Enhancing Alginate Hydrogels as Possible Wound-Healing Patches: The Synergistic Impact of Reduced Graphene Oxide and Tannins on Mechanical and Adhesive Properties
Abstract
:1. Introduction
2. Materials and Methods
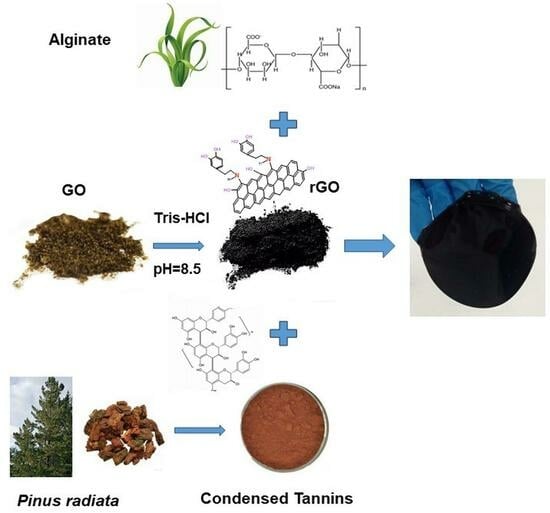
2.1. Hydrogels Synthesis
2.2. Characterization of the Hydrogels
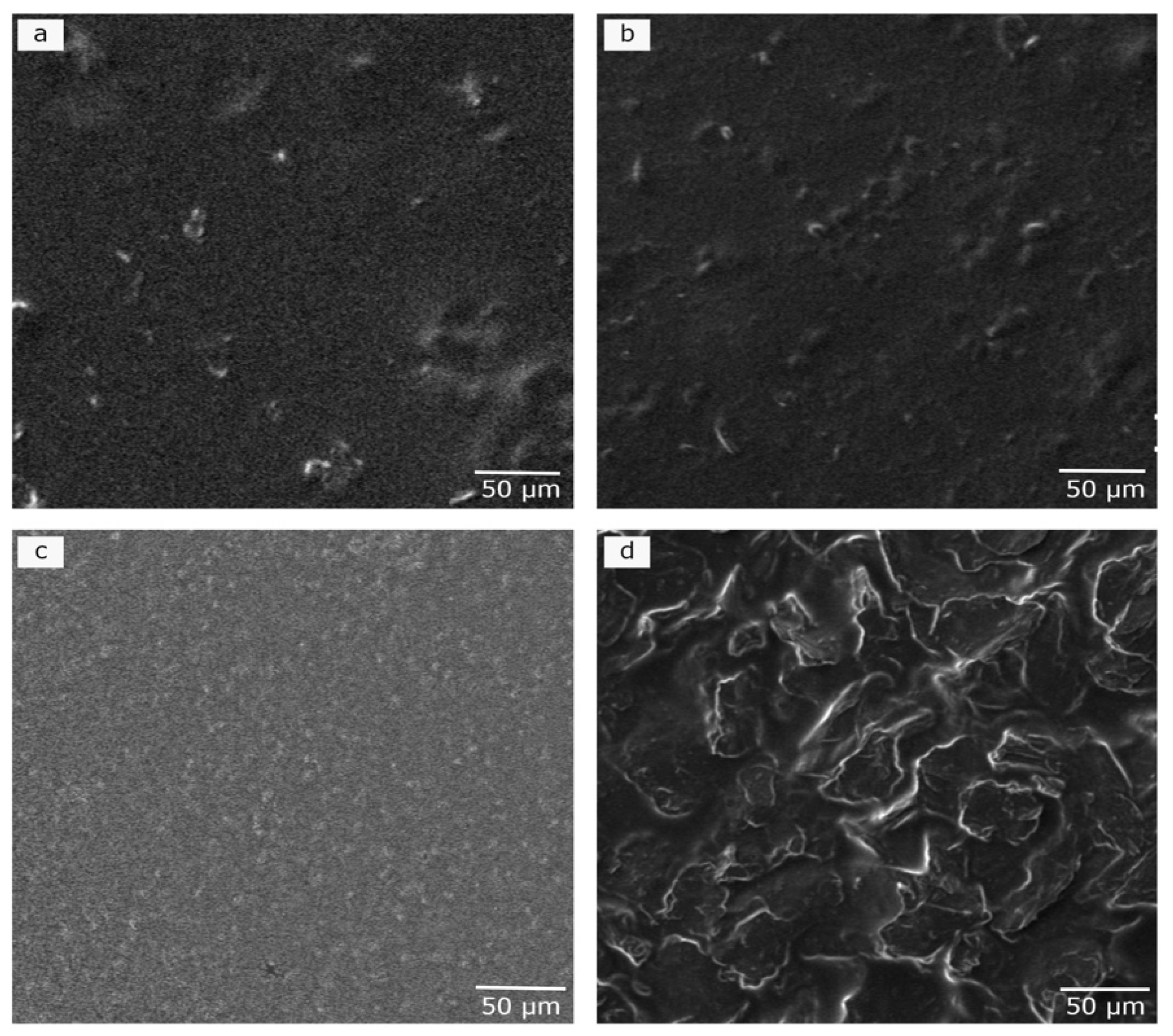
2.2.1. Scanning Electron Microscopy (SEM)
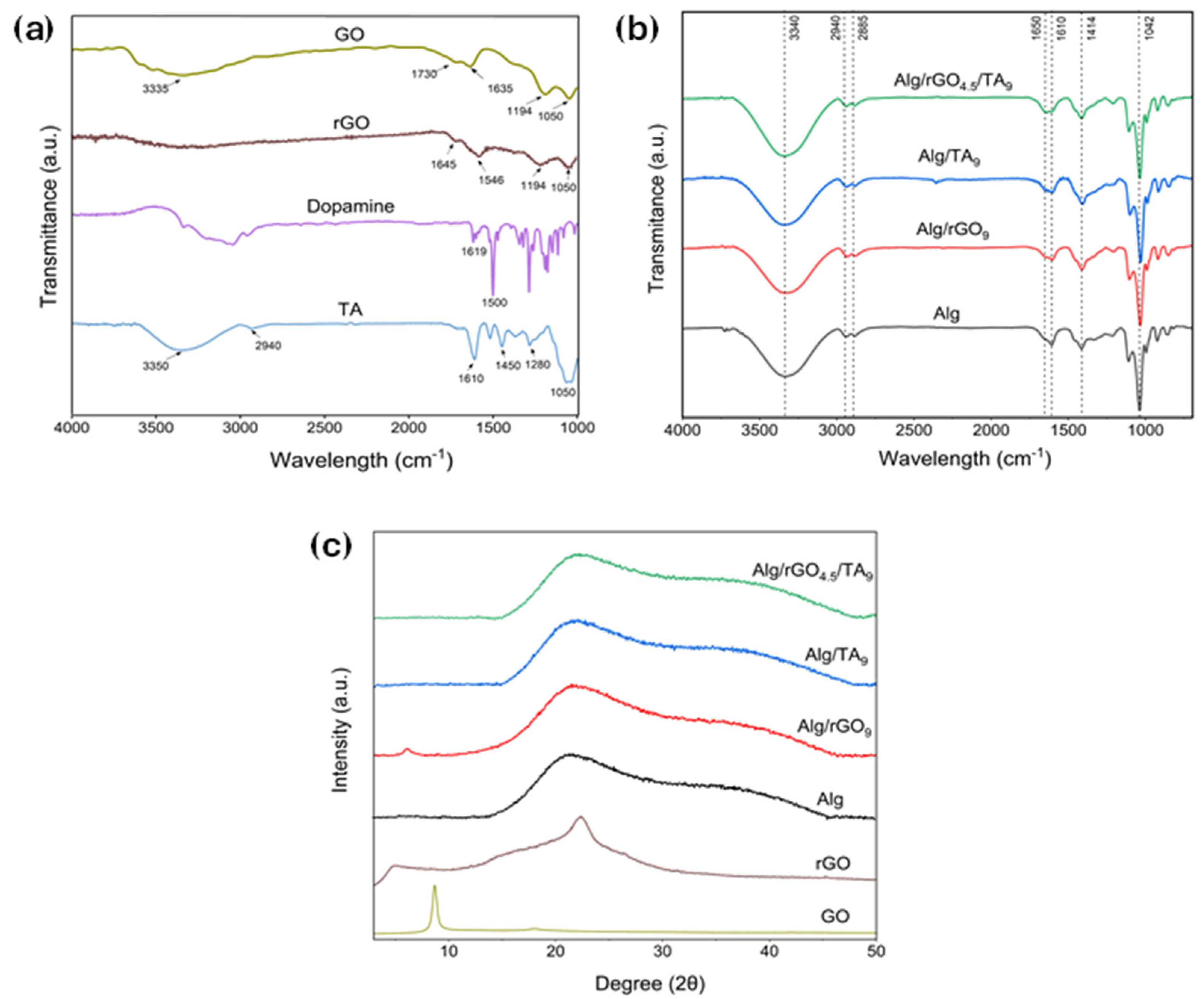
2.2.2. Fourier Transform Infrared Spectroscopy (FTIR)
2.2.3. X-ray Diffraction (XRD)
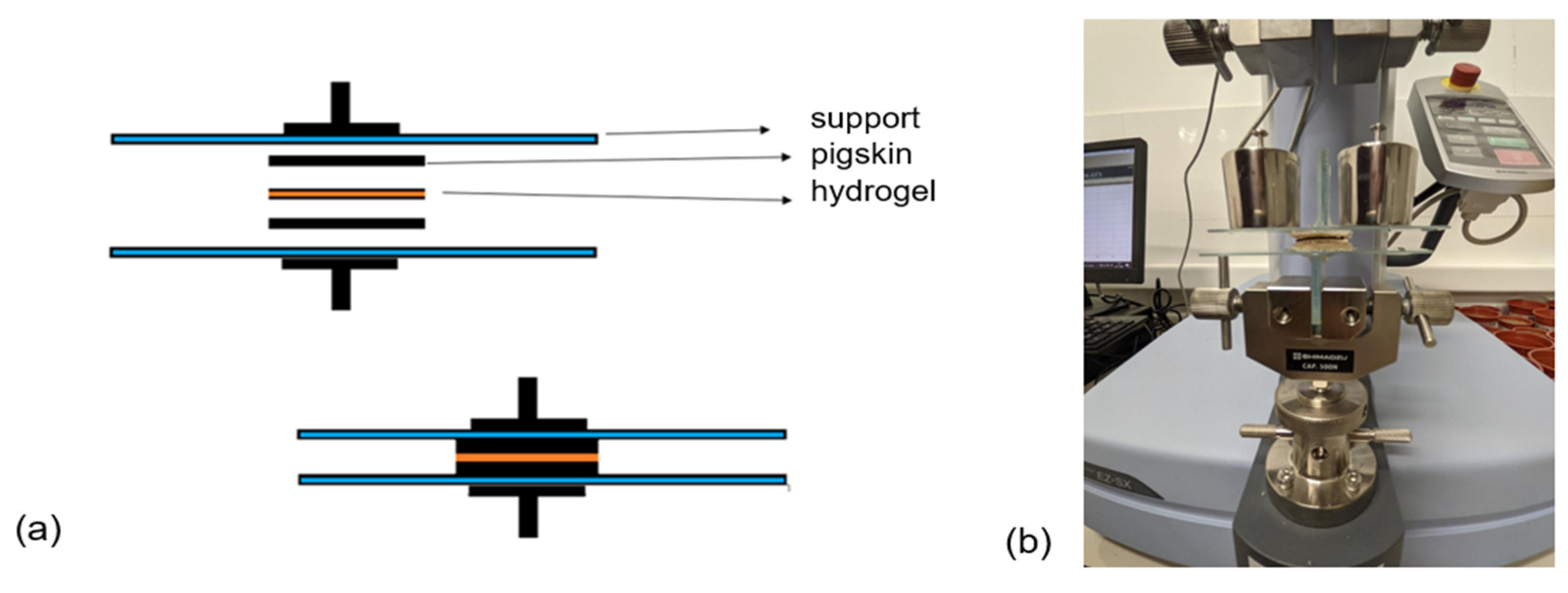
2.2.4. Mechanical Characterization
2.2.5. Adhesive Properties
2.2.6. Cytotoxicity Assay
2.2.7. In Vitro Wound-healing Assay (Scratch Test)
2.3. Statistical Analysis
3. Results and Discussion
3.1. Morphological Studies
3.2. Chemical Characterization of the Hydrogels
3.3. Mechanical Characterization of the Hydrogels
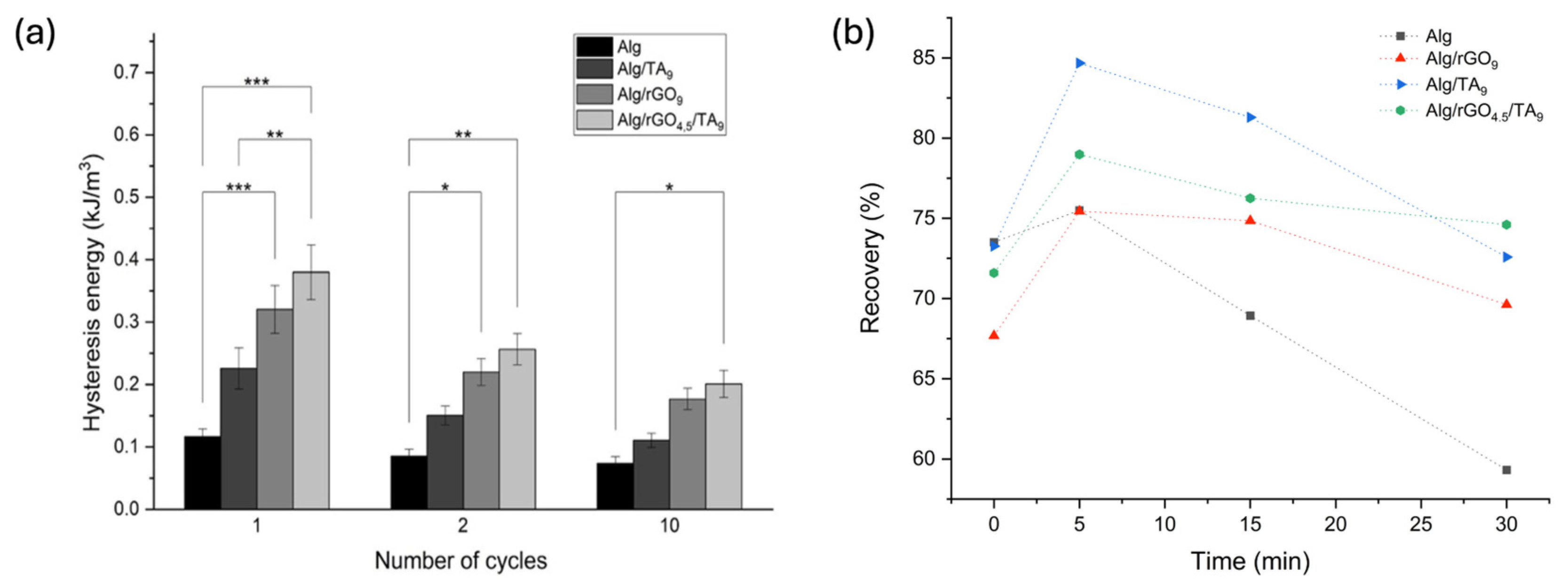
3.3.1. Hysteresis and Self-Recovery of the Hydrogels
3.3.2. Adhesive Properties of the Hydrogels
3.4. Biological Characterization
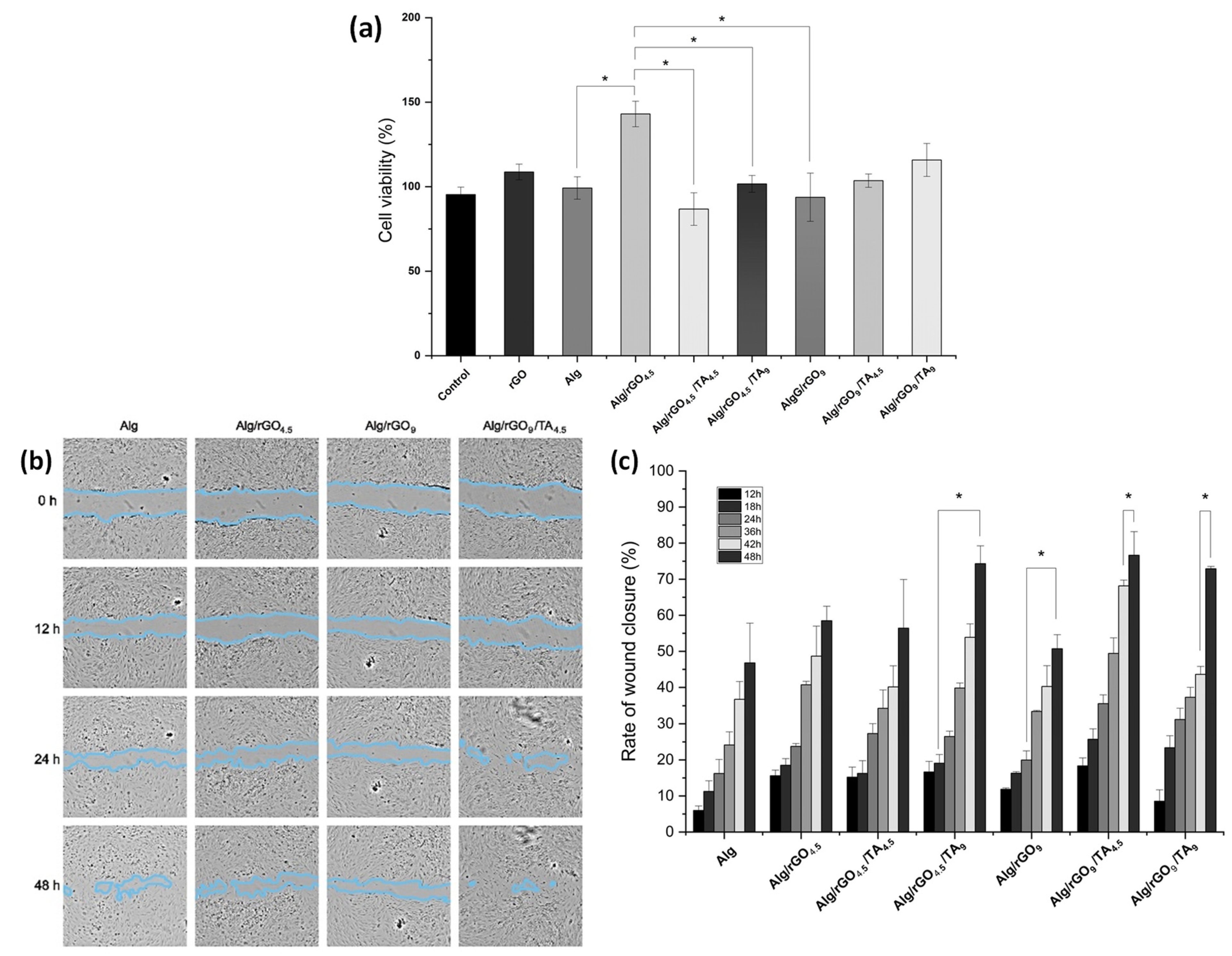
3.4.1. Cytotoxicity Assays
3.4.2. In Vitro Wound-healing Assay (Scratch Test)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chaudhary, S.; Chakraborty, E. Hydrogel based tissue engineering and its future applications in personalized disease modeling and regenerative therapy. Beni-Suef Univ. J. Basic Appl. Sci. 2022, 11, 3. [Google Scholar] [CrossRef] [PubMed]
- Mantha, S.; Pillai, S.; Khayambashi, P.; Upadhyay, A.; Zhang, Y.L.; Tao, O.; Pham, H.M.; Tran, S.D. Smart Hydrogels in Tissue Engineering and Regenerative Medicine. Materials 2019, 12, 3323. [Google Scholar] [CrossRef] [PubMed]
- Nath, J.; Chowdhury, A.; Dolui, S.K. Chitosan/graphene oxide-based multifunctional pH-responsive hydrogel with significant mechanical strength, self-healing property, and shape memory effect. Adv. Polym. Technol. 2018, 37, 3665–3679. [Google Scholar] [CrossRef]
- Raslan, A.; Ciriza, J.; Ochoa de Retana, A.M.; Sanjuan, M.L.; Toprak, M.S.; Galvez-Martin, P.; Saenz-del-Burgo, L.; Pedraz, J.L. Modulation of Conductivity of Alginate Hydrogels Containing Reduced Graphene Oxide through the Addition of Proteins. Pharmaceutics 2021, 13, 1473. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, S.; Yu, W. Functionalized Graphene Oxide-Reinforced Chitosan Hydrogel as Biomimetic Dressing for Wound Healing. Macromol. Biosci. 