Design and Application of Antifouling Bio-Coatings
Abstract
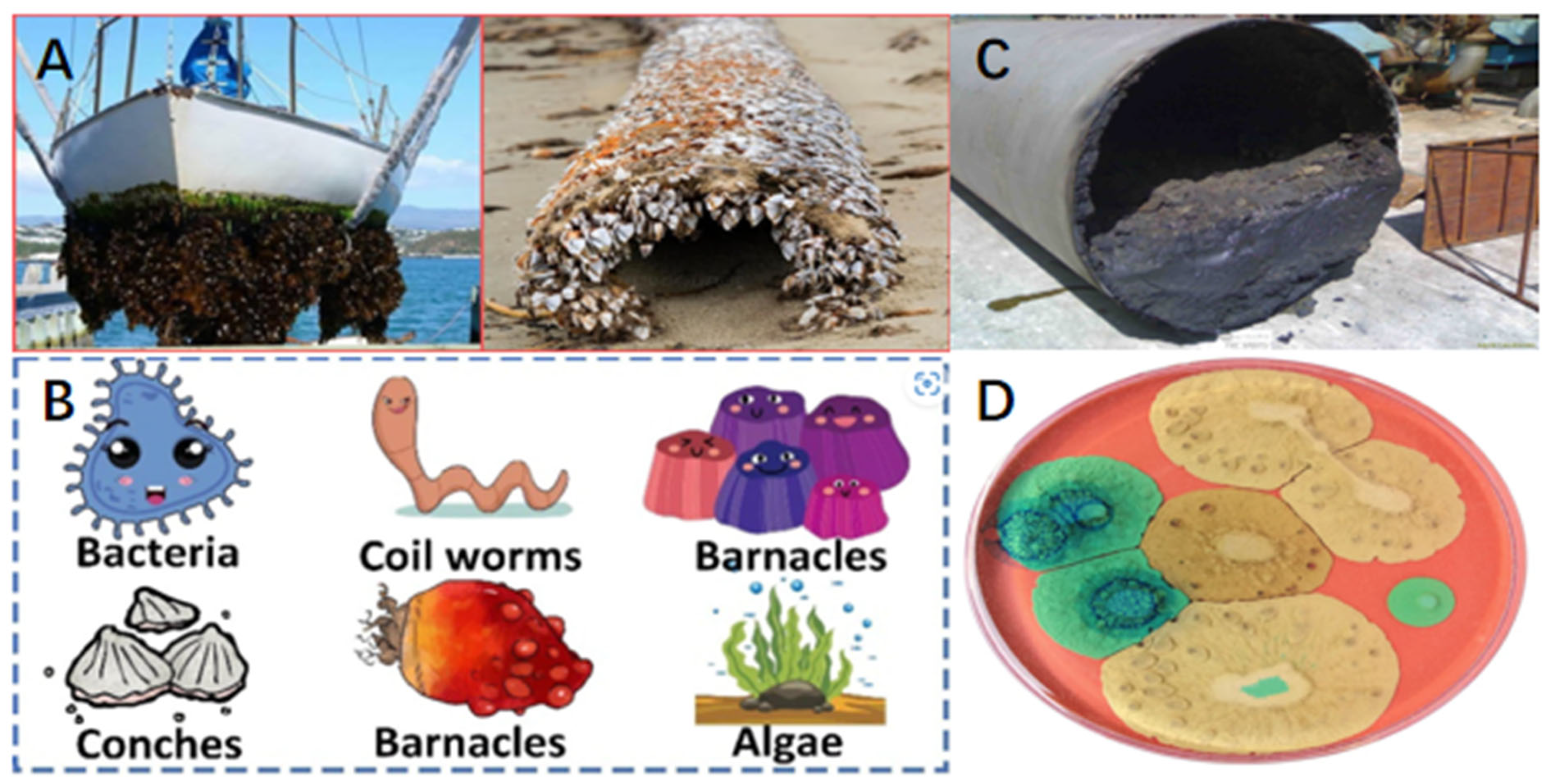
1. Introduction
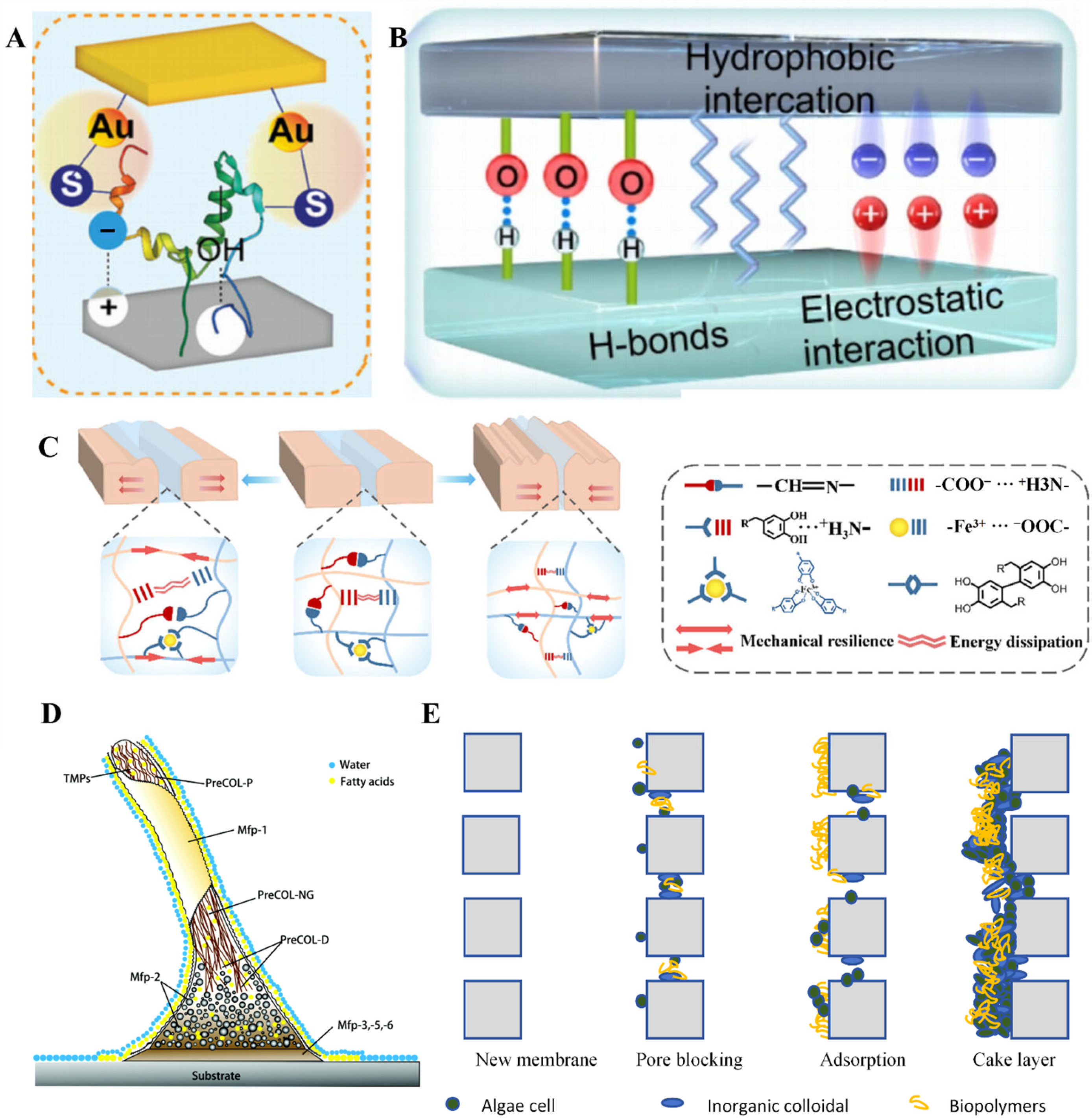
2. Mechanisms of Contaminant Interface Adhesion
2.1. The Interaction Between Contaminant and Interface
2.2. The Adhesion Process of Interface Contaminant
3. Antifouling Mechanisms
3.1. Surface Free Energy
3.2. Surface Wettability
3.3. Surface Microstructure
3.4. Surface Superslippery
4. Strategies for Constructing Antifouling Bio-Coatings
4.1. Superhydrophilic Interface
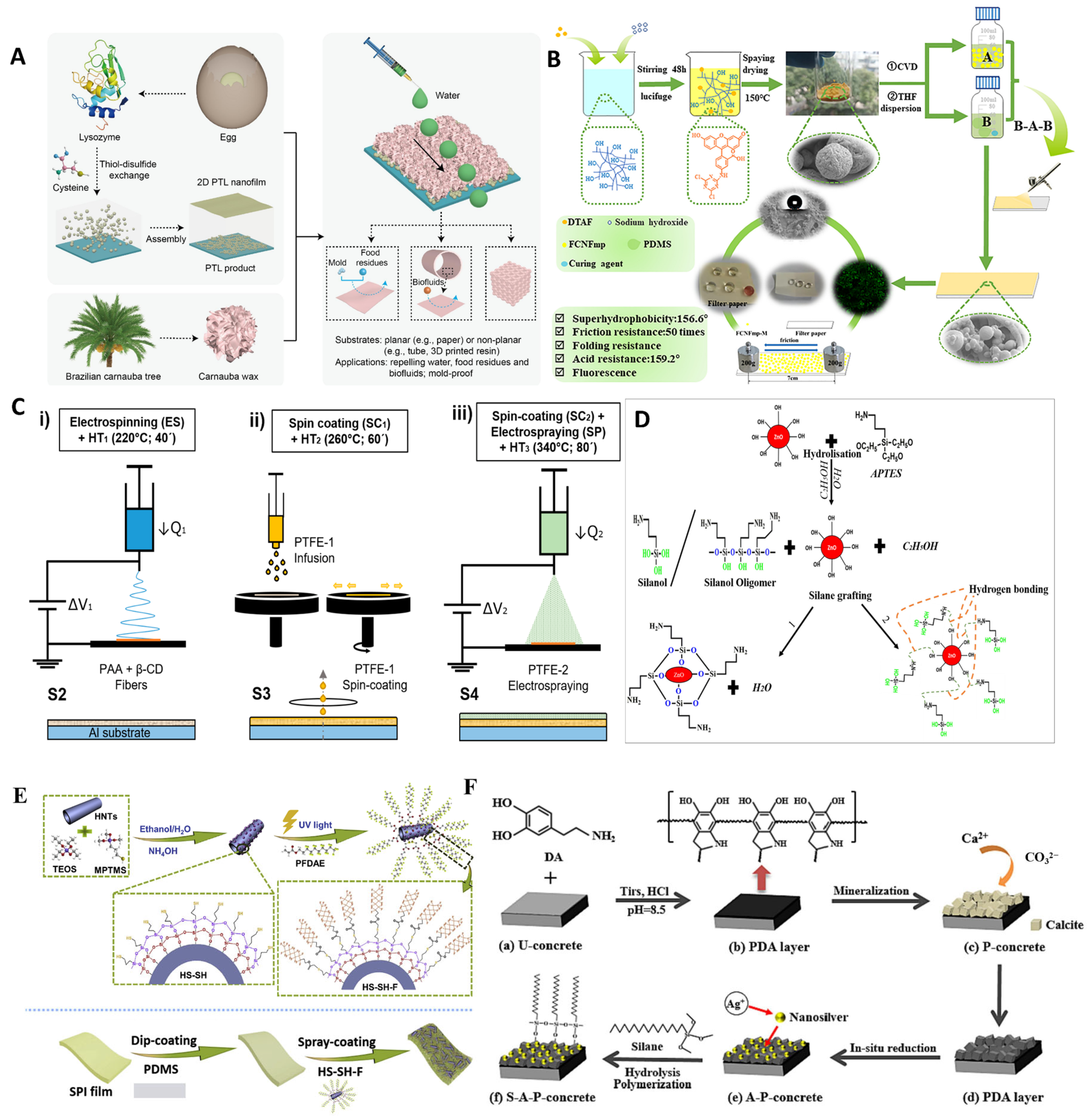
4.1.1. Polymer Blending Strategy
4.1.2. Protein Phase Transition Self-Assembly Strategy
4.1.3. Chemical Deposition Strategy
4.1.4. Spin-Coating and Dip-Coating Strategy
4.2. Superhydrophobic Surfaces
4.2.1. Spray Coating Strategy
4.2.2. Chemical Deposition Strategy
4.2.3. Sol–Gel Strategy
4.2.4. Organic–Inorganic Hybridization Strategy
4.3. Superlubric Interface
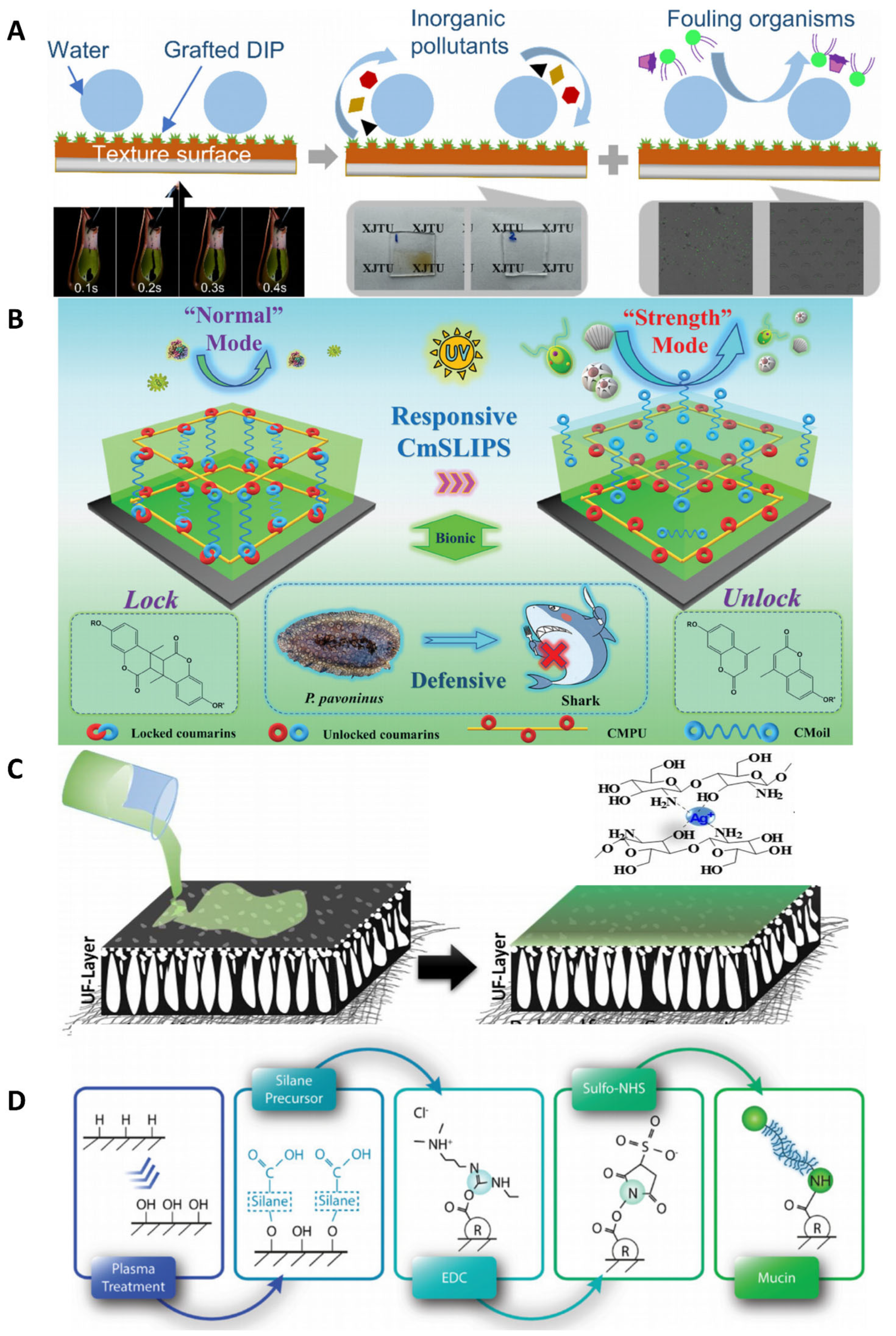
4.3.1. Slippery Liquid-Infused Porous Surfaces Strategy
4.3.2. Physical Deposition Strategy
4.3.3. Chemical Deposition Strategy
5. Application Directions of Antifouling Bio-Coatings
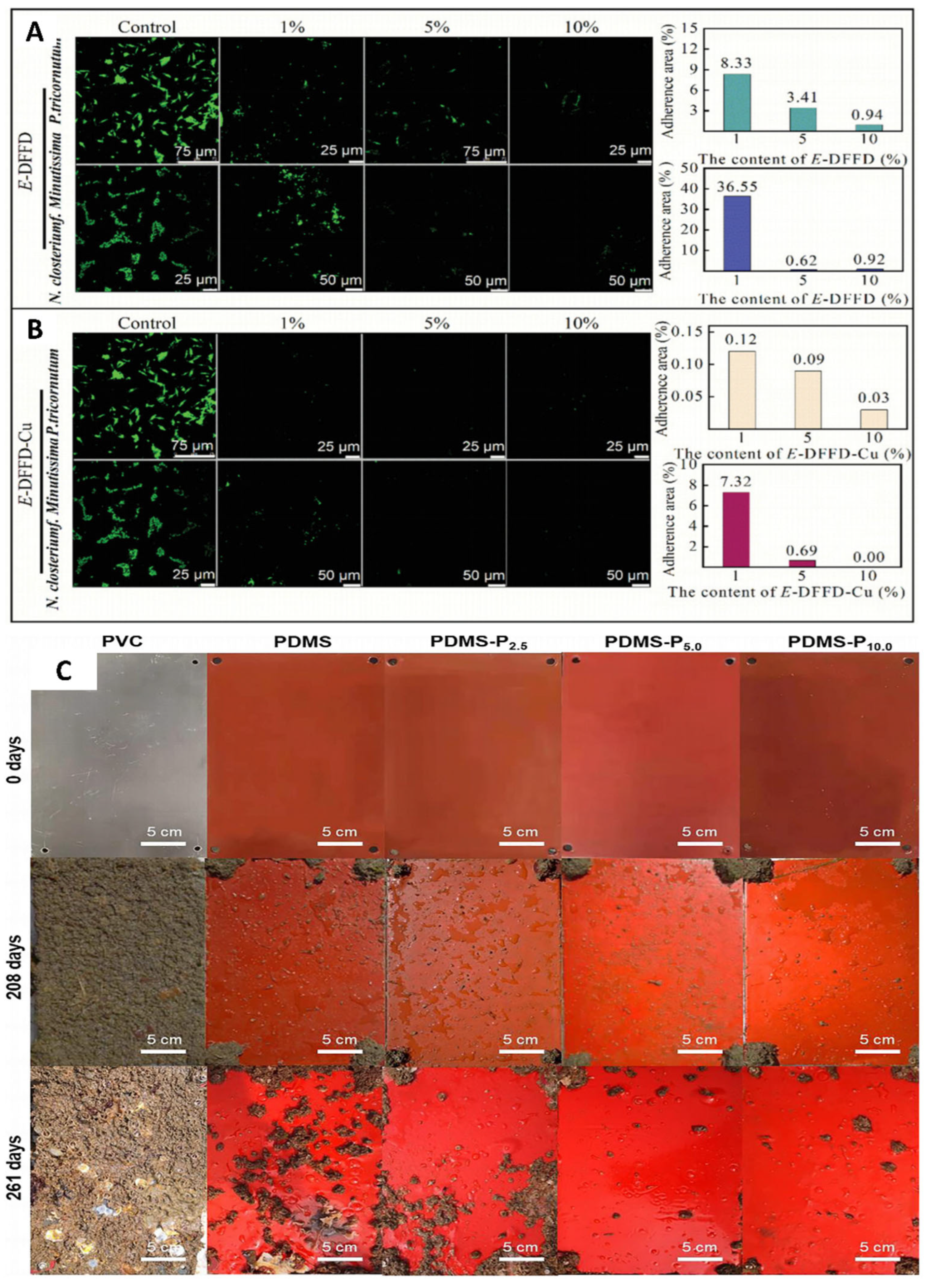
5.1. Marine Antifouling Bio-Coatings
5.1.1. Anti-Bio-Fouling on Ship Surfaces
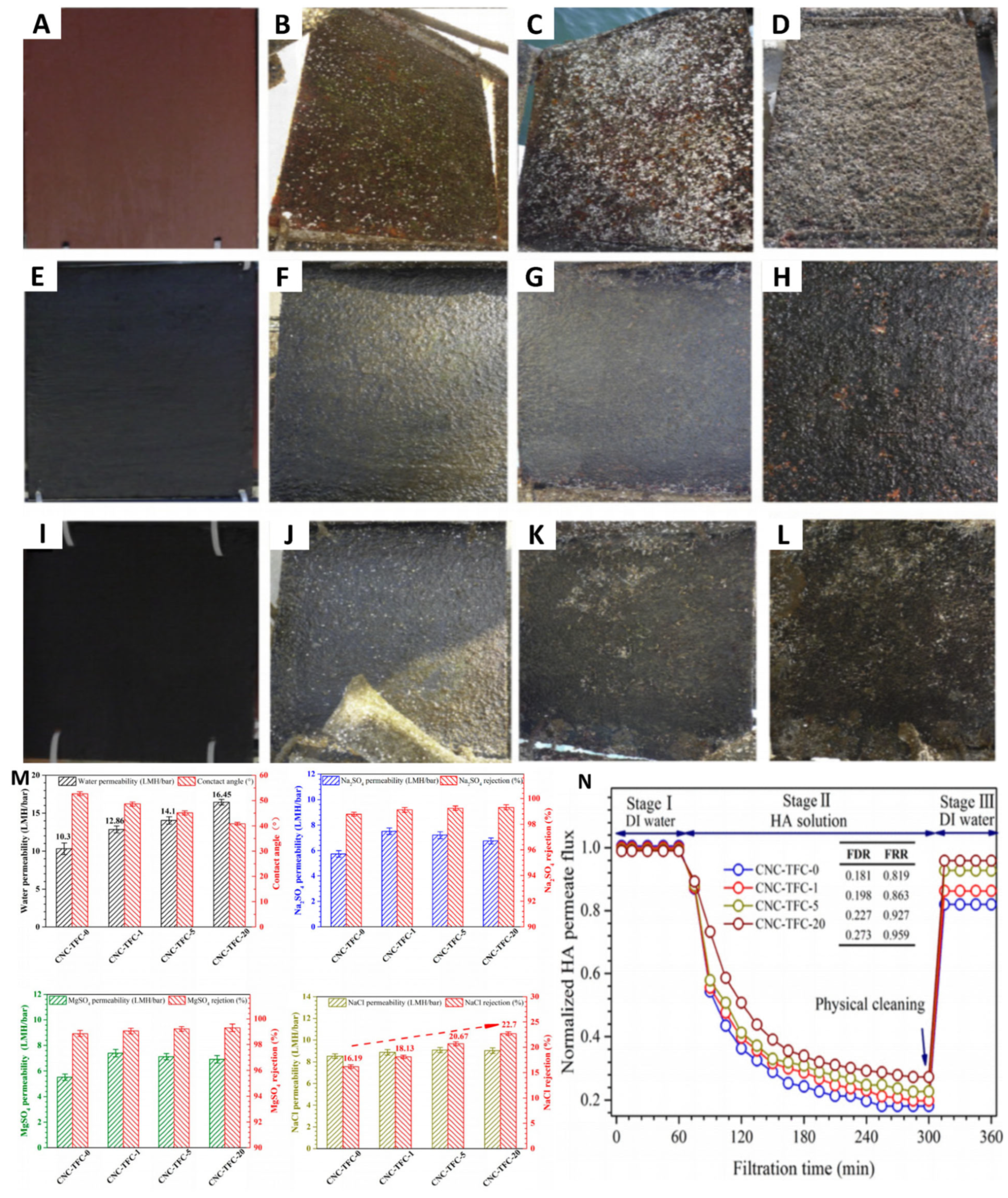
5.1.2. Seawater Desalination
5.2. Medical Antifouling Bio-Coatings
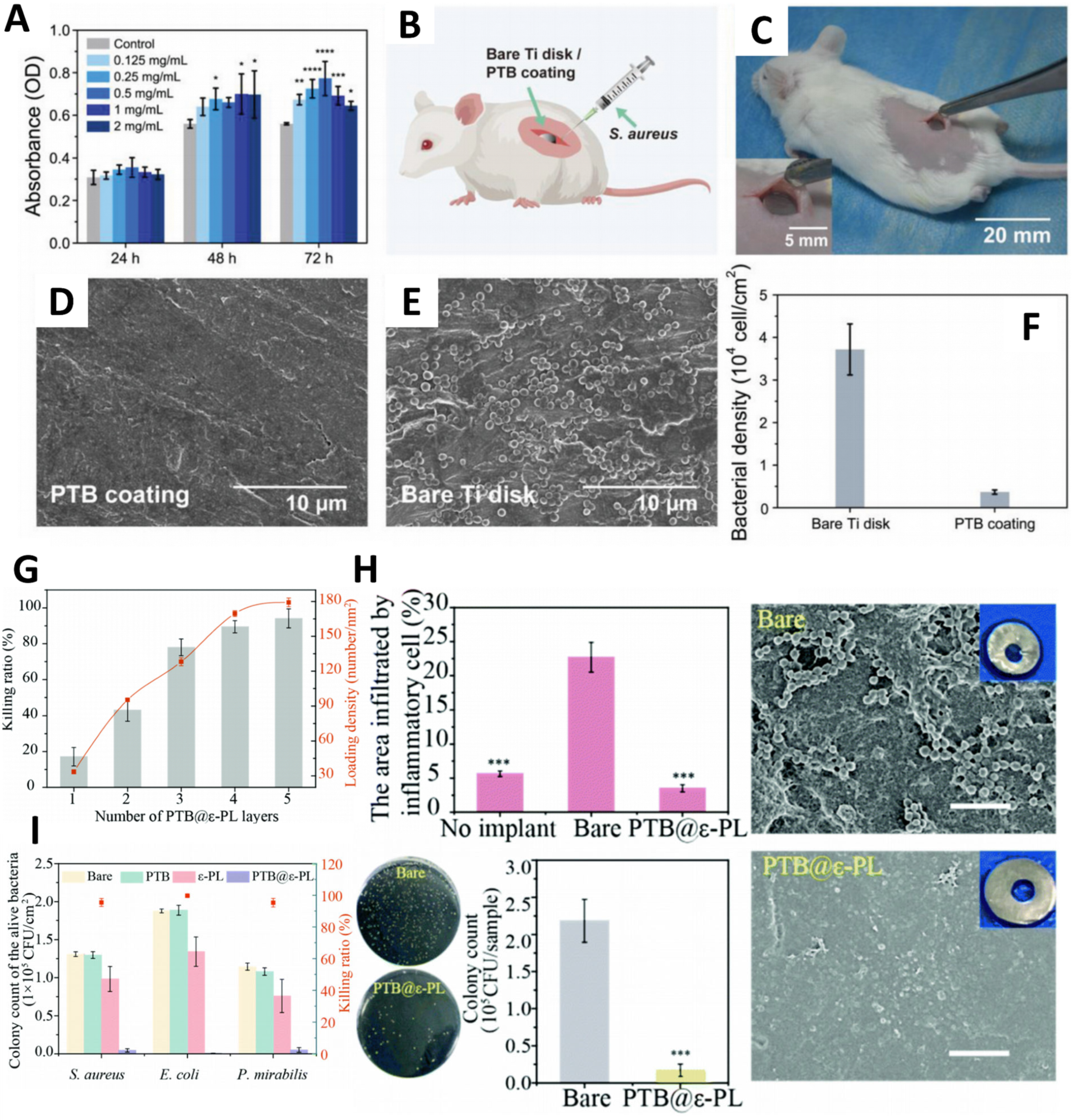
5.2.1. Antifouling of Implant Surfaces
5.2.2. Antifouling of Medical Device Surfaces
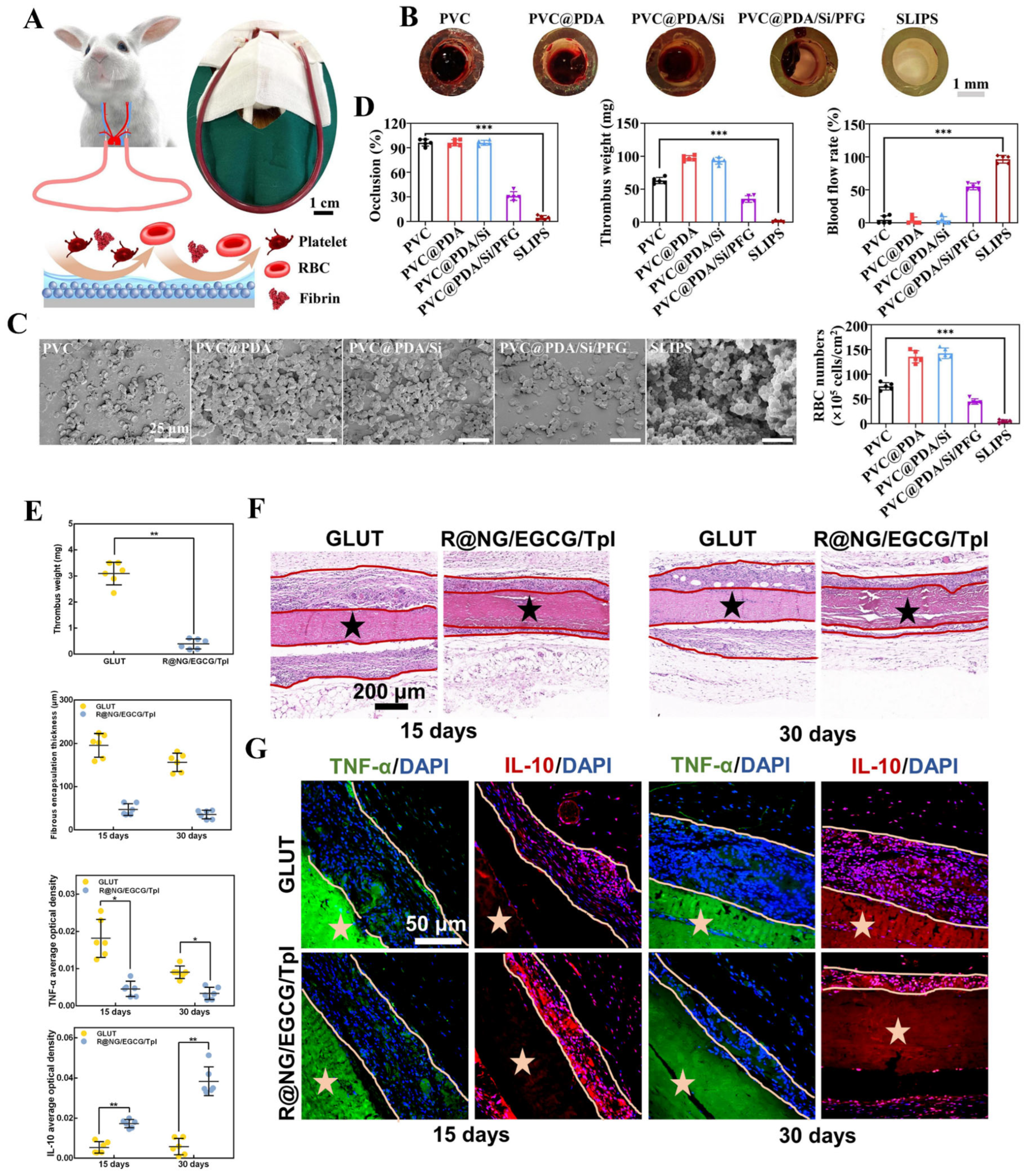
5.2.3. Anticoagulant and Anti-Inflammatory Coatings for Medical Devices
5.3. Antilipid Bio-Coatings
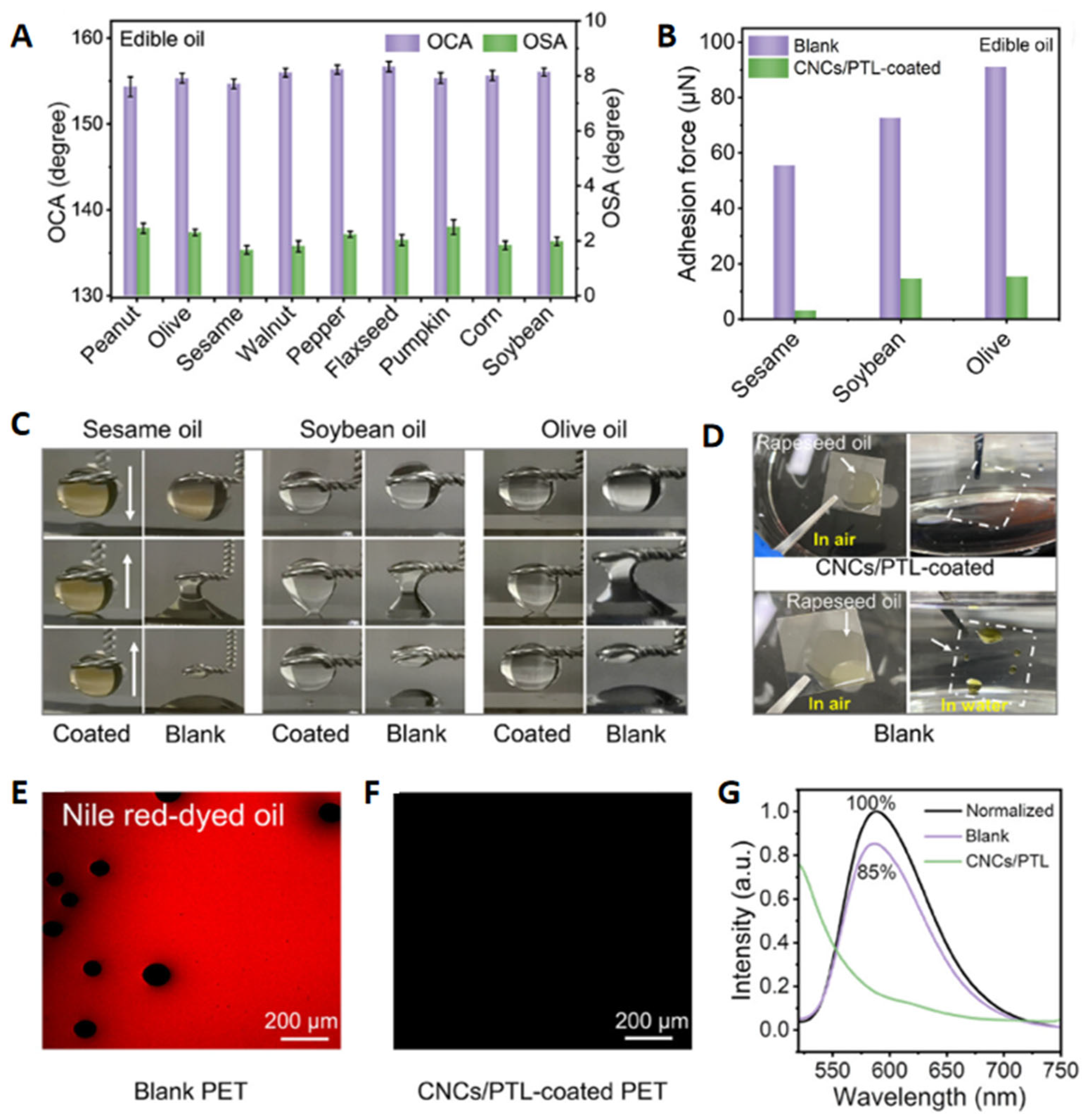
5.3.1. Antilipid Pollution Adhesion
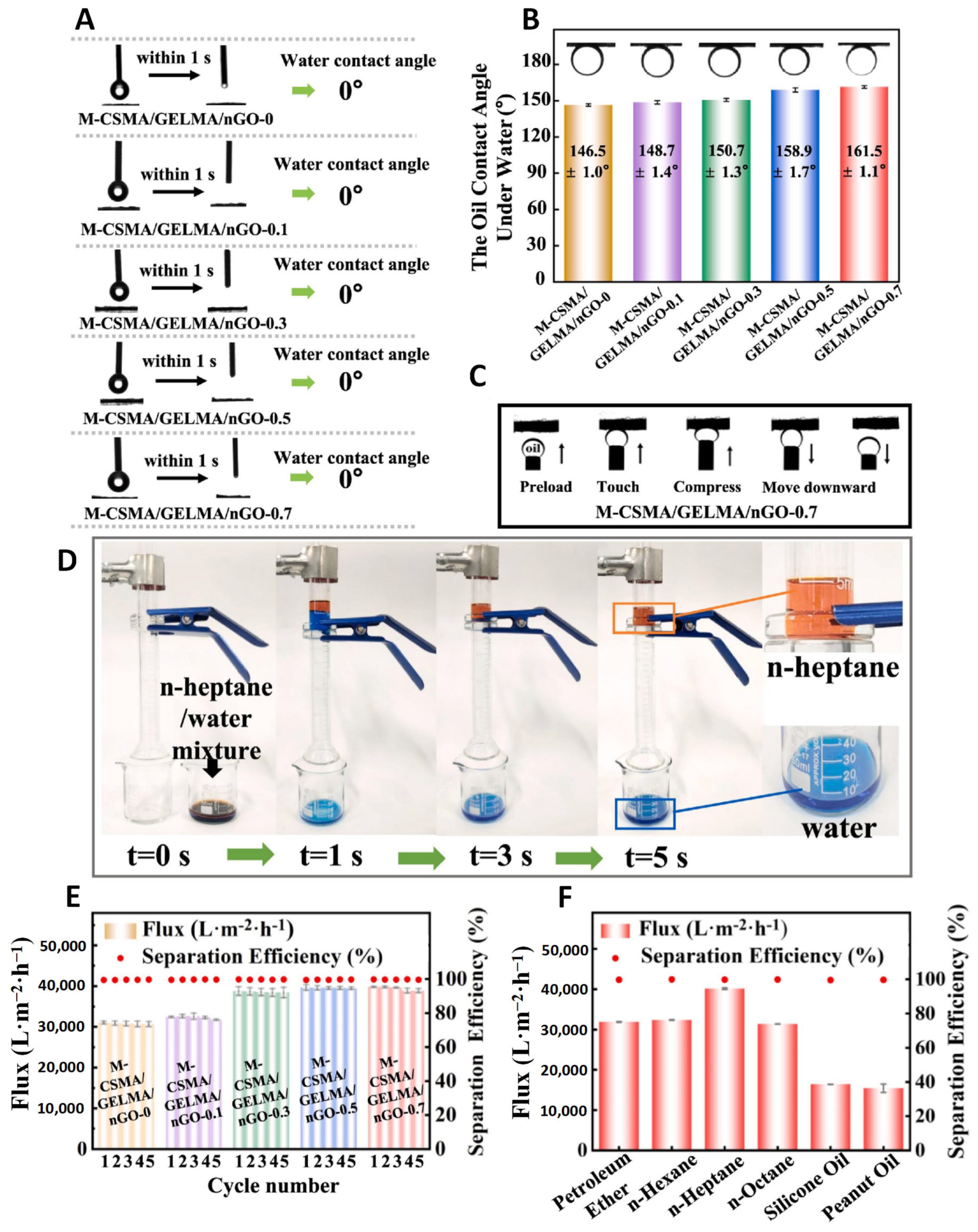
5.3.2. Oil–Water Separation
5.4. Antiscale and Anti-Ice Bio-Coating
5.4.1. Antiscale Coatings for Metal Surfaces
5.4.2. Antiscale Coatings
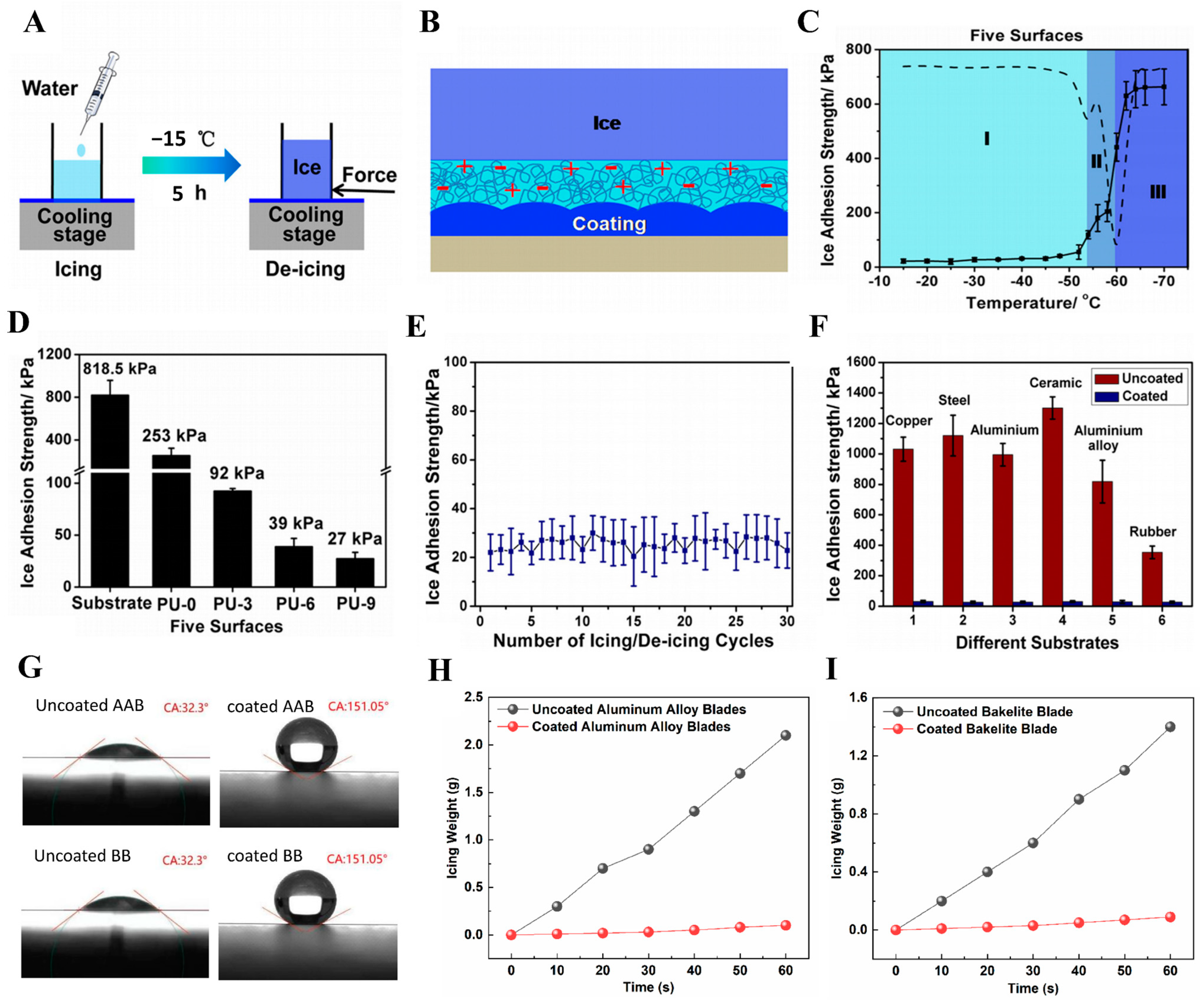
5.4.3. Anti-Icing Coatings for Metal Surfaces
5.4.4. Anti-Icing Coatings for Wind Turbine Facilities
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Banerjee, I.; Pangule, R.C.; Kane, R.S. Antifouling Coatings: Recent Developments in the Design of Surfaces That Prevent Fouling by Proteins, Bacteria, and Marine Organisms. Adv. Mater. 2011, 23, 690–718. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Guo, Z. Bioinspired surfaces with wettability for antifouling application. Nanoscale 2019, 11, 22636–22663. [Google Scholar] [CrossRef] [PubMed]
- Zang, X.; Ni, Y.; Wang, Q.; Cheng, Y.; Huang, J.; Cao, X.; Carmalt, C.J.; Lai, Y.; Ha Kim, D.; Liu, Y.; et al. Non-toxic evolution: Advances in multifunctional antifouling coatings. Mater. Today 2024, 75, 210–243. [Google Scholar] [CrossRef]
- Liu, S.; Guo, W. Anti-Biofouling and Healable Materials: Preparation, Mechanisms, and Biomedical Applications. Adv. Funct. Mater. 2018, 28, 1800596. [Google Scholar] [CrossRef]
- Amini, S.; Kolle, S.; Petrone, L.; Ahanotu, O.; Sunny, S.; Sutanto, C.N.; Hoon, S.; Cohen, L.; Weaver, J.C.; Aizenberg, J.; et al. Preventing mussel adhesion using lubricant-infused materials. Science 2017, 357, 668–673. [Google Scholar] [CrossRef]
- Francolini, I.; Vuotto, C.; Piozzi, A.; Donelli, G. Antifouling and antimicrobial biomaterials: An overview. APMIS 2017, 125, 392–417. [Google Scholar] [CrossRef]
- Filipović, U.; Dahmane, R.G.; Ghannouchi, S.; Zore, A.; Bohinc, K. Bacterial adhesion on orthopedic implants. Adv. Colloid Interface Sci. 2020, 283, 102228. [Google Scholar] [CrossRef]
- Yang, K.; Shi, J.; Wang, L.; Chen, Y.; Liang, C.; Yang, L.; Wang, L.-N. Bacterial anti-adhesion surface design: Surface patterning, roughness and wettability: A review. J. Mater. Sci. Technol. 2022, 99, 82–100. [Google Scholar] [CrossRef]
- Amin Yavari, S.; Castenmiller, S.M.; van Strijp, J.A.G.; Croes, M. Combating Implant Infections: Shifting Focus from Bacteria to Host. Adv. Mater. 2020, 32, 2002962. [Google Scholar] [CrossRef]
- Willers, A.; Arens, J.; Mariani, S.; Pels, H.; Maessen, J.G.; Hackeng, T.M.; Lorusso, R.; Swol, J. New Trends, Advantages and Disadvantages in Anticoagulation and Coating Methods Used in Extracorporeal Life Support Devices. Membranes 2021, 11, 617. [Google Scholar] [CrossRef]
