Black Soldier Fly Culture as a Source of Chitin and Chitosan for Its Potential Use in Concrete: An Overview
Abstract
1. Introduction
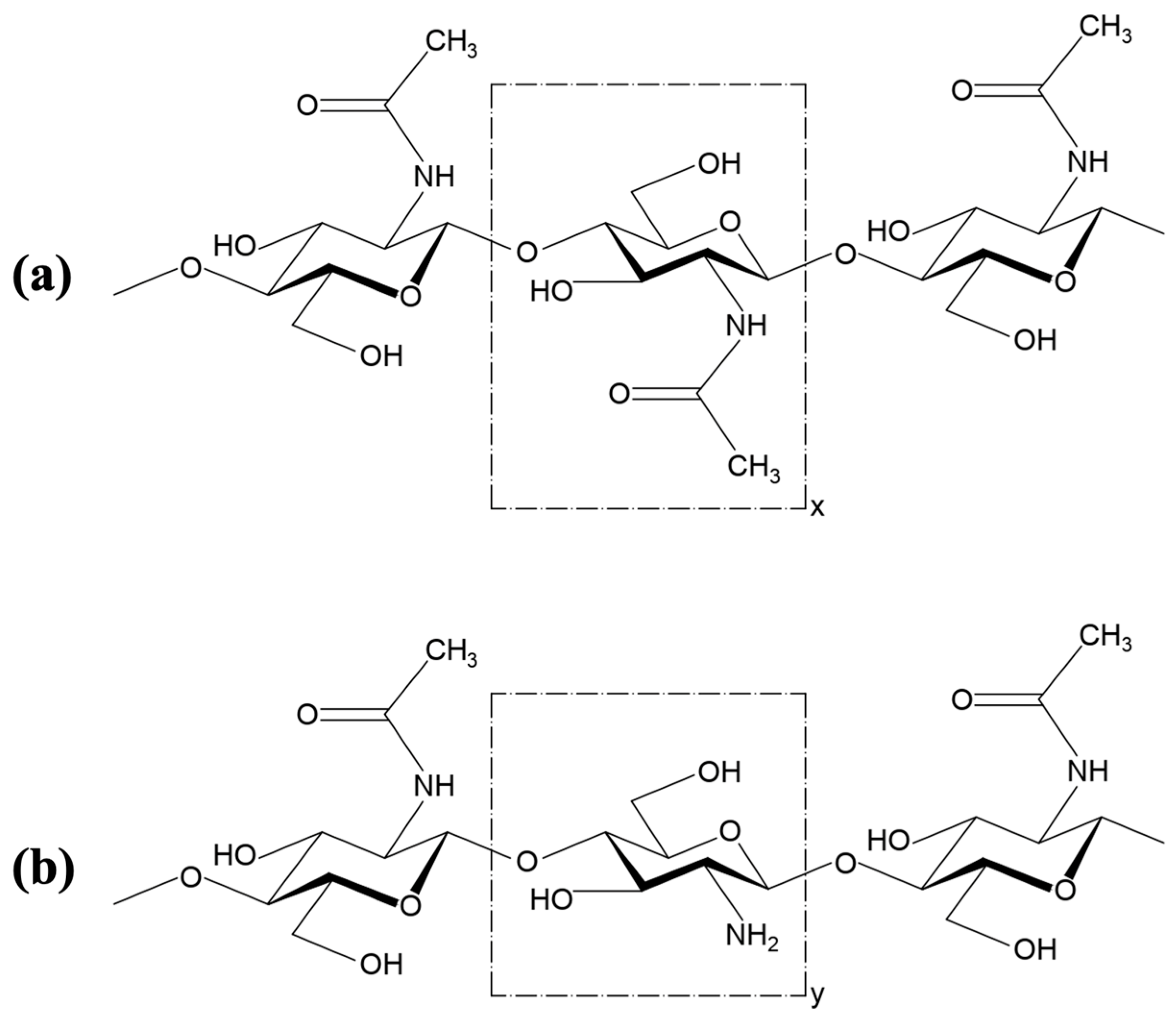
2. Chemical Description of Chitin and Chitosan
3. Rheological Properties of Chitin and Chitosan
4. Isolation of Chitin and Chitosan
4.1. Chemical
4.2. Bio-Based
4.2.1. Biological
4.2.2. Enzymatic
5. Chitin and Chitosan Content in Black Soldier Fly
6. Use of Biopolymers in Concrete
6.1. Chitin
6.2. Chitosan
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kayondo, M.; Combrinck, R.; Boshoff, W.P. State-of-the-Art Review on Plastic Cracking of Concrete. Constr. Build. Mater. 2019, 225, 886–899. [Google Scholar] [CrossRef]
- Larosche, C.J. Types and Causes of Cracking in Concrete Structures. Fail. Distress Repair Concr. Struct. 2009, 57–83. [Google Scholar] [CrossRef]
- Altera, A.Z.A.; Yavuz Bayraktar, O.; Bodur, B.; Kaplan, G. Investigation of the Usage Areas of Different Fiber Reinforced. Concrete. Kastamonu Univ. J. Eng. Sci. 2021, 7, 7–18. [Google Scholar]
- Olivia, M.; Jingga, H.; Toni, N.; Wibisono, G. Biopolymers to Improve Physical Properties and Leaching Characteristics of Mortar and Concrete: A Review. IOP Conf. Ser. Mater. Sci. Eng. 2018, 345, 012028. [Google Scholar] [CrossRef]
- Ozel, N.; Elibol, M. Chitin and Chitosan from Mushroom (Agaricus bisporus) Using Deep Eutectic Solvents. Int. J. Biol. Macromol. 2024, 262, 130110. [Google Scholar] [CrossRef]
- Shamshina, J.L.; Oldham, T.; Rogers, R.D. Applications of Chitin in Agriculture. In Sustainable Agriculture Reviews 36: Chitin and Chitosan: Applications in Food, Agriculture, Pharmacy, Medicine and Wastewater Treatment; Crini, G., Lichtfouse, E., Eds.; Springer International Publishing: Cham, The Netherlands, 2019; pp. 125–146. [Google Scholar] [CrossRef]
- Shou, K.; Huang, Y.; Qi, B.; Hu, X.; Ma, Z.; Lu, A.; Jian, C.; Zhang, L.; Yu, A. Induction of Mesenchymal Stem Cell Differentiation in the Absence of Soluble Inducer for Cutaneous Wound Regeneration by a Chitin Nanofiber-Based Hydrogel. J. Tissue Eng. Regen. Med. 2018, 12, e867–e880. [Google Scholar] [CrossRef]
- Ways, T.M.M.; Lau, W.M.; Khutoryanskiy, V.V. Chitosan and Its Derivatives for Application in Mucoadhesive Drug Delivery Systems. Polymers 2018, 10, 267. [Google Scholar] [CrossRef]
- Sarkar, S.; Das, D.; Dutta, P.; Kalita, J.; Wann, S.B.; Manna, P. Chitosan: A Promising Therapeutic Agent and Effective Drug Delivery System in Managing Diabetes Mellitus. Carbohydr. Polym. 2020, 247, 116594. [Google Scholar] [CrossRef]
