Amphiphilic Dendronized Copolymer-Encapsulated Au, Ag and Pd Nanoparticles for Catalysis in the 4-Nitrophenol Reduction and Suzuki–Miyaura Reactions
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Materials
2.2. Instruments
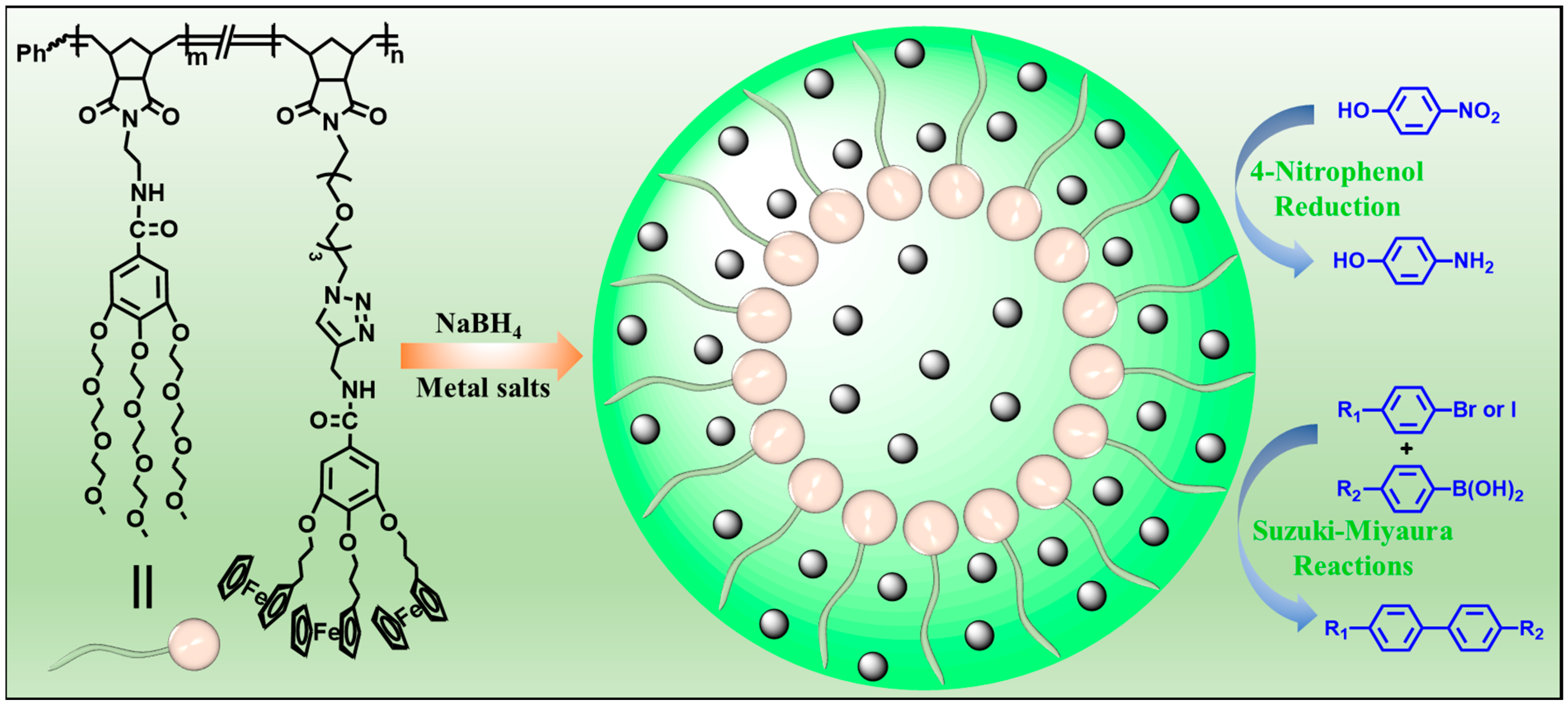
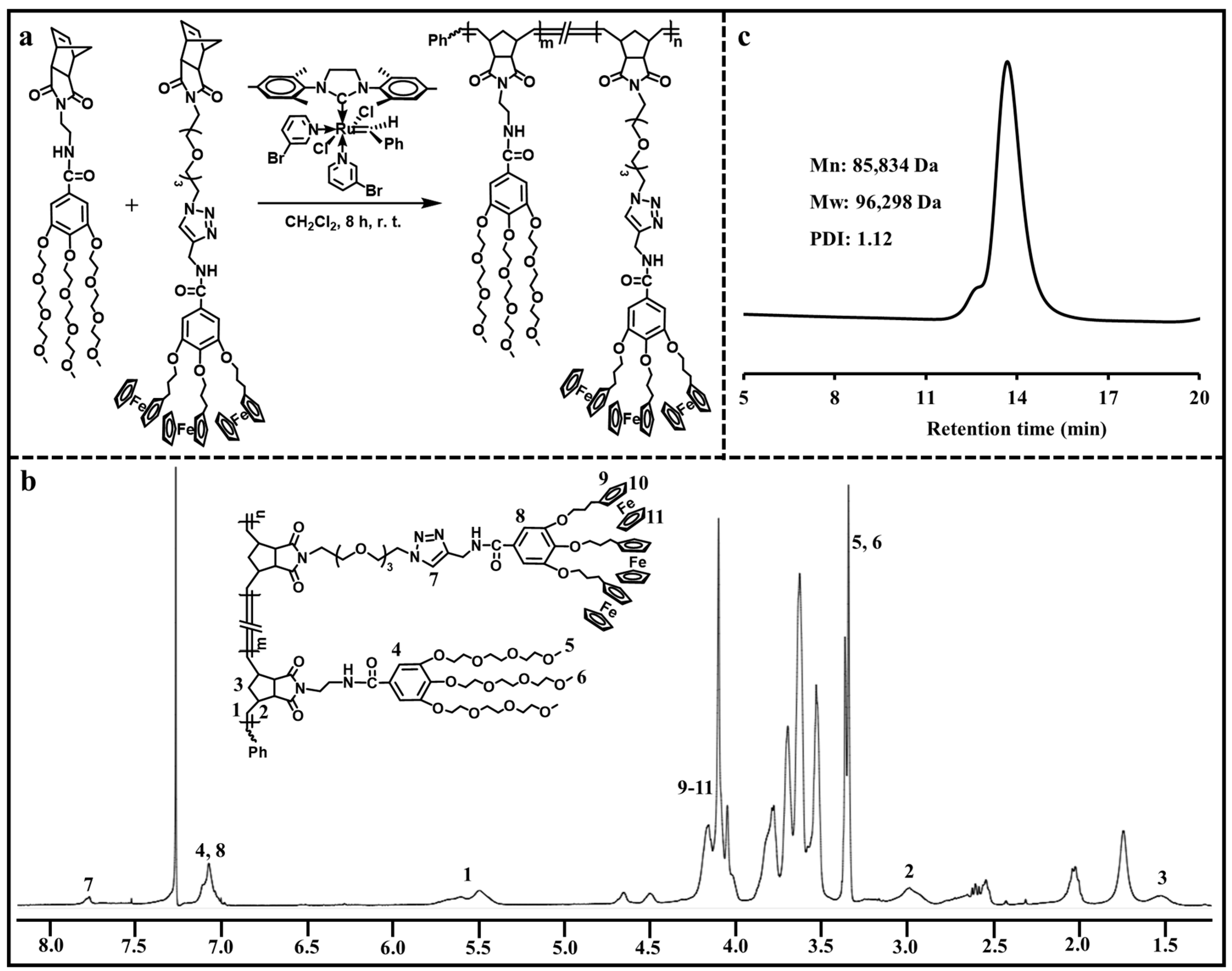
2.3. Synthesis of Amphiphilic Dendronized Copolymer
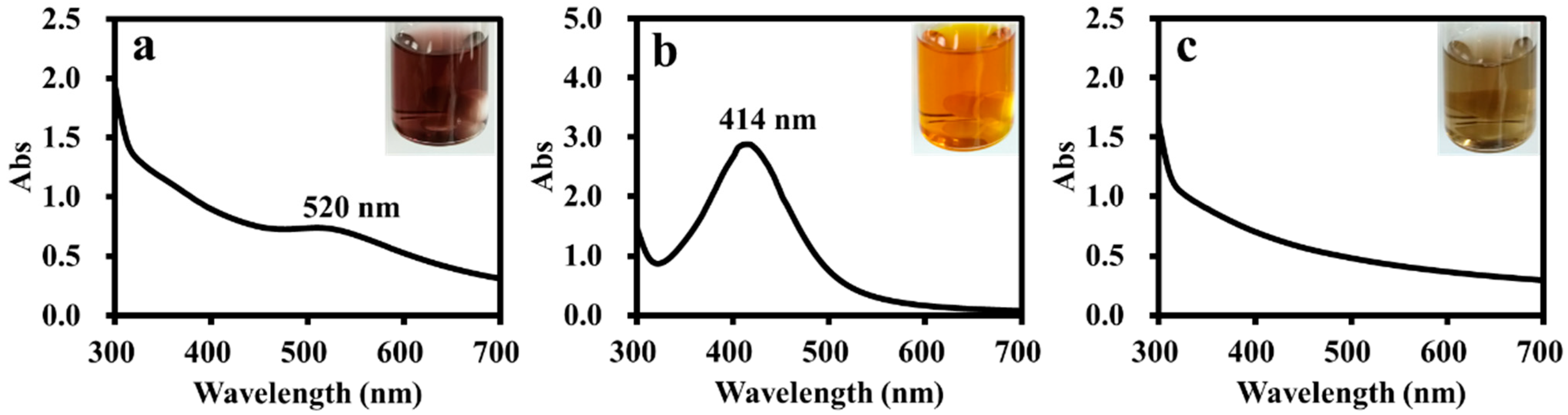
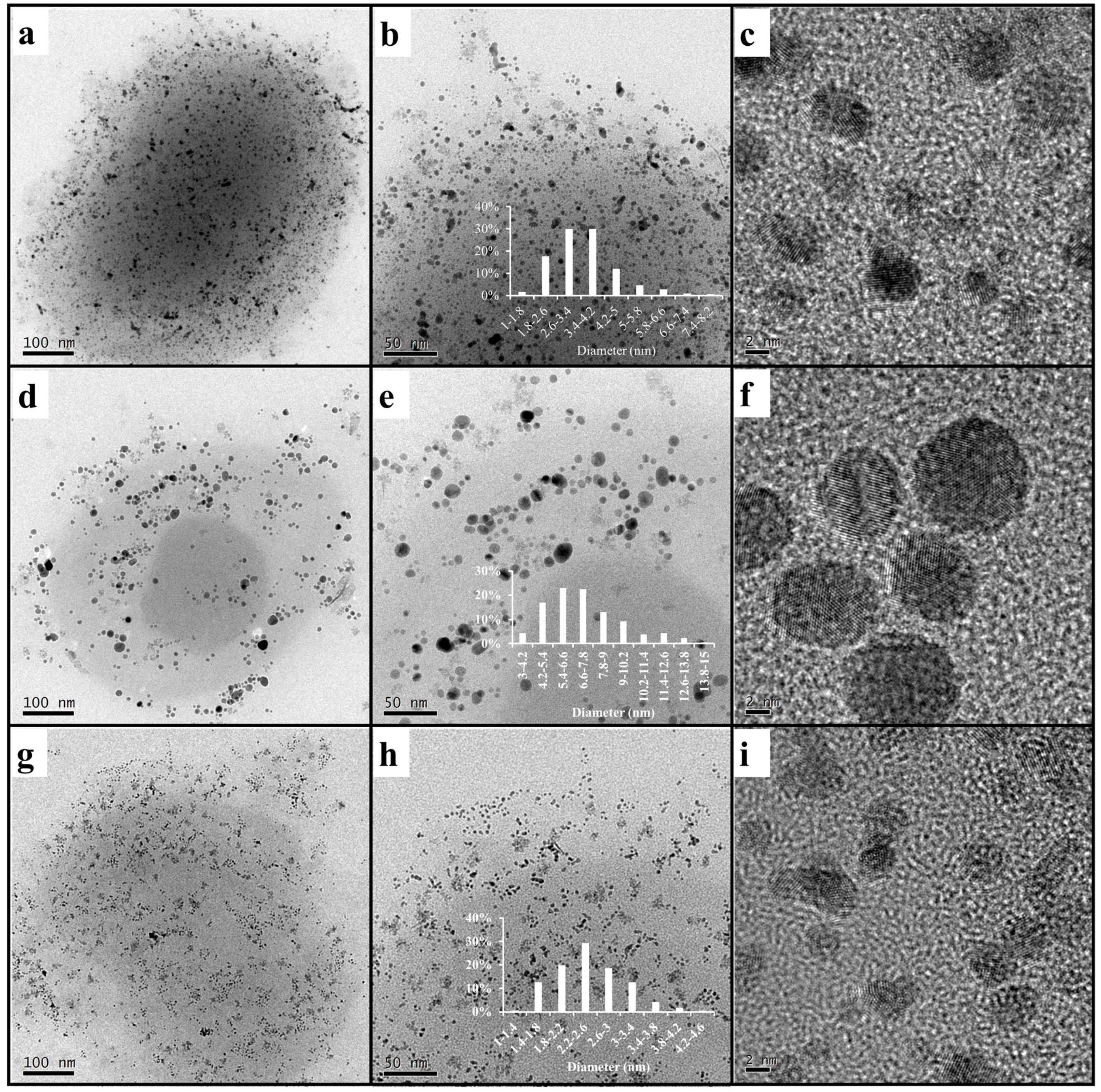
2.4. Preparation of Amphiphilic Dendronized Copolymer-Encapsulated Au, Ag and Pd Nanoparticles
2.5. General Process for the 4-Nitrophenol Reduction Using Amphiphilic Dendronized Copolymer-Encapsulated Au, Ag and Pd Nanoparticles as Catalysts
2.6. General Process for Suzuki–Miyaura Reactions Using Amphiphilic Dendronized Copolymer-Encapsulated Pd Nanoparticles as Catalyst
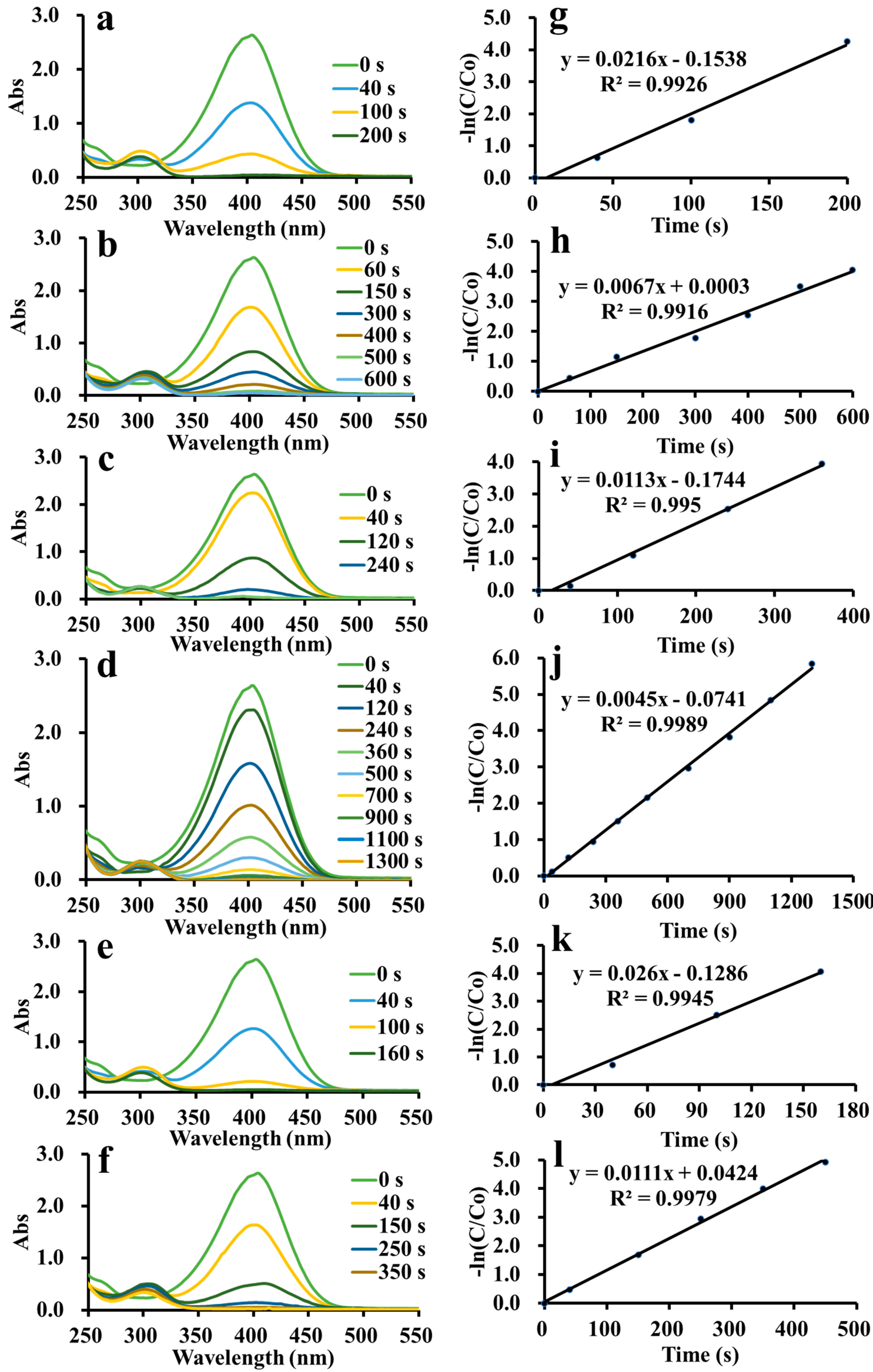
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gao, C.B.; Lyu, F.L.; Yin, Y.D. Encapsulated Metal Nanoparticles for Catalysis. Chem. Rev. 2021, 121, 834–881. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Astruc, D. State of the Art and Prospects in Metal–Organic Framework (MOF)-Based and MOF-Derived Nanocatalysis. Chem. Rev. 2020, 120, 1438–1511. [Google Scholar] [CrossRef] [PubMed]
- Axet, M.R.; Philippot, K. Catalysis with Colloidal Ruthenium Nanoparticles. Chem. Rev. 2020, 120, 1085–1145. [Google Scholar] [CrossRef] [PubMed]
- Trzeciak, A.M.; Augustyniak, A.W. The role of palladium nanoparticles in catalytic C–C cross-coupling reactions. Coord. Chem. Rev. 2019, 384, 1–20. [Google Scholar] [CrossRef]
- Shifrina, Z.B.; Matveeva, V.G.; Bronstein, L.M. Role of Polymer Structures in Catalysis by Transition Metal and Metal Oxide Nanoparticle Composites. Chem. Rev. 2020, 120, 1350–1396. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.Y.; Li, S.F.; Cao, J.Z.; Wang, Y.C.; Yang, W.B.; Zhang, L.J.; Liu, Y.J.; Qiu, J.S.; Tao, S.Y. A Hierarchical-Structured Impeller with Engineered Pd Nanoparticles Catalyzing Suzuki Coupling Reactions for High-Purity Biphenyl. ACS Appl. Mater. Interfaces 2021, 13, 17429–17438. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.L.; Shi, X.F.; Hua, R.; Zhang, R.; Yao, Y.J.; Zhao, B.; Liu, T.; Zheng, J.Z.; Lu, G. Remarkably catalytic activity in reduction of 4-nitrophenol and methylene blue by Fe3O4@COF supported noble metal nanoparticles. Appl. Catal. B Environ. 2020, 260, 118142. [Google Scholar] [CrossRef]
- Wang, J.C.; Liu, C.X.; Kan, X.; Wu, X.W.; Kan, J.L.; Dong, Y.B. Pd@COF-QA: A phase transfer composite catalyst for aqueous Suzuki–Miyaura coupling reaction. Green Chem. 2020, 22, 1150–1155. [Google Scholar] [CrossRef]
- Favier, I.; Pla, D.; Gomez, M. Palladium Nanoparticles in Polyols: Synthesis, Catalytic Couplings, and Hydrogenations. Chem. Rev. 2020, 120, 1146–1183. [Google Scholar] [CrossRef]
- Shi, Y.F.; Lyu, Z.H.; Zhao, M.; Chen, R.H.; Nguyen, Q.N.; Xia, Y.N. Noble-Metal Nanocrystals with Controlled Shapes for Catalytic and Electrocatalytic Applications. Chem. Rev. 2021, 121, 649–735. [Google Scholar] [CrossRef]
- Gan, W.P.; Xu, H.; Jin, X.Y.; Cao, X.S.; Gao, H.F. Recyclable Palladium-Loaded Hyperbranched Polytriazoles as Efficient Polymer Catalysts for Heck Reaction. ACS Appl. Energy Mater. 2020, 2, 677–684. [Google Scholar] [CrossRef]
