One-Pot Synthesis of Amphiphilic Linear and Hyperbranched Polyelectrolytes and Their Stimuli-Responsive Self-Assembly in Aqueous Solutions
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Amphiphilic Copolymers
2.3. Colloidal Dispersion Preparation
2.4. Methods
2.4.1. Size Exclusion Chromatography
2.4.2. Proton Nuclear Magnetic Resonance Spectroscopy
2.4.3. Fourier Transform Infrared Spectroscopy
2.4.4. Dynamic Light Scattering
2.4.5. Electrophoretic Light Scattering
2.4.6. UV–Vis Spectroscopy
2.4.7. Fluorescence Spectroscopy
2.4.8. Transmission Electron Microscopy
3. Results and Discussion
3.1. Size Exclusion Chromatography Analysis
3.2. Proton Nuclear Magnetic Resonance Spectroscopy Analysis
3.3. Fourier Transform Infrared Spectroscopy Analysis
3.4. Zeta Potential Analysis
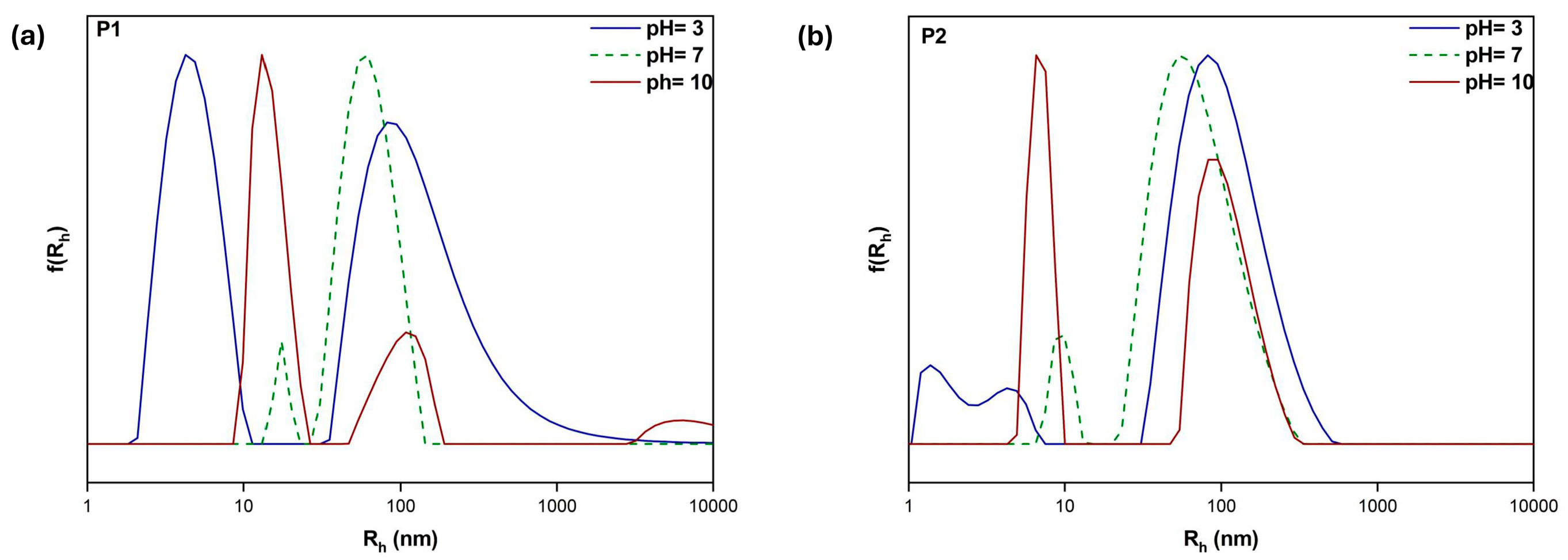
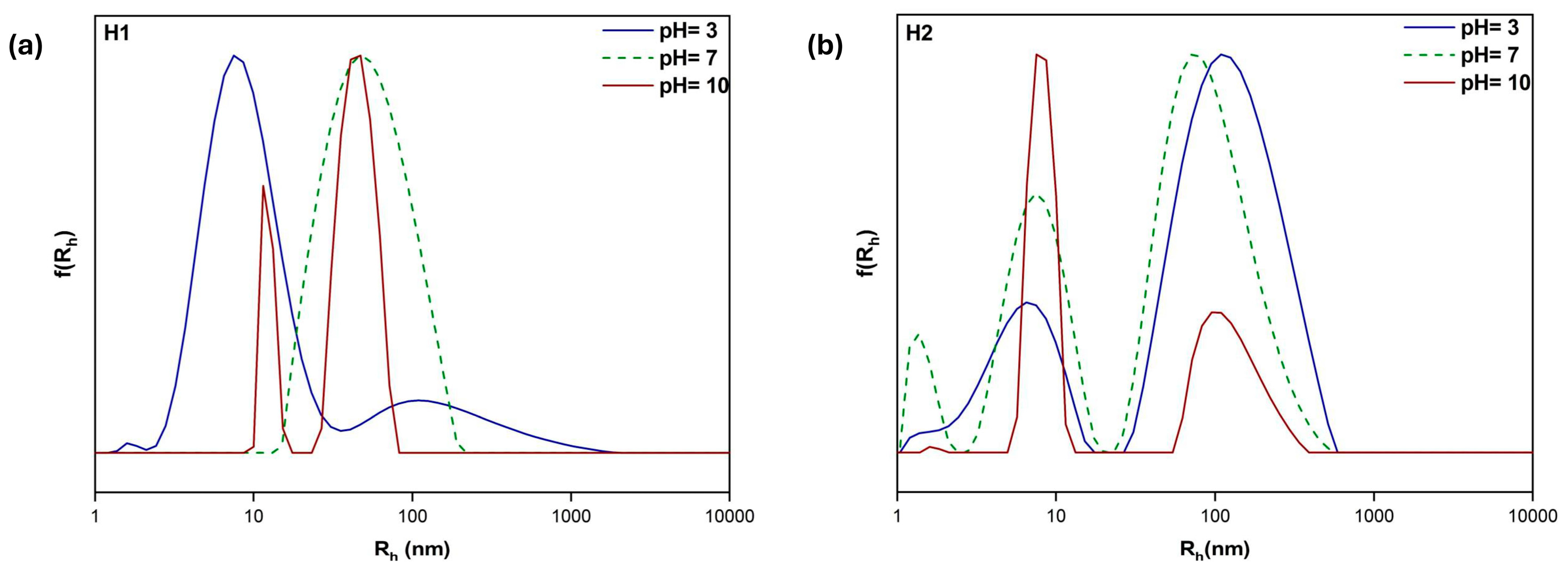
3.5. Response to Solution pH Changes
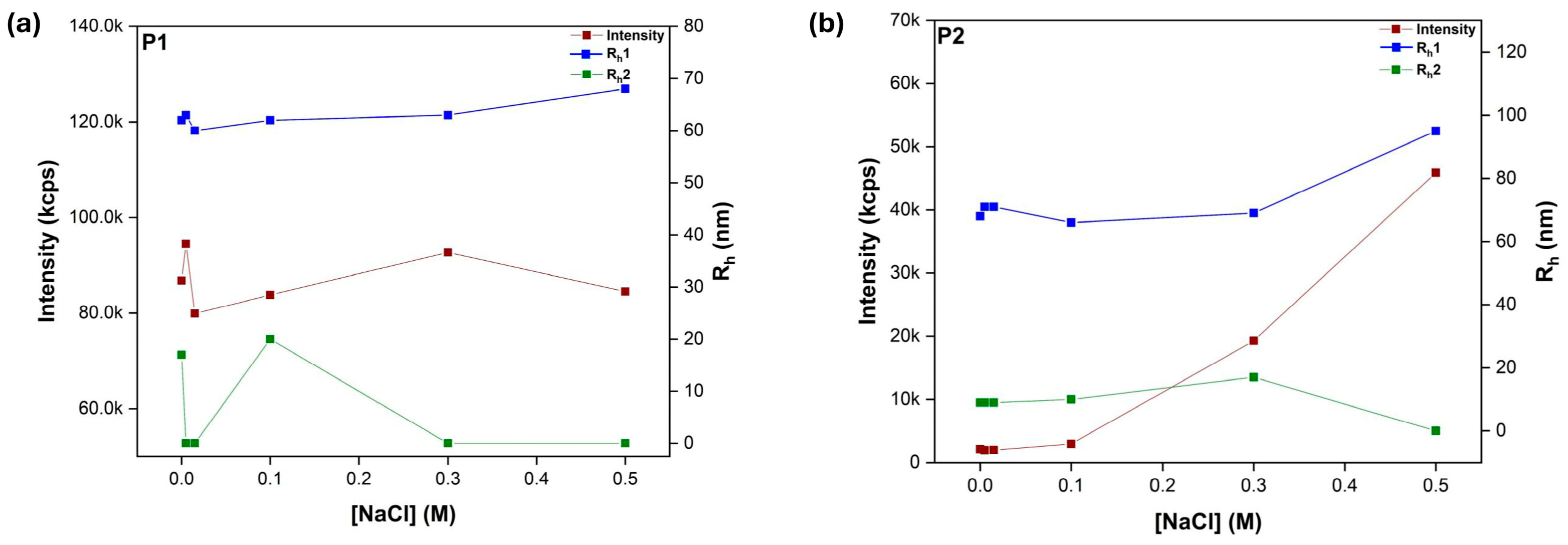
3.6. Behavior in Salt Solutions
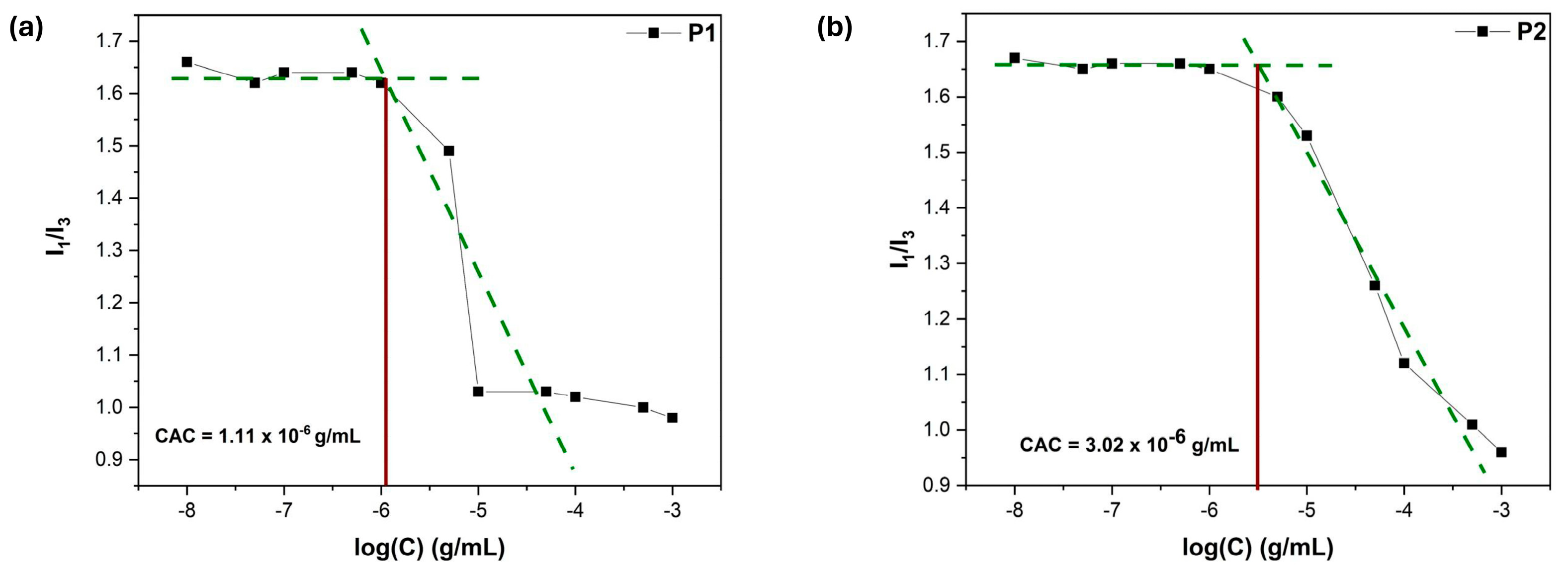
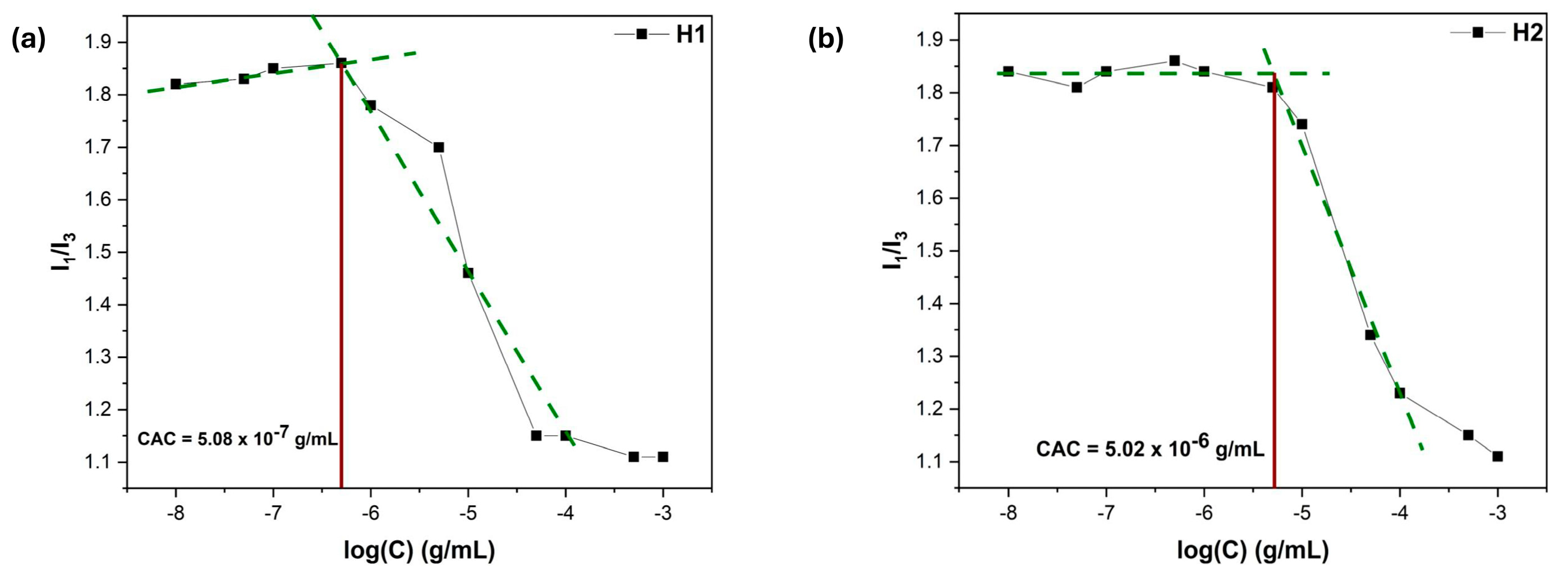
3.7. Microenvironment Polarity Through Pyrene Fluorescence
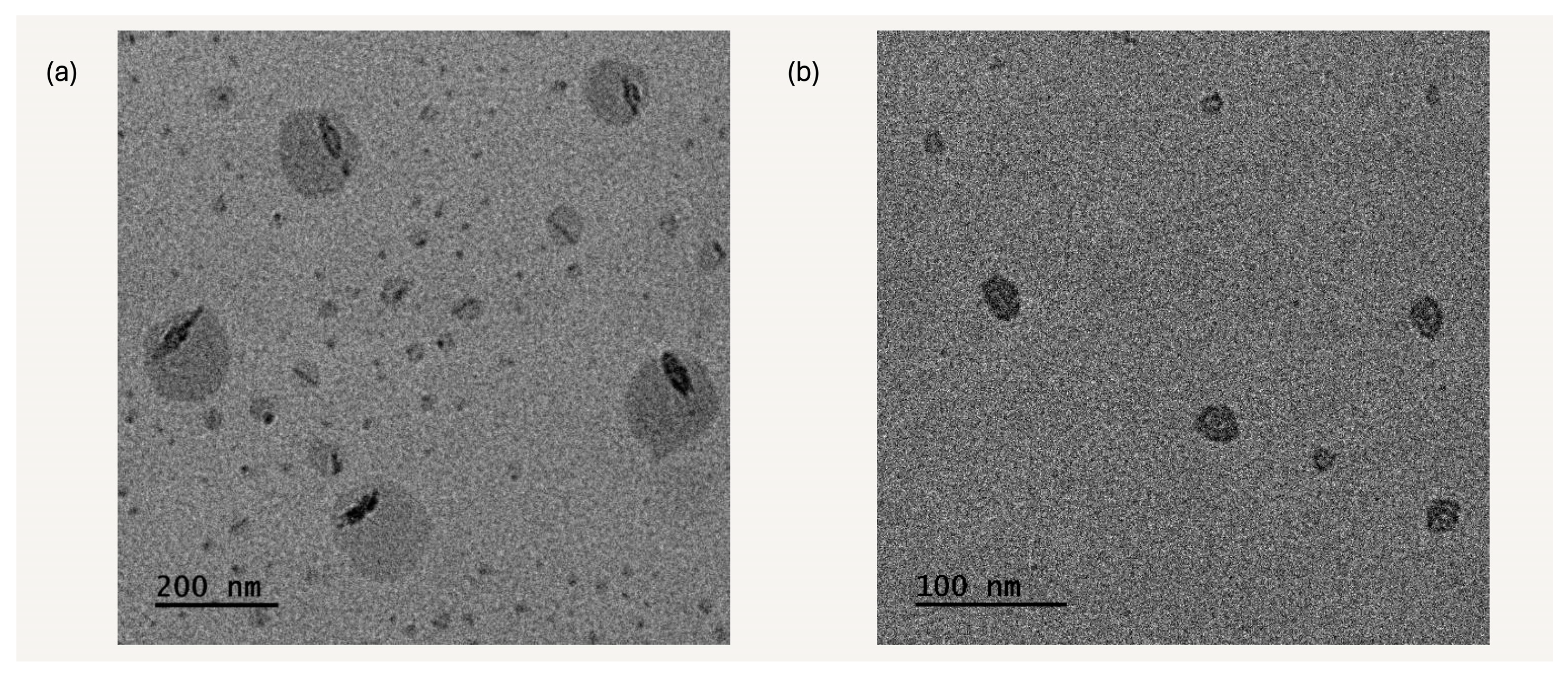
3.8. Transmission Electron Microscopy Imaging
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Li, J.C.; Reddy, V.S.; Jayathilaka, W.A.D.M.; Chinnappan, A.; Ramakrishna, S.; Ghosh, R. Intelligent Polymers, Fibers and Applications. Polymers 2021, 13, 1427. [Google Scholar] [CrossRef] [PubMed]
- Mu, M.; Ebara, M. Smart Polymers. In Polymer Science and Nanotechnology: Fundamentals and Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 257–279. [Google Scholar] [CrossRef]
