Characterization and Rheological Properties of a New Exopolysaccharide Overproduced by Rhizobium sp. L01
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation and Identification of Strain L01
2.2. Production and Preparation of REPS-L01
2.3. Extraction and Purification of REPS-L01
2.4. Analysis of REPS-L01’s Physical Properties
2.4.1. Chemical Composition
2.4.2. Fourier-Transform Infrared (FTIR) Spectroscopic Analysis
2.4.3. Molecular Weight (Mw)
2.4.4. Monosaccharide Composition Analysis
2.4.5. Determination of Substituent Content
2.5. Thermal Gravimetric Analysis (TGA-DTG)
2.6. Rheological Properties
2.7. Viscosity Tests
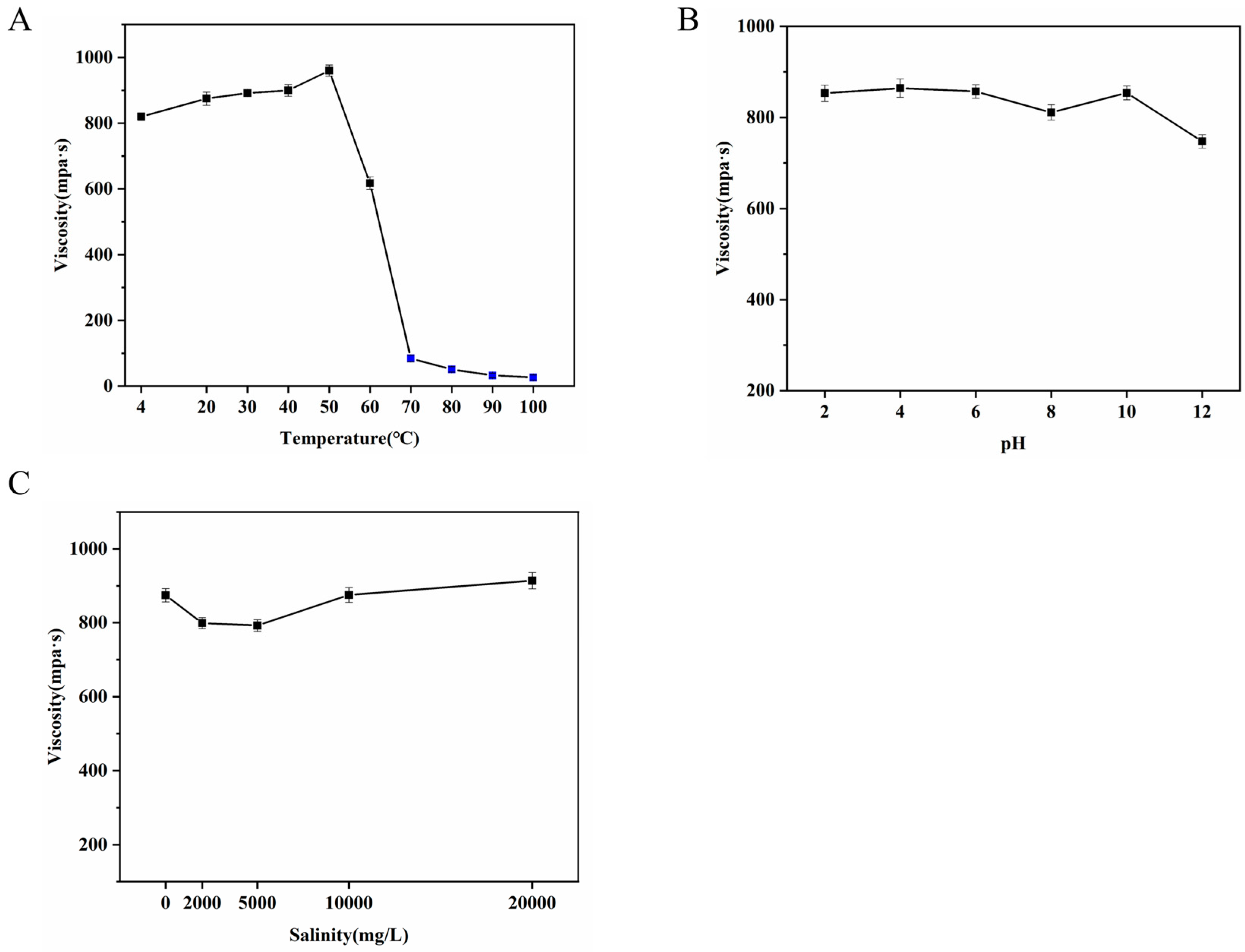
2.7.1. Effects of Temperature on Apparent Viscosity
2.7.2. Effects of pH on Apparent Viscosity
2.7.3. Effects of Salinity on Apparent Viscosity
2.8. Emulsification Index (E24)
2.9. Scanning Electron Microscopy (SEM) Studies
3. Results and Discussion
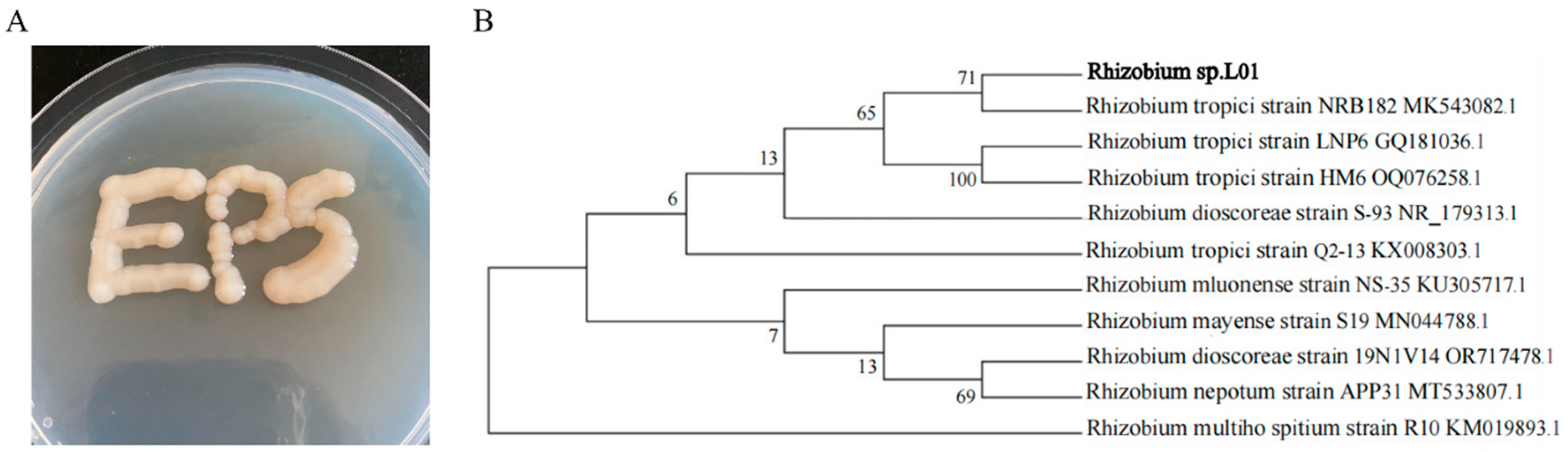
3.1. Identification of Rhizobium sp. L01
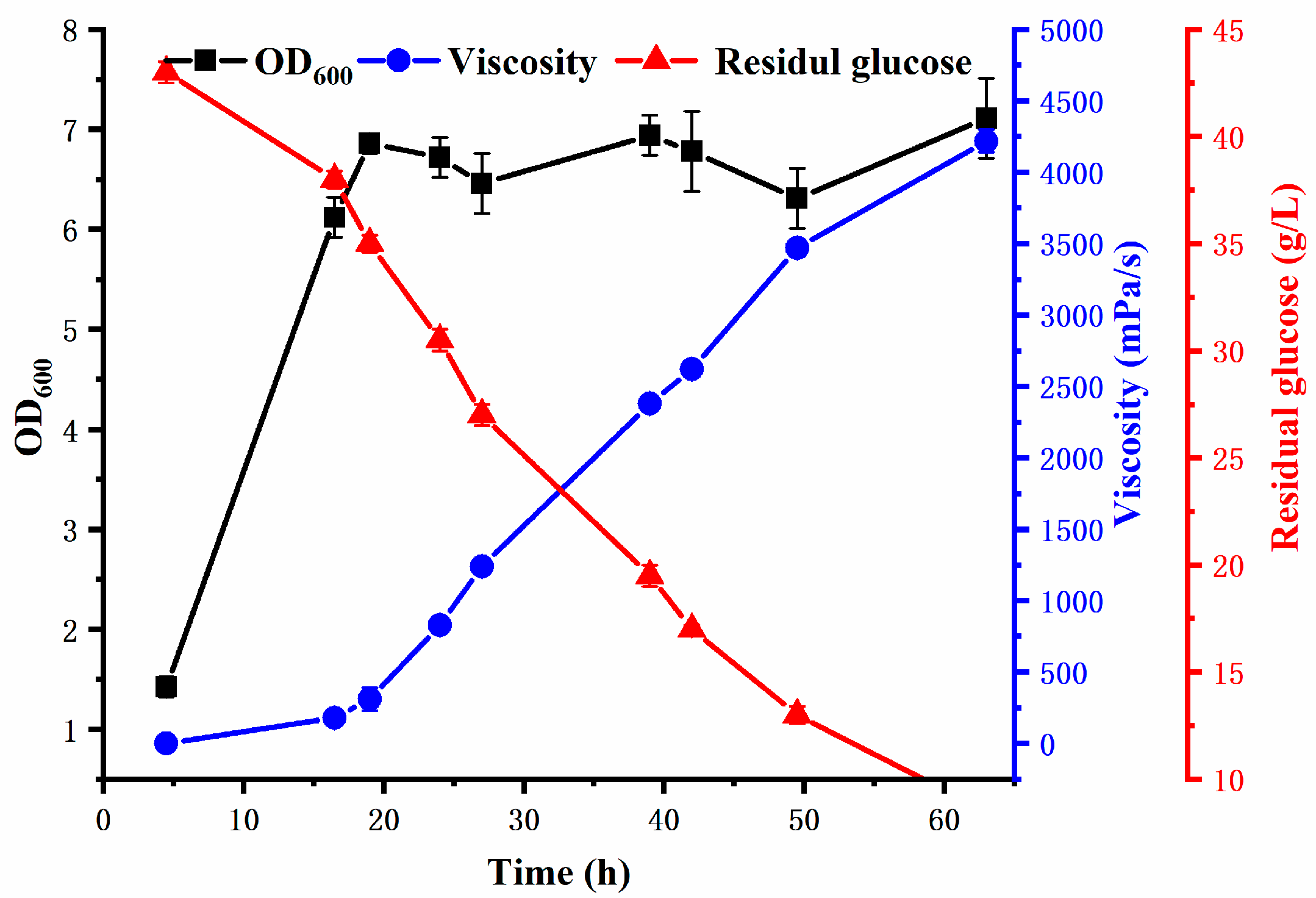
3.2. Production of REPS-L01
3.3. Determination of Monosaccharide Composition and Substituent Groups
| Strains | Monomer Composition (%) | Substituent | Yield (g/L) | Reference | |||||
|---|---|---|---|---|---|---|---|---|---|
| Glc | Gal | GlcA | GalA | Man | Rha | ||||
| Rhizobium sp. L01 | 74.7 | 25.3 | nd | nd | nd | nd | Succinate, pyruvate, and acetate | 22.8 | This work |
| Rhizobium tropici SEMIA 4077 | 59.59 | 31.29 | 4.58 | 0.08 | 0.74 | 3.72 | Acetate, carboxy | 7.45 | [8] |
| Rhizobium tropici SEMIA 4080 | 55.48 | 32.47 | 8.6 | tr | 0.86 | 2.58 | Succinate, pyruvate, and acetate | 2.52 | [30] |
| Rhizobium tropici MUTZC3 | 53.53 | 40.42 | tr | 2.60 | 0.74 | 2.60 | Succinate, pyruvate, and acetate | 4.39 | [30] |
| Rhizobium tropici LBMP-C01 | 314.7 μg/mL | 30.5 μg/mL | - | 1.0 μg/mL | 2.0 μg/mL | 2.0 μg/mL | Hydroxyl and carboxyl | 3.97 | [31] |
| Rhizobium tropici SRA1 | In a molar ratio of 3:1 | - | - | - | - | Hydroxyl, carboxyl, and methoxyl | 0.55 | [32] | |
| Rhizobium viscosum CECT908 | 35.0 | 43.7 | 17.1 | 2.0 | - | O-acetyl | 6.1 | [33] | |
| Rhizobium pusense ZB01 | 100 | - | - | - | - | - | O-acetyl | 3.18 | [34] |
| Rhizobium pusense KM7 | 85.3 | 12.2 | - | - | - | - | Hydroxyl, carboxyl, and succinyl | 21 | [5] |
| Sinorhizobium meliloti SMC1 | In a molar ratio of 7:1 | - | - | - | - | Succinate, pyruvate, and acetate | 22.3 | [6] | |
| Rhizobium radiobacter ATCC 18052 N-11 | 85.59 | 12.92 | - | - | 0.94 | - | Succinate, pyruvate, and acetate | 32.5 | [4] |
3.4. Fourier-Transform Infrared (FTIR) Spectroscopic Analysis