- Azuma, K.; Osaki, T.; Minami, S.; Okamoto, Y. Anticancer and Anti-Inflammatory Properties of Chitin and Chitosan Oligosaccharides. J. Funct. Biomater. 2015, 6, 33–49. [Google Scholar] [CrossRef]
- Avelelas, F.; Horta, A.; Pinto, L.F.V.; Cotrim Marques, S.; Marques Nunes, P.; Pedrosa, R.; Leandro, S.M. Antifungal and Antioxidant Properties of Chitosan Polymers Obtained from Nontraditional Polybius henslowii Sources. Mar. Drugs 2019, 17, 239. [Google Scholar] [CrossRef]
- Ke, C.-L.; Deng, F.-S.; Chuang, C.-Y.; Lin, C.-H. Antimicrobial Actions and Applications of Chitosan. Polymers 2021, 13, 904. [Google Scholar] [CrossRef] [PubMed]
- Shih, P.-Y.; Liao, Y.-T.; Tseng, Y.-K.; Deng, F.-S.; Lin, C.-H. A Potential Antifungal Effect of Chitosan Against Candida albicans Is Mediated via the Inhibition of SAGA Complex Component Expression and the Subsequent Alteration of Cell Surface Integrity. Front. Microbiol. 2019, 10, 602. [Google Scholar] [CrossRef]
- Ngasotter, S.; Xavier, K.; Meitei, M.M.; Waikhom, D.; Madhulika; Pathak, J.; Singh, S.K. Crustacean Shell Waste Derived Chitin and Chitin Nanomaterials for Application in Agriculture, Food, and Health—A Review. Carbohydr. Polym. Technol. Appl. 2023, 6, 100349. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and Chitosan: Properties and Applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Barrett, M.; Chia, S.Y.; Fischer, B.; Tomberlin, J.K. Welfare Considerations for Farming Black Soldier Flies, Hermetia illucens (Diptera: Stratiomyidae): A Model for the Insects as Food and Feed Industry. J. Insects Food Feed 2023, 9, 119–148. [Google Scholar] [CrossRef]
- Pedrazzani, C.; Righi, L.; Vescovi, F.; Maistrello, L.; Caligiani, A. Black Soldier Fly as a New Chitin Source: Extraction, Purification and Molecular/Structural Characterization. LWT 2024, 191, 115618. [Google Scholar] [CrossRef]
- Purkayastha, D.; Sarkar, S. Sustainable Waste Management Using Black Soldier Fly Larva: A Review. Int. J. Environ. Sci. Technol. 2022, 19, 12701–12726. [Google Scholar] [CrossRef]
- Moussian, B. Chitin: Structure, Chemistry and Biology. In Advances in Experimental Medicine and Biology; Springer New York LLC: New York, NY, USA, 2019; Volume 1142, pp. 5–18. [Google Scholar] [CrossRef]