- Mane, S.R.; Sathyan, A.; Shunmugam, R. Biomedical Applications of PH-Responsive Amphiphilic Polymer Nanoassemblies. ACS Appl. Nano Mater. 2020, 3, 2104–21117. [Google Scholar] [CrossRef]
- Perin, F.; Motta, A.; Maniglio, D. Amphiphilic Copolymers in Biomedical Applications: Synthesis Routes and Property Control. Mater. Sci. Eng. C 2021, 123, 111952. [Google Scholar] [CrossRef]
- Zhou, M.; Wen, L.; Wang, C.; Lei, Q.; Li, Y.; Yi, X. Recent Advances in Stimuli-Sensitive Amphiphilic Polymer-Paclitaxel Pro-drugs. Front. Bioeng. Biotechnol. 2022, 10, 875034. [Google Scholar] [CrossRef]
- Kashapov, R.; Gaynanova, G.; Gabdrakhmanov, D.; Kuznetsov, D.; Pavlov, R.; Petrov, K.; Zakharova, L.; Sinyashin, O. Self-Assembly of Amphiphilic Compounds as a Versatile Tool for Construction of Nanoscale Drug Carriers. Int. J. Mol. Sci. 2020, 21, 6961. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Liu, W.; Zeng, G.; Liu, Y.J. Microporous PDMAEMA-Based Stimuli-Responsive Hydrogel and Its Application in Drug Release. J. Appl. Polym. Sci. 2017, 134, 45326. [Google Scholar] [CrossRef]
- Saraiva, G.K.V.; de Souza, V.V.; de Oliveira, L.C.; Noronha, M.L.C.; Masini, J.C.; Chaimovich, H.; Salinas, R.K.; Florenzano, F.H.; Cuccovia, I.M. Characterization of PMMA-b-PDMAEMA Aggregates in Aqueous Solutions. Colloid. Polym. Sci. 2019, 297, 557–569. [Google Scholar] [CrossRef]
- Huang, Y.; Yong, P.; Chen, Y.; Gao, Y.; Xu, W.; Lv, Y.; Yang, L.; Reis, R.L.; Pirraco, R.P.; Chen, J. Micellization and Gelatinization in Aqueous Media of PH- and Thermo-Responsive Amphiphilic ABC (PMMA82-b-PDMAEMA150-b-PNIPAM65) Triblock Copolymer Synthesized by Consecutive RAFT Polymerization. RSC Adv. 2017, 7, 28711–28722. [Google Scholar] [CrossRef]