3.5. Thermal Analysis
3.6. Rheological Properties of REPS-L01
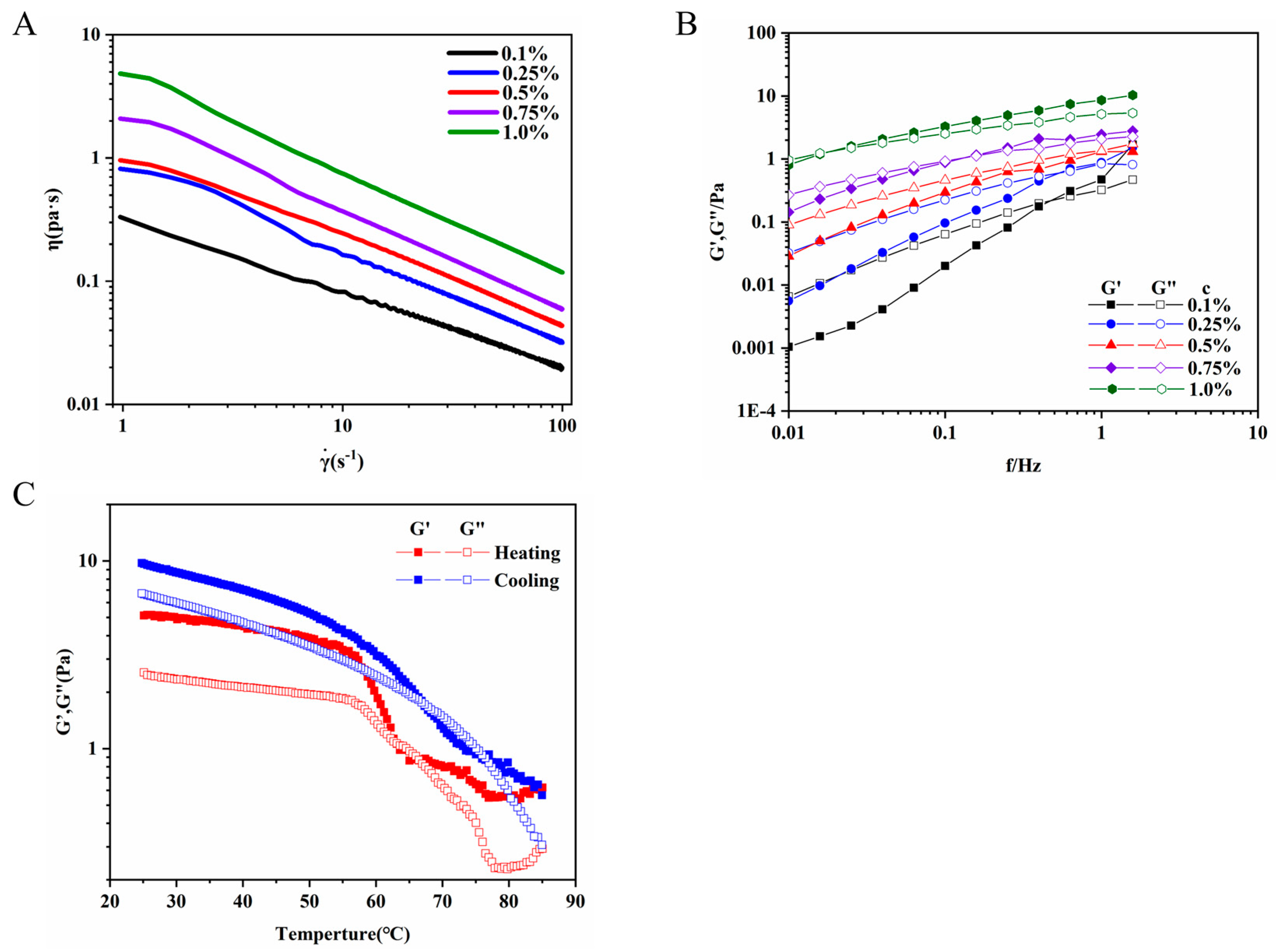
3.6.1. Steady Shear Measurement
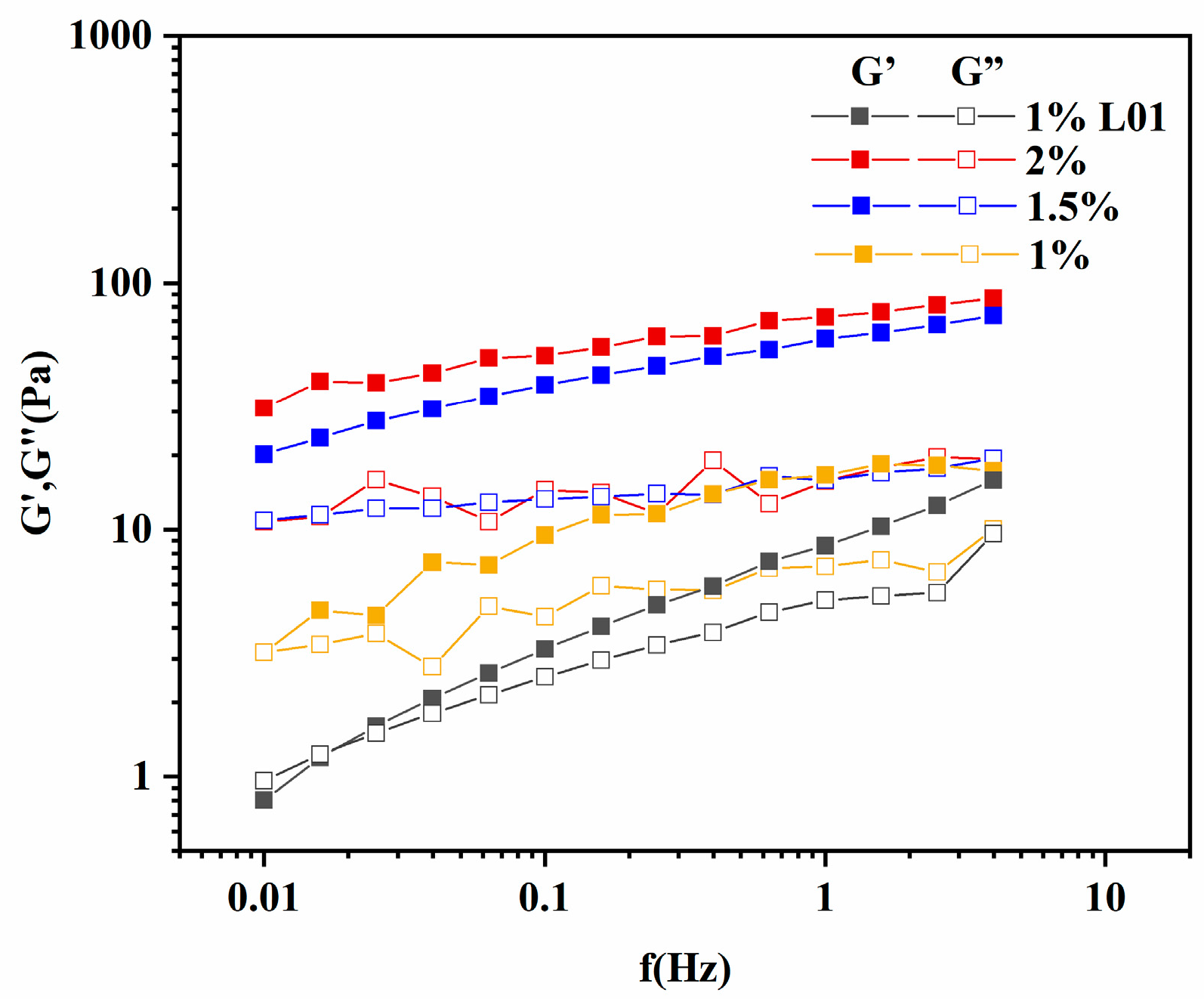
3.6.2. Dynamic Viscoelastic Behavior of REPS-L01
3.6.3. Temperature Sweeps
3.6.4. Stability Characteristics in Different Environments
3.7. The Emulsion Performance of REPS-L01
3.7.1. Emulsifying Activity Toward Different Hydrocarbons
3.7.2. Effect of REPS-L01 Concentration on Emulsion Activity
| Strain | REPS | Substrate | Concentration | E24 | Reference |
|---|---|---|---|---|---|
| Rhizobium sp. L01 | L01 | n-hexane | 2% | 100% | This work |
| 1% | 95% | ||||
| 0.5% | 75% | ||||
| Soybean oil | 1% | 100% | |||
| Paraffin liquid oil | 1% | 100% | |||
| Rhizobium radiobacter CAS | CAS | Soybean oil | 1% | 100% | [60] |
| 0.5% | 94% | ||||
| n-hexane | 1% | 100% | |||
| 0.5% | 65% | ||||
| Rhizobium radiobacter ATCC 18052 N-11 | SG-N | Soybean oil | 0.15% | 85.3% | [4] |
| Rhizobium tropici SEMIA 4080 | SEMIA 4080 | n-hexane | 5% | 34.67% | [12] |
| Paraffin liquid oil | 5% | 38.89% | |||
| Rhizobium tropici SEMIA 4077 | SEMIA 4077 | Soybean oil | 0.1% | 48.68% | [8] |
| 0.5% | 78.05% | ||||
| Diesel | 0.1% | 1.95% | |||
| 0.5% | 51.22% | ||||
| Rhizobium tropici SRA1 | SRA1 | n-hexane | 0.5% | 73.33% | [32] |
| Toluene | 0.5% | 76.66% |
3.7.3. Rheological Properties of Emulsion Gel
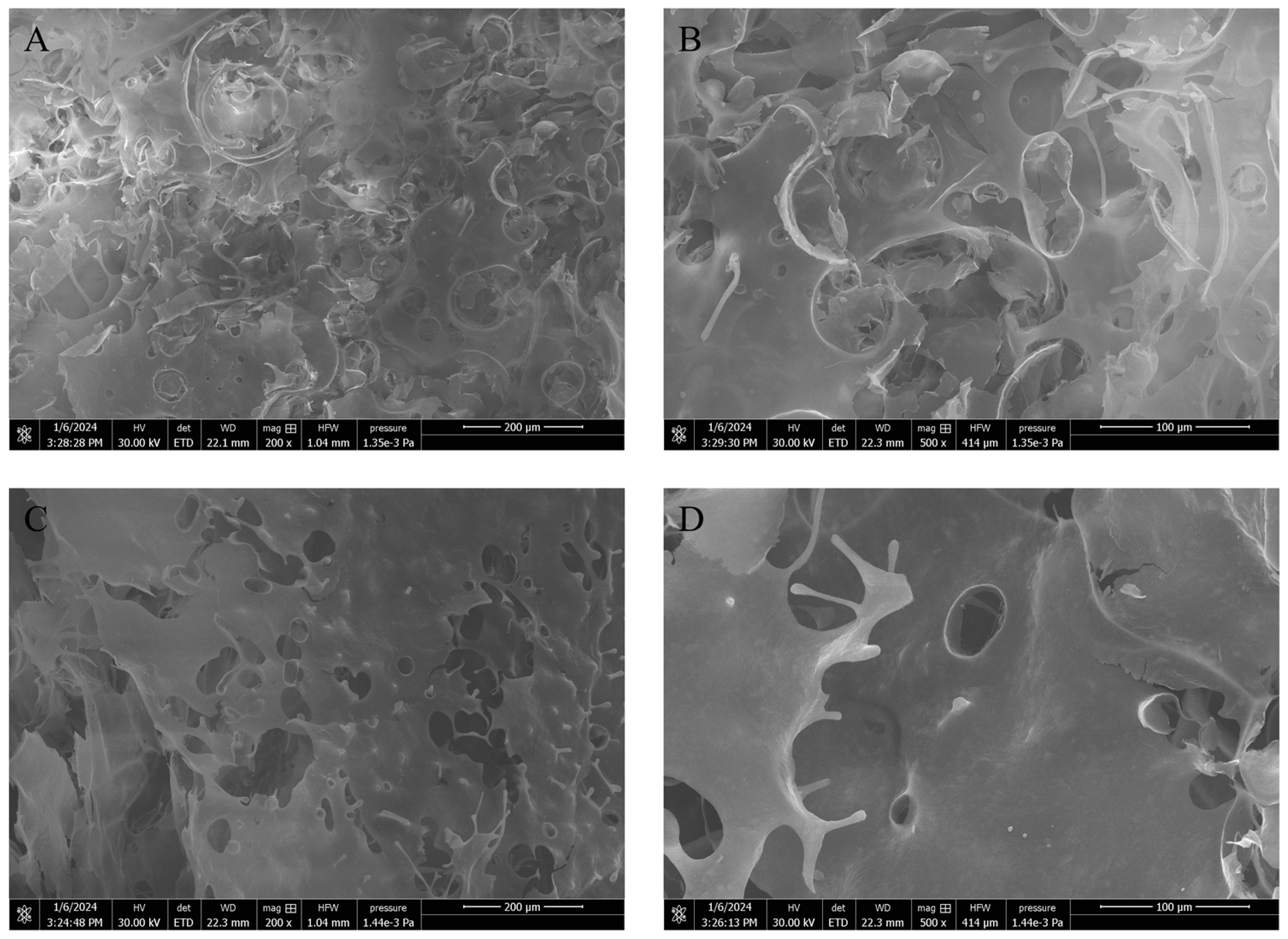
3.7.4. Micromorphology of Emulsion Gels
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Larson, S.L.; Ballard, J.H.; Runge, K.A.; Zhang, H.; Breland, B.R.; Nick, Z.H.; Weiss, C.A.; Han, F.X. Adsorption and characterization of exopolysaccharides from Rhizobium tropici on clay minerals. Carbohydr. Polym. Technol. Appl. 2023, 5, 100314. [Google Scholar] [CrossRef]
- Bhattacharyya, R.; Das, S.; Bhattacharya, R.; Chatterjee, M.; Dey, A. Rhizobial Exopolysaccharides: A Novel Biopolymer for Legume-Rhizobia Symbiosis and Environmental. In Microbes for Legume Improvement; Zaidi, A., Khan, M., Musarrat, J., Eds.; Springer: Cham, Switzerland, 2017; pp. 119–133. [Google Scholar]
- Lawson, C.J.; Sutherland, I.W. 9—Polysaccharides. In Economic Microbiology: Primary Products of Metabolism; Academic Press: Cambridge, MA, USA, 1978; pp. 327–392. [Google Scholar]
- Gao, H.; Li, Y.; Tian, J.; Huang, L.; Huang, D.; Zhang, W.; Xie, F.; Niu, Y.; Jin, M.; Jia, C.; et al. Characterization and rheological properties analysis of the succinoglycan produced by a high-yield mutant of Rhizobium radiobacter ATCC 19358. Int. J. Biol. Macromol. 2021, 166, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Mehta, K.; Shukla, A.; Saraf, M. Decoding the dynamics of enhanced succinoglycan biosynthesis from pretreated sugarcane molasses using Rhizobium pusense KM7: Kinetics and structural insights. Biocatal. Agric. Biotechnol. 2023, 53, 102884. [Google Scholar] [CrossRef]
