Transparent Poly(amide-imide)s with Low Coefficient of Thermal Expansion from Trifluoromethylated Trimellitic Anhydride
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.3. Monomer Synthesis
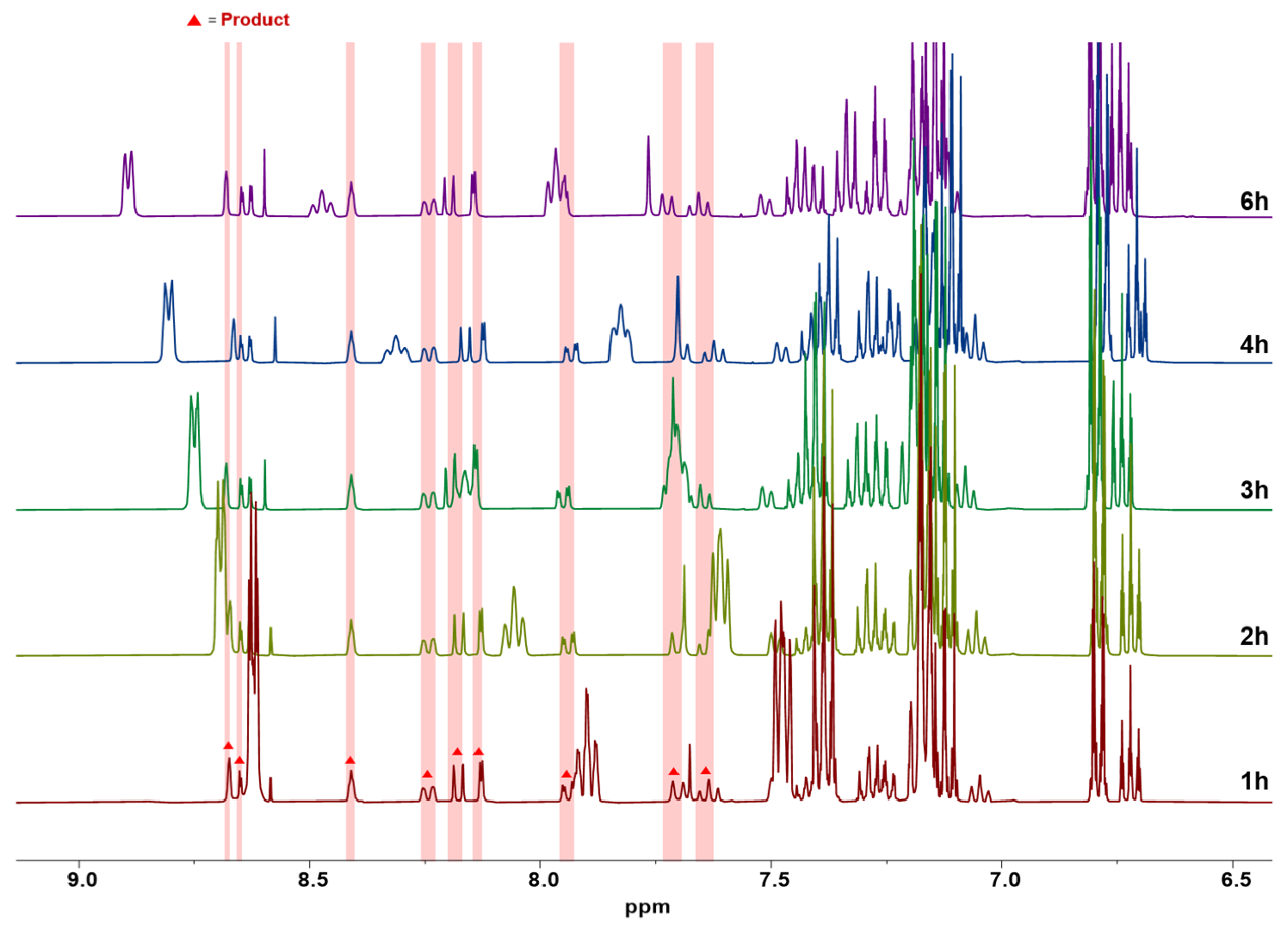
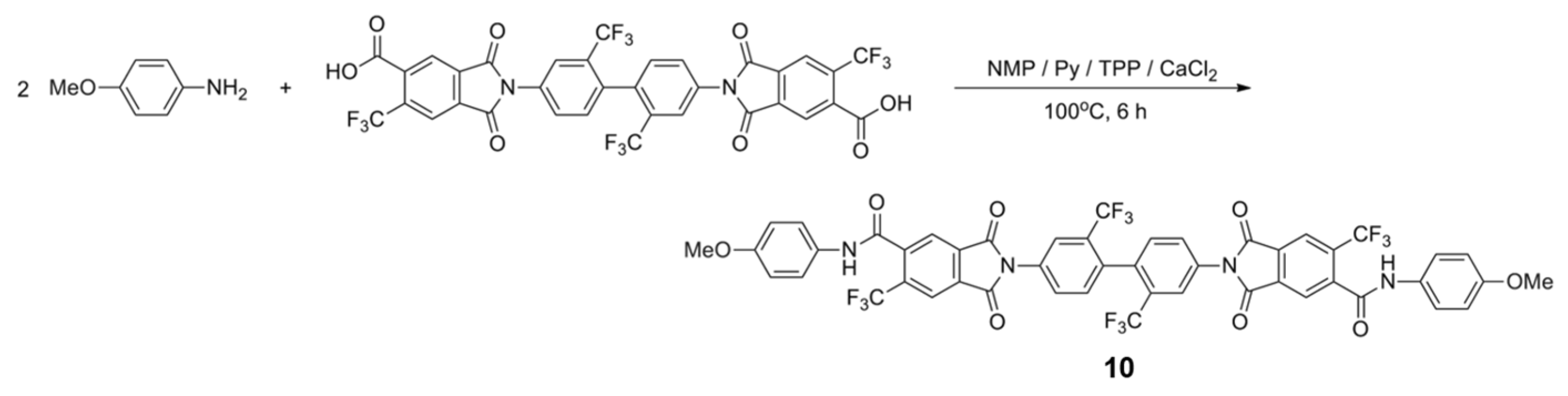
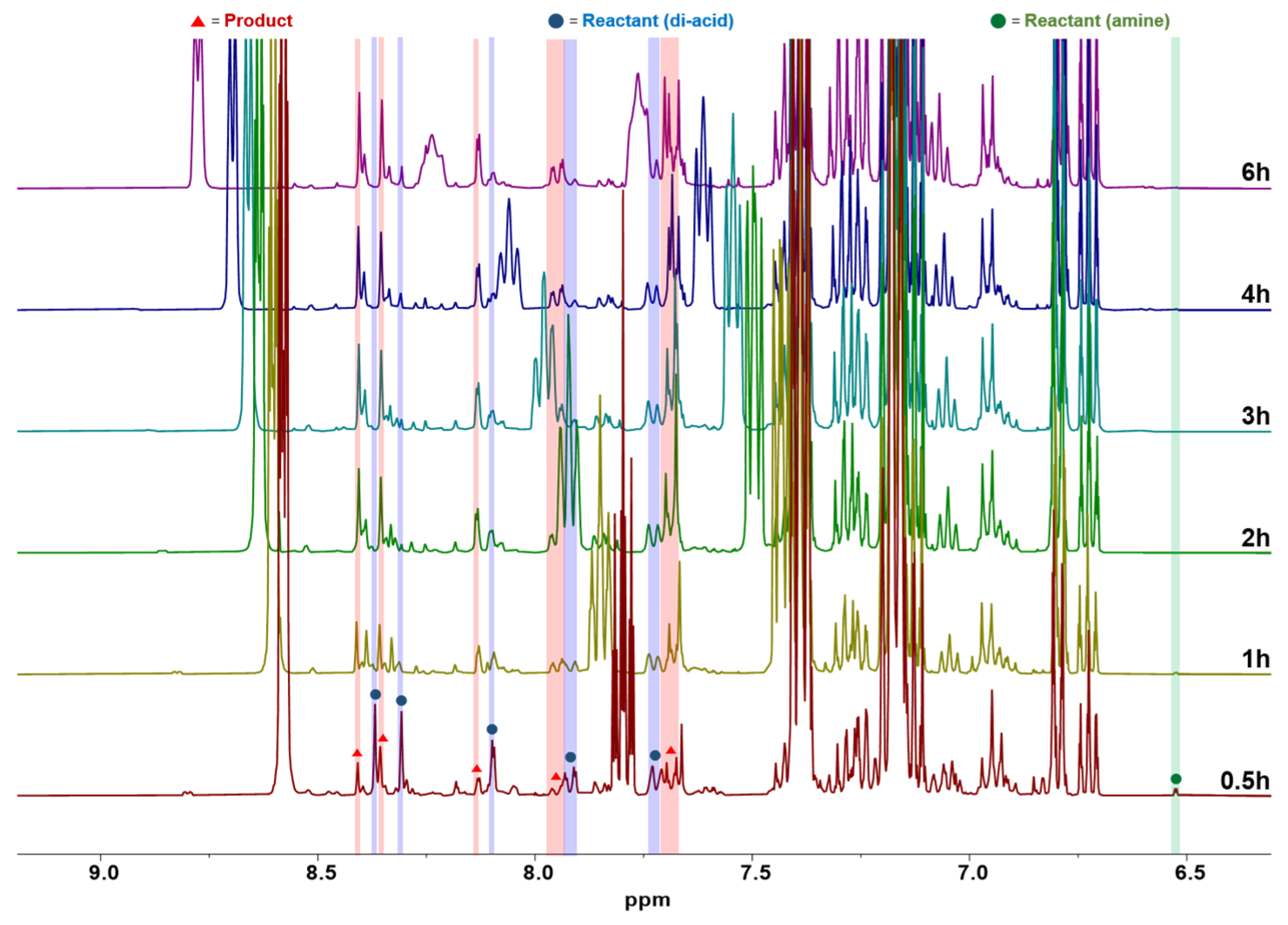
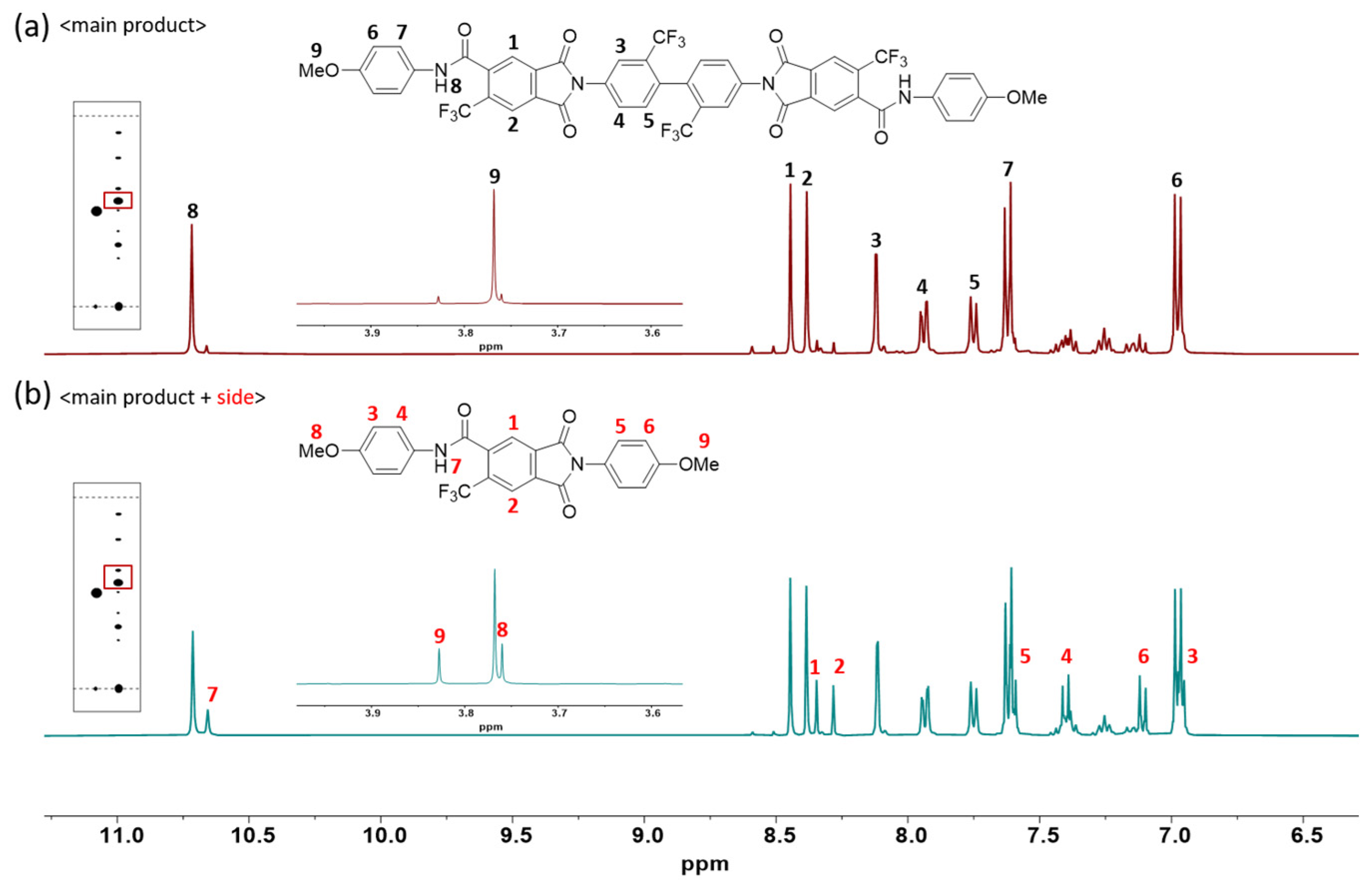
2.4. Model Reaction
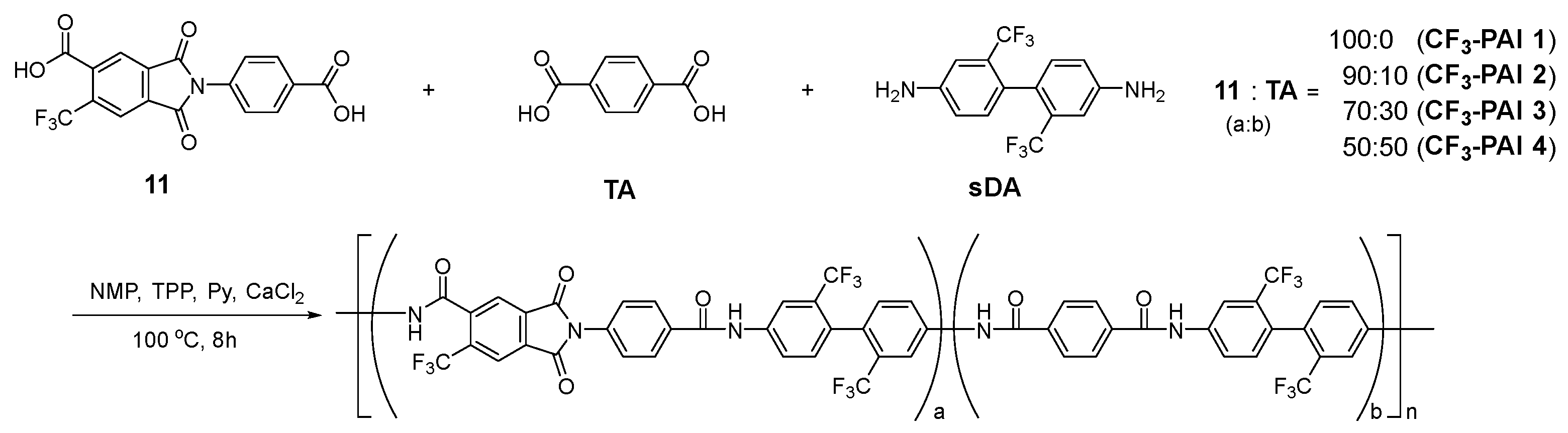
2.5. Polymerization
2.6. Preparation of Poly(amide-imide)s Films
3. Results and Discussion
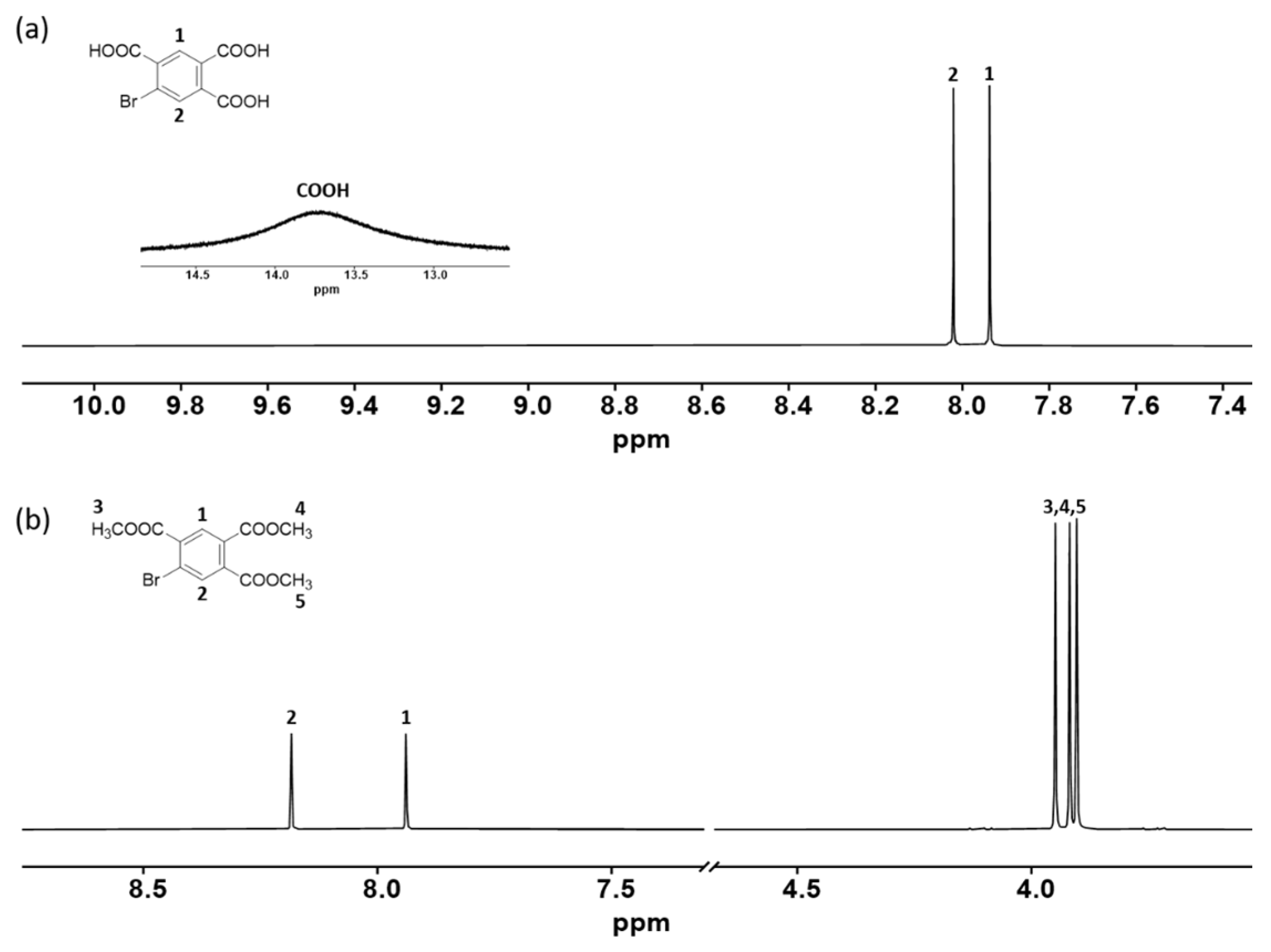
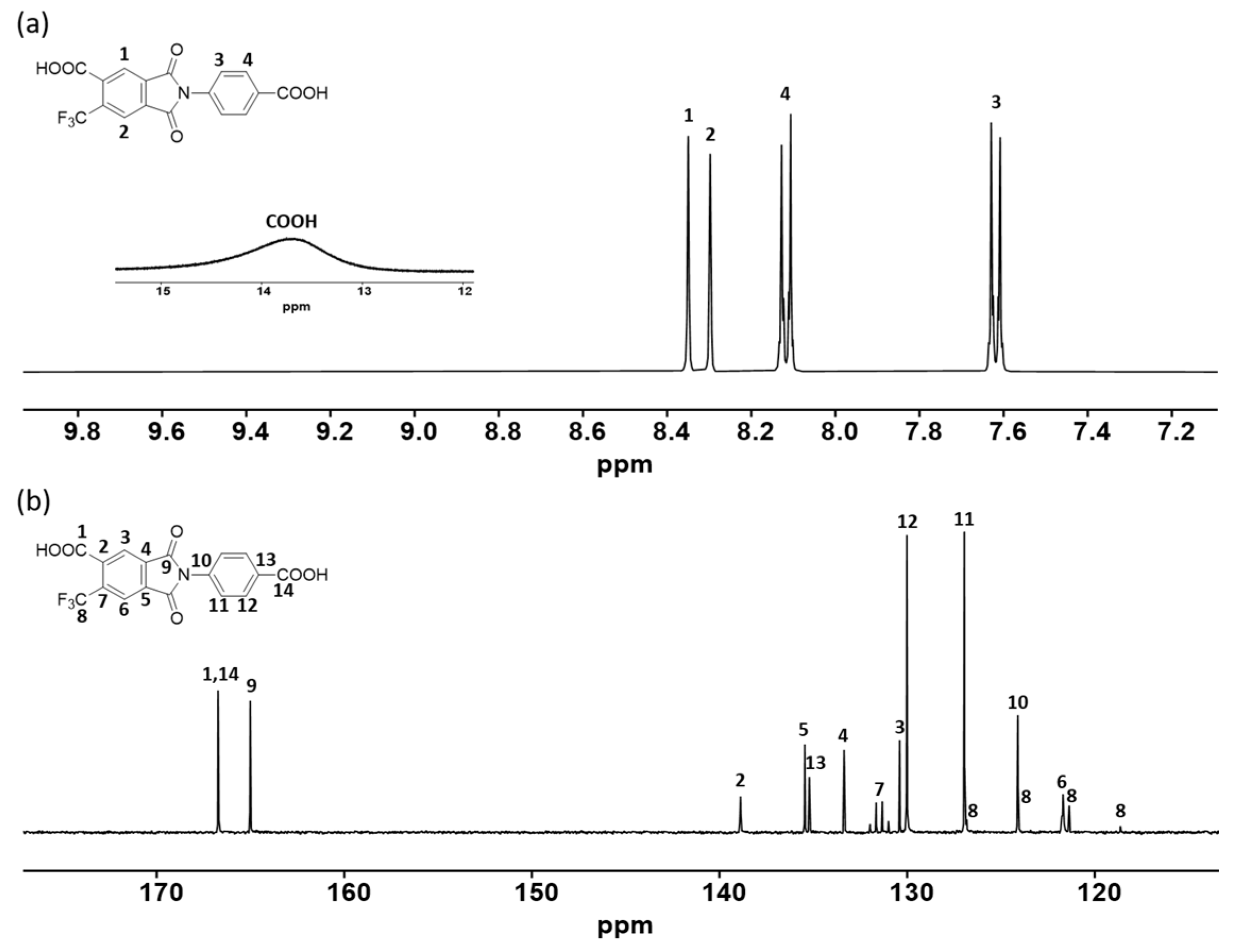
3.1. Monomer Synthesis
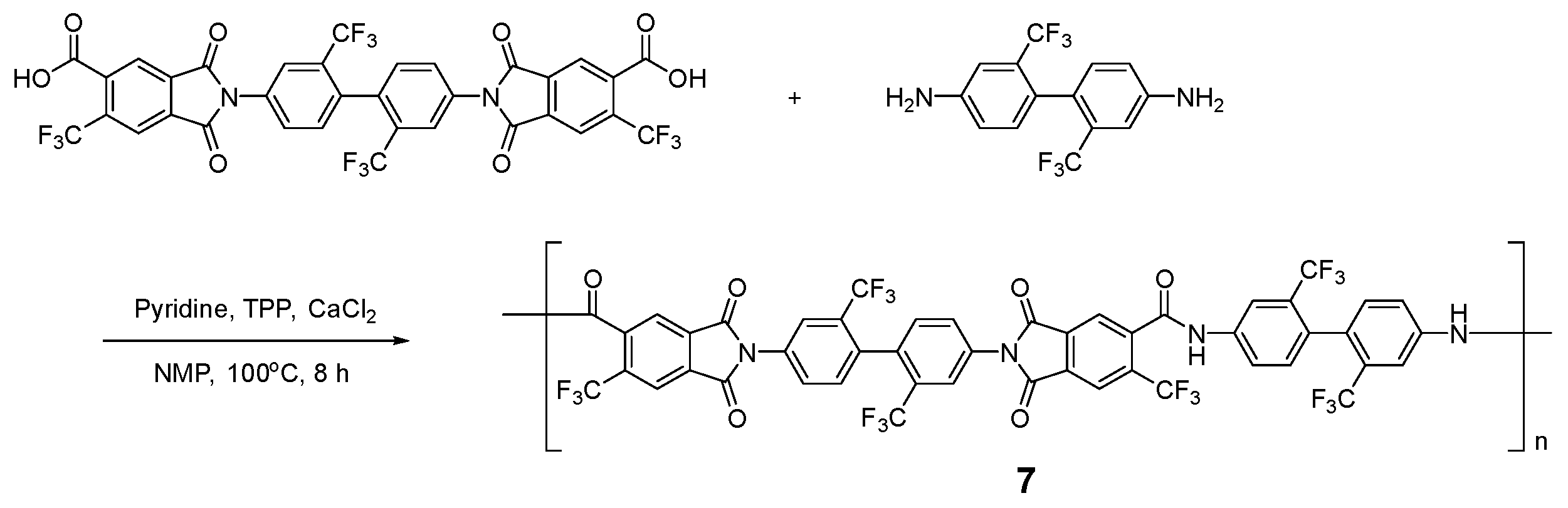
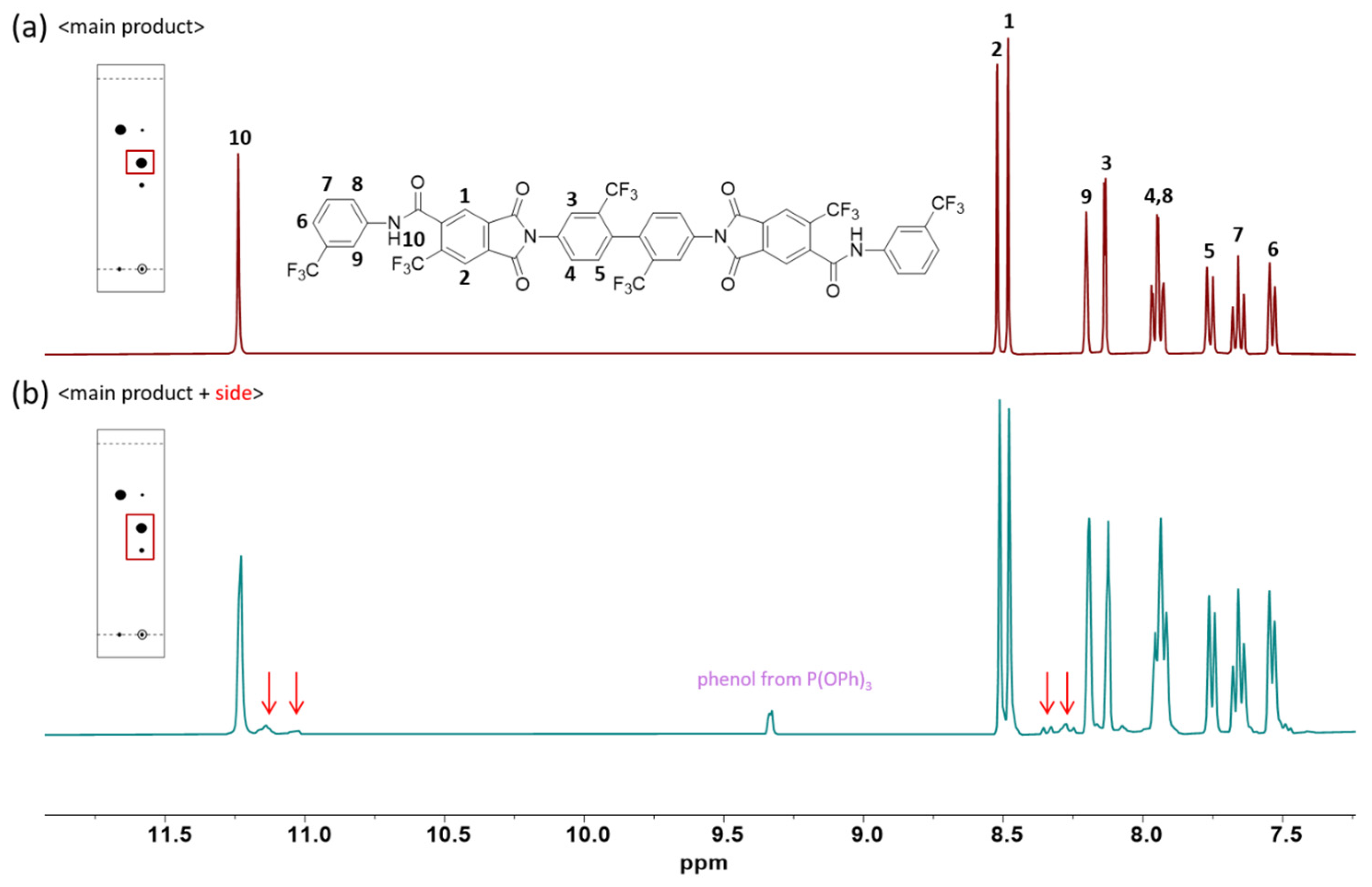
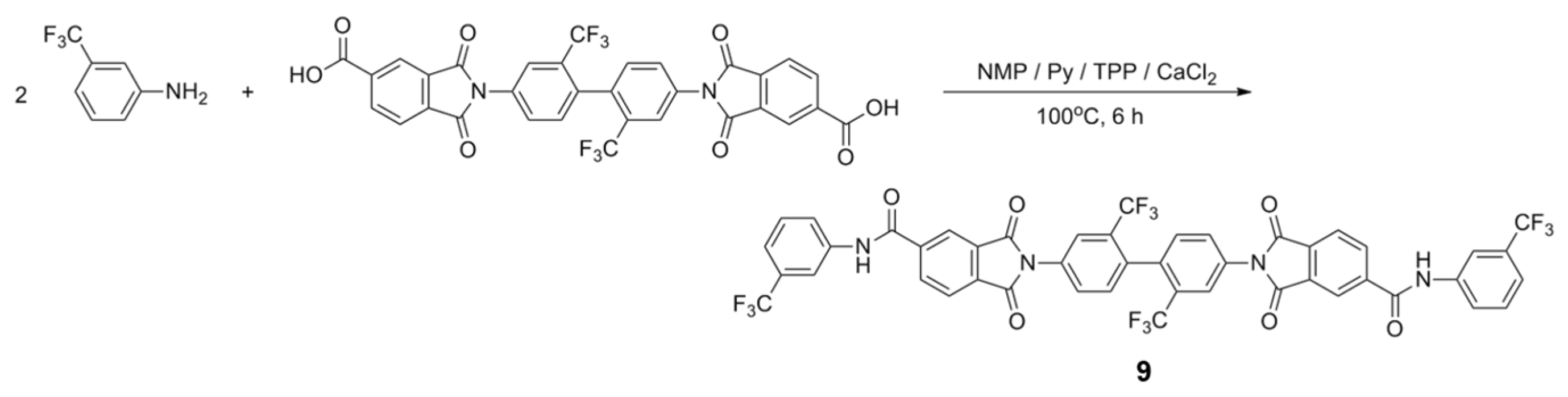
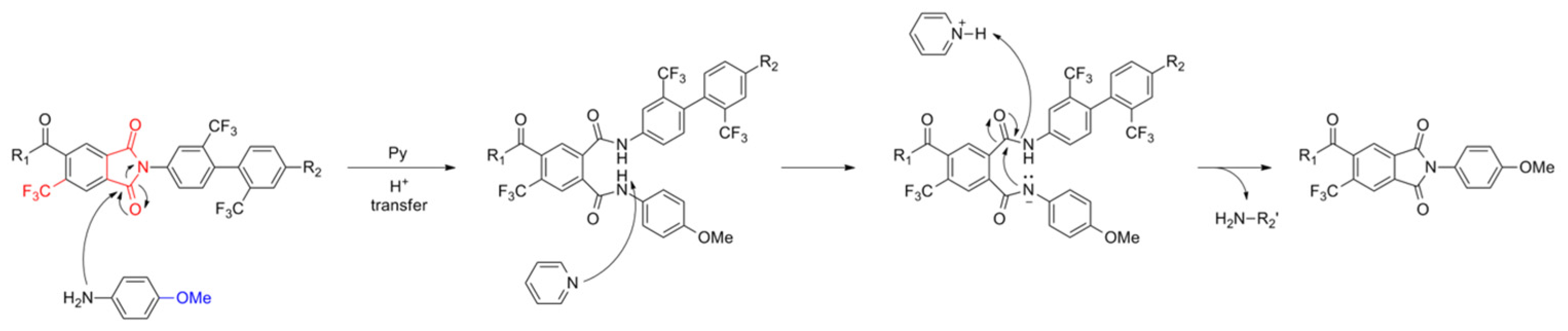
3.2. Model Reaction
3.3. Modification of Monomer Structure
3.4. Polymerization
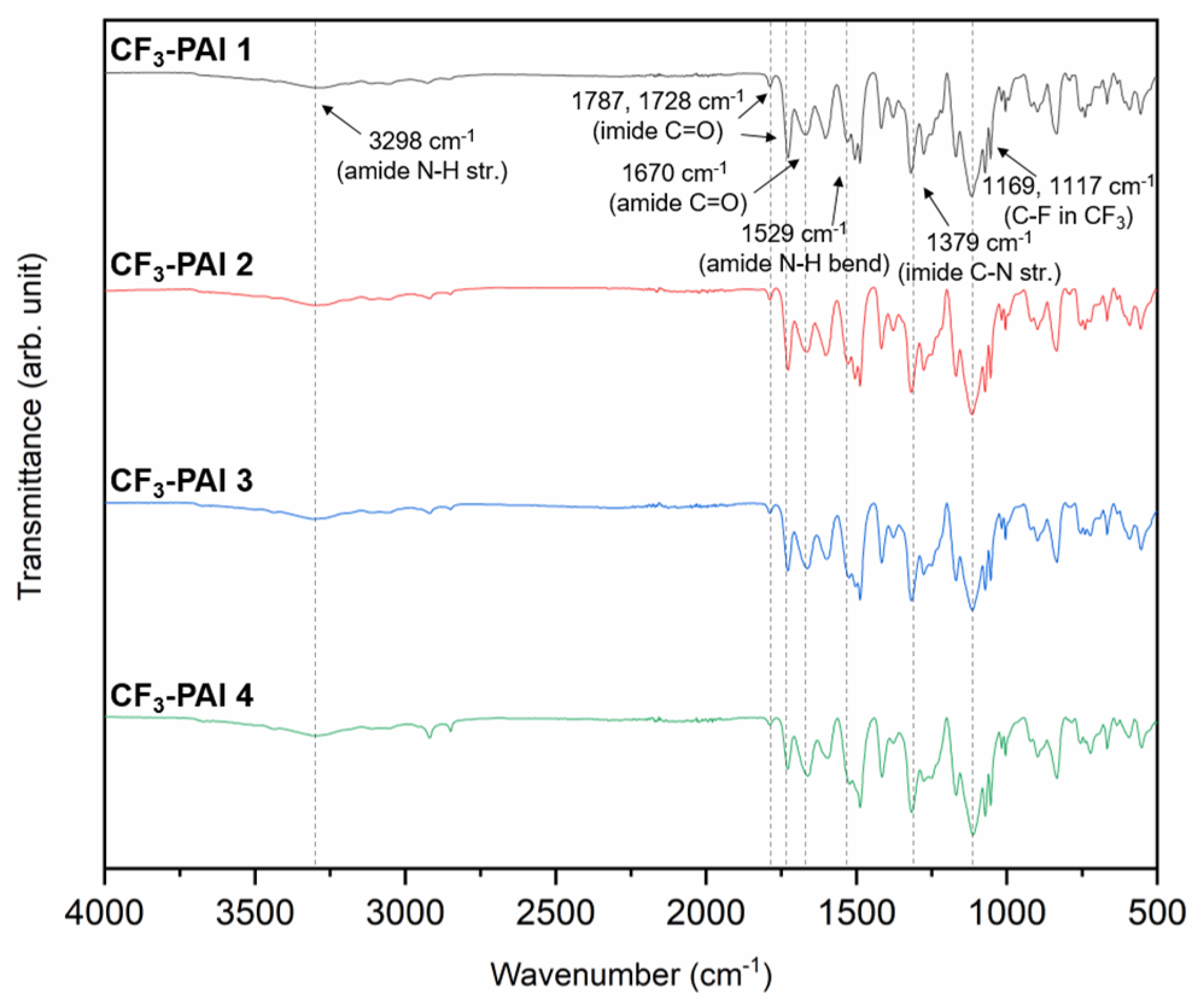
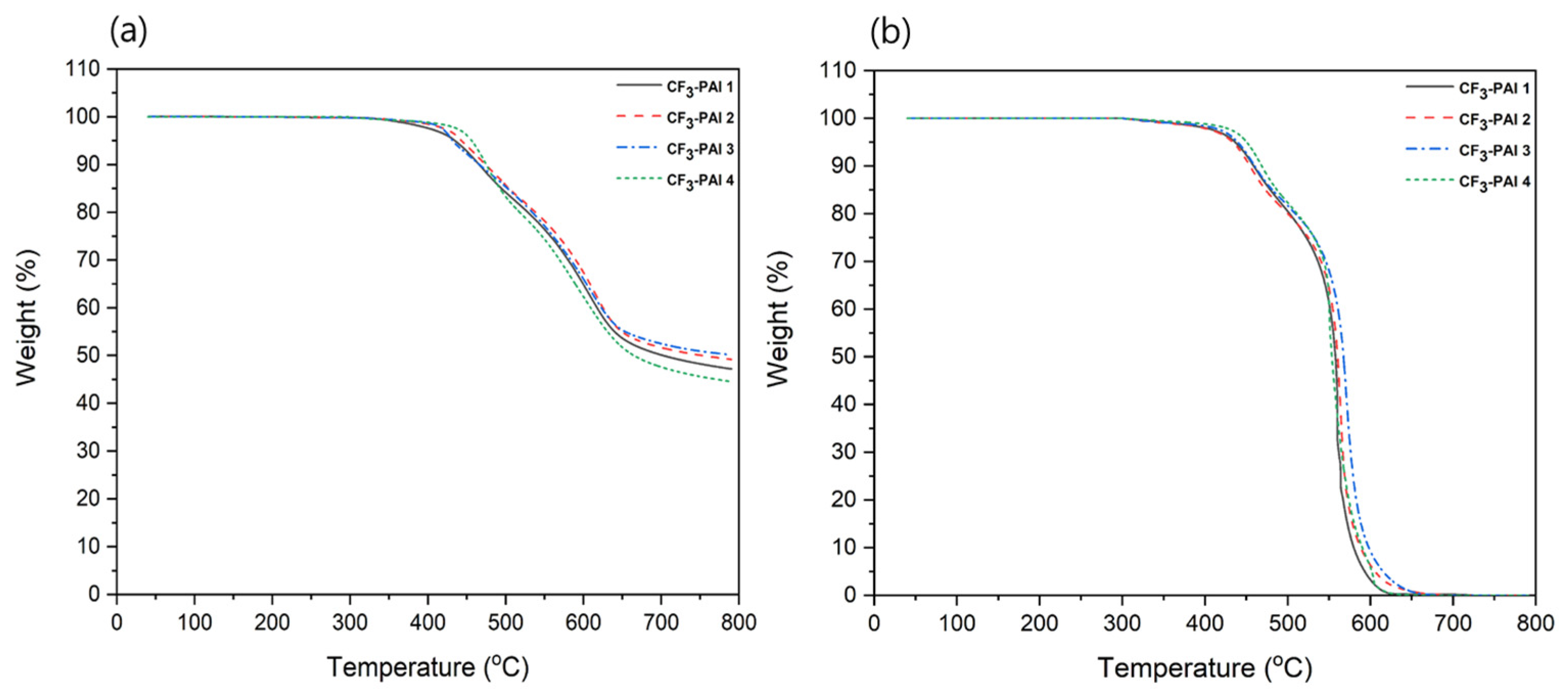
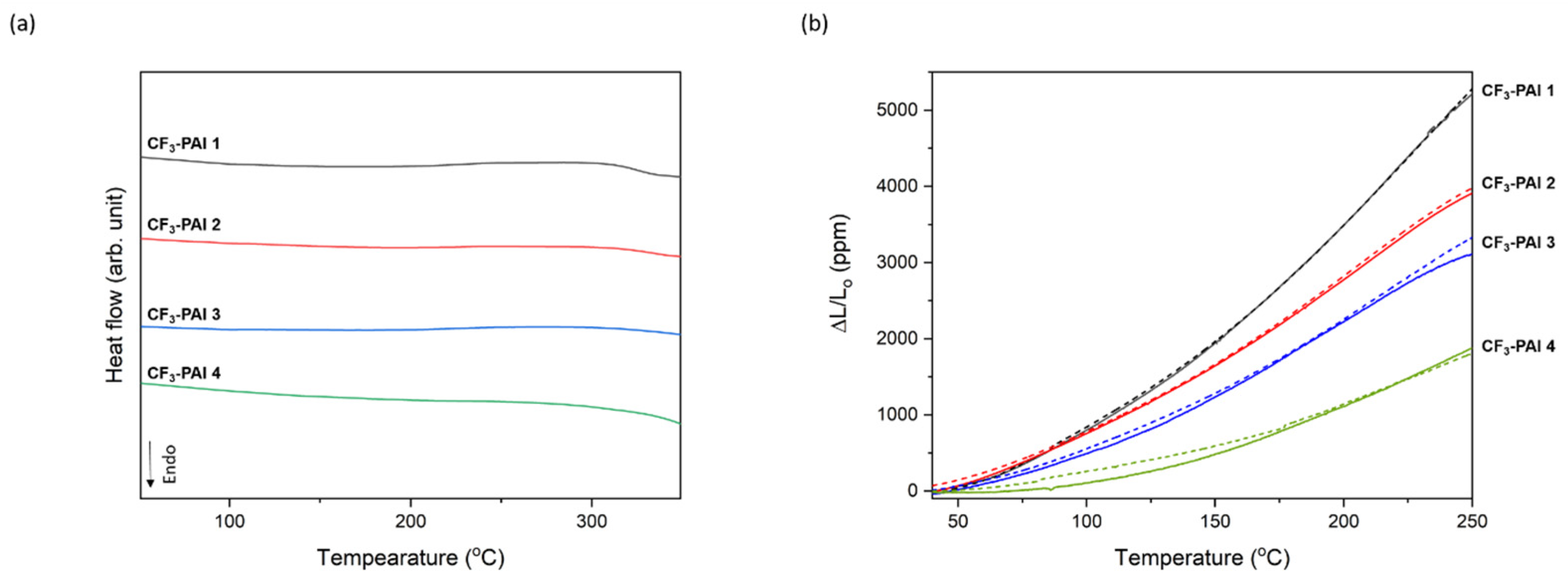
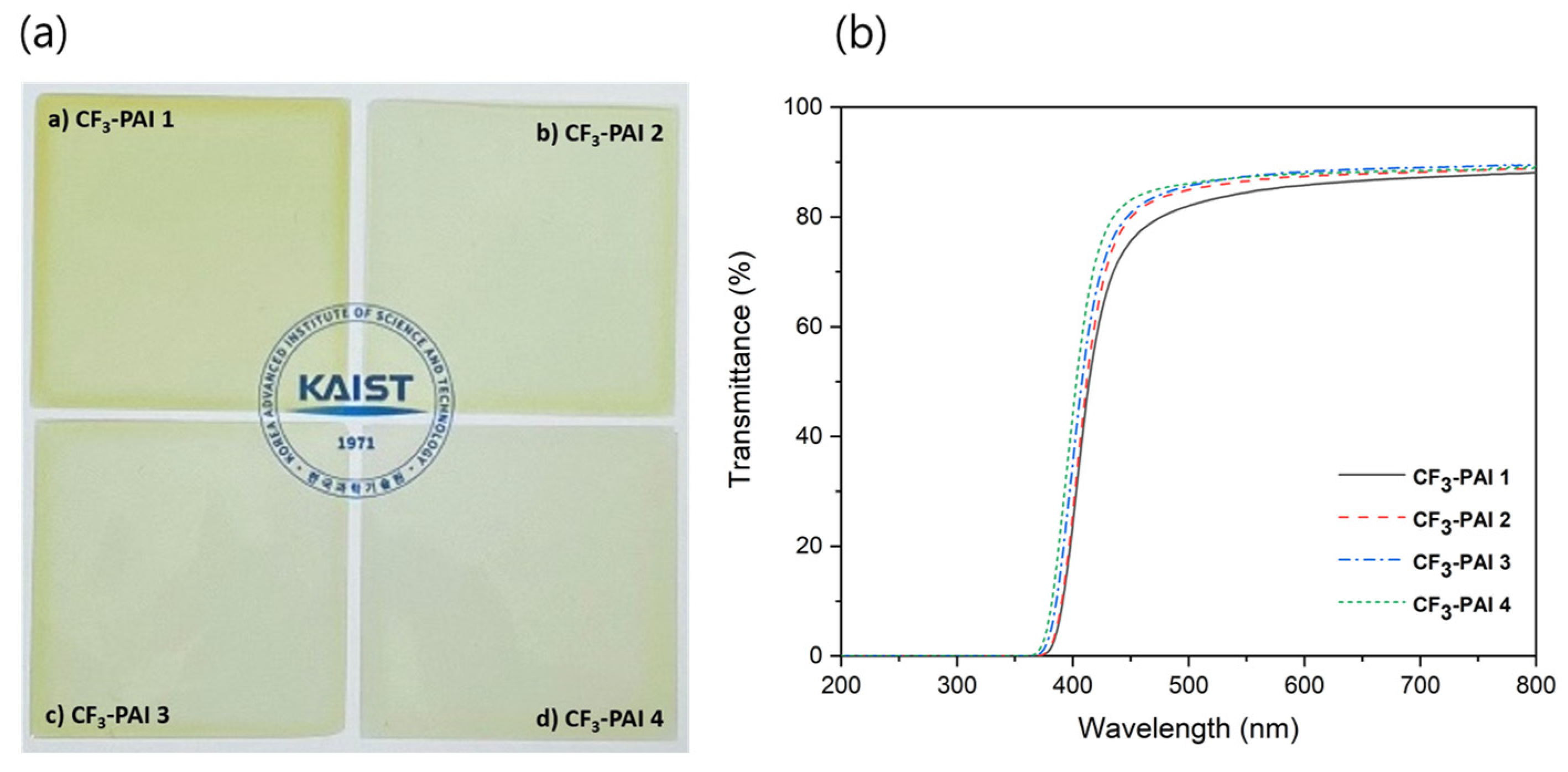
3.5. Properties of Polymers
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Choi, M.C.; Kim, Y.; Ha, C.S. Polymers for Flexible Displays: From Material Selection to Device Applications. Prog. Polym. Sci. 2008, 33, 581–630. [Google Scholar] [CrossRef]
- Logothetidis, S. Flexible Organic Electronic Devices: Materials, Process and Applications. Mater. Sci. Eng. B 2008, 152, 96–104. [Google Scholar] [CrossRef]
- Huang, J.J.; Chen, Y.P.; Lien, S.Y.; Weng, K.W.; Chao, C.H. High Mechanical and Electrical Reliability of Bottom-Gate Microcrystalline Silicon Thin Film Transistors on Polyimide Substrate. Curr. Appl. Phys. 2011, 11 (Suppl. S1), S266–S270. [Google Scholar] [CrossRef]