2021, 21, 2000432. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Zhang, H.; Zhang, X.; Ma, Q.; Teng, L.; Qiu, L. Hydrosoluble collagen based biodegradable hybrid hydrogel for biomedical scaffold. J. Biomater. Sci.-Polym. Ed. 2020, 31, 2199–2219. [Google Scholar] [CrossRef] [PubMed]
- Agnieszka, K.-P. Alginate-Based Hydrogels in Regenerative Medicine. In Alginates; Leonel, P., Ed.; IntechOpen: Rijeka, Croatia, 2019; Chapter 5. [Google Scholar]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.-Y.; Zhu, Y.-J.; Li, H.; Zhang, Y.-G.; Shen, Y.-Q.; Sun, T.-W.; Chen, F. Preparation and enhanced mechanical properties of hybrid hydrogels comprising ultralong hydroxyapatite nanowires and sodium alginate. J. Colloid Interface Sci. 2017, 497, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Drury, J.L.; Dennis, R.G.; Mooney, D.J. The tensile properties of alginate hydrogels. Biomaterials 2004, 25, 3187–3199. [Google Scholar] [CrossRef]
- Moghadam, M.N.; Pioletti, D.P. Improving hydrogels’ toughness by increasing the dissipative properties of their network. J. Mech. Behav. Biomed. Mater. 2015, 41, 161–167. [Google Scholar] [CrossRef]
- Bai, R.; Yang, J.; Suo, Z. Fatigue of hydrogels. Eur. J. Mech. A/Solids 2019, 74, 337–370. [Google Scholar] [CrossRef]
- Yao, H.; Wu, M.; Lin, L.; Wu, Z.; Bae, M.; Park, S.; Wang, S.; Zhang, W.; Gao, J.; Wang, D.; et al. Design strategies for adhesive hydrogels with natural antibacterial agents as wound dressings: Status and trends. Mater. Today Bio 2022, 16, 100429. [Google Scholar] [CrossRef]
- Jing, Z.; Dai, X.; Xian, X.; Du, X.; Liao, M.; Hong, P.; Li, Y. Tough, stretchable and compressive alginate-based hydrogels achieved by non-covalent interactions. RSC Adv. 2020, 10, 23592–23606. [Google Scholar] [CrossRef] [PubMed]
- Rafieian, S.; Mirzadeh, H.; Mandavi, H.; Masoumi, M.E. A review on nanocomposite hydrogels and their biomedical applications. Sci. Eng. Compos. Mater. 2019, 26, 154–174. [Google Scholar] [CrossRef]
- Georgakilas, V.; Otyepka, M.; Bourlinos, A.B.; Chandra, V.; Kim, N.; Kemp, K.C.; Hobza, P.; Zboril, R.; Kim, K.S. Functionalization of Graphene: Covalent and Non-Covalent Approaches, Derivatives and Applications. Chem. Rev. 2012, 112, 6156–6214. [Google Scholar] [CrossRef]
