Bicomponent Electrospinning of PVDF-Based Nanofiber Membranes for Air Filtration and Oil–Water Separation
Abstract
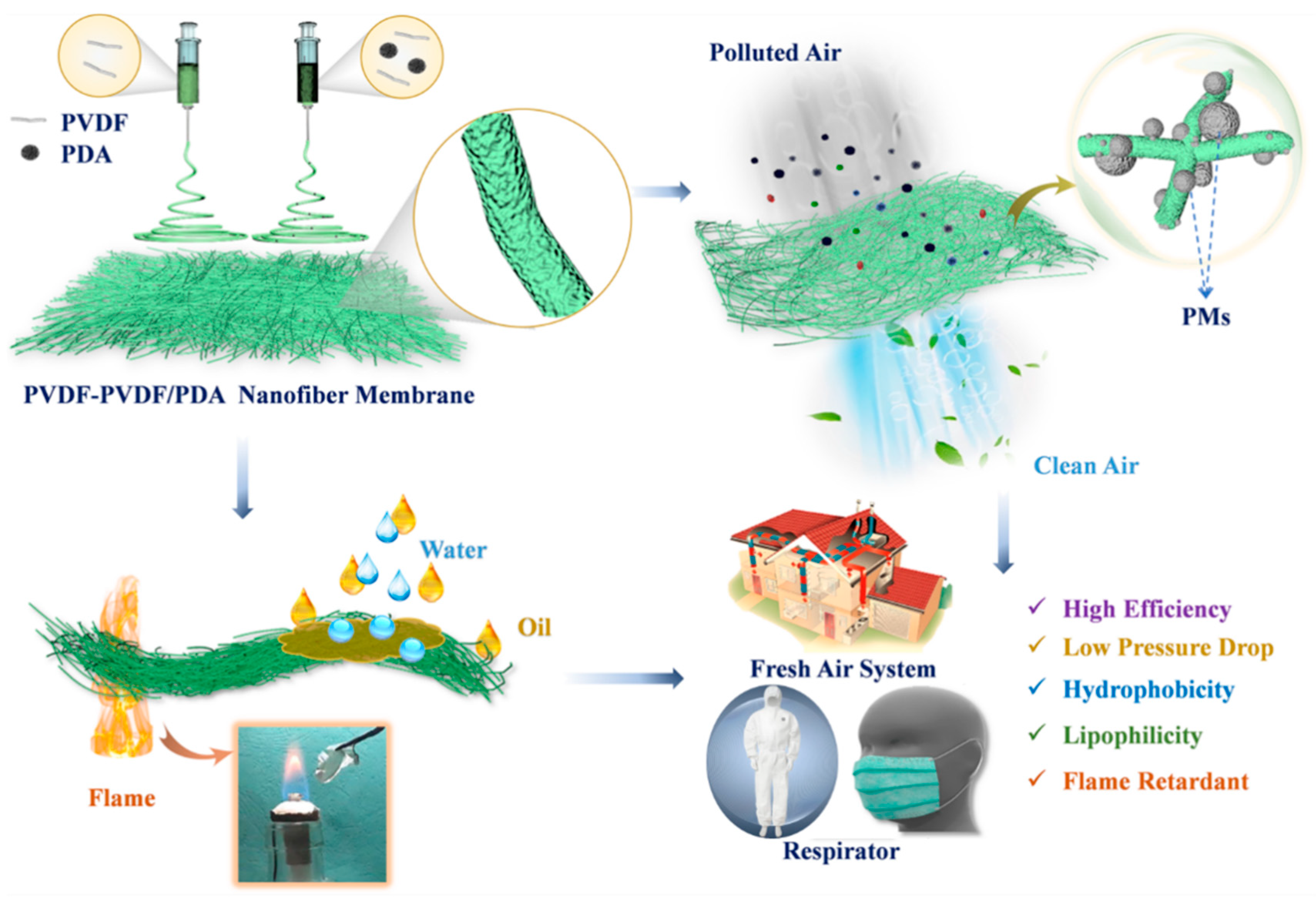
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Fabrication of Electrospinning PVDF/PDA and PVDF-PVDF/PDA NFMs
2.3. Measurement and Characterization
3. Results and Discussion
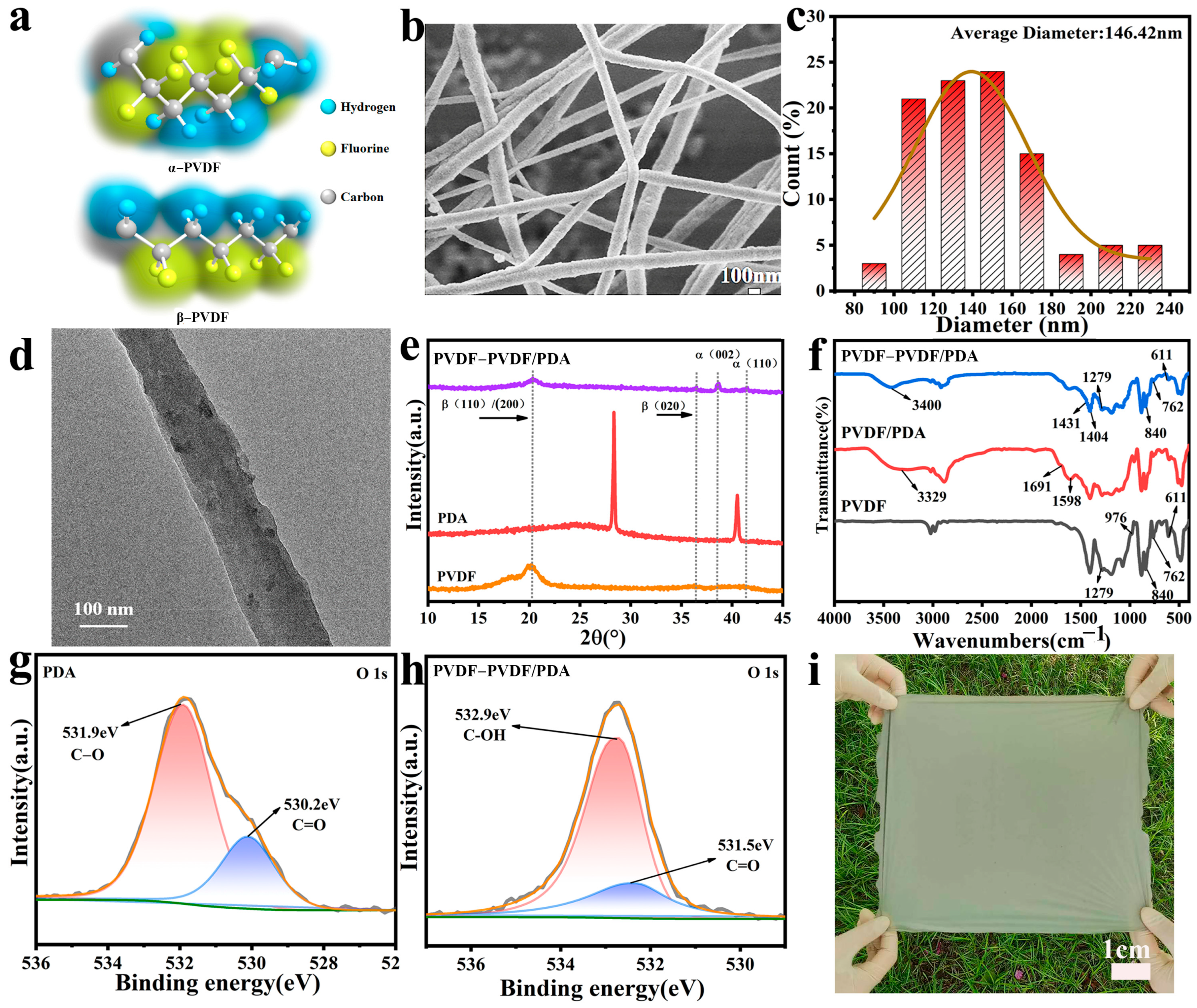
3.1. Morphology, Structure, and Component of PVDF-Based NFMs
3.2. Electrical Performance of PVDF-Based NFMs
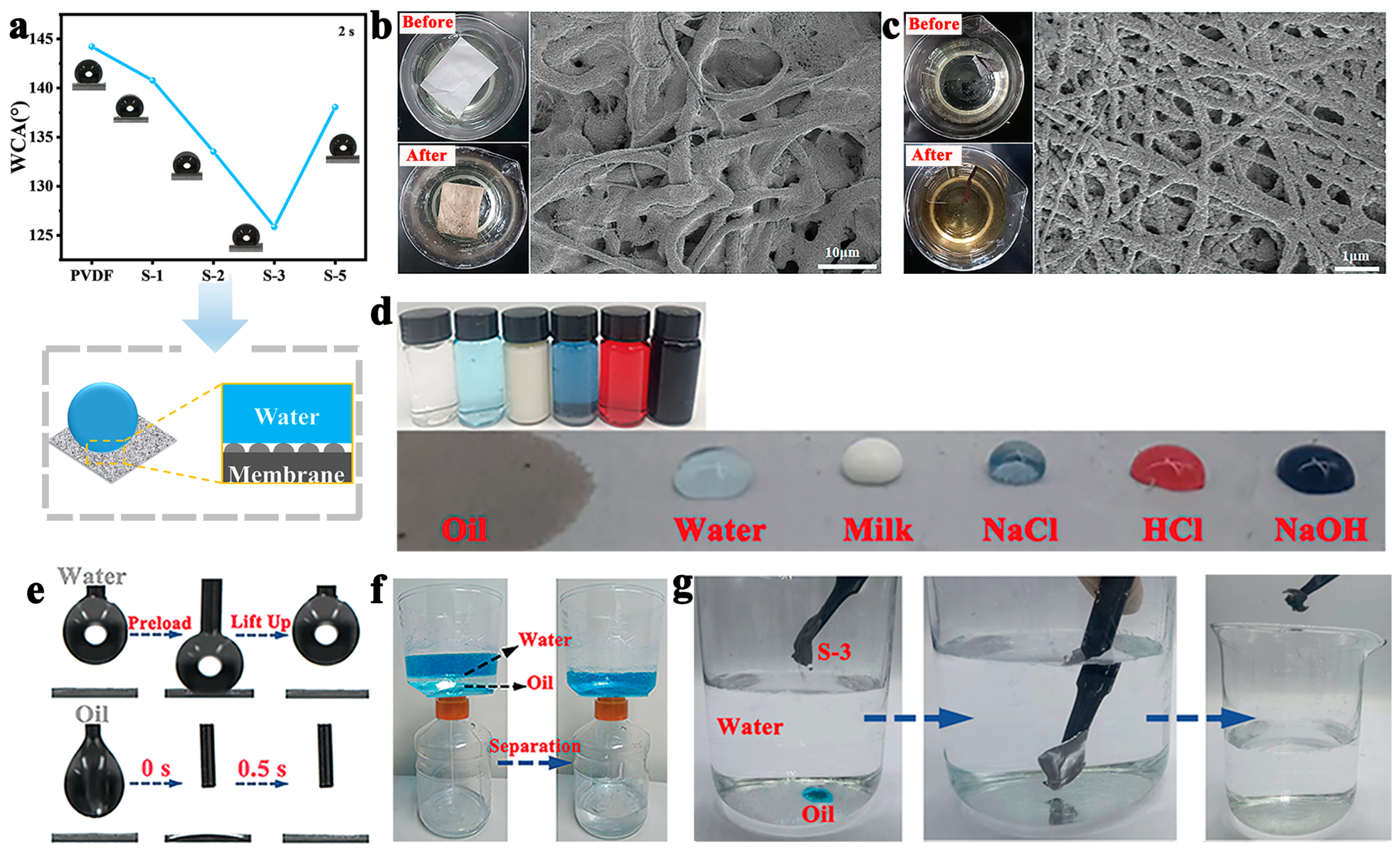
3.3. Air Filtration Performance and Oil–Water Separation of PVDF-Based NFMs
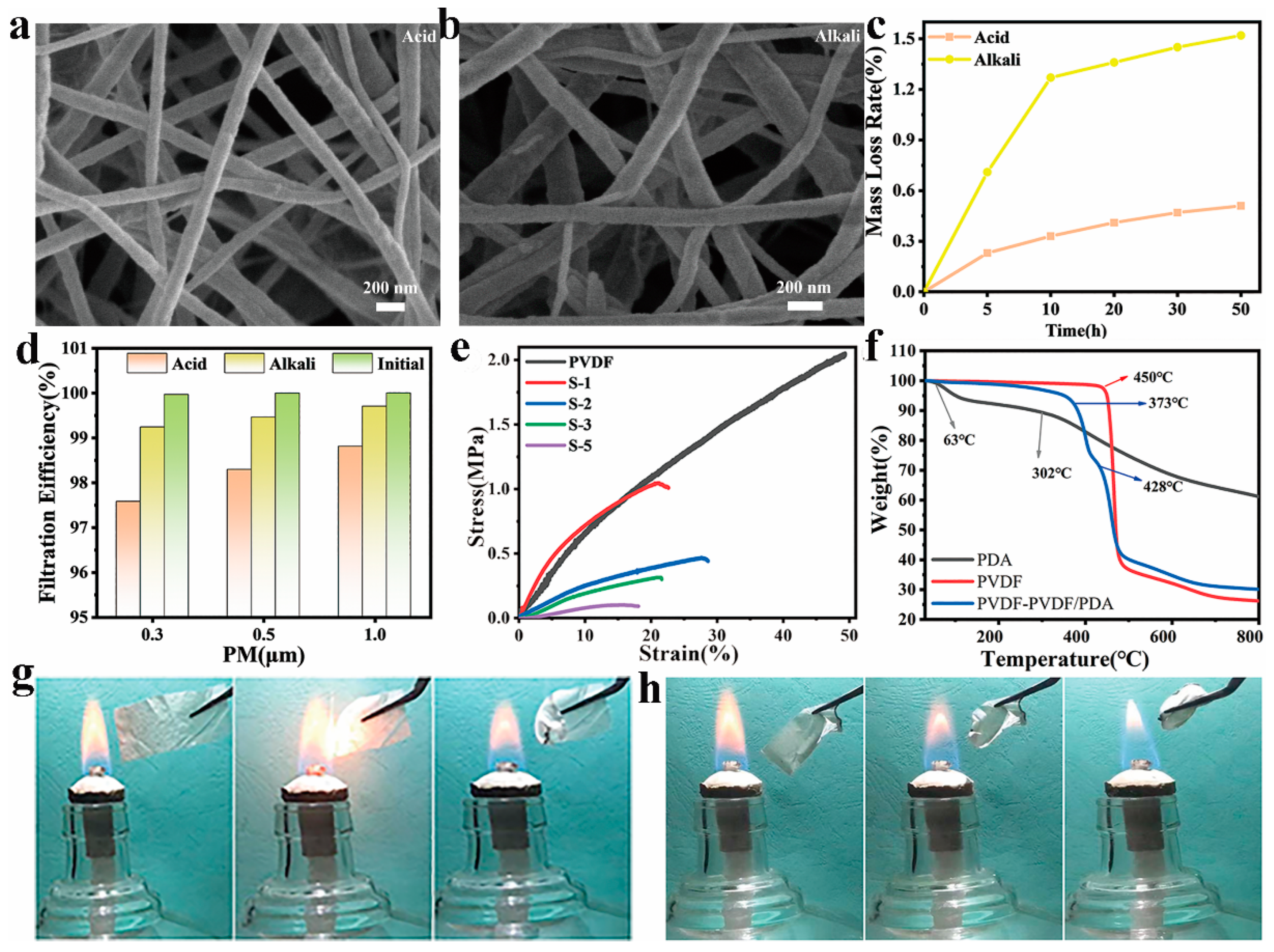
3.4. Acid and Alkali Resistance, Mechanical Property, Thermal Stability, and Flame Resistance of PVDF-Based NFMs
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Sanyal, A.; Sinha-Ray, S. Ultrafine PVDF nanofibers for filtration of air-borne particulate matters: A comprehensive review. Polymers 2021, 13, 1864. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Guo, C.; Zhang, Q.; Wu, X.; Zhong, L.; Zheng, Y. Preparation of transparent, amphiphobic and recyclable electrospun window screen air filter for high-efficiency particulate matters capture. J. Membr. Sci. 2023, 675, 121545. [Google Scholar] [CrossRef]
- Liang, G.; Zhang, W.; Zhang, X.; Yu, J.; Zhang, S.; Ding, B. Ultralight, superelastic, and antibacterial micro/nanofibrous sponges with dual-network interwoven structure for warmth retention. Compos. Commun. 2024, 50, 102006. [Google Scholar] [CrossRef]
- Li, P.; Jiang, D.; Xu, C.; Su, Z. A scanning manometry method to image the atomization pressure field for pre-debugging the nanocomposite spraying system. Compos. Commun. 2025, 53, 102189. [Google Scholar] [CrossRef]
- Zhou, N.; Gao, Y.; Huo, Y.; Zhang, K.; Zhu, J.; Chen, M.; Zhu, L.; Dong, Y.; Gao, H.; Soo Kim, I.; et al. Biodegradable micro-nanofiber medical tape with antibacterial and unidirectional moisture permeability. Chem. Eng. J. 2023, 474, 145793. [Google Scholar] [CrossRef]