- Nakano, S.; Saito, N.; Miura, K.; Sakano, T.; Ueda, T.; Sugi, K.; Yamaguchi, H.; Amemiya, I.; Hiramatsu, M.; Ishida, A. Highly Reliable a-IGZO TFTs on a Plastic Substrate for Flexible AMOLED Displays. J. Soc. Inf. Disp. 2012, 20, 493–498. [Google Scholar] [CrossRef]
- Dhara, M.G.; Banerjee, S. Fluorinated High-Performance Polymers: Poly(arylene Ether)s and Aromatic Polyimides Containing Trifluoromethyl Groups. Prog. Polym. Sci. 2010, 35, 1022–1077. [Google Scholar] [CrossRef]
- Ghosh, M.K.; Mittal, K.L. Polyimides: Fundamentals and Applications; Marcel Dekker: New York, NY, USA, 1996. [Google Scholar]
- Liaw, D.-J.; Wang, K.-L.; Huang, Y.-C.; Lee, K.-R.; Lai, J.-Y.; Ha, C.-S. Advanced polyimide materials: Syntheses, physical properties and applications. Prog. Polym. Sci. 2012, 37, 907–974. [Google Scholar] [CrossRef]
- Chung, I.S.; Kim, S.Y. Soluble Polyimides from Unsymmetrical Diamine with Trifluoromethyl Pendent Group. Macromolecules 2000, 33, 3190–3193. [Google Scholar] [CrossRef]
- Choi, H.; Chung, I.S.; Hong, K.; Park, C.E.; Kim, S.Y. Soluble polyimides from unsymmetrical diamine containing benzimidazole ring and trifluoromethyl pendent group. Polymer 2008, 49, 2644–2649. [Google Scholar] [CrossRef]
- Ke, F.; Song, N.; Liang, D.; Xu, H. A method to break charge transfer complex of polyimide: A study on solution behavior. J. Appl. Polym. Sci. 2013, 127, 797–803. [Google Scholar] [CrossRef]
- Zheng, H.B.; Wang, Z.Y. Polyimides Derived from Novel Unsymmetric Dianhydride. Macromolecules 2000, 33, 4310–4312. [Google Scholar] [CrossRef]
