Lignin from Plant-Based Agro-Industrial Biowastes: From Extraction to Sustainable Applications
Abstract
:1. Introduction
2. Lignin in Lignocellulosic Agro-Industrial Wastes
- 1.
- Ether bond (C-O-C) which can be originated either between two carbon atoms of different benzene rings or between the propane chain of a phenylpropane (elementary unit often referred to as monolignol) and a carbon of a different benzene ring. One type of bond related to the last situation is the -O-4 alkyl–aryl ether, considered the most common interaction in lignin (≃40–60% of connections [4,8]), but weaker than other bonds in lignin. A not-too-high dissociation energy for -O-4 alkyl–aryl ether (289 kJ/mol) [23] allows the biopolymer to break at that position during depolymerization processes. However, -aryl ether bonds are hydrolyzed around 100 times faster (due to a smaller bond dissociation energy, 215 KJ/mol) than -aryl ether bonds.
- 2.
- Carbon–carbon (C-C) bonds between aliphatic chains of different benzenes or between a benzene and another aliphatic chain. This type of link is more difficult to break which accentuates the recalcitrant nature of lignocellulose biomass, with 5–5′ and - bonds representing a high frequency of occurrence, 10–20% and 10–12% of connections, respectively [4].
3. Global Process for Lignin Extraction
3.1. Methods for Lignin Extraction
3.1.1. Physical Treatments
3.1.2. Chemical Treatments
- Kraft process: Known as sulfate pulping, this is a chemical treatment used in 85% of cellulose pulp production processes from lignocellulosic materials [170,172,173], with an annual lignin extraction volume in the range of 50–70 million tonnes [174,175]. This process aims to extract lignin by using an aqueous solution (white liquid) of sodium hydroxide (NaOH) and sodium sulfide (S), in the presence of sodium sulfate, although this is not the active form that reacts, and temperatures between 140 and 180 °C [176,177]. The sodium sulfate is used to obtain S. The initial pH of the process is close to 13, although this depends on the initial concentration of the alkaline solution. At this pH, more than half of the S is in the form of sulfide ions, which are hydrolyzed during the treatment to generate and ions to a pH of 10–12 at the end of the kraft process. Both ions ( and ) will attack the biomass, causing the recovery of lignin in the resulting black liquid [178,179], by lowering the pH with or sulfuric acid, among other techniques, until its precipitation. The lignins extracted will have a molecular weight between 1000 and 15,000 g , and will be soluble in both alkalis and organic solvents [177]. Other authors [180] state that the lignin content in the black liquor from the kraft pulp manufacturing process is in the range of 30–45% wt.Another more sustainable alternative, using oxygen in some stages of the kraft process, has been proposed in order to reduce the demand for other chemical agents such as sodium sulfide, bleaching agents or chlorine-derived compounds, and also recover some chemicals from effluents generated in the process, as this stream would be free of corrosive chloride ions [177].Some authors establish the composition of the white liquid by means of the concept “sulfidity” [25]. This technical term is an important parameter in the pulp and paper industry and affects the efficiency of the cooking process and the quality of the final product. It is calculated as the ratio between the amount of sodium sulfide and the amount of active alkali (NaOH + S) in percentage, Equation (2), and it is usually expressed on a O basis. A value of 20% for this parameter is recommended in the case of hardwoods (Eucalyptus), although this optimum value is strongly dependent on the type of biomass, alkali charge, treatment temperature and final product characteristics [178].Although the kraft process has very important advantages in generating a highly purified cellulose pulp (depending on the operating variables), offering easy reagent recovery, it also has drawbacks to overcome such as low yields in the biomass conversion into cellulose, high production costs and corrosiveness in digesters [178]. Apart from that, the lignin generated has 1–3% sulfur due to the incorporation of aliphatic thiol groups in its structure [181], causing a characteristic odor in this type of lignin [176] and environmental pollution; some studies indicate that 70–75% of the hydroxyl groups may be sulfonated [181]. Furthermore, kraft lignin attainment involves severe conditions producing fragmented but also repolymerizated (condensated) lignins, with higher amounts of recalcitrant carbon–carbon bonds and hydroxyl groups, and less ether linkages, making it difficult to break them up, and even less selective [170]. Finally, the precipitated cellulosic pulp could exhibit surface adherences of condensed lignin, decreasing the purity of the pulp obtained. Therefore, approximately 95% of all kraft lignin is currently burned as a low value fuel [170]. In other cases, the extracted kraft lignin can have annexed hemicellulose-related residues, leading to lignins with amphiphilic behavior like surfactants [177].
- Sulfite process: Together with hydrolysis processes, this accounts for 5% of commercial technical lignin [172], representing 1 million tonnes per year [175]. LAIWs can react with a mixture of alkaline earth metal sulfites at 120–180 °C and high pressure for 3–7 h [174]. Sulfite can be formed by reaction between and calcium carbonate or magnesium oxide at a pH of 1 to 5 [177]. This treatment allows the extraction of lignins of an amphiphilic nature, containing both hydrophilic groups such as sulfonates, (highly polar), or hydroxyl groups, with hydrogen bonding capability, but also hydrophobic groups, encompassing aromatic rings and alkyl groups that have relatively non-polar character. The balance between hydrophilic and hydrophobic groups in sulfite lignin determines its overall solubility and compatibility with other materials. It is considered that the high presence of sulfonate groups makes sulfite lignin more hydrophilic than other types of lignin, such as kraft lignin, which has fewer ionic groups. Therefore, sulfite lignins can form polyelectrolytes because they contain ionizable functional groups (sulfonic, carboxyl and phenolic hydroxyls) when dissolved in water, giving rise to a molecule with multiple negative electrical charges. The electrostatic repulsion between the negative charges of the sulfonate groups causes the lignin chain to expand, occupying a larger volume in the solution. This also enhances the solubility of lignin in water through ion–dipole interactions. Subsequent hydrolysis of the formed sulfonates allows lignin to be solubilized in water. These lignosulfonates will have a high molecular weight (1000–50,000 g ), sulfur content of 2.1–9.4%, mainly in the benzylic position, and high ash percentage (4.0–8.0%) [181,182]. On the other hand, polyelectrolytes could interact with cations to give rise to chemical complexes that modify the solution properties.
- Klason treatment: This is a chemical process that involves a first stage of treatment with concentrated sulfuric acid (72%) for 1 h at 30 °C, and a second hydrolysis stage of the residual solid with dilute sulfuric acid (4%) for 1 h at 121 °C [183]. The main drawback of the process is that the extracted lignin is highly altered, so it is only used as a quantitative method at an experimental level.
- Acid treatments: These processes, historically established and appealing due to their cost-effective and high efficiency, have limited utility for biomass delignification. This limitation arises because these pretreatments are optimized for hemicellulose recovery rather than maximizing solubilized lignin extraction. Several industrial companies carry out acid treatments of waste biomass to generate second generation biofuels, although this technology is not yet consolidated, which justifies that research is still ongoing [184]. Many studies use traditional mineral diluted acids such as hydrochloric, nitric, phosphoric and especially sulfuric acid (which is the most employed acid) considering acid concentrations in the range 0.2–8% (w/w), liquid:solid ratios from 5:1 to 20:1 (v/w), temperature range from 80 °C to 210 °C, and reaction times from 5 to 300 min [184,185] in order to preferentially solubilize hemicellulose, amorphous cellulose or soluble lignin, but leading to cellulignin solids in which lignin is still present to a considerable extent. Therefore, hydrolytic treatments with diluted inorganic acids could be of interest only in the case of LAIWs with a low lignin content; using diluted inorganic acids, only delignification percentages of 5–10% can be achieved [184]. Studies using more concentrated acids in the hydrolytic process tend to use lower temperatures (<60 °C), higher solid:liquid ratios from 1:2.5 to 1:10 (w/v) and acid concentration of more than 10% (w/v) [12,185,186]. Some authors mention in their studies acid concentrations as high as 65–86% (w/v) sulfuric acid, 41% (w/v) hydrochloric acid or 85% (w/w) phosphoric acid [187], and times in the range 2 to 10 h under atmospheric pressure [12]. Problems of equipment corrosivity, partial cellulose solubilization (especially amorphous fraction), mandatory need to recover the acid and production of undesirable compounds (e.g., inhibitors), in residual liquid streams, contribute to the environment pollution and cost increase. For these reasons, other environmentally friendly alternatives, employing weak organic acids such as formic, maleic, acetic, oxalic, citric, propionic or lactic acids, have been proposed. Some of these acids (formic, acetic, propionic or lactic) are considered “organosolv” because of their low toxicity, low cost and high recoverability. Specifically, Singh et al. [188] employed wheat straw and bagasse by applying a sequential treatment with concentrated sulfuric acid and then dilute acid, achieving lignin recovery percentages of 21.5% for one of the bagasse types. Table 2 presents the experimental conditions and delignification percentages of the most recent studies.
| LAIWs | Experimental Conditions | Delignification, % | Ref. |
|---|---|---|---|
| Sugarcane straw | Acetic acid 7.51 g LAIW-6.7 mL acid + 20 mg | 91.0 | [189] |
| Coffee husk | NaCl four times, 60 min, 80 °C | 80.0 | |
| Corn cob | p-Toluenesulfonic acid 70%, 70 °C, 30 min | 82.4 | [52] |
| Corn stover | Acetic acid 2%, 12 ± 2 h, 15 psi, 121 °C, 90 min 1 g LAIW-15 mL acid | 75.7 | [190] |
| Empty palm fruit bunch | 2% w/v, 150 °C, 30 min | 65.7 | [191] |
| Rice husk | 2% (v/v), 120 °C, 60 min 1 g LAIW-10 g acid | 18.8 | [192] |
| Rice straw | 0.75%
(v/v) +
Boric acid 1% (w/v) + glycerol 0.5% (v/v) 1 g LAIW-5 mL acid, 150 °C, 20 min | 44.0 | [193] |
| Acetic acid 99%, 80 °C, 24 h 1 g LAIW-10 mL acid | 4.7 | [29] | |
| p-Toluene sulfonic acid 60% (w/w), 80 °C, 45 min | 52.4 | [194] | |
| Sugarcane bagasse | Formic acid 80.0%, 130 °C, 90 min 1 g LAIW-10 mL acid | 90.6 | [195] |
| 1.5% (v/v), 121 °C, 20 min 1 g LAIW-10 mL acid | 33.1 | [196] | |
| Soybean hull | Citric acid 4.22%, 120 °C, 105 min 1 g LAIW-15 mL acid | 30.4 | [149] |
| Wheat straw | p-Toluenesulfonic acid 15%, 25%, 35% (w/w) 90 °C, 120 min | 24, 48, 66 | [197] |
| Maleic acid 60% (w/w) 110 °C, 60 min | 66.5 | [198] | |
| Sulfuric acid 1.5% (w/w) 121 °C, 15 lb/inch2, 75 min | 74.0 | [199] |
- Alkaline (AK) treatments: One of the best known hydrolytic processes is the so-called “soda pulping”, the preferred pulping process for agro-waste fractionation [174]. This chemical method uses NaOH solutions (10–16%) at high pressures and temperatures (140–170 °C) to produce the alkaline hydrolysis of LAIWs and extract the lignin (soda lignin) although anthraquinone is sometimes added in order to stabilize the hydrocelluloses [174,177]. It is traditionally applied to non-wood matrices like straw or bagasse [11] and allows obtaining 15% of the lignin marketed [172], with an annual extraction of 6000 tonnes [175]. In this case, the extracted lignin (soda lignin) is lighter in color, more soluble in water and has lower sulfur content compared with kraft lignin. Furthermore, it has a low molecular weight (generally 1000–3000 g ) and methane emissions are produced [201]. The application of soda extraction processes leads to efficient depolymerization of the material, extracting alkaline lignin (AL) with very high purity [202] and zero sulfur content [181]. Furthermore, soda lignins contain more hydroxyl groups and relatively more carbon–carbon bonds compared to native lignin [177]. The values of the combined severity factor for soda pulping are usually in the range of 0.3–4.5 for this type of process [170], so that an increase in the degree of delignification from 25.3% to 95.4% has been evidenced when the severity varies from 1 to 5, respectively. However, from combined severity factor values above 3, lignin starts to be degraded, undergoing changes in its structure as condensation reactions take place [170]. In this sense, poplar wood hydrolysis solid was subjected to alkaline lignin extraction (NaOH, 1 M at 75 °C, 3 h) recovering 48% lignin [203]. Ammonium hydroxide, anhydrous ammonia, sodium carbonate and sodium sulfite have also proven to exhibit LAIWs delignification, Table 3. Figure 3 resumes the conditions of the main alkaline treatments.
| LAIWs | Experimental Conditions | Delignification, % | Ref. |
|---|---|---|---|
| African oil palm fiber Coffee pulp waste Sugarcane bagasse | Ca(OH)2 0.1% (w/w)
40 °C, 1 g LAIW-28 mL solution | 31.3 35.0 32.8 | [204] |
| Banana stem | KOH 1–5% (w/w), 90 °C, 25 min 1 g LAIW-10 mL solution | 43.0 | [205] |
| Sugarcane bagasse Coconut husk Rice straw Corn stover | NaOH 3% 1:12 (w/v)
121 °C, 15 psi | 31.3 38.5 19.4 15.8 | [202] |
| Chestnut shell | NaOH or KOH: 1–5% (w/w) | 15.5–51.1 | [206] |
| Corncob | 0.5–4%
(w/v)
30–70 °C, 1–8 h 1 g LAIW-10 to 30 g solution | 46.8 | [205] |
| Corn stalk | NaOH 1.5%
(w/v), 121 °C, 30 min 1 g LAIW-10 g solution | 42.2 | [207] |
| Corn stover | OH 12%
(w/w)
130 °C, 40 min 1 g LAIW-10 mL AK solution | 75.1 | [208] |
| 29.5% room temperature, 60 days | 73.5 | [209] | |
| 15.0%
60 °C, 12 h | 62.0 | [209] | |
| 15.0%
90 °C, 1 g LAIW-12.5 mL solution | 68.9 | [210] | |
| 15.0% (w/w)
60 °C, 12 h 1 g LAIW-10 mL AK solution | 70.0 | [211] | |
| Groundnut shell | NaOH 1.75 N
80 °C, 6 h, 400 rpm 0.8 g LAIW-100 mL AK solution | 41.8 | [212] |
| Miscanthus giganteus | NaOH 0.16–1.0 mol/L
100–180 °C, 60–360 min, 360 rpm | 25.3–95.4 | [170] |
| Olive pomace | NaOH 8% (w/v), 50 °C, 24 h 1 g LAIW-59 mL AK solution | 68.0 | [213] |
| Pistachio shell | NaOH 1.75 N
80 °C, 6 h, 400 rpm 0.8 g LAIW-100 mL AK solution | 38.0 | [212] |
| Rice hull | 20%, 100 °C, 1 g LAIW-10 mL | 62.0 | [214] |
| Rice straw | 8% 140 °C, 1 g LAIW-6 g solution | 41.8 | [208] |
| : 5% 121 °C, 60 min, 15 psi 1 g LAIW-10 mL solution | 78.4 | [215] | |
| 2–10% (w/v)
80 °C, 180 min, 15 psi 1 g LAIW-10 mL solution | 28.3–60.5 | [216] | |
| Sugarcane bagasse | NaOH 5, 10, 15% KOH: 5, 10, 15% 98 °C, 90 min 1 g LAIW-10 mL AK solution | 5.0–17.4 9.7–18.3 | [217] |
| Urea/KOH (1:1 ratio) 3–12% −20 °C, 1–12 h 1 g LAIW-(20–50) g AK solution | 41.5 | [218] | |
| 1.5% (w/w) NaOH 1.5% (w/w) 121 °C, 20 min 1 g LAIW-10 mL solution | 35.3 38.3 | [196] | |
| NaOH 6% (w/w), 90 °C, 60 min 1 g LAIW-15 mL solution | 81.0 | [219] | |
| OH 20% (v/v) 70 °C, 24 h | 41.5 | [220] | |
| Sugarcane straw | NaOH 1% (w/v) 10% (w/v) OH 10% (w/v) 120 °C, 60 min, 1 g LAIW-10 g AK solution | 28.5 25.1 46.3 | [221] |
| Sweet sorghum residue | NaOH 2%
121 °C, 50 min, 15 psi 1 g LAIW-10 mL AK solution | 41.8 | [222] |
| Wheat straw | NaOH 0.1 M room temperature, 120 min 1 g LAIW-10 mL solution | 21.1 | [223] |
| OH 15% (w/w) 65 °C, 15 h 1 g LAIW-10 mL solution | 50.0 | [224] | |
| 15% (w/w) room temperature, 7 days 1 g LAIW-20 mL solution | 9.0 | [225] |
- Hydrotropic extraction: A novel development for the fractionation of LAIWs is the use of hydrotropic compounds in aqueous solution, that contain hydrophilic and hydrophobic groups (with very short chains), such as (sodium or potassium) alkyl benzene sulfonates, xylene sulfonates, toluene sulfonates, salicylates and urea. These compounds have the ability to increase the solubility of lignins, that are normally insoluble in water, during the initial treatment phase. Subsequently, the lignin may be precipitated by diluting the hydrotrope solution with water [226] so, in many experimental studies, quite acceptable delignification percentages of LAIWs have been obtained, Table 4.
| LAIWs | Hydrotropic Solvent | Experimental Conditions | Delignification, % | Ref. |
|---|---|---|---|---|
| Rice straw | Sodium cumene sulfonate 20% | 1 g LAIW-20 g acid, 121 °C, 1 h, 50% | 50.0 | [227] |
| Sodium xylene sulfonate 20% | 34.0 | |||
| Sugarcane bagasse | Sodium xylene sulfonate 40% | 170 °C, 1 h | 6.8 | [228] |
| Sodium xylene sulfonate 30% | 1 g LAIW-2 g hydrotrope 115 °C | 85.0 | [229] |
- Organosolv treatment: Process to extract technical lignin from LAIWs, using several organic solvents (30–100% w/w), solid–liquid ratios in the range 1:2 and 1:15, although 1:10 balance is the most common, temperatures in the range 100–250 °C (temperatures > 180 °C are required for higher delignification) and residence times from 30 to 60 min [177,230,231]. This treatment is implemented on a pilot scale for raw biomass with an annual organosolv lignin extraction of 1000 tonnes [175]. One such method is the “Alcell” process, which uses ethanol: water mixtures (1:1) at 180–210 °C and 2–3.5 MPa to solubilize lignin from lignocellulosic biomasses [232]; the “Alcell” process has evolved into the “Lignol” process whose main difference is the addition of an inorganic acid (e.g., sulfuric acid) to maintain the pH in the range 2–3.7 [233].Other organosolv pretreatment with the addition of catalysts is becoming the focus of much research for LAIWs [230]. Although ethanol is the most common solvent involved due to its advantageous properties (low cost and toxicity, high quality of the recovered lignin and high recyclability [231,234]), other organic solvents are involved, such as methanol, 1-butanol, isobutanol, 2-methyltetrahydrofuran (MeTHF), tetrahydrofuran (THF), glycerol, ethylene glycol, cyrene, triethylene glycol (TEG), polyethylene glycol (PEG), acetone, formic acid, acetic acid, dimethyl isosorbide, ethyl lactate and -valerolactone, being used for LAIWs depolymerization and subsequent lignin recovery. Although there is research based on dioxane treatments, its use in industrial processes has been severely restricted due to the risks it poses to human health and the environment. Organosolv treatment achieves, in many cases, high lignin removal rates (>70%) with low loss of cellulose (<2%) [235], but research needs to address their main disadvantages: high material cost, solvent volatility and solvent recovery cost [236]. Table 5 shows organosolv experimental conditions and grades of delignification for different LAIWs.Table 5. Experimental conditions for organosolv delignification using LAIWs.