- Tarcan, R.; Todor-Boer, O.; Petrovai, I.; Leordean, C.; Astilean, S.; Botiz, I. Reduced graphene oxide today. J. Mater. Chem. C 2020, 8, 1198–1224. [Google Scholar] [CrossRef]
- Tang, T.; Goossens, K.; Lu, S.J.; Meng, D.; Bielawski, C.W. Agar-reduced graphene oxide selectively adsorbs organic dyes and strengthens double-network hydrogels. RSC Adv. 2020, 10, 29287–29295. [Google Scholar] [CrossRef] [PubMed]
- Gupta, T.; Pradhan, A.; Bandyopadhyay-Ghosh, S.; Ghosh, S.B. Thermally exfoliated graphene oxide reinforced stress responsive conductive nanocomposite hydrogel. Polym. Adv. Technol. 2019, 30, 2392–2401. [Google Scholar] [CrossRef]
- Aparicio-Collado, J.L.; Garcia-San-Martin, N.; Molina-Mateo, J.; Torregrosa Cabanilles, C.; Donderis Quiles, V.; Serrano-Aroca, A.; Sabater i Serra, R. Electroactive calcium-alginate/polycaprolactone/reduced graphene oxide nanohybrid hydrogels for skeletal muscle tissue engineering. Colloids Surf. B-Biointerfaces 2022, 214, 112455. [Google Scholar] [CrossRef]
- Xiao, D.; He, M.; Liu, Y.; Xiong, L.; Zhang, Q.; Wei, L.; Li, L.; Yu, X. Strong alginate/reduced graphene oxide composite hydrogels with enhanced dye adsorption performance. Polym. Bull. 2020, 77, 6609–6623. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Kinloch, I.A.; Young, R.J. Graphene/elastomer nanocomposites. Carbon 2015, 95, 460–484. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Lu, L. Polydopamine and Its Derivative Materials: Synthesis and Promising Applications in Energy, Environmental, and Biomedical Fields. Chem. Rev. 2014, 114, 5057–5115. [Google Scholar] [CrossRef] [PubMed]
- Salazar, P.; Martín, M.; González-Mora, J.L. Polydopamine-modified surfaces in biosensor applications. In Polymer Science: Research Advances, Practical Applications and Educational Aspects; Formatex Research Center SL: Badajoz, Spain, 2016. [Google Scholar]
- Cheng, C.; Li, S.; Zhao, J.; Li, X.; Liu, Z.; Ma, L.; Zhang, X.; Sun, S.; Zhao, C. Biomimetic assembly of polydopamine-layer on graphene: Mechanisms, versatile 2D and 3D architectures and pollutant disposal. Chem. Eng. J. 2013, 228, 468–481. [Google Scholar] [CrossRef]
- Han, L.; Lu, X.; Wang, M.; Gan, D.; Deng, W.; Wang, K.; Fang, L.; Liu, K.; Chan, C.W.; Tang, Y.; et al. A Mussel-Inspired Conductive, Self-Adhesive, and Self-Healable Tough Hydrogel as Cell Stimulators and Implantable Bioelectronics. Small 2017, 13, 1601916. [Google Scholar] [CrossRef] [PubMed]
