Change in Thermodynamic Entropy and Free Volume of Epoxy Resin During Tensile Deformation
Abstract
1. Introduction
2. Materials and Experimental Procedure
2.1. Test Specimen Preparation and Dimensions
2.2. Tensile Test and Digital Image Correlation Method
2.3. Differential Scanning Calorimetry (DSC)
2.4. Positron Annihilation Lifetime Spectroscopy (PALS)
3. Results and Discussion
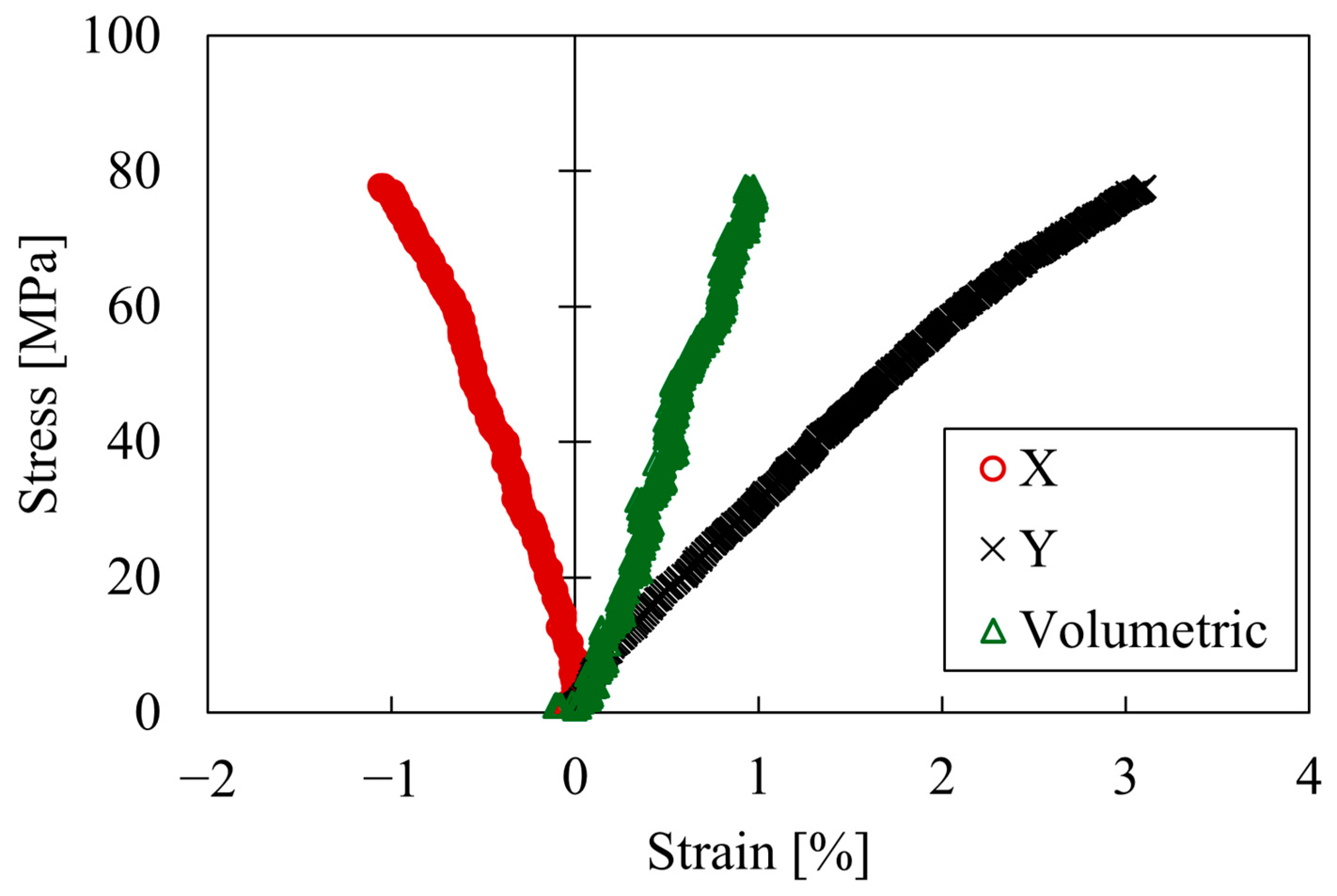
3.1. Results of Tensile Test
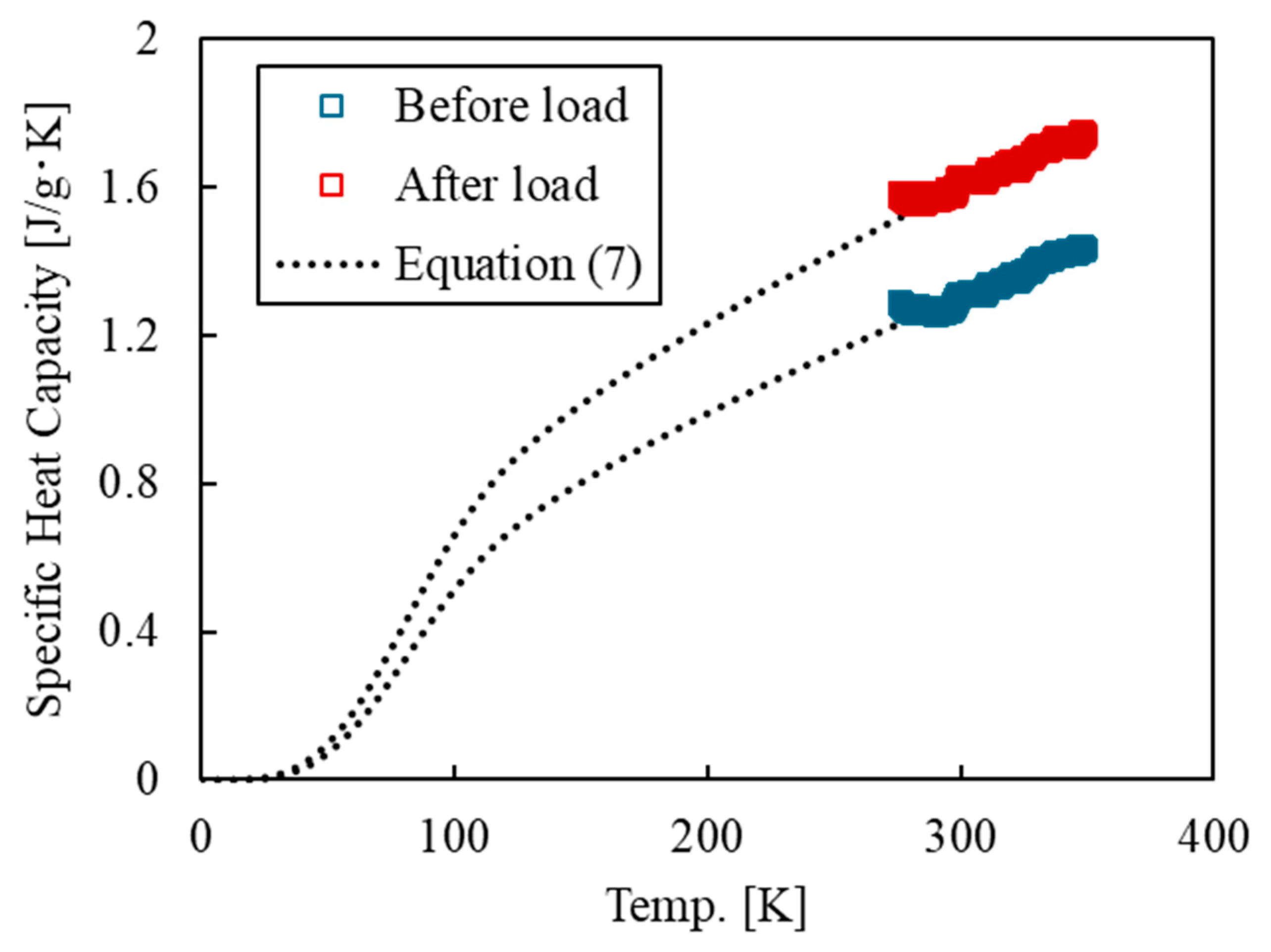
3.2. Thermodynamic Entropy Generation of Epoxy Resin with Deformation
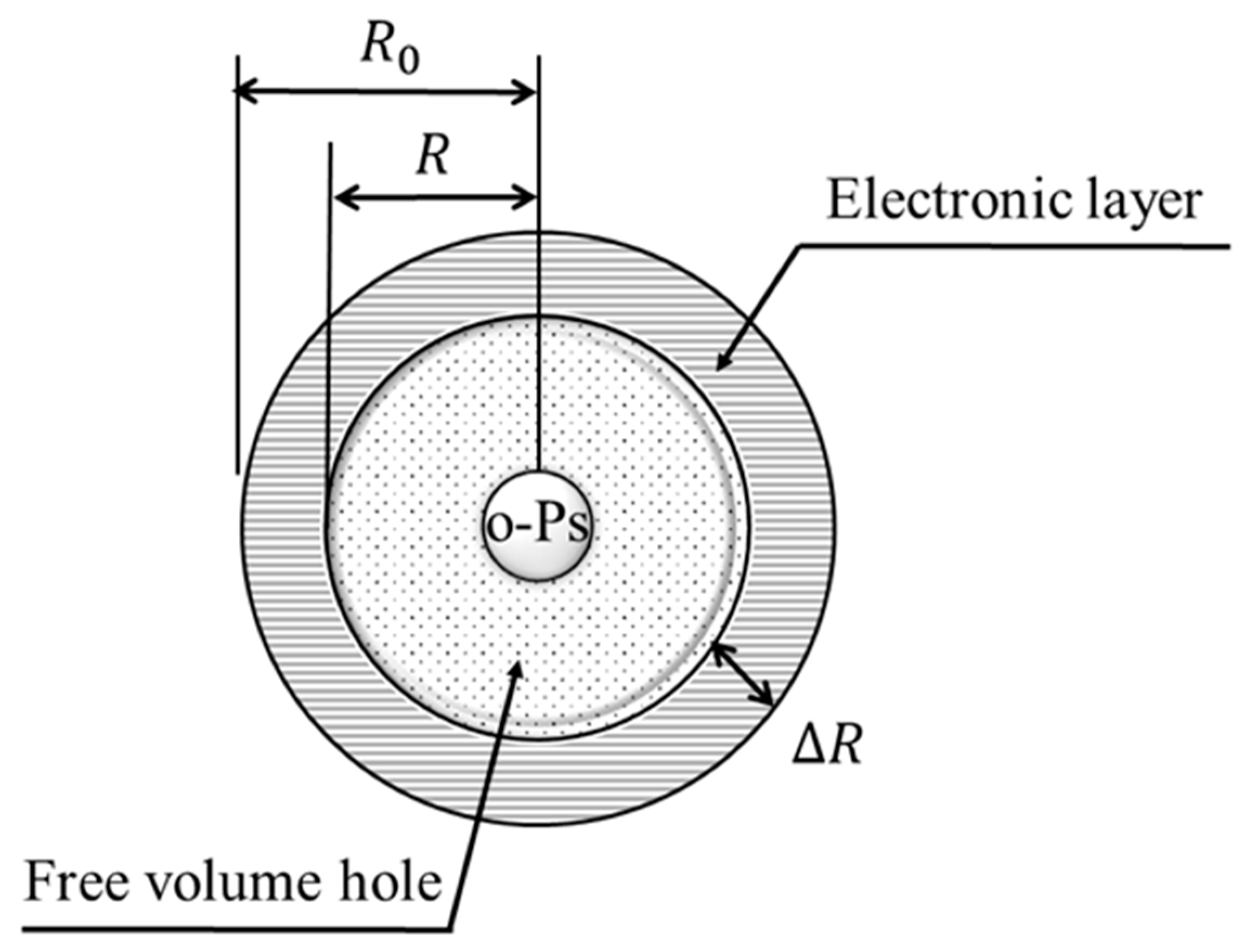
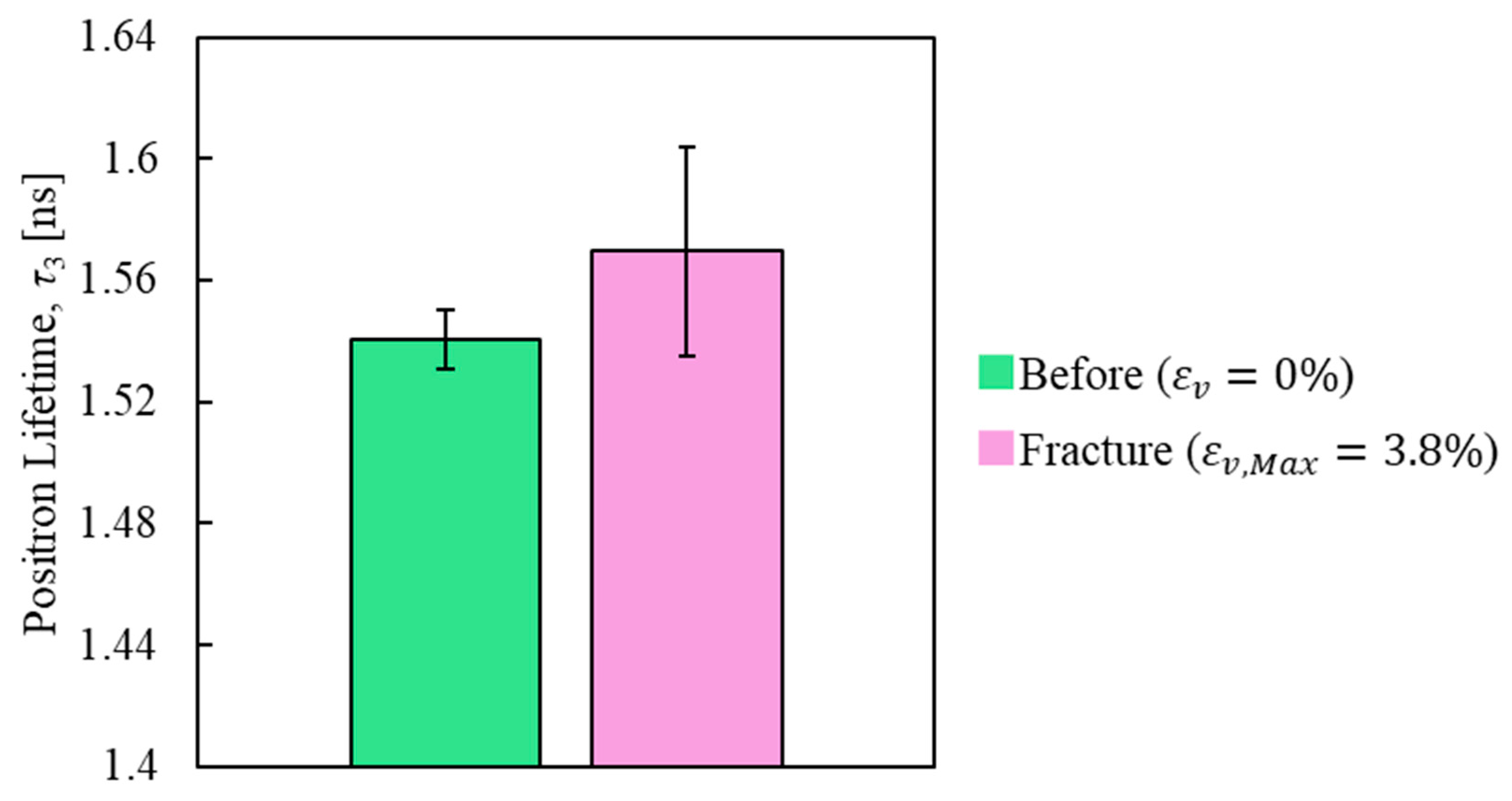
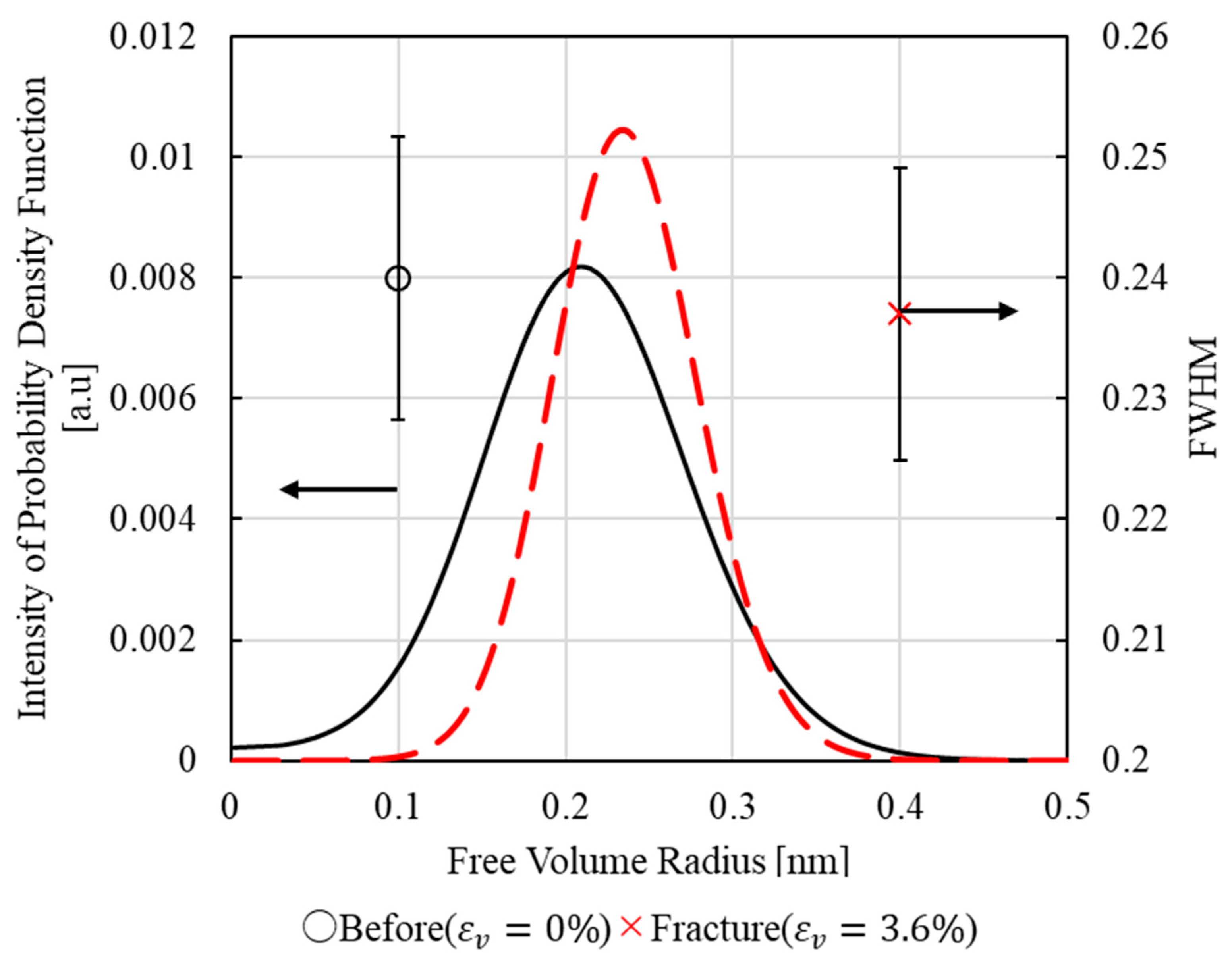
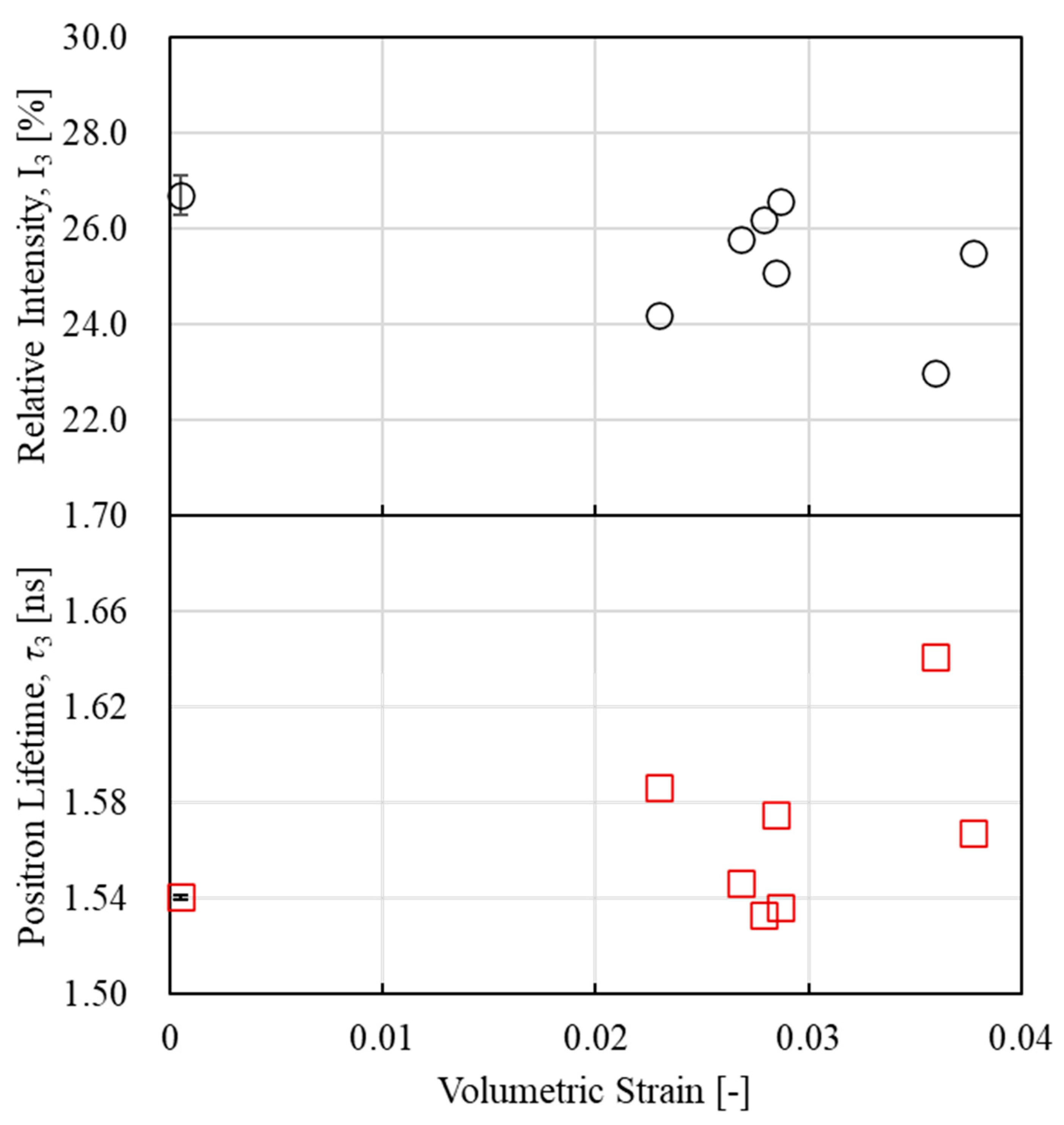
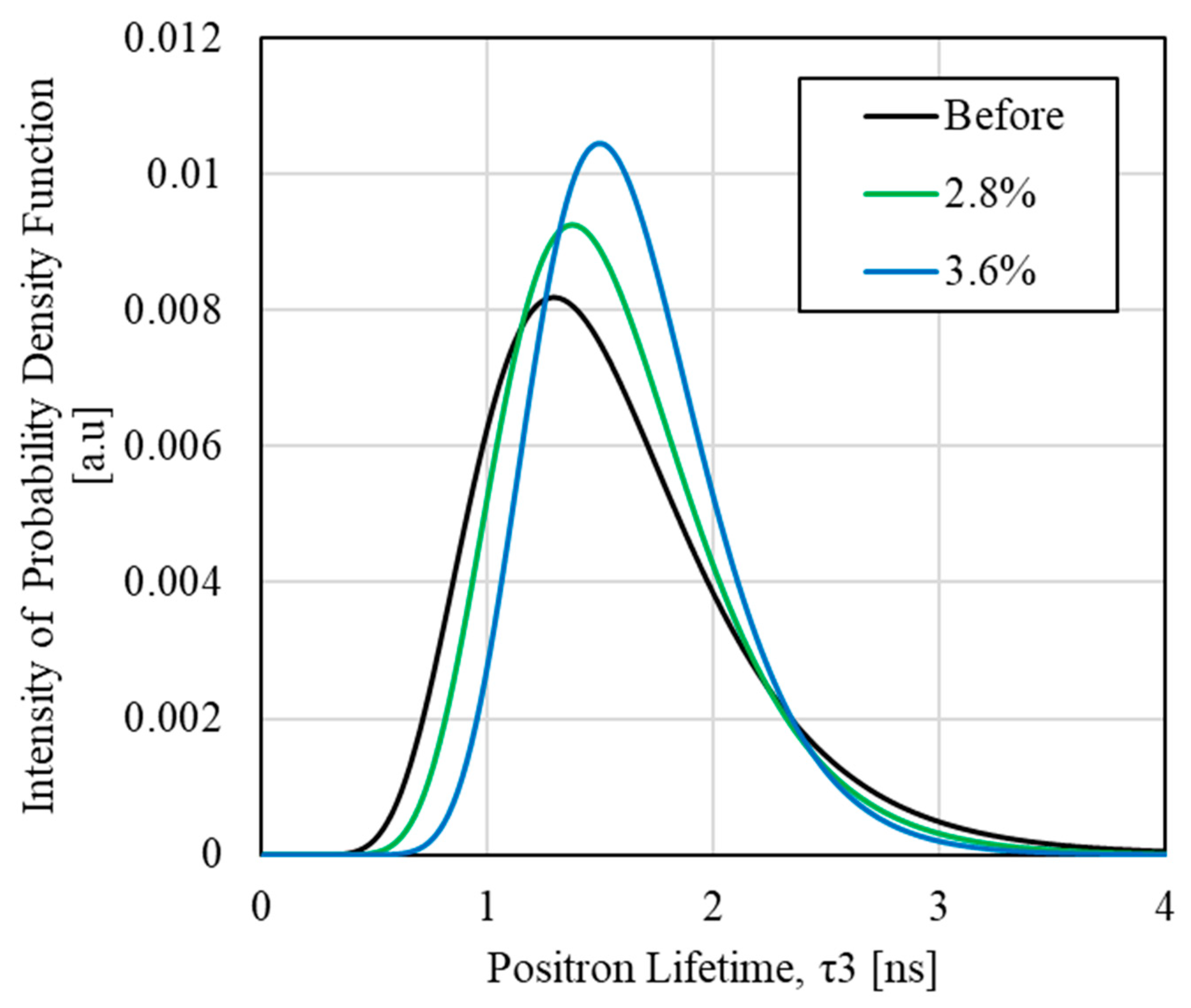
3.3. PALS Measurement Results
3.4. Relationship Between Thermodynamic Entropy Generation and Free Volume
3.5. Internal Changes in Materials During Entropy Generation Processes
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Witik, R.A.; Gaille, F.; Teuscher, R.; Ringwald, H.; Michaud, V.; Månson, J.A.E. Economic and environmental assessment of alternative production methods for composite aircraft components. J. Clean. Prod. 2012, 29–30, 91–102. [Google Scholar] [CrossRef]
- Kim, H.C.; Wallington, T.J. Life-cycle energy and greenhouse gas emission benefits of lightweighting in automobiles: Review and harmonization. Environ. Sci. Technol. 2013, 47, 6089–6097. [Google Scholar] [CrossRef] [PubMed]
