’s Modulus (GPa) | Recycling Condition | ||||
| ESO and rosin-based fumaropimaric acid | Transesterification | 65 | 16 | - | Ethanol solvent at 140 °C | [14] | 285–292 AUD/kg |
| ESO and camphoric acid | 40–48 | 0.56 | 0.0024 | Ethylene glycol at 190 °C | [29] | 986 AUD/kg | |
| Eugenol-derived epoxy and succinic acid | 42–47 | - | - | Ethanol at 160 °C | [59] | 1763–2030 AUD/kg | |
| Malonic ester and poly(hydroxyethyl) | - | 11.3–33.0 | 0.317–1.112 | - | [24] | 900 AUD/kg | |
| Vegetable-oil-based dimer acid and glycidylamine | −10–25 | 12–22 | 0.014–0.037 | Hydrothermal at 90 °C | [57] | 453–550 AUD/kg | |
| Hempseed-oil-based epoxy and diglycidyl bisphenol A | 40 | 54–75 | 1.02–1.29 | - | [27] | 397 AUD/kg | |
| Adipic acid and methane diamine | 72–86 | 57–75 | 1.9–2.3 | Ethanolamine at 60 °C | [26] | 1236–1436 AUD/kg | |
| Isosorbide-derived epoxy and 4,4′-methylenedianiline | Disulfide exchange | 37.3 | 11.37 | 2.36 | 5 wt.% NaOH solution | [65] | 169 AUD/kg |
| Isosorbide-derived epoxy and 4,4′-disulfanediyldianiline | 41.4 | 10.98 | 1.99 | 9110 AUD/kg | |||
| EVO—mono-unsaturated fatty acids and 2,2′-dithiodibenzoic acid | 17–40 | - | - | Mechanical recycling: grounded vitrimer powder was pressed and heated (120–170 °C; 10 min, 1–2 tons depending on fatty acid types) | [63] | 510–545 AUD/kg a* | |
| EVO—di-unsaturated fatty acids and 2,2′-dithiodibenzoic acid | 45–62 | ||||||
| EVO—tri-unsaturated fatty acids and 2,2′-dithiodibenzoic acid | 64–91 | ||||||
| ESO and vanillin | Polyimine exchange | 27.6 | 7.7 | 0.04 | HCl at RT | [25] | 436 AUD/kg |