- Dong, Z.; Wei, H.; Mao, J.; Wang, D.; Yang, M.; Bo, S.; Ji, X. Synthesis and Responsive Behavior of Poly(N,N-Dimethylami-noethyl Methacrylate) Brushes Grafted on Silica Nanoparticles and Their Quaternized Derivatives. Polymer 2012, 53, 2074–2084. [Google Scholar] [CrossRef]
- Kumar, K.; Mogha, N.K.; Yadav, R.; Venkatesu, P. Insulin-Induced Conformational Transition of Fluorescent Copolymers: A Perspective of Self-Assembly between Protein and Micellar Solutions of Smart Copolymers. Phys. Chem. Chem. Phys. 2020, 22, 9573–9586. [Google Scholar] [CrossRef]
- Cai, Y.; Ding, P.; Ni, J.; Zhou, L.; Ahmad, A.; Guo, X.; Cohen Stuart, M.A.; Wang, J. Regulated Polyelectrolyte Nanogels for Enzyme Encapsulation and Activation. Biomacromolecules 2021, 22, 4748–4757. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Zhang, Y.; Maji, S.; Greiner, A. PDMAEMA Based Gene Delivery Materials. Mater. Today 2012, 15, 388–393. [Google Scholar] [CrossRef]
- Qiu, X.; Lu, L.; Wang, J.; Tang, G.; Song, G. Fabrication, Thermal Properties and Thermal Stabilities of Microencapsulated n-Alkane with Poly(Lauryl Methacrylate) as Shell. Thermochim. Acta 2015, 620, 10–17. [Google Scholar] [CrossRef]
- Duc, N.T.; Ha, P.T.T.; Van Khoi, N.; Huy, N.Q.; Mien, N.T.; Hoan, D.C.; Chau, C.N.; Tung, N.T. Oil Absorbent Based on Lauryl Methacrylate Grafting onto Cellulose of Water Hyacinth (Eichhornia crassipes). Vietnam. J. Chem. 2024, 62, 169–178. [Google Scholar] [CrossRef]
- Suraj Belgaonkar, M.; Kandasubramanian, B. Hyperbranched Polymer-Based Nanocomposites: Synthesis, Progress, and Appli-cations. Eur. Polym. J. 2021, 147, 110301. [Google Scholar] [CrossRef]