- Akhtar, S.; McCash, L.B.; Nadeem, S.; Saleem, A. Scientific breakdown for physiological blood flow inside a tube with multi-thrombosis. Sci. Rep. 2021, 11, 6718. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Man, J.; Liu, J.; Li, J.; Song, X.; Wang, J.; Li, J.; Chen, Y. Construction of the Mussel-Inspired PDAM/Lysine/Heparin Composite Coating Combining Multiple Anticoagulant Strategies. ACS Appl. Mater. Interfaces 2023, 15, 27719–27731. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, J.; Wang, X.-w.; Huang, Y.; Li, R.-y.; Wang, Y.-x.; Ren, K.-f.; Ji, J. A Tough, Slippery, and Anticoagulant Double-Network Hydrogel Coating. ACS Appl. Polym. Mater. 2022, 4, 5941–5951. [Google Scholar] [CrossRef]
- Magin, C.M.; Cooper, S.P.; Brennan, A.B. Non-toxic antifouling strategies. Mater. Today 2010, 13, 36–44. [Google Scholar] [CrossRef]
- Chambers, L.D.; Stokes, K.R.; Walsh, F.C.; Wood, R.J.K. Modern approaches to marine antifouling coatings. Surf. Coat. Technol. 2006, 201, 3642–3652. [Google Scholar] [CrossRef]
- Saha, R.; Bhattacharya, D.; Mukhopadhyay, M. Advances in modified antimicrobial peptides as marine antifouling material. Colloids Surf. B Biointerfaces 2022, 220, 112900. [Google Scholar] [CrossRef]
- Lu, T.-C.; Lin, Y.-T.; Xiao, W.-B.; Qiu, Q.-Z.; Tian, H.-Y.; Lei, Y.; Liu, A.-L. Reagent-free anti-fouling electrochemical immunosensor based on AL-BSA/AuNPs/PANI coating for the point-of-care detection of C-reactive protein in plasma and whole blood. Biosens. Bioelectron. 2024, 264, 116667. [Google Scholar] [CrossRef]
- Kunam, P.K.; Ramakanth, D.; Akhila, K.; Gaikwad, K.K. Bio-based materials for barrier coatings on paper packaging. Biomass Convers. Biorefinery 2024, 14, 12637–12652. [Google Scholar] [CrossRef]
- Zhang, Y.; Ren, H.; Linghu, C.; Zhang, J.; Gao, A.; Su, H.; Miao, S.; Qin, R.; Hu, B.; Chen, X.; et al. Stabilizing Metal Coating on Flexible Devices by Ultrathin Protein Nanofilms. Adv. Mater. 2024, 36, 2412378. [Google Scholar] [CrossRef]
- Halake, K.; Cho, S.; Kim, J.; Lee, T.; Cho, Y.; Chi, S.; Park, M.; Kim, K.; Lee, D.; Ju, H.; et al. Applications Using the Metal Affinity of Polyphenols with Mussel-Inspired Chemistry. Macromol. Res. 2018, 26, 93–99. [Google Scholar] [CrossRef]
- Fuentes, C.; Brughmans, G.; Tran, L.; Dupont-Gillain, C.; Verpoest, I.; Van Vuure, A. Mechanical behaviour and practical adhesion at a bamboo composite interface: Physical adhesion and mechanical interlocking. Compos. Sci. Technol. 2015, 109, 40–47. [Google Scholar] [CrossRef]
- Wang, X.; Lei, R.; Li, L.; Fei, X.; Ju, R.; Sun, X.; Cao, H.; Zhang, Q.; Chen, C.; Wang, X. Rearrangement of protein structures on a gold nanoparticle surface is regulated by ligand adsorption modes. Nanoscale 2021, 13, 20425–20436. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Gao, Z.; Xu, T.; Zhu, X.; Miao, X.; Song, Y.; Ren, G.; Li, X. Robust Hydrogel Coating with Oil-Repellent Property in Air, Water, and Oil Surroundings. ACS Appl. Mater. Interfaces 2020, 12, 49138–49145. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Chen, G.Y.; Xu, H.; Liu, X. Substrate-Independent, Transparent Oil-Repellent Coatings with Self-Healing and Persistent Easy-Sliding Oil Repellency. ACS Nano 2016, 10, 1076–1085. [Google Scholar] [CrossRef]
- Lv, Y.; Cai, F.; He, Y.; Li, L.; Huang, Y.; Yang, J.; Zheng, Y.; Shi, X. Multi-crosslinked hydrogels with strong wet adhesion, self-healing, antibacterial property, reactive oxygen species scavenging activity, and on-demand removability for seawater-immersed wound healing. Acta Biomater. 2023, 159, 95–110. [Google Scholar] [CrossRef]
- Bandara, N.; Zeng, H.; Wu, J. Marine mussel adhesion: Biochemistry, mechanisms, and biomimetics. J. Adhes. Sci. Technol. 2013, 27, 2139–2162. [Google Scholar] [CrossRef]
- Singla, S.; Jain, D.; Zoltowski, C.M.; Voleti, S.; Stark, A.Y.; Niewiarowski, P.H.; Dhinojwala, A. Direct evidence of acid-base interactions in gecko adhesion. Sci. Adv. 2021, 7, eabd9410. [Google Scholar] [CrossRef]
- He, Y.; Sun, C.; Jiang, F.; Yang, B.; Li, J.; Zhong, C.; Zheng, L.; Ding, H. Lipids as integral components in mussel adhesion. Soft Matter 2018, 14, 7145–7154. [Google Scholar] [CrossRef]
- Gohad, N.V.; Aldred, N.; Hartshorn, C.M.; Jong Lee, Y.; Cicerone, M.T.; Orihuela, B.; Clare, A.S.; Rittschof, D.; Mount, A.S. Synergistic roles for lipids and proteins in the permanent adhesive of barnacle larvae. Nat. Commun. 2014, 5, 4414. [Google Scholar] [CrossRef]
- Hart, L.F.; Hertzog, J.E.; Rauscher, P.M.; Rawe, B.W.; Tranquilli, M.M.; Rowan, S.J. Material properties and applications of mechanically interlocked polymers. Nat. Rev. Mater. 2021, 6, 508–530. [Google Scholar] [CrossRef]
- Polyakov, Y.S.; Zydney, A.L. Ultrafiltration membrane performance: Effects of pore blockage/constriction. J. Membr. Sci. 2013, 434, 106–120. [Google Scholar] [CrossRef]