| Glycerol triglycidyl ether and vanillin | 70 | 62 | 1.62 | Amine solution at RT | [82] | 402 AUD/kg | |
| Vanillin and 4,4′-diaminodiphenylmethane | 102 | 80.3 | 2.89 | HCl at 60 °C | [83] | 744 AUD/kg | |
| Vanillin and isophorondiamine | 99 | 53.9 | 1.56 | 774 AUD/kg | |||
| Vanillin and diethyltriamine | 66 | 33.5 | 1.39 | 814 AUD/kg | |||
| Vanillin and 1,6-hexylenediamine | 83 | 73.2 | 1.96 | HCl at RT | [84] | 279 AUD/kg | |
| Vanillin and m-xylenediamine | 96 | 78.3 | 2.39 | 299 AUD/kg | |||
| Castor oil and DL-limonene | Vinylogous urethane | 39 | 5.5 | 0.027 | - | [42] | 25678 ADU/kg |
| β-ketoesters and vegetable-oil-based amine | 15–90 | 5.12 | 0.096 | Amine solution | [10] | 283 AUD/kg | |
| Methyloleate epoxy and tris(2-aminoethyl)amine | Transamidation | 7–21 | 1.5 | 0.017 | - | [81] | 5062 AUD/kg |
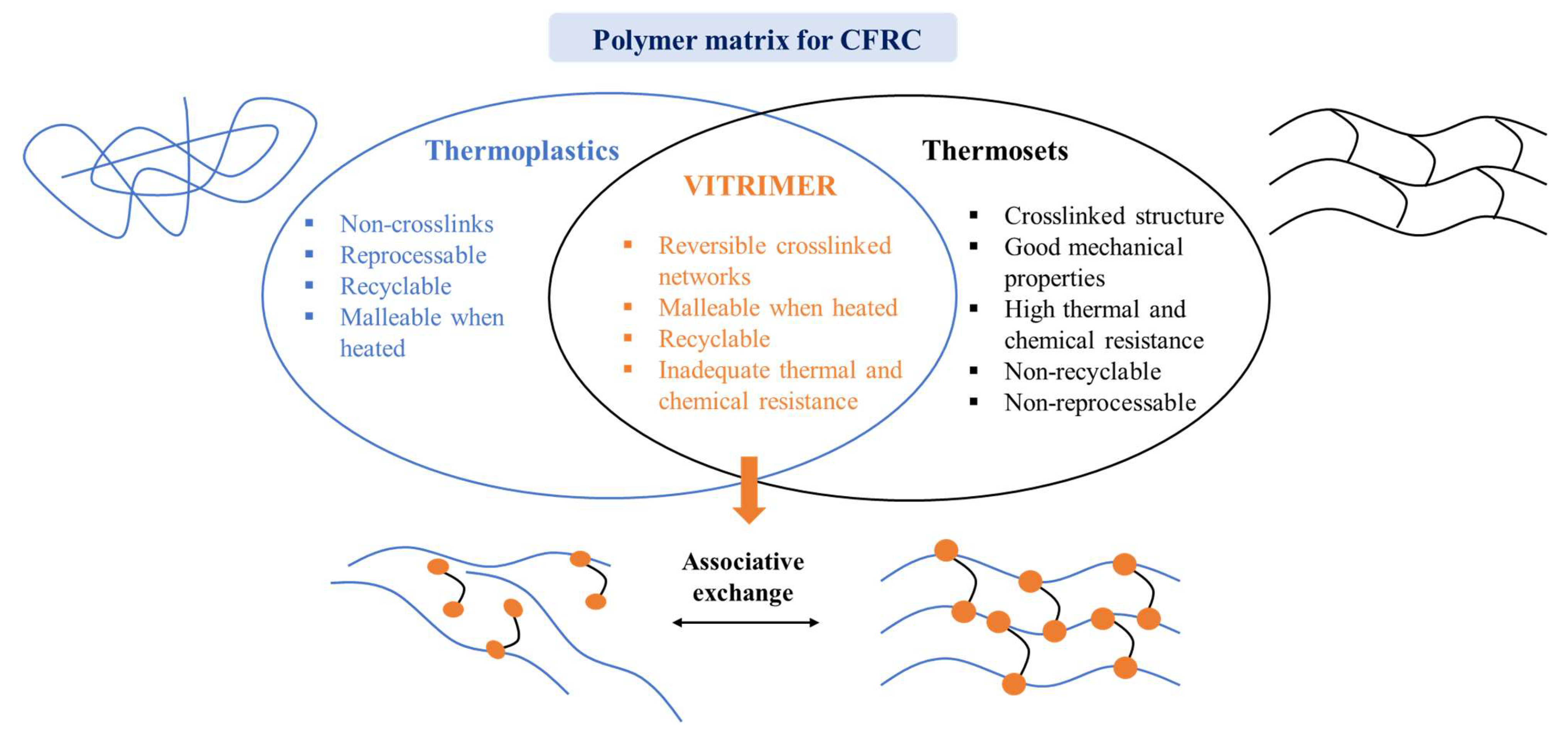
4. Bio-Vitrimer-Based Carbon Fibre Composite
4.1. Use of Bio-Based Vitrimer Matrix for Sustainable CFRCs
| Samples | Tg (°C) | Td5% (°C) | Tensile Strength (MPa) | Young’s Modulus (GPa) | Flexural Strength (MPa) and Modulus (GPa) | Recycling Condition | Reference |
|---|---|---|---|---|---|---|---|
| Epoxidized soybean oil & vanillin & 4-aminophenol | 27.6 | - | 145.4 ± 17.13 | 1.18 ± 0.14 | - | 0.1 M HCl for 20 h for the resin matrix to be completely dissolved. | [25] |
| Vanillin & 4-aminophenol & glycerol triglycidyl ether | 70 | 257 | 449 ± 12 | 12.9 ± 0.9 | - | 0.1 molL−1 ethylene-diamine (EDA) | [82] |
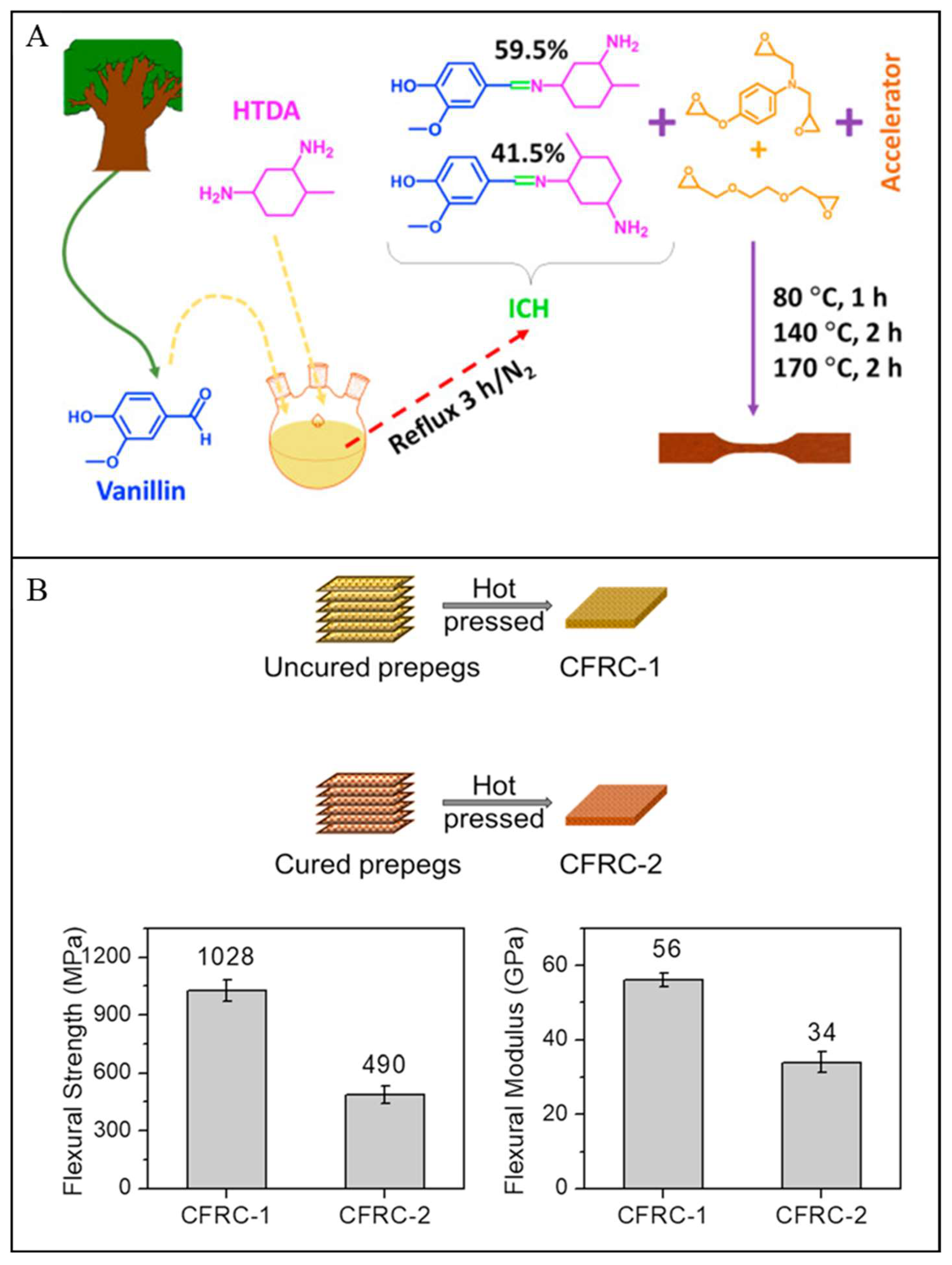
| Vanillin & methylcyclohexane-diamine | >131 | 267–284 | - | - | >490 & >34 | EDA, with epoxy resin:EDA ratio was 1:5 | [3] |
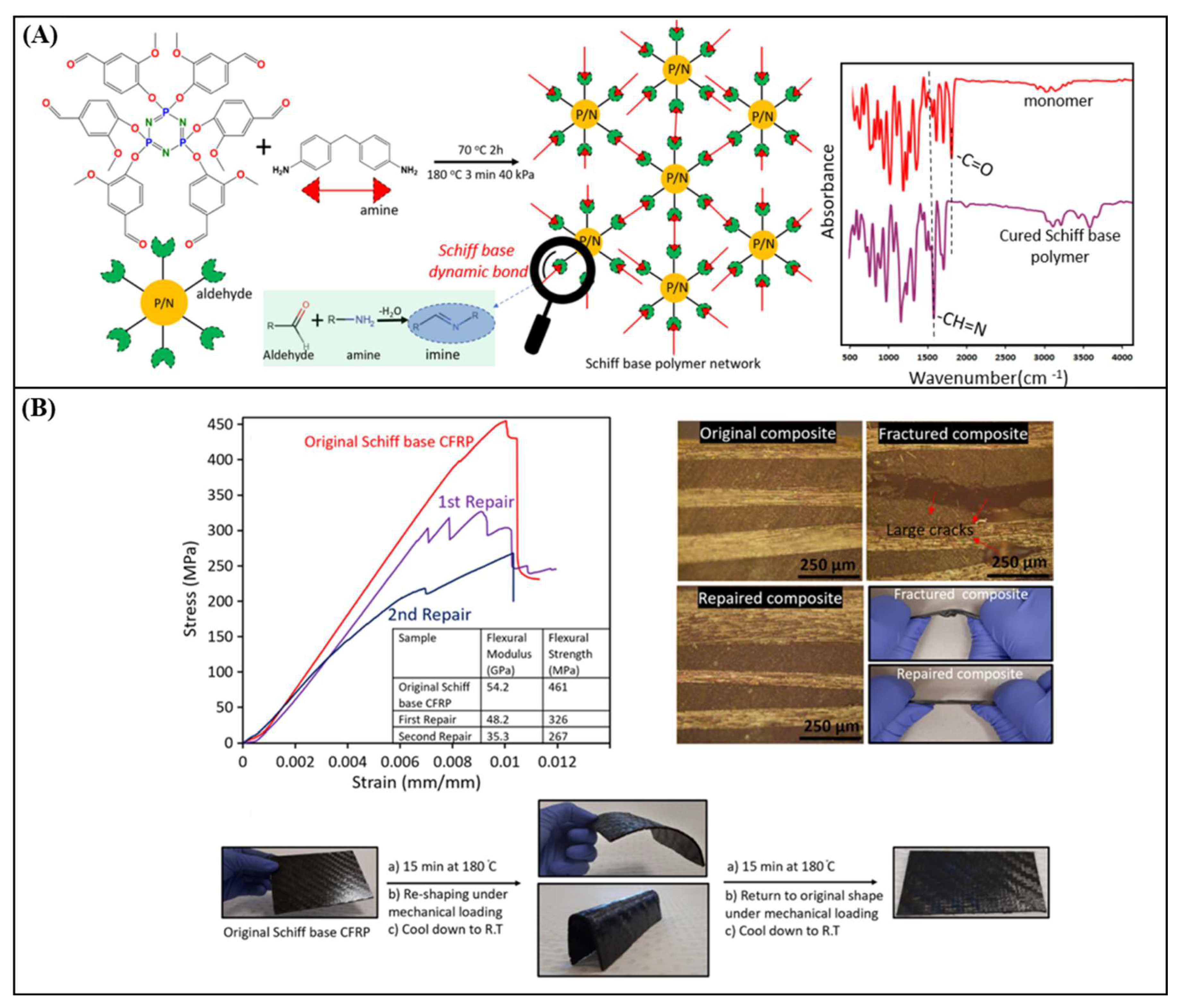