- Zhang, W.J.; Kong, H.M.; Wu, Z.N.; Yao, A.F.; Wang, L.A.; Quo, L.; He, Y.J.; Qiao, X.G.; Pang, X.C.; Xie, J.P. Confined Unimolecular Micelles for Precisely Controlled In Situ Synthesis of Stable Ultrasmall Metal Nanocluster Assemblies. Chem. Mater. 2021, 33, 5067–5075. [Google Scholar] [CrossRef]
- Huang, T.F.; Sheng, G.; Manchanda, P.; Emwas, A.H.; Lai, Z.P.; Nunes, S.P.; Peinemann, K.V. Cyclodextrin polymer networks decorated with subnanometer metal nanoparticles for high-performance low-temperature catalysis. Sci. Adv. 2019, 5, eaax6976. [Google Scholar] [CrossRef] [PubMed]
- Frauenrath, H. Dendronized polymers—Building a new bridge from molecules to nanoscopic objects. Prog. Polym. Sci. 2005, 30, 325–384. [Google Scholar] [CrossRef]
- Liu, X.; Liu, F.F.; Liu, W.T.; Gu, H.B. ROMP and MCP as Versatile and Forceful Tools to Fabricate Dendronized Polymers for Functional Applications. Polym. Rev. 2020, 61, 1–53. [Google Scholar] [CrossRef]
- Chen, Y.M.; Xiong, X.Q. Tailoring dendronized polymers. Chem. Commun. 2010, 46, 5049–5060. [Google Scholar] [CrossRef]
- Liang, C.O.; Helms, B.; Hawker, C.J.; Fréchet, J.M.J. Dendronized cyclocopolymers with a radial gradient of polarity and their use to catalyze a difficult esterification. Chem. Commun. 2003, 20, 2524–2525. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Lin, W.; Astruc, D.; Gu, H.B. Syntheses and applications of dendronized polymers. Prog. Polym. Sci. 2019, 96, 43–105. [Google Scholar] [CrossRef]
- Liu, F.F.; Liu, X.; Astruc, D.; Gu, H.B. Dendronized triazolyl-containing ferrocenyl polymers as stabilizers of gold nanoparticles for recyclable two-phase reduction of 4-nitrophenol. J. Colloid Interface Sci. 2019, 533, 161–170. [Google Scholar] [CrossRef]
- Lu, J.H.; Yang, Y.; Gao, J.F.; Duan, H.C.; Lu, C.L. Thermoresponsive Amphiphilic Block Copolymer-Stablilized Gold Nanoparticles: Synthesis and High Catalytic Properties. Langmuir 2018, 34, 8205–8214. [Google Scholar] [CrossRef]
- Liu, X.; Liu, F.F.; Astruc, D.; Lin, W.; Gu, H.B. Highly-branched amphiphilic organometallic dendronized diblock copolymer: ROMP synthesis, self-assembly and long-term Au and Ag nanoparticle stabilizer for high-efficiency catalysis. Polymer 2019, 173, 1–10. [Google Scholar] [CrossRef]
- Liu, X.; Ling, Q.J.; Zhao, L.; Qiu, G.R.; Wang, Y.H.; Song, L.X.; Zhang, Y.; Ruiz, J.; Astruc, D.; Gu, H.B. New ROMP synthesis of ferrocenyl dendronized polymers. Macromol. Rapid Commun. 2017, 38, 1700448. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Qiu, G.R.; Zhang, L.; Liu, F.F.; Mu, S.D.; Long, Y.R.; Zhao, Q.X.; Liu, Y.; Gu, H.B. Controlled ROMP Synthesis of Ferrocene-Containing Amphiphilic Dendronized Diblock Copolymers as Redox-Controlled Polymer Carriers. Macromol. Chem. Phys. 2018, 219, 1800273. [Google Scholar] [CrossRef]
- Ogba, O.M.; Warner, N.C.; O’Leary, D.J.; Grubbs, R.H. Recent Advances in Ruthenium-Based Olefin Metathesis. Chem. Soc. Rev. 2018, 47, 4510–4544. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Rapakousiou, A.; Deraedt, C.; Ciganda, R.; Wang, Y.L.; Ruiz, J.; Gu, H.B.; Astruc, D. Multiple Applications of Polymers Containing Electron-reservoir Metal-sandwich Complexes. Chem. Commun. 2020, 56, 11374–11385. [Google Scholar] [CrossRef]