- Liao, Y.; Bokhary, A.; Maleki, E.; Liao, B. A review of membrane fouling and its control in algal-related membrane processes. Bioresour. Technol. 2018, 264, 343–358. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Vance, T.D.R.; Stevens, C.A.; Voets, I.; Davies, P.L. RTX Adhesins are Key Bacterial Surface Megaproteins in the Formation of Biofilms. Trends Microbiol. 2019, 27, 453–467. [Google Scholar] [CrossRef]
- Ali, A.; Culliton, D.; Fahad, S.; Ali, Z.; Kang, E.-T.; Xu, L. Nature-inspired anti-fouling strategies for combating marine biofouling. Prog. Org. Coat. 2024, 189, 108349. [Google Scholar] [CrossRef]
- Ma, Y.; Zhang, B.; Frenkel, I.; Zhang, Z.; Pei, X.; Zhou, F.; He, X. Mussel-Inspired Underwater Adhesives-from Adhesion Mechanisms to Engineering Applications: A Critical Review. In Progress in Adhesion and Adhesives; Wiley Online Books: Hoboken, NJ, USA, 2021; pp. 739–759. [Google Scholar]
- Li, K.; Xu, Z.; Liu, X.; He, Y.; Tian, X.; Xu, X.; Bo, G.; Yuan, S.; Xu, L.; Yang, M.; et al. Mussel foot inspired bionic adhesive material enhanced by a reconstructed in vitro system for interfacial adhesion. Chem. Eng. J. 2023, 452, 139580. [Google Scholar] [CrossRef]
- Tarakhovskaya, E.R. Mechanisms of bioadhesion of macrophytic algae. Russ. J. Plant Physiol. 2014, 61, 19–25. [Google Scholar] [CrossRef]
- Lebret, K.; Thabard, M.; Hellio, C. 4—Algae as marine fouling organisms: Adhesion damage and prevention. In Advances in Marine Antifouling Coatings and Technologies; Hellio, C., Yebra, D., Eds.; Woodhead Publishing: Cambridge, UK, 2009; pp. 80–112. [Google Scholar]
- Liang, C.; Strickland, J.; Ye, Z.; Wu, W.; Hu, B.; Rittschof, D. Biochemistry of Barnacle Adhesion: An Updated Review. Front. Mar. Sci. 2019, 6, 565. [Google Scholar] [CrossRef]
- Edelman, G.M. Cell Adhesion Molecules. Science 1983, 219, 450–457. [Google Scholar] [CrossRef]
- Nicolas, A.; Safran, S.A. Limitation of Cell Adhesion by the Elasticity of the Extracellular Matrix. Biophys. J. 2006, 91, 61–73. [Google Scholar] [CrossRef]
- Xu, X.R.; Carrim, N.; Neves, M.A.D.; McKeown, T.; Stratton, T.W.; Coelho, R.M.P.; Lei, X.; Chen, P.; Xu, J.; Dai, X.; et al. Platelets and platelet adhesion molecules: Novel mechanisms of thrombosis and anti-thrombotic therapies. Thromb. J. 2016, 14, 29. [Google Scholar] [CrossRef]
- Sang, Y.; Roest, M.; de Laat, B.; de Groot, P.G.; Huskens, D. Interplay between platelets and coagulation. Blood Rev. 2021, 46, 100733. [Google Scholar] [CrossRef] [PubMed]
- Berrier, A.L.; Yamada, K.M. Cell–matrix adhesion. J. Cell. Physiol. 2007, 213, 565–573. [Google Scholar] [CrossRef]
- Schlüter, F.; Schnöing, L.; Zettler, H.; Augustin, W.; Scholl, S. Measuring Local Crystallization Fouling in a Double-Pipe Heat Exchanger. Heat Transf. Eng. 2020, 41, 149–159. [Google Scholar] [CrossRef]
- Fernandes, S.; Gomes, I.B.; Simões, L.C.; Simões, M. Overview on the hydrodynamic conditions found in industrial systems and its impact in (bio)fouling formation. Chem. Eng. J. 2021, 418, 129348. [Google Scholar] [CrossRef]
- Meng, L.; Chen, X.; Cai, T.; Tong, X.; Wang, Z. Surface energy-induced anti-wetting and anti-fouling enhancement of Janus membrane for membrane distillation. Water Res. 2024, 263, 122176. [Google Scholar] [CrossRef]
- Prakash, C.G.J.; Prasanth, R. Approaches to design a surface with tunable wettability: A review on surface properties. J. Mater. Sci. 2021, 56, 108–135. [Google Scholar] [CrossRef]
- Gururani, R.; Pandit, S.K.; Kumari, P.; Kumar, A. Towards the Development of One-Step Scalable Self-Cleaning and Stain-Resistant Coating on Cellulosic Wood. Fibers Polym. 2024, 25, 1779–1788. [Google Scholar] [CrossRef]
- Lin, Y.-T.; Wu, C.-H.; Syu, W.-L.; Ho, P.-C.; Tseng, Z.-L.; Yang, M.-C.; Lin, C.-C.; Chen, C.-C.; Chen, C.-C.; Liu, T.-Y. Replica of Bionic Nepenthes Peristome-like and Anti-Fouling Structures for Self-Driving Water and Raman-Enhancing Detection. Polymers 2022, 14, 2465. [Google Scholar] [CrossRef]
- Jin, H.; Tian, L.; Bing, W.; Zhao, J.; Ren, L. Bioinspired marine antifouling coatings: Status, prospects, and future. Prog. Mater. Sci. 2022, 124, 100889. [Google Scholar] [CrossRef]
- Ober, C. Fifty years of the Baier curve: Progress in understanding antifouling coatings. Green Mater. 2017, 5, 1–3. [Google Scholar] [CrossRef]
- Tran, T.N. Enhancing Antifouling Properties of Ultrafiltration Membranes: Learning from Baier’s Curve. Ph.D. Dissertation, State University of New York at Buffalo, Buffalo, NY, USA, 2020. [Google Scholar]