| Vanillin & phosphonitrilic chloride trimer & 4,4-diaminodiphenyl methane | 129 | 215 | 461 ± 21 | 47.3 ± 2 | 455 ± 14 & 54.2 ± 4 | THF/HCl at RT for 24h | [85] |
| Hyperbranched polyesters (HBP) & rosin-derived fumaropimaric acid & glycerol triglycidyl ether | 75.6–90.5 | - | 585 ± 35 (with the 15 wt.% of HBP in the vitrimer system | - | - | Ethylene glycol (EG) at 180 °C for 12 h under N2 atmosphere | [88] |
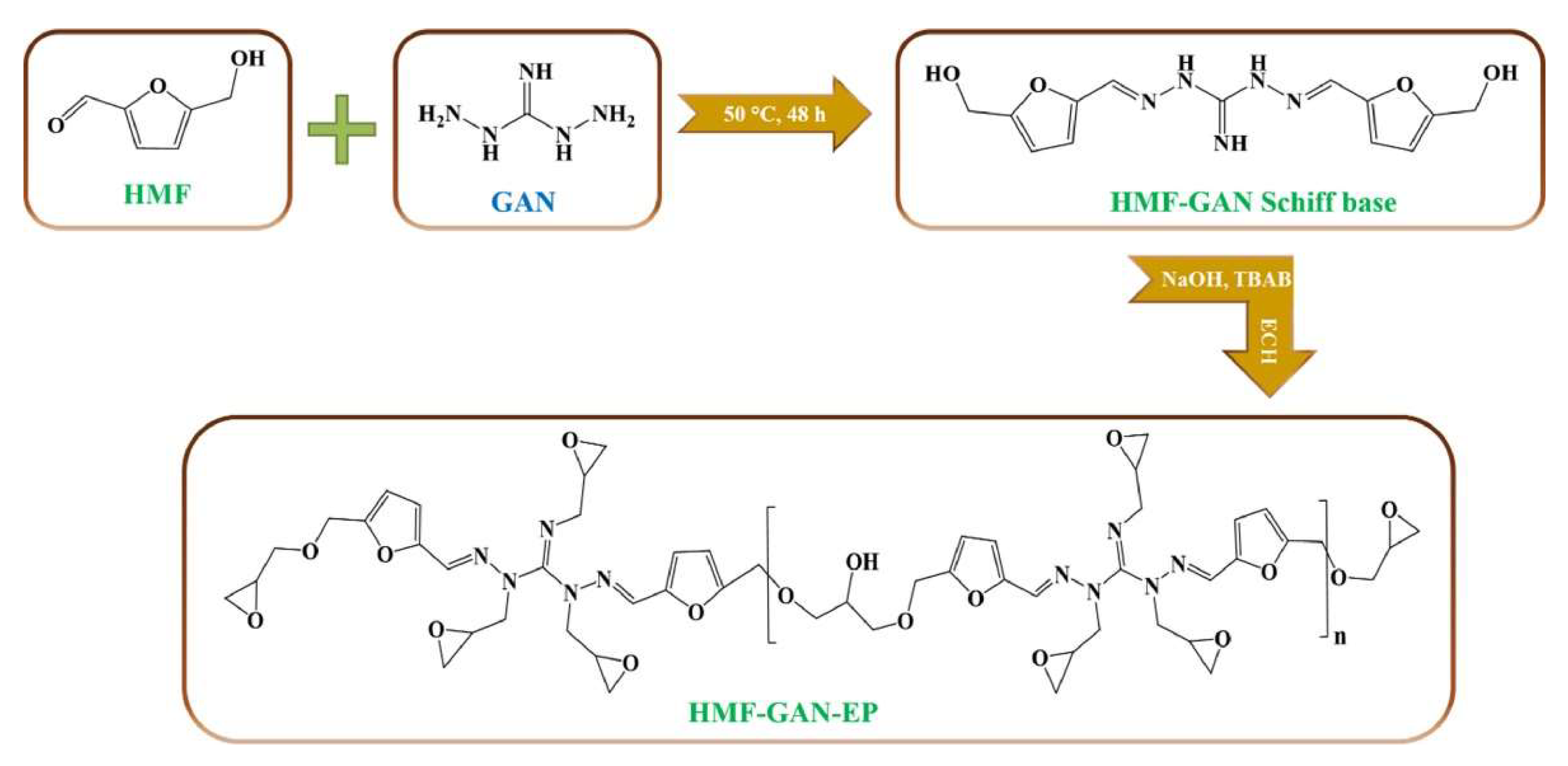
| 5-hydroxymethylfurfural (HMF) & 1,3-diaminoguanidine monohydrochloride (GAN) & furan-based curing agent (DIFFA) | 234.1 | - | - | - | - | THF:HCl (8:2, v/v) | [86] |
| Itaconic acid & maleic anhydride & glycerine | 49–56 | 285 | 417 ± 26 | 31.3 ± 1.8 | - | 1 M NaOH at RT for 5 h | [87] |
| Adipic acid & epoxidized menthane diamine | 72.1–86.4 | 282 (for Td10%) | 465 | - | - | Ethanolamine at 60 °C for 30 min | [26] |
4.2. Processability of Vitrimers by Their Flowable and Thermoformable Behaviours
5. Conclusions and Future Perspectives
- Properties: Keeping biobased material performance comparative to commercial synthetic products remains an open question. Many reported biobased vitrimers often exhibit reduced thermal stability and mechanical properties (e.g., tensile strength, flexural strength, hardness, etc.). Indeed, in many studies, bio-based vitrimers showed relatively low Tg due to the presence of vegetable oil monomers that often have low thermal stability, resulting in reduced mechanical properties. Therefore, most reported bio-based vitrimers have not yet reached sufficient standards to meet the requirements of the market.
