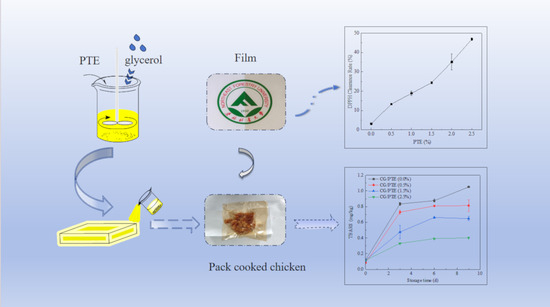
Cassia Seed Gum Films Incorporated with Partridge Tea Extract as an Edible Antioxidant Food Packaging Film for Preservation of Chicken Jerky
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of PTE
2.3. Preparation of Films
2.4. Characterization of Films
2.4.1. Scanning Electron Microscopy (SEM)
2.4.2. FTIR Analysis
2.4.3. X-ray Diffraction (XRD)
2.5. Performance Test
2.5.1. Color of Films
2.5.2. Haze
2.5.3. Thickness and Mechanical Properties
2.5.4. Light Transmittance
2.5.5. Water Vapor Permeability (WVP)
2.5.6. Determination of DPPH Free Radical Scavenging Ability
2.6. Packaging Application
2.7. Statistical Analysis
3. Results
3.1. SEM Observation
3.2. FTIR Analysis
3.3. XRD Analysis
3.4. Color Analysis
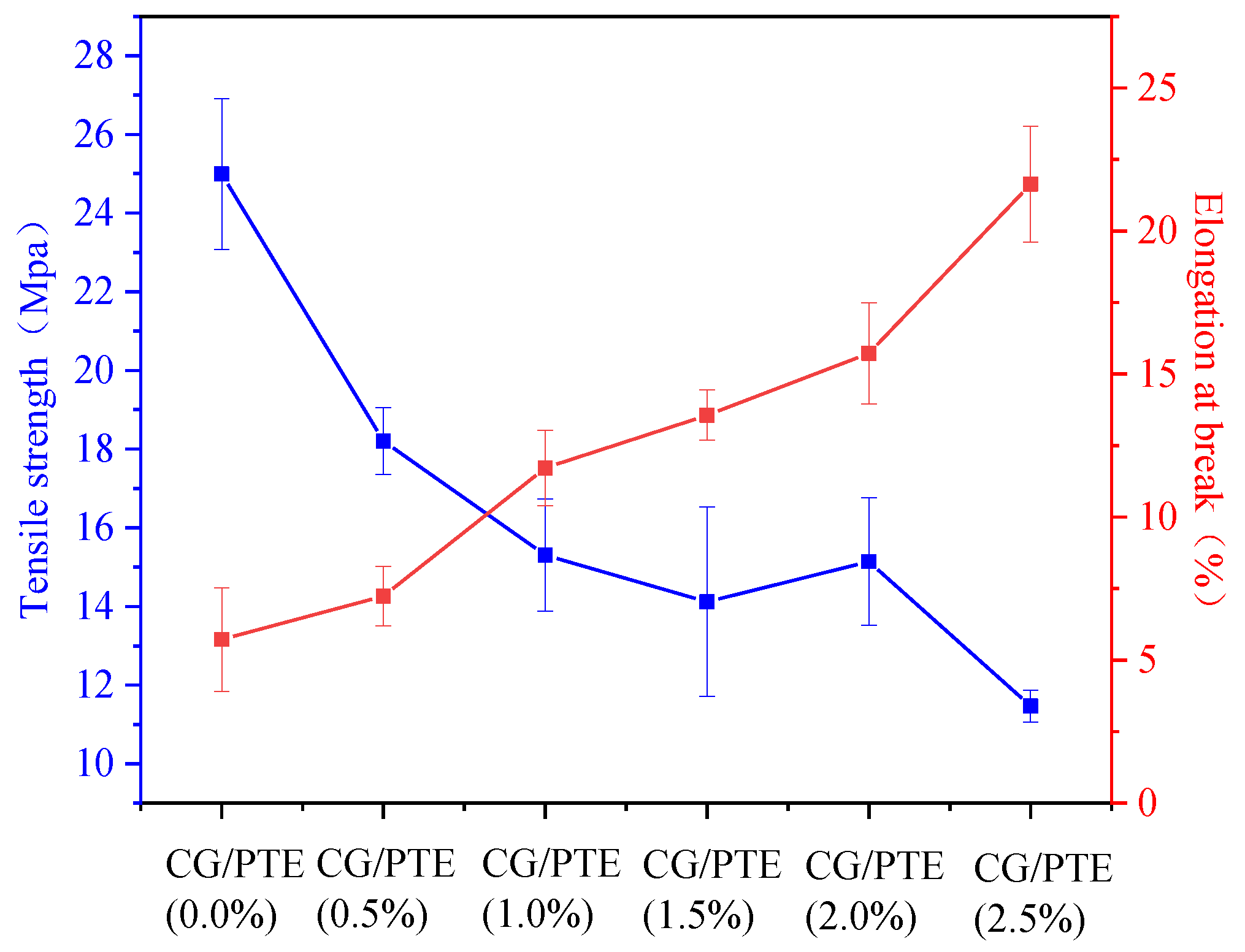
3.5. Mechanical Properties of CG/PTE Films
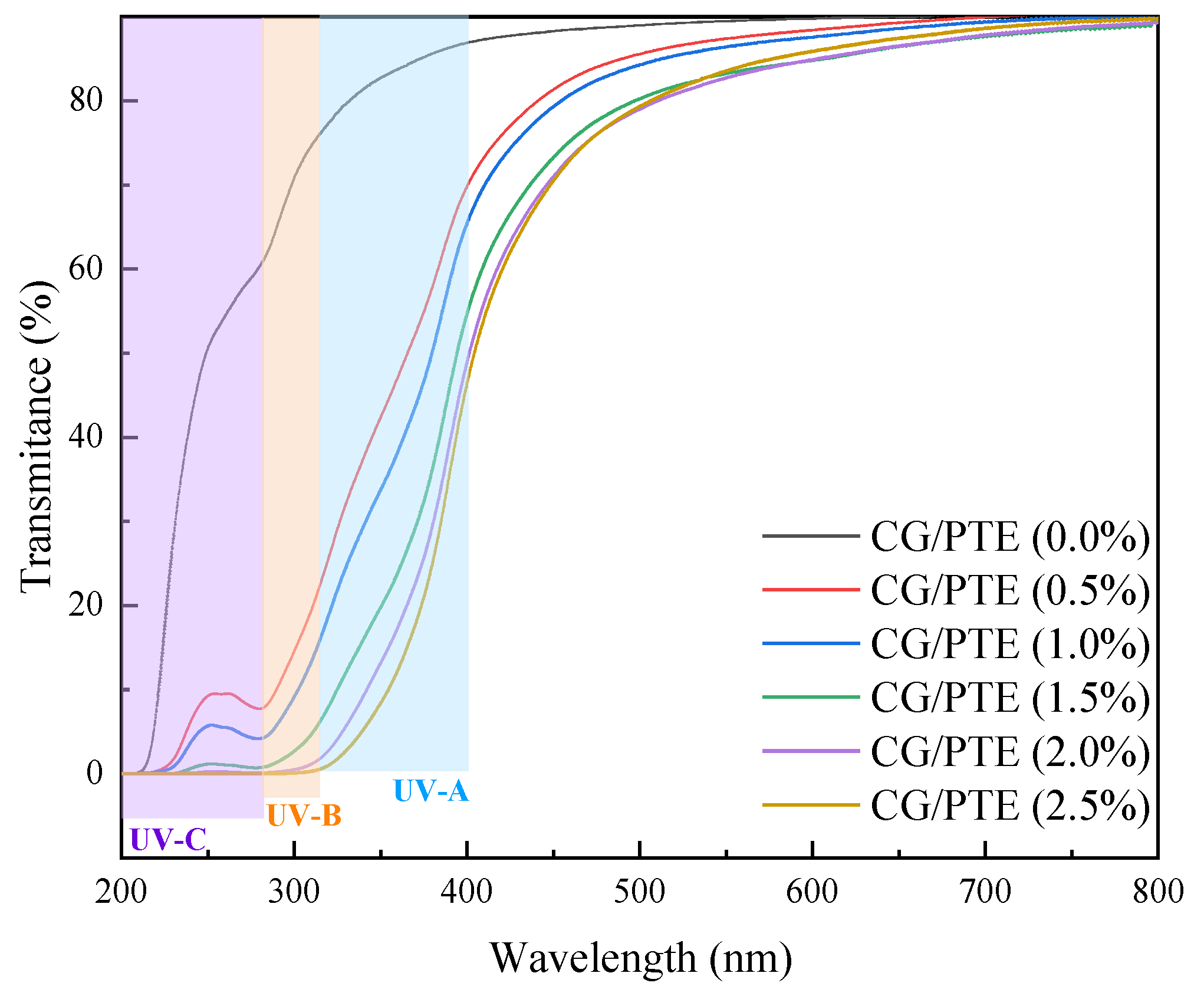
3.6. Optical Performance
3.7. Water Vapor Permeability
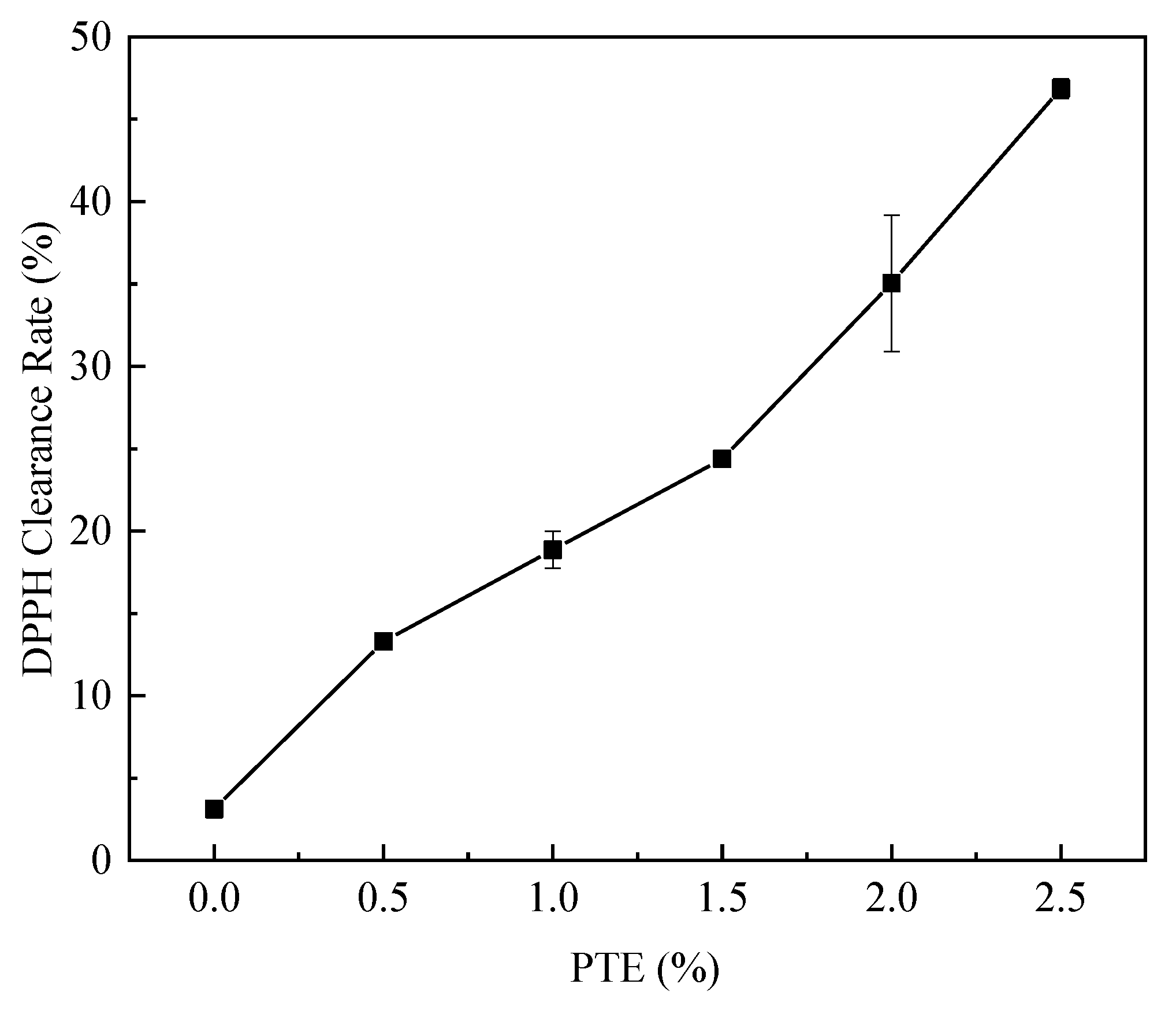
3.8. Antioxidant Activities of CG/PTE Films
3.9. Application in Chicken Jerk Preservation
3.9.1. Appearance
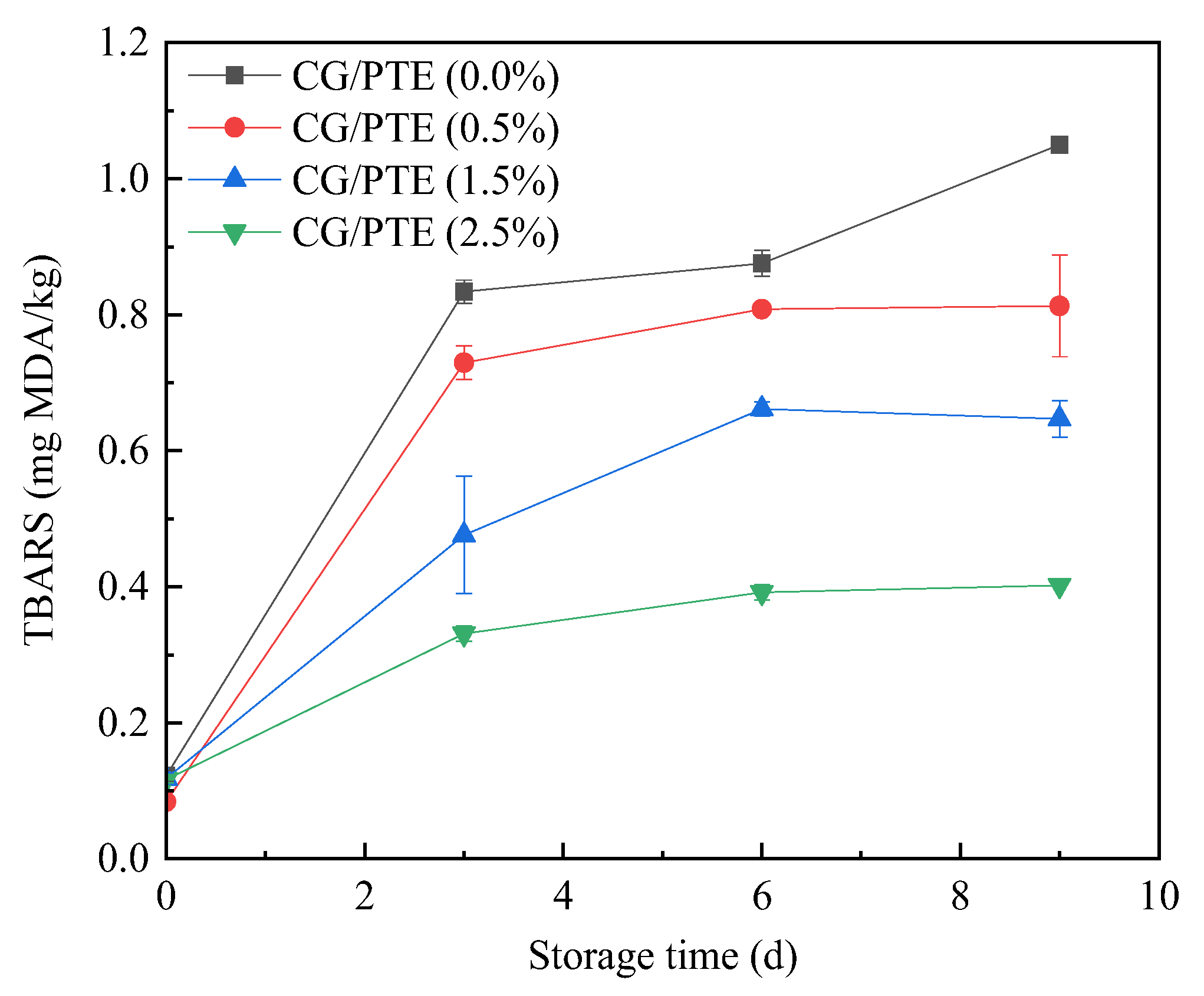