- Ren, X.; Liu, H.; Wang, J.; Yu, J. Electrospinning-Derived functional carbon-based materials for energy conversion and storage. Chin. Chem. Lett. 2024, 35, 109282. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, L.; Liu, Y.; Zhang, F.; Leng, J. Recent progress in shape memory polymer composites: Driving modes, forming technologies, and applications. Compos. Commun. 2024, 51, 102062. [Google Scholar] [CrossRef]
- Fan, C.; Long, Z.; Zhang, Y.; Mensah, A.; He, H.; Wei, Q.; Lv, P. Robust integration of energy harvesting with daytime radiative cooling enables wearing thermal comfort self-powered electronic devices. Nano Energy 2023, 116, 108842. [Google Scholar] [CrossRef]
- Wang, C.; He, X.; Zhu, G.; Li, X.; Zhu, X.; Chen, R.; Tian, S.; Li, X.; Zhu, J.; Shao, J.; et al. Extreme orientation of stereocomplexed poly (lactic acid) induced ultrafine electroactive nanofibers for respiratory healthcare and intelligent diagnosis. ACS Sustain. Chem. Eng. 2024, 12, 9290–9300. [Google Scholar] [CrossRef]
- Li, Y.; Wang, D.; Xu, G.; Qiao, L.; Li, Y.; Gong, H.; Shi, L.; Li, D.; Gao, M.; Liu, G.; et al. ZIF-8/PI Nanofibrous Membranes With High-Temperature Resistance for Highly Efficient PM0.3 Air Filtration and Oil-Water Separation. Front. Chem. 2021, 9, 810861. [Google Scholar] [CrossRef]
- Singh, R.K.; Lye, S.W.; Miao, J. Holistic investigation of the electrospinning parameters for high percentage of β-phase in PVDF nanofibers. Polymer 2021, 214, 123366. [Google Scholar] [CrossRef]
- Chen, J.; Ayranci, C.; Tang, T. Piezoelectric performance of electrospun PVDF and PVDF composite fibers: A review and machine learning-based analysis. Mater. Today Chem. 2023, 30, 101571. [Google Scholar] [CrossRef]
- Dai, G.; Chu, J.C.H.; Chan, C.K.W.; Choi, C.H.J.; Ng, D.K.P. Reactive oxygen species-responsive polydopamine nanoparticles for targeted and synergistic chemo and photodynamic anticancer therapy. Nanoscale 2021, 13, 15899–15915. [Google Scholar] [CrossRef]
- Zhang, Y.; Zeng, Z.; Ma, X.Y.D.; Zhao, C.; Ang, J.M.; Ng, B.F.; Wan, M.P.; Wong, S.-C.; Wang, Z.; Lu, X. Mussel-Inspired approach to cross-linked functional 3D nanofibrous aerogels for energy-efficient filtration of ultrafine airborne particles. Appl. Surf. Sci. 2019, 479, 700–708. [Google Scholar] [CrossRef]
- Ma, F.; Zhang, N.; Wei, X.; Yang, J.; Wang, Y.; Zhou, Z. Blend-electrospun poly (vinylidene fluoride)/polydopamine membranes: Self-polymerization of dopamine and the excellent adsorption/separation abilities. J. Mater. Chem. A 2017, 5, 14430–14443. [Google Scholar] [CrossRef]