- Durability: Limited solvent resistance is another crucial challenge for the development of bio-based vitrimers. For example, many polyimine-based vitrimers exhibit low acid and water resistance due to the imine chemistry in their structure, restricting their application in harsh conditions.
- Processing: Compared to traditional polymer matrix composites, most vitrimers and biobased vitrimers have unique processing requirements depending on their dynamic chemistry, which, in turn, limits their widespread application in CFRC manufacturing.
- Scalability: Industrial scale is another big challenge for biobased vitrimers, as the current production methods are typically not suitable for large-scale industrial applications.
- Cost: Biobased vitrimers are still more expensive than conventional polymer matrix composites, which is mostly related to the pre-treatment process of the raw bio-resources to obtain the bio-based monomer. Indeed, due to complex and heterogeneous structures, most bio-based monomers are still obtained from multiple modifications involving petrochemical reagents and produce large by-product waste. An example of this is natural aromatic compounds (e.g., vanillin, eugenol, etc.) obtained from the modification of lignin macromolecules and/or from biomass pyrolysis pre-treatments, which is an energy-intensive process.
- Environmental impact: In most studies reported in this review or elsewhere, vitrimers are only partially bio-based, and often require copolymerization with petroleum-based monomers or involve toxic and non-natural catalysts/solvents. For instance, the number of bio-based vitrimers is limited by the availability of bio-based diamines and/or aromatic diamines, leading to the reliance on petroleum-based amines (e.g., diethylenetriamine, tris(2-aminoethyl)amine, etc.). Additionally, many reported bio-based vitrimers obtained from epoxidized vegetable oils through transesterification reactions often involve diglycidyl ether of the bisphenol A monomer, which is very toxic for all living things. This also weakens the sustainability of these bio-based materials.
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CFRC | Carbon fibre-reinforced composite |
| 5-HMF | 5-hydroxymethylfurfural |
| CA | Carboxylation |
| CANs | Covalent adaptable networks |
| CF | Carbon fibre |
| CMC | Carboxymethyl cellulose |
| CPA | Camphoric acid |
| DA | Dimer acid |
| DETA | Diethylenetriamine |
| DIFFA | Difurfurylamine |
| DSs | Degrees of substitution |
| EL | Enzymatic lignin |
| ELV | Enzymatic lignin-based vitrimer |
| ENR | Epoxy natural rubber |
| EP | Epoxidation |
| ESO | Epoxidized soybean oil |
| EVO | Epoxidized vegetable oil |
| FPA | Fumaropimaric acid |
| GAN | 1,3-diaminoguanidine monohydrochloride |
| HCl | Hydrochloric acid |
| LEVs | Lignin-based epoxy vitrimers |
| NaOH | Sodium hydroxide |
| S/G | Syringyl/Guacyl |
| SAA | Starch acetoacetate |
| SA | Sebacic acid |
| TBD | 1,5,7-triazabicyclo[4.4.0]dec-5-ene |
| Tg | Glass transition temperature |
| TGDDM | N,N,N′,N′-tetraglycidyl-4,4′-diaminodiphenylmethane |
| THF | Tetrahydrofuran |
| VA | Vanillin |
References
- Blythe, A.; Fox, B.; Nikzad, M.; Eisenbart, B.; Chai, B.X. Stiffness Degradation under Cyclic Loading Using Three-Point Bending of Hybridised Carbon/Glass Fibres with a Polyamide 6,6 Nanofibre Interlayer. J. Compos. Sci. 2022, 6, 270. [Google Scholar] [CrossRef]
- Blythe, A.; Fox, B.; Nikzad, M.; Eisenbart, B.; Chai, B.X.; Blanchard, P.; Dahl, J. Evaluation of the Failure Mechanism in Polyamide Nanofibre Veil Toughened Hybrid Carbon/Glass Fibre Composites. Materials 2022, 15, 8877. [Google Scholar] [CrossRef] [PubMed]
- Memon, H.; Wei, Y.; Zhang, L.; Jiang, Q.; Liu, W. An imine-containing epoxy vitrimer with versatile recyclability and its application in fully recyclable carbon fiber reinforced composites. Compos. Sci. Technol. 2020, 199, 108314. [Google Scholar] [CrossRef]
- Chai, B.; Eisenbart, B.; Nikzad, M.; Fox, B.; Blythe, A.; Blanchard, P.; Dahl, J. A novel heuristic optimisation framework for radial injection configuration for the resin transfer moulding process. Compos. Part A Appl. Sci. Manuf. 2023, 165, 107352. [Google Scholar] [CrossRef]
- Chai, B.X.; Eisenbart, B.; Nikzad, M.; Fox, B.; Blythe, A.; Bwar, K.H.; Wang, J.; Du, Y.; Shevtsov, S. Application of KNN and ANN Metamodeling for RTM Filling Process Prediction. Materials 2023, 16, 6115. [Google Scholar] [CrossRef]
- Fei, M.; Chang, Y.-C.; Hao, C.; Shao, L.; Liu, W.; Zhao, B.; Zhang, J. Highly engineerable Schiff base polymer matrix with facile fiber composite manufacturability and hydrothermal recyclability. Compos. Part B Eng. 2023, 248, 110366. [Google Scholar] [CrossRef]
- Giorgini, L.; Benelli, T.; Brancolini, G.; Mazzocchetti, L. Recycling of carbon fiber reinforced composite waste to close their life cycle in a cradle-to-cradle approach. Curr. Opin. Green Sustain. Chem. 2020, 26, 100368. [Google Scholar] [CrossRef]