- Liu, X.; Ren, Z.J.; Liu, F.F.; Zhao, L.; Ling, Q.J.; Gu, H.B. Multifunctional Self-Healing Dual Network Hydrogels Constructed via Host–Guest Interaction and Dynamic Covalent Bond as Wearable Strain Sensors for Monitoring Human and Organ Motions. ACS Appl. Mater. Interfaces 2021, 13, 14612–14622. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.F.; Liu, X.; Gu, H.B. Multi-Network Poly(β-cyclodextrin)/PVA/Gelatin/Carbon Nanotubes Composite Hydrogels Constructed by Multiple Dynamic Crosslinking as Flexible Electronic Devices. Macromol. Mater. Eng. 2022, 307, 2100724. [Google Scholar] [CrossRef]
- Deraedt, C.; Salmon, L.; Etienne, L.; Ruiz, J.; Astruc, D. “Click” dendrimers as efficient nanoreactors in aqueous solvent: Pd nanoparticle stabilization for sub-ppm Pd catalysis of Suzuki-Miyaura reactions of aryl bromides. Chem. Commun. 2013, 49, 8169–8171. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.L.; Ciganda, R.; Salmon, L.; Gregurec, D.; Irigoyen, J.; Moya, S.; Ruiz, J.; Astruc, D. Highly Efficient Transition Metal Nanoparticle Catalysts in Aqueous Solutions. Angew. Chem. Int. Ed. 2016, 55, 3091–3095. [Google Scholar] [CrossRef]
- Huang, T.; Meng, F.; Qi, L.M. Facile Synthesis and One-Dimensional Assembly of Cyclodextrin-Capped Gold Nanoparticles and Their Applications in Catalysis and Surface-Enhanced Raman Scattering. J. Phys. Chem. C 2009, 113, 13636–13642. [Google Scholar] [CrossRef]
- Yoshida, H.; Kuwauchi, Y.; Jinschek, J.R.; Sun, K.J.; Tanaka, S.; Kohyama, M.; Shimada, S.; Haruta, M.; Takeda, S. Visualizing Gas Molecules Interacting with Supported Nanoparticulate Catalysts at Reaction Conditions. Science 2012, 335, 317–319. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Liu, C.Y.; Liu, Y. Au/graphene hydrogel: Synthesis, characterization and its use for catalytic reduction of 4-nitrophenol. J. Mater. Chem. 2012, 22, 8426–8430. [Google Scholar] [CrossRef]
- Yuwen, L.H.; Xu, F.; Xue, B.; Luo, Z.M.; Zhang, Q.; Bao, B.Q.; Su, S.; Weng, L.X.; Huang, W.; Wang, L.H. General synthesis of noble metal (Au, Ag, Pd, Pt) nanocrystal modified MoS2 nanosheets and the enhanced catalytic activity of Pd–MoS2 for methanol oxidation. Nanoscale 2014, 6, 5762–5769. [Google Scholar] [CrossRef] [PubMed]
- Ogasawara, S.; Kato, S. Palladium Nanoparticles Captured in Microporous Polymers: A Tailor-Made Catalyst for Heterogeneous Carbon Cross-Coupling Reactions. J. Am. Chem. Soc. 2010, 132, 4608–4613. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.W.; Lee, Y.W.; Kim, M.; Kang, S.W.; Han, S.W. One-pot synthesis and electrocatalytic activity of octapodal Au-Pd nanoparticles. Chem. Commun. 2011, 47, 2553–2555. [Google Scholar] [CrossRef] [PubMed]
- Hervés, P.; Pérez-Lorenzo, M.; Liz-Marzán, L.M.; Dzubiella, J.; Lubc, Y.; Ballauff, M. Catalysis by metallic nanoparticles in aqueous solution: Model reactions. Chem. Soc. Rev. 2012, 41, 5577–5587. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.L.; Akita, T.; Ishida, T.; Haruta, M.; Xu, Q. Synergistic catalysis of Au@Ag core-shell nanoparticles stabilized on metal-organic framework. J. Am. Chem. Soc. 2011, 133, 1304–1306. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Zhang, Q.; Chen, J.Y.; Xia, Y.N. A comparison study of the catalytic properties of Au-based nanocages, nanoboxes, and nanoparticles. Nano Lett. 2010, 10, 30–35. [Google Scholar] [CrossRef] [PubMed]
- Gangula, A.; Podila, R.; Karanam, L.; Janardhana, C.; Rao, A.M. Catalytic reduction of 4-nitrophenol using biogenic gold and silver nanoparticles derived from Breynia rhamnoides. Langmuir 2011, 27, 15268–15274. [Google Scholar] [CrossRef]