- Hou, J.; Aydemir, B.E.; Dumanli, A.G. Understanding the Structural Diversity of Chitins as a Versatile Biomaterial. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2021, 379, 20200331. [Google Scholar] [CrossRef] [PubMed]
- Mohan, K.; Muralisankar, T.; Jayakumar, R.; Rajeevgandhi, C. A Study on Structural Comparisons of α-Chitin Extracted from Marine Crustacean Shell Waste. Carbohydr. Polym. Technol. Appl. 2021, 2, 100037. [Google Scholar] [CrossRef]
- Wu, Q.; Jungstedt, E.; Šoltésová, M.; Mushi, N.E.; Berglund, L.A. High Strength Nanostructured Films Based on Well-Preserved β-Chitin Nanofibrils. Nanoscale 2019, 11, 11001–11011. [Google Scholar] [CrossRef]
- Kaya, M.; Mujtaba, M.; Ehrlich, H.; Salaberria, A.M.; Baran, T.; Amemiya, C.T.; Galli, R.; Akyuz, L.; Sargin, I.; Labidi, J. On Chemistry of γ-Chitin. Carbohydr. Polym. 2017, 176, 177–186. [Google Scholar] [CrossRef]
- Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Caballero, A.H.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. [Google Scholar] [CrossRef] [PubMed]
- Aranaz, I.; Mengíbar, M.; Harris, R.; Paños, I.; Miralles, B.; Acosta, N.; Galed, G.; Heras, Á. Functional Characterization of Chitin and Chitosan. Curr. Chem. Biol. 2009, 3, 203–230. [Google Scholar]
- Novikov, V.Y.; Derkach, S.R.; Konovalova, I.N.; Dolgopyatova, N.V.; Kuchina, Y.A. Mechanism of Heterogeneous Alkaline Deacetylation of Chitin: A Review. Polymers 2023, 15, 1729. [Google Scholar] [CrossRef]
- Xu, J.; Selling, G.W.; Liu, S.X. Effect of Jet-Cooking on Rheological Properties of Navy Bean Flour Suspensions. Food Chem. Adv. 2023, 2, 115618. [Google Scholar] [CrossRef]
- Triunfo, M.; Tafi, E.; Guarnieri, A.; Salvia, R.; Scieuzo, C.; Hahn, T.; Zibek, S.; Gagliardini, A.; Panariello, L.; Coltelli, M.B.; et al. Characterization of Chitin and Chitosan Derived from Hermetia illucens, a Further Step in a Circular Economy Process. Sci. Rep. 2022, 12, 6613. [Google Scholar] [CrossRef] [PubMed]
- Lucas, A.J.d.S.; Oreste, E.Q.; Costa, H.L.G.; López, H.M.; Saad, C.D.M.; Prentice, C. Extraction, Physicochemical Characterization, and Morphological Properties of Chitin and Chitosan from Cuticles of Edible Insects. Food Chem. 2021, 343, 128550. [Google Scholar] [CrossRef] [PubMed]
- Luo, Q.; Wang, Y.; Han, Q.; Ji, L.; Zhang, H.; Fei, Z.; Wang, Y. Comparison of the Physicochemical, Rheological, and Morphologic Properties of Chitosan from Four Insects. Carbohydr. Polym. 2019, 209, 266–275. [Google Scholar] [CrossRef]