- Li, Y.; Yuan, D.; Geng, Q.; Yang, X.; Wu, H.; Xie, Y.; Wang, L.; Ning, X.; Ming, J. MOF-Embedded bifunctional composite nanofiber membranes with a tunable hierarchical structure for high-efficiency PM0.3 purification and oil/water separation. ACS Appl. Mater. Interfaces 2021, 13, 39831–39843. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, S.; Xie, X.; Li, Z.; Wang, P.; Lu, B.; Liang, S.; Tang, Y.; Zhou, J. A functionalized separator enables dendrite-free Zn anode via metal-polydopamine coordination chemistry. InfoMat 2022, 5, 12374. [Google Scholar] [CrossRef]
- Wang, Z.; Sahadevan, R.; Crandall, C.; Menkhaus, T.J.; Fong, H. Hot-Pressed PAN/PVDF hybrid electrospun nanofiber membranes for ultrafiltration. J. Membr. Sci. 2020, 611, 118327. [Google Scholar] [CrossRef]
- Yang, Y.; Huang, E.; Dansawad, P.; Li, Y.; Qing, Y.; Lv, C.; Cao, L.; You, S.; Li, Y.; Li, W. Superhydrophilic and underwater superoleophobic PVDF-PES nanofibrous membranes for highly efficient surfactant-stabilized oil-in-water emulsions separation. J. Membr. Sci. 2023, 687, 122044. [Google Scholar] [CrossRef]
- Li, X.; Wang, C.; Huang, X.; Zhang, T.; Wang, X.; Min, M.; Wang, L.; Huang, H.; Hsiao, B.S. Anionic surfactant-triggered steiner geometrical poly (vinylidene fluoride) nanofiber/nanonet air filter for efficient particulate matter removal. ACS Appl. Mater. Interfaces 2018, 10, 42891–42904. [Google Scholar] [CrossRef]
- Zhao, K.; Wei, S.; Cao, M.; Wang, M.; Li, P.; Li, H.; Zhang, X.; Zhang, Y.; Chen, Y. Dielectric polyimide composites with enhanced thermal conductivity and excellent electrical insulation properties by constructing 3D oriented heat transfer network. Compos. Sci. Technol. 2024, 245, 110323. [Google Scholar] [CrossRef]
- Wu, X.; Wu, X.; Wang, T.; Zhao, L.; Truong, Y.B.; Ng, D.; Zheng, Y.; Xie, Z. Omniphobic surface modification of electrospun nanofiber membrane via vapor deposition for enhanced anti-wetting property in membrane distillation. J. Membr. Sci. 2020, 606, 118075. [Google Scholar] [CrossRef]