- Rahimnejad, M.; Zhong, W. Mussel-inspired hydrogel tissue adhesives for wound closure. RSC Adv. 2017, 7, 47380–47396. [Google Scholar] [CrossRef]
- Han, L.; Wang, M.; Li, P.; Gan, D.; Yan, L.; Xu, J.; Wang, K.; Fang, L.; Chan, C.W.; Zhang, H.; et al. Mussel-Inspired Tissue-Adhesive Hydrogel Based on the Polydopamine-Chondroitin Sulfate Complex for Growth-Factor-Free Cartilage Regeneration. ACS Appl. Mater. Interfaces 2018, 10, 28015–28026. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, N.; Elissetche, J.; Pereira, M.; Fernandez, K. Extraction of polyphenols from Eucalyptus nitens and Eucalyptus globulus: Experimental kinetics, modeling and evaluation of their antioxidant and antifungical activities. Ind. Crops Prod. 2017, 109, 737–745. [Google Scholar] [CrossRef]
- Molino, S.; Andrea Casanova, N.; Rufian Henares, J.A.; Fernandez Miyakawa, M.E. Natural Tannin Wood Extracts as a Potential Food Ingredient in the Food Industry. J. Agric. Food Chem. 2020, 68, 2836–2848. [Google Scholar] [CrossRef] [PubMed]
- Soledad Parada, M.; Fernandez, K. Modelling the hydrophilic extraction of the bark of Eucalyptus nitens and Eucalyptus globulus: Adsorption isotherm and thermodynamic studies. Ind. Crops Prod. 2017, 109, 558–569. [Google Scholar] [CrossRef]
- Koopmann, A.-K.; Schuster, C.; Torres-Rodriguez, J.; Kain, S.; Pertl-Obermeyer, H.; Petutschnigg, A.; Huesing, N. Tannin-Based Hybrid Materials and Their Applications: A Review. Molecules 2020, 25, 4910. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, Z.; Yin, J. Facile Synthesis of Soluble Graphene via a Green Reduction of Graphene Oxide in Tea Solution and Its Biocomposites. ACS Appl. Mater. Interfaces 2011, 3, 1127–1133. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Shi, Z.; Xu, H.; Ma, X.; Yin, J.; Tian, M. Fabrication of Super Extensible and Highly Tough Graphene Composite Hydrogels by Thermal Treatment Strategy for the Mixture of Tannin and Graphene Oxide. Macromol. Chem. Phys. 2017, 218, 1600549. [Google Scholar] [CrossRef]