LAIWs Organosolv Solvent Experimental Conditions Delignification, % Ref. Bamboo culms
Formic acid:acetic acid:water
30:50:20 v/v/v1 g LAIW-20 mL OS
60 °C, 1 h + 107 °C, 3 h31.5
[237]
Corn stalks
Formic acid:acetic acid:water
60:30:10 v/v/v1 g LAIW-5 g OS
90 °C, 180 min18.6
[238]
Corn Methanol 0–2.5% w/v 1 g LAIW-2 mL OS 55.0 [239] stover (alkali catalyzed) 80 °C, 60 min 70.7 [240] Corn straw Cyrene 50% w/w 120 °C, 60 min 78.0 [231] Cotton stalks
Formic acid 100%
2% w/w1 g LAIW-10 g OS
80 °C, 60 min81.0
[207]
Rice straw
Glycerol 90% w/w (salt catalyzed) 1 g LAIW-20 mL OS 150 °C, 20 min 71.9
[241]
Sugarcane
bagasseEthylene glycol 90% v/v
(acid catalyzed)1 g LAIW-10 mL OS
150 °C, 60 min67.1
[242]
Ethylene glycol 90% v/v
(acid catalyzed)1 g LAIW-20 mL OS
120 °C, 60 min, 1% HCl61.2
[243]
Ethanol 60% v/v
(acid catalyzed)1 g LAIW-10 mL OS
170 °C, 15 min, 25 g86.0
[244]
Ethanol 60% v/v
(alkali catalyzed)1 g LAIW-10 mL OS
180 °C, 30 min, 8% NaOH82.6
[245]
TEG 5% w/v
(alkali catalyzed)1 g LAIW-20 mL OS
90 °C, 120 min, 1% NaOH80.0
[246]
Wheat straw
Water–ethanol 3:7 to 1:9 v/v
1 g LAIW-25 mL OS
90 °C, 120 min50.0
[247]
Glycerol 70%
1 g LAIW-10 g OS
220 °C, 3 h65.0
[248]
Recently, the role of certain organic compounds to prevent lignin repolymerization processes has been discovered. Thus, compounds such as 2-naphthol and dimethyl phloroglucinol have been used to stimulate delignification processes, protecting lignin from condensation reactions [249].These compounds can yield lignin practically free of sulfur (0–0.3% [181]), molecular weight of 500 to 5000 g and also having high purity and reactivity, preserving almost completely the chemical structure of the natural lignin [177] without condensation. Furthermore, organosolv lignin has a high hydrophobic character, so it is practically insoluble in water favoring lignin precipitation processes for its recovery. Research is currently prioritizing the application of this method due to the low environmental impact of the process and the high purity of the lignin recovered, although further study is needed to improve the economic aspects of the process, for example, towards the recovery of the solvents used. In this sense, only solvents with low boiling points are easy to recycle by evaporation, but they are volatile and highly flammable with requirements of expensive high-pressure equipment. On the other hand, solvents with high boiling points present recovery problems; impurities can appear frequently [231]. - Ionosolv treatment (IL): Chemical treatment that intends to use ionic liquids, salts with low melting point (usually <100 °C) [4], to recover lignin (and hemicellulose) from LAIWs, leaving the cellulose unaltered. Other advantageous properties of ionic solvents, apart from being in a liquid state at a low temperature, encompass their non-volatility and non-flammability, high conductivity and thermal stability [250], but some drawbacks are also presented concerning the high cost for the production of these solvents [251]. Although some ionic solvents used in the past were labeled as toxic and corrosive (contain anions such as , , , [ and [), there is now a shift towards more environmentally friendly liquids. According to some studies, the constituent cations of the salts belong to different groups (quaternary ammonium or phosphine salts, imidazole and pyridine), while the most advisable anions are [ (acetate), [ (glycinate), [ (citrate), [ (hydrogen phosphate), [ (dihydrogen phosphate), [ (tosylate), [ (mesylate), [CH(OH) (lactate), etc. [251]. When treatments are carried out at high temperatures, phosphonium-based ionic solvents are preferred to imidazolium- and ammonium-based ionic solvents, as the stability of phosphonium salts is higher.
- Deep eutectic solvent (DES) treatment: This is considered an environmentally friendly chemical alternative for lignin extraction as solvents are required have low toxicity, high recyclability and biodegradability; other properties are non-flammability, being 20% cheaper than ionic solvents, high chemical and thermal stability, low vapor pressure, low volatility and biocompatibility [252,253,254]. This process generally consists of a binary system based on two organic components (solids under normal conditions): one of them is a hydrogen bond donor (HBD) and the other a hydrogen bond acceptor (HBA). Both compounds result in a mixture with a melting point much lower than the one for each compound separately [255], due to charge delocalization via hydrogen bonds between molecules of both organic compounds [256]. The main groups of HBA are quaternary ammonium, phosphonium or sulfonium halide salts [257], choline chloride (ChCl) being the most common natural deep eutectic solvent because of the low toxicity of cholinium, although mixtures consisting of choline chloride:glycerol and betaine: amino acid are also frequently reported. Concerning HBDs, the most relevant chemicals are amide, carboxylic acid [257] and polyols [258], but other solvents may also be used. In summary, Table 6 shows various HBAs or HBDs reported in recent studies with DESs. The most commonly used DESs for biomass processing are usually based on the following types of mixtures: quaternary ammonium salt with hydrated metal halide, due to their resistance to air and moisture, and lower melting point compared to non-hydrated forms, as well as quaternary ammonium salts and carboxylic acids for their low cost and easy preparation. Additionally, inorganic transition metals and some HBDs such as urea can also be used. Recently, incipient research is underway considering only non-ionic molecules with ambiguous HBD/HBA roles in DESs [259], as is the case of the combination of N-methylacetamide and a long-chain carboxylic acid (lauric acid) [223]. Microwave radiation-assisted DES treatments have sometimes been used, so that solvents with high electrical conductivity and high dielectric constant favored collisions with lignocellulosic biomass, promoting delignification processes [259].Table 6. Main hydrogen bond acceptors and donors in deep eutectic solvent technology.
Hydrogen bond acceptors (HBAs) Choline chloride (ChCl), N,N-diethyl-2-hydroxy-N-(2-hydroxyethyl)ethan-1-aminium chloride, Ammonium chloride, Tetra-hydroxymethyl phosphonium chloride (TPC), Tetramethyl-ammonium chloride (TMAC), Tetraethyl ammonium chloride (TAC), Tetrabutylammonium chloride, Tetrabutylammonium hydroxide (TAH), Methyltriphenylphosphonium bromide, Triethyl benzyl ammonium chloride (TEBAC), N-benzyl-2-hydroxy-N,N-dimethylethanamine, Tetraethylammonium chloride, Benzyltriethylammonium chloride (BTEAC), 2-acetate-N,N,N-trimethylethanaminium chloride, Glyoxylic acid (GA), Betaine (Be), Hydrated metal halide: AlCl3·6H2O; FeCl3·6H2O; CrCl3·6H2O, FeCl2·4H2O [253,256,258,259,260,261,262,263] Hydrogen bond donors (HBDs) Urea, Formamide, Thiourea ethanolamine (EA), Diethanolamine (DEA), Methyldiethanolamine, 3-aminopropanol (AP), Isopropanolamine (IPA), Triethanolamine (TEA), N-methyldiethanolamine (NMDEA), 1,3-diamino-2-propanol (DAP), 2-amino-1,3-propanediol (ADP), 4-hydroxy benzyl alcohol, Ethylene glycol (EG), Propylene glycol 1.4-butenediol, Acetamide (AC), Benzamide, Glycerol, Imidazole, Boric acid (BA), Monocarboxylic acids: glycolic, lactic (LA), levulinic, acetic (AA), formic (FA), propionic, butyric, octanoic, decanoic, Di or tricarboxylic acids: malonic, oxalic (OA), succinic, malic (MA), maleic, glutaric and citric (CA) acids, Glucose, Fructose, Sucrose, Xylitol, Salicylic acid, Gallic acid, p-coumaric acid, Vanillin, p-hydroxybenzoic acid, p-hydroxybenzaldehyde, p-hydroxybenzyl alcohol, Catechol, Resorcinol, Glycerol (Gly) Both HBDs and HBAs Menthol (Men)
2,6-dimethoxyphenol (Dmp)[99] DESs have a high viscosity, due to the existence of hydrogen bonding, electrostatic interactions and van der Waal forces between HBAs and HBDs. Some studies have linked a higher viscosity for those DESs with a higher number of hydroxyl groups, in the chemical composition of HBDs, due to the formation of a higher number of hydrogen bonds [259]. Nevertheless, viscosity can be lowered by both increasing the temperature or dilution with water to break the existing bonds, originating HBD–water interactions, although dilutions above 30% are not advisable [257]. Density is also an important factor for DES mobility and activity: the more hydroxyl groups and the shorter chain length of the HBDs, the higher density of the DESs [264]. In addition, the chemical nature of the HBDs could also condition the effectiveness of the lignin fractionation process. Effectiveness in DESs can be improved if some remarks are considered such as shortening alkyl chains in carboxylic acids, using monocarboxylic acid-based HBDs instead of di- or tri-carboxylic acids, increasing the presence of hydroxyl groups in a-hydroxyl acid-based DES, increasing the amount of amine/amide groups in HBDs and enhancing HBD:HBA molar ratio in DES [259]. In general, the molar ratio between HBDs and HBAs will affect the physicochemical properties (density, viscosity, polarity, melting point, etc.) of the generated mixture [265].Recent research has shown the suitability of incorporating co-solvents into the initial eutectic mixtures to increase the efficiency of biomass fractionation. In this sense, the incorporation of substances such as O or small amounts of water to DES systems would favor the fractionation and cleavage of lignin bonds, as a large amount of acid protons could be generated. Apart from that, the addition of water would decrease the viscosity and increase the diffusivity and interactions of DESs in the biomass [259]. Other advances in the field of fractionation with DESs are based on the use of simultaneous technologies, in particular the use of DESs combined with physical treatments such as microwave radiation and ultrasound techniques, promoting both the breaking of chemical bonds and the appearance of cavities or micropores, that favor the action of eutectic solvents.Lignins from DES treatment are characterized by a large number of phenolic hydroxyl groups and small molecular size [266]. Although the effectiveness of the use of DESs has been demonstrated, one of the main concerns related to eutectic solvents is that it results in more condensated lignin extraction. Although condensated lignins imply positive aspects for both the manufacture of composite materials and activated carbon production (increased surface area, more functional groups and less rigid structure), they also have serious drawbacks when soluble lignin is required, also hindering enzymatic hydrolysis as undesirable lignin could be deposited on the cellulose surface, hindering the cellulose activity [253]. Several studies have shown that deep eutectic solvents used for lignocellulosic material delignification have resulted in lignins with a large number of hydroxyl groups and high antioxidant activity, therefore promoting their reactivity and use [254]. To minimize the undesired effect of lignin condensation, recent research points to the use of ternary mixtures, e.g., by adding polyol-based solvents, such as ethylene glycol, to certain binary mixtures (choline chloride–oxalic acid). These polyols would react with benzylic intermediates in a -position with respect to the -O-4 ether bonds, inhibiting lignin condensation [267]. The precise mechanisms involving DES biomass delignification are still under investigation but the high capacity for DESs to form hydrogen bonds, interacting with lignin functional groups (hydroxyl, phenolic, methoxyl, etc.) against groups present in cellulose and hemicellulose could explain LAIW depolymerization, interacting and leading selectively to the preferential dissolution of lignin [268]. Table 7 summarizes some of the most recent research with DESs for different LAIWs. Although dependent on the type of DES used and the extraction conditions, the lignins extracted will, in general, have greater thermal stability, due to a lower presence of labile functional groups in their structure, with higher molecular weight and more conserved structure, as they are extracted with milder solvents, compared to lignins extracted by traditional basic or acidic methods.On the other hand, since around 2020, much research work has focused specifically on the use of lignin extraction methods adapted to preserve the structural characteristics of the lignin in the original material, so that it undergoes as few modifications as possible during the polymer extraction process. In this way, the field of application of the lignins obtained could be extended by applying specific depolymerization processes according to the applications to be given to the lignins obtained.
| LAIWs | DES | Experimental Conditions | Delignification, % | Ref. |
|---|---|---|---|---|
| Boehmeria nivea stalks | Be:GA 1:6 molar ratio | 1 g LAIW-10 g DES 130 °C, 2 h | 81.9 | [263] |
| Brewer’s spent grains | ChCl-LA/ChCl-Gly
1:10; 1:2 molar ratio | 1 g LAIW-20 g DES 60–80 °C, 24 h | 34.5–39.3 | [262] |
| Camellia oleifera shell | ChCl-EA/ChCl-AP/ChCl-IPA 1:6 molar ratio | 1 g LAIW-20 mL DES 90 °C, 1–12 h | 59.9 | [261] |
| Corncob | ChCl-Gly/ChCl-urea/ChCl- Imidazole 1:2; 1:2; 3:7 molar ratio | 1 g LAIW-16 g DES 115 °C, 15 h | 88.0 | [269] |
| Be-Lysine/Be-Arginine/ Be-Histidine 1:1 molar ratio | 1 g LAIW-10 to 25 g DES 60 °C, 5 h | 24.4–57.0 | [270] | |
| Corn stover | ChCl-LA/TMAC-LA 1:2 molar ratio | 1 g LAIW-10 g DES 130 °C, 2 h | 62.6 | [271] |
| Corn straw | ChCl-LA/Be-LA 1:2 molar ratio | 1 g LAIW-20 mL DES 120 °C, 6 h | 32.3–89.7 | [272] |
| Industrial xylose residues | Guanidinium hydrochloride-LA 2:1 to 1:2 molar ratio | 1 g LAIW-20 mL DES 120 °C, 2 h | 57.5–73.5 | [273] |
| Oil palm empty fruit brunch | ChCl-different
carboxylic acids | 1 g LAIW-10 g DES 120 °C, 8 h | >60.0 | [274] |
| ChCl-LA 1:2 molar ratio | 1 g LAIW-10 mL DES 100–120 °C, 10-30 min | 9.1–57.1 | [275] | |
| Reed straw | BTEAC-FA 1:2 to 1:6 molar ratio | 1 g LAIW-10 g DES 130 °C, 3 h | 78.1 | [276] |
| Be:LA:H2O 1:5:5 molar ratio | 1 g LAIW-20 g DES 80 °C, 24 h | 52.0 | [277] | |
| Rice straw | MA-proline/ChCl-OA/ChCl-urea 1:3; 2:1; 1:2 molar ratio | 1 g LAIW-10 g DES 120 °C, 4–12 h | 35.0–75.0 | [278] |
| ChCl-glycerol 1:3 to 1:9 molar ratio | 1 g LAIW-19 g DES 80–150 °C, 3–24 h | 53.3–74.2 | [279] | |
| Sugarcane bagasse | ChCl-LA/ChCl-CA/ChCl-AA
1:4 to 4:1 molar ratio | 1 g LAIW-15 mL DES 130 °C, 90 min | 2.7–54.5 | [280] |
| ChCl-OA/ChCl-trifluoracetic acid 1:2 to 1:1.5 molar ratio | 1 g LAIW-19 g DES | 51.0–56.0 | [281] | |
| ChCl-LA/Be:LA 10–5% w/w | 1 g LAIW-9 g DES 140 °C, 2 h | 16.6–39.4 | [282] | |
| Wheat straw | ChCl-CA-EG; ChCl-MA-EG;
ChCl-FA-EG; ChCl-AA-EG; ChCl-BA-EG; ChCl-LA-EG | 1 g LAIW-20 g DES 80–140 °C, 3–24 h | 35.7–92.4 | [253] |
| TEBAC-Gly-Al chloride 1:2:0.05 molar ratio | 10 g LAIW-3% DES 100 °C, 16 h | 1.2–7.8 | [283] | |
| Gly-K2CO3 1:5 molar ratio | 1 g LAIW-10 g DES 100–140 °C, 1 h | 70.5–83.0 | [284] |
- Oxidative treatments: These promote delignification processes using chemical oxidants, Table 8, such as alkaline hydrogen peroxide (), wet oxidation, organic peracid pretreatments, Fenton oxidation and ozone technology [234]. As this process takes place under conditions of medium severity, the formation of inhibitory compounds is more unlikely than other promising technologies with options to be implemented on an industrial scale (such as steam explosion).Hydrogen peroxide is considered by many authors to be an effective treatment that promotes sustainability [285]. This oxidative compound, in strongly alkaline media, generates hydroxyl (·OH) and superoxide radicals (), with a strong oxidizing character, encouraging lignin depolymerization. While medium-severity conditions in oxidative treatments with allow attacking mainly the hydrocarbon chains of lignin, more severe treatments would oxidize aromatic rings. It has been noted that, at pH higher than 10, the effectiveness of the hydrogen peroxide is high but no delignification was achieved below this value [286].These hydroxyl radicals are also produced by the Fenton process based on the oxidation, in a more efficient way, of LAIWs using and hydrogen peroxide under acidic conditions, to avoid the loss of reagent by the formation of complexes of this cation or the formation of Fe(OH)3, in the case of basic conditions [287]. Treatment by wet oxidation implies using oxygen (or air) and water at 120–238 °C and 3–35 air bars with lower production of inhibitors but higher production of collateral products, compared to other techniques (e.g., steam explosion) [234].
| LAIWs | Oxidative Treatment | Experimental Conditions | Delignification, % | Ref. |
|---|---|---|---|---|
| Brewer spent grains | Hydrogen peroxide
(alkaline conditions) | 1–8% , 20 °C, 0–12 h, pH = 11.5 1 g LAIW-12.5–50 g oxidant | 17–36 | [288] |
| Chinese hickory shell | Hydrogen
peroxide (alkaline conditions) | 0–3 mL (30%), 20 °C, 300 rpm, 3 h 1 g LAIW-15 mL oxidant | 50.0 | [289] |
| Oil palm empty fruit bunch | Fenton | 200 mM , 24 h, room temperature 1 g LAIW-50 mL oxidant | 71.2 | [287] |
| Rice husk | Hydrogen peroxide (alkaline conditions) | 1% , 5.3% NaOH, 20 °C 0–12 h, 150 rpm | 59.6 | [290] |
| Sisal waste | Ultraviolet-catalyzed hydrogen peroxide (alkaline conditions) | 0.05–0.4 g /g LAIW, pH = 10 1 g LAIW-20 mL oxidant | 76.2 | [291] |
| Toonna sinensis branches | Hydrogen
peroxide + acetic acid (100 mM as catalyzer) | 12% , 37.6% acetic acid, 170 °C, 90 min | 77.0 | [292] |
| Wheat straw | Hydrogen peroxide (alkaline conditions) | 4% , 110 °C, 2 h, pH = 11.5, 400 rpm 1 g LAIW-40 mL oxidant | 70.7 | [293] |
3.1.3. Physicochemical Treatments
- Liquid hot water (also known as autohydrolysis [294]) is a hydrothermal method focused on the solubilization mainly of hemicellulose (with the generation of oligosaccharides and monosaccharides), although the solubilization of smaller amounts of lignin can also be achieved; in particular, delignification percentages of 20–30% can be reached [295]. Pressurized water is used to carry out the hydrolysis of LAIWs involving an auto-catalyzed process by both hydronium ions, generated by the ionization of water, and the action of acetic acid formed from the acetyl groups, released from the hemicelluloses. Additionally, the way in which lignin depolymerization takes place is strongly dependent on the experimental conditions (mainly temperature, reaction time and solid:liquid ratio). Although more severe conditions would imply higher degrees of delignification of residual biomasses, bioproducts that are difficult to control would also be extracted, so typical conditions (140–240 °C and liquid:solid ratios of 10, [12,294,296]) are geared towards biofuel more than lignin extraction. Frequently, the terms “subcritical water” and “liquid hot water” are often used interchangeably to describe water that is at temperatures above its normal boiling point but below its critical point. From a more general point of view, this treatment consists of using water at temperatures between 100 °C and 374 °C and pressures high enough to keep it in a liquid state (under 1–20 MPa for 10–50 min) [296]. Some researchers have shown that the use of subcritical water as a one-stage method is not effective the for delignification of LAIWs [297] and they are frequently used for both bioactive compounds and sugars production. Nevertheless, delignification data (13.73%) have been obtained using mango seed shell (180 °C, 15 min, 2.5 MPa) [298]. More recent studies employ subcritical water in combination with other techniques such as deep eutectic or ionic solvents [299], obtaining significant delignification, especially by the contribution of other solvents apart from water [300], Table 9.