- Su, C.; Zhang, L.; Zhang, Y.; Huang, X.; Ye, Y.; Xia, Y.; Gong, Z.; Qin, X.; Liu, Y.; Guo, S. P(VDF-TrFE)/BaTiO3 nanofibrous membrane with enhanced piezoelectricity for high PM0.3 filtration and reusable face masks. ACS Appl. Mater. Interfaces 2023, 15, 5845–5855. [Google Scholar] [CrossRef]
- Wang, T.; Wang, P.; Pan, L.; He, Z.; Dai, L.; Wang, L.; Liu, S.; Jun, S.C.; Lu, B.; Liang, S.; et al. Stabling zinc metal anode with polydopamine regulation through dual effects of fast desolvation and ion confinement. Adv. Energy Mater. 2022, 13, 2203523. [Google Scholar] [CrossRef]
- Wang, M.; Cheng, X.; Jiang, G.; Xie, J.; Cai, W.; Li, J.; Wang, Y. Preparation and pervaporation performance of PVA membrane with biomimetic modified silica nanoparticles as coating. J. Membr. Sci. 2022, 653, 120535. [Google Scholar] [CrossRef]
- Zhang, M.; Tan, Z.; Zhang, Q.; Shen, Y.; Mao, X.; Wei, L.; Sun, R.; Zhou, F.; Liu, C. Flexible self-powered friction piezoelectric sensor based on structured PVDF-based composite nanofiber membranes. ACS Appl. Mater. Interfaces 2023, 15, 30849–30858. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Song, X.; Li, T.; Zhu, X.; Yang, S.; Zhu, J.; He, X.; Gao, J.; Xu, H. Biodegradable electroactive banofibrous air filters for long-term respiratory healthcare and self-powered monitoring. ACS Appl. Mater. Interfaces 2023, 15, 37580–37592. [Google Scholar] [CrossRef]
- Deng, H.; Zhao, N.; You, J.; Pan, Z.; Xing, B.; Ye, Y.; Lai, B.; Wang, Y.; Lu, T.; Liu, X. Removal of bisphenol A through peroxymonosulfate activation with N-doped graphite carbon spheres coated cobalt nanoparticles catalyst: Synergy of nonradicals. Chin. Chem. Lett. 2024, 110650, in press. [Google Scholar] [CrossRef]
- Kim, S.; Lee, H. Piezoelectric ceramics with high d33 constants and their application to film speakers. Materials 2021, 14, 5795. [Google Scholar] [CrossRef]
- Ding, S.; Cao, Y.; Huang, F.; Wang, Y.; Li, J.; Chen, S. Spontaneous polarization induced electrostatic charge in washable electret composite fabrics for reusable air-filtering application. Compos. Sci. Technol. 2022, 217, 109093. [Google Scholar] [CrossRef]
- Chen, M.; Jiang, J.; Feng, S.; Low, Z.-X.; Zhong, Z.; Xing, W. Graphene oxide functionalized polyvinylidene fluoride nanofibrous membranes for efficient particulate matter removal. J. Membr. Sci. 2021, 635, 119463. [Google Scholar] [CrossRef]
