Recent Advances in Polymers Bearing Activated Esters for the Synthesis of Glycopolymers by Postpolymerization Modification
Abstract
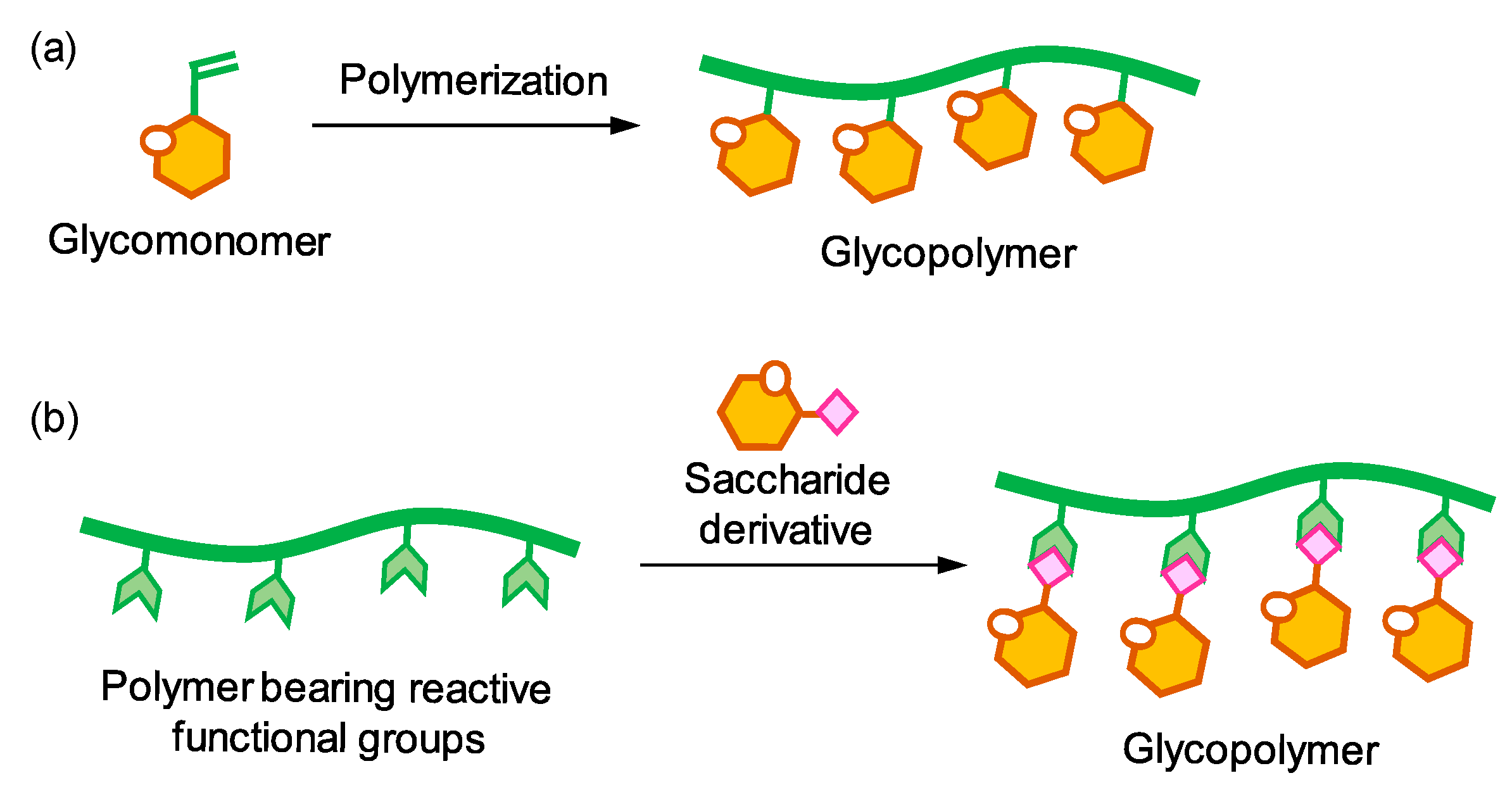
1. Introduction
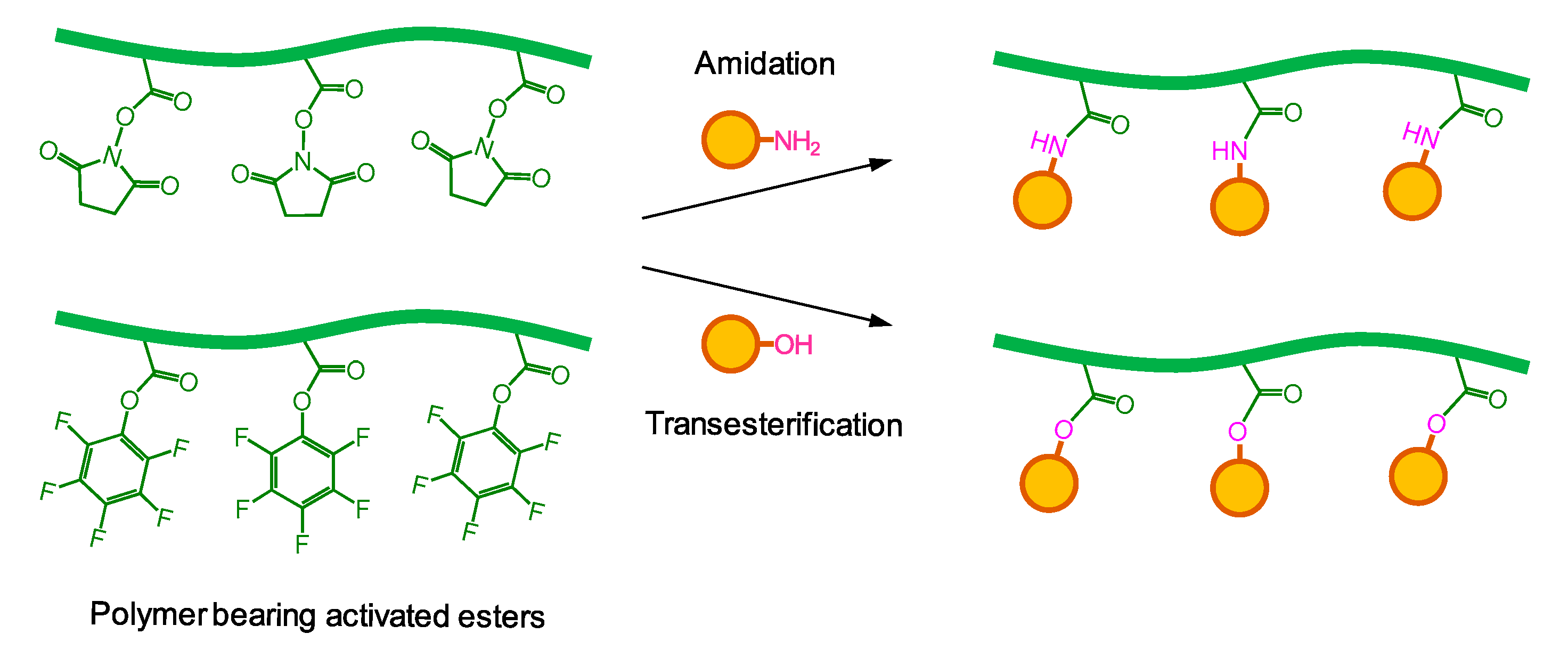
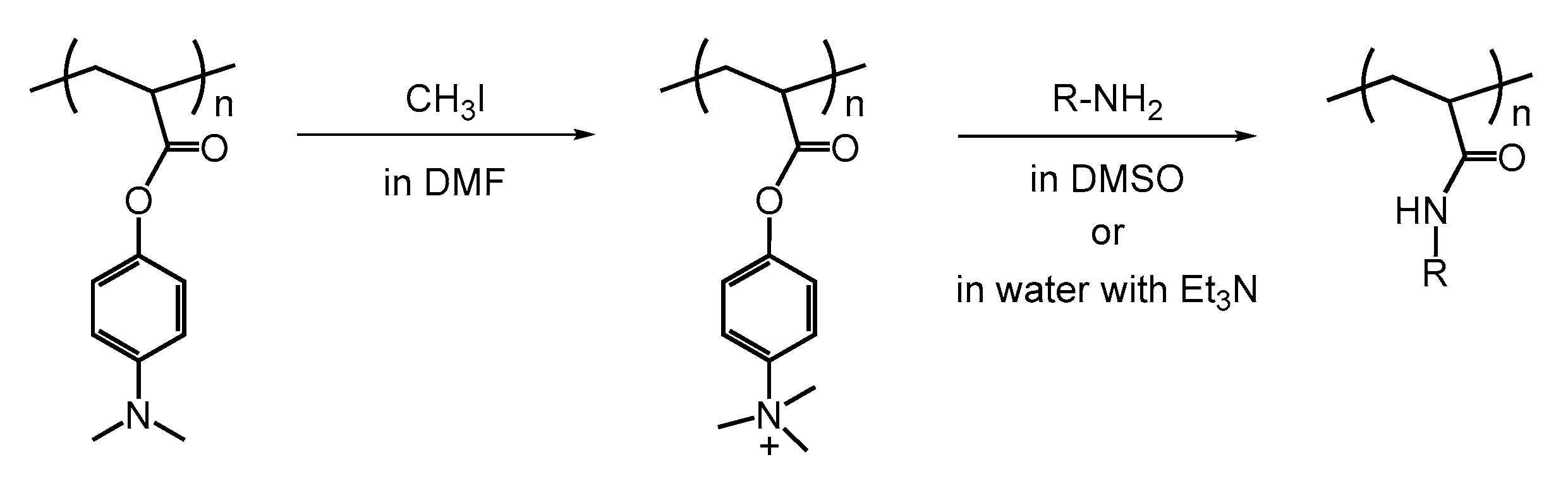
2. Activated Esters in Postpolymerization Modification
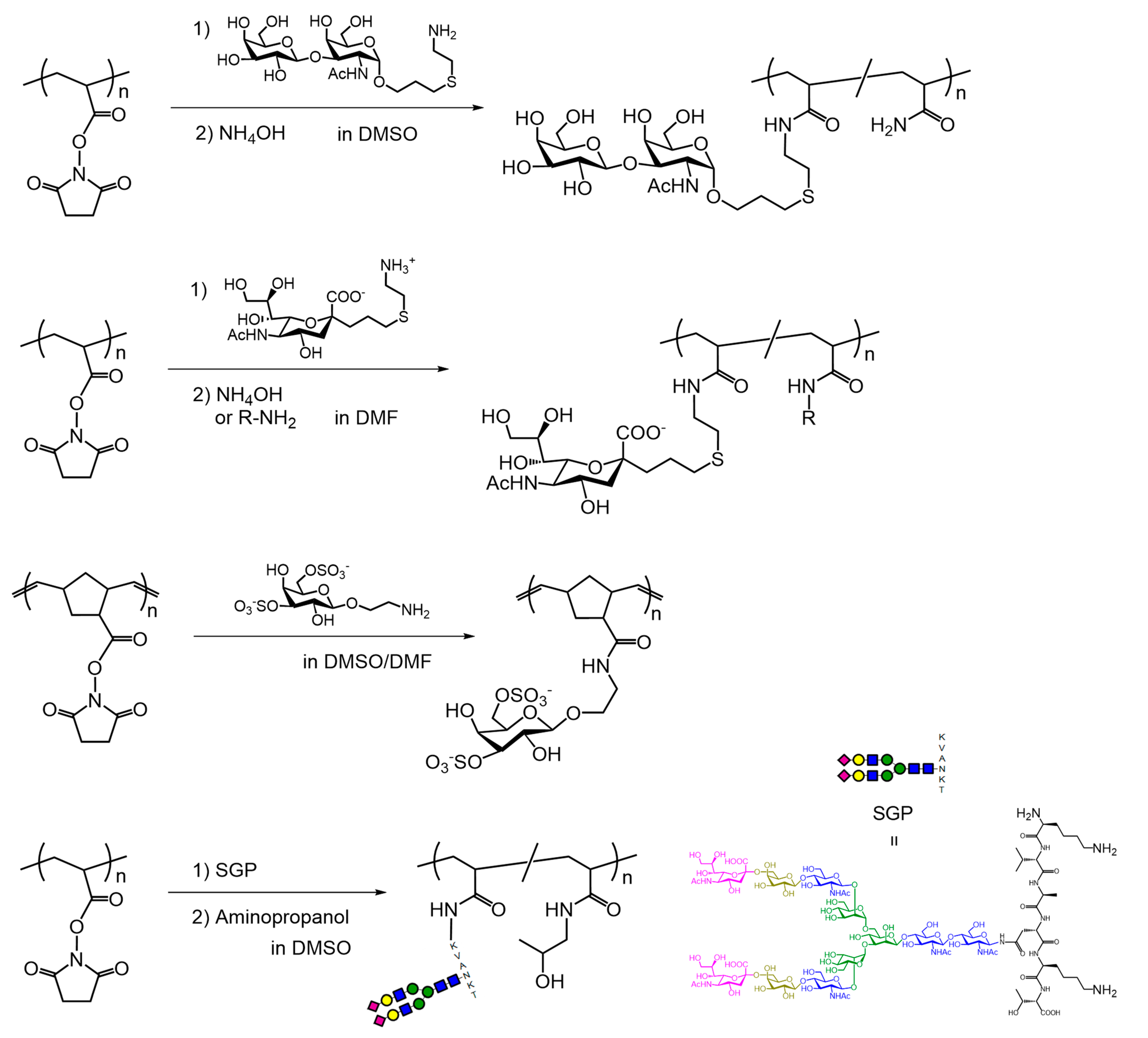
3. Synthesis of Glycopolymers Using Polymers Bearing Activated Esters
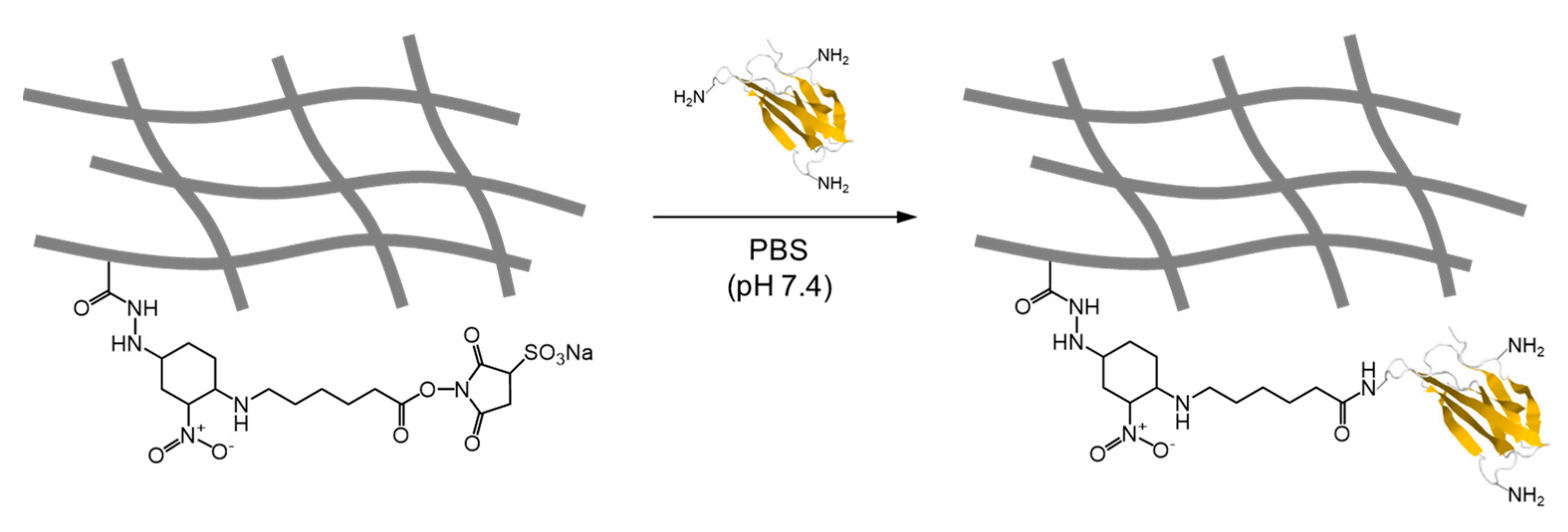
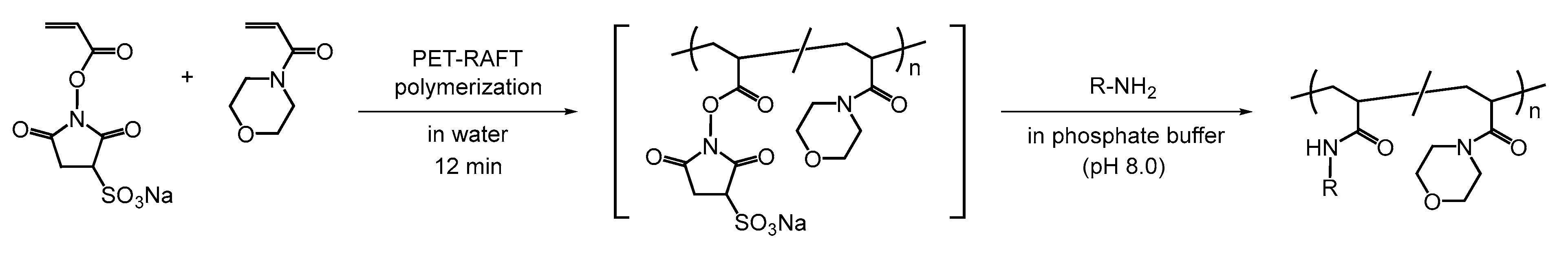
4. Synthesis of Glycopolymers Using Polymers Bearing Water-Soluble Activated Esters in Water
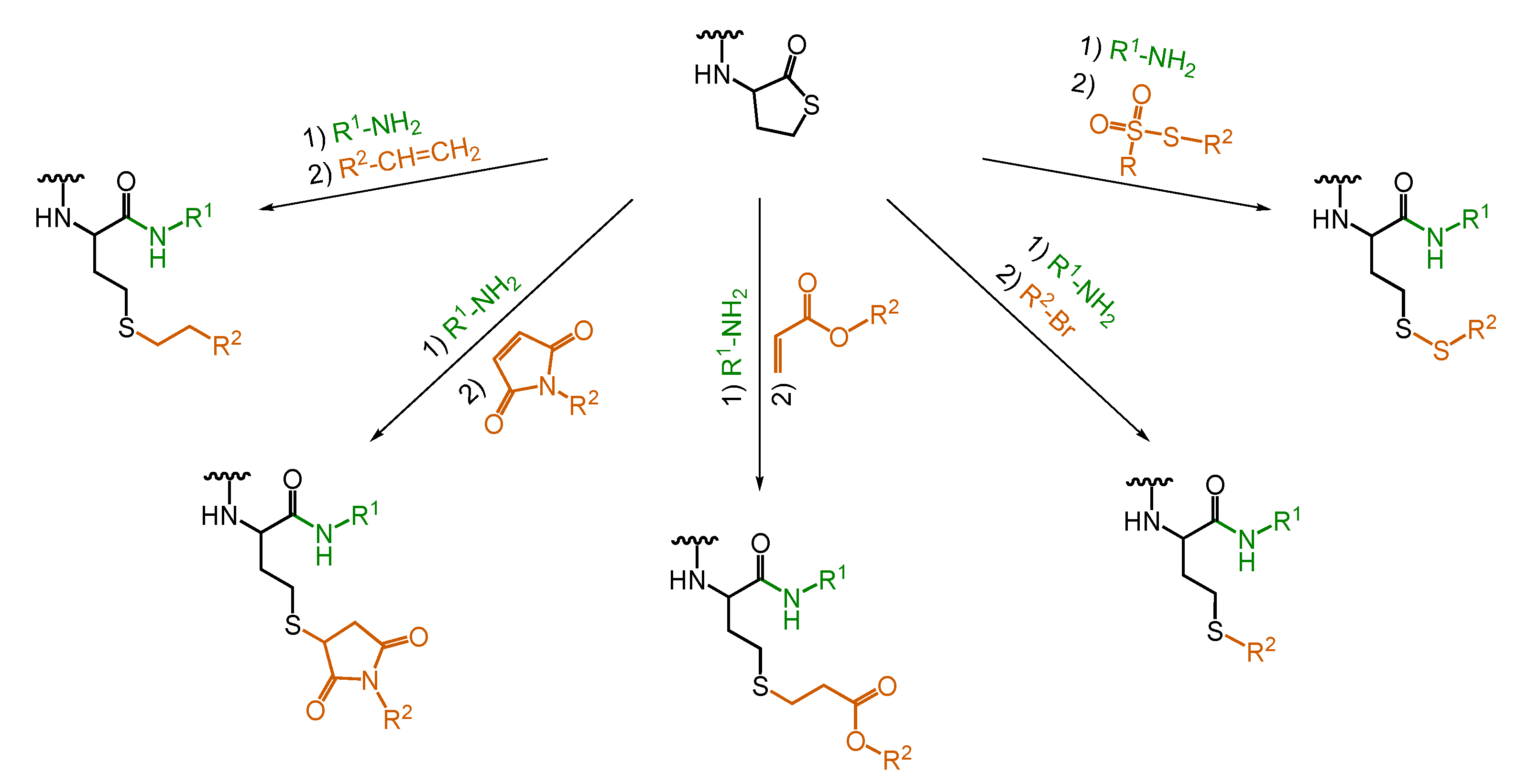
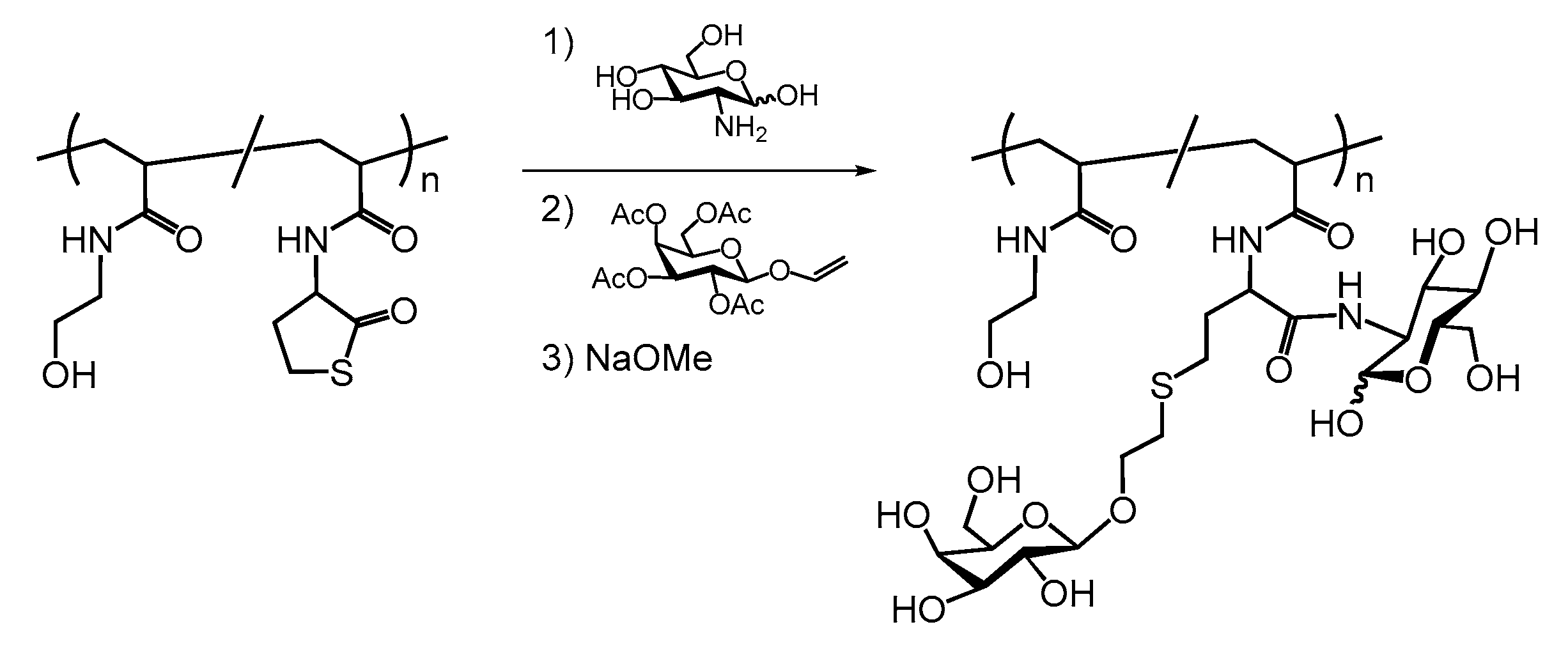
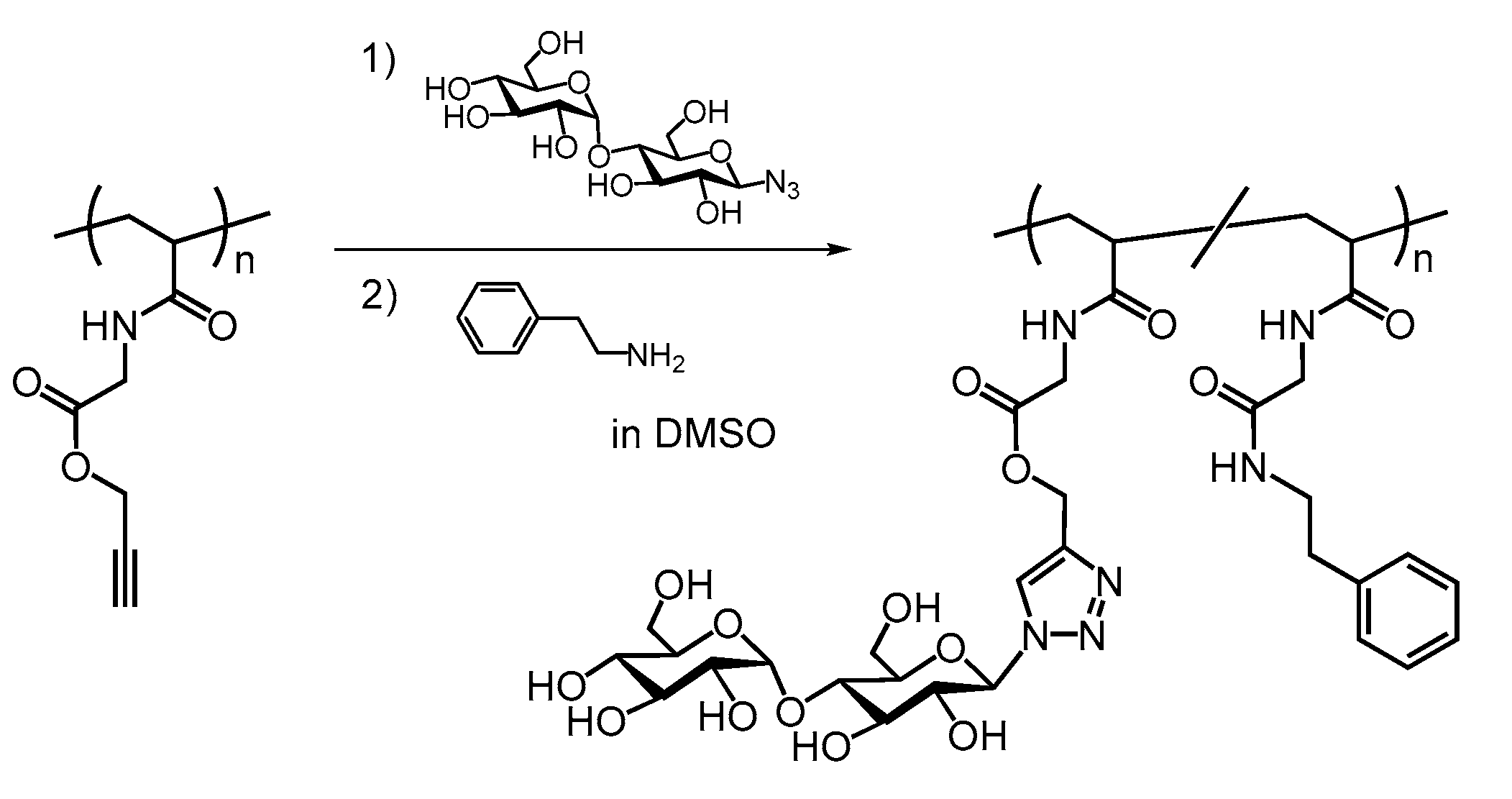
5. Dual Postpolymerization Modification
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Su, L.; Feng, Y.; Wei, K.; Xu, X.; Liu, R.; Chen, G. Carbohydrate-Based Macromolecular Biomaterials. Chem. Rev. 2021, 121, 10950–11029. [Google Scholar] [CrossRef] [PubMed]
- Chen, G. The Past Ten Years of Carbohydrate Polymers in ACS Macro Letters. ACS Macro Lett. 2021, 10, 1145–1150. [Google Scholar] [CrossRef] [PubMed]
- Delbianco, M.; Bharate, P.; Varela-Aramburu, S.; Seeberger, P.H. Carbohydrates in Supramolecular Chemistry. Chem. Rev. 2016, 116, 1693–1752. [Google Scholar] [CrossRef] [PubMed]
- Le Droumaguet, B.; Nicolas, J. Recent Advances in the Design of Bioconjugates from Controlled/Living Radical Polymerization. Polym. Chem. 2010, 1, 563–598. [Google Scholar] [CrossRef]
- Vázquez-Dorbatt, V.; Lee, J.; Lin, E.; Maynard, H.D. Synthesis of Glycopolymers by Controlled Radical Polymerization Techniques and Their Applications. ChemBioChem 2012, 13, 2478–2487. [Google Scholar] [CrossRef]
- Miura, Y. Design and Synthesis of Well-Defined Glycopolymers for the Control of Biological Functionalities. Polym. J. 2012, 44, 679–689. [Google Scholar] [CrossRef]
- Sunasee, R.; Narain, R. Glycopolymers and Glyco-Nanoparticles in Biomolecular Recognition Processes and Vaccine Development. Macromol. Biosci. 2013, 13, 9–27. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.; Wattanaarsakit, P.; Narain, R. Recent Advances in the Preparation of Glycopolymer Bioconjugates. Eur. Polym. J. 2013, 49, 3010–3033. [Google Scholar] [CrossRef]
- Miura, Y.; Hoshino, Y.; Seto, H. Glycopolymer Nanobiotechnology. Chem. Rev. 2016, 116, 1673–1692. [Google Scholar] [CrossRef] [PubMed]
- Abdouni, Y.; Yilmaz, G.; Becer, C.R. Sequence and Architectural Control in Glycopolymer Synthesis. Macromol. Rapid Commun. 2017, 38, 1700500. [Google Scholar] [CrossRef]
- Pramudya, I.; Chung, H. Recent Progress of Glycopolymer Synthesis for Biomedical Applications. Biomater. Sci. 2019, 7, 4848–4872. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Zhu, X.X. Copolymers Containing Carbohydrates and Other Biomolecules: Design, Synthesis and Applications. J. Mater. Chem. B 2019, 7, 1361–1378. [Google Scholar] [CrossRef] [PubMed]
- Stenzel, M.H. Glycopolymers for Drug Delivery: Opportunities and Challenges. Macromolecules 2022, 55, 4867–4890. [Google Scholar] [CrossRef]
- Bianculli, R.H.; Mase, J.D.; Schulz, M.D. Antiviral Polymers: Past Approaches and Future Possibilities. Macromolecules 2020, 53, 9158–9186. [Google Scholar] [CrossRef]