- Perrier, S. 50th Anniversary Perspective: RAFT Polymerization—A User Guide. Macromolecules 2017, 50, 7433–7447. [Google Scholar] [CrossRef]
- Tian, X.; Ding, J.; Zhang, B.; Qiu, F.; Zhuang, X.; Chen, Y. Recent Advances in RAFT Polymerization: Novel Initiation Mecha-nisms and Optoelectronic Applications. Polymers 2018, 10, 318. [Google Scholar] [CrossRef]
- Chrysostomou, V.; Pispas, S. Stimuli-responsive amphiphilic PDMAEMA-b-PLMA copolymers and their cationic and zwitterionic analogs. J. Polym. Sci. Part A Polym. Chem. 2018, 56, 598–610. [Google Scholar] [CrossRef]
- Wang, J.; Ge, Y.; Wang, Y.; Sun, R.; Yang, X.; Xue, H.; Ma, X.; Liu, J.; Hu, K. Pd nanoparticles supported in PDMAEMA-b-PLMA micelles: A superb catalytic platform for Suzuki-Miyaura cross-coupling in water. Eur. Polym. J. 2024, 202, 112650. [Google Scholar] [CrossRef]
- Giaouzi, D.; Pispas, S. PDEGMA-b-PDMAEMA-b-PLMA triblock terpolymers and their cationic analogues: Synthesis, stimuli responsive self-assembly and micelleplex formation. Polym. Chem. 2024, 15, 1536–1551. [Google Scholar] [CrossRef]
- Skandalis, A.; Selianitis, D.; Sory, D.R.; Rankin, S.M.; Jones, J.R.; Pispas, S. Poly(2-(dimethylamino) ethyl methacrylate)-b-poly(lauryl methacrylate)-b-poly(oligo ethylene glycol methacrylate) triblock terpolymer micelles as drug delivery carriers for curcumin. J. Appl. Polym. Sci. 2022, 139, e52899. [Google Scholar] [CrossRef]
- Jung, F.A.; Panteli, P.A.; Ko, C.H.; Kang, J.-J.; Barnsley, L.C.; Tsitsilianis, C.; Patrickios, C.S.; Papadakis, C.M. Structural Properties of Micelles Formed by Telechelic Pentablock Quaterpolymers with pH-Responsive Midblocks and Thermoresponsive End Blocks in Aqueous Solution. Macromolecules 2019, 52, 9746–9758. [Google Scholar] [CrossRef]