- Steam explosion method: This is a hydrothermal treatment considered as an environmentally friendly technique for four relevant reasons: the main agent used is water vapor, an abundant and renewable resource, high energy efficiency, low use of chemical reagents (diluted acid or alkali) and recovery of almost intact lignin. The disintegration of the biomass occurs by abrupt decompression after reaching high temperatures and pressures, usually in the ranges (160–270 °C) and (10–50 bars), respectively [39,301]. Residence times (2–40 min) and biomass properties (moisture content or particle size) are also determining factors in the depolymerization process of LAIWs [301,302,303]. Considering the reduction in fermentation inhibitors, the combination of a low temperature and longer residence time may be a better choice. In contrast, the option of a short residence time at high catalyst concentrations is less ideal [163].Finally, the concentration of the main catalysts (, and ) used to increase efficiency is strongly dependent on the type of catalyst, nature of biomass and purpose of the treatments. Most of the research studies, using this hydrothermal treatment, have focused on experimental conditions to enrich the solid resulting from steam explosion treatment in cellulose, for subsequent enzymatic hydrolysis and biofuel obtainment, as well as treatments for xylo-oligosaccharides or anaerobic gas production. In this sense, considering sugarcane bagasse depolymerization by acid catalyzed steam explosion [304] uses phosphoric acid concentrations between 0.05 and 0.4% (w/v) to disintegrate the structure of sugarcane bagasse. However, when using , concentrations in the range of 0.9-3% are used to treat the same material.In the latest LAIW delignification studies, the steam explosion technique has been used in combination with other main chemical, physicochemical or biological processes [303], as shown in Table 9, concerning combined biomass treatments, to increase the efficiency of the global lignin removal process.Table 9. Parameters and delignification percentages for different LAIWs using hydrothermal processes.Table 9. Parameters and delignification percentages for different LAIWs using hydrothermal processes.
LAIWs Hydrothermal Method Experimental Conditions Delignification, % Ref. Barley straw
Deionized water
(subcritical)60% v/v, 180 °C, 50 bar
5 mL/min61.0
[305]
Canola straw
Deionized water
(subcritical)60% v/v, 180 °C, 50 bar, 5 mL/min
51.0
[305]
Chestnut shell
Liquid hot water
100 °C, 24 h
1 g LAIW-10 mL water18.8
[306]
Rice straw Subcritical water 170–200 °C, 4.67 mL/min 85.0 [307] Sugarcane
bagasseSteam explosion
(NaOH)0.1 M calatyst 180 °C, 5 min, 20 bar 65.0
[308]
Liquid hot water
180 °C, 20 min, 160 psi
1 g LAIW-9 mL water12.8
[309]
Sugarcane straw
Steam explosion
Deionized water soaked 2 h at 20 °C
210 °C, 15 min, 20 bar6.0
[310]
Sugarcane trash
Steam explosion
(NaOH)
Double impregnation:
1 g LAIW-10 mL water
1 g-20 mL NaOH
17%, 190 °C, 17 min70.5
[311]
Wheat bran
Steam explosion
0.05 (v/w)
218 °C, 8 min25.1
[228]
Wheat straw
Liquid hot water
180 °C, 40 min
1 g LAIW-10 mL water21.6
[312]
Liquid hot water 160–200 °C, 30–90 min 10–35 [313] Subcritical water 121 °C, 45 min, 15 lb/inch2 57.0 [199] - Supercritical or subcritical fluids: Supercritical fluids (carbon dioxide, ammonia, water, acetone, ethanol, methanol, and hydrocarbons such as propane and butane) are obtained when they are subjected to pressures and temperatures above their critical point, so that there is no transition phase and the liquid and solid phases become indistinguishable. Therefore, these fluids are chemical compounds that have properties of both liquids and gases [314] and advantageous characteristics such as low viscosity, low dielectric constant and high diffusivity [4], improving the solubility of hydrophobic compounds in water and encouraging lignin fractionation [315]. In the case of , one of the most commonly used fluids due to its low cost, non-toxicity and a relatively low critical point, temperatures above 31.7 °C and pressures of 7.38 MPa are required [186]; however, water requires conditions above 374 °C and 221 bar [314]. In some cellulose and biofuel research, the delignification conditions must be carefully chosen, so that the yields in carbohydrate fraction are high as well as the quality of the recovered collateral lignin [316].Recently, the use of supercritical fluids, especially , in combination with other delignification treatments, has been advocated, as it is possible to preserve the structure of the lignin to a greater extent. Some experimental conditions for one-step treatments are shown in Table 10, exceptionally collecting delignification results from research prior to the time interval for which the review has been conducted.Table 10. Parameters and delignification percentages for different LAIWs using critical fluids.
LAIWs Fluid Experimental Conditions Delignification, % Ref. Barley straw
Ethanol
60% v/v, 220 °C,
50 bar, 40 min
5 mL/min60.0
[52]
Ethanol
20% v/v, 180 °C,
50 bar
5 mL/min54.0
[305]
Canola straw
Ethanol
20% v/v, 180 °C,
50 bar
5 mL/min45.0
[305]
Corn stover
(supercritical)
ethanol-water
2:1 v/v
200 °C, 13 MPa,
80 min
1 g LAIW:40 mL90.0
[317]
Hemp fibers
(supercritical)
ethanol-water
2:1 molar ratio
180 °C, 6 MPa,
60 min
1 g LAIW:6 mL89.6
[99]
Rice husk
(supercritical)
ethanol-water,
80 °C, 270 bar,
10 min34–91
[192]
Sugarcane bagasse
(supercritical)190 °C, 16 MPa,
60 min88.4
[318]
(supercritical)60 °C,
200 kgf/cm28.1
[319]
- Ammonia Fiber Expansion (AFEX): This treatment of LAIWs, with liquid ammonia at pressures of 0.7–2.7 MPa, temperatures of 60–100 °C and residence times in the range 5–60 min, involves rapid ammonia expansion and release of pressure at the end of the treatment, causing swelling of the biomass, changes in the structure of the lignin, some solubilization of the hemicellulose and partial delignification [12,209,320,321]. When concentrated ammonia is brought into contact with water, ammonium and hydroxide ions appear in solution, through an exothermic reaction that increases the temperature of the reaction medium. The simplicity of ammonia recovery makes this treatment very useful. However, some research reports a lack of effectiveness of the delignification process for some LAIWs such as corn stover [322] or agave bagasse [323]. No additional information on recent delignification data of LAIWs has been found by applying this process apart from those listed in Table 11.A modification of the AFEX process is the ammonia recycle percolation ( in continuous recirculation and recycling in a percolation reactor) [324]. Experimental conditions for this process usually comprise 140–210 °C, residence time 14–90 min, 5–15% (w/w) of ammonia solution and velocity of 1 cm [324,325] for woods. Soaking aqueous ammonia is also considered a modification of the AFEX process by using batch reactors, but under milder temperature conditions (25–60 °C) and longer reaction times (10–60 days).
| LAIWs | AFEX Conditions | Delignification, % | Ref. |
|---|---|---|---|
| Corn stover | 58.7% w/w 30 °C, 10 min 0.5 H2O2 loading (30%) | 24.0 | [326] |
| Pine chips | 70% moisture 130 °C, 15 min 1 g LAIW: 1 g | 4.0 | [327] |
| Wheat straw | 10 g-7 mL deionized water 130 °C, 15 min 1 g LAIW: 1 g | 3.8 | [328] |
- Hydrodynamic cavitation: This treatment allows, using non-rotational systems (orifices, Venturis, vortex diodes, swirling jet), or advanced rotational reactors to provoke liquid contraction, the growth and collapse of vapor bubbles in the solvent in which the residual biomass is found, by a pressure drop below the saturation pressure of the liquid. In this process, pressures of ∼500 bar, temperatures of ∼5000 °C and oxidation processes (by hydroxyl radical formation) are presented [329], causing mechanical and chemical effects on the biomass, and producing its disintegration.A hydrodynamic system has been used [330] to assist different alkaline pretreatment processes for sugarcane bagasse, achieving 48.31% delignification using KOH (0.5 mol/L) at 60 °C, 20 min, dropping the pressure from 3 bar to 0.3 bar in the flowing fluid through the orifice plate. The same experimental conditions but using NaOH (0.3 mol/L) produced a delignification of 43.63%. On the other hand, information collected from Iskalieva et al. [331] summarizes experimental conditions for LAIWs treated by this technique, generally in conjunction with other chemical processes, showing percentages of lignin release, solubilization or removal in the range 2.2–78.5%.
- Non-thermal plasma (cold plasma) technique: Although there are also high temperature processes, low-temperature plasma (in the range 25–100 °C) [81] are regarded as being more environmentally friendly, especially more than those using chemical agents, although its novelty justifies the need for further studies to be optimized [332]. Cold plasma processes are generally used in combination with other chemical treatments. In addition, the biomass can undergo mechanical as well as chemical depolymerization, if there is the possibility of free radical formation which can oxidize LAIWs. This technique is characterized by the presence of electrically charged particles, a mixture of electrons, ions, radicals and neutral particles (in varying proportions depending on full or partial ionization) by the action of an electrical discharge with sufficient voltage on a gas (air, oxygen, helium, air–argon, ozone, etc). The interactions produced between the electrons (attaining temperatures of 105–5000 °C) and the gas molecules also generate active free radicals that attack the biomass and lead to its degradation [333]. The main advantage is that, although electrons reach extremely high temperatures, the gas surrounding them is at a relatively low temperature, so that no dissociation of the reaction products is observed and no cooling is necessary. In addition, this technique avoids problems of environmental damage, waste generation and inhibitors production, in contrast to many of the commonly used chemical treatments. Its proven efficiency in lignin extraction, low cost and its non-toxic character make it a promising strategy in lignocellulosic biomass fractionation processes.
3.1.4. Combined or Assisted Methods
3.1.5. Thermochemical Treatments
3.1.6. Biological Treatments
3.2. Lignin Recovery and Depolymerization
| STEP I: Precipitation → Separation | STEP II: Washing → Drying | LAIWs | Ref. | ||
|---|---|---|---|---|---|
| Precip./Extraction | Separation | Washing | Drying | ||
| POOL FROM ALKALINE PROCESS | |||||
| 20% w/w final pH = 2 | Centrifugation 4000 rpm 15 min | Hot water | 60 °C, 5 days | Bagasse Coconut husk Rice straw Corn stover | [202] |
| 24% w/w final pH = 4 + enzymatic hydrolysis | Centrifugation 3000 rpm 60 min | 5× with buffer to pH = 4 | 60 °C | Miscanthus giganteus | [170] |
final pH = 2 | Centrifugation 12,000 rpm 15 °C, 20 min | until pH = 7 | Sugarcane bagasse | [217] | |
| , latic and formic acid 4 M pH = 4 | Centrifugation | until pH = 3 | Sugarcane bagasse | [219] | |
| HCl 12% 70 °C, pH = 2–3 | Centrifugation 9000 rpm | until pH = 7 | 60 °C | Green sandalwood | [365] |
| 30% | Vacuum
filtration | Spray drying | Sugarcane bagasse | [373] | |
| POOL FROM KRAFT PULPING | |||||
| LignoBoost using pH = 9.5 | Centrifugation | HCl pH = 2.5 + acetic acid pH = 3.5 | Freeze-dried | Pine Acacia Eucalyptus | [25] |
| HCl 1 M pH = 2 Ethanol pH = 7, 8 h | Vacuum filtering | Dried 45 °C, 24 h | A. mangium | [374] | |
| POOL FROM DEEP EUTECTIC SOLVENT TREATMENT | |||||
| Acetone:water 1:1
Evaporation 60 °C + freezing −80 °C | Rotavapor 60 °C, 30 min | Freeze-dried | Reed | [277] | |
| Deionized water precipitation | Centrifugation rpm, 5 min | Ethanol:water 1:9 v/v | Freeze-dried | Wheat straw | [253] |
| Cooling + Deionized water precipitation | Centrifugation and decanting | Lyophilization | Camellia oleifera shell | [261] | |
| Ethanol:water 1:1 v/v | Distilled water to pH = 7 | Freeze-dried | Rice straw | [375] | |
| Ethanol:water 1:2 v/v | Centrifugation | Distilled water 2 times | Oven dried 40 °C | Oil palm fruit bunch | [274] |
| Ethanol 3 times | Rotavapor 40 °C deionized water filtration | Deionized water 2 times | Oven dried 40 °C | Reed straw | [276] |
| Ethanol:water 9:1 v/v | Rotavapor | Ultrapure water 3 times | 45 °C, 24 h | Brewer’s spent grains | [262] |
| NaOH 0.1 M room temperature | Centrifugation + decantation | Distilled water 3 times | Lyophilization | Corncob | [270] |
| Ethanol:water 1:9 v/v | Centrifugation | Ethanol:water 1:9 v/v | Corn straw | [272] | |
| Distilled water | Centrifugation | Freeze-dried 48 h | Ind. xylose residues | [273] | |
| Water | Centrifugation | Cold water | 49 °C, 48 h | Oil cane bagasse | [282] |
| Deionized water | Vacuum filtered | Deionized water, pH = 7 | 70 °C | Oil palm fruit bunch | [275] |
| POOL FROM COMBINED TREATMENT | |||||
| Acetone 96% | Rotavapor 35 °C | 40 °C | Defatted grape seed | [376] | |
| 20% | Centrifugation 3500 rpm | Water to pH = 2 | 50 °C, 24 h | Coconut husk | [335] |
| HCl pH = 2 | Centrifugation
8500 rpm | Distilled water, 60 °C | 60 °C | Rice straw | [343] |
| Ethanol or acetone used in previous organosolv
treatment | Filtration | Vacuum 80 °C 4 h | Apricot kernel shells | [334] | |
| POOL FROM ORGANOSOLV TREATMENT | |||||
| Ethanol | Centrifugation
1200 rpm | Freeze-dried | Corn stover | [337] | |
| Pure water | Centrifugation | Deionized water | Freeze-dried 50 °C, 48 h | Corn stover | [377] |
| Distilled water | Filtration | 60 °C, 24 h | Sugarcane bagasse | [244] | |
| Water | Centrifugation | Freeze drying | Bamboo culms | [237] | |
| Acidic deionized water pH = 3–4 | Centrifugation 4000 rpm | 45 °C | Wheat straw | [247] | |
| Ethyl acetate | Filtration +
rotavapor | Vacuum dried 60 °C | Corn stalks | [238] | |
| 95% pH = 1.5–2.0 | Centrifugation 3220× g | Acidified water pH = 2 | Persimmon tree pruning waste | [378] | |
| POOL FROM TREATMENT | |||||
| HCl 6 M | Centrifugation | Freeze-dried | Chinese hickory shell | [289] | |
| POOL FROM ACID/ENZYMATIC TREATMENT | |||||
| Deionized water pH = 2 | Centrifugation
4000 rpm | Lyophilization | Corn stover | [379] | |
| 2.5 M 70 °C, 1 h | Filtration | Water wash | 80 °C | Poplar wood hydrolysis solid | [203] |
| POOL FROM SUPERCRITICAL/SUBCRITICAL TREATMENT | |||||
| HCl pH = 2 | Vacuum filtration | Deionized water | Freeze-dried | Hemp fibers | [99] |
4. Characterization of Recovered Lignin
4.1. Lignin Characterization Methods
4.1.1. Differential Scanning Calorimetry (DSC)
4.1.2. Thermogravimetric Analysis (TGA)
4.1.3. X-Ray Diffraction (XRD)
4.1.4. Size-Exclusion Chromatography (SEC)
4.1.5. UV-Vis Spectroscopy
4.1.6. Fourier Transform Infrared (FTIR) Spectroscopy
- OH and CH Stretching Region (3460–2800 ): O-H stretching of phenolic and aliphatic structures (3410–3460 ) takes place, while C-H stretching is found in aromatic methoxyls, methyl and methylene groups (2938, 2842 ). A further example is additional aliphatic methylene bands in hemp, jute and flax lignins (2917, 2847 ), that can be originated from fatty acids in lignins.
- Carbonyl/Carboxyl Region (1750–1600 ): Weak bands that may be attributed to unconjugated carbonyl/carboxyl stretching (1705–1720 ) are found, with shoulder peaks attributed to conjugated carbonyl/carboxyl stretching (1680 ). Intensity variations and broadening at 1705–1600 in oxidized, solvent-extracted lignins, sulfur-free lignin depending on the treatment may be originated due to potential protein or water-related bands around 1650 .
- Fingerprint Region (1400–400 ): Complex region difficult to analyze due to band complexity with contributions of various vibration modes. This region provides information about monolignol units and linkages, including guaiacyl unit bands (1269 , G ring and CO stretch; 1140 , CH in-plane deformation; 854 and 817 , CH out-of-plane vibrations in position 2, 5 and 6 of guaiacyl units). In this region can also be found information on syringyl unit bands (1326, 843 ), that are typical of hardwood and all non-wood lignins e.g., wheat straw, acacia and eucalyptus. Lastly, phenolic OH and aliphatic C-H bands (1370–1375, 1215–1220 ), and potential polysaccharide-related bands (1000–1300 ) can also be detected.
4.1.7. Raman Spectroscopy
- Aliphatic and Aromatic C-H Stretching: Bands in the 2700–3200 region indicate C-H stretching vibrations in aliphatic and aromatic groups. The intensity of the 2940 band, associated with methoxyl groups, may be higher in hardwood due to its higher syringyl content.
- Aromatic Ring Vibrations and Carbonyl/Carboxyl Stretching: The 1350–1850 region shows bands related to aromatic ring vibrations, C=C and C=O stretching. Differences in band intensities can be found between softwood and hardwood due to variations in aromatic ring-conjugated structures [425].
- Fingerprint Region: The 250–1450 region reveals differences between softwood and hardwood, with certain bands being more intense in one type or the other. These differences can be attributed to variations in specific functional groups and linkages.