- Kim, Y.; Hyun, J.Y.; Shin, I. Multivalent Glycans for Biological and Biomedical Applications. Chem. Soc. Rev. 2021, 50, 10567–10593. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.-J.; Gibson, M.I. Toward Glycomaterials with Selectivity as Well as Affinity. JACS Au 2021, 1, 2089–2099. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Terracciano, R.; Becker, J.; Monaco, A.; Yilmaz, G.; Becer, C.R. Hierarchy of Complex Glycomacromolecules: From Controlled Topologies to Biomedical Applications. Biomacromolecules 2022, 23, 543–575. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, K.; Kalita, U.; Singha, N.K. Tailor-Made Glycopolymers via Reversible Deactivation Radical Polymerization: Design, Properties and Applications. Polym. Chem. 2022, 13, 1458–1483. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, J.; Ding, Y.; Hu, X.; Chen, Y. Glycopolymers Based on Carbohydrate or Vinyl Backbones and Their Biomedical Applications. Polym. Chem. 2023, 14, 2414–2434. [Google Scholar] [CrossRef]
- Martínez-Bailén, M.; Rojo, J.; Ramos-Soriano, J. Multivalent Glycosystems for Human Lectins. Chem. Soc. Rev. 2023, 52, 536–572. [Google Scholar] [CrossRef] [PubMed]
- Mei, R.; Heng, X.; Liu, X.; Chen, G. Glycopolymers for Antibacterial and Antiviral Applications. Molecules 2023, 28, 985. [Google Scholar] [CrossRef]
- Nagao, M.; Matsumoto, H.; Miura, Y. Design of Glycopolymers for Controlling the Interactions with Lectins. Chem. Asian J. 2023, 18, e202300643. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.C.; Lee, R.T. Carbohydrate-Protein Interactions: Basis of Glycobiology. Acc. Chem. Res. 1995, 28, 321–327. [Google Scholar] [CrossRef]
- Mammen, M.; Choi, S.K.; Whitesides, G.M. Polyvalent Interactions in Biological Systems: Implications for Design and Use of Multivalent Ligands and Inhibitors. Angew. Chem. Int. Ed. 1998, 37, 2755–2794. [Google Scholar] [CrossRef]
- Lundquist, J.J.; Toone, E.J. The Cluster Glycoside Effect. Chem. Rev. 2002, 102, 555–578. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, M.A.; Gibson, M.I.; Klok, H.A. Synthesis of Functional Polymers by Post-Polymerization Modification. Angew. Chem. Int. Ed. 2009, 48, 48–58. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Theato, P. Activated Ester Containing Polymers: Opportunities and Challenges for the Design of Functional Macromolecules. Chem. Rev. 2016, 116, 1434–1495. [Google Scholar] [CrossRef] [PubMed]
- Blasco, E.; Sims, M.B.; Goldmann, A.S.; Sumerlin, B.S.; Barner-Kowollik, C. 50th Anniversary Perspective: Polymer Functionalization. Macromolecules 2017, 50, 5215–5252. [Google Scholar] [CrossRef]
- Kubo, T.; Easterling, C.P.; Olson, R.A.; Sumerlin, B.S. Synthesis of Multifunctional Homopolymers via Sequential Post-Polymerization Reactions. Polym. Chem. 2018, 9, 4605–4610. [Google Scholar] [CrossRef]
- Chen, X.; Michinobu, T. Postpolymerization Modification: A Powerful Tool for the Synthesis and Function Tuning of Stimuli-Responsive Polymers. Macromol. Chem. Phys. 2022, 223, 2100370. [Google Scholar] [CrossRef]
- Kolb, H.C.; Finn, M.G.; Sharpless, K.B. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew. Chem. Int. Ed. 2001, 40, 2004–2021. [Google Scholar] [CrossRef]
- Dondoni, A. Triazole: The Keystone in Glycosylated Molecular Architectures Constructed by a Click Reaction. Chem. Asian J. 2007, 2, 700–708. [Google Scholar] [CrossRef] [PubMed]
- Witczak, Z.J.; Bielski, R. (Eds.) Click Chemistry in Glycoscience; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013. [Google Scholar]
- Hoyle, C.E.; Bowman, C.N. Thiol–Ene Click Chemistry. Angew. Chem. Int. Ed. 2010, 49, 1540–1573. [Google Scholar] [CrossRef] [PubMed]
- Lowe, A.B. Thiol-Ene “Click” Reactions and Recent Applications in Polymer and Materials Synthesis. Polym. Chem. 2010, 1, 17–36. [Google Scholar] [CrossRef]
- Dondoni, A.; Marra, A. Recent Applications of Thiol–Ene Coupling as a Click Process for Glycoconjugation. Chem. Soc. Rev. 2012, 41, 573–586. [Google Scholar] [CrossRef] [PubMed]
- Ahangarpour, M.; Kavianinia, I.; Harris, P.W.R.; Brimble, M.A. Photo-Induced Radical Thiol–Ene Chemistry: A Versatile Toolbox for Peptide-Based Drug Design. Chem. Soc. Rev. 2021, 50, 898–944. [Google Scholar] [CrossRef]