- Delogu, M.; Zanchi, L.; Dattilo, C.A.; Pierini, M. Innovative composites and hybrid materials for electric vehicles lightweight design in a sustainability perspective. Mater. Today Commun. 2017, 13, 192–209. [Google Scholar] [CrossRef]
- Nunes, A.O.; Viana, L.R.; Guineheuc, P.M.; da Silva Moris, V.A.; de Paiva, J.M.F.; Barna, R.; Soudais, Y. Life cycle assessment of a steam thermolysis process to recover carbon fibers from carbon fiber-reinforced polymer waste. Int. J. Life Cycle Assess. 2018, 23, 1825–1838. [Google Scholar] [CrossRef]
- Forcellese, A.; Marconi, M.; Simoncini, M.; Vita, A. Life cycle impact assessment of different manufacturing technologies for automotive CFRP components. J. Clean. Prod. 2020, 271, 122677. [Google Scholar] [CrossRef]
- Schapery, R.A. Nonlinear viscoelastic and viscoplastic constitutive equations based on thermodynamics. Mech. Time-Depend. Mater. 1997, 1, 209–240. [Google Scholar] [CrossRef]
- Sakai, T.; Somiya, S. Analysis of creep behavior in thermoplastics based on visco-elastic theory. Mech. Time-Depend. Mater. 2011, 15, 293–308. [Google Scholar] [CrossRef]
- Sakai, T.; Hirai, Y.; Somiya, S. Estimating the creep behavior of glass-fiber-reinforced polyamide considering the effects of crystallinity and fiber volume fraction. Mech. Adv. Mater. Mod. Process. 2018, 4, 5. [Google Scholar] [CrossRef]