4.1.8. Nuclear Magnetic Resonance (NMR) Spectroscopy
4.1.9. X-Ray Photoelectron Spectroscopy (XPS)
5. Industrial Applications of Lignin
- Heat and fuel production: Bio-oil obtained by pyrolysis, gasification or hydrothermal liquefaction can be used for heat production by direct burning. However, bio-oil contains significant amounts of water, acids and heavy oligomers, so it has a low energy content that makes it unviable as a vehicle fuel, unless it is subjected to hydrodeoxygenation processes [397]. Additionally, steam reforming of pyrolysis bio-oil from biomass is intensively being investigated to produce hydrogen. Apart from that, lignin pyrolysis processes give rise to significant amounts of phenols and phenolic compounds, depending on the experimental conditions employed. These phenolic compounds can form part of phenolic resins that are highly valued as binders for composite wood products, such as plywood and oriented strand boards. Another possible way of utilizing lignin would be through combustion processes, for example of waste black liquor from kraft pulp production [440], generating and O as the main products, which can be used to produce heat or electricity. Additionally, lignin can be also converted into suitable diesel, gasoline and aviation fuel [441]. Another study demonstrates that lignin from wheat straw can be converted, by catalytic depolymerization, into aromatic C6–C8 fractions and can subsequently give rise to aromatic C8–C15 fractions, by low-temperature alkylation using ionic liquids, with suitable characteristics for jet fuels [442]. Other studies focus on the harnessing of lignin from LAIWs (e.g., wheat straw) by different gasification technologies (fixed bed updraft, bubbling fluidized bed and indirect gasification) for the production of syngas that can be fermented to obtain bioalcohols [443].The techno-economic assessment of the application of lignin to heat and fuel production (e.g., by gasification or hydrothermal liquefaction) shows significant incentives such as those derived from life cycle analysis or those associated with the price of fossil fuels [444].
- Civil construction: Lignin is added to cements and mortars, to make lignin-based cement composites. In this way, the amount of water required is reduced, increasing the particle-to-particle adhesion, plasticity and workability. Lignin may adhere to the particles and act as dispersant, providing steric hindrance and electrostatic repulsions, that prevent the formation of aggregates when lignin is adsorbed or adhered on the cement particles through the formation of a film [445,446]. Accordingly, lignin-based polyurethanes can be produced by replacing polyols from urethane with lignin, originating composites with modified properties with applicability as foam, for thermal insulation, to improve the energy efficiency of buildings apart from gap filler and adhesive for materials such as wood, metal, concrete and ceramics [447]. It also has been shown that lignin molecules can adhere to the surface of the metal, forming a protective layer with an anti-corrosion effect [445]. These protective properties in coatings and adhesive properties have also been demonstrated in lignin-based epoxy resins [132]. Additionally, lignin-based phenol resins are utilized in the production of various building materials such as particleboards, laminates, coatings, plywood and adhesives.There are also studies that demonstrate the benefits of lignin in the field of pavement engineering, as lignin-modified asphalt has advantages over conventional asphalt in terms of improved properties of the resulting material [448]. In this sense, the addition of lignin can not only reduce the proportion of asphalt in the asphalt pavement, but also improve its properties. Moreover, from an economic point of view, the use of lignin in the asphalt binder industry can save costs, given the market price of lignin (about USD 297 per ton) compared to that of asphalt (USD 484 per ton), positioning lignin pavement as a promising material in upcoming applications [448]. In addition, lignin can also act as a protective coating of natural fiber geotextiles against degradation processes in materials [449]. The incorporation of lignin in geotextiles used in pavements can increase their durability and wear resistance, preventing blockages. Geotextiles containing lignin can also protect underground pipelines from damage caused by soil movement.
- Bio-based activated carbon attainment: Composite carbon nanofiber from lignin can be obtained considering four sequential stages: fiber spinning, fiber stabilization, fiber carbonization and surface treatment [450]. In particular, this promising porous material could be employed for energy storage or adsorption. Energy applications are based on capacitors and battery production while adsorption methods can also be conducted, through electrostatic interactions of compounds with the polarized surface of the bioadsorbent, offering advantages such as low-cost, high surface area, high thermal and electrical stability, suitable thermal properties and corrosion resistance [436]. The current reliance on polyacrylonitrile for the production of carbon nanofibers limits and makes their industrial applications more expensive, with a market price in the range of USD 5–44/kg. The incorporation of lignin would reduce these costs, expanding the current demand for carbon fibers (140,000 tonnes in 2020) [451].Concerning energy storage, lignin-based phenolic resin carbon microspheres have been used to produce capacitors [452]. Related to sustainable energy storage, the generation of hydrogels from lignin, used for the preparation of electrolytes, is seen as a promising alternative for organosolv lignin [453]. The combination of these hydrogels with lignin-derived electrodes results in supercapacitors with improved electrochemical properties. Likewise, activated carbons prepared from waste lignin, once thermochemically activated (microwave-assisted KOH treatment) were integrated together with polyvinyl alcohol to form electrodes in supercapacitors, using LiCl as the gel electrolyte [454]. These authors also obtained, through a carbonization process followed by microwave-assisted chemical activation of lignin, a superconductor with a low production cost.These energy applications of activated carbons from LAIWs are not restricted to the production of supercapacitors; empty fruit bunch and rice husk KOH activated carbons have been used to produce biomaterials for hydrogen storage [168] and residual lignin can also be integrated in nanocomposites to form battery anodes and cathodes for hydrogen storage. The large number of oxygen atoms in lignin favors the adsorption of electrolyte ions, and the occurrence of redox reactions, increasing the energy storage capacity in batteries and supercapacitors [437]. Other recent research into the use of lignin includes the use of consistent nanoporous carbon, generated by low-temperature carbonization (350 °C, 30 min) and physical activation with (1000 °C) of organosolv lignin from materials such as hemphurds, eucalyptus chips, flax straw and rice husk for hydrogen storage by adsorption [455].On the other hand, hydrocarbons from the hydrothermal carbonization of coconut shell powder, in the presence of zinc chloride, leads to a mesoporous material rich in functional groups suitable for contaminant removal [456]. In the same line, activated carbon, obtained by chemical activation of charred materials, has higher porosity and surface area than that obtained by physical processes. The chemical treatment of lignins to obtain activated carbon involves the use of high temperatures (500–900 °C), inert atmosphere of Ar or , in the presence of agents such as , NaOH, , or preferably KOH as it generates a large number of pores with a high specific surface area [436].In this line, porous carbon materials have been applied to water treatment, using for example alkali lignin to retain Congo red effectively by adsorption [457]. An important application of lignin is in the adsorption of chromophore groups present in dyes, through different types of interactions: through the hydrogen bonding of OH groups of lignin and polar functional groups of chromophore dyes (carbonyls or amines). Hydrophobic interactions (London dispersion forces) between aromatic rings of the lignin and hydrophobic portions of chromophore groups could also take place. In addition, ionic interactions of charged lignin groups (sulfonates and carboxyl groups) with charged chromophore groups could also occur.Kraft lignin is rich in hydroxyl and aliphatic groups, both polar, so it has a high affinity for polar aromatic groups and some metals. It has been successfully used for the removal of dyes by the formation of kraft lignin hydrogels [458]. With respect to sulfite lignin, it contains a high amount of sulfonate groups, which makes it highly soluble in water, limiting its capacity to retain heavy metals (lead, cadmium and chromium) from wastewater [459], although electrostatic interactions with sulfonate groups and the use of modified lignins could be promising alternatives to improve their adsorptive capacity. Concerning organosolv lignin, it has a more conserved and less condensed structure than other lignins and, in general, tends to be more hydrophobic than kraft or sulfite lignin due to its preserved structure rich in aromatic rings and alkyl groups, which are hydrophobic. Nevertheless, its adsorption capacity depends on the nature of the solvent used in the extraction and the reaction conditions. In general, organosolv lignin has a limited solubility in water, so it has a great potential for the removal of pollutants from water [459]. Finally, it is possible to modify the properties of lignin by oxidation, reduction or functionalization processes to improve its adsorption capacity for certain pollutants. The production of lignin composites is currently a promising alternative for the adsorption of metals in polluted water, for example, by introducing into the structure of certain LAIWs, such as wheat husks [460]. The structure of lignin, with the presence of a multitude of functional groups, favors interaction with particles dissolved in water, promoting their purification by flocculation processes [446].
- Antioxidants and hydrogels: Significant research efforts have been made in recent years to develop hydrogels with appropriate structures for drug delivery. The variability and complexity of lignin makes it difficult to interact with the three-dimensional matrices of these materials and to create porous scaffolds [461]. The high aromaticity of lignin and, in particular, the presence of phenolic compounds, determine the antioxidant power of lignin. This antioxidant capacity of lignin can be chemically enhanced, resulting in modified lignins. In this context, alkali lignin extracted from cornstalk was chemically modified by the Mannich reaction (using ethylenediamine aqueous solution and, sequentially, formaldehyde), achieving significant increases in their antioxidant activity. This antioxidant capacity could be employed in biomedicine, food packaging, cosmetics and medical devices through the production of lignin-based films as carriers or in biocompatible scaffolds [462]. On the other hand, hydrogels from lignin can be used to encapsulate medicines. The hydrophilic character of lignin allows it to retain water, which causes the three-dimensional network of the lignin to facilitate drug-controlled release at the site of injury [463].
- Packaging: Lignin-derived biomaterials can be substitutes for plastic, offering more sustainable applications. Thermoplastic and thermosetting lignin polyester has gained great attention [464] to produce flexible and tough lignin-based films for packaging. Regarding this application, oxypropylated kraft lignin (5%) with polylactic acid biocomposites have shown suitable characteristics for being used in food packaging [465]. In addition to antimicrobial and flame retardant properties, the use of lignin to generate UV-blocking materials has also been investigated, attributing this property to the phenolic and carbonyl groups in the lignin [368,466], absorbing light in the UV range (200–400 nm). Apart from that, lignin can also be useful to prepare lignin-based bio-finish in order to provide hydrophobic materials [467]. Transparency of the wrapper is a highly appreciated quality, so the amount of added lignin (which could give a brown coloring) should be optimized. Boarino and Klok [466] collected in their research information on biodegradable polymers based on different types of lignins (soda, kraft, organosolv and lignosulfonates) with different matrices (polylactic acid, polyvinyl alcohol and poly(3-hydroxybutyrate)/polyhydroxyalkanoates), showing how soda lignin biocomposites have antioxidant capabilities and UV barrier activity, only demonstrated for punctual kraft and organosolv lignins. Additionally, from lignin, different phenolic compounds can be obtained to be used in packaging applications. There are microorganisms (Actynomyces, Aspergillus, Clostridium and Lactobacillus species) capable of generating feruloyl esterase enzymes, necessary for the generation of phenolic compounds from lignocellulosic biomass. The addition of feruloyl esterase to the biomass (usually pretreated) allows the hydrolysis of the ester bonds, releasing ferulic acids and other phenolic compounds [196], acting as protective coatings on cardboard boxes, paper bags and other types of packaging. Ferulic acid has been used in blends with polymers such as low-density polyethylene and ethylene vinyl acetate to make effective packaging films [468]. Likewise, a recent study [469] was able to effectively incorporate lignin nanoparticles into starch polymeric matrices, obtaining biofilm composites that were used for soybean oil packaging, observing a delay in its oxidative deterioration.For food packaging applications, lignin can not only improve the mechanical properties of polymeric biofilms, but also antioxidant and anti-UV barriers. The main challenge is to achieve their compatibility with the surrounding matrix, through proper lignin functionalization. Another challenge is to improve the digestibility of biofilms in composting processes [466].
- Agriculture: The application of lignin-based materials for agricultural mulching increases the water and nutrient retention capacity of the soil, as well as its organic matter supply through the gradual decomposition of lignin [470]. Lignin can be transformed into agrochemicals such as slow-release nitrogen fertilizer, pesticides, soil amendments and plant growth regulators [471]. The use of lignin-coated urea reduces the rate at which urea dissolves in the soil, reducing soil losses and controlling nitrogen levels, implying a higher utility of mixed lignin compared to the use of urea alone, as it provides higher crop yields [471]. On the other hand, when lignin is added to pesticides, in some cases, lignin can act as a dispersant, reducing the attractive forces between pesticide particles and thus reducing viscosity. As pesticides can penetrate more quickly, increasing the effectiveness and decreasing the consumption of the product. Thus, the use of lignin in the development of innovative materials and formulations, compared to traditional agrochemicals, is one of the main challenges in agriculture, demonstrating a growing use, high efficacy and cost-effectiveness [472].
- Surfactants: Lignin has great potential as a low-cost surfactant because of its hydrophobic aromatic configuration and multifunctionality. In this sense, lignosulfonates have been used as a surfactant in industry, but their purity and limited efficiency have hindered their application. This justifies the need to advance in the knowledge regarding the performance and property correlation of lignin-based surfactants in order to be widely incorporated in the market [473]. Kraft lignin is an excellent starting material to obtain surfactants with similar behavior to synthetic ones, as it possesses a wide variety of functional groups such as hydroxyl, carbonyl and methoxy which give it an amphipathic character (there are hydrophilic and hydrophobic parts). In this context, lignin from kraft pulping was diluted in ammonia and then soaked in a dilute solution of polyvinyl alcohol for surfactant generation [374]. These surfactants could be used as foamers, dispersants and emulsifiers. Likewise, small concentrations of cationic kraft lignin surfactants successfully stabilized oil–water emulsions [474]. On the other hand, lignosulfonates have been used as anionic surfactants in concrete admixtures, although they have little efficacy in decreasing the surface tension of water or the interfacial tension of water–oil mixtures [473].
- Biotransformed phenolic and other compounds: Monomers from lignin phenols obtained after the depolymerization of lignocellulosic biomass involves vanillin (producing vanillin alcohol by reduction), vanillic acid (reduced to vanillin), syringic acid that can be reduced to syringaldehyde, guaiacols, (iso) eugenol, ferulic acid, p-coumaric acid and alkylphenols [475], used in food, beverages, cosmetics and pharmaceuticals (due to their anti-inflammatory, antioxidant and anti-tumor properties). The separation of vanillin after the depolymerization (alkaline oxidation) of lignin (Indulin AT) has been efficiently carried out by chromatography, using water and ethanol as eluents [476]. Additionally, pyrolysis, gasification, liquefaction, hydrogenolysis and biological routes are alternatives for the depolymerization of lignin to produce benzene, toluene and xylene. However, the production of these compounds accounts for only 5% of lignin-based chemicals, with the drawback of their low price compared to other chemical compounds such as vanillin [6]. Other researchers have succeeded in obtaining compounds such as sodium levulinate, sodium 4-hydroxyvalerate, sodium acetate and sodium formate from the dearomatization of lignin in aqueous media, using as an electrolyte, by an electrocatalytic process [394].Related to phenolic compound production, the most significant cost contributions are solvent consumption and maintenance due to the costs of the dissolution and transformation of lignin to polyphenols. Challenges mostly concern their selling price, as they are heavily dependent on the quality of the lignin-derived resin, which is not currently competitive with those derived from petroleum. The profit could also be increased by reducing costs regarding dissolution, which is a process that is still in its early stage and could have a crucial impact on the product’s final quality [477].
- Cosmetics: The main applications of lignin in this field are based on its high antioxidant and ultraviolet radiation absorption capacity. There are studies that support the suitability of the use of sugarcane bagasse alkaline lignin in topical applications [373], as ingredient of sunscreens and blemish balm or antiaging cream, temporary skin sensitization, mutagenicity and cytotoxicity tests. The benefits of lignin from hazelnut and walnut or coconut husk, as an ultraviolet radiation protector, have also been corroborated [478,479].The global lignin cosmetics market is projected to grow at a compound annual growth rate (CAGR) of 10.7%, reaching approximately USD 13.5 billion by 2032 [480].
- Textile industry: Lignin is drawn as a textile fiber by the melt spinning method and electrospinning method [481]. The property of lignin to generate char when burnt has been the starting point for the use of this polymer in flame retardants and intumescent polylactic acid textile components [4]. In addition, lignin–silica-based materials recovered from rice husk have also been used as a flame retardant, overcoming one of the most important drawbacks of cotton textiles, namely its high flammability [255]. Some studies reveal that lignin-based epoxy resins can generate highly thermally and mechanically stable materials [132] for creating water, stain and abrasion resistant finishes on a wide range of fabrics. A textile application was established by Abdel-Shakur et al. [482], using corn straw lignin to make single-use medical textiles with demonstrated antimicrobial activity. Lignin can also be used as a dispersant in the textile industry and is expected to grow at an annual rate of 6% between 2020–2026 [483].
- Food industry: Lignin-derived nanogels, lignin-based films and lignin nanoparticles can also be used in the food field for the delivery of pre-encapsulated vitamins and food dyes [484]. This encapsulation technique using nanoparticles from alkali lignin has also been used to preserve essential oils, increasing their thermal stability as well as their antibacterial properties against S. aureus and E. coli [485]. Furthermore, lignin–whey protein has been used for the microencapsulation of Lactobacillus probiotic bacteria, under similar conditions to those of the digestive system [486]. According to this, alginate microcapsules are frequently used as encapsulation materials although, in order to improve their properties, either alginate microspheres cross-linked with calcium ions or a combination of alginate with other compounds (chitosan, calcium carbonate, gelatin or whey protein) are usually used for better protection of bacterial cells [486].
- Therapeutic applications: Many monomeric compounds derived from lignin have medical or therapeutic applications: ferulic acid, vanillin, eugenol, syringic acid and coumaric acid. With the exception of coumaric acid, all of them have been shown to have antimicrobial properties. In addition, with the exception of eugenol, they are diabetes-controlling and anticarcinogenic. While syringic acid has anticoagulant properties, eugenol helps control neurodegenerative diseases as do the lignophenols (which also control cardiovascular diseases and diabetes) [487].For therapeutic applications, products isolated from lignin or lignocellulosic biomass need to be well characterized to meet regulatory requirements. In this respect, further research is needed on methods for the large-scale isolation of lignin-derived therapeutic products, as well as on the characterization and quantification of products and impurity profiles as a basis for regulatory approval [488].
- Tissue engineering and smart materials: The use of lignin coatings can promote cell adhesion to certain medical implants, improving their biocompatibility. In this way, the use of chitin–lignin hybrid systems as fillers in polyurethane and foams has been studied [489], including additional properties to polyurethanes such as antimicrobial activity, light weighting and increased environmental sustainability. The field of study of lignin incorporation to promote tissue regeneration is a very novel field that requires further research for the development of lignin-derived biomaterials with suitable properties [461].On the other hand, smart materials can also be produced on the basis of lignin. Some examples can be found in the literature [490], in particular in relation to the production of biosensors and shape-programmable materials. In biomedical systems, they are explored for drug and gene delivery, tissue engineering, wound dressing and as pharmaceutical excipients. These materials exhibit properties like antimicrobial behavior, biodegradability, biocompatibility and antioxidant properties.These smart materials can act as chemical sensors for detecting metal or chromate ions, formaldehyde or biological species. There are studies with magnetic adsorbents based on functionalized lignin (with an abundance of carboxyl and hydroxyl groups) for the removal of Cr (VI), Cr (III), Cu (II), Zn (II), Ni (II) and Pb (II) [491]. In addition, chitosan smart films containing lignin have been used as biomarkers to monitor food freshness [492]. Concerning biomedical applications, bio-based lignin materials are explored for drug delivery (based on the chemical modification of lignin and polymer grafting strategy), tissue engineering, wound dressing and as pharmaceutical excipients. These materials exhibit properties like antimicrobial behavior, biodegradability, biocompatibility and antioxidant properties. Additionally, as smart coatings, lignin-based graphene avoids the disruption of electron transmission, which is beneficial for use in electromagnetic interference shielding [493]. Finally, shape-programmable materials, due to conductive properties, could detect changes in resistance induced by changes in applied voltage, gaining applicability in human motion sensing [490]. Some main challenges for these smart materials focus on improvements for drug delivery systems, synthesizing materials carrying more than one active substance, with the potential for sequential or simultaneous controlled and site-specific release as well as improvements in the thermo-mechanical properties of these new materials.