- Vinson, N.; Gou, Y.Z.; Becer, C.R.; Haddleton, D.M.; Gibson, M.I. Optimised “click” Synthesis of Glycopolymers with Mono/Di- and Trisaccharides. Polym. Chem. 2011, 2, 107–113. [Google Scholar] [CrossRef]
- Slavin, S.; Burns, J.; Haddleton, D.M.; Becer, C.R. Synthesis of Glycopolymers via Click Reactions. Eur. Polym. J. 2011, 47, 435–446. [Google Scholar] [CrossRef]
- Zhang, Q.; Collins, J.; Anastasaki, A.; Wallis, R.; Mitchell, D.A.; Becer, C.R.; Haddleton, D.M. Sequence-Controlled Multi-Block Glycopolymers to Inhibit DC-SIGN-gp120 Binding. Angew. Chem. Int. Ed. 2013, 52, 4435–4439. [Google Scholar] [CrossRef] [PubMed]
- Gou, Y.; Geng, J.; Richards, S.; Burns, J.; Remzi Becer, C.; Haddleton, D.M. A Detailed Study on Understanding Glycopolymer Library and Con A Interactions. J. Polym. Sci. A Polym. Chem. 2013, 51, 2588–2597. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Wilson, P.; Anastasaki, A.; McHale, R.; Haddleton, D.M. Synthesis and Aggregation of Double Hydrophilic Diblock Glycopolymers via Aqueous SET-LRP. ACS Macro Lett. 2014, 3, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Zhang, W.; Richards, S.-J.; Gibson, M.I.; Chen, G. Glycopolymer-Coated Gold Nanorods Synthesised by a One Pot Copper(0) Catalyzed Tandem RAFT/Click Reaction. Polym. Chem. 2014, 5, 2326–2332. [Google Scholar] [CrossRef]
- Collis, D.W.P.; Yilmaz, G.; Yuan, Y.; Monaco, A.; Ochbaum, G.; Shi, Y.; O’Malley, C.; Uzunova, V.; Napier, R.; Bitton, R.; et al. Hyaluronan (HA)-Inspired Glycopolymers as Molecular Tools for Studying HA Functions. RSC Chem. Biol. 2021, 2, 568–576. [Google Scholar] [CrossRef] [PubMed]
- Nagao, M.; Kurebayashi, Y.; Seto, H.; Tanaka, T.; Takahashi, T.; Suzuki, T.; Hoshino, Y.; Miura, Y. Synthesis of Well-Controlled Glycopolymers Bearing Oligosaccharides and Their Interactions with Influenza Viruses. Polym. J. 2016, 48, 745–749. [Google Scholar] [CrossRef]
- Jono, K.; Nagao, M.; Oh, T.; Sonoda, S.; Hoshino, Y.; Miura, Y. Controlling the Lectin Recognition of Glycopolymers via Distance Arrangement of Sugar Blocks. Chem. Commun. 2018, 54, 82–85. [Google Scholar] [CrossRef] [PubMed]
- Terada, Y.; Hoshino, Y.; Miura, Y. Glycopolymers Mimicking GM1 Gangliosides: Cooperativity of Galactose and Neuraminic Acid for Cholera Toxin Recognition. Chem. Asian J. 2019, 14, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Nagao, M.; Matsubara, T.; Hoshino, Y.; Sato, T.; Miura, Y. Topological Design of Star Glycopolymers for Controlling the Interaction with the Influenza Virus. Bioconjug. Chem. 2019, 30, 1192–1198. [Google Scholar] [CrossRef]
- Kichize, M.; Nagao, M.; Hoshino, Y.; Miura, Y. Multi-Block and Sequence-Controlled Polymerization of Glycopolymers, and Interaction with Lectin. Eur. Polym. J. 2020, 140, 110044. [Google Scholar] [CrossRef]
- Nagao, M.; Uemura, T.; Horiuchi, T.; Hoshino, Y.; Miura, Y. Screening of a Glycopolymer Library for GM1 Mimetics Synthesized by the “Carbohydrate Module Method”. Chem. Commun. 2021, 57, 10871–10874. [Google Scholar] [CrossRef] [PubMed]
- Nagao, M.; Kichize, M.; Hoshino, Y.; Miura, Y. Influence of Monomer Structures for Polymeric Multivalent Ligands: Consideration of the Molecular Mobility of Glycopolymers. Biomacromolecules 2021, 22, 3119–3127. [Google Scholar] [CrossRef]
- Ishida, T.; Nagao, M.; Oh, T.; Mori, T.; Hoshino, Y.; Miura, Y. Synthesis of Glycopolymers Carrying 3′-Sialyllactose for Suppressing Inflammatory Reaction via Siglec-E. Chem. Lett. 2022, 51, 308–311. [Google Scholar] [CrossRef]