- Facchi, D.P.; Lima, A.C.; de Oliveira, J.H.; Lazarin-Bidoia, D.; Nakamura, C.V.; Canesin, E.A.; Bonafe, E.G.; Monteiro, J.P.; Visentainer, J.V.; Muniz, E.C.; et al. Polyelectrolyte complexes based on alginate/tanfloc: Optimization, characterization and medical application. Int. J. Biol. Macromol. 2017, 103, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Olad, A.; Gordani, R.; Eslamzadeh, M.; Mollaei, M. Graphene Oxide Nanocomposite Hydrogels Based on Mucilage Extracted from Ocimum basilicum Seeds Grafted by Acrylate Polymers; Assay on Physicochemical Properties. J. Polym. Environ. 2023, 31, 2399–2414. [Google Scholar] [CrossRef]
- Prasathkumar, M.; Sadhasivam, S. Chitosan/Hyaluronic acid/Alginate and an assorted polymers loaded with honey, plant, and marine compounds for progressive wound healing—Know-how. Int. J. Biol. Macromol. 2021, 186, 656–685. [Google Scholar] [CrossRef] [PubMed]
- Hidayah, N.M.S.; Liu, W.-W.; Lai, C.-W.; Noriman, N.Z.; Khe, C.-S.; Hashim, U.; Lee, H.C. Comparison on graphite, graphene oxide and reduced graphene oxide: Synthesis and characterization. AIP Conf. Proc. 2017, 1892, 150002. [Google Scholar] [CrossRef]
- Khosravi, H.; Naderi, R.; Ramezanzadeh, B. Designing an epoxy composite coating having dual-barrier-active self-healing anti-corrosion functions using a multi-functional GO/PDA/MO nano-hybrid. Mater. Today Chem. 2023, 27, 101282. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, Y.; Zhen, M.; Wu, Y.; Ma, M.; Cheng, Y.; Jin, Y. Effect of catechin and tannins on the structural and functional properties of sodium alginate/gelatin/poly(vinylalcohol) blend films. Food Hydrocoll. 2023, 135, 108141. [Google Scholar] [CrossRef]
- Das, A.K.; Islam, M.N.; Faruk, M.O.; Ashaduzzaman, M.; Dungani, R. Review on tannins: Extraction processes, applications and possibilities. South Afr. J. Bot. 2020, 135, 58–70. [Google Scholar] [CrossRef]
- Liu, H.; Xi, P.; Xie, G.; Shi, Y.; Hou, F.; Huang, L.; Chen, F.; Zeng, Z.; Shao, C.; Wang, J. Simultaneous Reduction and Surface Functionalization of Graphene Oxide for Hydroxyapatite Mineralization. J. Phys. Chem. C 2012, 116, 3334–3341. [Google Scholar] [CrossRef]
- Cui, W.; Li, M.; Liu, J.; Wang, B.; Zhang, C.; Jiang, L.; Cheng, Q. A Strong Integrated Strength and Toughness Artificial Nacre Based on Dopamine Cross-Linked Graphene Oxide. ACS Nano 2014, 8, 9511–9517. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Lee, J.U.; Jung, B.M.; Byun, J.-H.; Yi, J.-W.; Lee, S.-B.; Kim, B.-S. Simultaneous enhancement of mechanical, electrical and thermal properties of graphene oxide paper by embedding dopamine. Carbon 2013, 65, 296–304. [Google Scholar] [CrossRef]
