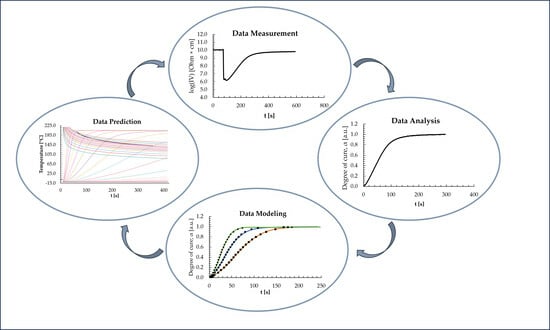
In Situ Monitoring of the Curing of Highly Filled Epoxy Molding Compounds: The Influence of Reaction Type and Silica Content on Cure Kinetic Models
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Dielectric Analysis (DEA) in Transfer Mold
2.3. Kinetic Analysis via Dielectric Analysis (DEA)
2.4. Differential Scanning Calorimetry (DSC)
3. Results and Discussion
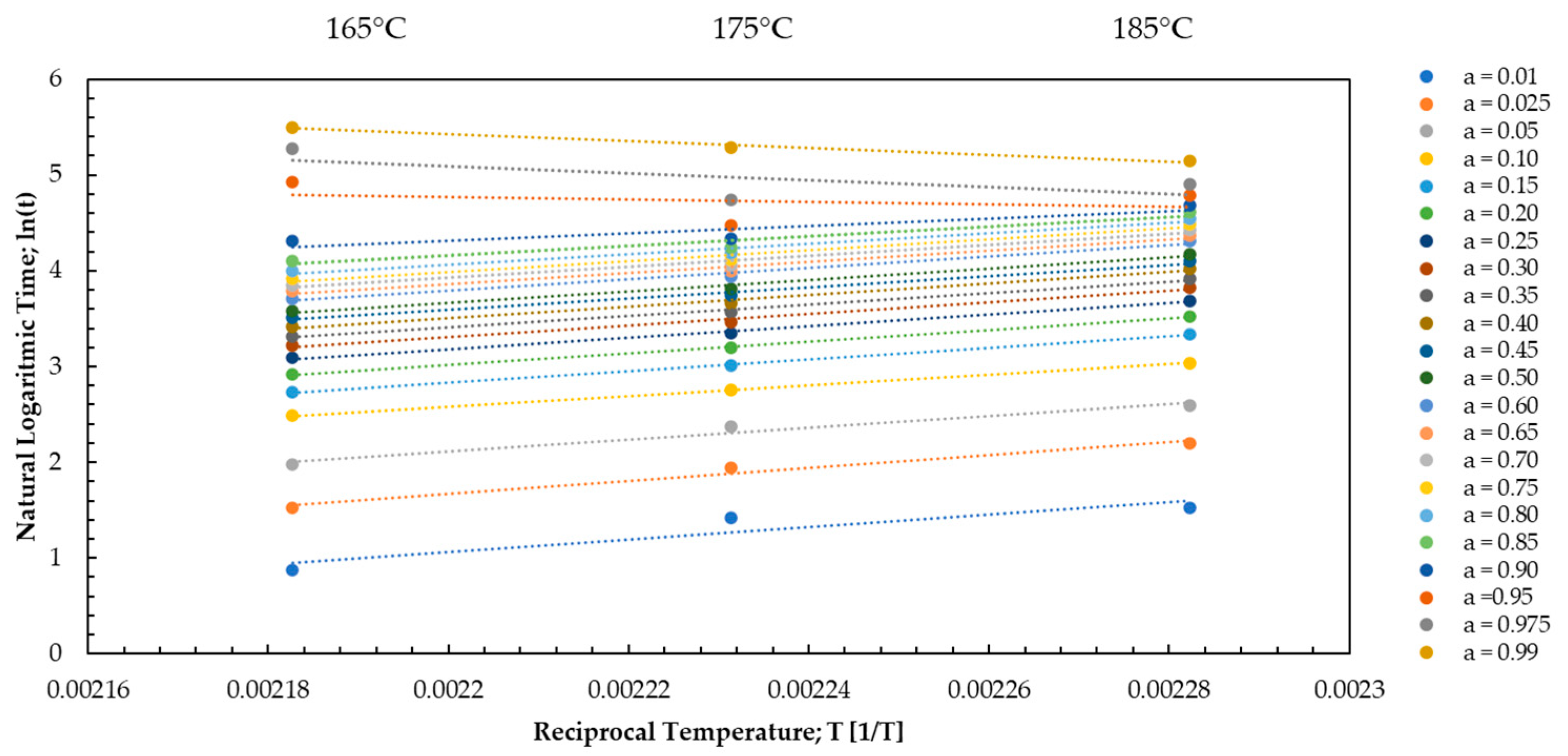
3.1. Kinetic Analysis via Dielectric Analysis (DEA)
3.2. Kinetic Analysis via Differential Scanning Calorimetry (DSC)
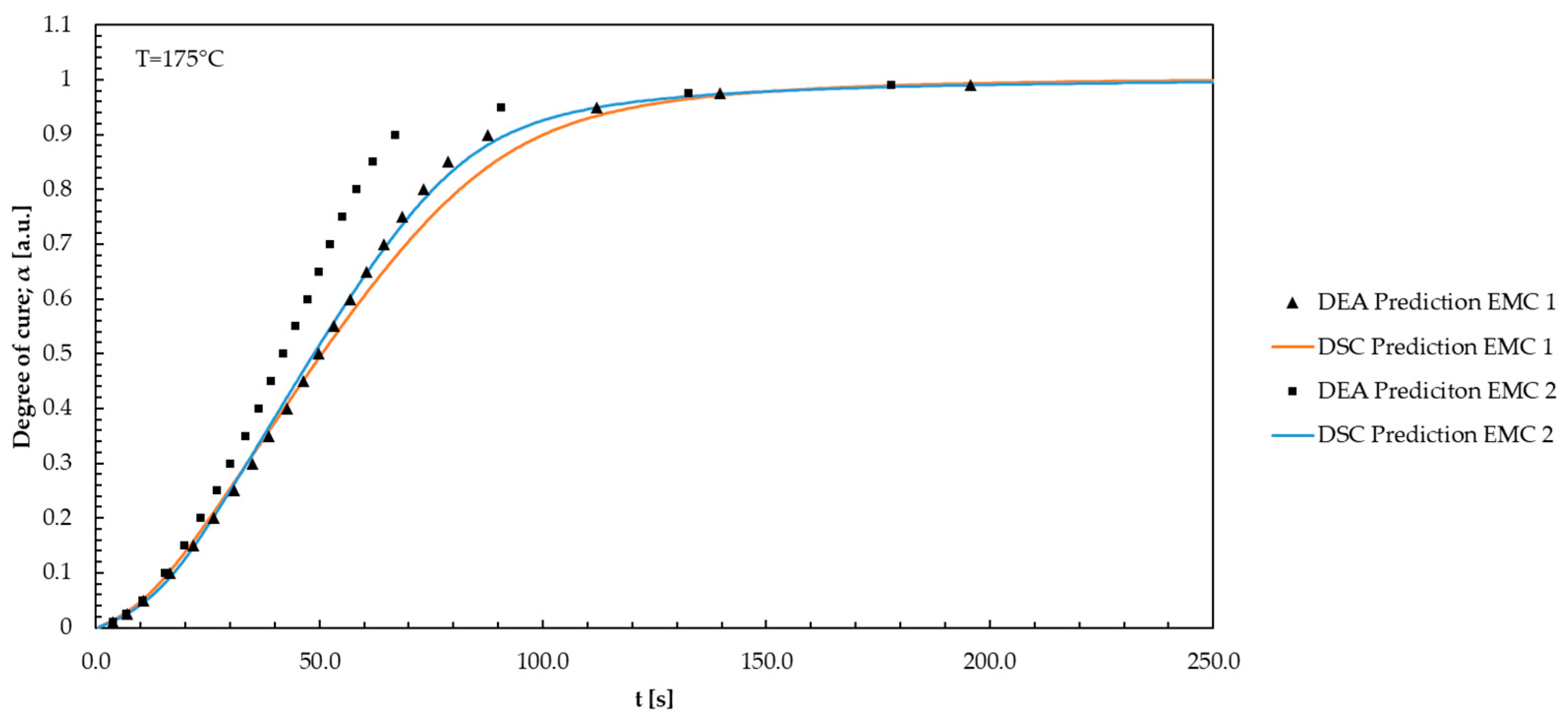
3.3. Comparison of DEA and DSC Kinetic Modelling
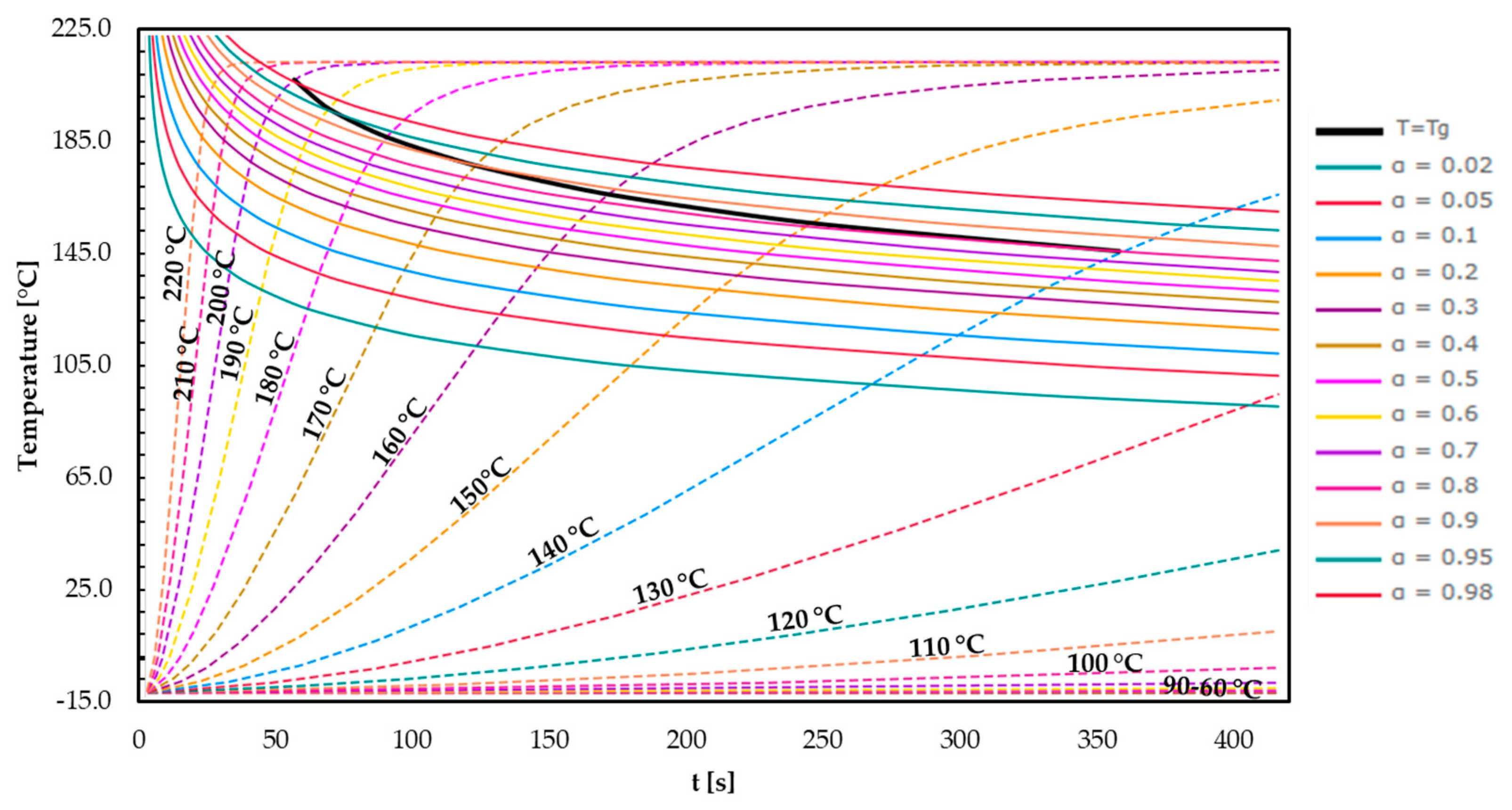
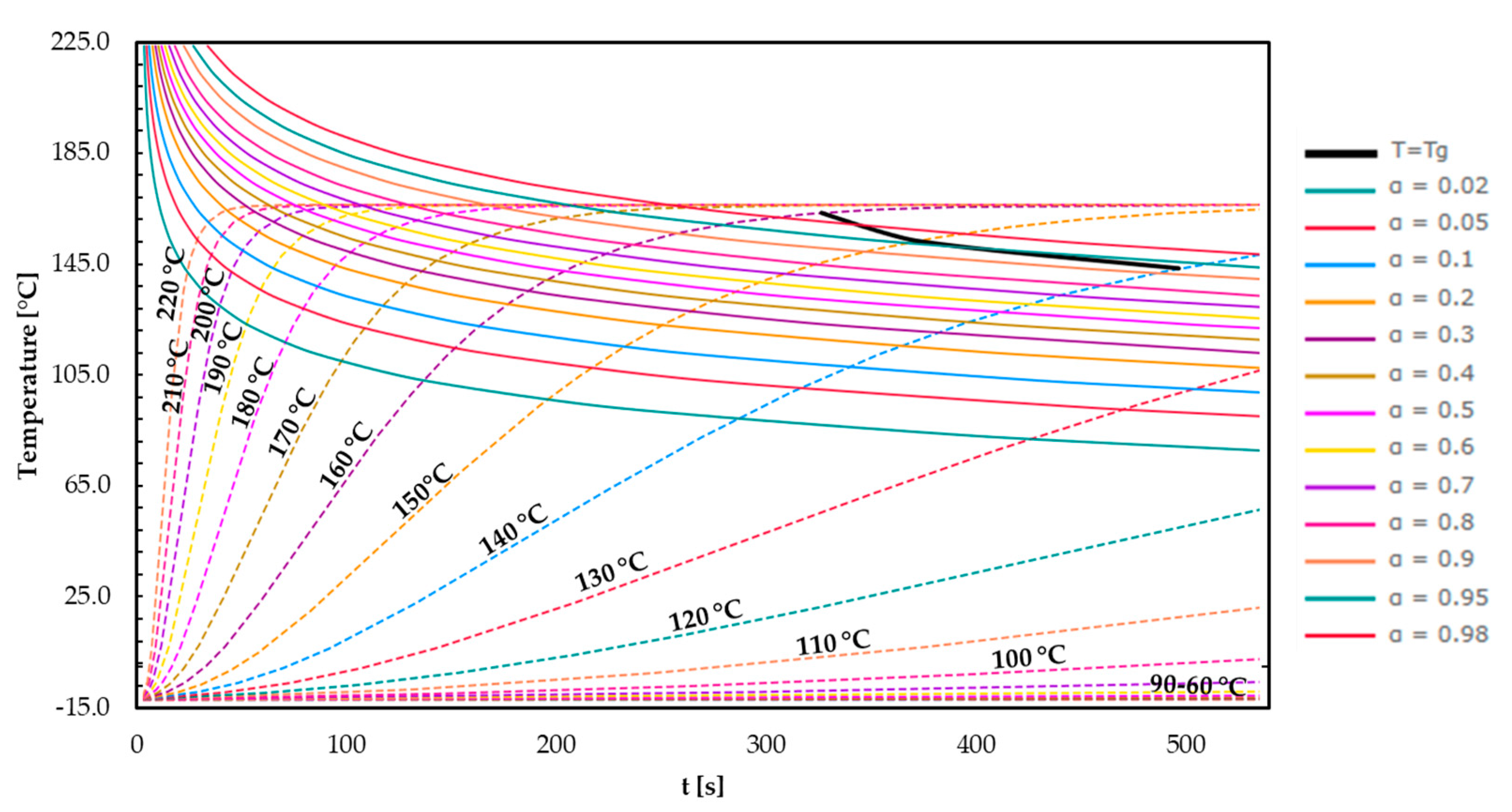
3.4. Time–Temperature–Transformation (TTT) Diagrams
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
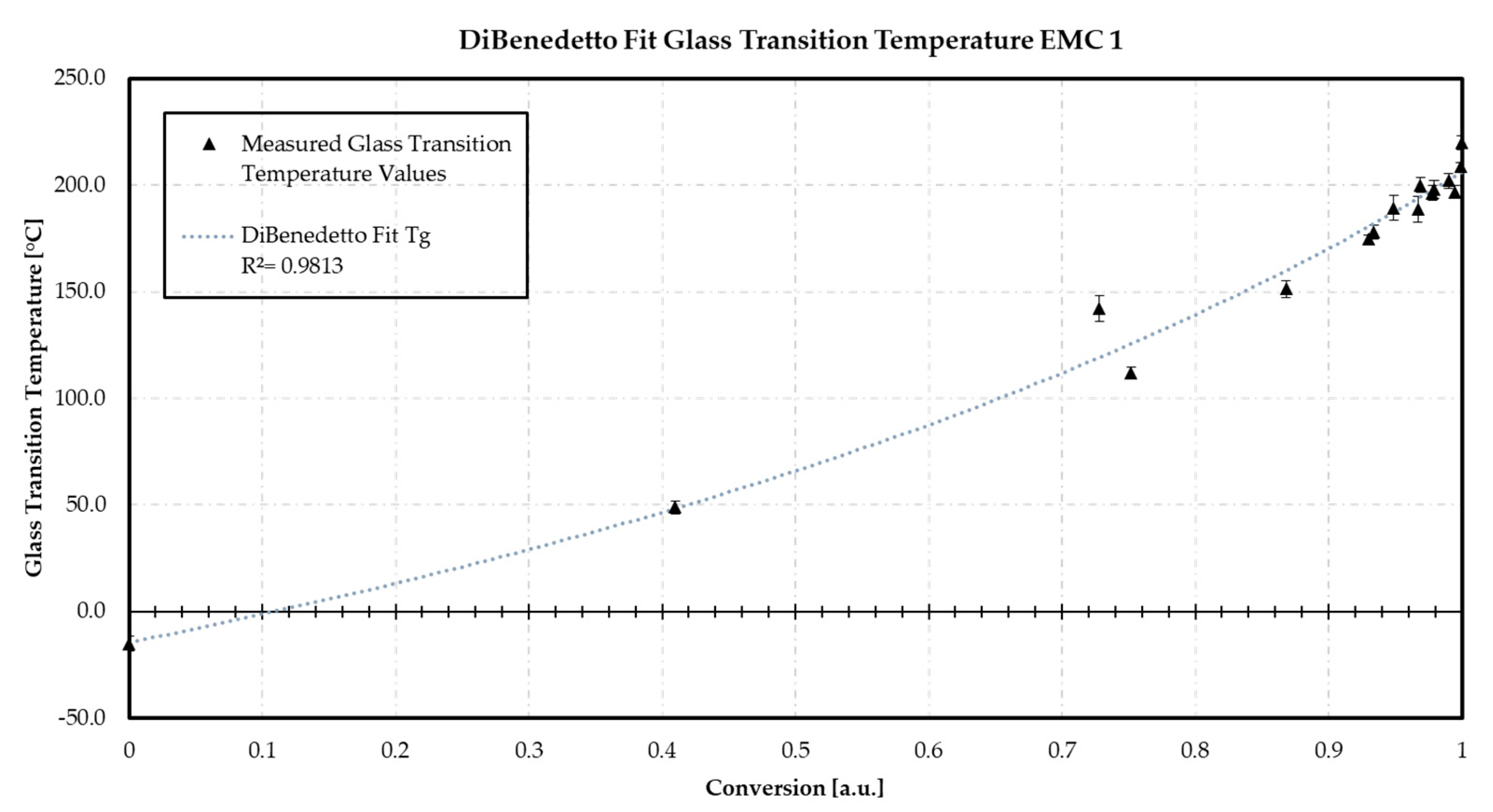
Appendix B

Appendix C
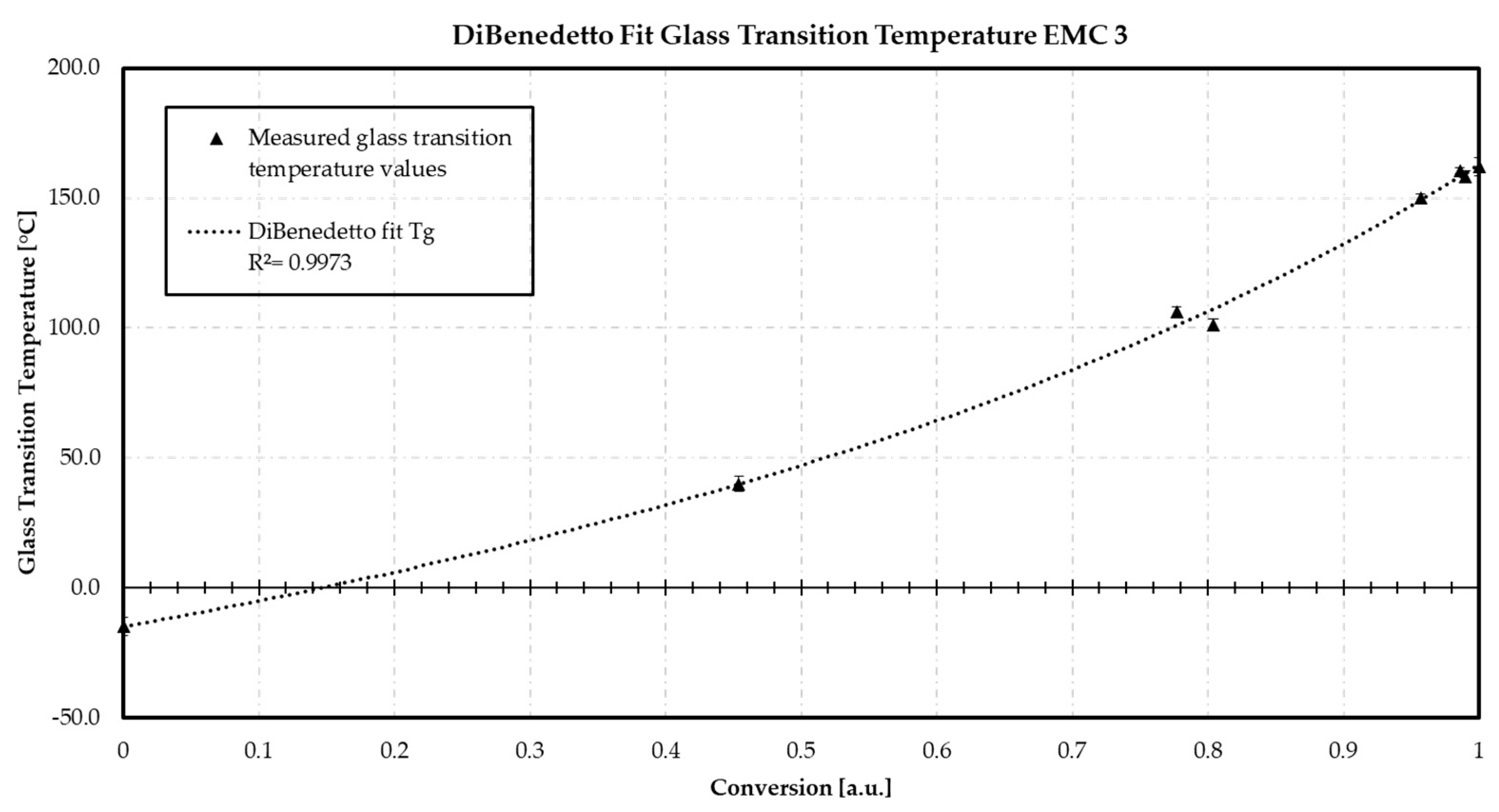
Appendix D
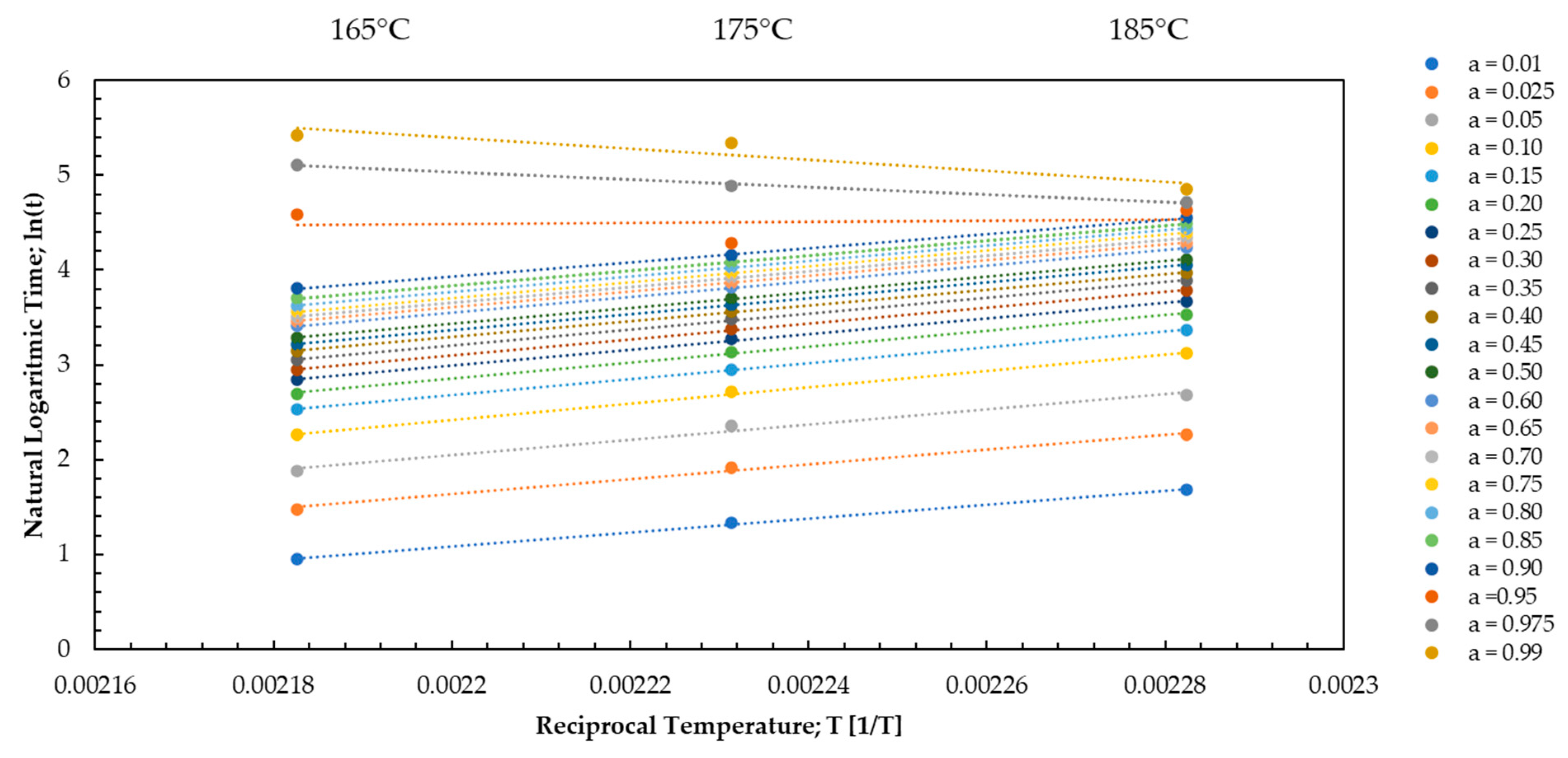
Appendix E
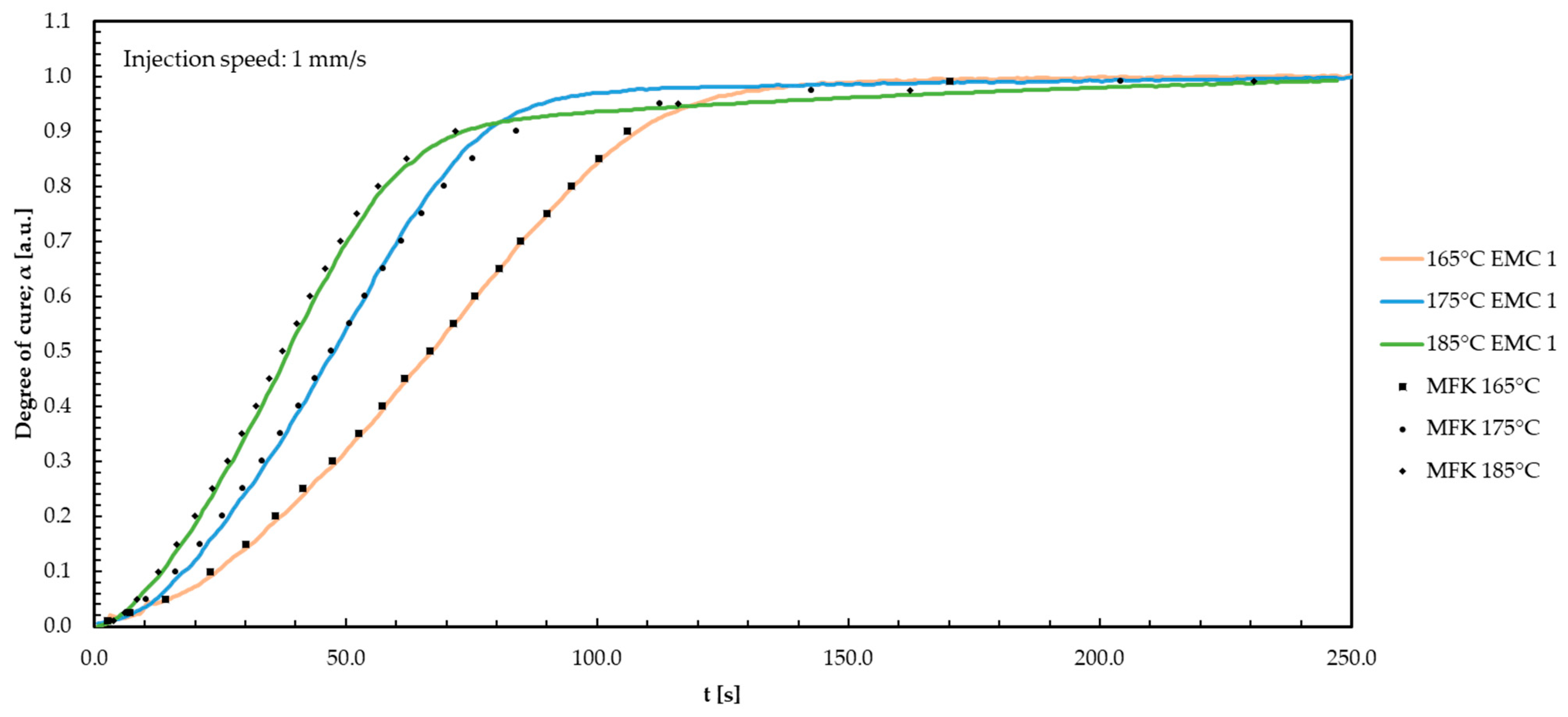
Appendix F
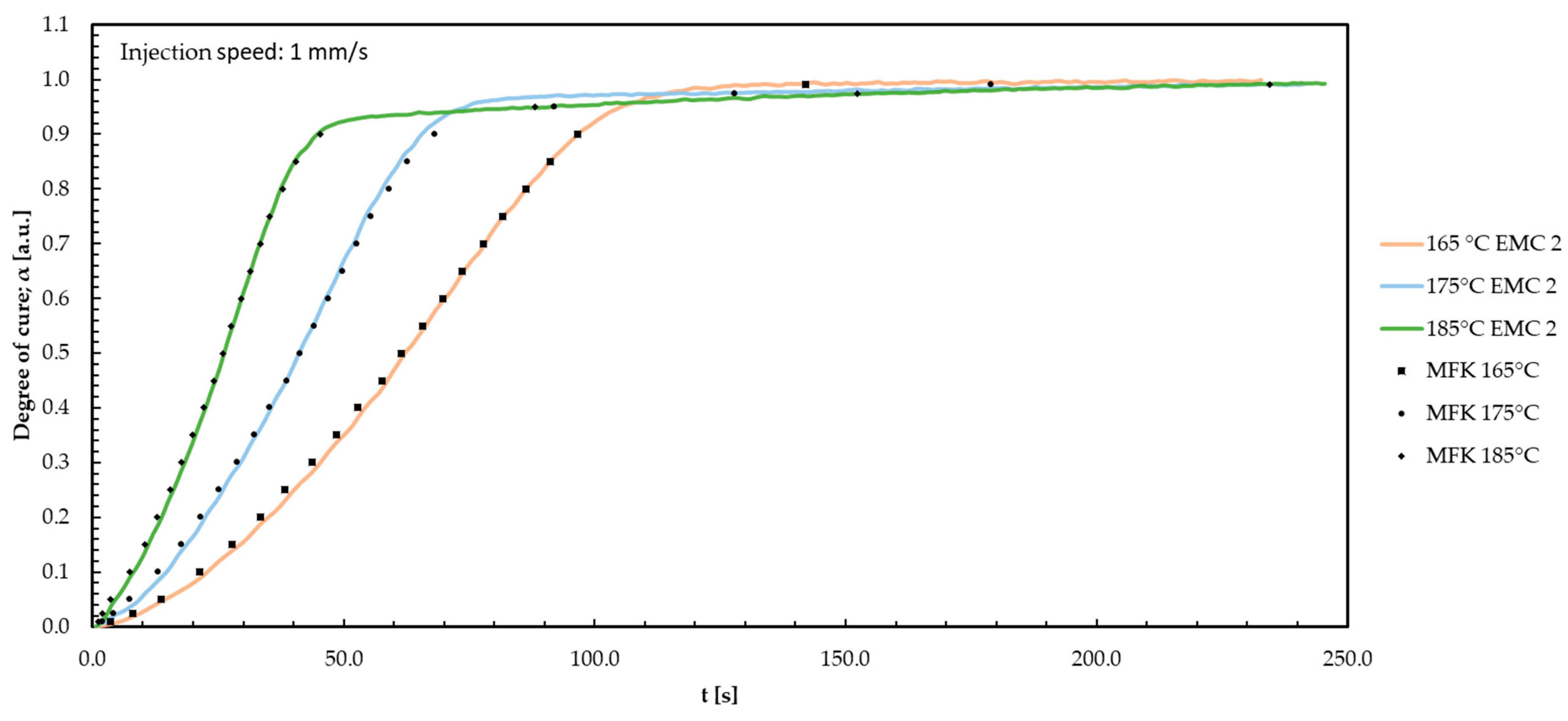
Appendix G
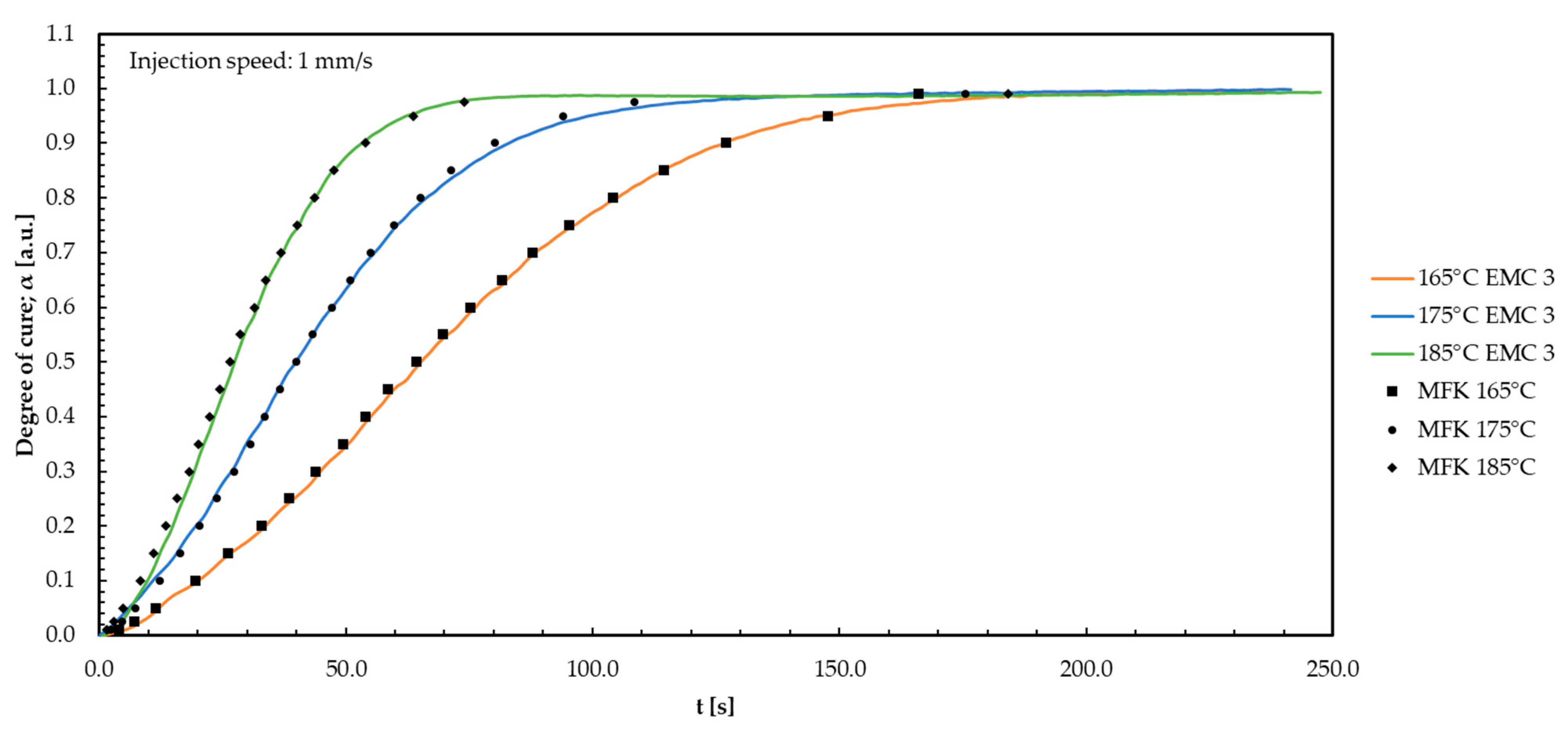
Appendix H

Appendix I
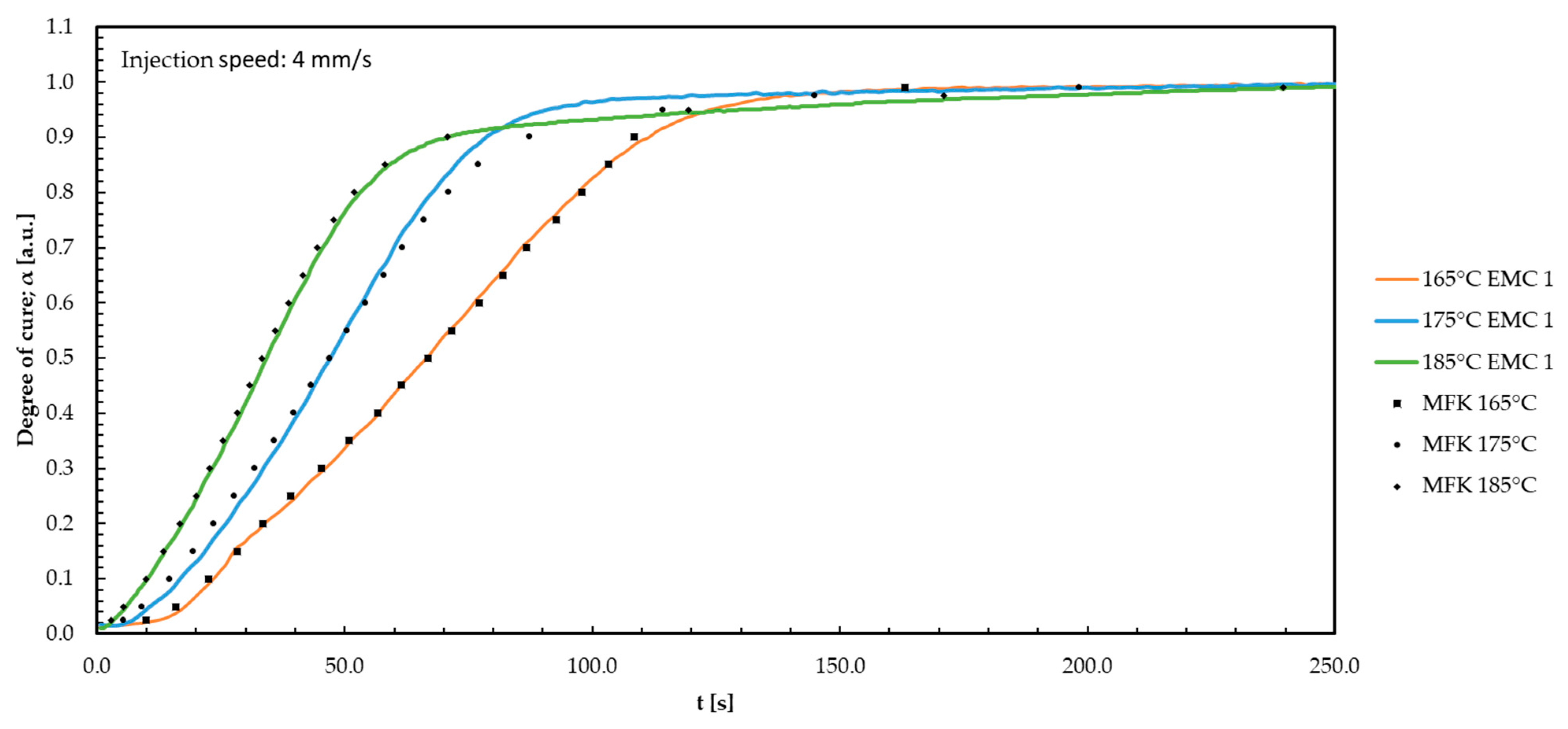
Appendix J

Appendix K
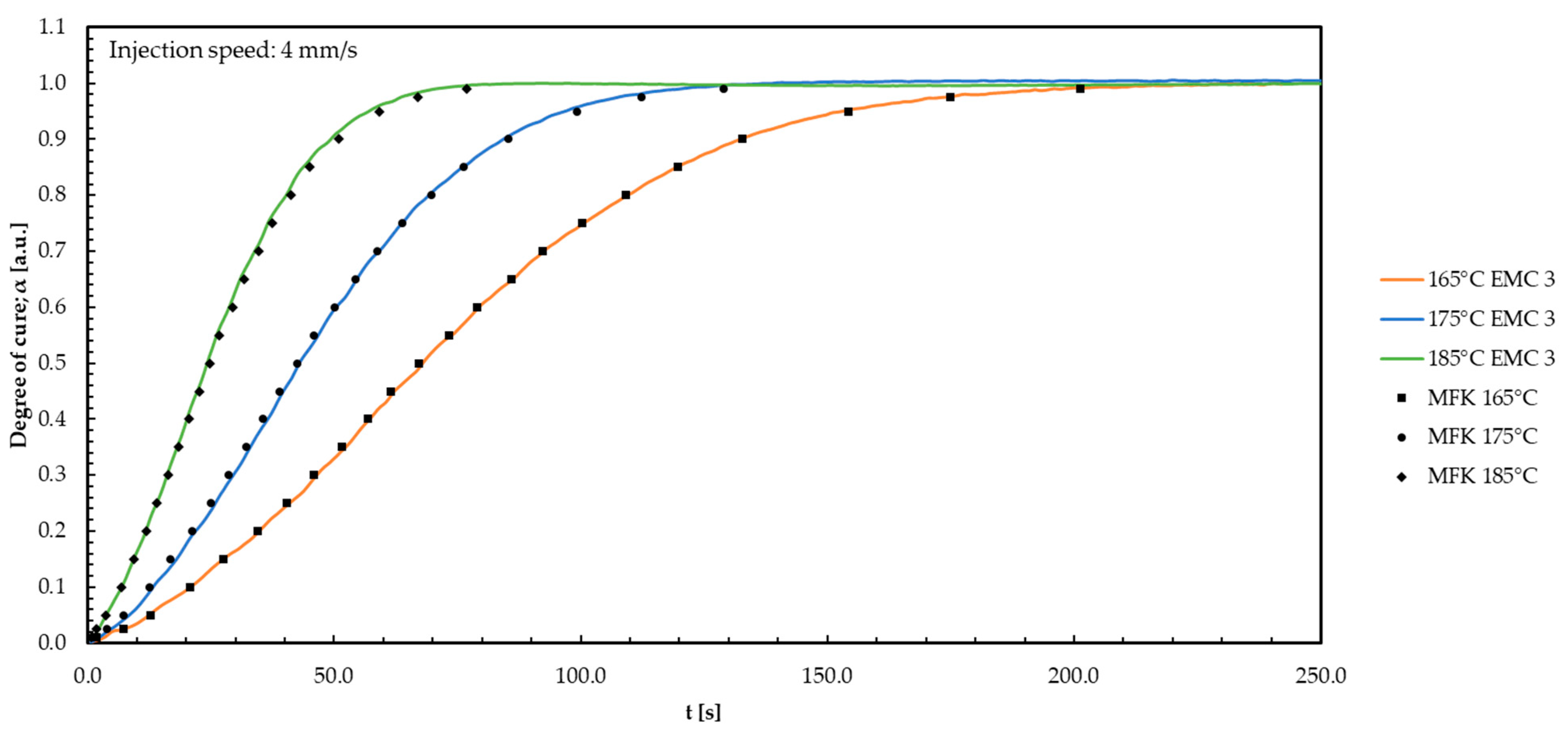
Appendix L
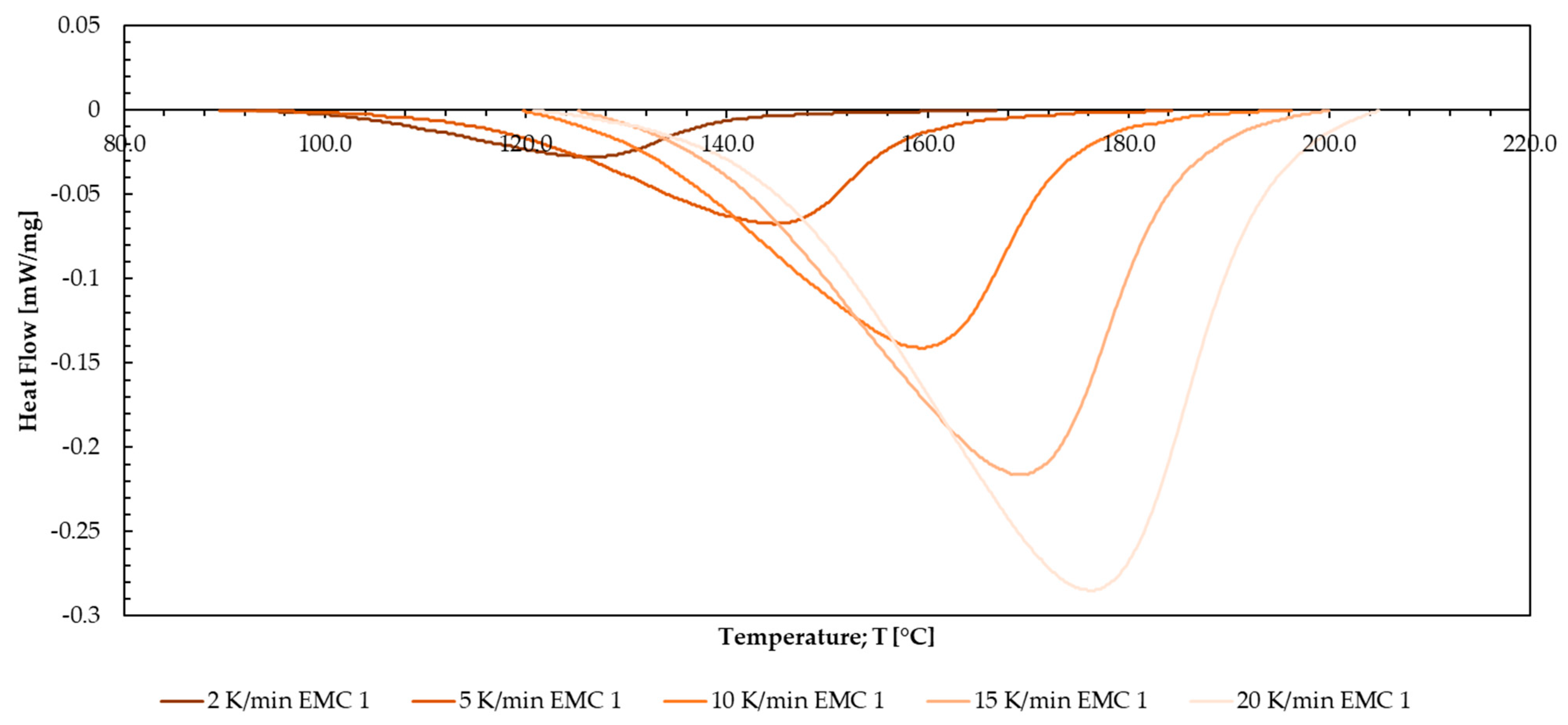
Appendix M

Appendix N
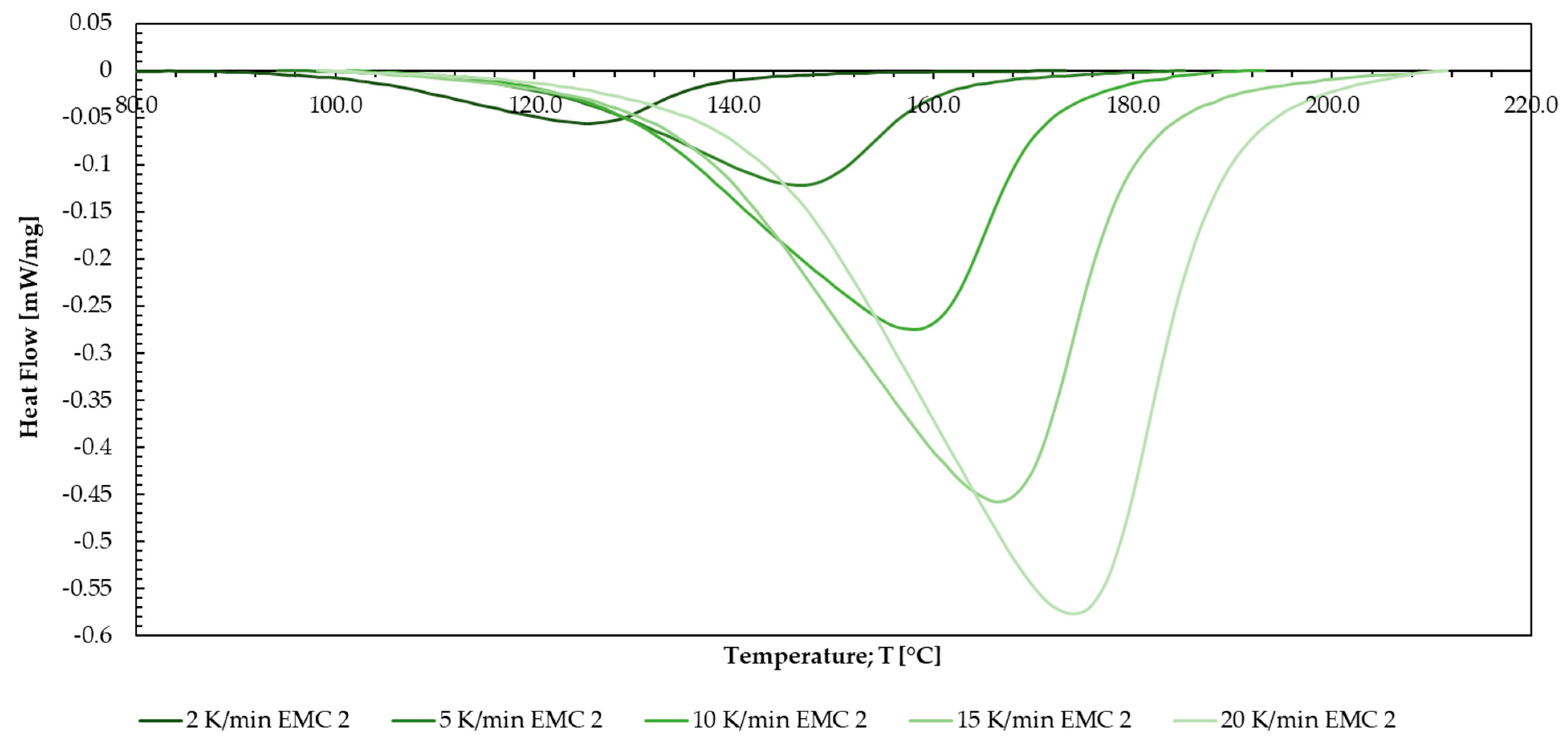
Appendix O
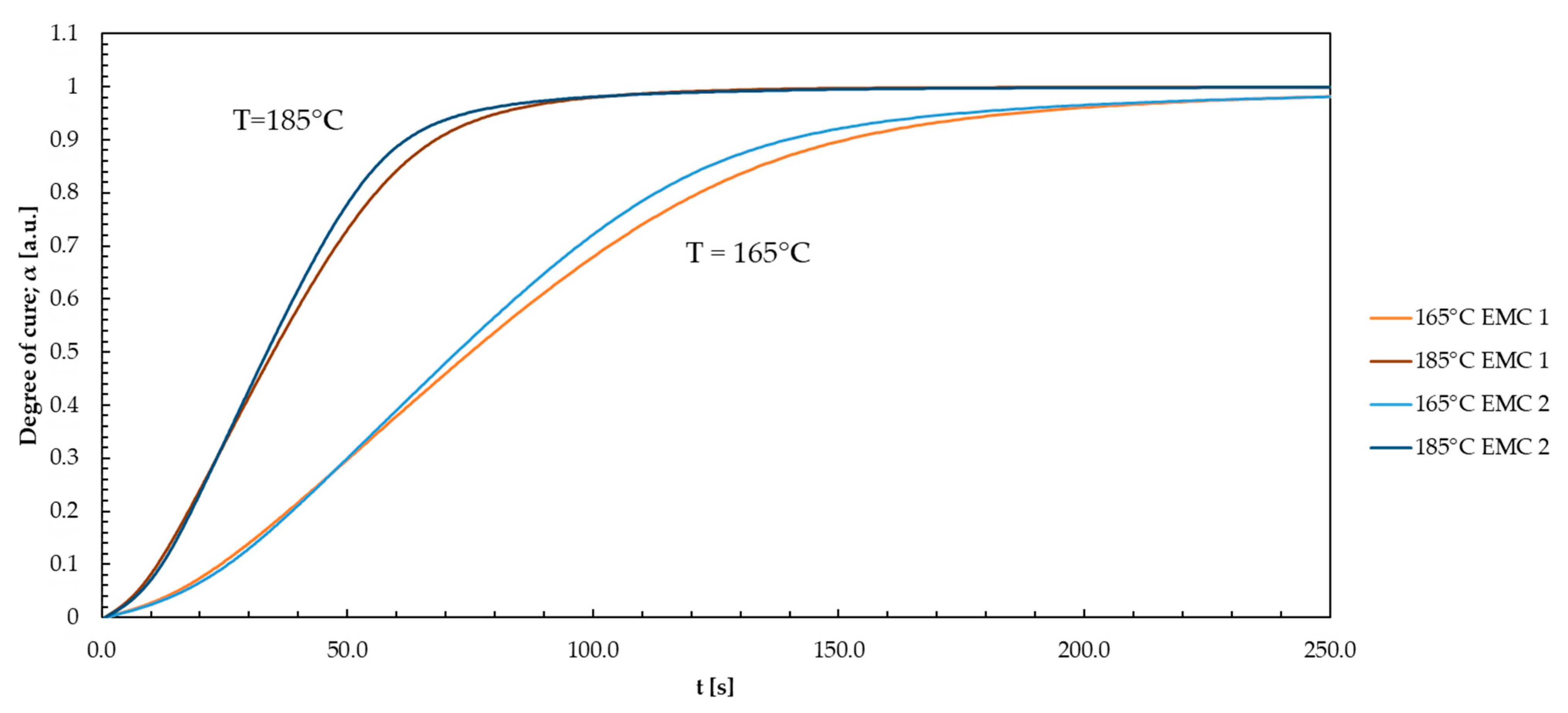
Appendix P
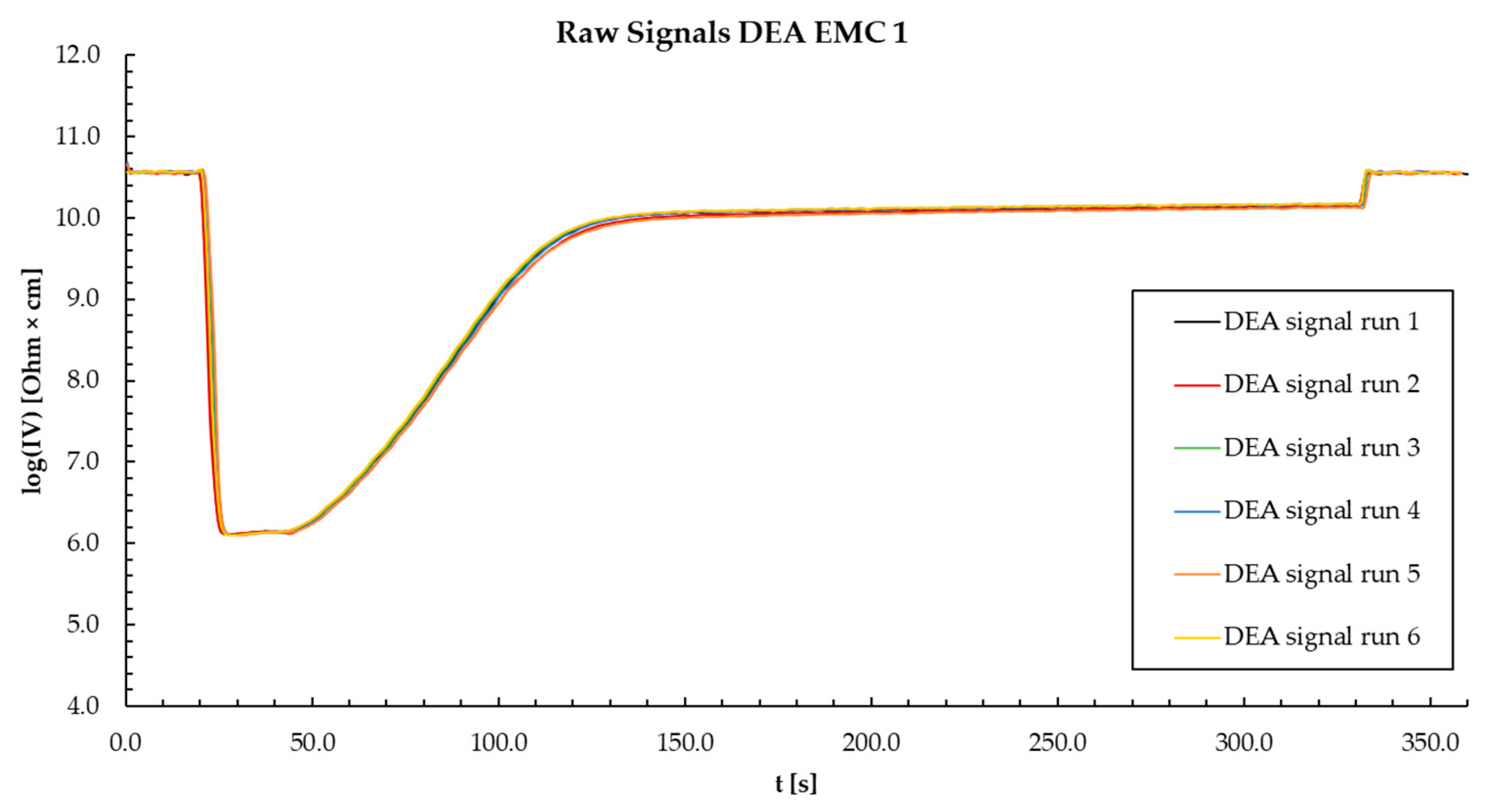
Appendix Q
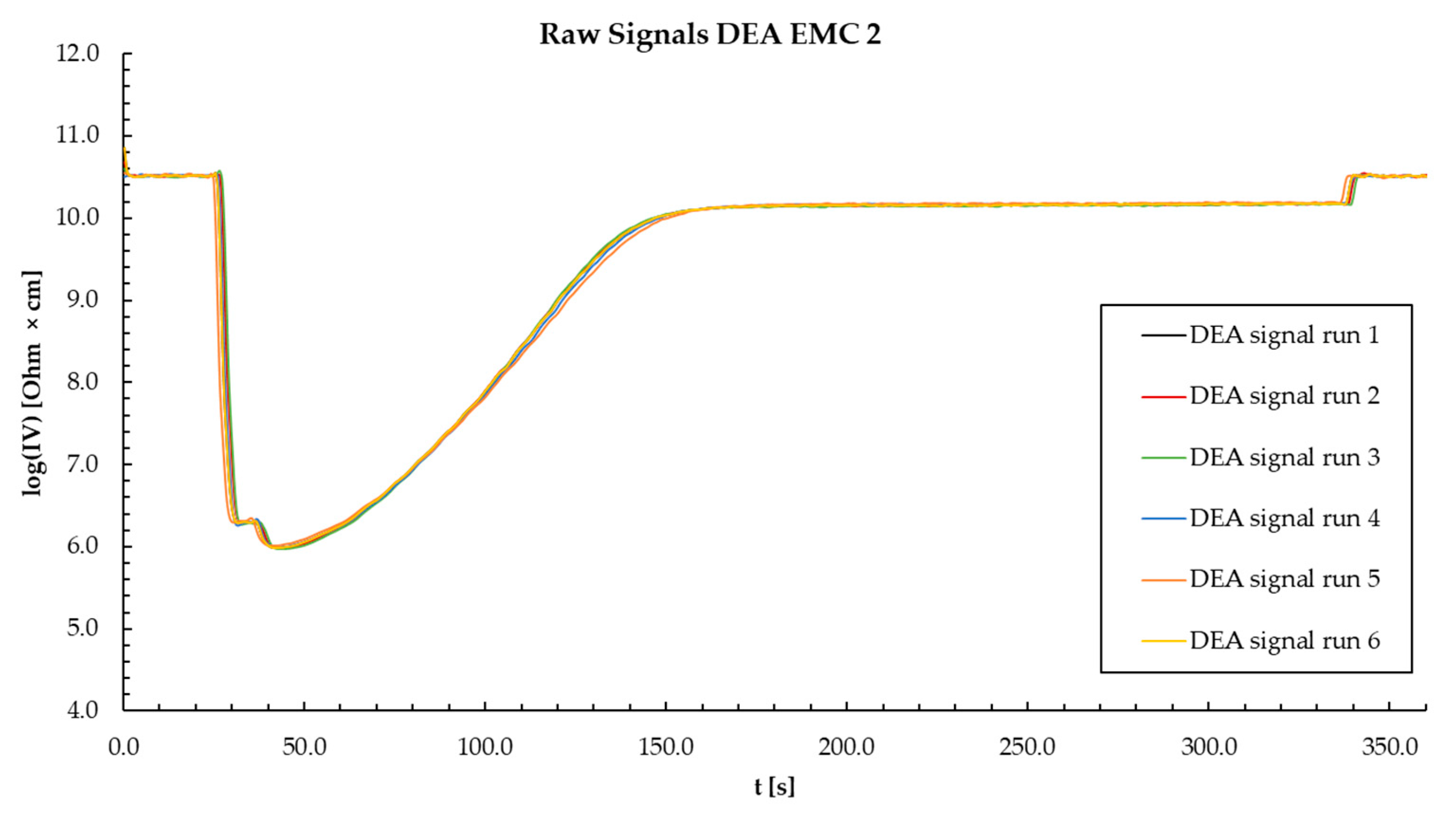
Appendix R
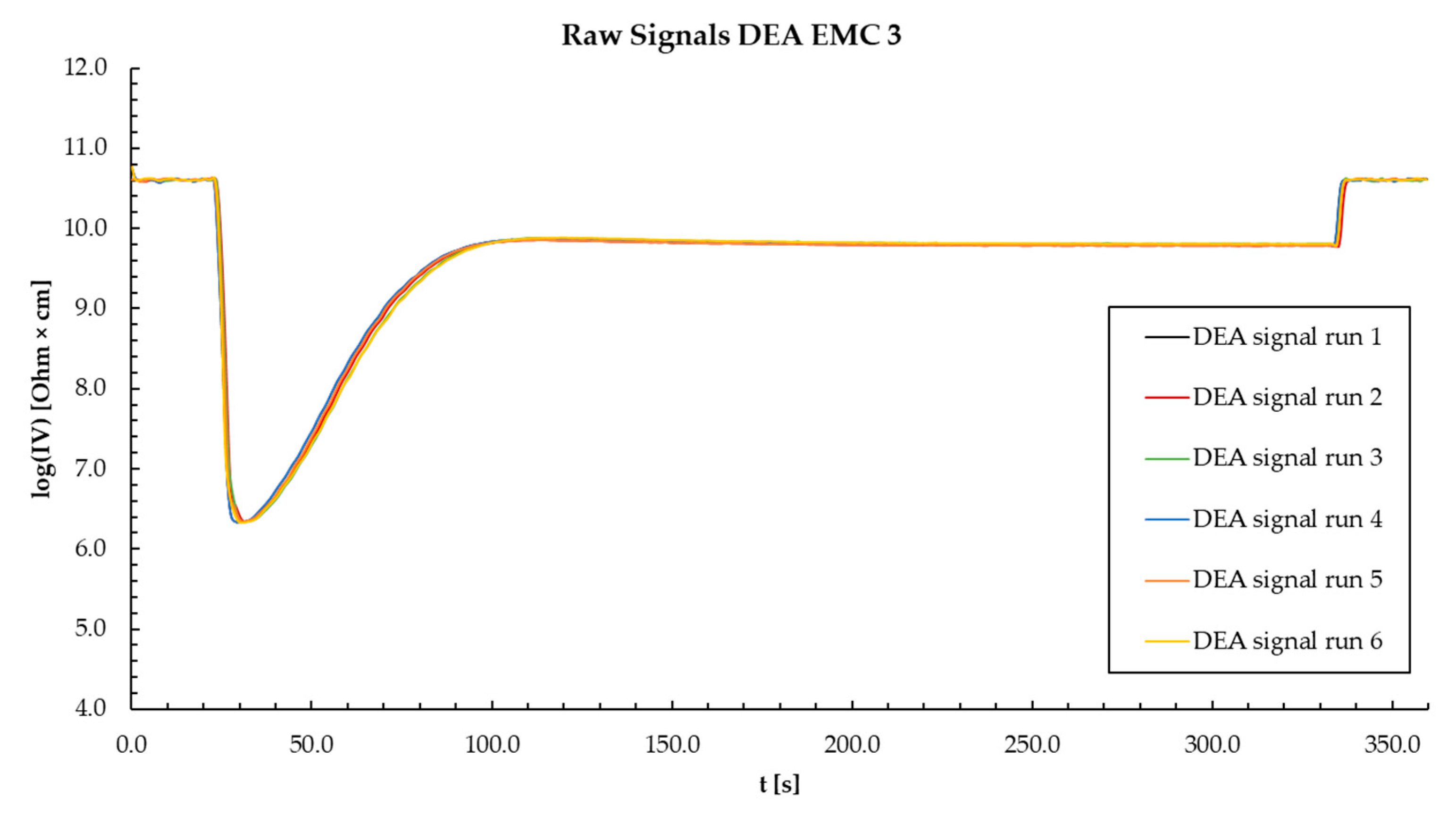
References
- Kinjo, N.; Ogata, M.; Nishi, K.; Kaneda, A.; Dušek, K. Epoxy molding compounds as encapsulation materials for microelectronic devices. In Speciality Polymers/Polymer Physics; Springer: Berlin/Heidelberg, Germany, 1989; pp. 1–48. [Google Scholar]