- Isa, A.; Nosbi, N.; Ismail, M.C.; Akil, H.M.; Ali, W.F.F.W.; Omar, M.F. A review on recycling of carbon fibres: Methods to reinforce and expected fibre composite degradations. Materials 2022, 15, 4991. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Olivetti, E.A.; Zhao, Y.; Chang, J.C.; Pickering, S.J.; McKechnie, J. Comparing Life Cycle Energy and Global Warming Potential of Carbon Fiber Composite Recycling Technologies and Waste Management Options. ACS Sustain. Chem. Eng. 2018, 6, 9854–9865. [Google Scholar] [CrossRef]
- Hajiali, F.; Tajbakhsh, S.; Marić, M. Thermally reprocessable bio-based polymethacrylate vitrimers and nanocomposites. Polymer 2021, 212, 123126. [Google Scholar] [CrossRef]
- Liang, K.; Zhang, G.; Zhao, J.; Shi, L.; Cheng, J.; Zhang, J. Malleable, Recyclable, and Robust Poly (amide–imine) Vitrimers Prepared through a Green Polymerization Process. ACS Sustain. Chem. Eng. 2021, 9, 5673–5683. [Google Scholar] [CrossRef]
- Si, H.; Zhou, L.; Wu, Y.; Song, L.; Kang, M.; Zhao, X.; Chen, M. Rapidly reprocessable, degradable epoxy vitrimer and recyclable carbon fiber reinforced thermoset composites relied on high contents of exchangeable aromatic disulfide crosslinks. Compos. Part B Eng. 2020, 199, 108278. [Google Scholar] [CrossRef]
- Zhao, W.; Feng, Z.; Liang, Z.; Lv, Y.; Xiang, F.; Xiong, C.; Duan, C.; Dai, L.; Ni, Y. Vitrimer-Cellulose Paper Composites: A New Class of Strong, Smart, Green, and Sustainable Materials. ACS Appl. Mater. Interfaces 2019, 11, 36090–36099. [Google Scholar] [CrossRef]
- Yang, X.; Guo, L.; Xu, X.; Shang, S.; Liu, H. A fully bio-based epoxy vitrimer: Self-healing, triple-shape memory and reprocessing triggered by dynamic covalent bond exchange. Mater. Des. 2020, 186, 108248. [Google Scholar] [CrossRef]
- Montarnal, D.; Capelot, M.; Tournilhac, F.; Leibler, L. Silica-like malleable materials from permanent organic networks. Science 2011, 334, 965–968. [Google Scholar] [CrossRef]
- Snyder, R.L.; Fortman, D.J.; De Hoe, G.X.; Hillmyer, M.A.; Dichtel, W.R. Reprocessable acid-degradable polycarbonate vitrimers. Macromolecules 2018, 51, 389–397. [Google Scholar] [CrossRef]
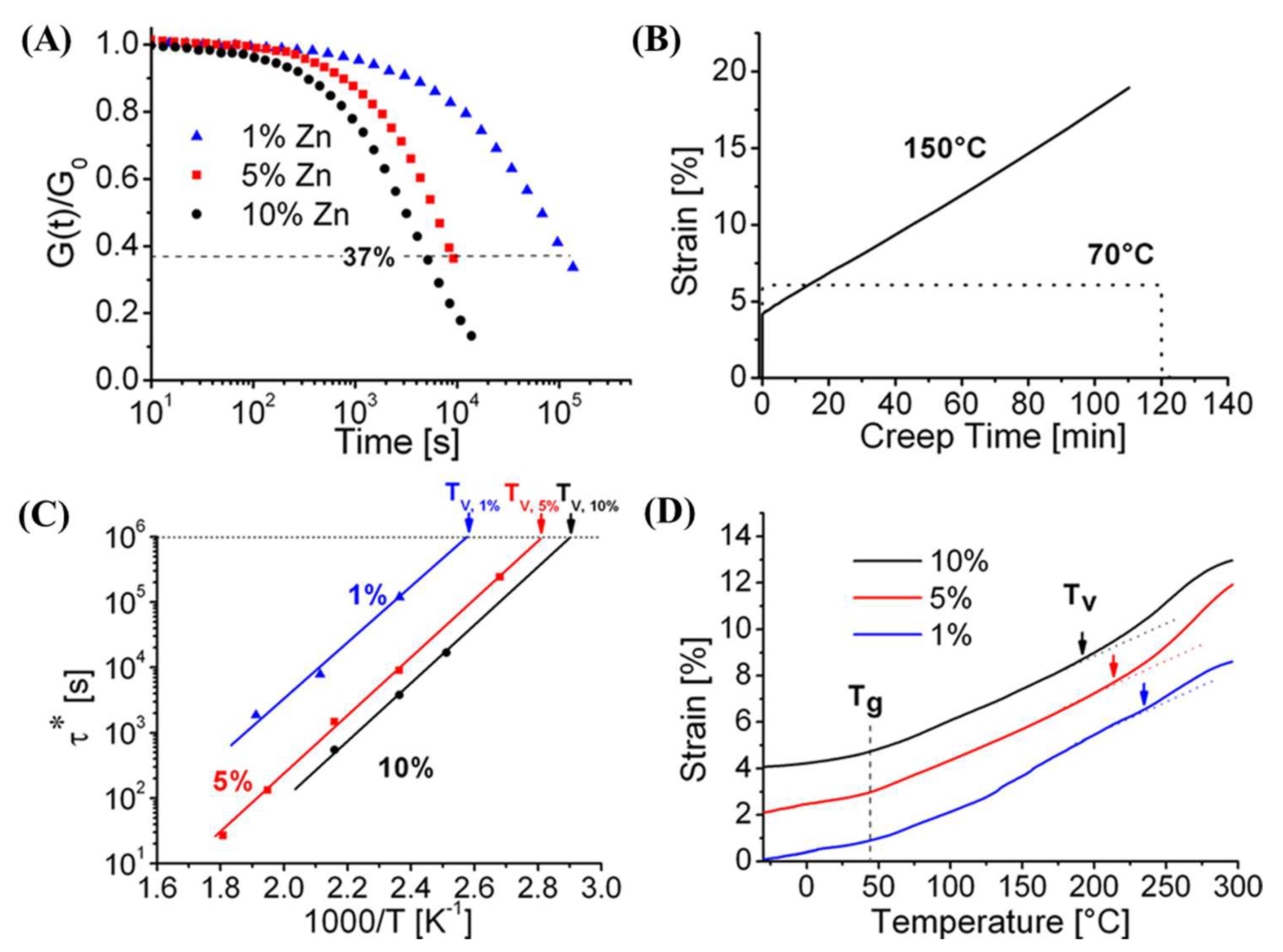
- Niu, X.; Wang, F.; Li, X.; Zhang, R.; Wu, Q.; Sun, P. Using Zn2+ ionomer to catalyze transesterification reaction in epoxy vitrimer. Ind. Eng. Chem. Res. 2019, 58, 5698–5706. [Google Scholar] [CrossRef]
- Altuna, F.I.; Hoppe, C.E.; Williams, R.J.J. Epoxy vitrimers: The effect of transesterification reactions on the network structure. Polymers 2018, 10, 43. [Google Scholar] [CrossRef]
- Kar, G.P.; Saed, M.O.; Terentjev, E.M. Scalable upcycling of thermoplastic polyolefins into vitrimers through transesterification. J. Mater. Chem. A 2020, 8, 24137–24147. [Google Scholar] [CrossRef]
- Denissen, W.; De Baere, I.; Van Paepegem, W.; Leibler, L.; Winne, J.; Prez, F.E.D. Vinylogous Urea Vitrimers and Their Application in Fiber Reinforced Composites. Macromolecules 2018, 51, 2054–2064. [Google Scholar] [CrossRef]
- Taynton, P.; Ni, H.; Zhu, C.; Yu, K.; Loob, S.; Jin, Y.; Qi, H.J.; Zhang, W. Repairable woven carbon fiber composites with full recyclability enabled by malleable polyimine networks. Adv. Mater. 2016, 28, 2904–2909. [Google Scholar] [CrossRef]
- de Luzuriaga, A.R.; Martin, R.; Markaide, N.; Rekondo, A.; Cabañero, G.; Rodríguez, J.; Odriozola, I. Epoxy resin with exchangeable disulfide crosslinks to obtain reprocessable, repairable and recyclable fiber-reinforced thermoset composites. Mater. Horiz. 2016, 3, 241–247. [Google Scholar] [CrossRef]
- Dhers, S.; Vantomme, G.; Avérous, L. A fully bio-based polyimine vitrimer derived from fructose. Green Chem. 2019, 21, 1596–1601. [Google Scholar] [CrossRef]
- Debnath, S.; Kaushal, S.; Ojha, U. Catalyst-Free Partially Bio-Based Polyester Vitrimers. ACS Appl. Polym. Mater. 2020, 2, 1006–1013. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; He, J.; Li, Y.-D.; Zhao, X.-L.; Zeng, J.-B. Biobased epoxy vitrimer from epoxidized soybean oil for reprocessable and recyclable carbon fiber reinforced composite. Compos. Commun. 2020, 22, 10044. [Google Scholar] [CrossRef]
- Xu, Y.; Dai, S.; Bi, L.; Jiang, J.; Zhang, H.; Chen, Y. Catalyst-free self-healing bio-based vitrimer for a recyclable, reprocessable, and self-adhered carbon fiber reinforced composite. Chem. Eng. J. 2022, 429, 132518. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, T.; Hao, C.; Mikkelsen, A.; Zhao, B.; Zhang, J. Hempseed Oil-Based Covalent Adaptable Epoxy-Amine Network and Its Potential Use for Room-Temperature Curable Coatings. ACS Sustain. Chem. Eng. 2020, 8, 14964–14974. [Google Scholar] [CrossRef]
- Liu, X.; Liang, L.; Lu, M.; Song, X.; Liu, H.; Chen, G. Water-resistant bio-based vitrimers based on dynamic imine bonds: Self-healability, remodelability and ecofriendly recyclability. Polymer 2020, 210, 123030. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, J.; Gao, L.; Zhang, B.; Jiang, J.; Hu, J. Recyclable, reprocessable, self-adhered and repairable carbon fiber reinforced polymers using full biobased matrices from camphoric acid and epoxidized soybean oil. Green Chem. 2021, 23, 2763–2772. [Google Scholar] [CrossRef]