- Koyanagi, J.; Nakada, M.; Miyano, Y. Tensile strength at elevated temperature and its applicability as an accelerated testing methodology for unidirectional composites. Mech. Time-Depend. Mater. 2012, 16, 19–30. [Google Scholar] [CrossRef]
- Blanco, M.; Ramos, J.A.; Goyanes, S.; Rubiolo, G.; Salgueiro, W.; Somoza, A.; Mondragon, I. Intermolecular interactions on amine-cured epoxy matrices with different crosslink densities. Influence on the hole and specific volumes and the mechanical behavior. J. Polym. Sci. Part B Polym. Phys. 2009, 47, 1240–1252. [Google Scholar] [CrossRef]
- Ravikumar, H.B.; Ranganathaiah, C.; Kumaraswamy, G.N.; Siddaramaiah. Influence of free volume on the mechanical properties of Epoxy/poly (methylmethacrylate) blends. J. Mater. Sci. 2005, 40, 6523–6527. [Google Scholar] [CrossRef]
- Liu, L.M.; Fang, P.F.; Zhang, S.P.; Wang, S.J. Effect of epoxide equivalent on microstructure of epoxy/rectorite nanocomposite studied by positrons. Mater. Chem. Phys. 2005, 92, 361–365. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, B.; Zheng, Y.; Zhu, Y.; Wang, J.; Qi, N. Effect of surface decoration of CNTs on the interfacial interaction and microstructure of epoxy/MWNT nanocomposites. ChemPhysChem 2008, 9, 1046–1052. [Google Scholar] [CrossRef]