- Li, H.; Han, L.; Cooperwhite, J.; Kim, I. Palladium nanoparticles decorated carbon nanotubes: Facile synthesis and their applications as highly efficient catalysts for the reduction of 4-nitrophenol. Green Chem. 2012, 14, 586–591. [Google Scholar] [CrossRef]
- Emmanuel, R.; Karuppiah, C.; Chen, S.M.; Palanisamy, S.; Padmavathy, S.; Prakash, P. Green synthesis of gold nanoparticles for trace level detection of a hazardous pollutant (nitrobenzene) causing Methemoglobinaemia. J. Hazard. Mater. 2014, 279, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Nemanashi, M.; Meijboom, R. Synthesis and characterization of Cu, Ag and Au dendrimer-encapsulated nanoparticles and their application in the reduction of 4-nitrophenol to 4-aminophenol. J. Colloid Interface Sci. 2013, 389, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.F.; Liu, X.; Chen, F.; Fu, Q. Tannic Acid: A green and efficient stabilizer of Au, Ag, Cu and Pd nanoparticles for the 4-Nitrophenol Reduction, Suzuki-Miyaura coupling reactions and click reactions in aqueous solution. J. Colloid Interface Sci. 2021, 604, 281–291. [Google Scholar] [CrossRef] [PubMed]
- Lan, T.X.; An, R.; Liu, Z.; Li, K.J.; Xiang, J.; Liu, G.Y. Facile fabrication of a biomass-based film with interwoven fibrous network structure as heterogeneous catalysis platform. J. Colloid Interface Sci. 2018, 532, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Xiao, M.; Bao, Z.; Wang, P.; Wang, J. A General Approach to Mesoporous Metal Oxide Microspheres Loaded with Noble Metal Nanoparticles. Angew. Chem. Int. Edit. 2012, 51, 6406–6410. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Su, X.T.; Ma, L.D.; Khan, A.; Wang, L.; Wang, J.D.; Maloletnev, A.S.; Yang, C. Promotion effects of halloysite nanotubes on catalytic activity of Co3O4 nanoparticles toward reduction of 4-nitrophenol and organic dyes. J. Hazard. Mater. 2021, 403, 123870. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.J.; Zhang, G.M.; Zhang, P.; Fu, Z.Y. Ag-Pd bimetallic hollow nanostructures with tunable compositions and structures for the reduction of 4-nitrophenol. J. Alloys Compd. 2022, 925, 166689. [Google Scholar] [CrossRef]
- Hunge, Y.M.; Yadav, A.A.; Kang, S.W.; Kim, H. Facile synthesis of multitasking composite of Silver nanoparticle with Zinc oxide for 4-nitrophenol reduction, photocatalytic hydrogen production, and 4-chlorophenol degradation. J. Alloys Compd. 2022, 928, 167133. [Google Scholar] [CrossRef]
- Tian, X.Q.; Muhammad, Z.; Li, J.; Sun, W.; Niu, X.Y.; Zhu, Y.J. Pd/Mo2N-TiO2 as efficient catalysts for promoted selective hydrogenation of 4-nitrophenol: A green bio-reducing preparation method. J. Catal. 2020, 391, 190–201. [Google Scholar] [CrossRef]
- Shu, F.; Wu, J.; Jiang, G.P.; Qiao, Y.Z.; Wang, Y.L.; Wu, D.D.; Zhong, Y.J.; Zhang, T.W.; Song, J.L.; Jin, Y.C.; et al. A hierarchically porous and hygroscopic carbon-based catalyst from natural wood for efficient catalytic reduction of industrial high-concentration 4-nitrophenol. Sep. Purif. Technol. 2022, 300, 121823. [Google Scholar] [CrossRef]
- Kong, Y.H.; Sun, Q.X.; Zhang, G.G.; Liu, F.; Liu, M.C.; Zheng, Y.Q. Stepwise synthesis of polyhedral AuAgPd nanoframes for plasmon-enhanced catalytic reduction of 4-nitrophenol. Mater. Lett. 2022, 325, 132808. [Google Scholar] [CrossRef]