- Atthi, N.; Sripumkhai, W.; Pattamang, P.; Thongsook, O.; Srihapat, A.; Meananeatra, R.; Supadech, J.; Klunngien, N.; Jeamsaksiri, W. Fabrication of robust PDMS micro-structure with hydrophobic and antifouling properties. Microelectron. Eng. 2020, 224, 111255. [Google Scholar] [CrossRef]
- Sigal, G.B.; Mrksich, M.; Whitesides, G.M. Effect of Surface Wettability on the Adsorption of Proteins and Detergents. J. Am. Chem. Soc. 1998, 120, 3464–3473. [Google Scholar] [CrossRef]
- Falde, E.J.; Yohe, S.T.; Colson, Y.L.; Grinstaff, M.W. Superhydrophobic materials for biomedical applications. Biomaterials 2016, 104, 87–103. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wang, Z.; Huang, S.; Pan, Y.; Zhao, X. Flexible, durable, and unconditioned superoleophobic/superhydrophilic surfaces for controllable transport and oil–water separation. Adv. Funct. Mater. 2018, 28, 1706867. [Google Scholar] [CrossRef]
- Hooda, A.; Goyat, M.S.; Pandey, J.K.; Kumar, A.; Gupta, R. A review on fundamentals, constraints and fabrication techniques of superhydrophobic coatings. Prog. Org. Coat. 2020, 142, 105557. [Google Scholar] [CrossRef]
- Simpson, J.T.; Hunter, S.R.; Aytug, T. Superhydrophobic materials and coatings: A review. Rep. Prog. Phys. 2015, 78, 086501. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, X. Enhanced Anti-Wetting Methods of Hydrophobic Membrane for Membrane Distillation. Adv. Sci. 2023, 10, 2300598. [Google Scholar] [CrossRef]
- Björneholm, O.; Hansen, M.H.; Hodgson, A.; Liu, L.-M.; Limmer, D.T.; Michaelides, A.; Pedevilla, P.; Rossmeisl, J.; Shen, H.; Tocci, G.; et al. Water at Interfaces. Chem. Rev. 2016, 116, 7698–7726. [Google Scholar] [CrossRef]
- Zhang, H.; Chiao, M. Anti-fouling Coatings of Poly(dimethylsiloxane) Devices for Biological and Biomedical Applications. J. Med. Biol. Eng. 2015, 35, 143–155. [Google Scholar] [CrossRef]
- Liu, Y.; He, X.; Yuan, C.; Cao, P.; Bai, X. Antifouling applications and fabrications of biomimetic micro-structured surfaces: A review. J. Adv. Res. 2024, 59, 201–221. [Google Scholar] [CrossRef]
- Yang, H.; Wang, S.; Li, C.; Li, H. Three-Dimensional Numerical Simulations and Antifouling Mechanism of Microorganisms on Microstructured Surfaces. Processes 2021, 9, 319. [Google Scholar] [CrossRef]
- Cheng, Y.T.; Rodak, D.E.; Wong, C.A.; Hayden, C.A. Effects of micro- and nano-structures on the self-cleaning behaviour of lotus leaves. Nanotechnology 2006, 17, 1359. [Google Scholar] [CrossRef]
- Pu, X.; Li, G.; Huang, H. Preparation, anti-biofouling and drag-reduction properties of a biomimetic shark skin surface. Biol. Open 2016, 5, 389–396. [Google Scholar] [CrossRef]
- Zhang, M.; Yu, J.; Chen, R.; Liu, Q.; Liu, J.; Song, D.; Liu, P.; Gao, L.; Wang, J. Highly transparent and robust slippery lubricant-infused porous surfaces with anti-icing and anti-fouling performances. J. Alloys Compd. 2019, 803, 51–60. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, R.; Liu, Q.; Liu, J.; Yu, J.; Song, D.; Liu, P.; Gao, L.; Wang, J. Long-Term Stability of a Liquid-Infused Coating with Anti-Corrosion and Anti-Icing Potentials on Al Alloy. ChemElectroChem 2019, 6, 3911–3919. [Google Scholar] [CrossRef]
- Wang, J.; Sun, L.; Zou, M.; Gao, W.; Liu, C.; Shang, L.; Gu, Z.; Zhao, Y. Bioinspired shape-memory graphene film with tunable wettability. Sci. Adv. 2017, 3, e1700004. [Google Scholar] [CrossRef]
- Zhang, D.; Huang, M.; Klausen, L.H.; Li, Q.; Li, S.; Dong, M. Liquid-Phase Friction of Two-Dimensional Molybdenum Disulfide at the Atomic Scale. ACS Appl. Mater. Interfaces 2023, 15, 21595–21601. [Google Scholar] [CrossRef]
- Yan, W.; Xue, S.; Zhao, X.; Zhang, W.; Li, J. Hexagonal boron nitride based slippery liquid infused porous surface with anti-corrosion, anti-contaminant and anti-icing properties for protecting magnesium alloy. Chin. Chem. Lett. 2024, 35, 109224. [Google Scholar] [CrossRef]
- Pawlak, Z.; Pai, R.; Bayraktar, E.; Kaldonski, T.; Oloyede, A. Lamellar lubrication in vivo and vitro: Friction testing of hexagonal boron nitride. Biosystems 2008, 94, 202–208. [Google Scholar] [CrossRef]
- Zuo, K.; Zhang, X.; Huang, X.; Oliveira, E.F.; Guo, H.; Zhai, T.; Wang, W.; Alvarez, P.J.J.; Elimelech, M.; Ajayan, P.M.; et al. Ultrahigh resistance of hexagonal boron nitride to mineral scale formation. Nat. Commun. 2022, 13, 4523. [Google Scholar] [CrossRef]
- Heydarian, S.; Jafari, R.; Momen, G. Recent progress in the anti-icing performance of slippery liquid-infused surfaces. Prog. Org. Coat. 2021, 151, 106096. [Google Scholar] [CrossRef]