- Malkoch, M.; Thibault, R.J.; Drockenmuller, E.; Messerschmidt, M.; Voit, B.; Russell, T.P.; Hawker, C.J. Orthogonal Approaches to the Simultaneous and Cascade Functionalization of Macromolecules Using Click Chemistry. J. Am. Chem. Soc. 2005, 127, 14942–14949. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Wang, X.; Miao, D.; Wang, C.; Deng, W. Synthesis of Well-Defined Heteroglycopolymers via Combining Sequential Click Reactions and PPM: The Effects of Linker and Heterogeneity on Con a Binding. Polym. Chem. 2020, 11, 3054–3065. [Google Scholar] [CrossRef]
- Tanaka, T.; Nagai, H.; Noguchi, M.; Kobayashi, A.; Shoda, S. One-Step Conversion of Unprotected Sugars to Beta-Glycosyl Azides Using 2-Chloroimidazolinium Salt in Aqueous Solution. Chem. Commun. 2009, 23, 3378–3379. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Ishitani, H.; Miura, Y.; Oishi, K.; Takahashi, T.; Suzuki, T.; Shoda, S.; Kimura, Y. Protecting-Group-Free Synthesis of Glycopolymers Bearing Sialyloligosaccharide and Their High Binding with the Influenza Virus. ACS Macro Lett. 2014, 3, 1074–1078. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Zhou, Y.; Tamoto, C.; Kurebayashi, Y.; Takahashi, T.; Suzuki, T. An α2,3-Linked Sialylglycopolymer as a Multivalent Glycoligand against Avian and Human Influenza Viruses. J. Appl. Glycosci. 2017, 64, 43–48. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tanaka, T.; Fukumoto, H.; Ishitani, H. Synthesis of Glycopolymer Gold Nanoparticles Decorated with Oligosaccharides via a Protecting-Group-Free Process and Their Specific Recognition by Lectins. Trans. Mater. Res. Soc. Jpn. 2017, 42, 113–118. [Google Scholar] [CrossRef][Green Version]
- Tanaka, T.; Okamoto, M. Reversible Temperature-Responsive and Lectin-Recognizing Glycosylated Block Copolymers Synthesized by RAFT Polymerization. Polym. J. 2018, 50, 523–531. [Google Scholar] [CrossRef]
- Tanaka, T.; Okamoto, M. Lectin and Temperature Dual-Responsive Glycosylated Block Copolymers Synthesized by Consecutive RAFT Polymerization Reactions. Bull. Chem. Soc. Jpn. 2018, 91, 772–777. [Google Scholar] [CrossRef]
- Tsuji, S.; Aoki, T.; Ushio, S.; Tanaka, T. Synthesis and Aggregation Behavior of Temperature- and pH-Responsive Glycopolymers as Sugar-Displaying Conjugates. Polymers 2020, 12, 956. [Google Scholar] [CrossRef]
- Tanaka, T.; Nakashima, K.; Tsuji, S.; Han, X.; Zhao, J.; Honda, Y.; Sakakibara, K.; Kurebayashi, Y.; Takahashi, T.; Suzuki, T. Controlled Synthesis of Glycopolymers with Pendant Complex-Type Sialylglycopeptides and Their Binding Affinity with a Lectin and an Influenza Virus. Polym. Chem. 2019, 10, 5124–5130. [Google Scholar] [CrossRef]
- Li, G.; Ma, W.; Mo, J.; Cheng, B.; Shoda, S.; Zhou, D.; Ye, X.-S. Influenza Virus Precision Diagnosis and Continuous Purification Enabled by Neuraminidase-Resistant Glycopolymer-Coated Microbeads. ACS Appl. Mater. Interfaces 2021, 13, 46260–46269. [Google Scholar] [CrossRef] [PubMed]
- Batz, H.; Franzmann, G.; Ringsdorf, H. Model Reactions for Synthesis of Pharmacologically Active Polymers by Way of Monomeric and Polymeric Reactive Esters. Angew. Chem. Int. Ed. Eng. 1972, 11, 1103–1104. [Google Scholar] [CrossRef] [PubMed]
- Theato, P. Synthesis of Well-Defined Polymeric Activated Esters. J. Polym. Sci. A Polym. Chem. 2008, 46, 6677–6687. [Google Scholar] [CrossRef]
- Das, A.; Theato, P. Multifaceted Synthetic Route to Functional Polyacrylates by Transesterification of Poly(Pentafluorophenyl Acrylates). Macromolecules 2015, 48, 8695–8707. [Google Scholar] [CrossRef]
- He, L.; Szameit, K.; Zhao, H.; Hahn, U.; Theato, P. Postpolymerization Modification Using Less Cytotoxic Activated Ester Polymers for the Synthesis of Biological Active Polymers. Biomacromolecules 2014, 15, 3197–3205. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Li, Z.; Lin, S.; Theato, P. Fibrous Materials Based on Polymeric Salicyl Active Esters as Efficient Adsorbents for Selective Removal of Anionic Dye. ACS Appl. Mater. Interfaces 2020, 12, 21100–21113. [Google Scholar] [CrossRef] [PubMed]