- Yan, J.; Wang, Z.; Gao, L.; Ding, M. Polyimides Derived from 3,3′-Bis(N-aminophthalimide). Macromolecules 2006, 39, 7555–7560. [Google Scholar] [CrossRef]
- Kim, M.; Kim, S.Y. Curable Aromatic Polyimides Containing Enaminonitrile Groups. Macromolecules 2002, 35, 4553–4555. [Google Scholar] [CrossRef]
- Liaw, D.J.; Chang, F.C.; Leung, M.K.; Chou, M.Y.; Muellen, K. High Thermal Stability and Rigid Rod of Novel Organosoluble Polyimides and Polyamides Based on Bulky and Noncoplanar Naphthalene-Biphenyldiamine. Macromolecules 2005, 38, 4024–4029. [Google Scholar] [CrossRef]
- Yang, C.P.; Su, Y.Y. Novel Organosoluble and Colorless Poly(ether imide)sbased on 3,3-Bis[4-(3,4-dicarboxyphenoxy)phenyl]phthalide Dianhydride and Aromatic Bis(ether amine)sBearing Pendent Trifluoromethyl Groups. J. Polym. Sci. Part A Polym. Chem. 2006, 44, 3140–3152. [Google Scholar] [CrossRef]
- Hasegawa, M. Development of Solution-Processable, Optically Transparent Polyimides with Ultra-Low Linear Coefficients of Thermal Expansion. Polymers 2017, 9, 520. [Google Scholar] [CrossRef]
- Ozawa, H.; Ishiguro, E.; Kyoya, Y.; Kikuchi, Y.; Matsumoto, T. Colorless Polyimides Derived from an Alicyclic Tetracarboxylic Dianhydride, CpODA. Polymers 2021, 13, 2824. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M.; Ichikawa, K.; Takahashi, S.; Ishii, J. Solution-Processable Colorless Polyimides Derived from Hydrogenated Pyromellitic Dianhydride: Strategies to Reduce the Coefficients of Thermal Expansion by Maximizing the Spontaneous Chain Orientation Behavior during Solution Casting. Polymers 2022, 14, 1131. [Google Scholar] [CrossRef]