- Drelich, J.; Chibowski, E. Superhydrophilic and superwetting surfaces: Definition and mechanisms of control. Langmuir 2010, 26, 18621–18623. [Google Scholar] [CrossRef]
- Drelich, J.; Chibowski, E.; Meng, D.D.; Terpilowski, K. Hydrophilic and superhydrophilic surfaces and materials. Soft Matter 2011, 7, 9804–9828. [Google Scholar] [CrossRef]
- Choi, M.; Xiangde, L.; Park, J.; Choi, D.; Heo, J.; Chang, M.; Lee, C.; Hong, J. Superhydrophilic coatings with intricate nanostructure based on biotic materials for antifogging and antibiofouling applications. Chem. Eng. J. 2017, 309, 463–470. [Google Scholar] [CrossRef]
- Wang, R.; Zhu, L.; Zhu, X.; Yan, Z.; Xia, F.; Zhang, J.; Liu, X.; Yu, J.; Xue, Q. A super-hydrophilic and underwater super-oleophobic membrane with robust anti-fouling performance of high viscous crude oil for efficient oil/water separation. Colloids Surf. A Physicochem. Eng. Asp. 2023, 658, 130662. [Google Scholar] [CrossRef]
- Li, C.; Lu, D.; Deng, J.; Zhang, X.; Yang, P. Amyloid-Like Rapid Surface Modification for Antifouling and In-Depth Remineralization of Dentine Tubules to Treat Dental Hypersensitivity. Adv. Mater. 2019, 31, 1903973. [Google Scholar] [CrossRef]
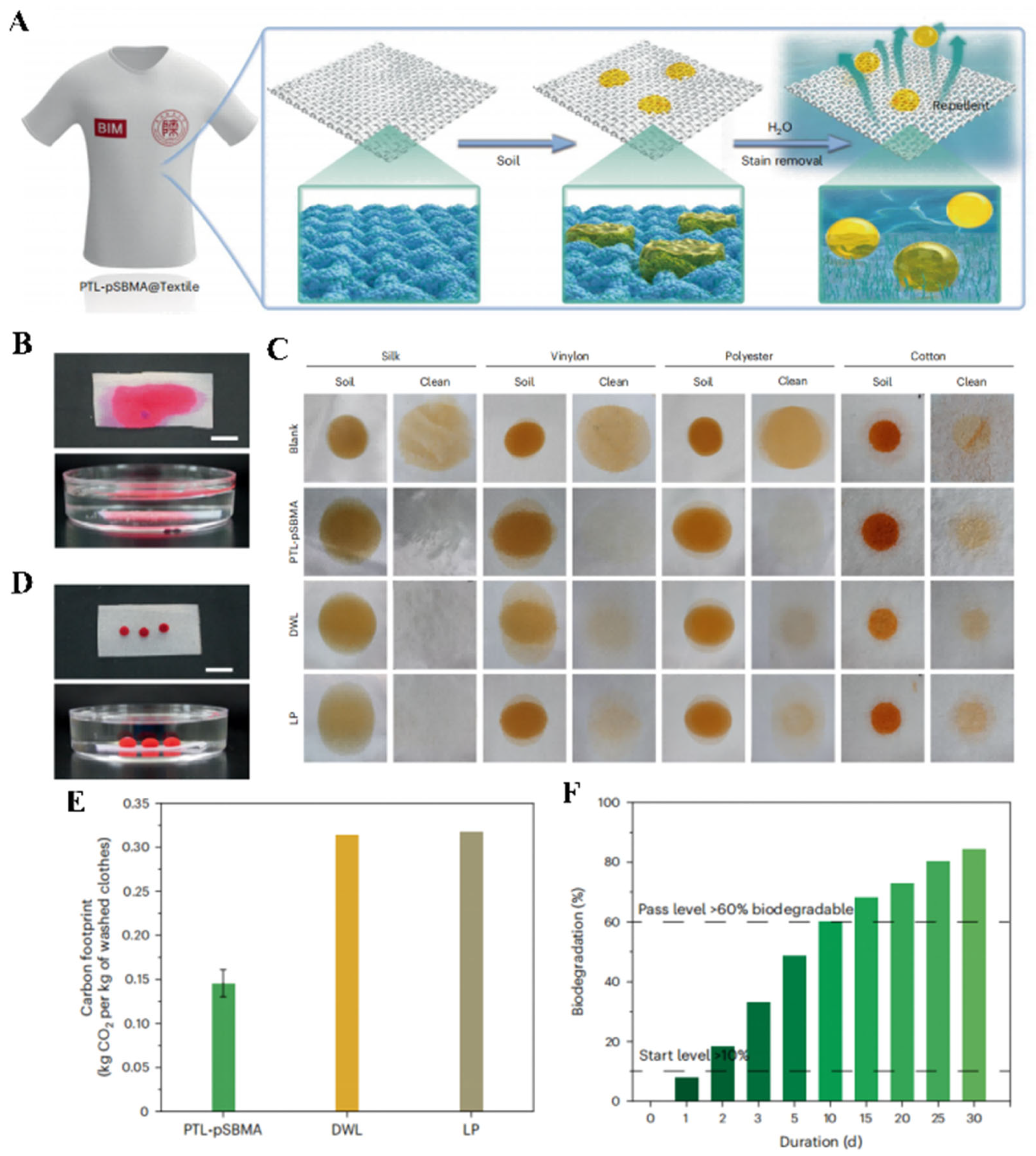
- Fu, C.; Wang, Z.; Gao, Y.; Zhao, J.; Liu, Y.; Zhou, X.; Qin, R.; Pang, Y.; Hu, B.; Zhang, Y.; et al. Sustainable polymer coating for stainproof fabrics. Nat. Sustain. 2023, 6, 984–994. [Google Scholar] [CrossRef]
- Zhang, G.; Liu, Y.; Chen, C.; Huang, C.; Long, L.; Zhang, S.; Yang, G.; Shen, F.; Zhang, X.; Zhang, Y. Green, robust self-cleaning superhydrophilic coating and on-demand oil–water separation. Appl. Surf. Sci. 2022, 595, 153472. [Google Scholar] [CrossRef]
- Li, L.; Yang, L.; Liao, Y.; Yu, H.; Liang, Z.; Zhang, B.; Lan, X.; Luo, R.; Wang, Y. Superhydrophilic versus normal polydopamine coating: A superior and robust platform for synergistic antibacterial and antithrombotic properties. Chem. Eng. J. 2020, 402, 126196. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Q.; Shao, T.; Miao, W.; You, C.; Wang, Z. Fabrication of anti-fouling and anti-bacterial hydrophilic coating through enzymatically-synthesized cellooligomers. Appl. Surf. Sci. 2022, 600, 154133. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, P.; Wang, X.; Yu, H. Designing Co-coordinated phytic acid 3D network structure for superhydrophilic property of TiO2 nanoparticles. Surf. Interfaces 2024, 51, 104684. [Google Scholar] [CrossRef]
- Nosonovsky, M. Multiscale roughness and stability of superhydrophobic biomimetic interfaces. Langmuir 2007, 23, 3157–3161. [Google Scholar] [CrossRef]
- Li, Z.; Cao, M.; Li, P.; Zhao, Y.; Bai, H.; Wu, Y.; Jiang, L. Surface-embedding of functional micro-/nanoparticles for achieving versatile superhydrophobic interfaces. Matter 2019, 1, 661–673. [Google Scholar] [CrossRef]
- Doshi, D.A.; Shah, P.B.; Singh, S.; Branson, E.D.; Malanoski, A.P.; Watkins, E.B.; Majewski, J.; van Swol, F.; Brinker, C.J. Investigating the interface of superhydrophobic surfaces in contact with water. Langmuir 2005, 21, 7805–7811. [Google Scholar] [CrossRef]
- Bayer, I.S. Superhydrophobic coatings from ecofriendly materials and processes: A review. Adv. Mater. Interfaces 2020, 7, 2000095. [Google Scholar] [CrossRef]
- Li, J.; Tian, J.; Gao, Y.; Qin, R.; Pi, H.; Li, M.; Yang, P. All-natural superhydrophobic coating for packaging and blood-repelling materials. Chem. Eng. J. 2021, 410, 128347. [Google Scholar] [CrossRef]
- Vicente, A.; Rivero, P.J.; Urdiroz, U.; García, P.; Mora, J.; Palacio, J.F.; Palomares, F.J.; Rodríguez, R. Novel Design of Superhydrophobic and Anticorrosive PTFE and PAA + β − CD Composite Coating Deposited by Electrospinning, Spin Coating and Electrospraying Techniques. Polymers 2022, 14, 4356. [Google Scholar] [CrossRef]
- Yi, K.; Fu, S.; Huang, Y. Nanocellulose-based superhydrophobic coating with acid resistance and fluorescence. Prog. Org. Coat. 2022, 168, 106911. [Google Scholar] [CrossRef]
- Liu, X.; Wang, K.; Zhang, W.; Zhang, J.; Li, J. Robust, self-cleaning, anti-fouling, superamphiphobic soy protein isolate composite films using spray-coating technique with fluorinated HNTs/SiO2. Compos. Part B Eng. 2019, 174, 107002. [Google Scholar] [CrossRef]
- Yin, B.; Xu, H.; Fan, F.; Qi, D.; Hua, X.; Xu, T.; Liu, C.; Hou, D. Superhydrophobic coatings based on bionic mineralization for improving the durability of marine concrete. Constr. Build. Mater. 2023, 362, 129705. [Google Scholar] [CrossRef]