- Kim, J.; Jeong, J.P.; Kim, Y.; Jung, S. Physicochemical and Rheological Properties of Succinoglycan Overproduced by Sinorhizobium meliloti 1021 Mutant. Polymers 2024, 16, 244. [Google Scholar] [CrossRef] [PubMed]
- Jeong, J.P.; Kim, Y.; Hu, Y.; Jung, S. Bacterial Succinoglycans: Structure, Physical Properties, and Applications. Polymers 2022, 14, 276. [Google Scholar] [CrossRef]
- Castellane, T.C.L.; Persona, M.R.; Campanharo, J.C.; Lemos, E.G.D. Production of exopolysaccharide from rhizobia with potential biotechnological and bioremediation applications. Int. J. Biol. Macromol. 2015, 74, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Asif, A.; Ali, M.; Qadir, M.; Karthikeyan, R.; Singh, Z.; Khagura, R.; Gioia, F.D.; Ahmed, Z.F.R. Enhancing crop resilience by harnessing the synergistic effects of biostimulants against abiotic stress. Front. Plant Sci. 2013, 14, 1276117. [Google Scholar] [CrossRef]
- Artenisa, C.R.; Claire, T.V.; Angelita, D.S.M.; Maria, V.S.S.; José, D.P.O.; Aurenivia, B.; Márcia, D.V.B.F. Rhizobium tropici exopolysaccharides as carriers improve the symbiosis of cowpea-Bradyrhizobium-Paenibacillus. Afr. J. Microbiol. Res. 2015, 9, 2037–2050. [Google Scholar] [CrossRef]
- Metuge, J.A.; Havugimana, E.; Rugandirababisha, J.; Senwo, Z.N.; Mutimawurugo, M.C. Evaluation of Rhizobium tropici-Derived Extracellular Polymeric Substances on Selected Soil Properties, Seed Germination, and Growth of Black-Eyed Peas (Vigna unguiculata). Agric. Sci. 2024, 15, 548–564. [Google Scholar]
- Castellane, T.C.L.; Campanharo, J.C.; Colnago, L.A.; Coutinho, I.D.; Lopes, É.M.; Lemos, M.V.F.; Lemos, E.G.D.M. Characterization of new exopolysaccharide production by Rhizobium tropici during growth on hydrocarbon substrate. Int. J. Biol. Macromol. 2017, 96, 361–369. [Google Scholar] [CrossRef]
- Wang, S.; Zhao, L.; Li, Q.; Liu, C.; Han, J.; Zhu, L.; Zhu, D.; He, Y.; Liu, H. Rheological properties and chain conformation of soy hull water-soluble polysaccharide fractions obtained by gradient alcohol precipitation. Food Hydrocoll. 2019, 91, 34–39. [Google Scholar] [CrossRef]
- Bahl, M.A.; Schultheis, E.; Hempel, D.C.; Nörtemann, B.; Franco-Lara, E. Recovery and purification of the exopolysaccharide PS-EDIV from Sphingomonas pituitosa DSM 13101. Carbohydr. Polym. 2010, 80, 1037–1041. [Google Scholar] [CrossRef]