- Yadav, D.; Awasthi, S.K. A Pd confined hierarchically conjugated covalent organic polymer for hydrogenation of nitroaromatics: Catalysis, kinetics, thermodynamics and mechanism. Green Chem. 2020, 22, 4295–4303. [Google Scholar] [CrossRef]
- Chen, C.S.; Chen, T.C.; Chiu, K.L.; Wu, H.C.; Pao, C.W.; Chen, C.L.; Hsu, H.C.; Kao, H.M. Silver particles deposited onto magnetic carbon nanofibers as highly active catalysts for 4-nitrophenol reduction. Appl. Catal. B Environ. 2022, 315, 121596. [Google Scholar] [CrossRef]
- Liu, T.; Sun, Y.H.; Jiang, B.; Guo, W.; Qin, W.; Xie, Y.M.; Zhao, B.; Zhao, L.; Liang, Z.Q.; Jiang, L. Pd Nanoparticle-Decorated 3D-Printed Hierarchically Porous TiO2 Scaffolds for the Efficient Reduction of a Highly Concentrated 4-Nitrophenol Solution. ACS Appl. Mater. Interfaces 2020, 12, 28100–28109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.D.; Ouyang, B.; Zhang, X.L.; Xia, G.Q.; Wang, N.T.; Ou, H.Y.; Ma, L.; Mao, P.X.; Ostrikov, K.K.; Di, L.B.; et al. Plasma-enabled synthesis of Pd/GO rich in oxygen-containing groups and defects for highly efficient 4-nitrophenol reduction. Appl. Surf. Sci. 2022, 597, 153727. [Google Scholar] [CrossRef]
- Li, X.Z.; Peng, W.; Li, L.; Chen, S.; Ye, L.; Peng, C. Simple synthesis of copper/MXene/polyacrylamide hydrogel catalyst for 4-nitrophenol reduction. Mater. Lett. 2022, 324, 132705. [Google Scholar] [CrossRef]
- Huang, X.J.; Lin, D.Y.; Duan, P.; Chen, H.P.; Zhao, Y.J.; Yang, W.T.; Pan, Q.H.; Tian, X.L. Space-confined growth of nanoscale metal-organic frameworks/Pd in hollow mesoporous silica for highly efficient catalytic reduction of 4-nitrophenol. J. Colloid Interface Sci. 2023, 629, 55–64. [Google Scholar] [CrossRef]
- Wang, Q.L.; Wei, Z.J.; Li, J.; Feng, D.Y.; Feng, A.; Zhang, H. Hierarchical-Structured Pd Nanoclusters Catalysts x-PdNCs/CoAl(O)/rGO-T by the Captopril-Capped Pd Cluster Precursor Method for the Highly Efficient 4-Nitrophenol Reduction. ACS Appl. Mater. Interfaces 2022, 14, 27775–27790. [Google Scholar] [CrossRef] [PubMed]
- Feng, D.Y.; Wei, Z.J.; Wang, Q.L.; Feng, A.; Zhang, H. Controllable Synthesis of Cobalt-Containing Nanosheet Array-Like Ternary CuCoAl-LDH/rGO Hybrids to Boost the Catalytic Efficiency for 4-Nitrophenol Reduction. ACS Appl. Mater. Interfaces 2022, 14, 24265–24280. [Google Scholar] [CrossRef]
- Wunder, S.; Lu, Y.; Albrecht, M.; Ballauff, M. Catalytic Activity of Faceted Gold Nanoparticles Studied by a Model Reaction: Evidence for Substrate-Induced Surface Restructuring. ACS Catal. 2011, 1, 908–916. [Google Scholar] [CrossRef]
- Ansar, S.M.; Kitchens, C.L. Impact of Gold Nanoparticle Stabilizing Ligands on the Colloidal Catalytic Reduction of 4-Nitrophenol. ACS Catal. 2016, 6, 5553–5560. [Google Scholar] [CrossRef]
- Wunder, S.; Polzer, F.; Lu, Y.; Mei, Y.; Ballauff, M. Kinetic analysis of catalytic reduction of 4-nitrophenol by metallic nanoparticles immobilized in spherical polyelectrolyte brushes. J. Phys. Chem. C 2010, 114, 8814–8820. [Google Scholar] [CrossRef]
- Beletskaya, I.P.; Alonso, F.; Tyurin, V. The Suzuki-Miyaura reaction after the Nobel prize. Coord. Chem. Rev. 2019, 385, 137–173. [Google Scholar] [CrossRef]
- Biffis, A.; Centomo, P.; Del Zotto, A.; Zecca, M. Pd Metal catalysts for cross-couplings and related reactions in the 21st century: A critical review. Chem. Rev. 2018, 118, 2249–2295. [Google Scholar] [CrossRef] [PubMed]