3.9.2. TBARS Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Šovljanski, O.; Pezo, L.; Tomić, A.; Ranitović, A.; Cvetković, D.; Markov, S. Formation of Predictive-Based Models for Monitoring the Microbiological Quality of Beef Meat Processed for Fast-Food Restaurants. Int. J. Environ. Res. Public Health 2022, 19, 16727. [Google Scholar] [CrossRef]
- Wickramasinghe, N.N.; Hlaing, M.M.; Ravensdale, J.T.; Coorey, R.; Chandry, P.S.; Dykes, G.A. Characterization of the Biofilm Matrix Composition of Psychrotrophic, Meat Spoilage Pseudomonads. Sci. Rep. 2020, 10, 16457. [Google Scholar] [CrossRef]
- Das, A.K.; Rajkumar, V.; Nanda, P.K.; Chauhan, P.; Pradhan, S.R.; Biswas, S. Antioxidant Efficacy of Litchi (Litchi chinensis Sonn) Pericarp Extract in Sheep Meat Nuggets. Antioxidants 2016, 5, 16. [Google Scholar] [CrossRef]
- Papuc, C.; Goran, G.V.; Predescu, C.N.; Nicorescu, V. Mechanisms of Oxidative Processes in Meat and Toxicity Induced by Postprandial Degradation Products: A Review. Comp. Rev. Food Sci. Food Safe 2017, 16, 96–123. [Google Scholar] [CrossRef]
- Zhou, G.H.; Xu, X.L.; Liu, Y. Preservation Technologies for Fresh Meat—A Review. Meat Sci. 2010, 86, 119–128. [Google Scholar] [CrossRef]