- Dubois, M.K.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F.A. A Colorimetric Method for the Determination of Sugars. Nature 1951, 168, 167. [Google Scholar] [CrossRef]
- Cesaretti, M.; Luppi, E.; Maccari, F.; Volpi, N. A 96-well assay for uronic acid carbazole reaction. Carbohydr. Polym. 2003, 54, 59–61. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Chen, A.; Ge, X.; Li, S.; Xu, H. Chain conformation and physicochemical properties of polysaccharide (glucuronoxylomannan) from Fruit Bodies of Tremella fuciformis. Carbohydr. Polym. 2020, 245, 116354. [Google Scholar] [CrossRef] [PubMed]
- Kanyuck, K.M.; Norton-Welch, A.B.; Mills, T.B.; Norton, I.T. Structural characterization of interpenetrating network formation of high acyl gellan and maltodextrin gels. Food Hydrocoll. 2021, 112, 106295. [Google Scholar] [CrossRef]
- Wu, C.; Yuan, C.; Chen, S.; Liu, D.; Ye, X.; Hu, Y. The effect of curdlan on the rheological properties of restructured ribbonfish (Trichiurus spp.) meat gel. Food Chem. 2015, 179, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Evans, L.R.; Linker, A.; Impallomeni, G. Structure of succinoglycan from an infectious strain of Agrobacterium radiobacter. Int. J. Biol. Macromol. 2000, 27, 319–326. [Google Scholar] [CrossRef]
- Torres, C.A.; Marques, R.; Ferreira, A.R.; Antunes, S.; Grandfils, C.; Freitas, F.; Reis, M.A. Impact of glycerol and nitrogen concentration on Enterobacter A47 growth and exopolysaccharide production. Int. J. Biol. Macromol. 2014, 71, 81–86. [Google Scholar] [CrossRef] [PubMed]
- Kurt, G.; Kasgoz, A. Effects of molecular weight and molecular weight distribution on creep properties of polypropylene homopolymer. J. Appl. Polym. Sci. 2021, 138, 50722. [Google Scholar] [CrossRef]
- Heyraud, A.; Rinaudo, M.; Courtois, B. Comparative studies of extracellular polysaccharide elaborated by Rhizobium meliloti strain M5N1 in defined medium and in non-growing cell suspensions. Int. J. Biol. Macromol. 1986, 8, 85–88. [Google Scholar] [CrossRef]
- Cheng, R.; Wang, L.; Li, J.; Fu, R.; Wang, S.; Zhang, J. In vitro and in vivo anti-inflammatory activity of a succinoglycan Riclin from Agrobacterium sp. ZCC3656. J. Appl. Microbiol. 2019, 127, 1716–1726. [Google Scholar] [CrossRef]
- Kwon, C.; Lee, S.; Jung, S. Matrix-a©ssisted laser desorption/ionization time-of-flight mass spectrometric behavior of succinoglycan monomers, dimers, and trimers isolated from Sinorhizobium meliloti 1021. Carbohydr. Res. 2011, 346, 2308–2314. [Google Scholar] [CrossRef]