- Zhang, H.J.; Sellaiyan, S.; Sako, K.; Uedono, A.; Taniguchi, Y.; Hayashi, K. Effect of free-volume holes on static mechanical properties of epoxy resins studied by positron annihilation and PVT experiments. Polymer 2020, 190, 122225. [Google Scholar] [CrossRef]
- Shoji, N.; Sasaki, K.; Uedono, A.; Taniguchi, Y.; Hayashi, K.; Matsubara, N.; Kobayashi, T.; Yamashita, T. Effect of conversion on epoxy resin properties: Combined molecular dynamics simulation and experimental study. Polymer 2022, 254, 125041. [Google Scholar] [CrossRef]
- Bowman, A.L.; Mun, S.; Nouranian, S.; Huddlestona, B.D.; Gwaltneyc, S.R.; Baskesd, M.I.; Horstemeyerg, M.F. Free volume and internal structural evolution during creep in model amorphous polyethylene by molecular dynamics simulations. Polymer 2019, 170, 85–100. [Google Scholar] [CrossRef]
- Takase, N.; Koyanagi, J.; Mori, K.; Sakai, T. Molecular dynamics simulation for evaluating fracture entropy of a polymer material under various combined stress states. Materials 2021, 14, 1884. [Google Scholar] [CrossRef]
- Iwamoto, S.; Oya, Y.; Koyanagi, J. Evaluation of microscopic damage of PEEK polymers under cyclic loadings using molecular dynamics simulations. Polymers 2022, 14, 4955. [Google Scholar] [CrossRef] [PubMed]
- Makarewicz, C.; Safandowska, M.; Idczak, R.; Rozanski, A. Positron annihilation lifetime spectroscopic analysis of plastic deformation of high-density polyethylene. Macromolecules 2021, 54, 9649–9662. [Google Scholar] [CrossRef]