- Constantinou, A.P.; Zhao, H.; McGilvery, C.M.; Porter, A.E.; Georgiou, T.K. A Comprehensive Systematic Study on Thermoresponsive Gels: Beyond the Common Architectures of Linear Terpolymers. Polymers 2017, 9, 31. [Google Scholar] [CrossRef]
- Fan, F.; Piao, J.-G.; Zhao, Y.; Jin, L.; Li, M.; Wang, Y.; Yang, L. Bioinspired Membrane-Disruptive Macromolecules as Drug-Free Therapeutics. ACS Appl. Bio Mater. 2020, 3, 1267–1275. [Google Scholar] [CrossRef]
- Skandalis, A.; Pispas, S. Synthesis of (AB) n-, AnBn-, and AxBy-type amphiphilic and double-hydrophilic star copolymers by RAFT polymerization. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 1771–1783. [Google Scholar] [CrossRef]
- Zielinska, A.; Carreiró, F.; Oliveira, A.M.; Neves, A.; Pires, B.; Nagasamy Venkatesh, D.; Durazzo, A.; Lucarini, M.; Eder, P.; Silva, A.M.; et al. Polymeric Nanoparticles: Production, Characterization, Toxicology and Ecotoxicology. Molecules 2020, 25, 3731. [Google Scholar] [CrossRef]
- Hartlieb, M.; Floyd, T.; Cook, A.B.; Sanchez-Cano, C.; Catrouillet, S.; Burns, J.A.; Perrier, S. Well-Defined Hyperstar Copolymers Based on a Thiol–Yne Hyperbranched Core and a Poly(2-Oxazoline) Shell for Biomedical Applications. Polym. Chem. 2017, 8, 2041–2054. [Google Scholar] [CrossRef]
- Salerno, A.; Diéguez, S.; Diaz-Gomez, L.; Fajrin, A.; Marliana, S.D.; Handayani, D.S. Synthesis of Eugenol–Lauryl Methacrylate Copolymers via Cationic Polymerization. IOP Conf. Ser. Mater. Sci. Eng. 2018, 349, 012003. [Google Scholar] [CrossRef]