- O’Neill, M.A.; Darvill, A.G.; Albersheim, P. The degree of esterification and points of substitution by O-acetyl and O-(3-hydroxybutanoyl) groups in the acidic extracellular polysaccharides secreted by Rhizobium leguminosarum biovars viciae, trifolii, and phaseoli are not related to host range. J. Biol. Chem. 1991, 266, 9549–9555. [Google Scholar] [CrossRef]
- Simsek, S.; Mert, B.; Campanella, O.H.; Reuhs, B. Chemical and rheological properties of bacterial succinoglycan with distinct structural characteristics. Carbohydr. Polym. 2009, 76, 320–324. [Google Scholar] [CrossRef]
- Radchenkova, N.; Vassilev, S.; Panchev, I.; Anzelmo, G.; Tomova, I.; Nicolaus, B.; Kuncheva, M.; Petrov, K.; Kambourova, M. Production and Properties of Two Novel Exopolysaccharides Synthesized by a Thermophilic Bacterium Aeribacillus pallidus 418. Appl. Biochem. Biotechnol. 2013, 171, 31–43. [Google Scholar] [CrossRef] [PubMed]
- Castellane, T.C.L.; Lemos, M.V.F.; Lemos, E.G.D.M. Evaluation of the biotechnological potential of Rhizobium tropici strains for exopolysaccharide production. Carbohydr. Polym. 2014, 111, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Leonel, T.F.; Moretto, C.; Castellane, T.C.L.; da Costa, P.I.; Lemos, E.G.D.M. The Influence of Cooper and Chromium Ions on the Production of Exopolysaccharide and Polyhydroxybutyrate by Rhizobium tropici LBMP-C01. J. Polym. Environ. 2019, 27, 445–455. [Google Scholar] [CrossRef]
- Das, S.; Sen, I.K.; Kati, A.; Some, S.; Mandal, A.K.; Islam, S.S.; Bhattacharyya, R.; Mukhopadhyay, A. Flocculating, emulsification and metal sorption properties of a partial characterized novel exopolysaccharide produced by Rhizobium tropici SRA1 isolated from Psophocarpus tetragonolobus (L.) D.C. Int. Microbiol. 2019, 22, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Gudiña, E.J.; Couto, M.R.; Silva, S.P.; Coelho, E.; Coimbra, M.A.; Teixeira, J.A.; Rodrigues, L.R. Sustainable Exopolysaccharide Production by Rhizobium viscosum CECT908 Using Corn Steep Liquor and Sugarcane Molasses as Sole Substrates. Polymers 2014, 15, 20. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Zhang, Z.; Lyu, X.; Zhao, W. Structural characterization of a water-soluble and antimicrobial β-glucan secreted by Rhizobium pusense. J. Biosci. Bioeng. 2022, 134, 318–325. [Google Scholar] [CrossRef]
- Chouly, C.; Colquhoun, I.J.; Jodelet, A.; York, G.; Walker, G.C. NMR studies of succinoglycan repeating-unit octasaccharides from Rhizobium meliloti and Agrobacterium radiobacter. Int. J. Biol. Macromol. 1995, 17, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Zevenhuizen, L.P.T.M. Succinoglycan and galactoglucan. Carbohydr. Polym. 1997, 33, 139–144. [Google Scholar] [CrossRef]