- Fihri, A.; Bouhrara, M.; Nekoueishahraki, B.; Basset, J.M.; Polshettiwar, V. Nanocatalysts for Suzuki cross-coupling reactions. Chem. Soc. Rev. 2011, 40, 5181–5203. [Google Scholar] [CrossRef]
- Veisi, H.; Ozturk, T.; Karmakar, B.; Tamoradi, T.; Hemmati, S. In situ decorated Pd NPs on chitosan-encapsulated Fe3O4/SiO2-NH2 as magnetic catalyst in Suzuki-Miyaura coupling and 4-nitrophenol reduction. Carbohydr. Polym. 2020, 235, 115966. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharjee, P.; Dewan, A.; Boruah, P.K.; Das, M.R.; Mahanta, S.P.; Thakur, A.J.; Bora, U. Bimetallic Pd–Ag nanoclusters decorated micro-cellulose bio-template towards efficient catalytic Suzuki–Miyaura coupling reaction of nitrogen-rich heterocycles. Green Chem. 2022, 24, 7208–7219. [Google Scholar] [CrossRef]
- Jasim, S.A.; Ansari, M.J.; Majdi, H.S.; Opulencia, M.J.C.; Uktamov, K.F. Nanomagnetic Salamo-based-Pd(0) Complex: An efficient heterogeneous catalyst for Suzuki–Miyaura and Heck cross-coupling reactions in aqueous medium. J. Mol. Struct. 2022, 1261, 132930. [Google Scholar] [CrossRef]
- Wang, Y.B.; Tao, J.H.; Wang, Y.N.; Huang, L.Z.; Ding, X.P. Remarkable reduction ability towards p-nitrophenol by a synergistic effect against the aggregation and leaching of palladium nanoparticles in dendritic supported catalysts. Appl. Surf. Sci. 2022, 574, 151702. [Google Scholar] [CrossRef]
- Chen, D.D.; Wei, L.S.; Yu, Y.H.; Zha, L.; Sun, Q.H.; Han, C.; Lu, J.M.; Nie, H.G.; Shao, L.X.; Qian, J.J.; et al. Size-Selective Suzuki–Miyaura Coupling Reaction over Ultrafine Pd Nanocatalysts in a Water-Stable Indium–Organic Framework. Inorg. Chem. 2022, 61, 15320–15324. [Google Scholar] [CrossRef]
- Ren, F.F.; Li, S.M.; Zheng, W.Q.; Song, Q.Y.; Jia, W.H.; Nan, Y.Q.; Jia, H.J.; Liu, J.; Li, Y.X. Preparation of a novel heterogeneous palladium nanocatalyst based on carboxyl modified magnetic nanoparticles and its applications in Suzuki-Miyaura coupling reactions. Colloids Surf. A Physicochem. Eng. Asp. 2022, 642, 128611. [Google Scholar] [CrossRef]
- Altan, O.; Kalay, E. The influence of band bending phenomenon on photocatalytic Suzuki-Miyaura coupling reaction: The case of AgPd alloy nanoparticles supported on graphitic carbon nitride. Appl. Surf. Sci. 2022, 580, 152287. [Google Scholar] [CrossRef]
- Căta, L.; Terenti, N.; Cociug, C.; Hădade, N.D.; Grosu, I.; Bucur, C.; Cojocaru, B.; Parvulescu, V.I.; Mazur, M.; Čejka, J. Sonogashira Synthesis of New Porous Aromatic Framework-Entrapped Palladium Nanoparticles as Heterogeneous Catalysts for Suzuki–Miyaura Cross-Coupling. ACS Appl. Mater. Interfaces 2022, 14, 10428–10437. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.G.; Zhan, H.; Wang, N.; Song, Y.P.; Wang, C.G.; Wang, X.M.; Ma, L.L.; Chen, L.G. Palladium Nanoparticles on Covalent Organic Framework Supports as Catalysts for Suzuki–Miyaura Cross-Coupling Reactions. ACS Appl. Nano Mater. 2021, 4, 6239–6249. [Google Scholar] [CrossRef]
- Wang, G.; Hao, P.C.; Chang, Y.J.; Zhang, Q.P.; Liu, W.Y.; Duan, B.; Zhan, H.J.; Bi, S.X. Copper and palladium bimetallic sub-nanoparticles were stabilized on modified polyaniline materials as an efficient catalyst to promote C–C coupling reactions in aqueous solution. Nanoscale 2022, 14, 2256–2265. [Google Scholar] [CrossRef]