- He, Z.; Ren, X.; Wang, Z.; Pan, Z.; Qi, Y.; Han, S.; Yu, H.; Liu, J. Preparation and Characterization of Light-Colored Polyimide Nanocomposite Films Derived from a Fluoro-Containing Semi-Alicyclic Polyimide Matrix and Colloidal Silica with Enhanced High-Temperature Dimensionally Stability. Polymers 2023, 15, 3015. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; He, Z.; Wang, Z.; Pan, Z.; Qi, Y.; Han, S.; Yu, H.; Liu, J. Design, Synthesis and Properties of Semi-Alicyclic Colorless and Transparent Polyimide Films with High Glass Transition Temperatures and Low Retardation for Potential Applications in Flexible Electronics. Polymers 2023, 15, 3408. [Google Scholar] [CrossRef]
- She, Y.; Wang, S.; Liao, Q.; Lin, M. Transparent and highly organosoluble aromatic polyimides with twisted backbone and bulky side substituents for flexible substrate materials. J. Polym. Sci. 2024, 62, 1061–1073. [Google Scholar] [CrossRef]
- Hasegawa, M.; Miyama, T.; Ishii, J.; Watanabe, D.; Uchida, A. Colorless Polyimides Derived from 5,5’-bis(2,3-norbornanedicarboxylic anhydride): Strategies to Reduce the Linear Coefficients of Thermal Expansion and Improve the Film Toughness. Polymers 2023, 15, 3838. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, J.; Zhao, F.; Li, F.; Dai, C.; Chen, C.; Yang, Z.; Tu, G. Synergistic Enhancement of Mechanical and Dielectric Properties in Transparent Polyimides by Regulating Hydrogen Bonding and Microbranched Cross-Linking Structure. ACS Appl. Polym. Mater. 2024, 6, 10738–10749. [Google Scholar] [CrossRef]
- Fang, Y.; Lu, X.; Xiao, J.; Zhang, S.; Lu, Q. Thermally Stable and Transparent Polyimide Derived from Side-Group-Regulated Spirobifluorene Unit for Substrate Application. Macromol. Rapid Commun. 2024, 45, 2400245–2400257. [Google Scholar] [CrossRef] [PubMed]