- Park, S.; Shin, Y.; Jung, S. Structural, rheological properties and antioxidant activities analysis of the exopolysaccharide produced by Rhizobium leguminosarum bv. viciae VF39. Int. J. Biol. Macromol. 2014, 257, 128811. [Google Scholar] [CrossRef] [PubMed]
- Kazachenko, A.S.; Vasilieva, N.Y.; Borovkova, V.S.; Fetisova, O.Y.; Issaoui, N.; Malyar, Y.N.; Elsuf’ev, E.V.; Karacharov, A.A.; Skripnikov, A.M.; Miroshnikova, A.V.; et al. Food Xanthan Polysaccharide Sulfation Process with Sulfamic Acid. Foods 2021, 10, 2571. [Google Scholar] [CrossRef] [PubMed]
- Hajji, M.; Hamdi, M.; Sellimi, S.; Ksouda, G.; Laouer, H.; Li, S.; Nasri, M. Structural characterization, antioxidant and antibacterial activities of a novel polysaccharide from Periploca laevigata root barks. Carbohydr. Polym. 2019, 206, 380–388. [Google Scholar] [CrossRef]
- Savi, A.; Calegari, G.C.; Santos, V.A.Q.; Pereira, E.A.; Teixeira, S.D. Chemical characterization and antioxidant of polysaccharide extracted from Dioscorea bulbifera. J. King Saud Univ. Sci. 2020, 32, 636–642. [Google Scholar] [CrossRef]
- Choppe, E.; Puaud, F.; Nicolai, T.; Benyahia, L. Rheology of xanthan solutions as a function of temperature, concentration and ionic strength. Carbohydr. Polym. 2010, 82, 1228–1235. [Google Scholar] [CrossRef]
- Morris, E.R.; Cutler, A.N.; Ross-Murphy, S.B.; Rees, D.A.; Price, J. Concentration and shear rate dependence of viscosity in random coil polysaccharide solutions. Carbohydr. Polym. 1981, 1, 5–21. [Google Scholar] [CrossRef]
- Qu, W.; Yu, P. Intelligent Gel as a High-performance Plugging Agent in Deep Wells. Acta Geol. Sin. Engl. Ed. 2019, 93, 192. [Google Scholar]
- Mortazavi, S.A.; Smart, J.D. Factors influencing gel-strengthening at the mucoadhesive-mucus interface. J. Pharm. Pharmacol. 2011, 46, 86–90. [Google Scholar] [CrossRef] [PubMed]
- Ilyin, S.O. Structural Rheology in the Development and Study of Complex Polymer Materials. Polymers 2024, 16, 2458. [Google Scholar] [CrossRef]
- Tang, X.; Xing, J.; Yan, X.; Ye, C.; Zhang, L.; Zhang, Y.; Shu, B.; Mu, J.; Li, W.; Wang, J.; et al. Metallocene Polyolefins Reinforced by Low-Entanglement UHMWPE through Interfacial Entanglements. Adv. Polym. Technol. 2022, 18, 9344096. [Google Scholar] [CrossRef]
- Tong, L.; Kang, X.; Fang, Q.; Yang, W.; Cen, S.; Lou, Q.; Huang, T. Rheological properties and interactions of fish gelatin–κ-carrageenan polyelectrolyte hydrogels: The effects of salt. J. Texture Stud. 2022, 53, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Sousa, B.F.S.D.; Castellane, T.C.L.; Campanharo, J.C.; Lemos, E.G.D.M. Rhizobium spp. exopolysaccharides production and xanthan lyase use on its structural modification. Int. J. Biol. Macromol. 2019, 136, 424–435. [Google Scholar] [CrossRef] [PubMed]