- Metal catalyst support: The wide variety of functional groups in lignin allows it to be used as a support for metal catalysts, as they can be fixed by strong coordination. The main applications of ligninic metal supports are in wastewater treatment, promoting degradation and reduction processes of toxic metals, dyes and phenolic compounds. They are also used in platform chemical transformation (for example in Fischer–Tropsch synthesis to obtain hydrocarbons from syngas, containing CO and , lignin depolymerization and energy conversion, mainly for hydrogen production. In the latter case, lignin-supported metal catalysts have proven to be effective in hydrogen production processes by water electrolysis or photocatalysis and they accelerate electrocatalysis processes in fuel cells [494].
- Abrasive: Lignin–alumina hybrid additive results in promising materials for phenolic binders, improving the mechanical properties of certain abrasive materials [495]. Furthermore, some authors [496] conclude that the conditions for obtaining abrasives with the best thermo-mechanical properties involve the incorporation of 5% magnesium lignosulfonate ( oxidized). In relation to advances in sustainable manufacturing, Bellinetto et al. [497] carry out a study expressing the correlation between structure and properties for the case of succinic-anhydride-modified lignin. Moreover, 2% kraft lignin added as an additive to tree-leaf pellets increases the abrasion resistance [498]. Thus, activated lignin can be a very promising renewable and environmentally friendly material for the sustainable development of the modern abrasives industry [496].
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Hoffmann, A.; Nong, J.P.; Porzel, A.; Bremer, M.; Fischer, S. Modification of lignoboost Kraft lignin from softwoods with dihydroxybenzenes. React. Funct. Polym. 2019, 142, 112–118. [Google Scholar] [CrossRef]
- Haq, I.; Mazumder, P.; Kalamdhad, A.S. Recent advances in removal of lignin from paper industry wastewater and its industrial applications—A review. Bioresour. Technol. 2020, 312, 123636. [Google Scholar] [CrossRef]
- Tardy, B.L.; Lizundia, E.; Guizani, C.; Hakkarainen, M.; Sipponen, M.H. Prospects for the integration of lignin materials into the circular economy. Mater. Today 2023, 65, 122–132. [Google Scholar] [CrossRef]
- Alam, M.M.; Greco, A.; Rajabimashhadi, Z.; Esposito Corcione, C. Efficient and environmentally friendly techniques for extracting lignin from lignocellulose biomass and subsequent uses: A review. Clean. Mater. 2024, 13, 100253. [Google Scholar] [CrossRef]
- Haile, A.; Gelebo, G.G.; Tesfaye, T.; Mengie, W.; Mebrate, M.A.; Abuhay, A.; Limeneh, D.Y. Pulp and paper mill wastes: Utilizations and prospects for high value-added biomaterials. Bioresour. Bioprocess. 2021, 8, 35. [Google Scholar] [CrossRef]
- Ramirez Brenes, R.G.; Alhadeff, E.M.; Bojorge, N.; Trales, L.E.M.; Pazos, G.A.D. BTX production by breaking down lignin: Current status and future prospects. Biofuels Bioprod. Biorefining 2022, 17, 664–681. [Google Scholar] [CrossRef]
- Lignin Market Size, Share & Trend Analysis Report by Product (Lignosulfonates, Kraft Lignin, Organosolv Lignin, Others), by Application, by Region, and Segment Forecasts, 2024–2030; Technical Report; Grand View Research: San Francisco, CA, USA, 2024.
- Cassoni, A.C.; Costa, P.; Vasconcelos, M.W.; Pintado, M. Systematic review on lignin valorization in the agro-food system: From sources to applications. J. Environ. Manag. 2022, 317, 115258. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, X.; Li, M.; Zheng, Y. Lignin biorefinery: Lignin source, isolation, characterization, and bioconversion. In Advances in Bioenergy; Elsevier: Amsterdam, The Netherlands, 2022; pp. 211–270. [Google Scholar] [CrossRef]
- Cazier, E.A.; Pham, T.N.; Cossus, L.; Abla, M.; Ilc, T.; Lawrence, P. Exploring industrial lignocellulosic waste: Sources, types, and potential as high-value molecules. Waste Manag. 2024, 188, 11–38. [Google Scholar] [CrossRef]
- Fontecha-Cámara, M.A.; Delgado-Blanca, I.; Mañas Villar, M.; Orriach-Fernández, F.J.; Soriano-Cuadrado, B. Extraction and depolymerization of lignin from different agricultural and forestry wastes to obtain building blocks in a circular economy framework. Polymers 2024, 16, 1981. [Google Scholar] [CrossRef]
- Tanis, M.H.; Wallberg, O.; Galbe, M.; Al-Rudainy, B. Lignin extraction by using two-step fractionation: A review. Molecules 2023, 29, 98. [Google Scholar] [CrossRef]
- Wang, T.P.; Li, H.; Yuan, J.M.; Li, W.X.; Li, K.; Huang, Y.B.; Xiao, L.P.; Lu, Q. Structures and pyrolytic characteristics of organosolv lignins from typical softwood, hardwood and herbaceous biomass. Ind. Crop. Prod. 2021, 171, 113912. [Google Scholar] [CrossRef]
- Liu, X.; Bouxin, F.P.; Fan, J.; Budarin, V.L.; Hu, C.; Clark, J.H. Recent advances in the catalytic depolymerization of lignin towards phenolic chemicals: A review. ChemSusChem 2020, 13, 4296–4317. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Runge, T.; Karlen, S.D.; Ralph, J.; Gonzales-Vigil, E.; Mansfield, S.D. Chemical pulping advantages of zip-lignin hybrid poplar. ChemSusChem 2017, 10, 3565–3573. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Pu, Y.; Ragauskas, A.J. Current understanding of the correlation of lignin structure with biomass recalcitrance. Front. Chem. 2016, 4, 45. [Google Scholar] [CrossRef] [PubMed]
- Herbaut, M.; Zoghlami, A.; Habrant, A.; Falourd, X.; Foucat, L.; Chabbert, B.; Paës, G. Multimodal analysis of pretreated biomass species highlights generic markers of lignocellulose recalcitrance. Biotechnol. Biofuels 2018, 11, 52. [Google Scholar] [CrossRef]
- Yoo, C.G.; Dumitrache, A.; Muchero, W.; Natzke, J.; Akinosho, H.; Li, M.; Sykes, R.W.; Brown, S.D.; Davison, B.; Tuskan, G.A.; et al. Significance of lignin S/G ratio in biomass recalcitrance of Popul. Trichocarpa Var. Bioethanol Prod. ACS Sustain. Chem. Eng. 2017, 6, 2162–2168. [Google Scholar] [CrossRef]
- Agarwal, U.P.; Ralph, S.A.; Padmakshan, D.; Liu, S.; Foster, C.E. Estimation of syringyl units in wood lignins by FT-Raman spectroscopy. J. Agric. Food Chem. 2019, 67, 4367–4374. [Google Scholar] [CrossRef]
- Vermaas, J.V.; Crowley, M.F.; Beckham, G.T. Molecular lignin solubility and structure in organic solvents. ACS Sustain. Chem. Eng. 2020, 8, 17839–17850. [Google Scholar] [CrossRef]
- Thoresen, M.; Malgas, S.; Gandla, M.L.; Jönsson, L.J.; Sithole, B.; Pletschke, B.I. he effects of chemical and structural factors on the enzymatic saccharification of Eucalyptus sp. samples pre-treated by various technologie. Ind. Crop. Prod. 2021, 166, 113449. [Google Scholar] [CrossRef]
- Harman-Ware, A.E.; Happs, R.M.; Davison, B.H.; Davis, M.F. The effect of coumaryl alcohol incorporation on the structure and composition of lignin dehydrogenation polymers. Biotechnol. Biofuels 2017, 10, 281. [Google Scholar] [CrossRef]
- Du, X.; Wu, S.; Li, P. Catalytic hydrogenolysis lignin to obtain phenols: A review of selective cleavage of ether bonds. BioResources 2023, 18, 4231. [Google Scholar] [CrossRef]
- Šelo, G.; Planinic, M.; Tišma, M.; Tomas, S.; Koceva Komlenic, D.; Bucic-Kojic, A. A comprehensive review on valorization of agro-food industrial residues by solid-state fermentation. Foods 2021, 10, 927. [Google Scholar] [CrossRef]
- Jardim, J.M.; Hart, P.W.; Lucia, L.A.; Jameel, H.; Chang, H.m. The effect of the Kraft pulping process, wood species, and pH on lignin recovery from black liquor. Fibers 2022, 10, 16. [Google Scholar] [CrossRef]
- Garedew, M.; Lin, F.; Song, B.; DeWinter, T.M.; Jackson, J.E.; Saffron, C.M.; Lam, C.H.; Anastas, P.T. Greener routes to biomass waste valorization: Lignin transformation through electrocatalysis for renewable chemicals and fuels production. ChemSusChem 2020, 13, 4214–4237. [Google Scholar] [CrossRef] [PubMed]
- Phiri, R.; Mavinkere Rangappa, S.; Siengchin, S. Agro-waste for renewable and sustainable green production: A review. J. Clean. Prod. 2024, 434, 139989. [Google Scholar] [CrossRef]
- Zhang, L.; Kamitakahara, H.; Murayama, H.; Ohsako, T.; Itai, A. Analysis of fruit lignin content, composition, and linkage types in pear cultivars and related species. J. Agric. Food Chem. 2020, 68, 2493–2505. [Google Scholar] [CrossRef]
- Peng, X.; Nie, S.; Li, X.; Huang, X.; Li, Q. Characteristics of the water and alkali-soluble Hemicelluloses fractionated by sequential acidification and graded-ethanol from sweet maize stems. Molecules 2019, 24, 212. [Google Scholar] [CrossRef] [PubMed]
- Kohli, K.; Katuwal, S.; Biswas, A.; Sharma, B.K. Effective delignification of lignocellulosic biomass by microwave assisted deep eutectic solvents. Bioresour. Technol. 2020, 303, 122897. [Google Scholar] [CrossRef]
- Kumar, A.; Gautam, A.; Dutt, D. Biotechnological transformation of lignocellulosic biomass into industrial products: An overview. Adv. Biosci. Biotechnol. 2016, 7, 149–168. [Google Scholar] [CrossRef]
- Mujtaba, M.; Fernandes Fraceto, L.; Fazeli, M.; Mukherjee, S.; Savassa, S.M.; Araujo de Medeiros, G.; do Espírito Santo Pereira, A.; Mancini, S.D.; Lipponen, J.; Vilaplana, F. Lignocellulosic biomass from agricultural waste to the circular economy: A review with focus on biofuels, biocomposites and bioplastics. J. Clean. Prod. 2023, 402, 136815. [Google Scholar] [CrossRef]
- Brum, F.L.; Martins, G.R.; Mohana-Borges, R.; da Silva, A.S. Acquiring thin histological sections of açaí (Euterpe oleracea Mart.) seeds for molecular mapping by mass spectrometry imaging. Mass Spectrom. 2022, 1, e9474. [Google Scholar] [CrossRef]
- Garcez, L.R.; dos Santos Lima, M.; Ribas, L.F.; Balestra, C.E.T.; Monteiro, N.B.R.; Melo Filho, J.d.A.; Gil, M.A.R. Characteristics of the açai seed (Euterpe precatoria Martius) after thermal processing and its potential in soil-cement brick. Case Stud. Constr. Mater. 2024, 20, e02816. [Google Scholar] [CrossRef]
- Leal, J.; Rambo, M.; Pedroza, M.; Cardoso, R.; Fagnani, H.; de Sousa, V.; Coêlho, A.; Rambo, M. Thinking about alternatives to açai waste in Amazonia communities. J. Braz. Chem. Soc. 2025, 36, e20240098. [Google Scholar] [CrossRef]
- An, J.; Liu, J.; Liang, Y.; Ma, Y.; Chen, C.; Cheng, Y.; Peng, P.; Zhou, N.; Zhang, R.; Addy, M.; et al. Characterization, bioavailability and protective effects of phenolic-rich extracts from almond hulls against pro-oxidant induced toxicity in Caco-2 cells. Food Chem. 2020, 322, 126742. [Google Scholar] [CrossRef]
- Salgado-Ramos, M.; Martí-Quijal, F.J.; Huertas-Alonso, A.J.; Sánchez-Verdú, M.P.; Barba, F.J.; Moreno, A. Almond hull biomass: Preliminary characterization and development of two alternative valorization routes by applying innovative and sustainable technologies. Ind. Crop. Prod. 2022, 179, 114697. [Google Scholar] [CrossRef]
- McNeill, D.C.; Pal, A.K.; Nath, D.; Rodriguez-Uribe, A.; Mohanty, A.K.; Pilla, S.; Gregori, S.; Dick, P.; Misra, M. Upcycling of ligno-cellulosic nutshells waste biomass in biodegradable plastic-based biocomposites uses—A comprehensive review. Compos. Part C Open Access 2024, 14, 100478. [Google Scholar] [CrossRef]
- Zhang, F.; Wang, T.; Wang, X.; Lü, X. Apple pomace as a potential valuable resource for full-components utilization: A review. J. Clean. Prod. 2021, 329, 129676. [Google Scholar] [CrossRef]
- Ghai, H.; Sakhuja, D.; Yadav, S.; Solanki, P.; Putatunda, C.; Bhatia, R.K.; Bhatt, A.K.; Varjani, S.; Yang, Y.H.; Bhatia, S.K.; et al. An overview on co-pyrolysis of biodegradable and non-biodegradable wastes. Energies 2022, 15, 4168. [Google Scholar] [CrossRef]
- Patelski, A.M.; Ciach, M.; Dziekonska-Kubczak, U.; Nowak, A.; Balcerek, M.; Pielech-Przybylska, K. Bioconversion of apple pomace to Meyerozyma Guilliermondii Scheffersomyces Stipitis Biomass. Appl. Sci. 2024, 14, 6108. [Google Scholar] [CrossRef]
- Hoque, M.; Janaswamy, S. Biodegradable packaging films from banana peel fiber. Sustain. Chem. Pharm. 2024, 37, 101400. [Google Scholar] [CrossRef]
- Klein, G.H.; Longo, V.D.; Romani, L.C.; Saldanha, L.F.; Fornari, A.C.; Bazoti, S.F.; Camargo, A.F.; Alves, S.L.; Treichel, H. Utilization of banana peel waste for the production of bioethanol and other high-value-added compounds. Food Humanit. 2024, 3, 100376. [Google Scholar] [CrossRef]
- Bhutto, S.; Abro, F.u.R.; Ali, M.; Buller, A.S.; Bheel, N.; Gamil, Y.; Najeh, T.; Deifalla, A.F.; Ragab, A.E.; Almujibah, H.R. Effect of banana tree leaves ash as cementitious material on the durability of concrete against sulphate and acid attacks. Heliyon 2024, 10, e29236. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Pandey, D.; Patil, T.; Sawarkar, A.N. Pyrolysis of banana leaves biomass: Physico-chemical characterization, thermal decomposition behavior, kinetic and thermodynamic analyses. Bioresour. Technol. 2020, 310, 123464. [Google Scholar] [CrossRef]
- Demirel, F.; Germec, M.; Coban, H.B.; Turhan, I. Optimization of dilute acid pretreatment of barley husk and oat husk and determination of their chemical composition. Cellulose 2018, 25, 6377–6393. [Google Scholar] [CrossRef]
- Sarkar, S.; Álvarez, V.H.; Saldaña, M.D. Relevance of ions in pressurized fluid extraction of carbohydrates and phenolics from barley hull. J. Supercrit. Fluids 2014, 93, 27–37. [Google Scholar] [CrossRef]
- Bledzki, A.K.; Mamun, A.A.; Volk, J. Barley husk and coconut shell reinforced polypropylene composites: The effect of fibre physical, chemical and surface properties. Compos. Sci. Technol. 2010, 70, 840–846. [Google Scholar] [CrossRef]
- Kim, T.H.; Taylor, F.; Hicks, K.B. Bioethanol production from barley hull using SAA (soaking in aqueous ammonia) pretreatment. Bioresour. Technol. 2008, 99, 5694–5702. [Google Scholar] [CrossRef]
- Serna-Díaz, M.; Mercado-Flores, Y.; Jiménez-González, A.; Anducho-Reyes, M.; Medina-Marín, J.; Seck Tuoh-Mora, J.; Téllez-Jurado, A. Use of barley straw as a support for the production of conidiospores of Trichoderma harzianum. Biotechnol. Rep. 2020, 26, e00445. [Google Scholar] [CrossRef]
- Pradhan, D.; Jaiswal, S.; Tiwari, B.K.; Jaiswal, A.K. Choline chloride oxalic acid dihydrate deep eutectic solvent pretreatment of Barley straw for production of cellulose nanofibers. Int. J. Biol. Macromol. 2024, 281, 136213. [Google Scholar] [CrossRef]
- Liu, Z.; Khurshid, K.; Saldaña, M.D. Biorefinery of barley straw using pressurized fluids: Biocompounds and biopolymers production. J. Supercrit. Fluids 2023, 203, 106078. [Google Scholar] [CrossRef]
- Kim, T.H.; Oh, K.K.; Ryu, H.J.; Lee, K.H.; Kim, T.H. Hydrolysis of hemicellulose from barley straw and enhanced enzymatic saccharification of cellulose using acidified zinc chloride. Renew. Energy 2014, 65, 56–63. [Google Scholar] [CrossRef]
- Raza, M.; Abu-Jdayil, B. Synergic interactions, kinetic and thermodynamic analyses of date palm seeds and cashew shell waste co-pyrolysis using Coats-Redfern method. Case Stud. Therm. Eng. 2023, 47, 103118. [Google Scholar] [CrossRef]
- Wei, M.; Zhu, W.; Xie, G.; Lestander, T.A.; Xiong, S. Cassava stem wastes as potential feedstock for fuel ethanol production: A basic parameter study. Renew. Energy 2015, 83, 970–978. [Google Scholar] [CrossRef]
- Bolonhesi, I.B.d.T.M.; Andreani, C.L.; de Melo, M.R.; Gomes, S.D.; Lopes, D.D. Biomass immobilization in hydrolyzed lignocellulosic material can enhance biohydrogen production from cassava residues? Biochem. Eng. J. 2023, 190, 108725. [Google Scholar] [CrossRef]
- Kamalini, A.; Muthusamy, S.; Ramapriya, R.; Muthusamy, B.; Pugazhendhi, A. Optimization of sugar recovery efficiency using microwave assisted alkaline pretreatment of cassava stem using response surface methodology and its structural characterization. J. Mol. Liq. 2018, 254, 55–63. [Google Scholar] [CrossRef]
- Kayiwa, R.; Kasedde, H.; Lubwama, M.; Kirabira, J. Characterization and pre-leaching effect on the peels of predominant cassava varieties in Uganda for production of activated carbon. Curr. Res. Green Sustain. Chem. 2021, 4, 100083. [Google Scholar] [CrossRef]