- Li, W.; Shang, T.; Yang, W.; Yang, H.; Lin, S.; Jia, X.; Cai, Q.; Yang, X. Effectively Exerting the Reinforcement of Dopamine Reduced Graphene Oxide on Epoxy-Based Composites via Strengthened Interfacial Bonding. ACS Appl. Mater. Interfaces 2016, 8, 13037–13050. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.-L.; Yan, X.-P.; Meng, K.; Wang, S.-F. Graphene Oxide Based Photoinduced Charge Transfer Label-Free Near-Infrared Fluorescent Biosensor for Dopamine. Anal. Chem. 2011, 83, 8787–8793. [Google Scholar] [CrossRef] [PubMed]
- Gonultas, O.; Ucar, M.B. Chemical Composition of Some Commercial Tannins Produced in Turkey. In Proceedings of the 55th International Convention of Society of Wood Science and Technology, Beijing, China, 27–31 August 2012. [Google Scholar]
- Fajardo, A.R.; Silva, M.B.; Lopes, L.C.; Piai, J.F.; Rubira, A.F.; Muniz, E.C. Hydrogel based on an alginate—Ca2+/chondroitin sulfate matrix as a potential colon-specific drug delivery system. RSC Adv. 2012, 2, 11095–11103. [Google Scholar] [CrossRef]
- Gu, R.P.; Xu, W.Z.; Charpentier, P.A. Synthesis of polydopamine-coated graphene-polymer nanocomposites via RAFT polymerization. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 3941–3949. [Google Scholar] [CrossRef]
- Rasyida, A.; Halimah, S.; Wijayanti, I.D.; Wicaksono, S.T.; Nurdiansah, H.; Silaen, Y.M.T.; Ni’mah, Y.L.; Ardhyananta, H.; Purniawan, A. A Composite of Hydrogel Alginate/PVA/r-GO for Scaffold Applications with Enhanced Degradation and Biocompatibility Properties. Polymers 2023, 15, 534. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wu, X.; Qin, Z.; Sun, X.; Zhang, H.; Yu, Q.; Yao, M.; He, S.; Dong, X.; Yao, F.; et al. Dual physically cross-linked carboxymethyl cellulose-based hydrogel with high stretchability and toughness as sensitive strain sensors. Cellulose 2020, 27, 9975–9989. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, Q.; Zhao, Y.; Yuan, J.; Zha, X.; Xie, H.; Kong, F.; Xiong, X. Strong and Tough PAm/SA Hydrogel with Highly Strain Sensitivity. J. Renew. Mater. 2022, 10, 415–430. [Google Scholar] [CrossRef]