- John, B.; Rajimol, P.R.; Rajan, T.P.D.; Sahoo, S.K. Design and fabrication of nano textured superhydrophobic and anti-corrosive silane-grafted ZnO/bio-based polyurethane bilayer coating. Surf. Coat. Technol. 2022, 451, 129036. [Google Scholar] [CrossRef]
- Hassan, M.M. Wool fabrics coated with an anionic Bunte salt-terminated polyether: Physicomechanical properties, stain resistance, and dyeability. ACS Omega 2018, 3, 17656–17667. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Mawignon, F.J.; Li, C.; Luo, Y.; Yu, J.; Li, G.; Zheng, Y.; Lu, S.; Wang, Z.; Sufyan, M.; et al. Biomimetic Slippery Surface with Exclusive Liquid-Repellent and Self-Cleaning Properties for Antifouling. Langmuir 2024, 40, 12443–12453. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.; Guo, H.; Di, Z.; Chen, S.; Song, L.; Hu, J.; Hou, Y.; Zhan, X.; Zhang, Q.P. pavoninus-Inspired Smart Slips Marine Antifouling Coating Based on Coumarin: Antifouling Durability and Adaptive Adjustability of Lubrication. Adv. Funct. Mater. 2024, 34, 2310702. [Google Scholar] [CrossRef]
- Maalige R., N.; Aruchamy, K.; Polisetti, V.; Halakarni, M.; Mahto, A.; Mondal, D.; Sanna Kotrappanavar, N. Restructuring thin film composite membrane interfaces using biopolymer as a sustainable alternative to prevent organic fouling. Carbohydr. Polym. 2021, 254, 117297. [Google Scholar] [CrossRef]
- Winkeljann, B.; Bauer, M.G.; Marczynski, M.; Rauh, T.; Sieber, S.A.; Lieleg, O. Covalent Mucin Coatings Form Stable Anti-Biofouling Layers on a Broad Range of Medical Polymer Materials. Adv. Mater. Interfaces 2020, 7, 1902069. [Google Scholar] [CrossRef]
- Xie, M.; Zhao, W.; Wu, Y. Preventing algae biofilm formation via designing long-term oil storage surfaces for excellent antifouling performance. Appl. Surf. Sci. 2021, 554, 149612. [Google Scholar] [CrossRef]
- Chen, J.; Lu, G.; Hao, Y.; Lu, Y.; Huang, L.-F.; Zhang, Y. Bio-Based Anti-Biofoulant with Copper(II) as Intramolecular Catalyst for Radicals Formation. Adv. Mater. Interfaces 2023, 10, 2201882. [Google Scholar] [CrossRef]
- Raman, S.; Karunamoorthy, L.; Doble, M.; Kumar, R.; Venkatesan, R. Barnacle adhesion on natural and synthetic substrates: Adhesive structure and composition. Int. J. Adhes. Adhes. 2013, 41, 140–143. [Google Scholar] [CrossRef]
- Khandeparker, L.; Anil, A.C. Underwater adhesion: The barnacle way. Int. J. Adhes. Adhes. 2007, 27, 165–172. [Google Scholar] [CrossRef]
- Zhang, H.; Li, Y.; Tian, S.; Qi, X.; Yang, J.; Li, Q.; Lin, C.; Zhang, J.; Zhang, L. A switchable zwitterionic ester and capsaicin copolymer for multifunctional marine antibiofouling coating. Chem. Eng. J. 2022, 436, 135072. [Google Scholar] [CrossRef]
- Silverman, H.G.; Roberto, F.F. Understanding Marine Mussel Adhesion. Mar. Biotechnol. 2007, 9, 661–681. [Google Scholar] [CrossRef] [PubMed]
- Waite, J.H. Mussel adhesion—Essential footwork. J. Exp. Biol. 2017, 220, 517–530. [Google Scholar] [CrossRef] [PubMed]
- Peres, R.S.; Armelin, E.; Moreno-Martínez, J.A.; Alemán, C.; Ferreira, C.A. Transport and antifouling properties of papain-based antifouling coatings. Appl. Surf. Sci. 2015, 341, 75–85. [Google Scholar] [CrossRef]
- Elsaid, K.; Kamil, M.; Sayed, E.T.; Abdelkareem, M.A.; Wilberforce, T.; Olabi, A. Environmental impact of desalination technologies: A review. Sci. Total Environ. 2020, 748, 141528. [Google Scholar] [CrossRef]
- Darre, N.C.; Toor, G.S. Desalination of Water: A Review. Curr. Pollut. Rep. 2018, 4, 104–111. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, P.; Liang, X.; Zhao, J.; Liu, Y.; Cao, Y.; Wang, H.; Chen, Y.; Zhang, Z.; Pan, F.; et al. Ultrafast seawater desalination with covalent organic framework membranes. Nat. Sustain. 2022, 5, 518–526. [Google Scholar] [CrossRef]
- Bai, L.; Liu, Y.; Bossa, N.; Ding, A.; Ren, N.; Li, G.; Liang, H.; Wiesner, M.R. Incorporation of Cellulose Nanocrystals (CNCs) into the Polyamide Layer of Thin-Film Composite (TFC) Nanofiltration Membranes for Enhanced Separation Performance and Antifouling Properties. Environ. Sci. Technol. 2018, 52, 11178–11187. [Google Scholar] [CrossRef]
- Li, J.; Taylor, M.; Zhang, Z. Anti-fouling Medical Coatings. In Antimicrobial Coatings and Modifications on Medical Devices; Zhang, Z., Wagner, V.E., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 189–214. [Google Scholar]
- Stark, K.; Massberg, S. Interplay between inflammation and thrombosis in cardiovascular pathology. Nat. Rev. Cardiol. 2021, 18, 666–682. [Google Scholar] [CrossRef]
- Zeng, Q.; Zhu, Y.; Yu, B.; Sun, Y.; Ding, X.; Xu, C.; Wu, Y.-W.; Tang, Z.; Xu, F.-J. Antimicrobial and Antifouling Polymeric Agents for Surface Functionalization of Medical Implants. Biomacromolecules 2018, 19, 2805–2811. [Google Scholar] [CrossRef]