- Yang, T.; Zhu, X.; Zhang, Y.; Ke, L.; Zhu, J.; Huang, R.; Li, S.; Zhu, Y.; Zhang, S.; Zhong, G.-J.; et al. Nanopatterning of beaded poly (lactic acid) nanofibers for highly electroactive, breathable, UV-shielding and antibacterial protective membranes. Int. J. Biol. Macromol. 2024, 260, 129566. [Google Scholar] [CrossRef]
- Wang, M.J.; Yang, J.; Peng, L.; Bai, Y.; Liu, Z.; Yang, X.; Lu, H.; Zhou, B.; Jiang, N.; He, G.; et al. Optimizing the size and electronic effects of core-shell heterostructures via well-constructed Ru clusters encapsulated in N-doped carbon layers. Chin. Chem. Lett. 2024, 110573, in press. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, C.; Zhou, Y.; Zhang, F.; Duan, X.; Liu, Y.; Zhao, X.; Liu, J.; Shuai, X.; Wang, J.; et al. MRI-Visible mesoporous polydopamine nanoparticles with enhanced antioxidant capacity for osteoarthritis therapy. Biomaterials 2023, 295, 122030. [Google Scholar] [CrossRef]
- Cheng, N.; Miao, D.; Wang, C.; Lin, Y.; Babar, A.A.; Wang, X.; Wang, Z.; Yu, J.; Ding, B. Nanosphere-Structured hierarchically porous PVDF-HFP fabric for passive daytime radiative cooling via one-step water vapor-induced phase separation. Chem. Eng. J. 2023, 460, 141581. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, S.; Liu, L.; Yu, J.; Ding, B. A fluffy dual-network structured nanofiber/net filter enables high-efficiency air fltration. Adv. Funct. Mater. 2019, 29, 1904108. [Google Scholar] [CrossRef]
- Zhu, G.; Li, X.; Li, X.-P.; Wang, A.; Li, T.; Zhu, X.; Tang, D.; Zhu, J.; He, X.; Li, H.; et al. Nanopatterned electroactive polylactic acid nanofibrous MOFilters for efficient PM0.3 filtration and bacterial inhibition. ACS Appl. Mater. Interfaces 2023, 15, 47145–47157. [Google Scholar] [CrossRef] [PubMed]
- Zong, D.; Bai, W.; Geng, M.; Yin, X.; Wang, F.; Yu, J.; Zhang, S.; Ding, B. Direct synthesis of elastic and stretchable hierarchical structured fiber and graphene-based sponges for noise reduction. ACS Nano 2023, 17, 17576–17586. [Google Scholar] [CrossRef]