- Hartulistiyoso, E.; Farobie, O.; Zaky, M. Delignification of cassava peel as bioethanol raw material using combined alkali and microwave heating methods. IOP Conf. Ser. Earth Environ. Sci. 2022, 1038, 012021. [Google Scholar] [CrossRef]
- Widiarto, S.; Pramono, E.; Suharso; Rochliadi, A.; Arcana, I.M. Cellulose nanofibers preparation from cassava peels via mechanical disruption. Fibers 2019, 7, 44. [Google Scholar] [CrossRef]
- Quiceno Suarez, A.; Cadena-Chamorro, E.M.; Ciro-Velásquez, H.J.; Arango-Tobón, J.C. By-products of the cocoa agribusiness: High value added materials based on their bromatological and chemical characterization. Rev. Fac. Nac. Agron. Medellín 2024, 77, 10585–10599. [Google Scholar] [CrossRef]
- Afedzi, A.E.K.; Obeng-Boateng, F.; Aduama-Larbi, M.S.; Zhou, X.; Xu, Y. Valorization of Ghanaian cocoa processing residues as extractives for value-added functional food and animal feed additives—A review. Biocatal. Agric. Biotechnol. 2023, 52, 102835. [Google Scholar] [CrossRef]
- Mendoza-Meneses, C.J.; Feregrino-Pérez, A.A.; Gutiérrez-Antonio, C. Potential use of industrial cocoa waste in biofuel production. J. Chem. 2021, 2021, 3388067. [Google Scholar] [CrossRef]
- Dahunsi, S.; Osueke, C.; Olayanju, T.; Lawal, A. Co-digestion of Theobroma cacao (Cocoa) pod husk and poultry manure for energy generation: Effects of pretreatment methods. Bioresour. Technol. 2019, 283, 229–241. [Google Scholar] [CrossRef] [PubMed]
- Sánchez, M.; Laca, A.; Laca, A.; Díaz, M. Cocoa bean shell: A by-product with high potential for nutritional and biotechnological applications. Antioxidants 2023, 12, 1028. [Google Scholar] [CrossRef]
- Salaenoi, J.; Jurejan, N.; Yokthongwattana, C.; Pluempanupat, W.; Boonprab, K. Characteristics of coconut husk cellulose and its effectiveness as a potassium permanganate absorbent for fishery applications. Case Stud. Chem. Environ. Eng. 2024, 10, 100975. [Google Scholar] [CrossRef]
- Fatmawati, A.; Nurtono, T.; Widjaja, A. Thermogravimetric kinetic-based computation of raw and pretreated coconut husk powder lignocellulosic composition. Bioresour. Technol. Rep. 2023, 22, 101500. [Google Scholar] [CrossRef]
- Costa, J.E.; Barbosa, A.S.; Melo, M.A.; Melo, D.M.; Medeiros, R.L.; Braga, R.M. Renewable aromatics through catalytic pyrolysis of coconut fiber (Cocos Nucifera Linn.) Using Low Cost HZSM-5. Renew. Energy 2022, 191, 439–446. [Google Scholar] [CrossRef]
- Gunawan, T.D.; Mariana.; Munawar, E.; Muchtar, S. Preparation and characterization of chemically activated adsorbent from the combination of coconut shell and fly ash. Mater. Today Proc. 2022, 63, S40–S45. [Google Scholar] [CrossRef]
- Anuchi, S.O.; Campbell, K.L.S.; Hallett, J.P. Effective pretreatment of lignin-rich coconut wastes using a low-cost ionic liquid. Sci. Rep. 2022, 12, 6108. [Google Scholar] [CrossRef]
- Tamilselvan, K.; Sundarajan, S.; Ramakrishna, S.; Amirul, A.A.A.; Vigneswari, S. Sustainable valorisation of coffee husk into value added product in the context of circular bioeconomy: Exploring potential biomass-based value webs. Food Bioprod. Process. 2024, 145, 187–202. [Google Scholar] [CrossRef]
- Amensisa, Y.E.; Demsash, H.D.; Tefera, M.E. Extraction and characterization of cellulose from coffee husk and breweryś spent grain fibers using alkali-hydrogen peroxide treatment method. Adv. Mater. Sci. Eng. 2024, 2024, 5101871. [Google Scholar] [CrossRef]
- Nguyen, D.V.; Duong, C.T.T.; Vu, C.N.M.; Nguyen, H.M.; Pham, T.T.; Tran-Thuy, T.M.; Nguyen, L.Q. Data on chemical composition of coffee husks and lignin microparticles as their extracted product. Data Brief 2023, 51, 109781. [Google Scholar] [CrossRef]
- Elegbede, J.; Ajayi, V.; Lateef, A. Microbial valorization of corncob: Novel route for biotechnological products for sustainable bioeconomy. Environ. Technol. Innov. 2021, 24, 102073. [Google Scholar] [CrossRef]
- Selvakumar, P.; Adane, A.; Zelalem, T.; Hunegnaw, B.; Karthik, V.; Kavitha, S.; Jayakumar, M.; Karmegam, N.; Govarthanan, M.; Kim, W. Optimization of binary acids pretreatment of corncob biomass for enhanced recovery of cellulose to produce bioethanol. Fuel 2022, 321, 124060. [Google Scholar] [CrossRef]
- Zou, H.; Jiang, Q.; Zhu, R.; Chen, Y.; Sun, T.; Li, M.; Zhai, J.; Shi, D.; Ai, H.; Gu, L.; et al. Enhanced hydrolysis of lignocellulose in corn cob by using food waste pretreatment to improve anaerobic digestion performance. J. Environ. Manag. 2020, 254, 109830. [Google Scholar] [CrossRef]
- Mishra, O.; Tripathi, S.; Bhardwaj, N.K. Suitability of corn stalk pulp for improving physical strength properties of agro-residue pulp. Cellul. Chem. Technol. 2020, 54, 65–71. [Google Scholar]
- Zhu, Q.; Dong, H.; Yan, D.; Gao, D.; Xu, K.; Cheng, X.; Xin, J.; Lu, X. High concentration bioethanol production from corn stalk via enhanced pretreatment with ionic liquids. Chem. Eng. Sci. 2024, 283, 119375. [Google Scholar] [CrossRef]
- Adekunle, A.E.; Rabeya, T.; Jehadin, F.; Asad, M.A.; Ayodele, O.O.; Islam, M.S. Compressed hot water pretreatment enhanced bioethanol production from corn stalk. Bioresour. Technol. Rep. 2020, 12, 100595. [Google Scholar] [CrossRef]
- Zabed, H.M.; Akter, S.; Yun, J.; Zhang, G.; Zhao, M.; Mofijur, M.; Awasthi, M.K.; Kalam, M.; Ragauskas, A.; Qi, X. Towards the sustainable conversion of corn stover into bioenergy and bioproducts through biochemical route: Technical, economic and strategic perspectives. J. Clean. Prod. 2023, 400, 136699. [Google Scholar] [CrossRef]
- Abolore, R.S.; Jaiswal, S.; Jaiswal, A.K. Green and sustainable pretreatment methods for cellulose extraction from lignocellulosic biomass and its applications: A review. Carbohydr. Polym. Technol. Appl. 2024, 7, 100396. [Google Scholar] [CrossRef]
- Zhong, X.; Yuan, R.; Zhang, B.; Wang, B.; Chu, Y.; Wang, Z. Full fractionation of cellulose, hemicellulose, and lignin in pith-leaf containing corn stover by one-step treatment using aqueous formic acid. Ind. Crop. Prod. 2021, 172, 113962. [Google Scholar] [CrossRef]
- Carmona-Cabello, M.; Herreros, J.M.; Molero, E.; Lopez-Uceda, A.; Pinzi, S.; Dorado, M.P.; Romero, P.E. Upcycling of agricultural residues for additive manufacturing: Corn straw waste as reinforcing agent in acrylonitrile-butadiene-styrene composite matrix. Biomass Conver. Biorefinery 2024, 15, 7869–7880. [Google Scholar] [CrossRef]
- Du, Y.P.; Tian, X.Y.; Zheng, X.P.; Chai, Y.; Zhang, Y.C.; Zheng, Y.Z. One-pot direct conversion of passion fruit hull into 5-hydroxymethylfurfural catalysed by halogenated metal ionic liquids and organic acids. J. Mol. Liq. 2024, 398, 124273. [Google Scholar] [CrossRef]
- Glinska, K.; Gitalt, J.; Torrens, E.; Plechkova, N.; Bengoa, C. Extraction of cellulose from corn stover using designed ionic liquids with improved reusing capabilities. Process Saf. Environ. Prot. 2021, 147, 181–191. [Google Scholar] [CrossRef]
- Prakash, S.; Radha.; Sharma, K.; Dhumal, S.; Senapathy, M.; Deshmukh, V.P.; Kumar, S.; Madhu.; Anitha, T.; Balamurugan, V.; et al. Unlocking the potential of cotton stalk as a renewable source of cellulose: A review on advancements and emerging applications. Int. J. Biol. Macromol. 2024, 261, 129456. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Wang, H.; Tu, Z.; Hu, J.; Wu, F. Renewable fuel and value-added chemicals potential of reed straw waste (RSW) by pyrolysis: Kinetics, thermodynamics, products characterization, and biochar application for malachite green removal. Renew. Energy 2024, 229, 120724. [Google Scholar] [CrossRef]
- Liu, J.; Wang, C.; Zhao, X.; Yin, F.; Yang, H.; Wu, K.; Liang, C.; Yang, B.; Zhang, W. Bioethanol production from corn straw pretreated with deep eutectic solvents. Electron. J. Biotechnol. 2023, 62, 27–35. [Google Scholar] [CrossRef]
- Chowdhary, P.; Gupta, A.; Gnansounou, E.; Pandey, A.; Chaturvedi, P. Current trends and possibilities for exploitation of Grape pomace as a potential source for value addition. Environ. Pollut. 2021, 278, 116796. [Google Scholar] [CrossRef] [PubMed]
- Filippi, K.; Georgaka, N.; Alexandri, M.; Papapostolou, H.; Koutinas, A. Valorisation of grape stalks and pomace for the production of bio-based succinic acid by Actinobacillus succinogenes. Ind. Crop. Prod. 2021, 168, 113578. [Google Scholar] [CrossRef]
- Coelho, C.C.; Michelin, M.; Cerqueira, M.A.; Gonçalves, C.; Tonon, R.V.; Pastrana, L.M.; Freitas-Silva, O.; Vicente, A.A.; Cabral, L.M.; Teixeira, J.A. Cellulose nanocrystals from grape pomace: Production, properties and cytotoxicity assessment. Carbohydr. Polym. 2018, 192, 327–336. [Google Scholar] [CrossRef]
- Salgado-Ramos, M.; Tabasso, S.; Calcio Gaudino, E.; Moreno, A.; Mariatti, F.; Cravotto, G. An innovative, green cascade protocol for grape stalk valorization with process intensification technologies. Appl. Sci. 2022, 12, 7417. [Google Scholar] [CrossRef]
- Ollani, S.; Peano, C.; Sottile, F. Recent innovations on the reuse of almond and hazelnut by-products: A review. Sustainability 2024, 16, 2577. [Google Scholar] [CrossRef]
- Chaowana, P.; Hnoocham, W.; Chaiprapat, S.; Yimlamai, P.; Chitbanyong, K.; Wanitpinyo, K.; Chaisan, T.; Paopun, Y.; Pisutpiched, S.; Khantayanuwong, S.; et al. Utilization of hemp stalk as a potential resource for bioenergy. Mater. Sci. Energy Technol. 2024, 7, 19–28. [Google Scholar] [CrossRef]
- Martínez, B.; Bernat-Maso, E.; Gil, L. Applications and properties of hemp stalk-based insulating biomaterials for buildings: Review. Materials 2023, 16, 3245. [Google Scholar] [CrossRef]
- Ban, Q.; Wang, J.; Guo, P.; Yue, J.; Zhang, L.; Li, J. Improved biohydrogen production by co-fermentation of corn straw and excess sludge: Insights into biochemical process, microbial community and metabolic genes. Environ. Res. 2024, 256, 119171. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yu, Y.; Yi, F.; Qiang, J.; Li, C.; Zhao, N.; Lu, J.; Jia, Z.; Zhou, L.; Mperejekumana, P.; et al. Characteristics and formation of nitrogen-containing products from the pyrolysis of maple wood and maize straw. J. Anal. Appl. Pyrolysis 2022, 163, 105462. [Google Scholar] [CrossRef]
- Liao, H.; Feng, B.; Ying, W.; Lian, Z.; Zhang, J. Novel approach for corn straw biorefineries: Production of xylooligosaccharides, lignin and ethanol by nicotinic acid hydrolysis and pentanol pretreatment. Bioresour. Technol. 2024, 395, 130352. [Google Scholar] [CrossRef]
- Zhang, G.; Li, Z.; Liu, L.; Xiang, Q. Road to valorisation of melon seeds (Cucumis Melo L.): A Compr. Rev. Nutr. Profiles, Biol. Act. Food Appl. Sustain. Food Technol. 2024, 2, 1166–1182. [Google Scholar] [CrossRef]
- Fadeyi, A.E.; Akiode, S.O.; Emmanuel, S.A.; Falayi, O.E. Compositional analysis and characterization of lignocellulosic biomass from selected agricultural wastes. J. Sci. Math. Lett. 2020, 8, 48–56. [Google Scholar] [CrossRef]
- Zhou, L.; Sun, J.; Xu, X.; Ma, M.; Li, Y.; Chen, Q.; Su, H. Full quantitative resource utilization of raw mustard waste through integrating a comprehensive approach for producing hydrogen and soil amendments. Microb. Cell Factories 2024, 23, 27. [Google Scholar] [CrossRef]
- Ruviaro, A.S.; dos Santos Lima, G.T.; Silvestro, L.; Barraza, M.T.; Rocha, J.C.; de Brito, J.; Gleize, P.J.P.; Pelisser, F. Characterization and investigation of the use of oat husk ash as supplementary cementitious material as partial replacement of Portland cement: Analysis of fresh and hardened properties and environmental assessment. Constr. Build. Mater. 2023, 363, 129762. [Google Scholar] [CrossRef]
- Arzami, A.N.; Ho, T.M.; Mikkonen, K.S. Valorization of cereal by-product hemicelluloses: Fractionation and purity considerations. Food Res. Int. 2022, 151, 110818. [Google Scholar] [CrossRef] [PubMed]
- Ábrego Gacía, A.; Poggi-Varaldo, H.M.; Mendoza-Vargas, A.; Mercado-Valle, F.G.; Ríos-Leal, E.; Ponce-Noyola, T.; Calva-Calva, G. Effects of fermented oat straw as a lovastatin carrier on in vitro methane production and rumen microbiota. Front. Energy Res. 2021, 9, 630701. [Google Scholar] [CrossRef]
- Rencoret, J.; Marques, G.; Rosado, M.J.; Benito, J.; Barro, F.; Gutiérrez, A.; del Río, J.C. Variations in the composition and structure of the lignins of oat (Avena sativa L.) straws according to variety and planting season. Int. J. Biol. Macromol. 2023, 242, 124811. [Google Scholar] [CrossRef] [PubMed]
- Raksodewanto, A.A.; Sudarmanta, B.; Setiyawan, A.; Priyanto, U.; Fariza, O.; Yarsono, S.; Zufri, M.F.; Puspitasari, S. Pyrolysis of oil palm trunk biomass using a fixed bed reactor to produce raw material for bio-carbon black. IOP Conf. Ser. Earth Environ. Sci. 2024, 1344, 012006. [Google Scholar] [CrossRef]
- Eom, I.Y.; Yu, J.H.; Jung, C.D.; Hong, K.S. Efficient ethanol production from dried oil palm trunk treated by hydrothermolysis and subsequent enzymatic hydrolysis. Biotechnol. Biofuels 2015, 8, 83. [Google Scholar] [CrossRef]
- Zaini, L.H.; Gindl-Altmutter, W.; Gusenbauer, C.; Rahayu, I.S.; Lubis, M.A.R.; Mautner, A.; Veigel, S. Nanofibrils from oil palm trunk: Effect of delignification and fibrillation technique. J. Wood Sci. 2024, 70, 19. [Google Scholar] [CrossRef]
- Miranda, I.; Simões, R.; Medeiros, B.; Nampoothiri, K.M.; Sukumaran, R.K.; Rajan, D.; Pereira, H.; Ferreira-Dias, S. Valorization of lignocellulosic residues from the olive oil industry by production of lignin, glucose and functional sugars. Bioresour. Technol. 2019, 292, 121936. [Google Scholar] [CrossRef]
- Gómez-Cruz, I.; Cara, C.; Romero, I.; Castro, E.; Gullón, B. Valorisation of exhausted olive pomace by an eco-friendly solvent extraction process of natural antioxidants. Antioxidants 2020, 9, 1010. [Google Scholar] [CrossRef]
- Manzanares, P.; Ruiz, E.; Ballesteros, M.; Negro, M.J.; Gallego, F.J.; López-Linares, J.C.; Castro, E. Residual biomass potential in olive tree cultivation and olive oil industry in Spain: Valorization proposal in a biorefinery context. Span. J. Agric. Res. 2017, 15, e0206. [Google Scholar] [CrossRef]
- Manzanares, P.; Ballesteros, I.; Negro, M.; González, A.; Oliva, J.; Ballesteros, M. Processing of extracted olive oil pomace residue by hydrothermal or dilute acid pretreatment and enzymatic hydrolysis in a biorefinery context. Renew. Energy 2020, 145, 1235–1245. [Google Scholar] [CrossRef]
- Bai, Y.; Arulrajah, A.; Horpibulsuk, S.; Chu, J. Gasified olive stone biochar as a green construction fill material. Constr. Build. Mater. 2023, 403, 133003. [Google Scholar] [CrossRef]
- Mateo, S.; Moya, A.J.; Hodaifa, G.; Sánchez, S.; Cuevas, M. Valorization of olive endocarp from olive oil and table olive processing as a low-cost bioadsorbent for the removal of furfural from aqueous solutions. J. Water Process Eng. 2021, 44, 102442. [Google Scholar] [CrossRef]
- Ismail, H.Y.; Fayyad, S.; Ahmad, M.N.; Leahy, J.J.; Naushad, M.; Walker, G.M.; Albadarin, A.B.; Kwapinski, W. Modelling of yields in torrefaction of olive stones using artificial intelligence coupled with kriging interpolation. J. Clean. Prod. 2021, 326, 129020. [Google Scholar] [CrossRef]
- Kartal, F.; Özveren, U. An improved machine learning approach to estimate hemicellulose, cellulose, and lignin in biomass. Carbohydr. Polym. Technol. Appl. 2021, 2, 100148. [Google Scholar] [CrossRef]
- Cláudio, A.F.M.; Cognigni, A.; de Faria, E.L.; Silvestre, A.J.; Zirbs, R.; Freire, M.G.; Bica, K. Valorization of olive tree leaves: Extraction of oleanolic acid using aqueous solutions of surface-active ionic liquids. Sep. Purif. Technol. 2018, 204, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Ben Mabrouk, A.; Putaux, J.L.; Boufi, S. Valorization of olive leaf waste as a new source of fractions containing cellulose nanomaterials. Ind. Crop. Prod. 2023, 202, 116996. [Google Scholar] [CrossRef]
- Mateo, S.; Mateo, P.; Barbanera, M.; Buratti, C.; Moya, A.J. Acid hydrolysis of olive tree leaves: Preliminary study towards biochemical conversion. Processes 2020, 8, 886. [Google Scholar] [CrossRef]
- Fonseca, B.G.; Mateo, S.; Roberto, I.C.a.; Sánchez, S.; Moya, A.J. Bioconversion in batch bioreactor of olive-tree pruning biomass optimizing treatments for ethanol production. Biochem. Eng. J. 2020, 164, 107793. [Google Scholar] [CrossRef]