- Komori, S.; Sakamoto, Y. Development trend of epoxy molding compound for encapsulating semiconductor chips. In Materials for Advanced Packaging; Springer: Berlin/Heidelberg, Germany, 2009; pp. 339–363. [Google Scholar]
- Tong, K.; Kwong, C.; Ip, K. Optimization of process conditions for the transfer molding of electronic packages. J. Mater. Process. Technol. 2003, 138, 361–365. [Google Scholar] [CrossRef]
- Kamal, M.R.; Isayev, A.I. Injection Molding: Technology and Fundamentals; Carl Hanser Verlag GmbH Co KG: Munich, Germany, 2012. [Google Scholar]
- Zhao, Y.; Drummer, D. Influence of filler content and filler size on the curing kinetics of an epoxy resin. Polymers 2019, 11, 1797. [Google Scholar] [CrossRef]
- Dallaev, R.; Pisarenko, T.; Papež, N.; Sadovský, P.; Holcman, V. A Brief Overview on Epoxies in Electronics: Properties, Applications, and Modifications. Polymers 2023, 15, 3964. [Google Scholar] [CrossRef]
- Tarrío-Saavedra, J.; López-Beceiro, J.; Naya, S.; Artiaga, R. Effect of silica content on thermal stability of fumed silica/epoxy composites. Polym. Degrad. Stab. 2008, 93, 2133–2137. [Google Scholar] [CrossRef]
- Linec, M.; Mušič, B. The Effects of Silica-Based Fillers on the Properties of Epoxy Molding Compounds. Materials 2019, 12, 1811. [Google Scholar] [CrossRef]
- Altmann, N.; Halley, P.J.; Cooper-White, J.; Lange, J. The effects of silica fillers on the gelation and vitrification of highly filled epoxy-amine thermosets. In Macromolecular Symposia; WILEY-VCH Verlag GmbH: Weinheim, Germany, 2001; Volume 169, pp. 171–177. [Google Scholar]
- Dutta, A.; Ryan, M. Effect of fillers on kinetics of epoxy cure. J. Appl. Polym. Sci. 1979, 24, 635–649. [Google Scholar] [CrossRef]