- Wang, J.L.; Wang, L.L.; Cai, X.P.; Karmakar, B.; Zangeneh, M.M.; Liu, H.R. Pd nanoparticles fabricated cyano-functionalized mesoporous SBA-15: A novel heterogeneous catalyst for Suzuki-Miyaura coupling reactions and anti-human lung cancer effects. Mater. Chem. Phys. 2021, 257, 123375. [Google Scholar] [CrossRef]
- Veisi, H.; Joshani, Z.; Karmakar, B.; Tamoradi, T.; Heravi, M.M.; Gholami, J. Ultrasound assisted synthesis of Pd NPs decorated chitosan-starch functionalized Fe3O4 nanocomposite catalyst towards Suzuki-Miyaura coupling and reduction of 4-nitrophenol. Int. J. Biol. Macromol. 2021, 172, 104–113. [Google Scholar] [CrossRef]
- Palem, R.R.; Shimoga, G.; Kim, S.Y.; Bathula, C.; Ghodake, G.S.; Lee, S.H. Biogenic palladium nanoparticles: An effectual environmental benign catalyst for organic coupling reactions. J. Ind. Eng. Chem. 2022, 106, 52–68. [Google Scholar] [CrossRef]
- Fan, M.Y.; Wang, W.D.; Wang, X.Y.; Zhu, Y.Y.; Dong, Z.P. Ultrafine Pd Nanoparticles Modified on Azine-Linked Covalent Organic Polymers for Efficient Catalytic Suzuki–Miyaura Coupling Reaction. Ind. Eng. Chem. Res. 2022, 59, 12677–12685. [Google Scholar] [CrossRef]
- Heidari, F.; Hekmati, M.; Veisi, H. Magnetically separable and recyclable Fe3O4@SiO2/isoniazide/Pd nanocatalyst for highly efficient synthesis of biaryls by Suzuki coupling reactions. J. Colloid Interface Sci. 2017, 501, 175–184. [Google Scholar] [CrossRef]


| Catalyst | Amount (mol%) | Diameter (nm) | K (s−1) | TOF (h−1) |
|---|---|---|---|---|
| AuNPs | 2.0 | 3.5 ± 3.0 | 2.16 × 10−2 | 900 |
| 0.5 | 6.70 × 10−3 | 1200 | ||
| AgNPs | 2.0 | 7.2 ± 4.0 | 1.13 × 10−2 | 750 |
| 0.5 | 4.50 × 10−3 | 550 | ||
| PdNPs | 2.0 | 2.5 ± 1.0 | 2.60 × 10−2 | 1130 |
| 0.5 | 1.11 × 10−2 | 2060 |
| Catalyst | Amount | Diameter (nm) | K (s−1) | TOF (h−1) | Reference |
|---|---|---|---|---|---|
| Co3O4/HNTs | 0.1 mg | 9.4 | 4.42 × 10−3 | 262 | [46] |
| AgPd-120 | - | 27.6 ± 3.1 | 4.07 × 10−3 | 765 | [47] |
| Ag/ZnO | 1 mg | - | 4.92 × 10−3 | - | [48] |
| Pd/Mo2N-TiO2 | 0.8 wt% | 2.4 ± 0.33 | 1.96 × 10−2 | 698 | [49] |
| Pd/3D-AC | 0.1 g | 2.97 | 1.50 × 10−2 | 101 | [50] |
| AuAgPd PNFs | 40 μg | - | 1.04 × 10−3 | - | [51] |
| Pd@CCTP | 2.0 mg | 20–25 | 2.08 × 10−2 | 883 | [52] |
| Ag0.5/C15h | 0.51 wt% | 3.5 ± 1.1 | 1.22 × 10−3 | - | [53] |
| Pd/TiO2 | 0.11 wt% | 2.0–5.0 | 1.22 × 10−2 | 161 | [54] |
| Pd/GO-P | 0.00065 mg | 3.9 ± 0.4 | 2.32 × 10−2 | - | [55] |
| Cu/MXene/PAM | 1.0 mg | - | 1.26 × 10−2 | - | [56] |
| UiO-66-NH2/Pd@HMSN | 0.05 mg | 5.07 | 9.24 × 10−3 | 214 | [57] |
| ADC-PdNPs | 2.0 mol% | 2.5 ± 1.0 | 2.60 × 10−2 | 1130 | This work |
| ADC-PdNPs | 0.5 mol% | 2.5 ± 1.0 | 1.11 × 10−2 | 2060 | This work |
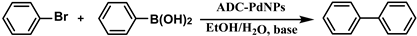 | |||||
|---|---|---|---|---|---|
| Entry | Catalyst (ppm) | Base | Time (h) | Isolated Yield (%) | TOF (h−1) |
| 1 | 500 | NaOH | 10 | 66 | 132 |
| 2 | 500 | K3PO4 | 10 | 48 | 96 |
| 3 | 500 | KOH | 10 | 69 | 138 |
| 4 | 500 | K2CO3 | 10 | 99 | 325 a |
| 5 | 400 | K2CO3 | 10 | 99 | 394 a |
| 6 | 300 | K2CO3 | 10 | 99 | 483 a |
| 7 | 200 | K2CO3 | 10 | 99 | 700 a |
| 8 | 100 | K2CO3 | 10 | 99 | 1257 a |
| 9 | 50 | K2CO3 | 10 | 97 | 2250 a |
| 10 | 10 | K2CO3 | 20 | 77 | 3850 |
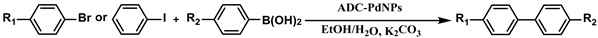 | ||||
|---|---|---|---|---|
| Entry | Aryl Halide | Aryl Boronic Acid | Isolated Yield (%) | TOF (h−1) |
| 1 |  |  | 99 | 1980 |