- Lang, X.; Xu, Z.; Li, Q.; Yuan, L.; Thumu, U.; Zhao, H. Modulating the Reactivity of Polymer with Pendant Ester Groups by Methylation Reaction for Preparing Functional Polymers. Polym. Chem. 2022, 13, 5905–5911. [Google Scholar] [CrossRef]
- Won, S.; Hindmarsh, S.; Gibson, M.I. Triggerable Multivalent Glyconanoparticles for Probing Carbohydrate–Carbohydrate Interactions. ACS Macro Lett. 2018, 7, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.-J.; Baker, A.N.; Walker, M.; Gibson, M.I. Polymer-Stabilized Sialylated Nanoparticles: Synthesis, Optimization, and Differential Binding to Influenza Hemagglutinins. Biomacromolecules 2020, 21, 1604–1612. [Google Scholar] [CrossRef] [PubMed]
- Baker, A.N.; Richards, S.-J.; Pandey, S.; Guy, C.S.; Ahmad, A.; Hasan, M.; Biggs, C.I.; Georgiou, P.G.; Zwetsloot, A.J.; Straube, A.; et al. Glycan-Based Flow-Through Device for the Detection of SARS-COV-2. ACS Sens. 2021, 6, 3696–3705. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, P.G.; Guy, C.S.; Hasan, M.; Ahmad, A.; Richards, S.-J.; Baker, A.N.; Thakkar, N.V.; Walker, M.; Pandey, S.; Anderson, N.R.; et al. Plasmonic Detection of SARS-CoV-2 Spike Protein with Polymer-Stabilized Glycosylated Gold Nanorods. ACS Macro Lett. 2022, 11, 317–322. [Google Scholar] [CrossRef] [PubMed]
- Mammen, M.; Dahmann, G.; Whitesides, G.M. Effective Inhibitors of Hemagglutination by Influenza Virus Synthesized from Polymers Having Active Ester Groups. Insight into Mechanism of Inhibition. J. Med. Chem. 1995, 38, 4179–4190. [Google Scholar] [CrossRef] [PubMed]
- Sigal, G.B.; Mammen, M.; Dahmann, G.; Whitesides, G.M. Polyacrylamides Bearing Pendant α-Sialoside Groups Strongly Inhibit Agglutination of Erythrocytes by Influenza Virus: The Strong Inhibition Reflects Enhanced Binding through Cooperative Polyvalent Interactions. J. Am. Chem. Soc. 1996, 118, 3789–3800. [Google Scholar] [CrossRef]
- Baek, M.-G.; Roy, R. Relative Lectin Binding Properties of T-Antigen-Containing Glycopolymers: Copolymerization of N-Acryloylated T-Antigen Monomer vs. Graft Conjugation of Aminated T-Antigen Ligands onto Poly(N-Acryloxysuccinimide). Macromol. Biosci. 2001, 1, 305–311. [Google Scholar] [CrossRef]
- Gordon, E.J.; Gestwicki, J.E.; Strong, L.E.; Kiessling, L.L. Synthesis of End-Labeled Multivalent Ligands for Exploring Cell-Surface-Receptor–Ligand Interactions. Chem. Biol. 2000, 7, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Eissa, A.M.; Abdulkarim, A.; Sharples, G.J.; Cameron, N.R. Glycosylated Nanoparticles as Efficient Antimicrobial Delivery Agents. Biomacromolecules 2016, 17, 2672–2679. [Google Scholar] [CrossRef] [PubMed]
- Scherer, M.; Kappel, C.; Mohr, N.; Fischer, K.; Heller, P.; Forst, R.; Depoix, F.; Bros, M.; Zentel, R. Functionalization of Active Ester-Based Polymersomes for Enhanced Cell Uptake and Stimuli-Responsive Cargo Release. Biomacromolecules 2016, 17, 3305–3317. [Google Scholar] [CrossRef] [PubMed]
- Boyer, C.; Davis, T.P. One-Pot Synthesis and Biofunctionalization of Glycopolymers via RAFT Polymerization and Thiol-Ene Reactions. Chem. Commun. 2009, 40, 6029–6031. [Google Scholar] [CrossRef] [PubMed]
- Boyer, C.; Whittaker, M.; Davis, T.P. Synthesis and Postfunctionalization of Well-defined Star Polymers via “Double” Click Chemistry. J. Polym. Sci. A Polym. Chem. 2011, 49, 5245–5256. [Google Scholar] [CrossRef]
- Utama, R.H.; Jiang, Y.; Zetterlund, P.B.; Stenzel, M.H. Biocompatible Glycopolymer Nanocapsules via Inverse Miniemulsion Periphery RAFT Polymerization for the Delivery of Gemcitabine. Biomacromolecules 2015, 16, 2144–2156. [Google Scholar] [CrossRef] [PubMed]