- Puentes, J.G.; Mateo, S.; Fonseca, B.G.; Roberto, I.C.; Sánchez, S.; Moya, A.J. Monomeric carbohydrates production from olive tree pruning biomass: Modeling of dilute acid hydrolysis. Bioresour. Technol. 2013, 149, 149–154. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, B.; Romaní, A.; Gullón, B.; Yáñez, R. Introduction: State of the art of fruit and vegetable waste management. In Fruit and Vegetable Waste Utilization and Sustainability; Elsevier: Amsterdam, The Netherlands, 2023; pp. 1–18. [Google Scholar] [CrossRef]
- Sohni, S.; Begum, S.; Hashim, R.; Khan, S.B.; Mazhar, F.; Syed, F.; Khan, S.A. Physicochemical characterization of microcrystalline cellulose derived from underutilized orange peel waste as a sustainable resource under biorefinery concept. Bioresour. Technol. Rep. 2024, 25, 101731. [Google Scholar] [CrossRef]
- Rosado, M.J.; Rencoret, J.; Gutiérrez, A.; del Río, J.C. Structural Characterization of the Milled-Wood Lignin Isolated from Sweet Orange Tree (Citrus sinensis) Pruning Residue. Polymers 2023, 15, 1840. [Google Scholar] [CrossRef] [PubMed]
- González, Z.; Vargas, F.; Jiménez, L.; Rodríguez Pascual, A. Orange tree prunings as raw material for cellulose production by the kraft process. Cellul. Chem. Technol. 2013, 47, 603–6116. [Google Scholar]
- Espinach, F.X.; Espinosa, E.; Reixach, R.; Rodríguez, A.; Mutjé, P.; Tarrés, Q. Study on the macro and micromechanics tensile strength properties of orange tree pruning fiber as sustainable reinforcement on bio-polyethylene compared to oil-derived polymers and its composites. Polymers 2020, 12, 2206. [Google Scholar] [CrossRef]
- Pawlak-Kruczek, H.; Arora, A.; Moscicki, K.; Krochmalny, K.; Sharma, S.; Niedzwiecki, L. A transition of a domestic boiler from coal to biomass. Emissions from combustion of raw and torrefied Palm Kernel shells (PKS). Fuel 2020, 263, 116718. [Google Scholar] [CrossRef]
- Sangthong, S.; Phetwarotai, W.; Bakar, M.S.A.; Cheirsilp, B.; Phusunti, N. Phenol-rich bio-oil from pyrolysis of palm kernel shell and its isolated lignin. Ind. Crop. Prod. 2022, 188, 115648. [Google Scholar] [CrossRef]
- Muñoz, P.; Letelier, V.; Zamora, D.; Morales, M. Feasibility of using paper pulp residues into fired clay bricks. J. Clean. Prod. 2020, 262, 121464. [Google Scholar] [CrossRef]
- Souza, A.G.d.; Rocha, D.B.; Kano, F.S.; Rosa, D.d.S. Valorization of industrial paper waste by isolating cellulose nanostructures with different pretreatment methods. Resour. Conserv. Recycl. 2019, 143, 133–142. [Google Scholar] [CrossRef]
- Infante-Neta, A.A.; de Carvalho, A.A.O.; D’Almeida, A.P.; Gonçalves, L.R.B.; de Albuquerque, T.L. Xylitol production from passion fruit peel hydrolysate: Optimization of hydrolysis and fermentation processes. Bioresour. Technol. 2024, 414, 131628. [Google Scholar] [CrossRef]
- Lu, A.; Yu, X.; Ji, Q.; Chen, L.; Yagoub, A.E.G.; Olugbenga, F.; Zhou, C. Preparation and characterization of lignin-containing cellulose nanocrystals from peanut shells using a deep eutectic solvent containing lignin-derived phenol. Ind. Crop. Prod. 2023, 195, 116415. [Google Scholar] [CrossRef]
- Kumar, N.V.; Sawargaonkar, G.L.; Rani, C.S.; Singh, A.; Prakash, T.R.; Triveni, S.; Kamdi, P.J.; Pasumarthi, R.; Karthik, R.; Venkatesh, B. Comparative analysis of pigeonpea stalk biochar characteristics and energy use under different biochar production methods. Sustainability 2023, 15, 14394. [Google Scholar] [CrossRef]
- Sahoo, S.S.; Vijay, V.K.; Chandra, R.; Kumar, H. Production and characterization of biochar produced from slow pyrolysis of pigeon pea stalk and bamboo. Clean. Eng. Technol. 2021, 3, 100101. [Google Scholar] [CrossRef]
- Sahu, P.; Gangil, S.; Bhargav, V.K. Biopolymeric transitions under pyrolytic thermal degradation of Pigeon pea stalk. Renew. Energy 2023, 206, 157–167. [Google Scholar] [CrossRef]
- Mala, T.; Piayura, S.; Itthivadhanapong, P. Characterization of dried pineapple (Ananas Comosus L.) Peel Powder Its Appl. A Nov. Funct. Food Ingred. Cracker Prod. Future Foods 2024, 9, 100322. [Google Scholar] [CrossRef]
- Chen, W.H.; Liu, L.X.; Sheen, H.K.; Culaba, A.B.; Shiong Khoo, K.; Lim, S. Binary energy production from pineapple peel waste and optimized by statistical and machine learning approaches. Fuel 2024, 372, 132275. [Google Scholar] [CrossRef]
- Pereira, P.H.F.; Arantes, V.; Pereira, B.; Ornaghi, H.L.; de Oliveira, D.M.; Santagneli, S.H.; Cioffi, M.O.H. Effect of the chemical treatment sequence on pineapple peel fiber: Chemical composition and thermal degradation behavior. Cellulose 2022, 29, 8587–8598. [Google Scholar] [CrossRef]
- Makepa, D.C.; Chihobo, C.H.; Ruziwa, W.R.; Musademba, D. Microwave-assisted pyrolysis of pine sawdust: Process modelling, performance optimization and economic evaluation for bioenergy recovery. Heliyon 2023, 9, e14688. [Google Scholar] [CrossRef]
- Stoffel, R.B.; Neves, P.V.; Felissia, F.E.; Ramos, L.P.; Gassa, L.M.; Area, M.C. Hemicellulose extraction from slash pine sawdust by steam explosion with sulfuric acid. Biomass Bioenergy 2017, 107, 93–101. [Google Scholar] [CrossRef]
- Corsi, L.; Mateo, S.; Spaccini, F.; Buratti, C.; Moya, A.J. Optimization of microwave-assisted organosolv pretreatment for enzymatic hydrolysis of cherry tree pruning and pistachio shells: A step to bioethanol production. BioEnergy Res. 2023, 17, 294–308. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, H.L.; Her, J.Y. Isolation of microcrystalline cellulose (MCC) from pistachio shells and preparation of carrageenan-based composite films. Carbohydr. Polym. Technol. Appl. 2024, 7, 100423. [Google Scholar] [CrossRef]
- Sathish, T.; Muthukumar, K.; Saravanan, R.; Giri, J. Optimized thermal pretreatment for lignocellulosic biomass of pigeon pea stalks to augment quality and quantity of biogas production. Int. J. Thermofluids 2024, 24, 100911. [Google Scholar] [CrossRef]
- Jasmine, A.; Rajendran, M.; Thirunavukkarasu, K.; Abinandan, S.; Vaidyanathan, V.K.; Krishnamurthi, T. Microwave-assisted alkali pre-treatment medium for fractionation of rice straw and catalytic conversion to value-added 5-hydroxymethyl furfural and lignin production. Int. J. Biol. Macromol. 2023, 236, 123999. [Google Scholar] [CrossRef] [PubMed]
- Otero-Jiménez, V.; Carreño Carreño, J.d.P.; Barreto-Hernandez, E.; van Elsas, J.D.; Uribe-Vélez, D. Impact of rice straw management strategies on rice rhizosphere microbiomes. Appl. Soil Ecol. 2021, 167, 104036. [Google Scholar] [CrossRef]
- Domanski, J.; Borowski, S.; Marchut-Mikolajczyk, O.; Kubacki, P. Pretreatment of rye straw with aqueous ammonia for conversion to fermentable sugars as a potential substrates in biotechnological processes. Biomass Bioenergy 2016, 91, 91–97. [Google Scholar] [CrossRef]
- Tinôco, D.; Genier, H.L.A.; da Silveira, W.B. Technology valuation of cellulosic ethanol production by Kluyveromyces marxianus CCT 7735 from sweet sorghum bagasse at elevated temperatures. Renew. Energy 2021, 173, 188–196. [Google Scholar] [CrossRef]
- Martins, R.P.; Schmatz, A.A.; de Freita, L.A.; Mutton, M.J.R.; Brienzo, M. Solubilization of hemicellulose and fermentable sugars from bagasse, stalks, and leaves of sweet sorghum. Ind. Crop. Prod. 2021, 170, 113813. [Google Scholar] [CrossRef]
- Bittencourt, G.A.; Vandenberghe, L.P.d.S.; Valladares-Diestra, K.K.; Soccol, C.R. Soybean hull valorization for sugar production through the optimization of citric acid pretreatment and enzymatic hydrolysis. Ind. Crop. Prod. 2022, 186, 115178. [Google Scholar] [CrossRef]
- Tummino, M.L.; Laurenti, E.; Bracco, P.; Cecone, C.; Parola, V.L.; Vineis, C.; Testa, M.L. Antibacterial properties of functionalized cellulose extracted from deproteinized soybean hulls. Cellulose 2023, 30, 7805–7824. [Google Scholar] [CrossRef]
- Martelli-Tosi, M.; Assis, O.B.; Silva, N.C.; Esposto, B.S.; Martins, M.A.; Tapia-Blácido, D.R. Chemical treatment and characterization of soybean straw and soybean protein isolate/straw composite films. Carbohydr. Polym. 2017, 157, 512–520. [Google Scholar] [CrossRef]
- Wu, W.; Zhu, P.; Luo, L.; Lin, H.; Tao, Y.; Ruan, L.; Wang, L.; Qing, Q. p-Toluenesulfonic acid enhanced neutral deep eutectic solvent pretreatment of soybean straw for efficient lignin removal and enzymatic hydrolysis. Bioresour. Technol. 2024, 395, 130338. [Google Scholar] [CrossRef]
- Franca, A.S.; Oliveira, L.S. Potential Uses of Spent Coffee Grounds in the Food Industry. Foods 2022, 11, 2064. [Google Scholar] [CrossRef]
- Ricciulli, M.O.; Arce, G.L.; Vieira, E.C.; Ávila, I. Interaction among lignocellulosic biomass components in thermochemical processes. Biomass Bioenergy 2024, 182, 107073. [Google Scholar] [CrossRef]
- Wang, J.; Li, X.; Huang, W.; Wu, L.; Cui, J.; Bai, H.; Zeng, F. Mathematical model for bale-density prediction in large steel roller-type round balers during bale rolling. Bioresour. Technol. 2024, 396, 130445. [Google Scholar] [CrossRef]
- Tang, Z.; Wu, C.; Tang, W.; Huang, M.; Ma, C.; He, Y.C. Enhancing enzymatic saccharification of sunflower straw through optimal tartaric acid hydrothermal pretreatment. Bioresour. Technol. 2023, 385, 129279. [Google Scholar] [CrossRef]
- Santana, M.B.; Gama, F.A.; Pereira, I.O.; Tramontina, R.; Squina, F.M.; Ambrosi, A.; Zielinski, A.; Poletto, P.; Ienczak, J.L. Harnessing tobacco stem biomass for eco-friendly xylo-oligomers production via hydrothermal treatment and succinic acid via fermentation. J. Clean. Prod. 2024, 456, 142305. [Google Scholar] [CrossRef]
- Kossatz, H.L.; Rose, S.H.; Viljoen-Bloom, M.; van Zyl, W.H. Production of ethanol from steam exploded triticale straw in a simultaneous saccharification and fermentation process. Process Biochem. 2017, 53, 10–16. [Google Scholar] [CrossRef]
- Aghili Mehrizi, A.; Tangestaninejad, S.; Denayer, J.F.; Karimi, K.; Shafiei, M. The critical impacts of anion and cosolvent on morpholinium ionic liquid pretreatment for efficient renewable energy production from triticale straw. Renew. Energy 2023, 202, 686–698. [Google Scholar] [CrossRef]
- Tibenderana, P.; Bainomugisha, J.; Abdulkadir, T.S.; Agwe, T.M.; Twesigye-omwe, M.N.; Ako, T. Performance evaluation of open air burnt sorghum and wheat straw ashes in hard water treatment. Int. J. Agric. Technol. 2024, 20, 399–410. [Google Scholar]
- Zhan, Q.; Lin, Q.; Wu, Y.; Liu, Y.; Wang, X.; Ren, J. A fractionation strategy of cellulose, hemicellulose, and lignin from wheat straw via the biphasic pretreatment for biomass valorization. Bioresour. Technol. 2023, 376, 128887. [Google Scholar] [CrossRef]
- Joshi, M.; Manjare, S. Chemical approaches for the biomass valorisation: A comprehensive review of pretreatment strategies. Environ. Sci. Pollut. Res. 2024, 31, 48928–48954. [Google Scholar] [CrossRef]
- Chaudhary, G.; Chaudhary, N.; Saini, S.; Gupta, Y.; Vivekanand, V.; Panghal, A. Assessment of pretreatment strategies for valorization of lignocellulosic biomass: Path forwarding towards lignocellulosic biorefinery. Waste Biomass Valorization 2023, 15, 1–36. [Google Scholar] [CrossRef]
- Arce, C.; Kratky, L. Mechanical pretreatment of lignocellulosic biomass toward enzymatic/fermentative valorization. iScience 2022, 25, 104610. [Google Scholar] [CrossRef] [PubMed]
- Romero-García, J.; Solarte-Toro, J.; Galán-Martín, A.; Ruiz, E.; Castro, E.; Ortiz-Sánchez, M.; Cardona Alzate, C. Olive leaves upgrading applying a novel two-stage organosolv pretreatment: Techno-economic and environmental assessment. Biochem. Eng. J. 2024, 207, 109317. [Google Scholar] [CrossRef]
- Ong, V.Z.; Wu, T.Y. An application of ultrasonication in lignocellulosic biomass valorisation into bio-energy and bio-based products. Renew. Sustain. Energy Rev. 2020, 132, 109924. [Google Scholar] [CrossRef]
- Fia, A.; Amorim, J. Microwave pretreatment of biomass for conversion of lignocellulosic materials into renewable biofuels. J. Energy Inst. 2023, 106, 101146. [Google Scholar] [CrossRef]
- Lobato-Peralta, D.R.; Duque-Brito, E.; Villafán-Vidales, H.I.; Longoria, A.; Sebastian, P.; Cuentas-Gallegos, A.K.; Arancibia-Bulnes, C.A.; Okoye, P.U. A review on trends in lignin extraction and valorization of lignocellulosic biomass for energy applications. J. Clean. Prod. 2021, 293, 126123. [Google Scholar] [CrossRef]
- Pedersen, M.; Meyer, A.S. Lignocellulose pretreatment severity relating pH to biomatrix opening. New Biotechnol. 2010, 27, 739–750. [Google Scholar] [CrossRef]
- De Saegher, T.; Deroma, M.; Atanasova, B.; Van Geem, K.M.; De Clercq, J.; Lauwaert, J.; Verberckmoes, A. Maximizing the valorization potential of lignin through optimization of the soda pulping conditions. Sep. Purif. Technol. 2025, 354, 128900. [Google Scholar] [CrossRef]
- Álvarez, C.; Duque, A.; Sánchez-Monedero, A.; González, E.J.; González-Miquel, M.; Cañadas, R. Exploring Recent Advances in Lignocellulosic Biomass Waste Delignification Through the Combined Use of Eutectic Solvents and Intensification Techniques. Processes 2024, 12, 2514. [Google Scholar] [CrossRef]
- Ruwoldt, J.; Syverud, K.; Tanase-Opedal, M. Purification of soda lignin. Sustain. Chem. Environ. 2024, 6, 100102. [Google Scholar] [CrossRef]
- Gaudenzi, E.; Cardone, F.; Lu, X.; Canestrari, F. The use of lignin for sustainable asphalt pavements: A literature review. Constr. Build. Mater. 2023, 362, 129773. [Google Scholar] [CrossRef]
- Bajwa, D.; Pourhashem, G.; Ullah, A.; Bajwa, S. A concise review of current lignin production, applications, products and their environmental impact. Ind. Crop. Prod. 2019, 139, 111526. [Google Scholar] [CrossRef]
- Beaucamp, A.; Muddasar, M.; Amiinu, I.S.; Moraes Leite, M.; Culebras, M.; Latha, K.; Gutiérrez, M.C.; Rodriguez-Padron, D.; del Monte, F.; Kennedy, T.; et al. Lignin for energy applications: State of the art, life cycle, technoeconomic analysis and future trends. Green Chem. 2022, 24, 8193–8226. [Google Scholar] [CrossRef]
- Chio, C.; Sain, M.; Qin, W. Lignin utilization: A review of lignin depolymerization from various aspects. Renew. Sustain. Energy Rev. 2019, 107, 232–249. [Google Scholar] [CrossRef]
- Trovagunta, R.; Marquez, R.; Tolosa, L.; Barrios, N.; Zambrano, F.; Suarez, A.; Pal, L.; Gonzalez, R.; Hubbe, M.A. Lignin self-assembly phenomena and valorization strategies for pulping, biorefining, and materials development: Part 1. The physical chemistry of lignin self-assembly. Adv. Colloid Interface Sci. 2024, 332, 103247. [Google Scholar] [CrossRef] [PubMed]
- Gomes, R.M.; da Silva, F.G., Jr. Impact of sulfidity on the kraft pulping of eucalyptus. BioResources 2020, 15, 3945–3961. [Google Scholar] [CrossRef]
- Fernández-Rodríguez, J.; Erdocia, X.; Hernández-Ramos, F.; Gordobil, O.; González Alriols, M.; Labidi, J. Direct lignin depolymerization process from sulfur-free black liquors. Fuel Process. Technol. 2020, 197, 106201. [Google Scholar] [CrossRef]
- Wu, Y.; Gao, X.; Nguyen, T.T.; Wu, J.; Guo, M.; Liu, W.; Du, C. Green and Low–Cost Natural Lignocellulosic Biomass–Based Carbon Fibers–Processing, Properties, and Applications in Sports Equipment: A Review. Polymers 2022, 14, 2591. [Google Scholar] [CrossRef] [PubMed]
- Covinich, L.G.; Area, M.C. Trends and limitations of lignin as a starting material. BioResources 2023, 19, 6–9. [Google Scholar] [CrossRef]
- Borges Gomes, F.J.; de Souza, R.E.; Brito, E.O.; Costa Lelis, R.C. A review on lignin sources and uses. J. Appl. Biotechnol. Bioeng. 2020, 7, 100–105. [Google Scholar] [CrossRef]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of Structural Carbohydrates and Lignin in Biomass; Laboratory Analytical Procedure NREL/TP-510-42618; National Renewable Energy Laboratory (USA): Golden, CO, USA, 2012. [Google Scholar]
- du Pasquier, J.; Paës, G.; Perré, P. Principal factors affecting the yield of dilute acid pretreatment of lignocellulosic biomass: A critical review. Bioresour. Technol. 2023, 369, 128439. [Google Scholar] [CrossRef]