| 2 | 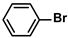 | 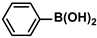 | 97 | 1940 |
| 3 | 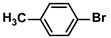 |  | 95 | 1900 |
| 4 | 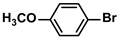 | 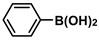 | 96 | 1920 |
| 5 | 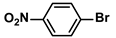 | 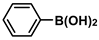 | 93 | 1860 |
| 6 | 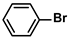 |  | 96 | 1920 |
| 7 |  | 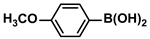 | 98 | 1960 |
| 8 |  | 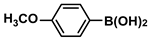 | 94 | 1880 |
| 9 | 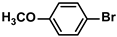 | 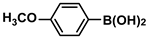 | 95 | 1900 |
| 10 | 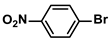 | 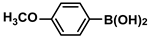 | 92 | 1840 |
| 11 |  | 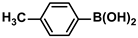 | 91 | 1820 |
| 12 | 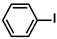 | 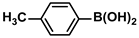 | 96 | 1920 |
| 13 | 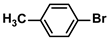 | 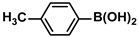 | 92 | 1840 |
| 14 | 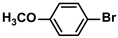 |  | 95 | 1900 |
| 15 | 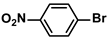 |  | 93 | 1860 |
| Catalyst | Amount | Aryl Halide | Aryl Boronic Acid | Diameter (nm) | Yield (%) | TOF (h−1) | Reference |
|---|---|---|---|---|---|---|---|
| Fe3O4/SiO2-NH2@CS/Pd | 0.1 mol% |  | 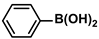 | 5.0 | 98 | 980 | [66] |
| Pd–Ag@PMFC | 0.008 g |  | 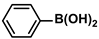 | 52.0 | 93 | - | [67] |
| Fe3O4@H2L-Pd(0) | 0.8 mol% |  | 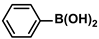 | 12.0–19.0 | 99 | - | [68] |
| DMSTNs-SH-Pd | 0.2 mol% | 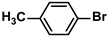 | 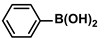 | 3.1 | 78 | 780 | [69] |
| Pd@InOF-1 | 10.0 mg | 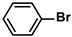 |  | 3.14 | 94 | - | [70] |
| Fe3O4@SiO2-DTPA-Pd | 1.7 µmol |  |  | - | 95 | 186 | [71] |
| g-CN(G)-AgPd | 5.0 mg | 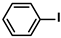 | 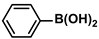 | 2.54 ± 0.76 | 94 | - | [72] |
| PAF-SP@Pd | 20 mg | 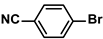 |  | 1–10 | 98 | 12 | [73] |
| Pd/COF-SMC2 | 0.5 mol % | 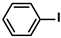 |  | 5 | 96 | 192 | [74] |
| Cu/Pd@Mod-PANI-3OH | 0.094 mol% |  | 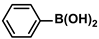 | <1 | 93 | 659 | [75] |
| SBA-15/Et-CN/Pd | 0.1 mol% | 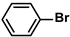 | 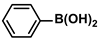 | - | 95 | 950 | [76] |
| PdNPs | 50 ppm | 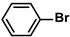 |  | 2.0 ± 1.0 | 98 | 2450 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, F.; Liu, X. Amphiphilic Dendronized Copolymer-Encapsulated Au, Ag and Pd Nanoparticles for Catalysis in the 4-Nitrophenol Reduction and Suzuki–Miyaura Reactions. Polymers 2024, 16, 1080. https://doi.org/10.3390/polym16081080
Liu F, Liu X. Amphiphilic Dendronized Copolymer-Encapsulated Au, Ag and Pd Nanoparticles for Catalysis in the 4-Nitrophenol Reduction and Suzuki–Miyaura Reactions. Polymers. 2024; 16(8):1080. https://doi.org/10.3390/polym16081080
Chicago/Turabian StyleLiu, Fangfei, and Xiong Liu. 2024. "Amphiphilic Dendronized Copolymer-Encapsulated Au, Ag and Pd Nanoparticles for Catalysis in the 4-Nitrophenol Reduction and Suzuki–Miyaura Reactions" Polymers 16, no. 8: 1080. https://doi.org/10.3390/polym16081080