- Makarewicz, C.; Safandowska, M.; Idczak, R.; Rozanski, A. Plastic deformation of polypropylene studied by positron annihilation lifetime spectroscopy. Macromolecules 2022, 55, 10062–10076. [Google Scholar] [CrossRef]
- Huang, D.; Dong, Y.; Guo, H.; Yang, E.; Chen, Y.; Luo, M.; Pan, Z.; Liu, J.; Li, L.; Zhang, H.; et al. Minute-scale evolution of free-volume holes in polyethylenes during the continuous stretching process observed by in situ positron annihilation lifetime experiments. Macromolecules 2023, 56, 4748–4759. [Google Scholar] [CrossRef]
- Uedono, A.; Sako, K.; Ueno, W.; Kimura, M. Free volumes introduced by fractures of CFRP probed using positron annihilation. Compos. Part A Appl. Sci. Manuf. 2019, 122, 54–58. [Google Scholar] [CrossRef]
- Naderi, M.; Amiri, M.; Khonsari, M.M. On the thermodynamic entropy of fatigue fracture. Proc. R. Soc. A Math. Phys. Eng. Sci. 2010, 466, 423–438. [Google Scholar] [CrossRef]
- Nourian-Avval, A.; Khonsari, M.M. A new model for fatigue life prediction under multiaxial loadings based on energy dissipation. Int. J. Fatigue 2021, 151, 106255. [Google Scholar] [CrossRef]
- Feng, E.S.; Wang, X.G.; Jiang, C. A new multiaxial fatigue model for life prediction based on energy dissipation evaluation. Int. J. Fatigue 2019, 122, 1–8. [Google Scholar] [CrossRef]