- Zdanowicz, M.; Jędrzejewski, R.; Pilawka, R. Deep Eutectic Solvents as Simultaneous Plasticizing and Crosslinking Agents for Starch. Int. J. Biol. Macromol. 2019, 129, 1040–1046. [Google Scholar] [CrossRef]
- Feng, G.; Hu, L.; Ma, Y.; Jia, P.; Hu, Y.; Zhang, M.; Liu, C.; Zhou, Y. An Efficient Bio-Based Plasticizer for Poly (Vinyl chloride) from Waste Cooking Oil and Citric Acid: Synthesis and Evaluation in PVC Films. J. Clean. Prod. 2018, 189, 334–343. [Google Scholar] [CrossRef]
- Chen, H.L.; Nath, T.K.; Chong, S.; Foo, V.; Gibbins, C.; Lechner, A.M. The Plastic Waste Problem in Malaysia: Management, Recycling and Disposal of Local and Global Plastic Waste. SN Appl. Sci. 2021, 3, 437. [Google Scholar] [CrossRef]
- Mamun, A.A.; Prasetya, T.A.E.; Dewi, I.R.; Ahmad, M. Microplastics in Human Food Chains: Food Becoming a Threat to Health Safety. Sci. Total Environ. 2023, 858, 159834. [Google Scholar] [CrossRef]
- Wu, X.; Zhou, J.; Liu, Z.; Liu, J.; He, S.; Shao, W. Constructing a Biodegradable Carrageenan Based Food Packaging Film According to the Synergistic Strategies between Peppermint Essential Oil and Thymol. Int. J. Biol. Macromol. 2023, 253, 127537. [Google Scholar] [CrossRef]