- Wang, S.; Ma, S.; Li, Q.; Xu, X.; Wang, B.; Yuan, W.; Zhou, S.; You, S.; Zhu, J. Facile in situ preparation of high-performance epoxy vitrimer from renewable resources and its application in nondestructive recyclable carbon fiber composite. Green Chem. 2019, 21, 1484–1497. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, Y.; Ji, Y.; Wei, Y. Functional epoxy vitrimers and composites. Prog. Mater. Sci. 2020, 120, 100710. [Google Scholar] [CrossRef]
- Wang, S.; Ma, S.; Li, Q.; Xu, X.; Wang, B.; Huang, K.; Liu, Y.; Zhu, J. Facile Preparation of Polyimine Vitrimers with Enhanced Creep Resistance and Thermal and Mechanical Properties via Metal Coordination. Macromolecules 2020, 53, 2919–2931. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, T.; Hao, C.; Wang, L.; Han, J.; Liu, H.; Zhang, J. Preparation of a lignin-based vitrimer material and its potential use for recoverable adhesives. Green Chem. 2018, 20, 2995–3000. [Google Scholar] [CrossRef]
- Salaeh, S.; Das, A.; Wießner, S.; Stapor, M. Vitrimer-like material based on a biorenewable elastomer crosslinked with a dimeric fatty acid. Eur. Polym. J. 2021, 151, 110452. [Google Scholar] [CrossRef]
- Di Mauro, C.; Malburet, S.; Genua, A.; Graillot, A.; Mija, A. Sustainable Series of New Epoxidized Vegetable Oil-Based Thermosets with Chemical Recycling Properties. Biomacromolecules 2020, 21, 3923–3935. [Google Scholar] [CrossRef] [PubMed]
- Zych, A.; Tellers, J.; Bertolacci, L.; Ceseracciu, L.; Marini, L.; Mancini, G.; Athanassiou, A. Biobased, Biodegradable, Self-Healing Boronic Ester Vitrimers from Epoxidized Soybean Oil Acrylate. ACS Appl. Polym. Mater. 2021, 3, 1135–1144. [Google Scholar] [CrossRef]
- Omonov, T.S.; Curtis, J.M. 7—Plant Oil-Based Epoxy Intermediates for Polymers. In Bio-Based Plant Oil Polymers and Composites; Madbouly, S.A., Zhang, C., Kessler, M.R., Eds.; William Andrew Publishing: Norwich, NY, USA, 2016; pp. 99–125. [Google Scholar]
- Çaylı, G.; Küsefoğlu, S. Biobased polyisocyanates from plant oil triglycerides: Synthesis, polymerization, and characterization. J. Appl. Polym. Sci. 2008, 109, 2948–2955. [Google Scholar] [CrossRef]
- Wu, J.; Yu, X.; Zhang, H.; Guo, J.; Hu, J.; Li, M.-H. Fully Biobased Vitrimers from Glycyrrhizic Acid and Soybean Oil for Self-Healing, Shape Memory, Weldable, and Recyclable Materials. ACS Sustain. Chem. Eng. 2020, 8, 6479–6487. [Google Scholar] [CrossRef]
- Zhao, X.-L.; Liu, Y.-Y.; Weng, Y.; Li, Y.-D.; Zeng, J.-B. Sustainable Epoxy Vitrimers from Epoxidized Soybean Oil and Vanillin. ACS Sustain. Chem. Eng. 2020, 8, 15020–15029. [Google Scholar] [CrossRef]
- Xu, Y.-Z.; Fu, P.; Dai, S.-L.; Zhang, H.-B.; Bi, L.-W.; Jiang, J.-X.; Chen, Y.-X. Catalyst-free self-healing fully bio-based vitrimers derived from tung oil: Strong mechanical properties, shape memory, and recyclability. Ind. Crops Prod. 2021, 171, 113978. [Google Scholar] [CrossRef]
- Zhu, Y.; Gao, F.; Zhong, J.; Shen, L.; Lin, Y. Renewable castor oil and DL-limonene derived fully bio-based vinylogous urethane vitrimers. Eur. Polym. J. 2020, 135, 109865. [Google Scholar] [CrossRef]
- Ren, J.; Xuan, H.; Ge, L. Double network self-healing chitosan/dialdehyde starch-polyvinyl alcohol film for gas separation. Appl. Surf. Sci. 2018, 469, 213–219. [Google Scholar] [CrossRef]
- Liu, H.; Guo, L.; Tao, S.; Huang, Z.; Qi, H. Freely Moldable Modified Starch as a Sustainable and Recyclable Plastic. Biomacromolecules 2021, 22, 2676–2683. [Google Scholar] [CrossRef] [PubMed]
- Jouyandeh, M.; Tikhani, F.; Hampp, N.; Yazdi, D.A.; Zarrintaj, P.; Ganjali, M.R.; Saeb, M.R. Highly curable self-healing vitrimer-like cellulose-modified halloysite nanotube/epoxy nanocomposite coatings. Chem. Eng. J. 2020, 396, 125196. [Google Scholar] [CrossRef]
- Vidil, T.; Llevot, A. Fully Biobased Vitrimers: Future Direction toward Sustainable Cross-Linked Polymers. Macromol. Chem. Phys. 2022, 223, 2100494. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, J.; Qi, H.J.; Wang, T.; Naguib, H.E. Green and Sustainable Layered Chitin–Vitrimer Composite with Enhanced Modulus, Reprocessability, and Smart Actuator Function. ACS Sustain. Chem. Eng. 2020, 8, 15168–15178. [Google Scholar] [CrossRef]
- Li, C.; Ju, B.; Zhang, S. Construction of a new green vitrimer material: Introducing dynamic covalent bond into carboxymethyl cellulose. Cellulose 2021, 28, 2879–2888. [Google Scholar] [CrossRef]
- Lossada, F.; Guo, J.; Jiao, D.; Groeer, S.; Bourgeat-Lami, E.; Montarnal, D.; Walther, A. Vitrimer chemistry meets cellulose nanofibrils: Bioinspired nanopapers with high water resistance and strong adhesion. Biomacromolecules 2018, 20, 1045–1055. [Google Scholar] [CrossRef]
- Tratnik, N.; Tanguy, N.R.; Yan, N. Recyclable, self-strengthening starch-based epoxy vitrimer facilitated by exchangeable disulfide bonds. Chem. Eng. J. 2023, 451, 138610. [Google Scholar] [CrossRef]
- Agarwal, S. Major factors affecting the characteristics of starch based biopolymer films. Eur. Polym. J. 2021, 160, 110788. [Google Scholar] [CrossRef]