- Mehdizadeh, M.; Khonsari, M.M. On the application of fracture fatigue entropy to multiaxial loading. Int. J. Fatigue 2021, 150, 106321. [Google Scholar] [CrossRef]
- Karimian, S.F.; Bruck, H.A.; Modarres, M. Thermodynamic entropy to detect fatigue crack initiation using digital image correlation, and effect of overload spectrums. Int. J. Fatigue 2019, 129, 105256. [Google Scholar] [CrossRef]
- Mahmoudi, A.; Khosravani, M.R.; Khonsari, M.M.; Reinicke, T. On the evaluation of entropy threshold for debonding during crack propagation using DIC technique. Eng. Fract. Mech. 2023, 288, 109361. [Google Scholar] [CrossRef]
- Liakat, M.; Khonsari, M.M. On the anelasticity and fatigue fracture entropy in high-cycle metal fatigue. Mater. Des. 2015, 82, 18–27. [Google Scholar] [CrossRef]
- Hajshirmohammadi, B.; Khonsari, M.M. On the entropy of fatigue crack propagation. Int. J. Fatigue 2020, 133, 105413. [Google Scholar] [CrossRef]
- Liakat, M.; Khonsari, M.M. Rapid estimation of fatigue entropy and toughness in metals. Mater. Des. 2014, 62, 149–157. [Google Scholar] [CrossRef]
- Nourian-Avval, A.; Khonsari, M.M. Rapid prediction of fatigue life based on thermodynamic entropy generation. Int. J. Fatigue 2021, 145, 106105. [Google Scholar] [CrossRef]
- Sakai, T.; Takase, N.; Oya, Y.; Koyanagi, J. A possibility for quantitative detection of mechanically-induced invisible damage by thermal property measurement via entropy generation for a polymer material. Materials 2022, 15, 737. [Google Scholar] [CrossRef] [PubMed]