- Wang, X.; Jin, J.; Song, M.; Lin, Y. Effect of graphene oxide sheet size on the curing kinetics and thermal stability of epoxy resins. Mater. Res. Express 2016, 3, 105303. [Google Scholar] [CrossRef]
- Jouyandeh, M.; Paran, S.M.R.; Jannesari, A.; Saeb, M.R. ‘Cure Index’ for thermoset composites. Prog. Org. Coat. 2019, 127, 429–434. [Google Scholar] [CrossRef]
- Lee, C.-L.; Wei, K.-H. Curing kinetics and viscosity change of a two-part epoxy resin during mold filling in resin-transfer molding process. J. Appl. Polym. Sci. 2000, 77, 2139–2148. [Google Scholar] [CrossRef]
- Soares, L.L.; Amico, S.C.; Isoldi, L.A.; Souza, J.A. Modeling of the resin transfer molding process including viscosity dependence with time and temperature. Polym. Compos. 2021, 42, 2795–2807. [Google Scholar] [CrossRef]
- Wisanrakkit, G.; Gillham, J.; Enns, J. The glass transition temperature (Tg) as a parameter for monitoring the cure of an amine/epoxy system at constant heating rates. J. Appl. Polym. Sci. 1990, 41, 1895–1912. [Google Scholar] [CrossRef]
- Enns, J.B.; Gillham, J.K. Time–temperature–transformation (TTT) cure diagram: Modeling the cure behavior of thermosets. J. Appl. Polym. Sci. 1983, 28, 2567–2591. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Y.; Liu, Y. Construction of improved isothermal TTT cure diagram based on an epoxy–amine thermoset. J. Appl. Polym. Sci. 2019, 136, 47279. [Google Scholar] [CrossRef]
- Aronhime, M.T.; Gillham, J.K. Time-temperature-transformation (TTT) cure diagram of thermosetting polymeric systems. In Epoxy Resins and Composites III; Springer: Berlin/Heidelberg, Germany, 2005; pp. 83–113. [Google Scholar]
- Nasseri, L.; Rosenfeld, C.; Solt-Rindler, P.; Mitter, R.; Moser, J.; Kandelbauer, A.; Konnerth, J.; Van Herwijnen, H.W. Comparison between cure kinetics by means of dynamic rheology and DSC of formaldehyde-based wood adhesives. J. Adhes. 2024, 1–22. [Google Scholar] [CrossRef]