- Gaballa, H.; Theato, P. Glucose-Responsive Polymeric Micelles via Boronic Acid–Diol Complexation for Insulin Delivery at Neutral pH. Biomacromolecules 2019, 20, 871–881. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.; Jones, M.W.; Hunaban, M.; Haddleton, D.M.; Gibson, M.I. Probing Bacterial-Toxin Inhibition with Synthetic Glycopolymers Prepared by Tandem Post-Polymerization Modification: Role of Linker Length and Carbohydrate Density. Angew. Chem. Int. Ed. 2012, 51, 7812–7816. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Miao, D.; Wang, X.; Wang, C.; Deng, W. Precise Synthesis of Heterogeneous Glycopolymers with Well-defined Saccharide Motifs in the Side Chain via Post-polymerization Modification and Recognition with Lectin. J. Polym. Sci. 2020, 58, 2074–2087. [Google Scholar] [CrossRef]
- Chen, L.; Leman, D.; Williams, C.R.; Durie, K.; Krause, D.C.; Locklin, J. Versatile Methodology for Glycosurfaces: Direct Ligation of Nonderivatized Reducing Saccharides to Poly(Pentafluorophenyl Acrylate) Grafted Surfaces via Hydrazide Conjugation. Langmuir 2017, 33, 8821–8828. [Google Scholar] [CrossRef] [PubMed]
- Hong, V.; Steinmetz, N.F.; Manchester, M.; Finn, M.G. Labeling Live Cells by Copper-Catalyzed Alkyne–Azide Click Chemistry. Bioconjug. Chem. 2010, 21, 1912–1916. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, D.C.; McKay, C.S.; Legault, M.C.B.; Danielson, D.C.; Blake, J.A.; Pegoraro, A.F.; Stolow, A.; Mester, Z.; Pezacki, J.P. Cellular Consequences of Copper Complexes Used to Catalyze Bioorthogonal Click Reactions. J. Am. Chem. Soc. 2011, 133, 17993–18001. [Google Scholar] [CrossRef] [PubMed]
- Moghaddam, M.J.; Matsuda, T. Molecular Design of Three-dimensional Artificial Extracellular Matrix: Photosensitive Polymers Containing Cell Adhesive Peptide. J. Polym. Sci. A Polym. Chem. 1993, 31, 1589–1597. [Google Scholar] [CrossRef]
- Nuhn, L.; Gietzen, S.; Mohr, K.; Fischer, K.; Toh, K.; Miyata, K.; Matsumoto, Y.; Kataoka, K.; Schmidt, M.; Zentel, R. Aggregation Behavior of Cationic Nanohydrogel Particles in Human Blood Serum. Biomacromolecules 2014, 15, 1526–1533. [Google Scholar] [CrossRef]
- Leber, N.; Nuhn, L.; Zentel, R. Cationic Nanohydrogel Particles for Therapeutic Oligonucleotide Delivery. Macromol. Biosci. 2017, 17, 1700092. [Google Scholar] [CrossRef] [PubMed]
- Spicer, C.D.; Davis, B.G. Selective Chemical Protein Modification. Nat. Commun. 2014, 5, 4740. [Google Scholar] [CrossRef] [PubMed]
- Boutureira, O.; Bernardes, G.J.L. Advances in Chemical Protein Modification. Chem. Rev. 2015, 115, 2174–2195. [Google Scholar] [CrossRef] [PubMed]
- Chari, R.V.J.; Miller, M.L.; Widdison, W.C. Antibody–Drug Conjugates: An Emerging Concept in Cancer Therapy. Angew. Chem. Int. Ed. 2014, 53, 3796–3827. [Google Scholar] [CrossRef]
- Messina, M.S.; Messina, K.M.M.; Bhattacharya, A.; Montgomery, H.R.; Maynard, H.D. Preparation of Biomolecule-Polymer Conjugates by Grafting-from Using ATRP, RAFT, or ROMP. Prog. Polym. Sci. 2020, 100, 101186. [Google Scholar] [CrossRef] [PubMed]
- Heredia, K.L.; Maynard, H.D. Synthesis of Protein–Polymer Conjugates. Org. Biomol. Chem. 2007, 5, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Staros, J.V. N-Hydroxysulfosuccinimide Active Esters: Bis(N-Hydroxysulfosuccinimide) Esters of Two Dicarboxylic Acids Are Hydrophilic, Membrane-Impermeant, Protein Cross-Linkers. Biochemistry 1982, 21, 3950–3955. [Google Scholar] [CrossRef] [PubMed]