- Balafouti, A.; Pispas, S. Hyperbranched Copolymers of Methacrylic Acid and Lauryl Methacrylate H-P(MAA-Co-LMA): Syn-thetic Aspects and Interactions with Biorelevant Compounds. Pharmaceutics 2023, 15, 1198. [Google Scholar] [CrossRef] [PubMed]
- Onugwu, A.L.; Nwagwu, C.S.; Onugwu, O.S.; Echezona, A.C.; Agbo, C.P.; Ihim, S.A.; Emeh, P.; Nnamani, P.O.; Attama, A.A.; Khutoryanskiy, V.V. Nanotechnology Based Drug Delivery Systems for the Treatment of Anterior Segment Eye Diseases. J. Control. Release 2023, 354, 465–488. [Google Scholar] [CrossRef]
- Bhattacharjee, S. DLS Zeta Potential—What They Are What They Are Not? J. Control. Release 2016, 235, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Shukla, P.; Misra, A.; Mishra, P.R. Interfacial and Colloidal Properties of Emulsified Systems: Pharmaceutical and Biological Perspective. In Colloid and Interface Science in Pharmaceutical Research and Development; Elsevier: Amsterdam, The Netherlands, 2014; pp. 149–172. [Google Scholar] [CrossRef]
- Van De Wetering, P.; Moret, E.E.; Schuurmans-Nieuwenbroek, N.M.E.; Van Steenbergen, M.J.; Hennink, W.E. Structure-Activ-ity Relationships of Water-Soluble Cationic Methacrylate/Methacrylamide Polymers for Nonviral Gene Delivery. Bioconjugate Chem. 1999, 10, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Xiao, Z.; Chen, G.; Li, T.; Peng, Y.; Shuai, X. A PH-Sensitive Nanomedicine Incorporating Catalase Gene and Photo-sensitizer Augments Photodynamic Therapy and Activates Antitumor Immunity. Nano Today 2022, 43, 101390. [Google Scholar] [CrossRef]
- Car, A.; Baumann, P.; Duskey, J.T.; Chami, M.; Bruns, N.; Meier, W. PH-Responsive PDMS-b-PDMAEMA Micelles for Intracel-lular Anticancer Drug Delivery. Biomacromolecules 2014, 15, 3235–3245. [Google Scholar] [CrossRef]