- Nasseri, L.; Rosenfeld, C.; Solt, P.; Mihalic, M.; Kandelbauer, A.; Konnerth, J.; Van Herwijnen, H.W. Determination of the gel point of formaldehyde-based wood adhesives by using a multiwave technique. ACS Appl. Polym. Mater. 2023, 5, 6354–6363. [Google Scholar] [CrossRef]
- Areny, N.; Konuray, O.; Fernàndez-Francos, X.; Salla, J.M.; Morancho, J.M.; Ramis, X. Time-temperature-transformation (TTT) diagram of a dual-curable off-stoichiometric epoxy-amine system with latent reactivity. Thermochim. Acta 2018, 666, 124–134. [Google Scholar] [CrossRef]
- Di Benedetto, A. Prediction of the glass transition temperature of polymers: A model based on the principle of corresponding states. J. Polym. Sci. Part B Polym. Phys. 1987, 25, 1949–1969. [Google Scholar] [CrossRef]
- Stutz, H.; Illers, K.; Mertes, J. A generalized theory for the glass transition temperature of crosslinked and uncrosslinked polymers. J. Polym. Sci. Part B Polym. Phys. 1990, 28, 1483–1498. [Google Scholar] [CrossRef]
- Lechner, M.D.; Gehrke, K.; Nordmeier, E.H. Makromolekulare Chemie: Ein Lehrbuch für Chemiker. In Physiker, Materialwissenschaftler und Verfahrenstechniker, 4th ed.; Birkhäuser Verlag: Basel, Switzerland, 2003. [Google Scholar]
- Kaya, B. Concept Development and Implementation of Online Monitoring Methods in the Transfer Molding Process for Electronic Packages. Ph.D. Thesis, Technische Universität Berlin, Berlin, Germany, 2018. [Google Scholar]
- Vyazovkin, S.; Burnham, A.K.; Criado, J.M.; Pérez-Maqueda, L.A.; Popescu, C.; Sbirrazzuoli, N. ICTAC Kinetics Committee recommendations for performing kinetic computations on thermal analysis data. Thermochim. Acta 2011, 520, 1–19. [Google Scholar] [CrossRef]