- Bao, F.; Qiu, L.; Zou, B.; Lei, H.; Peng, W.; Cheng, S.Z.D.; Huang, M. Development of Fluorinated Colorless Polyimides of Restricted Dihedral Rotation toward Flexible Substrates with Thermal Robustness. Macromolecules 2024, 57, 3568–3579. [Google Scholar] [CrossRef]
- Li, L.; Jiang, W.; Yang, X.; Meng, Y.; Hu, P.; Huang, C.; Liu, F. From Molecular Design to Practical Applications: Strategies for Enhancing the Optical and Thermal Performance of Polyimide Films. Polymers 2024, 16, 2315. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Wang, X.; Yuan, S.; Ren, X.; Yang, C.; Han, S.; Qi, Y.; Li, D.; Liu, J. Preparation and Characterization of Atomic Oxygen-Resistant, Optically Transparent and Dimensionally Stable Copolyimide Films from Fluorinated Monomers and POSS-Substituted Diamine. Polymers 2024, 16, 2845. [Google Scholar] [CrossRef] [PubMed]
- Dodda, J.M.; Bělský, P. Progress in designing poly(amide imide)s (PAI) in terms of chemical structure, preparation methods and processability. Eur. Polym. J. 2016, 84, 514–537. [Google Scholar] [CrossRef]
- Lee, Y.D.; Kim, K.; Ok, Y.; Kim, M.; Chang, J.H. Syntheses and Characterizations of Colorless and Transparent Poly(amide imide) Copolymer. Polymer 2016, 40, 298–305. [Google Scholar] [CrossRef][Green Version]
- Kim, S.D.; Lee, B.; Byun, T.; Chung, I.S.; Park, J.; Shin, I.; Ahn, N.Y.; Seo, M.; Lee, Y.; Kim, Y.; et al. Poly(amide-imide) materials for transparent and flexible displays. Sci. Adv. 2018, 4, eaau1956. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.; Kim, S.D.; Park, J.; Byun, T.; Kim, S.J.; Seo, M.; Kim, S.Y. Transparent Poly(Amide-Imide)s Containing Trifluoromethyl Groups with High Glass Transition Temperature. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 1782–1786. [Google Scholar] [CrossRef]