- Tiozon, R.J.N.; Bonto, A.P.; Sreenivasulu, N. Enhancing the functional properties of rice starch through biopolymer blending for industrial applications: A review. Int. J. Biol. Macromol. 2021, 192, 100–117. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Jin, X.; Qian, G.; Yang, W.; Su, L.; Ma, Y.; Ren, S.; Li, S. Lignin-based vitrimer for circulation in plastics, coatings, and adhesives with tough mechanical properties, catalyst-free and good chemical solvent resistance. Ind. Crops Prod. 2022, 187, 115439. [Google Scholar] [CrossRef]
- Tang, R.; Xue, B.; Tan, J.; Guan, Y.; Wen, J.; Li, X.; Zhao, W. Regulating Lignin-Based Epoxy Vitrimer Performance by Fine-Tuning the Lignin Structure. ACS Appl. Polym. Mater. 2022, 4, 1117–1125. [Google Scholar] [CrossRef]
- Quader, M.A.; Ahmed, S. Chapter Four—Bioenergy With Carbon Capture and Storage (BECCS): Future Prospects of Carbon-Negative Technologies. In Clean Energy for Sustainable Development; Rasul, M.G., Azad, A.K., Sharma, S.C., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 91–140. [Google Scholar]
- Liu, Y.; Tang, Z.; Chen, Y.; Zhang, C.; Guo, B. Engineering of β-Hydroxyl Esters into Elastomer–Nanoparticle Interface toward Malleable, Robust, and Reprocessable Vitrimer Composites. ACS Appl. Mater. Interfaces 2018, 10, 2992–3001. [Google Scholar] [CrossRef]
- Li, Y.; Liu, T.; Zhang, S.; Shao, L.; Fei, M.; Yu, H.; Zhang, J. Catalyst-free vitrimer elastomers based on a dimer acid: Robust mechanical performance, adaptability and hydrothermal recyclability. Green Chem. 2020, 22, 870–881. [Google Scholar] [CrossRef]
- Feng, Z.; Hu, J.; Zuo, H.; Ning, N.; Zhang, L.; Yu, B.; Tian, M. Photothermal-Induced Self-Healable and Reconfigurable Shape Memory Bio-Based Elastomer with Recyclable Ability. ACS Appl. Mater. Interfaces 2019, 11, 1469–1479. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Hao, C.; Wang, L.; Li, Y.; Liu, W.; Xin, J.; Zhang, J. Eugenol-Derived Biobased Epoxy: Shape Memory, Repairing, and Recyclability. Macromolecules 2017, 50, 8588–8597. [Google Scholar] [CrossRef]
- Liu, T.; Zhao, B.; Zhang, J. Recent development of repairable, malleable and recyclable thermosetting polymers through dynamic transesterification. Polymer 2020, 194, 122392. [Google Scholar] [CrossRef]
- Altuna, F.I.; Pettarin, V.; Williams, R.J. Self-healable polymer networks based on the cross-linking of epoxidised soybean oil by an aqueous citric acid solution. Green Chem. 2013, 15, 3360–3366. [Google Scholar] [CrossRef]
- Zeng, R.-T.; Wu, Y.; Li, Y.-D.; Wang, M.; Zeng, J.-B. Curing behavior of epoxidized soybean oil with biobased dicarboxylic acids. Polym. Test. 2017, 57, 281–287. [Google Scholar] [CrossRef]
- Di Mauro, C.; Malburet, S.; Graillot, A.; Mija, A. Recyclable, Repairable, and Reshapable (3R) Thermoset Materials with Shape Memory Properties from Bio-Based Epoxidized Vegetable Oils. ACS Appl. Bio Mater. 2020, 3, 8094–8104. [Google Scholar] [CrossRef] [PubMed]
- di Mauro, C.; Tran, T.-N.; Graillot, A.; Mija, A. Enhancing the Recyclability of a Vegetable Oil-Based Epoxy Thermoset through Initiator Influence. ACS Sustain. Chem. Eng. 2020, 8, 7690–7700. [Google Scholar] [CrossRef]
- Ma, Z.; Wang, Y.; Zhu, J.; Yu, J.; Hu, Z. Bio-based epoxy vitrimers: Reprocessibility, controllable shape memory, and degradability. J. Polym. Sci. Part A Polym. Chem. 2017, 55, 1790–1799. [Google Scholar] [CrossRef]
- Yang, X.; Ke, Y.; Chen, Q.; Shen, L.; Xue, J.; Quirino, R.L.; Yan, Z.; Luo, Y.; Zhang, C. Efficient transformation of renewable vanillin into reprocessable, acid-degradable and flame retardant polyimide vitrimers. J. Clean. Prod. 2022, 333, 130043. [Google Scholar] [CrossRef]
- Taynton, P.; Yu, K.; Shoemaker, R.K.; Jin, Y.; Qi, H.J.; Zhang, W. Heat- or water-driven malleability in a highly recyclable covalent network polymer. Adv. Mater. 2014, 26, 3938–3942. [Google Scholar] [CrossRef] [PubMed]
- Zheng, H.; Liu, Q.; Lei, X.; Chen, Y.; Zhang, B.; Zhang, Q. Performance-modified polyimine vitrimers: Flexibility, thermal stability and easy reprocessing. J. Mater. Sci. 2019, 54, 2690–2698. [Google Scholar] [CrossRef]
- Taplan, C.; Guerre, M.; Winne, J.M.; Prez, F.E.D. Fast processing of highly crosslinked, low-viscosity vitrimers. Mater. Horiz. 2020, 7, 104–110. [Google Scholar] [CrossRef]
- Hajj, R.; Duval, A.; Dhers, S.; Avérous, L. Network Design to Control Polyimine Vitrimer Properties: Physical Versus Chemical Approach. Macromolecules 2020, 53, 3796–3805. [Google Scholar] [CrossRef]
- Zhou, Z.; Su, X.; Liu, J.; Liu, R. Synthesis of Vanillin-Based Polyimine Vitrimers with Excellent Reprocessability, Fast Chemical Degradability, and Adhesion. ACS Appl. Polym. Mater. 2020, 2, 5716–5725. [Google Scholar] [CrossRef]
- Ricarte, R.G.; Tournilhac, F.; Cloître, M.; Leibler, L. Linear Viscoelasticity and Flow of Self-Assembled Vitrimers: The Case of a Polyethylene/Dioxaborolane System. Macromolecules 2020, 53, 1852–1866. [Google Scholar] [CrossRef]
- Wu, J.; Gao, L.; Guo, Z.; Zhang, H.; Zhang, B.; Hu, J.; Li, M.-H. Natural glycyrrhizic acid: Improving stress relaxation rate and glass transition temperature simultaneously in epoxy vitrimers. Green Chem. 2021, 23, 5647–5655. [Google Scholar] [CrossRef]
- Tao, Y.; Fang, L.; Zhou, J.; Wang, C.; Sun, J.; Fang, Q. Gel–Sol Transition of Vanillin-Based Polyimine Vitrimers: Imparting Vitrimers with Extra Welding and Self-Healing Abilities. ACS Appl. Polym. Mater. 2020, 2, 295–303. [Google Scholar] [CrossRef]