- Liao, M.; Zhao, Y.; Pan, Y.; Pan, J.; Yao, Q.; Zhang, S.; Zhao, H.; Hu, Y.; Zheng, W.; Zhou, W.; et al. A good adhesion and antibacterial double-network composite hydrogel from PVA, sodium alginate and tannic acid by chemical and physical cross-linking for wound dressings. J. Mater. Sci. 2023, 58, 5756–5772. [Google Scholar] [CrossRef]
- Chen, T.; Chen, Y.; Rehman, H.U.; Chen, Z.; Yang, Z.; Wang, M.; Li, H.; Liu, H. Ultratough, Self-Healing, and Tissue-Adhesive Hydrogel for Wound Dressing. ACS Appl. Mater. Interfaces 2018, 10, 33523–33531. [Google Scholar] [CrossRef]
- Obagi, Z.; Damiani, G.; Grada, A.; Falanga, V. Principles of Wound Dressings: A Review. Surg. Technol. Int. Int. Dev. Surg. Surg. Res. 2019, 35, 1–8. [Google Scholar]
- Marrella, A.; Lagazzo, A.; Barberis, F.; Catelani, T.; Quarto, R.; Scaglione, S. Enhanced mechanical performances and bioactivity of cell laden-graphene oxide/alginate hydrogels open new scenario for articular tissue engineering applications. Carbon 2017, 115, 608–616. [Google Scholar] [CrossRef]
- Wang, W.; Lin, S.; Ye, Z.; Zhou, Y.; Zou, Q.; Zheng, T.; Ding, M. Electrospun egg white protein/polyvinyl alcohol/graphene oxide fibrous wound dressing: Fabrication, antibacterial, cytocompatibility and wound healing assay. Colloids Surf. A-Physicochem. Eng. Asp. 2023, 658, 130658. [Google Scholar] [CrossRef]
- Jafari, H.; Ghaffari-bohlouli, P.; Podstawczyk, D.; Nie, L.; Shavandi, A. Tannic acid post-treatment of enzymatically crosslinked chitosan-alginate hydrogels for biomedical applications. Carbohydr. Polym. 2022, 295, 119844. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.L.; Teng, Y.Y.; Wu, J.J.; Liu, S.Y.; Tang, X.Y.; Jia, Y.; Chen, Z.H.; Zhang, K.W.; Sun, Z.L.; Li, X.; et al. Fibroblasts: Heterogeneous Cells With Potential in Regenerative Therapy for Scarless Wound Healing. Front. Cell Dev. Biol. 2021, 9, 713605. [Google Scholar] [CrossRef]
- Wan, L.; He, Y.; Wang, A.; Pan, J.; Xu, C.; Fu, D.; Ye, Q.; Wu, F. Development of an integrated device utilizing exosome-hyaluronic acid-based hydrogel and investigation of its osteogenic and angiogenic characteristics. Mater. Des. 2024, 237, 112565. [Google Scholar] [CrossRef]
- Yang, R.; Huang, J.; Zhang, W.; Xue, W.; Jiang, Y.; Li, S.; Wu, X.; Xu, H.; Ren, J.; Chi, B. Mechanoadaptive injectable hydrogel based on poly(γ-glutamic acid) and hyaluronic acid regulates fibroblast migration for wound healing. Carbohydr. Polym. 2021, 273, 118607. [Google Scholar] [CrossRef]