- Gonil, P.; Sajomsang, W. Applications of Magnetic Resonance Spectroscopy to Chitin from Insect Cuticles. Int. J. Biol. Macromol. 2012, 51, 514–522. [Google Scholar] [CrossRef]
- Ivanov, V.; Stabnikov, V. Basic Concepts on Biopolymers and Biotechnological Admixtures for Eco-Efficient Construction Materials. Biopolym. Biotech Admix. Eco-Effic. Constr. Mater. 2016, 13–35. [Google Scholar] [CrossRef]
- Haider, M.; Jian, G.; Zhong, T.; Li, H.; Fernandez, C.A.; Fifield, L.S.; Wolcott, M.; Nassiri, S. Insights into Setting Time, Rheological and Mechanical Properties of Chitin Nanocrystals- and Chitin Nanofibers-Cement Paste. Cem. Concr. Compos. 2022, 132, 104623. [Google Scholar] [CrossRef]
- Brigode, C.; Hobbi, P.; Jafari, H.; Verwilghen, F.; Baeten, E.; Shavandi, A. Isolation and Physicochemical Properties of Chitin Polymer from Insect Farm Side Stream as a New Source of Renewable Biopolymer. J. Clean. Prod. 2020, 275, 122924. [Google Scholar] [CrossRef]
- Soetemans, L.; Uyttebroek, M.; Bastiaens, L. Characteristics of Chitin Extracted from Black Soldier Fly in Different Life Stages. Int. J. Biol. Macromol. 2020, 165, 3206–3214. [Google Scholar] [CrossRef]
- Abidin, N.A.Z.; Kormin, F.; Abidin, N.A.Z.; Anuar, N.A.F.M.; Abu Bakar, M.F. The Potential of Insects as Alternative Sources of Chitin: An Overview on the Chemical Method of Extraction from Various Sources. Int. J. Mol. Sci. 2020, 21, 4978. [Google Scholar] [CrossRef]
- Dayakar, B.; Xavier, K.A.M.; Das, O.; Porayil, L.; Balange, A.K.; Nayak, B.B. Application of Extreme Halophilic Archaea as Biocatalyst for Chitin Isolation from Shrimp Shell Waste. Carbohydr. Polym. Technol. Appl. 2021, 2, 100093. [Google Scholar] [CrossRef]
- Xiong, A.; Ruan, L.; Ye, K.; Huang, Z.; Yu, C. Extraction of Chitin from Black Soldier Fly (Hermetia illucens) and Its Puparium by Using Biological Treatment. Life 2023, 13, 1424. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.S.; Liang, S.H.; Lai, W.L.; Lee, J.X.; Wang, Y.P.; Liu, Y.T.; Wang, S.H.; Lee, M.H. Sustainable Extraction of Chitin from Spent Pupal Shell of Black Soldier Fly. Processes 2021, 9, 976. [Google Scholar] [CrossRef]
- Dhanabalan, V.; Xavier, K.A.M.; Eppen, S.; Joy, A.; Balange, A.; Asha, K.K.; Murthy, L.N.; Nayak, B.B. Characterization of Chitin Extracted from Enzymatically Deproteinized Acetes Shell Residue with Varying Degree of Hydrolysis. Carbohydr. Polym. 2021, 253, 117203. [Google Scholar] [CrossRef]