- Chandrasekar, C.M.; Krishnamachari, H.; Farris, S.; Romano, D. Development and Characterization of Starch-Based Bioactive Thermoplastic Packaging Films Derived from Banana Peels. Carbohydr. Polym. Technol. Appl. 2023, 5, 100328. [Google Scholar] [CrossRef]
- Rajput, G.; Pandey, I.P.; Joshi, G. Carboxymethylation of Cassia Angustifolia Seed Gum: Synthesis and Rheological Study. Carbohydr. Polym. 2015, 117, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Liu, W.; Wang, L. Developing a Green and Edible Film from Cassia Gum: The Effects of Glycerol and Sorbitol. J. Clean. Prod. 2018, 175, 276–282. [Google Scholar] [CrossRef]
- Sharma, D.; Sharma, P. Synergistic Studies of Cassia Tora Gum with Xanthan and Guar Gum: Carboxymethyl Synthesis of Cassia Gum-Xanthan Synergistic Blend and Characterization. Carbohydr. Res. 2023, 523, 108723. [Google Scholar] [CrossRef] [PubMed]
- Zhao, P.; Yan, X.; Cheng, M.; Wang, Y.; Wang, Y.; Wang, K.; Wang, X.; Wang, J. Effect of Pickering Emulsion on the Physical Properties, Microstructure and Bioactivity of Corn Starch/Cassia Gum Composite Films. Food Hydrocoll. 2023, 141, 108713. [Google Scholar] [CrossRef]