- Khanna, S.; Roy, S.; Bagchi, D.; Bagchi, M.; Sen, C.K. Upregulation of oxidant-induced VEGF expression in cultured keratinocytes by a grape seed proanthocyanidin extract. Free. Radic. Biol. Med. 2001, 31, 38–42. [Google Scholar] [CrossRef]


| Nomenclature | 0% rGO | 4.5% rGO | 9% rGO |
|---|---|---|---|
| 0% TA | Alg | Alg/rGO4.5 | Alg/rGO9 |
| 4.5% TA | Alg/TA4.5 | Alg/rGO4.5/TA4.5 | Alg/rGO9/TA4.5 |
| 9% TA | Alg/TA9 | Alg/rGO4.5/TA9 | Alg/rGO9/TA9 |
| Hydrogels | Tensile Strength (kPa) | Elongation (%) | Elastic Modulus (kPa) | Toughness (kJ/m3) |
|---|---|---|---|---|
| Alg | 84.9 ± 17.6 a | 63.8 ± 4.9 a | 169.2 ± 41.5 a | 22.4 ± 4.5 a |
| Alg/rGO9 | 168.8 ± 12.4 b | 53.9 ± 4.2 a | 412 ± 45.7 b | 42.9 ± 3.2 a,b |
| Alg/TA9 | 179.4 ± 30.5 b | 81.8 ± 8.1 b | 298.7 ± 49.3 b | 72.4 ± 17.9 b |
| Alg/rGO4.5/TA9 | 170.7 ± 23.16 b | 74.8 ± 11.4 b | 310.5 ± 57.5 b | 64.3 ± 15 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carrasco, S.; González, L.; Tapia, M.; Urbano, B.F.; Aguayo, C.; Fernández, K. Enhancing Alginate Hydrogels as Possible Wound-Healing Patches: The Synergistic Impact of Reduced Graphene Oxide and Tannins on Mechanical and Adhesive Properties. Polymers 2024, 16, 1081. https://doi.org/10.3390/polym16081081
Carrasco S, González L, Tapia M, Urbano BF, Aguayo C, Fernández K. Enhancing Alginate Hydrogels as Possible Wound-Healing Patches: The Synergistic Impact of Reduced Graphene Oxide and Tannins on Mechanical and Adhesive Properties. Polymers. 2024; 16(8):1081. https://doi.org/10.3390/polym16081081
Chicago/Turabian StyleCarrasco, Sebastián, Luisbel González, Mauricio Tapia, Bruno F. Urbano, Claudio Aguayo, and Katherina Fernández. 2024. "Enhancing Alginate Hydrogels as Possible Wound-Healing Patches: The Synergistic Impact of Reduced Graphene Oxide and Tannins on Mechanical and Adhesive Properties" Polymers 16, no. 8: 1081. https://doi.org/10.3390/polym16081081
APA StyleCarrasco, S., González, L., Tapia, M., Urbano, B. F., Aguayo, C., & Fernández, K. (2024). Enhancing Alginate Hydrogels as Possible Wound-Healing Patches: The Synergistic Impact of Reduced Graphene Oxide and Tannins on Mechanical and Adhesive Properties. Polymers, 16(8), 1081. https://doi.org/10.3390/polym16081081