- Peng, L.; Hung, C.-T.; Wang, S.; Zhang, X.; Zhu, X.; Zhao, Z.; Wang, C.; Tang, Y.; Li, W.; Zhao, D. Versatile nanoemulsion assembly approach to synthesize functional mesoporous carbon nanospheres with tunable pore sizes and architectures. J. Am. Chem. Soc. 2019, 141, 7073–7080. [Google Scholar] [CrossRef]
- Liang, C.; Li, J.; Chen, Y.; Ke, L.; Zhu, J.; Zheng, L.; Li, X.-P.; Zhang, S.; Li, H.; Zhong, G.-J.; et al. Self-charging, breathable, and antibacterial poly (lactic acid) nanofibrous air filters by surface engineering of ultrasmall electroactive nanohybrids. ACS Appl. Mater. Interfaces 2023, 15, 57636–57648. [Google Scholar] [CrossRef]
- Li, J.; Yin, J.; Wee, M.G.V.; Chinnappan, A.; Ramakrishna, S. A self-Powered piezoelectric nanofibrous membrane as wearable tactile sensor for human body motion monitoring and recognition. Adv. Fiber Mater. 2023, 5, 1417–1430. [Google Scholar] [CrossRef]
- Zhu, Y.; Yan, J.; Liu, J.; Chen, H.; Gui, J.; Wu, C.; Zhu, X.; Yin, P.; Liu, M.; Zhang, Y.; et al. Multi-mimic activities of Co3O4 nanopolyhedrons and application in regulating the content of intracellular hydrogen peroxide/oxygen. ACS Appl. Nano Mater. 2022, 5, 15102–15114. [Google Scholar] [CrossRef]
- Dong, W.; Zhao, Z.; Liu, F.; Li, P.; Wang, L.; Zhou, Y.; Shen, Y.; Lang, C.; Deng, B.; Li, H.; et al. PVDF nanofiber modified with ZnO nanowires/polydopamine for the treatment of sewage containing heavy metals, organic dyes, and bacteria. ACS Appl. Mater. Interfaces 2023, 15, 58994–59004. [Google Scholar] [CrossRef]
- Zhi, Q.; Li, D.; Zhang, Z.; Fu, L.; Zhu, W. High-Content continuous carbon fiber reinforced multifunctional prepreg filaments suitable for direct 3D-printing. Compos. Commun. 2023, 44, 101726. [Google Scholar] [CrossRef]
- Xue, M.; Qin, R.; Peng, C.; Xia, L.; Xu, Y.; Luo, W.; Chen, G.; Zeng, B.; Liu, X.; Dai, L. o-Vanillin based MOFs as phosphorus-free flame retardant for reinforced epoxy resin. Compos. Commun. 2024, 46, 101821. [Google Scholar] [CrossRef]
- Si, Y.; Yang, J.; Wang, D.; Shi, S.; Zhi, C.; Huang, K.; Hu, J. Bioinspired hierarchical multi-protective membrane for extreme environments via co-electrospinning-electrospray strategy. Small 2023, 20, 2304705. [Google Scholar] [CrossRef]