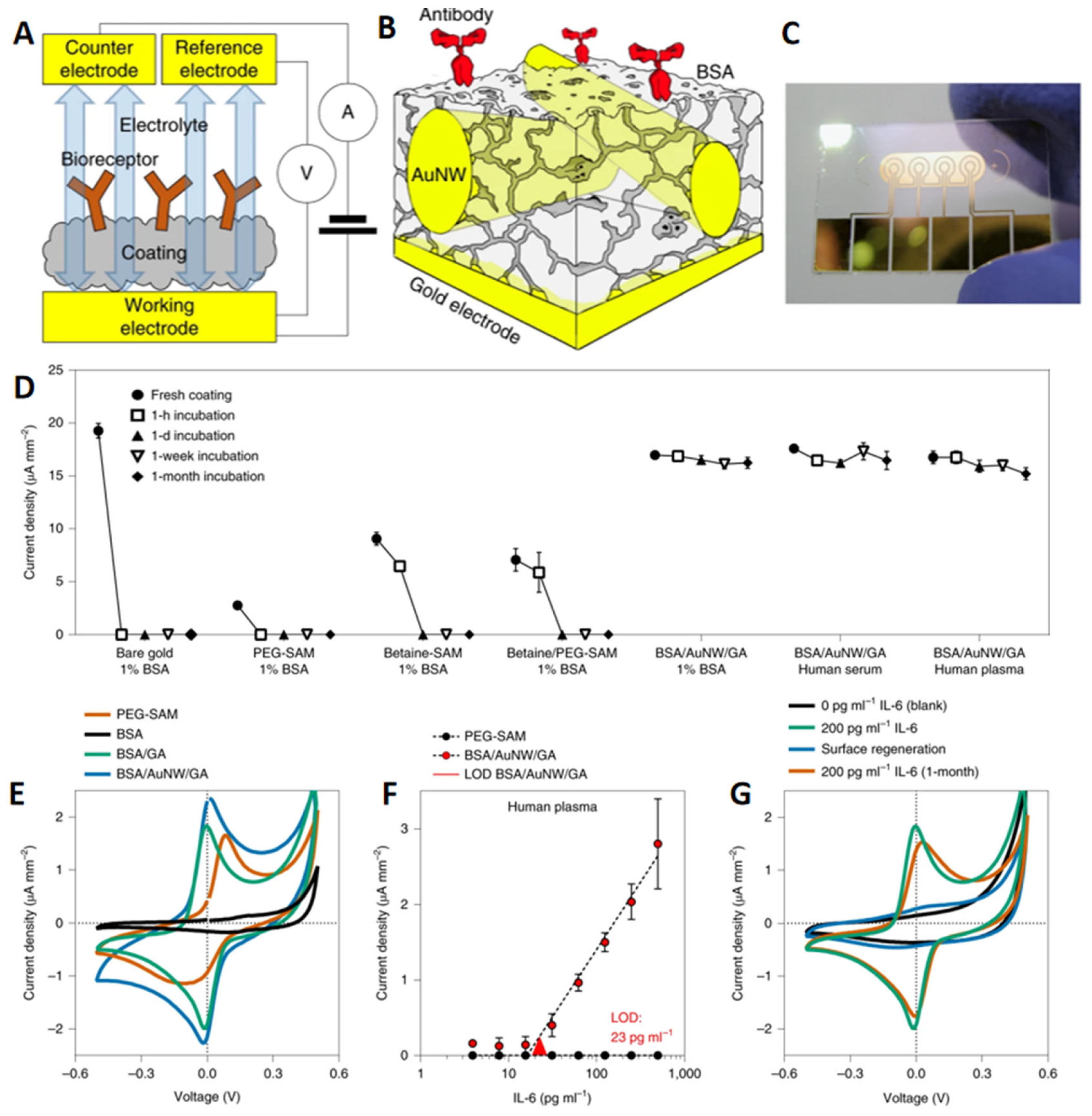
- Xu, J.; Lee, H. Anti-Biofouling Strategies for Long-Term Continuous Use of Implantable Biosensors. Chemosensors 2020, 8, 66. [Google Scholar] [CrossRef]
- Ribeiro, M.; Monteiro, F.J.; Ferraz, M.P. Infection of orthopedic implants with emphasis on bacterial adhesion process and techniques used in studying bacterial-material interactions. Biomatter 2012, 2, 176–194. [Google Scholar] [CrossRef] [PubMed]
- Pye, A.D.; Lockhart, D.E.A.; Dawson, M.P.; Murray, C.A.; Smith, A.J. A review of dental implants and infection. J. Hosp. Infect. 2009, 72, 104–110. [Google Scholar] [CrossRef] [PubMed]
- Silva-Bermudez, P.; Rodil, S.E. An overview of protein adsorption on metal oxide coatings for biomedical implants. Surf. Coat. Technol. 2013, 233, 147–158. [Google Scholar] [CrossRef]
- Hu, X.; Tian, J.; Li, C.; Su, H.; Qin, R.; Wang, Y.; Cao, X.; Yang, P. Amyloid-Like Protein Aggregates: A New Class of Bioinspired Materials Merging an Interfacial Anchor with Antifouling. Adv. Mater. 2020, 32, 2000128. [Google Scholar] [CrossRef]
- Tian, J.; Liu, Y.; Miao, S.; Yang, Q.; Hu, X.; Han, Q.; Xue, L.; Yang, P. Amyloid-like protein aggregates combining antifouling with antibacterial activity. Biomater. Sci. 2020, 8, 6903–6911. [Google Scholar] [CrossRef]
- Barfidokht, A.; Gooding, J.J. Approaches Toward Allowing Electroanalytical Devices to be Used in Biological Fluids. Electroanalysis 2014, 26, 1182–1196. [Google Scholar] [CrossRef]
- Dugas, V.; Elaissari, A.; Chevalier, Y. Surface Sensitization Techniques and Recognition Receptors Immobilization on Biosensors and Microarrays. In Recognition Receptors in Biosensors; Zourob, M., Ed.; Springer: New York, NY, USA, 2010; pp. 47–134. [Google Scholar]
- Sabaté del Río, J.; Henry, O.Y.F.; Jolly, P.; Ingber, D.E. An antifouling coating that enables affinity-based electrochemical biosensing in complex biological fluids. Nat. Nanotechnol. 2019, 14, 1143–1149. [Google Scholar] [CrossRef]
- Wang, Y.; Li, Z.; Wang, J.; Wang, J.; Li, X.; Kuang, C. Superhydrophobic coating constructed from rosin acid and TiO2 used as blood repellent dressing. Mater. Des. 2022, 222, 111072. [Google Scholar] [CrossRef]
- Sahar, H.; Akhtar, J.; Aamir, M.; Shah Gilani, M.R.H.; Jabeen, U. Chapter 10—Self-cleaning coating materials. In Antiviral and Antimicrobial Smart Coatings; Kumar, A., Behera, A., Nguyen, T.A., Bilal, M., Gupta, R.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2023; pp. 289–308. [Google Scholar]
- Li, S.; Zhao, F.; Bai, Y.; Ye, Z.; Feng, Z.; Liu, X.; Gao, S.; Pang, X.; Sun, M.; Zhang, J.; et al. Slippery liquid-infused microphase separation surface enables highly robust anti-fouling, anti-corrosion, anti-icing and anti-scaling coating on diverse substrates. Chem. Eng. J. 2022, 431, 133945. [Google Scholar] [CrossRef]
- Wang, Y.; Wu, H.; Zhou, Z.; Maitz, M.F.; Liu, K.; Zhang, B.; Yang, L.; Luo, R.; Wang, Y. A thrombin-triggered self-regulating anticoagulant strategy combined with anti-inflammatory capacity for blood-contacting implants. Sci. Adv. 2022, 8, eabm3378. [Google Scholar] [CrossRef] [PubMed]
- Giagnorio, M.; Amelio, A.; Grüttner, H.; Tiraferri, A. Environmental impacts of detergents and benefits of their recovery in the laundering industry. J. Clean. Prod. 2017, 154, 593–601. [Google Scholar] [CrossRef]
- Gulzar, M.; Masjuki, H.H.; Kalam, M.A.; Varman, M.; Rizwanul Fattah, I.M. Oil filter modification for biodiesel–fueled engine: A pathway to lubricant sustainability and exhaust emissions reduction. Energy Convers. Manag. 2015, 91, 168–175. [Google Scholar] [CrossRef]
- Feng, N.; Chen, X.; Han, Q.; Liu, Y.; Yan, L.; Gao, A.; Yang, P. Detaching adhesive oil staining from a surface by water. Aggregate 2024, 5, e465. [Google Scholar] [CrossRef]
- Ron, E.Z.; Rosenberg, E. Enhanced bioremediation of oil spills in the sea. Curr. Opin. Biotechnol. 2014, 27, 191–194. [Google Scholar] [CrossRef]
- Zhang, B.; Matchinski, E.J.; Chen, B.; Ye, X.; Jing, L.; Lee, K. Chapter 21—Marine Oil Spills—Oil Pollution, Sources and Effects. In World Seas: An Environmental Evaluation, 2nd ed.; Sheppard, C., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 391–406. [Google Scholar]
- Feng, Z.; Xu, Y.; Ding, W.; Li, Q.; Zhao, X.; Wei, X.; Hakkarainen, M.; Wu, M. Nano graphene oxide creates a fully biobased 3D-printed membrane with high-flux and anti-fouling oil/water separation performance. Chem. Eng. J. 2024, 485, 149603. [Google Scholar] [CrossRef]