- Yin, J.; Mao, D.; Fan, B. Copolyamide-Imide Membrane with Low CTE and CME for Potential Space Optical Applications. Polymers 2021, 13, 1001. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Jiang, G.; Zhang, Y.; Wu, L.; Jia, Y.; Tan, Y.; Liu, J.; Zhang, X. Enhancement of Flame Retardancy of Colorless and Transparent Semi-Alicyclic Polyimide Film from Hydrogenated-BPDA and 4,4′-oxydianiline via the Incorporation of Phosphazene Oligomer. Polymers 2020, 12, 90. [Google Scholar] [CrossRef] [PubMed]
- Liaw, D.J.; Chang, F.C. Highly Organosoluble and Flexible Polyimides with Color Lightness and Transparency Based on 2,2-Bis4-(2-Trifluoromethyl-4-Aminophenoxy)-3,5- Dimethylphenyllpropane. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 5766–5774. [Google Scholar] [CrossRef]
- Liaw, D.J.; Chen, W.H. High Glass Transitions of Novel Organosoluble Polyamide-Imides Based on Noncoplanar and Rigid Diimide-Dicarboxylic Acid. Polym. Degrad. Stab. 2006, 91, 1731–1739. [Google Scholar] [CrossRef]
- Liaw, D.J.; Huang, C.C.; Chen, W.H. Color Lightness and Highly Organosoluble Fluorinated Polyamides, Polyimides and Poly(amide-Imide)s Based on Noncoplanar 2,2′-Dimethyl-4,4′- Biphenylene Units. Polymer 2006, 47, 2337–2348. [Google Scholar] [CrossRef]
- Kim, S.D.; Ka, D.; Chung, I.S.; Kim, S.Y. Poly(arylene Ether)s with Low Refractive Indices: Poly(biphenylene Oxide)s with Trifluoromethyl Pendant Groups via a Meta-Activated Nitro Displacement Reaction. Macromolecules 2012, 45, 3023–3031. [Google Scholar] [CrossRef]