- Fagioli, L.; Pavoni, L.; Logrippo, S.; Pelucchini, C.; Rampoldi, L.; Cespi, M.; Bonacucina, G.; Casettari, L. Linear Viscoelastic Properties of Selected Polysaccharide Gums as Function of Concentration, pH, and Temperature. J. Food Sci. 2019, 84, 65–72. [Google Scholar] [CrossRef]
- Ribeiro, V.A.; Burkert, C.A.V. Exopolysaccharides Produced by Rhizobium: Production, Composition and Rheological Properties. J. Polym. Biopolym. Phys. Chem. 2016, 4, 1–6. [Google Scholar]
- Cai, W.; Hu, T.; Huang, Q. A polysaccharide from Lignosus rhinocerotis sclerotia: Self-healing properties and the effect of temperature on its rheological behavior. Carbohydr. Polym. 2021, 267, 118223. [Google Scholar] [CrossRef] [PubMed]
- Funami, T. In vivo and rheological approaches for characterizing food oral processing and usefulness of polysaccharides as texture modifiers—A review. Food Hydrocoll. 2017, 68, 2–14. [Google Scholar] [CrossRef]
- Liu, W.; Wang, R.; Li, J.; Xiao, W.; Rong, L.; Yang, J.; Wen, H.; Xie, J. Effects of different hydrocolloids on gelatinization and gels structure of chestnut starch. Food Hydrocoll. 2021, 120, 106925. [Google Scholar] [CrossRef]
- Sow, L.C.; Tan, S.J.; Yang, H. Rheological properties and structure modification in liquid and gel of tilapia skin gelatin by the addition of low acyl gellan. Food Hydrocoll. 2019, 90, 9–18. [Google Scholar] [CrossRef]
- Ma, S.; Zhu, P.; Wang, M. Effects of konjac glucomannan on pasting and rheological properties of corn starch. Food Hydrocoll. 2019, 89, 234–240. [Google Scholar] [CrossRef]
- Lopes, E.M.; Castellane, T.C.L.; Moretto, C.; Lemos, E.G.; Souza, J.A.M. Emulsification Properties of Bioemulsifiers Produced by Wild-Type and Mutant Bradyrhizobium elkanii Strains. J. Bioremediat. Biodegrad. 2014, 5, 1–6. [Google Scholar]
- Toren, A.; Navon-Venezia, S.; Ron, E.Z.; Rosenberg, E. Emulsifying activities of purified Alasan proteins from Acinetobacter radioresistens KA53. Appl. Environ. Microbiol. 2001, 67, 1102–1106. [Google Scholar] [CrossRef]
- Bach, H.; Gutnick, D.L. Novel polysaccharide–protein-based amphipathic formulations. Appl. Microbiol. Biotechno. 2006, 71, 34–38. [Google Scholar] [CrossRef]
- Freitas, F.; Alves, V.D.; Carvalheira, M.; Costa, N.; Oliveira, R.; Reis, M.A.M. Emulsifying behaviour and rheological properties of the extracellular polysaccharide produced by Pseudomonas oleovorans grown on glycerol byproduct. Carbohydr. Polym. 2009, 78, 549–556. [Google Scholar] [CrossRef]