- Kandelbauer, A.; Wuzella, G.; Mahendran, A.; Taudes, I.; Widsten, P. Using isoconversional kinetic analysis of liquid melamine–formaldehyde resin curing to predict laminate surface properties. J. Appl. Polym. Sci. 2009, 113, 2649–2660. [Google Scholar] [CrossRef]
- Kamal, M.R.; Sourour, S. Kinetics and thermal characterization of thermoset cure. Polym. Eng. Sci. 1973, 13, 59–64. [Google Scholar] [CrossRef]
- Wuzella, G.; Kandelbauer, A.; Mahendran, A.R.; Teischinger, A. Thermochemical and isoconversional kinetic analysis of a polyester–epoxy hybrid powder coating resin for wood based panel finishing. Prog. Org. Coat. 2011, 70, 186–191. [Google Scholar] [CrossRef]
- Mahendran, A.R.; Wuzella, G.; Kandelbauer, A. Thermal characterization of kraft lignin phenol-formaldehyde resin for paper impregnation. J. Adhes. Sci. Technol. 2010, 24, 1553–1565. [Google Scholar] [CrossRef]
- Alig, I.; Fischer, D.; Lellinger, D.; Steinhoff, B. Combination of NIR, Raman, ultrasonic and dielectric spectroscopy for in-line monitoring of the extrusion process. In Macromolecular Symposia; WILEY-VCH Verlag GmbH: Weinheim, Germany, 2005; Volume 230, pp. 51–58. [Google Scholar]
- Hardis, R.; Jessop, J.L.; Peters, F.E.; Kessler, M.R. Cure kinetics characterization and monitoring of an epoxy resin using DSC, Raman spectroscopy, and DEA. Compos. Part A Appl. Sci. Manuf. 2013, 49, 100–108. [Google Scholar] [CrossRef]
- Kandelbauer, A. Processing and process control. In Handbook of Thermoset Plastics; Elsevier: Amsterdam, The Netherlands, 2022; pp. 1045–1070. [Google Scholar]
- Lee, H.L. The Handbook of Dielectric Analysis and Cure Monitoring; Lambient Technology LLC: Boston, MA, USA, 2014; Volume 156. [Google Scholar]
- Weiss, S.; Urdl, K.; Mayer, H.A.; Zikulnig-Rusch, E.M.; Kandelbauer, A. IR spectroscopy: Suitable method for determination of curing degree and crosslinking type in melamine–formaldehyde resins. J. Appl. Polym. Sci. 2019, 136, 47691. [Google Scholar] [CrossRef]