- Lewis, M.R.; Raubitschek, A.; Shively, J.E. A Facile, Water-Soluble Method for Modification of Proteins with DOTA. Use of Elevated Temperature and Optimized pH To Achieve High Specific Activity and High Chelate Stability in Radiolabeled Immunoconjugates. Bioconjug. Chem. 1994, 5, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Suleiman, E.; Mayer, J.; Lehner, E.; Kohlhauser, B.; Katholnig, A.; Batzoni, M.; Damm, D.; Temchura, V.; Wagner, A.; Überla, K.; et al. Conjugation of Native-Like HIV-1 Envelope Trimers onto Liposomes Using EDC/Sulfo-NHS Chemistry: Requirements and Limitations. Pharmaceutics 2020, 12, 979. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Sarode, A.; Kokoroskos, N.; Ukidve, A.; Zhao, Z.; Guo, S.; Flaumenhaft, R.; Gupta, A.S.; Saillant, N.; Mitragotri, S. A Polymer-Based Systemic Hemostatic Agent. Sci. Adv. 2020, 6, eaba0588. [Google Scholar] [CrossRef] [PubMed]
- Kumai, J.; Sasagawa, S.; Horie, M.; Yui, Y. A Novel Method for Polyacrylamide Gel Preparation Using N-Hydroxysuccinimide-Acrylamide Ester to Study Cell-Extracellular Matrix Mechanical Interactions. Front. Mater. 2021, 8, 637278. [Google Scholar] [CrossRef]
- Gee, K.R.; Archer, E.A.; Kang, H.C. 4-Sulfotetrafluorophenyl (STP) Esters: New Water-Soluble Amine-Reactive Reagents for Labeling Biomolecules. Tetrahedron Lett. 1999, 40, 1471–1474. [Google Scholar] [CrossRef]
- Charoenpattarapreeda, J.; Tan, Y.S.; Iegre, J.; Walsh, S.J.; Fowler, E.; Eapen, R.S.; Wu, Y.; Sore, H.F.; Verma, C.S.; Itzhaki, L.; et al. Targeted Covalent Inhibitors of MDM2 Using Electrophile-Bearing Stapled Peptides. Chem. Commun. 2019, 55, 7914–7917. [Google Scholar] [CrossRef] [PubMed]
- Poellmann, M.J.; Wagoner Johnson, A.J. Characterizing and Patterning Polyacrylamide Substrates Functionalized with N-Hydroxysuccinimide. Cell. Mol. Bioeng. 2013, 6, 299–309. [Google Scholar] [CrossRef]
- Trappmann, B.; Gautrot, J.E.; Connelly, J.T.; Strange, D.G.T.; Li, Y.; Oyen, M.L.; Cohen Stuart, M.A.; Boehm, H.; Li, B.; Vogel, V.; et al. Extracellular-Matrix Tethering Regulates Stem-Cell Fate. Nat. Mater. 2012, 11, 642–649. [Google Scholar] [CrossRef]
- Niu, J.; Page, Z.A.; Dolinski, N.D.; Anastasaki, A.; Hsueh, A.T.; Soh, H.T.; Hawker, C.J. Rapid Visible Light-Mediated Controlled Aqueous Polymerization with in Situ Monitoring. ACS Macro Lett. 2017, 6, 1109–1113. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, S.; Aso, Y.; Ohara, H.; Tanaka, T. Polymeric Water-Soluble Activated Esters: Synthesis of Polymer Backbones with Pendant N-Hydoxysulfosuccinimide Esters for Post-Polymerization Modification in Water. Polym. J. 2019, 51, 1015–1022. [Google Scholar] [CrossRef]
- Tsuji, S.; Kobayashi, K.; Fujii, T.; Imoto, H.; Naka, K.; Aso, Y.; Ohara, H.; Tanaka, T. Polymers with Pendant Water-Soluble Tetrafluorobenzene Sulfonic Acid Activated Esters: Synthesis, Stability, and Use for Glycopolymers in Water. Macromol. Chem. Phys. 2022, 223, 2200072. [Google Scholar] [CrossRef]
- Tsuji, S.; Aso, Y.; Ohara, H.; Tanaka, T. Aqueous Synthesis of Sialylglycopeptide-Grafted Glycopolymers with High Affinity for the Lectin and the Influenza Virus Hemagglutinin. J. Polym. Sci. 2020, 58, 548–556. [Google Scholar] [CrossRef]
- Tsuji, S.; Aso, Y.; Tanaka, T. Aqueous One-Pot and Oxygen-Tolerant Synthesis of Glycopolymers Using Polymer-Backbone-Bearing Water-Soluble Activated Esters. Chem. Lett. 2023, 52, 67–70. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhao, H. Water-Soluble and Hydrolysis-Resistant Activated Ester Polymers: Synthesis, Characterization, and Application for Preparing Functional Polymers. J. Macromol. Sci. A 2023, 60, 358–366. [Google Scholar] [CrossRef]
- Breuck, J.D.; Streiber, M.; Ringleb, M.; Schröder, D.; Herzog, D.; Schubert, U.S.; Zechel, S.; Traeger, A.; Leiske, M.N. Amino-Acid-Derived Anionic Polyacrylamides with Tailored Hydrophobicity–Physicochemical Properties and Cellular Interactions. ACS Polym. Au 2024, in press. [Google Scholar] [CrossRef]
- Archer, W.R.; Gallagher, C.M.B.; Welborn, V.V.; Schulz, M.D. Exploring the role of polymer hydrophobicity in polymer–metal binding thermodynamics. Phys. Chem. Chem. Phys. 2022, 24, 3579–3585. [Google Scholar] [CrossRef] [PubMed]