- Wang, H.; Wang, Z.; Shi, Y.; Liu, M.; Yao, A.; Feng, Y.; Fu, L.; Lv, Y.; Yang, F.; Yu, B. Supramolecular engineered ultrathin MXene towards fire safe polylactic acid composites. Compos. Commun. 2023, 37, 101405. [Google Scholar] [CrossRef]
- Peng, Z.; Shi, J.; Xiao, X.; Hong, Y.; Li, X.; Zhang, W.; Cheng, Y.; Wang, Z.; Li, W.J.; Chen, J.; et al. Self-charging electrostatic face masks leveraging triboelectrification for prolonged air filtration. Nat. Commun. 2022, 13, 7835. [Google Scholar] [CrossRef]
- Al-Attabi, R.; She, F.; Zhao, S.; Dumée, L.F.; Schütz, J.A.; Xing, W.; Zhong, Z.; Kong, L. Durable and comfortable electrospun nanofiber membranes for face mask applications. Sep. Purif. Technol. 2023, 322, 124370. [Google Scholar] [CrossRef]
- Wang, H.; Bao, Y.; Yang, X.; Lan, X.; Guo, J.; Pan, Y.; Huang, W.; Tang, L.; Luo, Z.; Zhou, B.; et al. Study on filtration performance of PVDF/PUL composite air filtration membrane based on far-field electrospinning. Polymers 2022, 14, 3294. [Google Scholar] [CrossRef]
- Bui, T.T.; Shin, M.K.; Jee, S.Y.; Long, D.X.; Hong, J.; Kim, M.-G. Ferroelectric PVDF nanofiber membrane for high-efficiency PM0.3 air filtration with low air flow resistance. Colloids Surf. A 2022, 640, 128418. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Zhou, X.; Wang, B.; Dai, F.; Liu, J. Modified PVDF/PMMA/SiO2 composite nanofibrous membrane in airborne filtration: Transparency, mechanical properties and filtration performance. J. Environ. Chem. Eng. 2024, 12, 114109. [Google Scholar] [CrossRef]
- Moon, J.; Bui, T.T.; Jang, S.; Ji, S.; Park, J.T.; Kim, M.-G. A highly efficient nanofibrous air filter membrane fabricated using electrospun amphiphilic PVDF-g-POEM double comb copolymer. Sep. Purif. Technol. 2021, 279, 119625. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Li, Z.-J.; Xu, X.-F.; Wang, N.; Yang, M.-Y.; Long, Y.-Z.; Zhang, H.-D. Electrospinning dual energy-saving design of PVDF-HFP nanofiber films for passive radiant cooling and air filtration. AIP Adv. 2024, 14, 015349. [Google Scholar] [CrossRef]
- Wu, Y.; Li, X.; Zhong, Q.; Wang, F.; Yang, B. Preparation and filtration performance of antibacterial PVDF/SiO2/Ag composite nanofiber membrane. J. Build. Eng. 2023, 74, 106864. [Google Scholar] [CrossRef]