- Franieck, E.; Fleischmann, M.; Hölck, O.; Kutuzova, L.; Kandelbauer, A. Cure Kinetics Modeling of a High Glass Transition Temperature Epoxy Molding Compound (EMC) Based on Inline Dielectric Analysis. Polymers 2021, 13, 1734. [Google Scholar] [CrossRef]
- Franieck, E.; Fleischmann, M.; Hölck, O.; Schneider-Ramelow, M. Inline cure monitoring of epoxy resin by dielectric analysis. In Proceedings of the 2021 23rd European Microelectronics and Packaging Conference Exhibition (EMPC), Gothenburg, Sweden, 13–16 September 2021; pp. 1–8. [Google Scholar] [CrossRef]
- Rosentritt, M.; Behr, M.; Knappe, S.; Handel, G. Dielectric analysis of light-curing dental restorative materials—A pilot study. J. Mater. Sci. 2006, 41, 2805–2810. [Google Scholar] [CrossRef]
- Friedman, H.L. Kinetics of thermal degradation of char-forming plastics from thermogravimetry. Application to a phenolic plastic. J. Polym. Sci. Part C Polym. Symp. 1964, 6, 183–195. [Google Scholar] [CrossRef]
- Vyazovkin, S. Isoconversional Kinetics of Thermally Stimulated Processes; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Vogelwaid, J.; Bayer, M.; Walz, M.; Kutuzova, L.; Kandelbauer, A.; Jacob, T. Process optimization of the morphological properties of epoxy resin molding compounds using response surface design. Polymers, 2024; accepted. [Google Scholar]
- Rauschendorfer, J.E.; Möckelmann, J.; Vana, P. Enhancing the Mechanical Properties of Matrix-Free Poly (Methyl Acrylate)-Grafted Montmorillonite Nanosheets by Introducing Star Polymers and Hydrogen Bonding Moieties. Macromol. Mater. Eng. 2021, 306, 2100054. [Google Scholar] [CrossRef]
- Kim, W.G.; Lee, J.Y. Contributions of the network structure to the cure kinetics of epoxy resin systems according to the change of hardeners. Polymer 2002, 43, 5713–5722. [Google Scholar] [CrossRef]