- Cao, L.; Sun, G.; Zhang, C.; Liu, W.; Li, J.; Wang, L. An Intelligent Film Based on Cassia Gum Containing Bromothymol Blue-Anchored Cellulose Fibers for Real-Time Detection of Meat Freshness. J. Agric. Food Chem. 2019, 67, 2066–2074. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, X.; Huo, D.; Cao, C.; Li, Y.; Liang, Y.; Li, B.; Li, L. Preliminary Characterization, Antioxidant and α-Glucosidase Inhibitory Activities of Polysaccharides from Mallotus furetianus. Carbohydr. Polym. 2019, 215, 307–315. [Google Scholar] [CrossRef]
- Wang, R.; He, R.; Li, Z.; Li, S.; Li, C.; Wang, L. Tailor-Made Deep Eutectic Solvents-Based Green Extraction of Natural Antioxidants from Partridge Leaf-Tea (Mallotus furetianus L.). Sep. Purif. Technol. 2021, 275, 119159. [Google Scholar] [CrossRef]
- Huang, X.; Xu, M.; Shirahata, T.; Li, W.; Koike, K.; Kojima-Yuasa, A.; Yuasa, I.; Kobayashi, Y. Anti-Steatosis Compounds from Leaves of Mallotus furetianus. Nat. Prod. Res. 2018, 32, 1459–1462. [Google Scholar] [CrossRef]
- Yueli, L.; Liqun, W.; Haitao, W.; Lianbo, L.; Xinan, Y. Comparison of Anti–Atherosclerotic Effects of Two Different Extracts from Leaves of Mallotus furetianus. Asian Pac. J. Trop. Med. 2011, 4, 878–882. [Google Scholar] [CrossRef]