- Geng, Q.; Dong, S.; Li, Y.; Wu, H.; Yang, X.; Ning, X.; Yuan, D. High-performance photoinduced antimicrobial membrane toward efficient PM2.5-0.3 capture and oil-water separation. Sep. Purif. Technol. 2022, 284, 120267. [Google Scholar] [CrossRef]
- Toptaş, A.; Çalışır, M.D.; Kılıç, A. Production of ultrafine PVDF nanofiber/nanonet-based air filters via the electroblowing technique by employing PEG as a pore-forming agent. ACS Omega 2023, 8, 38557–38565. [Google Scholar] [CrossRef]
- Liu, F.; Li, M.; Li, F.; Weng, K.; Qi, K.; Liu, C.; Ni, Q.; Tao, X.; Zhang, J.; Shao, W.; et al. Preparation and properties of PVDF/Fe3O4 nanofibers with magnetic and electret effects and their application in air filtration. Macromol. Mater. Eng. 2020, 305, 1900856. [Google Scholar] [CrossRef]


| Samples | Surface Area (m2/g) | Pore Volume (cm3/g) | Microspore Volume (cm3/g) |
|---|---|---|---|
| PVDF | 7.337 | 0.032 | 0.002 |
| S-1 | 2.824 | 0.009 | 0.001 |
| S-2 | 8.654 | 0.050 | 0.002 |
| S-3 | 11.484 | 0.043 | 0.003 |
| S-5 | 9.176 | 0.049 | 0.002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Feng, T.; Fu, L.; Mu, Z.; Wei, W.; Li, W.; Liang, X.; Ma, L.; Wu, Y.; Wang, X.; Wu, T.; et al. Bicomponent Electrospinning of PVDF-Based Nanofiber Membranes for Air Filtration and Oil–Water Separation. Polymers 2025, 17, 703. https://doi.org/10.3390/polym17050703
Feng T, Fu L, Mu Z, Wei W, Li W, Liang X, Ma L, Wu Y, Wang X, Wu T, et al. Bicomponent Electrospinning of PVDF-Based Nanofiber Membranes for Air Filtration and Oil–Water Separation. Polymers. 2025; 17(5):703. https://doi.org/10.3390/polym17050703
Chicago/Turabian StyleFeng, Tianxue, Lin Fu, Zhimei Mu, Wenhui Wei, Wenwen Li, Xiu Liang, Liang Ma, Yitian Wu, Xiaoyu Wang, Tao Wu, and et al. 2025. "Bicomponent Electrospinning of PVDF-Based Nanofiber Membranes for Air Filtration and Oil–Water Separation" Polymers 17, no. 5: 703. https://doi.org/10.3390/polym17050703
APA StyleFeng, T., Fu, L., Mu, Z., Wei, W., Li, W., Liang, X., Ma, L., Wu, Y., Wang, X., Wu, T., Gao, M., Xu, G., & Zhang, X. (2025). Bicomponent Electrospinning of PVDF-Based Nanofiber Membranes for Air Filtration and Oil–Water Separation. Polymers, 17(5), 703. https://doi.org/10.3390/polym17050703