- Kaisler, M.; Van Den Broek, L.A.M.; Boeriu, C.G. Chitin and Chitosan as Sources of Bio-Based Building Blocks and Chemicals; Wiley: Hoboken, NJ, USA, 2020. [Google Scholar] [CrossRef]
- Silva, M.B.d.O.; de Oliveira, S.A.; Rosa, D.d.S. Comparative Study on Microwave-Assisted and Conventional Chitosan Production from Shrimp Shell: Process Optimization, Characterization, and Environmental Impacts. J. Clean. Prod. 2024, 440, 140726. [Google Scholar] [CrossRef]
- Arbia, W.; Arbia, L.; Adour, L.; Amrane, A. Chitin Extraction from Crustacean Shells Using Biological Methods-A Review. Food Technol. Biotechnol. 2013, 51, 12–25. [Google Scholar]
- Hamed, I.; Özogul, F.; Regenstein, J.M. Industrial Applications of Crustacean By-Products (Chitin, Chitosan, and Chitooligosaccharides): A Review. Trends Food Sci. Technol. 2016, 48, 40–50. [Google Scholar] [CrossRef]
- Mshayisa, V.V.; Van Wyk, J.; Zozo, B. Nutritional, Techno-Functional and Structural Properties of Black Soldier Fly (Hermetia illucens) Larvae Flours and Protein Concentrates. Foods 2022, 11, 724. [Google Scholar] [CrossRef] [PubMed]
- Fawole, F.J.; Labh, S.N.; Hossain, M.S.; Overturf, K.; Small, B.C.; Welker, T.L.; Hardy, R.W.; Kumar, V. Insect (Black Soldier Fly Larvae) Oil as a Potential Substitute for Fish or Soy Oil in the Fish Meal-Based Diet of Juvenile Rainbow Trout (Oncorhynchus mykiss). Anim. Nutr. 2021, 7, 1360–1370. [Google Scholar] [CrossRef]
- Peguero, D.A.; Gold, M.; Vandeweyer, D.; Zurbrügg, C.; Mathys, A. A Review of Pretreatment Methods to Improve Agri-Food Waste Bioconversion by Black Soldier Fly Larvae. Front. Sustain. Food Syst. 2022, 5, 745894. [Google Scholar] [CrossRef]
- Green, T. A Biochemical Analysis of Black Soldier Fly (Hermetia illucens) Larval Frass Plant Growth Promoting Activity. PLoS ONE 2023, 18, e0288913. [Google Scholar] [CrossRef]
- Guidini Lopes, I.; Wiklicky, V.; Ermolaev, E.; Lalander, C. Dynamics of Black Soldier Fly Larvae Composting—Impact of Substrate Properties and Rearing Conditions on Process Efficiency. Waste Manag. 2023, 172, 25–32. [Google Scholar] [CrossRef]
- Rampure, S.M.; Velayudhannair, K.; Marimuthu, N. Characteristics of Chitin Extracted from Different Growth Phases of Black Soldier Fly, Hermetia illucens, Fed with Different Organic Wastes. Int. J. Trop. Insect Sci. 2023, 43, 979–987. [Google Scholar] [CrossRef]
- Khayrova, A.; Lopatin, S.; Varlamov, V. Black Soldier Fly Hermetia illucens as a Novel Source of Chitin and Chitosan. Int. J. Sci. 2019, 8, 81–86. [Google Scholar] [CrossRef]