- Kim, S.D.; Kim, S.Y.; Chung, I.S. Soluble and Transparent Polyimides from Unsymmetrical Diamine Containing Two Trifluoromethyl Groups. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 4413–4422. [Google Scholar] [CrossRef]
- Salamone, J.C. Polymeric Materials Encyclopedia, Twelve Volume Set; CRC Press: Boca Raton, FL, USA, 1996. [Google Scholar]
- Yamazaki, N.; Matsumoto, M.; Higashi, F. Studies on Reactions of the N-Phosphonium Salts of Pyridines. XIV. Wholly Aromatic Polyamides by the Direct Polycondensation Reaction by Using Phosphites in the Presence of Metal Salts. J. Polym. Sci. Part A Polym. Chem. 1975, 13, 1373–1380. [Google Scholar] [CrossRef]
- Byun, T. Synthesis and Characterization of Soluble Aromatic Polyamides and Poly(amide-imide)s Containing Two Trifluoromethyl Groups. Doctoral Dissertation, Korea Advanced Institute of Science and Technology, Daejeon, Republic of Korea, 2021. [Google Scholar]







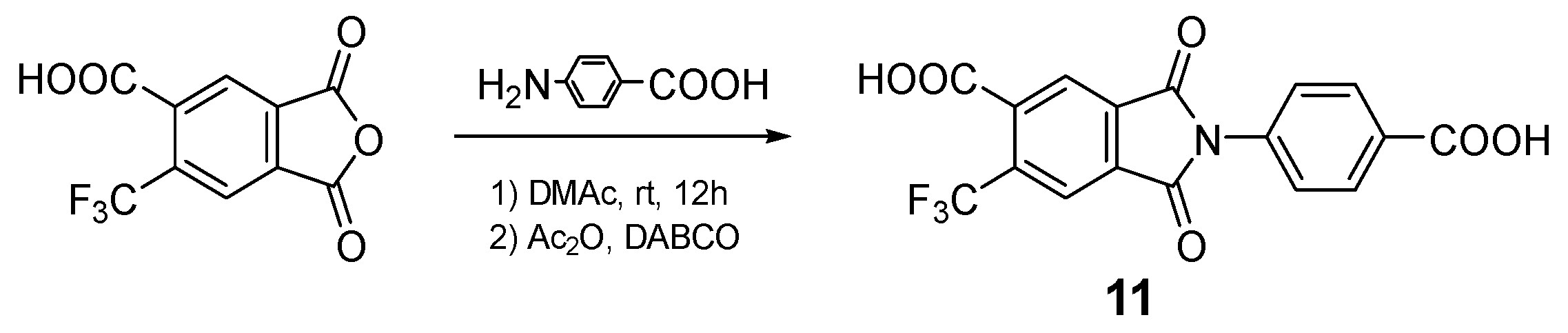

| Polymer Code | Yield (%) | Inherent Viscosity (ηinh) 1 (dL/g) |
|---|---|---|
| CF3-PAI 1 | 92.6 | 1.57 |
| CF3-PAI 2 | 95.1 | 1.86 |
| CF3-PAI 3 | 94.7 | 1.75 |
| CF3-PAI 4 | 96.4 | 2.90 |
| sDA-TA 1 2 | 99.9 | 4.39 |
| Solvents | CF3-PAI 1 | CF3-PAI 2 | CF3-PAI 3 | CF3-PAI 4 |
|---|---|---|---|---|
| NMP | ++ | ++ | ++ | ++ |
| DMAc | ++ | ++ | ++ | ++ |
| DMF | ++ | ++ | ++ | ++ |
| DMSO | ++ | ++ | ++ | + |
| m-cresol | −s | −s | −s | + |
| THF | ++ | + | −s | −s |
| Acetone | −s | −s | −s | −s |
| Ethyl acetate | − | − | − | − |
| Polymer Code | Tg (°C) 1 | Td5 (°C) 2 | CTE (ppm/°C) 3 | ||