- Granado, L.; Kempa, S.; Gregoriades, L.J.; Brüning, F.; Genix, A.-C.; Fréty, N.; Anglaret, E. Kinetic regimes in the curing process of epoxy-phenol composites. Thermochim. Acta 2018, 667, 185–192. [Google Scholar] [CrossRef]
- Rabearison, N.; Jochum, C.; Grandidier, J.-C. A cure kinetics, diffusion controlled and temperature dependent, identification of the Araldite LY556 epoxy. J. Mater. Sci. 2011, 46, 787–796. [Google Scholar] [CrossRef]
- Li, Q.; Li, X.; Meng, Y. Curing of DGEBA epoxy using a phenol-terminated hyperbranched curing agent: Cure kinetics, gelation, and the TTT cure diagram. Thermochim. Acta 2012, 549, 69–80. [Google Scholar] [CrossRef]
- Jahani, Y.; Baena, M.; Barris, C.; Perera, R.; Torres, L. Influence of curing, post-curing and testing temperatures on mechanical properties of a structural adhesive. Constr. Build. Mater. 2022, 324, 126698. [Google Scholar] [CrossRef]
- Palmese, G.; Gillham, J. Time–temperature–transformation (TTT) cure diagrams: Relationship between Tg and the temperature and time of cure for a polyamic acid/polyimide system. J. Appl. Polym. Sci. 1987, 34, 1925–1939. [Google Scholar] [CrossRef]
- Peng, X.; Gillham, J. Time–temperature–transformation (TTT) cure diagrams: Relationship between Tg and the temperature and time of cure for epoxy systems. J. Appl. Polym. Sci. 1985, 30, 4685–4696. [Google Scholar] [CrossRef]
- Fernando, S.; Vandi, L.-J.; Heitzmann, M.; Fernando, D. Cure cycle optimisation of structural epoxies used in composite civil infrastructure through the use of TTT diagrams. Constr. Build. Mater. 2023, 383, 131332. [Google Scholar] [CrossRef]
- Corezzi, S.; Fioretto, D.; Santucci, G.; Kenny, J.M. Modeling diffusion-control in the cure kinetics of epoxy-amine thermoset resins: An approach based on configurational entropy. Polymer 2010, 51, 5833–5845. [Google Scholar] [CrossRef]