- Wang, H.; Rehman, K.U.; Feng, W.; Yang, D.; Rehman, R.U.; Cai, M.; Zhang, J.; Yu, Z.; Zheng, L. Physicochemical Structure of Chitin in the Developing Stages of Black Soldier Fly. Int. J. Biol. Macromol. 2020, 149, 901–907. [Google Scholar] [CrossRef]
- Mirwandhono, E.; Nasution, M.I.A.; Yunilas. Extraction of Chitin and Chitosan Black Soldier Fly (Hermetia illucens) Prepupa Phase on Characterization and Yield. IOP Conf. Ser. Earth Environ. Sci. 2022, 1114, 012019. [Google Scholar] [CrossRef]
- Hahn, T.; Tafi, E.; von Seggern, N.; Falabella, P.; Salvia, R.; Thomä, J.; Febel, E.; Fijalkowska, M.; Schmitt, E.; Stegbauer, L.; et al. Purification of Chitin from Pupal Exuviae of the Black Soldier Fly. Waste Biomass Valorization 2022, 13, 1993–2008. [Google Scholar] [CrossRef]
- Aher, P.D.; Patil, Y.D.; Waysal, S.M.; Bhoi, A.M. Critical Review on Biopolymer Composites Used in Concrete. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Shanmugavel, D.; Selvaraj, T.; Ramadoss, R.; Raneri, S. Interaction of a Viscous Biopolymer from Cactus Extract with Cement Paste to Produce Sustainable Concrete. Constr. Build. Mater. 2020, 257, 119585. [Google Scholar] [CrossRef]
- Mohesson, H.M.; Abbas, W.A. Effect of Biopolymer Alginate on Some Properties of Concrete. J. Eng. 2020, 26, 121–131. [Google Scholar] [CrossRef]
- Mohamed, A.M.; Ariffin, M.A.M.; Smaoui, H.; Osman, M.H. Performance Evaluation of Concrete with Arabic Gum Biopolymer. Mater. Today Proc. 2019, 39, 983–987. [Google Scholar] [CrossRef]
- Depan, D.; Khattak, M. Development of Green Concrete Reinforced with Renewable Chitin Nanowhiskers; No. FHWA/LA. 17; Louisiana Department of Transportation and Development Louisiana Transportation Research Center: Baton Rouge, LA, USA, 2021. [Google Scholar]
- Baykara, H.; Riofrio, A.; Garcia-Troncoso, N.; Cornejo, M.; Tello-Ayala, K.; Flores Rada, J.; Caceres, J. Chitosan-Cement Composite Mortars: Exploring Interactions, Structural Evolution, Environmental Footprint and Mechanical Performance. ACS Omega 2024, 9, 24978–24986. [Google Scholar] [CrossRef]
- Nisticò, R.; Lavagna, L.; Versaci, D.; Ivanchenko, P.; Benzi, P. Chitosan and Its Char as Fillers in Cement-Base Composites: A Case Study. Bol. Soc. Esp. Ceram. Vidr. 2020, 59, 186–192. [Google Scholar] [CrossRef]
- Choi, S.J.; Bae, S.H.; Lee, J.I.; Bang, E.J.; Ko, H.M. Strength, Carbonation Resistance, and Chloride-ion Penetrability of Cement Mortars Containing Catechol-functionalized Chitosan Polymer. Materials 2021, 14, 6395. [Google Scholar] [CrossRef]