- Borisova, O.; Billon, L.; Zaremski, M.; Grassl, B.; Bakaeva, Z.; Lapp, A.; Stepanek, P.; Borisov, O. Synthesis and PH- and Salinity-Controlled Self-Assembly of Novel Amphiphilic Block-Gradient Copolymers of Styrene and Acrylic Acid. Soft Matter 2012, 8, 7649–7659. [Google Scholar] [CrossRef]
- Yuan, J.; Xu, J. Synthesis of Amphiphilic Block Copolymer and Its Application in Pigment-Based Ink. Materials 2024, 17, 330. [Google Scholar] [CrossRef]
- Yan, M.; Li, B.; Zhao, X. Determination of Critical Aggregation Concentration and Aggregation Number of Acid-Soluble Colla-gen from Walleye Pollock (Theragra chalcogramma) Skin Using the Fluorescence Probe Pyrene. Food Chem. 2010, 122, 1333–1337. [Google Scholar] [CrossRef]
- Bürger, J.; Kunnathully, V.S.; Kool, D.; Lindner, J.K.N.; Brassat, K. Characterisation of the PS-PMMA Interfaces in Microphase Separated Block Copolymer Thin Films by Analytical (S)TEM. Nanomaterials 2020, 10, 141. [Google Scholar] [CrossRef]

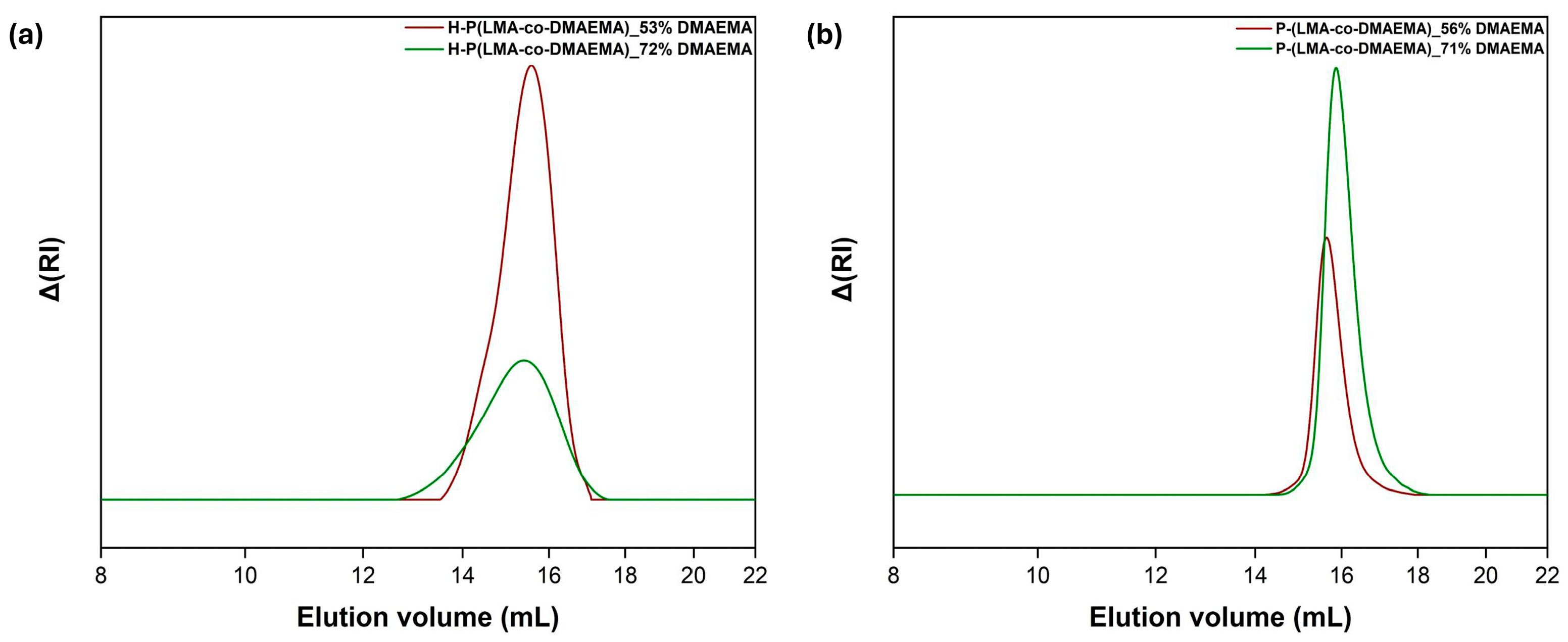




| Polymer | Code | mmol Ratios a | Mw (g/mol) (×104) b | Mw/Mn b | %wt DMAEMA c |
|---|---|---|---|---|---|
| P-(LMA-co-DMAEMA) | P1 | 3.9:6.4:0.2:0.1 | 9.7 | 1.17 | 56 |
| P-(LMA-co-DMAEMA) | P2 | 2.4:8.9:0.2:0.1 | 7.6 | 1.2 | 70 |
| H-P(LMA-co-DMAEMA) | H1 | 3.9:6.4:0.24:0.1:0.2 | 14.9 | 1.4 | 54 |
| H-P(LMA-co-DMAEMA) | H2 | 2.4:8.9:0.24:0.1:0.2 | 20.1 | 1.9 | 70 |
| Sample | ζ-Potential (mV) |
|---|---|
| P1 | +49 |
| P2 | +44 |
| H1 | +50 |
| H2 | +38 |
| Sample | pH | Intensity (kcps) | PDI | Rh (nm) |
|---|---|---|---|---|
| P1 | 3 | 282 | 0.472 | 4 133 |
| 7 | 86,800 | 0.257 | 17 62 | |
| 10 | 3064 | 0.426 | 14 100 | |