- Wu, H.; Jin, B.; Wang, H.; Wu, W.; Cao, Z.; Wu, J.; Huang, G. A Degradable and Self-Healable Vitrimer Based on Non-isocyanate Polyurethane. Front. Chem. 2020, 8, 585569. (In English) [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Lamb, K.J.; North, M. Recent developments in organocatalysed transformations of epoxides and carbon dioxide into cyclic carbonates. Green Chem. 2021, 23, 77–118. [Google Scholar] [CrossRef]
- Welch, A.J.; Dunn, E.; DuChene, J.S.; Atwater, H.A. Bicarbonate or carbonate processes for coupling carbon dioxide capture and electrochemical conversion. ACS Energy Lett. 2020, 5, 940–945. [Google Scholar] [CrossRef]
- Alabiso, W.; Schlögl, S. The impact of vitrimers on the industry of the future: Chemistry, properties and sustainable forward-looking applications. Polymers 2020, 12, 1660. [Google Scholar] [CrossRef] [PubMed]
- Denissen, W.; Rivero, G.; Nicolaÿ, R.; Leibler, L.; Winne, J.M.; Prez, F.E.D. Vinylogous urethane vitrimers. Adv. Funct. Mater. 2015, 25, 2451–2457. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, C.; Shi, Z.; Yin, J.; Tian, M. Tailoring vinylogous urethane chemistry for the cross-linked polybutadiene: Wide freedom design, multiple recycling methods, good shape memory behavior. Polymer 2018, 148, 202–210. [Google Scholar] [CrossRef]
- Pettazzoni, L.; Leonelli, F.; Martinelli, A.; Migneco, L.M.; Alfano, S.; Di Luca, D.; Celio, L.; Di Lisio, V. Transamidation-based vitrimers from renewable sources. J. Appl. Polym. Sci. 2022, 139, 52408. [Google Scholar] [CrossRef]
- Liu, Y.-Y.; Liu, G.-L.; Li, Y.-D.; Weng, Y.; Zeng, J.-B. Biobased High-Performance Epoxy Vitrimer with UV Shielding for Recyclable Carbon Fiber Reinforced Composites. ACS Sustain. Chem. Eng. 2021, 9, 4638–4647. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, A.; Zhang, L.; Chen, Z.; Qin, R.; Liu, Y.; Jiang, X.; Ye, D.; Liu, Z. Recyclable Carbon Fiber Reinforced Vanillin-Based Polyimine Vitrimers: Degradation and Mechanical Properties Study. Macromol. Mater. Eng. 2022, 307, 2100893. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, E.; Feng, Z.; Liu, J.; Chen, B.; Liang, L. Degradable bio-based epoxy vitrimers based on imine chemistry and their application in recyclable carbon fiber composites. J. Mater. Sci. 2021, 56, 15733–15751. [Google Scholar] [CrossRef]
- Zamani, P.; Zabihi, O.; Ahmadi, M.; Mahmoodi, R.; Kannangara, T.; Joseph, P.; Naebe, M. Biobased Carbon Fiber Composites with Enhanced Flame Retardancy: A Cradle-to-Cradle Approach. ACS Sustain. Chem. Eng. 2021, 10, 1059–1069. [Google Scholar] [CrossRef]
- Nabipour, H.; Wang, X.; Kandola, B.; Song, L.; Kan, Y.; Chen, J.; Hu, Y. A bio-based intrinsically flame-retardant epoxy vitrimer from furan derivatives and its application in recyclable carbon fiber composites. Polym. Degrad. Stab. 2023, 207, 110206. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, B.; Ma, S.; Yu, T.; Xu, X.; Li, Q.; Wang, S.; Han, Y.; Yu, Z.; Zhu, J. Catalyst-free malleable, degradable, bio-based epoxy thermosets and its application in recyclable carbon fiber composites. Compos. Part B Eng. 2021, 211, 108654. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, H.; Dai, S.; Xu, S.; Wang, J.; Bi, L.; Jiang, J.; Chen, Y. Hyperbranched polyester catalyzed self-healing bio-based vitrimer for closed-loop recyclable carbon fiber-reinforced polymers. Compos. Sci. Technol. 2022, 228, 109676. [Google Scholar] [CrossRef]
- Lucherelli, M.A.; Duval, A.; Avérous, L. Biobased vitrimers: Towards sustainable and adaptable performing polymer materials. Prog. Polym. Sci. 2022, 127, 101515. [Google Scholar] [CrossRef]
- Van Zee, N.J.; Nicolaÿ, R. Vitrimers: Permanently crosslinked polymers with dynamic network topology. Prog. Polym. Sci. 2020, 104, 101233. [Google Scholar] [CrossRef]
- El-Zaatari, B.M.; Ishibashi, J.S.; Kalow, J.A. Cross-linker control of vitrimer flow. Polym. Chem. 2020, 11, 5339–5345. [Google Scholar] [CrossRef]
- Capelot, M.; Unterlass, M.M.; Tournilhac, F.; Leibler, L. Catalytic Control of the Vitrimer Glass Transition. ACS Macro Lett. 2012, 1, 789–792. [Google Scholar] [CrossRef] [PubMed]
- Weidmann, S.; Volk, P.; Mitschang, P.; Markaide, N. Investigations on thermoforming of carbon fiber reinforced epoxy vitrimer composites. Compos. Part A Appl. Sci. Manuf. 2022, 154, 106791. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tran, H.T.T.; Nisha, S.S.; Radjef, R.; Nikzad, M.; Bjekovic, R.; Fox, B. Recyclable and Biobased Vitrimers for Carbon Fibre-Reinforced Composites—A Review. Polymers 2024, 16, 1025. https://doi.org/10.3390/polym16081025
Tran HTT, Nisha SS, Radjef R, Nikzad M, Bjekovic R, Fox B. Recyclable and Biobased Vitrimers for Carbon Fibre-Reinforced Composites—A Review. Polymers. 2024; 16(8):1025. https://doi.org/10.3390/polym16081025
Chicago/Turabian StyleTran, Hoang T. T., Shammi Sultana Nisha, Racim Radjef, Mostafa Nikzad, Robert Bjekovic, and Bronwyn Fox. 2024. "Recyclable and Biobased Vitrimers for Carbon Fibre-Reinforced Composites—A Review" Polymers 16, no. 8: 1025. https://doi.org/10.3390/polym16081025