- Kudo, N.; Fujita, R.; Oya, Y.; Sakai, T.; Nagano, H.; Koyanagi, J. Identification of invisible fatigue damage of thermosetting epoxy resin by non-destructive thermal measurement using entropy generation. Adv. Compos. Mater. 2024, 33, 233–249. [Google Scholar] [CrossRef]
- Sakai, T.; Oya, Y.; Koyanagi, J. Evaluation of volumetric strain on polyamide 6 by thermodynamic entropy generation. Exp. Mech. 2024, 64, 105–111. [Google Scholar] [CrossRef]
- Sato, M.; Hasegawa, K.; Koyanagi, J.; Higuchi, R.; Ishida, Y. Residual strength prediction for unidirectional CFRP using a nonlinear viscoelastic constitutive equation considering entropy damage. Compos. Part A Appl. Sci. Manuf. 2021, 141, 106178. [Google Scholar] [CrossRef]
- Huang, J.; Li, C.; Liu, W. Investigation of internal friction and fracture fatigue entropy of CFRP laminates with various stacking sequences subjected to fatigue loading. Thin-Walled Struct. 2020, 155, 106978. [Google Scholar] [CrossRef]
- Huang, J.; Yang, H.; Liu, W.; Zhang, K.; Huang, A. Confidence level and reliability analysis of the fatigue life of CFRP laminates predicted based on fracture fatigue entropy. Int. J. Fatigue 2022, 156, 106659. [Google Scholar] [CrossRef]
- Eldrup, M.; Lightbody, D.; Sherwood, J.N. The temperature dependence of positron lifetimes in solid pivalic acid. Chem. Phys. 1981, 63, 51–58. [Google Scholar] [CrossRef]