- Illy, N.; Mongkhoun, E. Thiolactone Chemistry, a Versatile Platform for Macromolecular Engineering. Polym. Chem. 2022, 13, 4592–4614. [Google Scholar] [CrossRef]
- Reinicke, S.; Espeel, P.; Stamenović, M.M.; Du Prez, F.E. One-Pot Double Modification of p(NIPAAm): A Tool for Designing Tailor-Made Multiresponsive Polymers. ACS Macro Lett. 2013, 2, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, L.E.; Badi, N.; Du Prez, F.; Gibson, M.I. Double-Modified Glycopolymers from Thiolactones to Modulate Lectin Selectivity and Affinity. ACS Macro Lett. 2018, 7, 1498–1502. [Google Scholar] [CrossRef] [PubMed]
- Baysak, E.; Daglar, O.; Saim Gunay, U.; Hizal, G.; Tunca, U.; Durmaz, H. Study on Post-Polymerization Modification of Ring-Opening Metathesis Polymers Involving Pendant Thiolactone Units. J. Polym. Sci. A Polym. Chem. 2018, 56, 2145–2153. [Google Scholar] [CrossRef]
- Baysak, E.; Gunay, U.S.; Daglar, O.; Durmaz, H. Synthesis and Post-Polymerization Modification of Polyester Containing Pendant Thiolactone Units. Eur. Polym. J. 2019, 112, 241–247. [Google Scholar] [CrossRef]
- Reese, C.M.; Thompson, B.J.; Logan, P.K.; Stafford, C.M.; Blanton, M.; Patton, D.L. Sequential and One-Pot Post-Polymerization Modification Reactions of Thiolactone-Containing Polymer Brushes. Polym. Chem. 2019, 10, 4935–4943. [Google Scholar] [CrossRef] [PubMed]
- Misztalewska-Turkowicz, I.; Coutelier, O.; Destarac, M. Two Pathways of Thiolactone Incorporation into Polyurethanes and Their One-Pot Double Postfunctionalization. Macromolecules 2020, 53, 10785–10795. [Google Scholar] [CrossRef]
- Kim, J.S.; Sirois, A.R.; Vazquez Cegla, A.J.; Jumai’an, E.; Murata, N.; Buck, M.E.; Moore, S.J. Protein–Polymer Conjugates Synthesized Using Water-Soluble Azlactone-Functionalized Polymers Enable Receptor-Specific Cellular Uptake toward Targeted Drug Delivery. Bioconjug. Chem. 2019, 30, 1220–1231. [Google Scholar] [CrossRef]
- Kakuchi, R.; Theato, P. Three-Component Reactions for Post-Polymerization Modifications. ACS Macro Lett. 2013, 2, 419–422. [Google Scholar] [CrossRef]
- Vong, K.K.H.; Maeda, S.; Tanaka, K. Propargyl-Assisted Selective Amidation Applied in C-Terminal Glycine Peptide Conjugation. Chem. Eur. J. 2016, 22, 18865–18872. [Google Scholar] [CrossRef] [PubMed]
- Vong, K.K.H.; Tsubokura, K.; Nakao, Y.; Tanei, T.; Noguchi, S.; Kitazume, S.; Taniguchi, N.; Tanaka, K. Cancer Cell Targeting Driven by Selective Polyamine Reactivity with Glycine Propargyl Esters. Chem. Commun. 2017, 53, 8403–8406. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Iwamoto, S.; Aso, Y. Accelerated Amidation of a Glycine Propargyl Ester on a Polymer and the Two-Way Use of One-Pot Double Postpolymerization Modification with Click Chemistry. Bull. Chem. Soc. Jpn. 2023, 96, 624–630. [Google Scholar] [CrossRef]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanaka, T. Recent Advances in Polymers Bearing Activated Esters for the Synthesis of Glycopolymers by Postpolymerization Modification. Polymers 2024, 16, 1100. https://doi.org/10.3390/polym16081100
Tanaka T. Recent Advances in Polymers Bearing Activated Esters for the Synthesis of Glycopolymers by Postpolymerization Modification. Polymers. 2024; 16(8):1100. https://doi.org/10.3390/polym16081100
Chicago/Turabian StyleTanaka, Tomonari. 2024. "Recent Advances in Polymers Bearing Activated Esters for the Synthesis of Glycopolymers by Postpolymerization Modification" Polymers 16, no. 8: 1100. https://doi.org/10.3390/polym16081100
APA StyleTanaka, T. (2024). Recent Advances in Polymers Bearing Activated Esters for the Synthesis of Glycopolymers by Postpolymerization Modification. Polymers, 16(8), 1100. https://doi.org/10.3390/polym16081100