|---|---|---|---|---|---|
| In N2 | In Air | 2nd Run | 3rd Run | ||
| CF3-PAI 1 | 325 | 437 | 428 | 26.7 | 26.6 |
| CF3-PAI 2 | 336 | 445 | 434 | 19.9 | 19.7 |
| CF3-PAI 3 | - | 434 | 440 | 16.6 | 16.8 |
| CF3-PAI 4 | - | 452 | 446 | 9.8 | 9.0 |
| Polymer Code | Cutoff Wavelength (nm) | Transmittance at 400 nm (%) | Transmittance at 550 nm (%) | Film Thickness (μm) |
|---|---|---|---|---|
| CF3-PAI 1 | 362 | 24.2 | 84.5 | 30 |
| CF3-PAI 2 | 361 | 26.2 | 86.5 | 30 |
| CF3-PAI 3 | 358 | 35.3 | 87.4 | 30 |
| CF3-PAI 4 | 356 | 44.2 | 87.2 | 30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.J.; Jeong, S.; Byun, T.; Kim, J.S.; Lee, H.; Kim, S.Y. Transparent Poly(amide-imide)s with Low Coefficient of Thermal Expansion from Trifluoromethylated Trimellitic Anhydride. Polymers 2025, 17, 309. https://doi.org/10.3390/polym17030309
Kim SJ, Jeong S, Byun T, Kim JS, Lee H, Kim SY. Transparent Poly(amide-imide)s with Low Coefficient of Thermal Expansion from Trifluoromethylated Trimellitic Anhydride. Polymers. 2025; 17(3):309. https://doi.org/10.3390/polym17030309
Chicago/Turabian StyleKim, Seong Jong, SeongUk Jeong, Taejoon Byun, Jun Sung Kim, Haeshin Lee, and Sang Youl Kim. 2025. "Transparent Poly(amide-imide)s with Low Coefficient of Thermal Expansion from Trifluoromethylated Trimellitic Anhydride" Polymers 17, no. 3: 309. https://doi.org/10.3390/polym17030309
APA StyleKim, S. J., Jeong, S., Byun, T., Kim, J. S., Lee, H., & Kim, S. Y. (2025). Transparent Poly(amide-imide)s with Low Coefficient of Thermal Expansion from Trifluoromethylated Trimellitic Anhydride. Polymers, 17(3), 309. https://doi.org/10.3390/polym17030309