- Kavitake, D.; Marchawala, F.Z.; Delattre, C.; Shetty, P.H.; Pathak, H.; Andhare, P. Biotechnological potential of exopolysaccharide as a bioemulsifier produced by Rhizobium radiobacter CAS isolated from curd. Bioact. Carbohydr. Diet. Fibre 2019, 20, 100202. [Google Scholar] [CrossRef]
- Hu, Y.; Jeong, D.; Kim, Y.; Kim, S.; Jung, S. Preparation of Succinoglycan Hydrogel Coordinated with Fe3+ Ions for Controlled Drug Delivery. Polymers 2020, 12, 977. [Google Scholar] [CrossRef] [PubMed]
- Rendleman, J.A. Metal-polysaccharide complexes—Part II. Food Chem. 1978, 3, 127–162. [Google Scholar] [CrossRef]
- Boutebba, A.; Milas, M.; Rinaudo, M. On the interchain associations in aqueous solutions of a succinoglycan polysaccharide. Int. J. Biol. Macromol. 1999, 24, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Tang, Q.; Huang, G. Improving method, properties and application of polysaccharide as emulsifier. Food Chem. 2021, 376, 131937. [Google Scholar] [CrossRef] [PubMed]


| Composition (mg/L) | Total Salinity (mg/L) | ||
|---|---|---|---|
| NaCl | CaCl2 | MgCl2 | |
| 1516.6 | 180.1 | 303.3 | 2000 |
| 3791.5 | 450.2 | 758.3 | 5000 |
| 7582.9 | 900.5 | 1516.6 | 10,000 |
| 15,165.8 | 1801.0 | 3033.2 | 20,000 |
| Parameters | REPS-L01 |
|---|---|
| Total carbohydrate (%) | 83.03 |
| Uronic acid (%) | 0 |
| Protein (%) | 0.68 |
| Molar mass moments (g/mol) | |
| Mw a | 3.04 × 106 |
| Mn b | 2.63 × 106 |
| Dispersity index | 1.155 |
| Monosaccharide content (molar ratio, %) | |
| Glucose | 74.7 |
| Galactose | 25.3 |
| Substituent content (mg/g) | |
| Pyruvic acid | 66.1 |
| Succinic acid | 10.2 |
| Acetic acid | 28.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, H.; Wen, Y.; Li, Z.; Wang, B.; Li, S. Characterization and Rheological Properties of a New Exopolysaccharide Overproduced by Rhizobium sp. L01. Polymers 2025, 17, 592. https://doi.org/10.3390/polym17050592
Huang H, Wen Y, Li Z, Wang B, Li S. Characterization and Rheological Properties of a New Exopolysaccharide Overproduced by Rhizobium sp. L01. Polymers. 2025; 17(5):592. https://doi.org/10.3390/polym17050592
Chicago/Turabian StyleHuang, Haolin, Yaolan Wen, Zhuangzhuang Li, Biao Wang, and Shuang Li. 2025. "Characterization and Rheological Properties of a New Exopolysaccharide Overproduced by Rhizobium sp. L01" Polymers 17, no. 5: 592. https://doi.org/10.3390/polym17050592
APA StyleHuang, H., Wen, Y., Li, Z., Wang, B., & Li, S. (2025). Characterization and Rheological Properties of a New Exopolysaccharide Overproduced by Rhizobium sp. L01. Polymers, 17(5), 592. https://doi.org/10.3390/polym17050592