- Zhao, J.; Deng, J.W.; Chen, Y.W.; Li, S.P. Advanced Phytochemical Analysis of Herbal Tea in China. J. Chromatogr. A 2013, 1313, 2–23. [Google Scholar] [CrossRef]
- Gao, H.-M.; Wang, T.; Hu, H.-T.; Yue, J.-N.; Shen, T.; Lou, H.-X.; Wang, X.-N. Three New Triterpenoids from Mallotus macrostachyus. Fitoterapia 2020, 142, 104498. [Google Scholar] [CrossRef]
- GB/T1040.3-2006; Plastics Determination of Tensile Properties Part 3: Test Conditions for Films and Sheets. Standard Press of China: Beijing, China, 2006.
- Shojaee-Aliabadi, S.; Hosseini, H.; Mohammadifar, M.A.; Mohammadi, A.; Ghasemlou, M.; Hosseini, S.M.; Khaksar, R. Characterization of κ-Carrageenan Films Incorporated Plant Essential Oils with Improved Antimicrobial Activity. Carbohydr. Polym. 2014, 101, 582–591. [Google Scholar] [CrossRef]
- Yu, S.-H.; Hsieh, H.-Y.; Pang, J.-C.; Tang, D.-W.; Shih, C.-M.; Tsai, M.-L.; Tsai, Y.-C.; Mi, F.-L. Active Films from Water-Soluble Chitosan/Cellulose Composites Incorporating Releasable Caffeic Acid for Inhibition of Lipid Oxidation in Fish Oil Emulsions. Food Hydrocoll. 2013, 32, 9–19. [Google Scholar] [CrossRef]
- GB/5009.181-2016; Determination of Malondialdehyde in Food. Standard Press of China: Beijing, China, 2016.