| P2 | 3 | 119 | 0.558 | 1 4 98 |
| 7 | 2091 | 0.391 | 9 68 | |
| 10 | 816 | 0.519 | 7 106 | |
| H1 | 3 | 195 | 0.401 | 9 108 |
| 7 | 71,400 | 0.249 | 50 | |
| 10 | 1409 | 0.354 | 12 45 | |
| H2 | 3 | 198 | 0.547 | 6 122 |
| 7 | 212 | 0.515 | 1 7 89 | |
| 10 | 607 | 0.473 | 8 122 |
| Sample | pH | I1/I3 |
|---|---|---|
| P1 | 3 | 1.29 |
| 7 | 0.98 | |
| 10 | 1.11 | |
| P2 | 3 | 1.43 |
| 7 | 0.96 | |
| 10 | 1.07 | |
| H1 | 3 | 1.26 |
| 7 | 1.11 | |
| 10 | 1.10 | |
| H2 | 3 | 1.44 |
| 7 | 1.11 | |
| 10 | 1.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gerardos, A.M.; Forys, A.; Trzebicka, B.; Pispas, S. One-Pot Synthesis of Amphiphilic Linear and Hyperbranched Polyelectrolytes and Their Stimuli-Responsive Self-Assembly in Aqueous Solutions. Polymers 2025, 17, 701. https://doi.org/10.3390/polym17050701
Gerardos AM, Forys A, Trzebicka B, Pispas S. One-Pot Synthesis of Amphiphilic Linear and Hyperbranched Polyelectrolytes and Their Stimuli-Responsive Self-Assembly in Aqueous Solutions. Polymers. 2025; 17(5):701. https://doi.org/10.3390/polym17050701
Chicago/Turabian StyleGerardos, Angelica Maria, Aleksander Forys, Barbara Trzebicka, and Stergios Pispas. 2025. "One-Pot Synthesis of Amphiphilic Linear and Hyperbranched Polyelectrolytes and Their Stimuli-Responsive Self-Assembly in Aqueous Solutions" Polymers 17, no. 5: 701. https://doi.org/10.3390/polym17050701
APA StyleGerardos, A. M., Forys, A., Trzebicka, B., & Pispas, S. (2025). One-Pot Synthesis of Amphiphilic Linear and Hyperbranched Polyelectrolytes and Their Stimuli-Responsive Self-Assembly in Aqueous Solutions. Polymers, 17(5), 701. https://doi.org/10.3390/polym17050701