- Yu, X.-L.; Wang, B.-B.; Xu, Z.-M.; Yan, W.-M. Study on Anti-Scale and Anti-Corrosion of Polydopamine Coating on Metal Surface. Coatings 2023, 13, 306. [Google Scholar] [CrossRef]
- Wang, R.; Peng, W.; Liu, J.; Wang, D.; Yan, X. A highly efficient and low Ca2+ dissolving limescale based material modified by Zn-Al composite oxides for F− removal. Desalination 2024, 585, 117769. [Google Scholar] [CrossRef]
- Georgiou, D.; Bendos, D.; Kalis, M.; Koutis, C. Removal and/or prevention of limescale in plumbing tubes by a radio-frequency alternating electric field inductance device. J. Water Process Eng. 2018, 22, 34–40. [Google Scholar] [CrossRef]
- Zang, R.; Wang, Y.; Meng, J.; Chen, W.; Wang, B.; Xu, X.; He, X.; Yang, H.; Li, K.; Wang, S. Sustainable scale resistance on a bioinspired synergistic microspine coating with a collectible liquid barrier. Mater. Horiz. 2022, 9, 2872–2880. [Google Scholar] [CrossRef]
- Liu, S.; Wang, C.; Liu, S.; Li, K.; Luo, H.; Fan, W.; Yin, Q.; Wang, H. pH-responsive smart composite coating with active anticorrosion and efficient scale inhibition properties. Prog. Org. Coat. 2022, 170, 106973. [Google Scholar] [CrossRef]
- Russian Maritime Register of Shipping. Rules for the Classification and Construction of Marine Vessels 2020, II—Hull; Russian Maritime Register of Shipping: Saint Petersburg, Russia, 2017. [Google Scholar]
- Chen, J.; Luo, Z.; Fan, Q.; Lv, J.; Wang, J. Anti-Ice Coating Inspired by Ice Skating. Small 2014, 10, 4693–4699. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.; Wong, T.-S.; Alvarenga, J.; Kreder, M.J.; Adorno-Martinez, W.E.; Aizenberg, J. Liquid-Infused Nanostructured Surfaces with Extreme Anti-Ice and Anti-Frost Performance. ACS Nano 2012, 6, 6569–6577. [Google Scholar] [CrossRef] [PubMed]
- Dou, R.; Chen, J.; Zhang, Y.; Wang, X.; Cui, D.; Song, Y.; Jiang, L.; Wang, J. Anti-icing Coating with an Aqueous Lubricating Layer. ACS Appl. Mater. Interfaces 2014, 6, 6998–7003. [Google Scholar] [CrossRef]
- Feng, F.; Wang, R.; Yuan, W.; Li, Y. Study on Anti-Icing Performance of Biogas-Residue Nano-Carbon Coating for Wind-Turbine Blade. Coatings 2023, 13, 814. [Google Scholar] [CrossRef]




| Strategy | Structural Accuracy | Potential for Large-Scale Development | Applicable Scenarios |
|---|---|---|---|
| Polymer blending strategies, | Middle | High | Industrial antifouling coatings, medical devices |
| Phase transition self-assembly strategies, | High | Middle | Catalytic carrier, precision sensor |
| Chemical deposition strategies | High | Low | Electronic devices, corrosion-resistant coatings |
| Spin-coating strategies | Low | Middle | Laboratory research, optical coating |
| Types | Example | Antifouling Mechanism | Advantages and Disadvantages | |
|---|---|---|---|---|
| Natural bio-based coating | Polysaccharide | Cellulose coating | superhydrophobic | Advantage: raw materials are readily available; disadvantage: Insufficient stability |
| Protein | Amyloid-like coating | Superhydrophilic | Advantages: there are various functionalization methods; disadvantages: the utilization rate of the reaction is poor | |
| lipid | Zwitterion coating | Superhydrophilic | Advantages: excellent antifouling performance; disadvantages: weak resistance to salt environment | |
| Synthetic bio-based coating | Biodegradable polymers | Polylactic acid | superhydrophobic | Advantages: degradability is beneficial for environmental protection; disadvantages: the antifouling effect is relatively weak |
| Bio-inspired synthetic materials | Polydopamine | superhydrophobic | Advantages: simple preparation; disadvantages: limited antifouling effect. | |
| Composite coating | Inorganic-biological hybrid coating | Titanium dioxide/chitosan | superhydrophobic | Advantages: high strength and wear resistance; disadvantages: limited effect |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Li, L.; Wu, Y.; Liu, Y. Design and Application of Antifouling Bio-Coatings. Polymers 2025, 17, 793. https://doi.org/10.3390/polym17060793
Wang J, Li L, Wu Y, Liu Y. Design and Application of Antifouling Bio-Coatings. Polymers. 2025; 17(6):793. https://doi.org/10.3390/polym17060793
Chicago/Turabian StyleWang, Jinglin, Ling Li, Yage Wu, and Yongchun Liu. 2025. "Design and Application of Antifouling Bio-Coatings" Polymers 17, no. 6: 793. https://doi.org/10.3390/polym17060793
APA StyleWang, J., Li, L., Wu, Y., & Liu, Y. (2025). Design and Application of Antifouling Bio-Coatings. Polymers, 17(6), 793. https://doi.org/10.3390/polym17060793