- Lv, X.; Zhang, W.; Liu, Y.; Zhao, Y.; Zhang, J.; Hou, M. Hygroscopicity Modulation of Hydrogels Based on Carboxymethyl Chitosan/Alginate Polyelectrolyte Complexes and Its Application as pH-Sensitive Delivery System. Carbohydr. Polym. 2018, 198, 86–93. [Google Scholar] [CrossRef]
- Cao, L.; Ge, T.; Meng, F.; Xu, S.; Li, J.; Wang, L. An Edible Oil Packaging Film with Improved Barrier Properties and Heat Sealability from Cassia Gum Incorporating Carboxylated Cellulose Nano Crystal Whisker. Food Hydrocoll. 2020, 98, 105251. [Google Scholar] [CrossRef]
- Tripathi, S.; Kumar, P.; Gaikwad, K.K. UV- Shielding and Antioxidant Properties of Chitosan Film Impregnated with Acacia Catechu Modified with Calcium Carbonate for Food Packaging. Int. J. Biol. Macromol. 2024, 257, 128790. [Google Scholar] [CrossRef]
- Ji, Q.; Jin, Z.; Ding, W.; Wu, Y.; Liu, C.; Yu, K.; Zhang, N.; Jin, G.; Lu, P.; Bao, D.; et al. Chitosan Composite Films Based on Tea Seed Oil Nano-Microcapsules: Antibacterial, Antioxidant and Physicochemical Properties. Food Packag. Shelf Life 2023, 40, 101212. [Google Scholar] [CrossRef]
- Alqahtani, N.; Alnemr, T.; Ali, S. Development of Low-Cost Biodegradable Films from Corn Starch and Date Palm Pits (Phoenix dactylifera). Food Biosci. 2021, 42, 101199. [Google Scholar] [CrossRef]
- Suderman, N.; Isa, M.I.N.; Sarbon, N.M. The Effect of Plasticizers on the Functional Properties of Biodegradable Gelatin-Based Film: A Review. Food Biosci. 2018, 24, 111–119. [Google Scholar] [CrossRef]
- Gao, H.-X.; He, Z.; Sun, Q.; He, Q.; Zeng, W.-C. A Functional Polysaccharide Film Forming by Pectin, Chitosan, and Tea Polyphenols. Carbohydr. Polym. 2019, 215, 1–7. [Google Scholar] [CrossRef]
- Guo, H.; Jiang, K.; Ou, J.; Huang, C.; Liu, F.; Zheng, J.; Ou, S. Preparation of Acrolein/Resveratrol-Grafted Chitosan-Sodium Alginate Bilayer Films and Their Antibacterial and Antioxidant Activities. Food Hydrocoll. 2024, 149, 109601. [Google Scholar] [CrossRef]
- Roy, S.; Ramakrishnan, R.; Goksen, G.; Singh, S.; Łopusiewicz, Ł. Recent Progress on UV-Light Barrier Food Packaging Films—A Systematic Review. Innov. Food Sci. Emerg. Technol. 2024, 91, 103550. [Google Scholar] [CrossRef]
- Wang, H.; Li, S.; Wang, S.; Zhou, J.; Liu, C.; Chen, C.; Xie, J. Development of Controlled-Release Antioxidant Poly (Lactic Acid) Bilayer Active Film with Different Distributions of α-Tocopherol and Its Application in Corn Oil Preservation. Food Chem. 2024, 439, 138094. [Google Scholar] [CrossRef]
- Yu, H.; Zhou, Q.; He, D.; Yang, J.; Wu, K.; Chai, X.; Xiang, Y.; Duan, X.; Wu, X. Enhanced Mechanical and Functional Properties of Chitosan/Polyvinyl Alcohol/Hydroxypropyl Methylcellulose/Alizarin Composite Film by Incorporating Cinnamon Essential Oil and Tea Polyphenols. Int. J. Biol. Macromol. 2023, 253, 126859. [Google Scholar] [CrossRef]
- Ben, Z.Y.; Samsudin, H.; Yhaya, M.F. Glycerol: Its Properties, Polymer Synthesis, and Applications in Starch Based Films. Eur. Polym. J. 2022, 175, 111377. [Google Scholar] [CrossRef]
- Han, H.-S.; Song, K.B. Antioxidant Properties of Watermelon (Citrullus lanatus) Rind Pectin Films Containing Kiwifruit (Actinidia chinensis) Peel Extract and Their Application as Chicken Thigh Packaging. Food Packag. Shelf Life 2021, 28, 100636. [Google Scholar] [CrossRef]