- Choi, S.J.; Bae, S.H.; Choi, H.Y.; Ko, H.M. Engineering Characteristics of Cement Composites Containing a Chitosan-Based Polymer and Steel Slag Aggregates. Polymers 2022, 14, 626. [Google Scholar] [CrossRef]
- Yehia, S.; Ibrahim, A.M.; Ahmed, D.F. The Impact of Using Natural Waste Biopolymer Cement on the Properties of Traditional/Fibrous Concrete. Innov. Infrastruct. Solut. 2023, 8, 287. [Google Scholar] [CrossRef]
- Qin, Z.; Wu, J.; Hei, Z.; Wang, L.; Lei, D.; Liu, K.; Li, Y. Study on the Effect of Citric Acid-Modified Chitosan on the Mechanical Properties, Shrinkage Properties, and Durability of Concrete. Materials 2024, 17, 2053. [Google Scholar] [CrossRef] [PubMed]
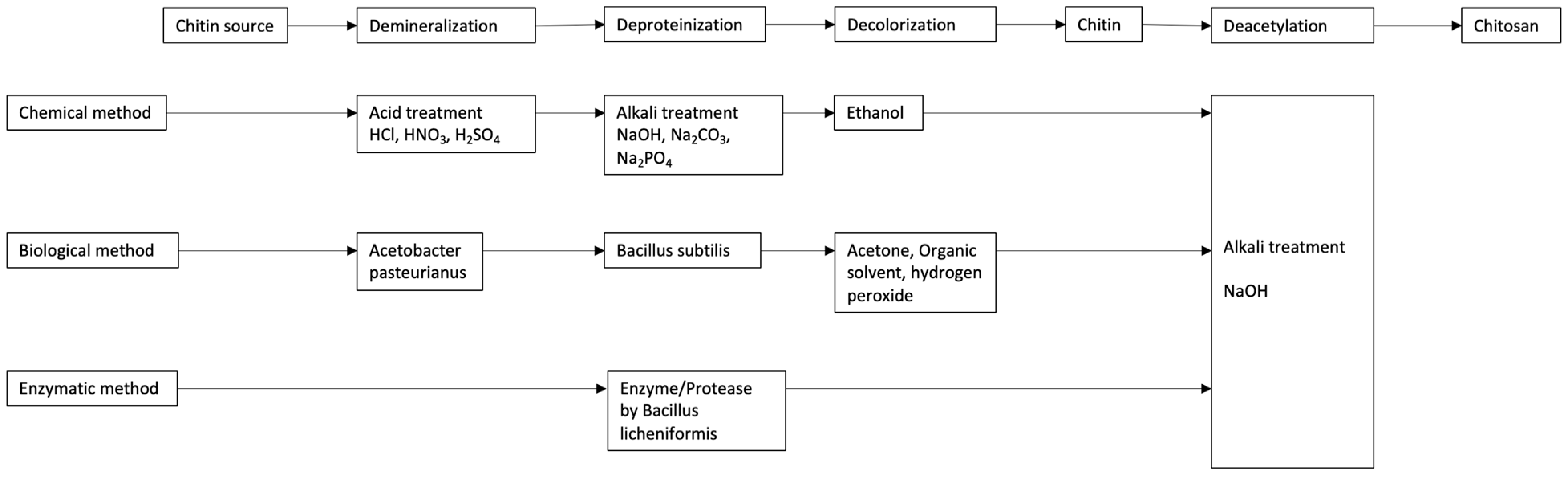
| Method | Process Description | Advantages | Disadvantages | References |
|---|---|---|---|---|
| Chemical | Acid and alkali treatment for demineralization, deproteinization, and deacetylation | High-efficiency and fast process | Degradation of polymer structure, harsh chemicals, and environmental concerns | [14,34,35,36] |
| Bio-based | Biological use of microorganisms (bacteria or fungi) for demineralization and deproteinization. | It is an eco-friendly, mild process and retains polymer quality | Slower process and variability in yield | [37,38,39] |
| Enzyme-based deproteinization using proteases (e.g., papain, trypsin) | Selective reaction and preserve polymer integrity | It is expensive and requires optimization for large-scale use | [17,41] |
| Growth Stage | Isolation Method | Chitin Content (%) | Degree Deacetylation (DDA%) | Crystallinity Index (%) | Reference |
|---|---|---|---|---|---|
| Larva | Chemical | 96.3 | 92 | 89 | [35] |
| Chemical | 84.0 | 92 | 84 | [28] | |
| Chemical | 3.6 | - | 33.09 | [52] | |
| Prepupa | Chemical | 94.5 | 77.9 | 94 | [35] |
| Chemical | 79.9 | - | - | [17] | |
| Chemical | 18.05 | - | - | [53] | |
| Chemical | 3.1 | - | 35.14 | [52] | |
| Enzymatic | 47.6 | - | 27 | [17] | |
| Pupa | Chemical | 93.9 | 96.7 | 93 | [35] |
| Sheddings | Chemical | 75.7 | 93.4 | 90 | [35] |
| Cocoons | Chemical | 96.8 | 89.8 | 94 | [35] |
| Chemical | 86.8 | 90 | 62 | [28] | |
| Chemical | 23.82 | - | - | [38] | |
| Chemical | 14.1 | - | 68.44 | [52] | |
| Chemical/organic | 85 | 96 | 74 | [54] | |
| Biological | 59.9 | 18.52 | - | [38] | |
| Biological | 12.4 | 81.5 | 52.8 | [39] | |