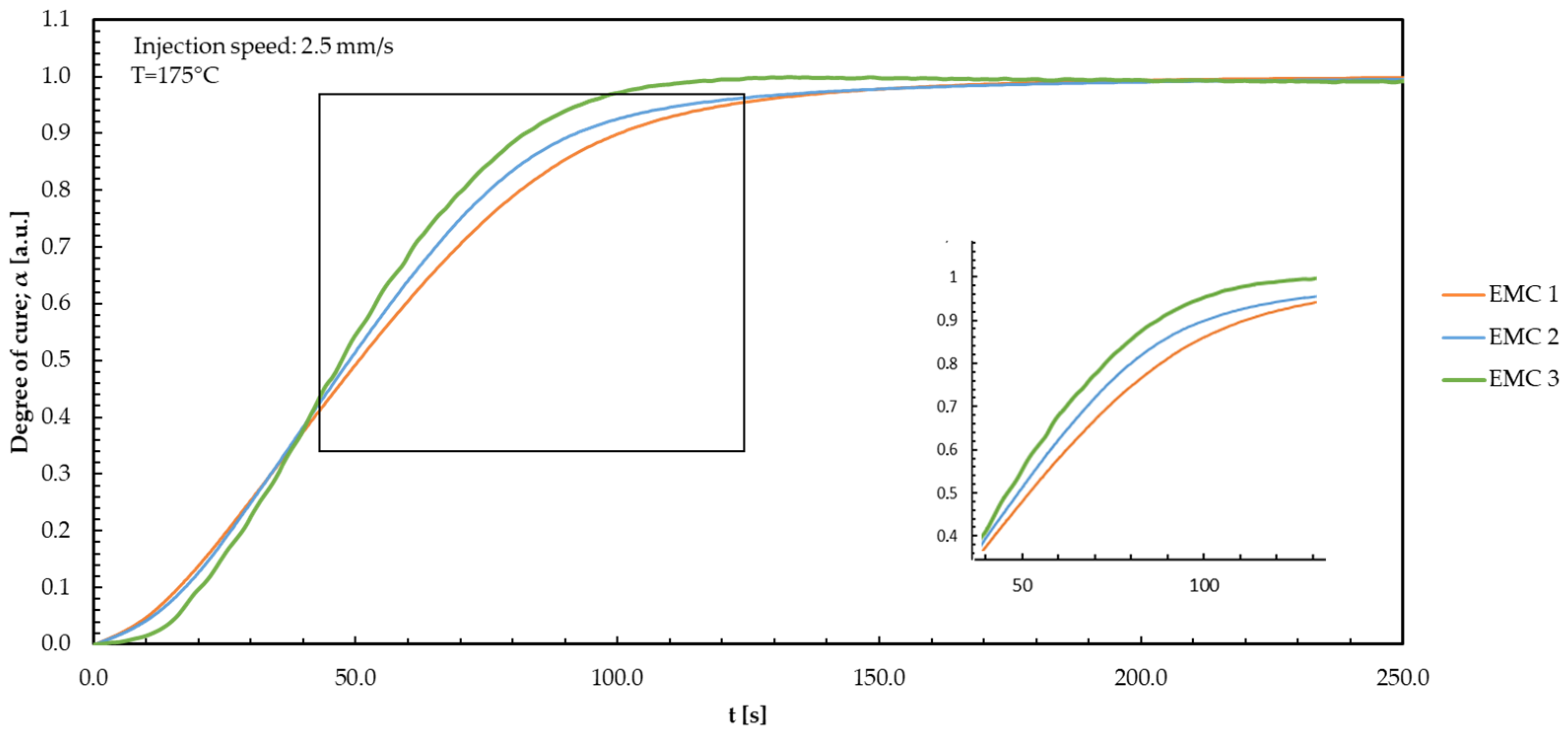
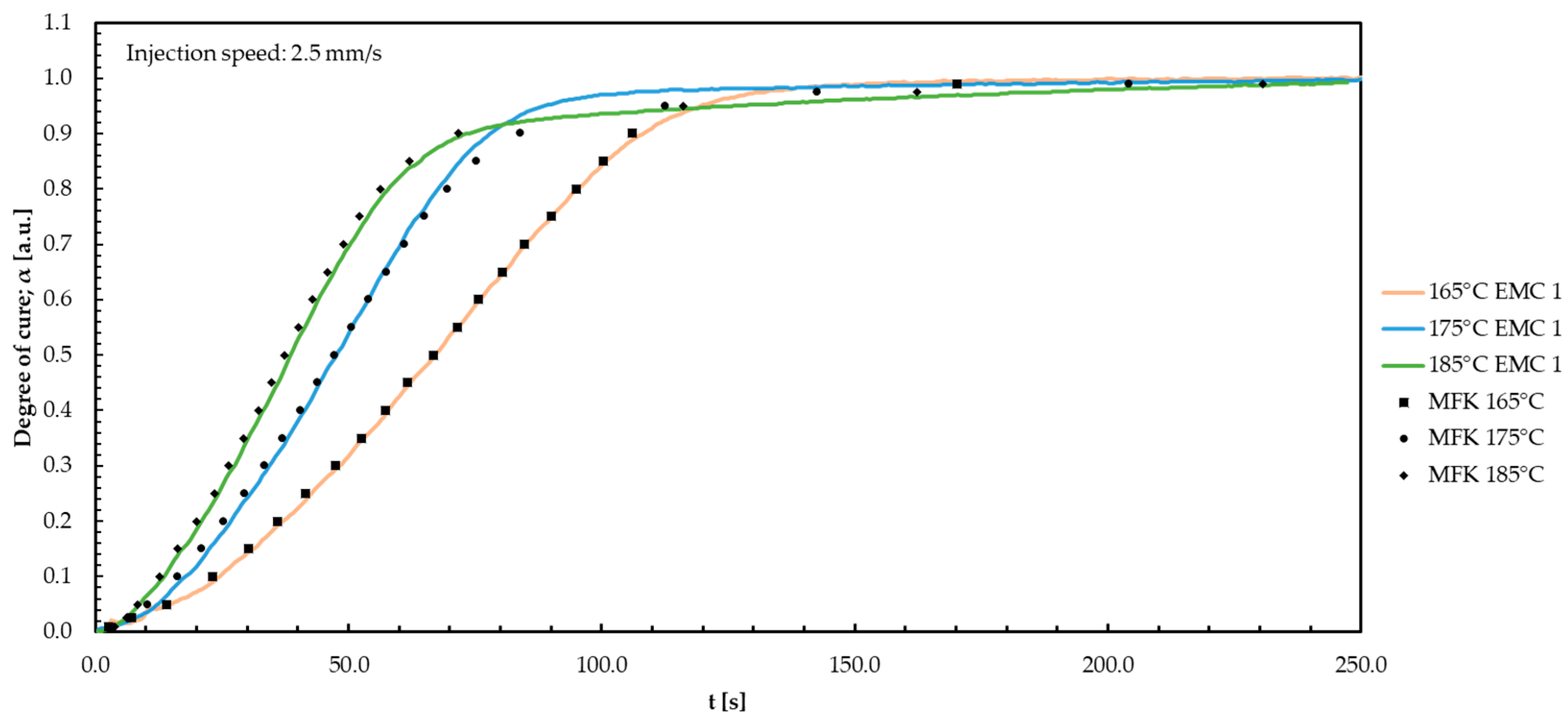
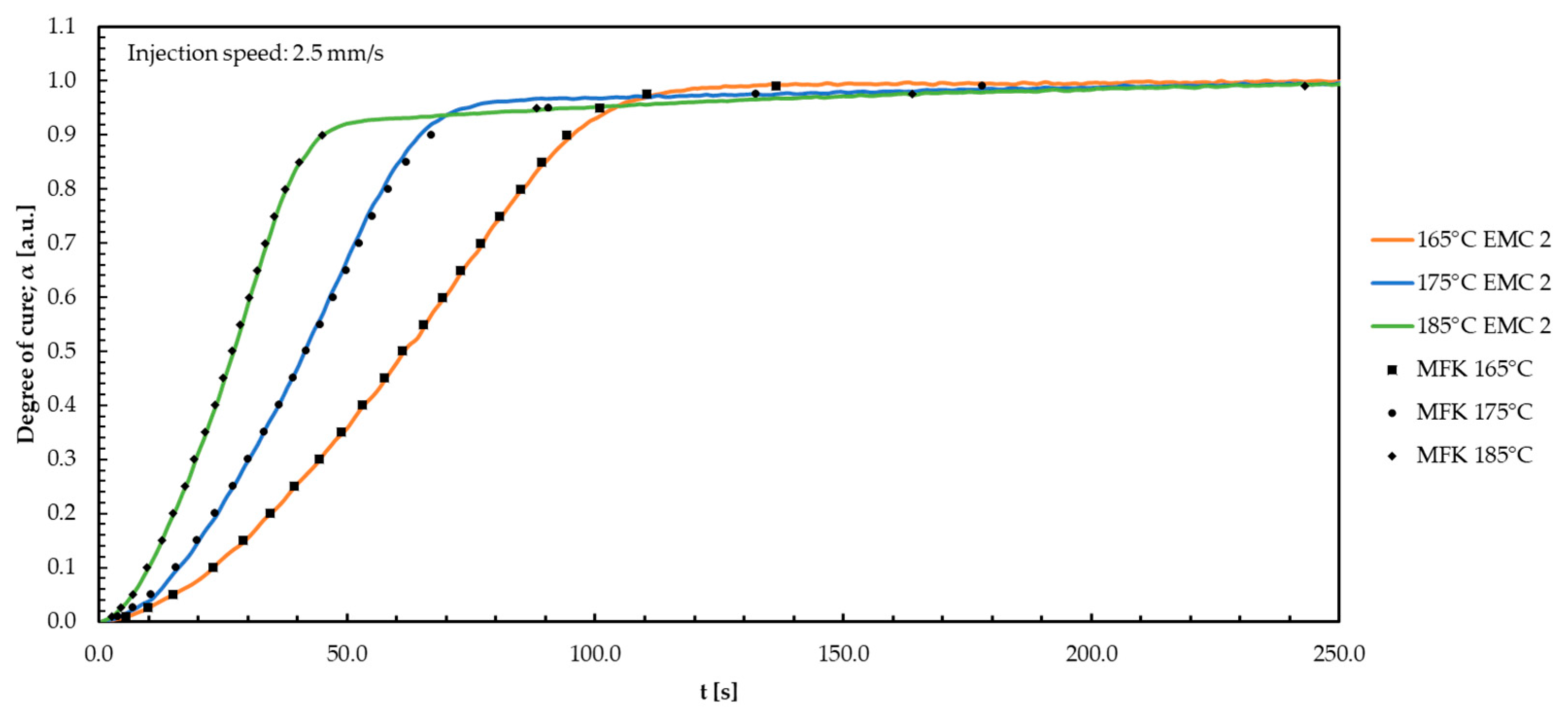
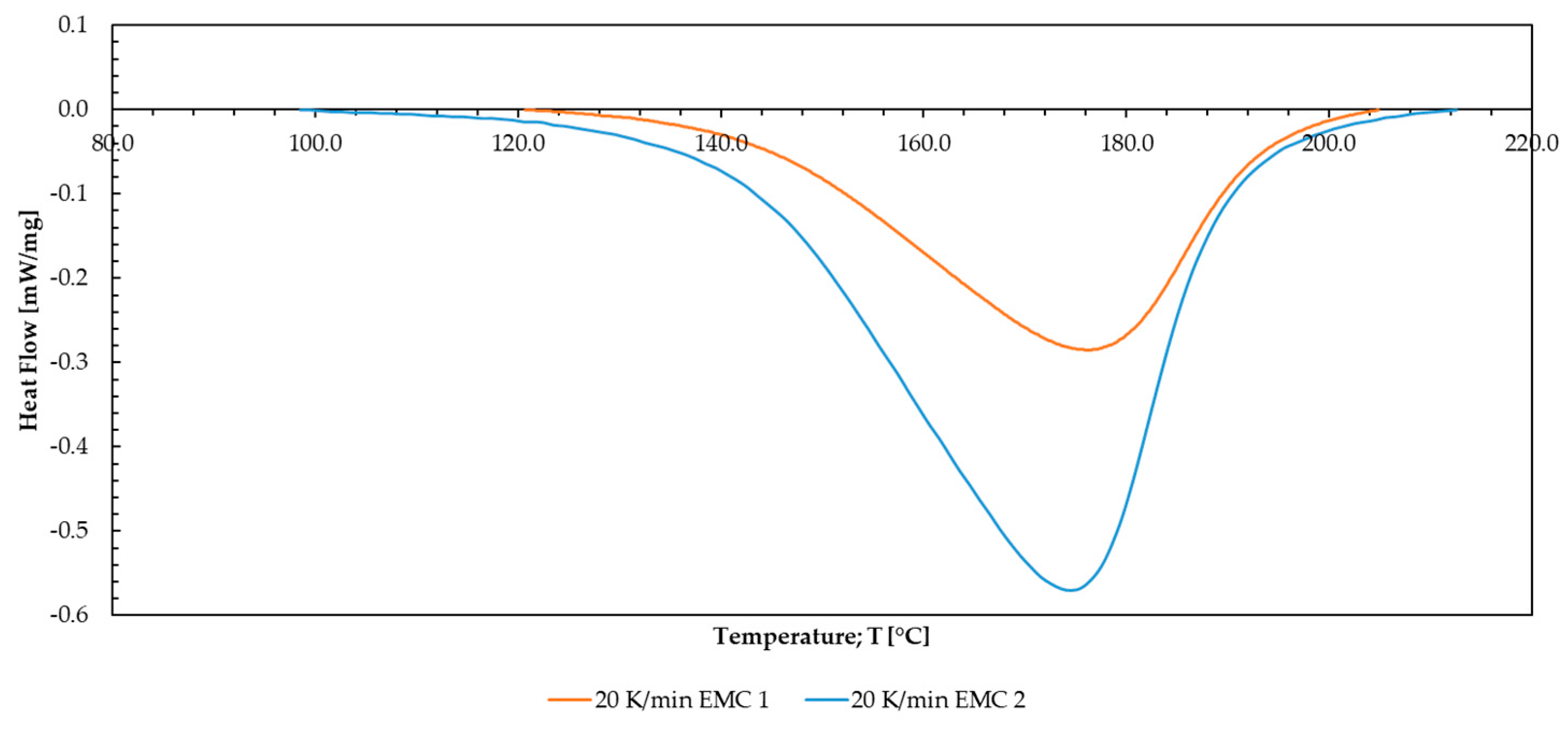


| Material | DSC | DEA 1.0 mm/s | DEA 2.5 mm/s | DEA 4.0 mm/s |
|---|---|---|---|---|
| EMC 1 | 65.5 ± 1.7 | 54.9 ± 1.8 | 57.4 ± 1.6 | 57.5 ± 1.4 |
| EMC 2 | 66.6 ± 2.3 | 72.2 ± 2.9 | 67.5 ± 1.5 | 71.2 ± 1.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vogelwaid, J.; Hampel, F.; Bayer, M.; Walz, M.; Kutuzova, L.; Lorenz, G.; Kandelbauer, A.; Jacob, T. In Situ Monitoring of the Curing of Highly Filled Epoxy Molding Compounds: The Influence of Reaction Type and Silica Content on Cure Kinetic Models. Polymers 2024, 16, 1056. https://doi.org/10.3390/polym16081056
Vogelwaid J, Hampel F, Bayer M, Walz M, Kutuzova L, Lorenz G, Kandelbauer A, Jacob T. In Situ Monitoring of the Curing of Highly Filled Epoxy Molding Compounds: The Influence of Reaction Type and Silica Content on Cure Kinetic Models. Polymers. 2024; 16(8):1056. https://doi.org/10.3390/polym16081056
Chicago/Turabian StyleVogelwaid, Julian, Felix Hampel, Martin Bayer, Michael Walz, Larysa Kutuzova, Günter Lorenz, Andreas Kandelbauer, and Timo Jacob. 2024. "In Situ Monitoring of the Curing of Highly Filled Epoxy Molding Compounds: The Influence of Reaction Type and Silica Content on Cure Kinetic Models" Polymers 16, no. 8: 1056. https://doi.org/10.3390/polym16081056
APA StyleVogelwaid, J., Hampel, F., Bayer, M., Walz, M., Kutuzova, L., Lorenz, G., Kandelbauer, A., & Jacob, T. (2024). In Situ Monitoring of the Curing of Highly Filled Epoxy Molding Compounds: The Influence of Reaction Type and Silica Content on Cure Kinetic Models. Polymers, 16(8), 1056. https://doi.org/10.3390/polym16081056