- Umaraw, P.; Munekata, P.E.S.; Verma, A.K.; Barba, F.J.; Singh, V.P.; Kumar, P.; Lorenzo, J.M. Edible Films/Coating with Tailored Properties for Active Packaging of Meat, Fish and Derived Products. Trends Food Sci. Technol. 2020, 98, 10–24. [Google Scholar] [CrossRef]
- Freitas, P.A.V.; González-Martínez, C.; Chiralt, A. Using Rice Straw Fractions to Develop Reinforced, Active PLA-Starch Bilayers for Meat Preservation. Food Chem. 2023, 405, 134990. [Google Scholar] [CrossRef]
- Hassan, A.H.A.; Korany, A.M.; Zeinhom, M.M.A.; Mohamed, D.S.; Abdel-Atty, N.S. Effect of Chitosan-Gelatin Coating Fortified with Papaya Leaves and Thyme Extract on Quality and Shelf Life of Chicken Breast Fillet and Kareish Cheese during Chilled Storage. Int. J. Food Microbiol. 2022, 371, 109667. [Google Scholar] [CrossRef]
- Zhang, A.; Fan, X.; Zeng, X.; Xu, J.; Zhou, C.; Xia, Q.; Sun, Y.; Wu, Z.; Pan, D. Enhancing Physicochemical, Antimicrobial, and Release Properties of Fish Skin Gelatin Films Using Dual-Layer Nanoparticles Loaded with Tea Polyphenols/Kojic Acid for Air-Dried Chicken Preservation. Food Hydrocoll. 2024, 149, 109580. [Google Scholar] [CrossRef]







| Film Samples | Thickness (cm) | L* | a* | b* | Haze (%) |
|---|---|---|---|---|---|
| CG/PTE (0.0%) | 0.127 ± 0.005 a | 91.42 ± 0.03 a | 2.28 ± 0.04 a | −12.32 ± 0.14 f | 1.24 ± 0.01 d |
| CG/PTE (0.5%) | 0.128 ± 0.001 a | 89.29 ± 0.03 b | 0.45 ± 0.05 b | −4.02 ± 0.09 e | 1.32 ± 0.01 c |
| CG/PTE (1.0%) | 0.07 ± 0.002 d | 88.06 ± 0.02 c | −0.55 ± 0.02 c | 1.44 ± 0.14 d | 1.49 ± 0.05 b |
| CG/PTE (1.5%) | 0.084 ± 0.003 c | 83.55 ± 0.13 d | −1.64 ± 0.06 d | 10.62 ± 0.55 c | 1.56 ± 0.03 a |
| CG/PTE (2.0%) | 0.114 ± 0.003 b | 82.69 ± 0.30 e | −1.96 ± 0.04 e | 14.48 ± 0.73 b | 1.62 ± 0.02 a |
| CG/PTE (2.5%) | 0.084 ± 0.001 c | 80.54 ± 0.35 f | −1.08 ± 0.15 f | 21.07 ± 0.95 a | 1.62 ± 0.02 a |
| Color Parameters | Day 0 | Day 9 | |||
|---|---|---|---|---|---|
| CG/PTE (0.0%) | CG/PTE (0.5%) | CG/PTE (1.5%) | CG/PTE (2.5%) | ||
| L* | 76.58 ± 0.29 a | 22.76 ± 0.09 d | 32.51 ± 0.52 c | 48.60 ± 0.30 b | 48.94 ± 0.21 b |
| a* | 1.20 ± 0.10 c | 9.49 ± 0.03 a | 6.52 ± 0.57 b | 6.90 ± 0.06 b | 6.18 ± 0.36 b |
| b* | 17.16 ± 1.40 c | 28.49 ± 3.58 a | 23.53 ± 1.44 b | 28.26 ± 0.38 a | 31.94 ± 0.53 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, N.; Pan, Z.; Ning, Y.; Liu, W.; Wen, X.; Yang, C.; Wang, L. Cassia Seed Gum Films Incorporated with Partridge Tea Extract as an Edible Antioxidant Food Packaging Film for Preservation of Chicken Jerky. Polymers 2024, 16, 1086. https://doi.org/10.3390/polym16081086
Wei N, Pan Z, Ning Y, Liu W, Wen X, Yang C, Wang L. Cassia Seed Gum Films Incorporated with Partridge Tea Extract as an Edible Antioxidant Food Packaging Film for Preservation of Chicken Jerky. Polymers. 2024; 16(8):1086. https://doi.org/10.3390/polym16081086
Chicago/Turabian StyleWei, Na, Zijing Pan, Yuping Ning, Wenhua Liu, Xin Wen, Chen Yang, and Lijuan Wang. 2024. "Cassia Seed Gum Films Incorporated with Partridge Tea Extract as an Edible Antioxidant Food Packaging Film for Preservation of Chicken Jerky" Polymers 16, no. 8: 1086. https://doi.org/10.3390/polym16081086
APA StyleWei, N., Pan, Z., Ning, Y., Liu, W., Wen, X., Yang, C., & Wang, L. (2024). Cassia Seed Gum Films Incorporated with Partridge Tea Extract as an Edible Antioxidant Food Packaging Film for Preservation of Chicken Jerky. Polymers, 16(8), 1086. https://doi.org/10.3390/polym16081086