| Adult fly | Chemical | 95.7 | - | 89 | [35] |
| Chemical | 85.3 | 93 | 93 | [28] | |
| Chemical | 11.99 | - | - | [38] | |
| Chemical | 2.9 | - | 87.92 | [52] | |
| Biological | 47.31 | 37.38 | - | [38] |
| Property | Chitin | Chitosan | References |
|---|---|---|---|
| Compressive Strength | High | Low | [32,57,64] |
| Tensile Strength (MPa) | Higher due to crystallinity | Lower than chitin; it depends on the degree of deacetylation | [61,62,65] |
| Elasticity | Brittle and rigid | More flexible compared to chitin | [56,59,63] |
| Solubility | Insoluble in water and most solvents | Soluble in acidic solutions (pH < 6.5) | [58,60] |
| Biodegradability | Slow | Faster than chitin | [39] |
| Biopolymer | Source | Advantages | Disadvantages | Reference |
|---|---|---|---|---|
| - | Cactus (Opuntia Ficus Indica) |
|
| [56] |
| Alginate | - |
|
| [57] |
| Arabic gum | - |
|
| [58] |
| Chitin nanowhiskers | - |
|
| [59] |
| Chitin | Shrimp shells |
|
| [33] |
| Chitosan | Shellfish |
|
| [61] |
| Chitosan | - |
|
| [62] |
| Chitosan | - |
|
| [63] |
| Chitosan | Shrimp shells |
|
| [64] |
| Chitosan | Shrimp shells |
|
| [60] |
| Chitosan | - |
|
| [65] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-Lara, H.; Parra-Pacheco, B.; Rico-García, E.; Aguirre-Becerra, H.; Feregrino-Pérez, A.A.; García-Trejo, J.F. Black Soldier Fly Culture as a Source of Chitin and Chitosan for Its Potential Use in Concrete: An Overview. Polymers 2025, 17, 717. https://doi.org/10.3390/polym17060717
González-Lara H, Parra-Pacheco B, Rico-García E, Aguirre-Becerra H, Feregrino-Pérez AA, García-Trejo JF. Black Soldier Fly Culture as a Source of Chitin and Chitosan for Its Potential Use in Concrete: An Overview. Polymers. 2025; 17(6):717. https://doi.org/10.3390/polym17060717
Chicago/Turabian StyleGonzález-Lara, Hugo, Benito Parra-Pacheco, Enrique Rico-García, Humberto Aguirre-Becerra, Ana Angélica Feregrino-Pérez, and Juan Fernando García-Trejo. 2025. "Black Soldier Fly Culture as a Source of Chitin and Chitosan for Its Potential Use in Concrete: An Overview" Polymers 17, no. 6: 717. https://doi.org/10.3390/polym17060717
APA StyleGonzález-Lara, H., Parra-Pacheco, B., Rico-García, E., Aguirre-Becerra, H., Feregrino-Pérez, A. A., & García-Trejo, J. F. (2025). Black Soldier Fly Culture as a Source of Chitin and Chitosan for Its Potential Use in Concrete: An Overview. Polymers, 17(6), 717. https://doi.org/10.3390/polym17060717