- Li, C.; Strachan, A. Free volume evolution in the process of epoxy curing and its effect on mechanical properties. Polymer 2016, 97, 456–464. [Google Scholar] [CrossRef]
- Uematsu, Y.; Kakiuchi, T.; Nishizawa, H.; Nakai, A.; Ohtani, A.; Uesugi, N.; Hattori, K. Non-destructive fatigue damage detection of carbon fiber-reinforced thermoplastics using positron annihilation method. Fatigue Fract. Eng. Mater. Struct. 2022, 45, 2784–2793. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inoue, T.; Oya, Y.; Koyanagi, J.; Sakai, T. Change in Thermodynamic Entropy and Free Volume of Epoxy Resin During Tensile Deformation. Polymers 2025, 17, 477. https://doi.org/10.3390/polym17040477
Inoue T, Oya Y, Koyanagi J, Sakai T. Change in Thermodynamic Entropy and Free Volume of Epoxy Resin During Tensile Deformation. Polymers. 2025; 17(4):477. https://doi.org/10.3390/polym17040477
Chicago/Turabian StyleInoue, Takuma, Yutaka Oya, Jun Koyanagi, and Takenobu Sakai. 2025. "Change in Thermodynamic Entropy and Free Volume of Epoxy Resin During Tensile Deformation" Polymers 17, no. 4: 477. https://doi.org/10.3390/polym17040477
APA StyleInoue, T., Oya, Y., Koyanagi, J., & Sakai, T. (2025). Change in Thermodynamic Entropy and Free Volume of Epoxy Resin During Tensile Deformation. Polymers, 17(4), 477. https://doi.org/10.3390/polym17040477