- Oriez, V.; Peydecastaing, J.; Pontalier, P.Y. Lignocellulosic biomass fractionation by mineral acids and resulting extract purification processes: Conditions, yields, and purities. Molecules 2019, 24, 4273. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Liu, J.; Geng, W.; Tang, W.; Yong, Q. A review of lignocellulosic biomass pretreatment technologies. Pap. Biomater. 2021, 6, 61–76. [Google Scholar] [CrossRef]
- Rashid, T.; Sher, F.; Rasheed, T.; Zafar, F.; Zhang, S.; Murugesan, T. Evaluation of current and future solvents for selective lignin dissolution: A review. J. Mol. Liq. 2021, 321, 114577. [Google Scholar] [CrossRef]
- Singh, S.K.; Matsagar, B.M.; Dhepe, P.L. Lignocellulosic biomass analysis: Acidic lignin recovery, characterisation, and depolymerisation. Biomass Convers. Biorefinery 2022, 14, 5239–5249. [Google Scholar] [CrossRef]
- Ávila, P.F.; Goldbeck, R. Fractionating process of lignocellulosic biomass for the enzymatic production of short chain cello-oligosaccharides. Ind. Crop. Prod. 2022, 178, 114671. [Google Scholar] [CrossRef]
- Gill, M.K.; Kocher, G.S.; Panesar, A.S. Optimization of acid-mediated delignification of corn stover, an agriculture residue carbohydrate polymer for improved ethanol production. Carbohydr. Polym. Technol. Appl. 2021, 2, 100029. [Google Scholar] [CrossRef]
- Chakraborty, M.S.; Lali, A.M. Separation and catalytic depolymerization of empty palm fruit bunch lignin. Ind. Crop. Prod. 2021, 174, 114183. [Google Scholar] [CrossRef]
- Daza Serna, L.; Orrego Alzate, C.; Cardona Alzate, C. Supercritical fluids as a green technology for the pretreatment of lignocellulosic biomass. Bioresour. Technol. 2016, 199, 113–120. [Google Scholar] [CrossRef]
- Chiranjeevi, T.; Mattam, A.J.; Vishwakarma, K.K.; Uma, A.; Peddy, V.C.R.; Gandham, S.; Ravindra Velankar, H. Assisted single-step acid pretreatment process for enhanced delignification of rice straw for bioethanol production. ACS Sustain. Chem. Eng. 2018, 6, 8762–8774. [Google Scholar] [CrossRef]
- Yin, C.; Wang, M.; Ma, Q.; Bian, H.; Ren, H.; Dai, H.; Cheng, J. Valorization of rice straw via hydrotropic lignin extraction and its characterization. Molecules 2021, 26, 4123. [Google Scholar] [CrossRef]
- Pham, T.A.; Ngo, D.S.; To, K.A. Formic acid-based organosolv delignification of sugarcane bagasse for efficient enzymatic saccharification. Sugar Tech 2022, 24, 779–787. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, N.; Chaudhary, G.; Saini, S.; Singh, C. Development of a two-step hydrothermal pretreatment for sugarcane bagasse delignification surpassing acidic and chemical methods. Waste Biomass Valorization 2024, 15, 4613–4628. [Google Scholar] [CrossRef]
- Ma, Q.; Zhu, J.; Gleisner, R.; Yang, R.; Zhu, J. Valorization of wheat straw using a recyclable hydrotrope at low temperatures (≤90 ∘C). ACS Sustain. Chem. Eng. 2018, 6, 14480–14489. [Google Scholar] [CrossRef]
- Su, C.; Hirth, K.; Liu, Z.; Cao, Y.; Zhu, J.Y. Maleic acid hydrotropic fractionation of wheat straw to facilitate value added multi-product biorefinery at atmospheric pressure. GCB Bioenergy 2021, 13, 1407–1424. [Google Scholar] [CrossRef]
- Khokhar, Z.; Syed, Q.; Nadeem, M.; Baig, S.; Irfan, M.; Gull, I.; Tipu, I.; Aslam, S.; Samra, Z.Q.; Athar, M.A. Delignification of wheat straw with acid and hydro-steam under pressure. World Appl. Sci. J. 2010, 11, 1524–15308. [Google Scholar]
- Roy, R.; Rahman, M.S.; Raynie, D.E. Recent advances of greener pretreatment technologies of lignocellulose. Curr. Res. Green Sustain. Chem. 2020, 3, 100035. [Google Scholar] [CrossRef]
- Lorenci Woiciechowski, A.; Dalmas Neto, C.J.; Porto de Souza Vandenberghe, L.; de Carvalho Neto, D.P.; Novak Sydney, A.C.; Letti, L.A.J.; Karp, S.G.; Zevallos Torres, L.A.; Soccol, C.R. Lignocellulosic biomass: Acid and alkaline pretreatments and their effects on biomass recalcitrance—Conventional processing and recent advances. Bioresour. Technol. 2020, 304, 122848. [Google Scholar] [CrossRef] [PubMed]
- Rattana-Amron, T.; Laosiripojana, N.; Kangwansupamonkon, W. Thermal oxidative degradation behavior of extracted lignins from agricultural wastes: Kinetic and thermodynamic analysis. Ind. Crop. Prod. 2024, 219, 119096. [Google Scholar] [CrossRef]
- Svensson, I.; Roncal, T.; De Winter, K.; Van Canneyt, A.; Tamminen, T.; Mikkelson, A.; Barrio, A. Valorisation of hydrolysis lignin rest from bioethabol pilot plant: Process development and upscaling. Ind. Crop. Prod. 2020, 156, 112869. [Google Scholar] [CrossRef]
- Fuertez Cordoba, J.M.; Ruiz Colorado, A.A.; Alvarez Zapata, H.D.; Molina Ochoa, A. Integrated dynamic model of the alkaline delignification process of lignocellulosic biomass. Dyna 2011, 170, 175–184. [Google Scholar]
- Novia, N.; Soniato, A.A.A.; Ramadhan, I.M.; Sari, A.; Hasanah, U.; Hermansyah, H.; Hasanudin, H.; Fudholi, A. Delignification and enzymatic hydrolysis kinetics of KOH microwave-assisted pretreated banana stem for bioethanol production. Int. J. Hydrogen Energy 2024, 85, 931–946. [Google Scholar] [CrossRef]
- Ma, L.; Cui, Y.; Cai, R.; Liu, X.; Zhang, C.; Xiao, D. Optimization and evaluation of alkaline potassium permanganate pretreatment of corncob. Bioresour. Technol. 2015, 180, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Dimos, K.; Paschos, T.; Louloudi, A.; Kalogiannis, K.G.; Lappas, A.A.; Papayannakos, N.; Kekos, D.; Mamma, D. Effect of various pretreatment methods on bioethanol pProduction from cotton stalks. Fermentation 2019, 5, 5. [Google Scholar] [CrossRef]
- An, S.; Li, W.; Liu, Q.; Xia, Y.; Zhang, T.; Huang, F.; Lin, Q.; Chen, L. Combined dilute hydrochloric acid and alkaline wet oxidation pretreatment to improve sugar recovery of corn stover. Bioresour. Technol. 2019, 271, 283–288. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Shao, Q.; Chundawat, S.P. Recent advances on ammonia-based pretreatments of lignocellulosic biomass. Bioresour. Technol. 2020, 298, 122446. [Google Scholar] [CrossRef]
- Akus-Szylberg, F.; Antczak, A.; Zawadzki, J. Effects of soaking aqueous ammonia pretreatment on chemical composition and enzymatic hydrolysis of corn stover. Ann. WULS For. Wood Technol. 2021, 115, 29–36. [Google Scholar] [CrossRef]
- Yoo, C.G.; Kim, H.; Lu, F.; Azarpira, A.; Pan, X.; Oh, K.K.; Kim, J.S.; Ralph, J.; Kim, T.H. Understanding the physicochemical characteristics and the improved enzymatic saccharification of corn stover pretreated with aqueous and gaseous ammonia. BioEnergy Res. 2015, 9, 67–76. [Google Scholar] [CrossRef]
- Subhedar, P.B.; Ray, P.; Gogate, P.R. Intensification of delignification and subsequent hydrolysis for the fermentable sugar production from lignocellulosic biomass using ultrasonic irradiation. Ultrason. Sonochem. 2018, 40, 140–150. [Google Scholar] [CrossRef]
- Elalami, D.; Carrere, H.; Abdelouahdi, K.; Garcia-Bernet, D.; Peydecastaing, J.; Vaca-Medina, G.; Oukarroum, A.; Zeroual, Y.; Barakat, A. Mild microwaves, ultrasonic and alkaline pretreatments for improving methane production: Impact on biochemical and structural properties of olive pomace. Bioresour. Technol. 2020, 299, 122591. [Google Scholar] [CrossRef]
- Novia, N.; Said, M.; Jannah, A.; Pebriantoni, P.; Bayu, M. Aqueous ammonia soaking-dilute acid pretreatment to produce bioethanol from rice hull. Technol. Rep. Kansai Univ. 2020, 62, 891–900. [Google Scholar]
- Pasha, S.M.; Navya, P.; Musfera, S.; Goutham, Y.; Pasha, C. Bioethanol production from ammonia pretreated rice straw. J. Res. Appl. Sci. Biotechnol. 2022, 1, 120–124. [Google Scholar] [CrossRef]
- Shen, Z.; Zhang, K.; Si, M.; Liu, M.; Zhuo, S.; Liu, D.; Ren, L.; Yan, X.; Shi, Y. Synergy of lignocelluloses pretreatment by sodium carbonate and bacterium to enhance enzymatic hydrolysis of rice straw. Bioresour. Technol. 2018, 249, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Mennani, M.; Ait Benhamou, A.; Kasbaji, M.; Boussetta, A.; Ablouh, E.H.; Kassab, Z.; El Achaby, M.; Boussetta, N.; Grimi, N.; Moubarik, A. Insights on the physico-chemical properties of alkali lignins from different agro-industrial residues and their use in phenol-formaldehyde wood adhesive formulation. Int. J. Biol. Macromol. 2022, 221, 149–162. [Google Scholar] [CrossRef]
- Zahoor; Madadi, M.; Nazar, M.; Shah, S.W.A.; Li, N.; Imtiaz, M.; Zhong, Z.; Zhu, D. Green alkaline fractionation of sugarcane bagasse at cold temperature improves digestibility and delignification without the washing processes and release of hazardous waste. Ind. Crop. Prod. 2023, 200, 116815. [Google Scholar] [CrossRef]
- Mota, I.F.; da Silva Burgal, J.a.; Antunes, F.; Pintado, M.E.; Costa, P.S. High value-added lignin extracts from sugarcane by-products. Int. J. Biol. Macromol. 2023, 230, 123144. [Google Scholar] [CrossRef] [PubMed]
- Chandel, A.K.; Antunes, F.A.; Silva, M.B.; da Silva, S. Unraveling the structure of sugarcane bagasse after soaking in concentrated aqueous ammonia (SCAA) and ethanol production by Scheffersomyces (Pichia) stipitis. Biotechnol. Biofuels 2013, 6, 102. [Google Scholar] [CrossRef]
- Jiménez, I.M.; Chandel, A.K.; Marcelino, P.R.; Anjos, V.; Batesttin Costa, C.; Jose, V.; Bell, M.; Pereira, B.; da Silva, S.S. Comparative data on effects of alkaline pretreatments and enzymatic hydrolysis on bioemulsifier production from sugarcane straw by Cutaneotrichosporon mucoides. Bioresour. Technol. 2020, 301, 122706. [Google Scholar] [CrossRef]
- Punia, P.; Singh, L. Optimization of alkali pre-treatment of sweet sorghum [Sorghum bicolor (L.) Moench] residue to improve enzymatic hydrolysis for fermentable sugars. Waste Manag. Bull. 2024, 2, 131–141. [Google Scholar] [CrossRef]
- Chen, X.; Cui, Y.; Gobeze, H.B.; Kuroda, D.G. Assessing the location of ionic and molecular solutes in a molecularly heterogeneous and nonionic deep eutectic solvent. J. Phys. Chem. B 2020, 124, 4762–4773. [Google Scholar] [CrossRef]
- Zhang, X.; Nghiem, N.P. Pretreatment and fractionation of wheat straw for production of fuel ethanol and value-added co-products in a biorefinery. AIMS Bioeng. 2014, 1, 40–52. [Google Scholar] [CrossRef]
- Lymperatou, A.; Gavala, H.N.; Skiadas, I.V. Aqueous ammonia soaking of wheat straw at ambient temperature for enhancing the methane yield: Process optimization by response surface methodology. Waste Biomass Valorization 2019, 11, 4821–4835. [Google Scholar] [CrossRef]
- Hussain, N.I.A.M.; Salim, N.; Mustapha, S.N.H.; Misnon, I.I.; Rahim, M.H.A.; Roslan, R. Lignocellulose biomass delignification using acid hydrotrope as green solvent: A mini-review. Cellul. Chem. Technol. 2023, 57, 1017–1028. [Google Scholar] [CrossRef]
- Devendra, L.P.; Pandey, A. Hydrotropic pretreatment on rice straw for bioethanol production. Renew. Energy 2016, 98, 2–8. [Google Scholar] [CrossRef]
- Glaser, S.J.; Al-Rudainy, B.; Hatti-Kaul, R.; Galbe, M. Wheat bran fractionation: Effect of steam explosion and hydrotropic extraction conditions on the recovery of sugars and lignin. Ind. Crop. Prod. 2023, 195, 116405. [Google Scholar] [CrossRef]
- Ansari, K.B.; Gaikar, V.G. Green hydrotropic extraction technology for delignification of sugarcane bagasse by using alkybenzene sulfonates as hydrotropes. Chem. Eng. Sci. 2014, 115, 157–166. [Google Scholar] [CrossRef]
- Ferreira, J.A.; Taherzadeh, M.J. Improving the economy of lignocellulose-based biorefineries with organosolv pretreatment. Bioresour. Technol. 2020, 299, 122695. [Google Scholar] [CrossRef] [PubMed]
- Rabelo, S.C.; Nakasu, P.Y.S.; Scopel, E.; Araújo, M.F.; Cardoso, L.H.; Costa, A.C.d. Organosolv pretreatment for biorefineries: Current status, perspectives, and challenges. Bioresour. Technol. 2023, 369, 128331. [Google Scholar] [CrossRef]
- Yong, K.J.; Wu, T.Y. Recent advances in the application of alcohols in extracting lignin with preserved β-O-4 content from lignocellulosic biomass. Bioresour. Technol. 2023, 384, 129238. [Google Scholar] [CrossRef]
- Tofani, G.; Jasiukaityte-Grojzdek, E.; Grilc, M.; Likozar, B. Organosolv biorefinery: Resource-based process optimisation, pilot technology scale-up and economics. Green Chem. 2024, 26, 186–201. [Google Scholar] [CrossRef]
- Zhou, Z.; Ouyang, D.; Liu, D.; Zhao, X. Oxidative pretreatment of lignocellulosic biomass for enzymatic hydrolysis: Progress and challenges. Bioresour. Technol. 2023, 367, 128208. [Google Scholar] [CrossRef]
- Rinaldi, R.; Jastrzebski, R.; Clough, M.T.; Ralph, J.; Kennema, M.; Bruijnincx, P.C.A.; Weckhuysen, B.M. Paving the way for lignin valorisation: Recent advances in bioengineering, biorefining and catalysis. Angew. Chem. Int. Ed. 2016, 55, 8164–8215. [Google Scholar] [CrossRef]
- Wong, J.L.; Khadaroo, S.N.B.A.; Cheng, J.L.Y.; Chew, J.J.; Khaerudini, D.S.; Sunarso, J. Green solvent for lignocellulosic biomass pretreatment: An overview of the performance of low transition temperature mixtures for enhanced bio-conversion. Next Mater. 2023, 1, 100012. [Google Scholar] [CrossRef]
- Wen, J.L.; Sun, S.L.; Xue, B.L.; Sun, R.C. Recent advances in characterization of lignin polymer by solution-state nuclear magnetic resonance (NMR) methodology. Materials 2013, 6, 359–391. [Google Scholar] [CrossRef]
- Feng, S.; Shui, T.; Wang, H.; Ai, X.; Kuboki, T.; Xu, C.C. Properties of phenolic adhesives formulated with activated organosolv lignin derived from cornstalk. Ind. Crop. Prod. 2021, 161, 113225. [Google Scholar] [CrossRef]
- Yuan, W.; Gong, Z.; Wang, G.; Zhou, W.; Liu, Y.; Wang, X.; Zhao, M. Alkaline organosolv pretreatment of corn stover for enhancing the enzymatic digestibility. Bioresour. Technol. 2018, 265, 464–470. [Google Scholar] [CrossRef] [PubMed]
- Gong, Z.; Wang, X.; Yuan, W.; Wang, Y.; Zhou, W.; Wang, G.; Liu, Y. Fed-batch enzymatic hydrolysis of alkaline organosolv-pretreated corn stover facilitating high concentrations and yields of fermentable sugars for microbial lipid production. Biotechnol. Biofuels 2020, 13, 13. [Google Scholar] [CrossRef]
- Tang, S.; Dong, Q.; Fang, Z.; Cong, W.j.; Zhang, H. Microbial lipid production from rice straw hydrolysates and recycled pretreated glycerol. Bioresour. Technol. 2020, 312, 123580. [Google Scholar] [CrossRef]
- Wei, W.; Wang, B.; Wang, X.; Ling, R.; Jin, Y. Comparison of acid and alkali catalyzed ethylene glycol organosolv pretreatment for sugar production from bagasse. Bioresour. Technol. 2021, 320, 124293. [Google Scholar] [CrossRef]
- Ling, R.; Wei, W.; Jin, Y. Pretreatment of sugarcane bagasse with acid catalyzed ethylene glycol-water to improve the cellulose enzymatic conversion. Bioresour. Technol. 2022, 361, 127723. [Google Scholar] [CrossRef]
- Hermsdorff, G.B.; Escobar, E.L.N.; da Silva, T.A.; Filho, A.Z.; Corazza, M.L.; Ramos, L.P. Ethanol organosolv pretreatment of sugarcane bagasse assisted by organic acids and supercritical carbon dioxide. Carbohydr. Polym. 2023, 300, 120263. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, J.; Xie, J.; Qin, Y. Effects of NaOH-catalyzed organosolv pretreatment and surfactant on the sugar production from sugarcane bagasse. Bioresour. Technol. 2020, 312, 123601. [Google Scholar] [CrossRef]
- Li, W.; Wang, Y.; Tan, X.; Miao, C.; Ragauskas, A.J.; Zhuang, X. Biocompatible organosolv fractionation via a novel alkaline lignin-first strategy towards lignocellulose valorization. Chem. Eng. J. 2024, 481, 148695. [Google Scholar] [CrossRef]
- Zeng, S.; Ma, Q.; Zhang, S.; Shen, C.; Li, J.; Zhao, H.; Guo, D.; Zhang, Y.; Yang, H. Evaluation of oxy-organosolv pretreatment on lignin extraction from wheat straw. Int. J. Biol. Macromol. 2023, 229, 861–872. [Google Scholar] [CrossRef]
- Sun, F.F.; Wang, L.; Hong, J.; Ren, J.; Du, F.; Hu, J.; Zhang, Z.; Zhou, B. The impact of glycerol organosolv pretreatment on the chemistry and enzymatic hydrolyzability of wheat straw. Bioresour. Technol. 2015, 187, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Cai, C.; Zhang, C.; Li, N.; Liu, H.; Xie, J.; Lou, H.; Pan, X.; Zhu, J.; Wang, F. Changing the role of lignin in enzymatic hydrolysis for a sustainable and efficient sugar platform. Renew. Sustain. Energy Rev. 2023, 183, 113445. [Google Scholar] [CrossRef]
- Kaur, G.; Kumar, H.; Singla, M. Diverse applications of ionic liquids: A comprehensive review. J. Mol. Liq. 2022, 351, 118556. [Google Scholar] [CrossRef]
- Lin, X.; Jiang, K.; Liu, X.; Han, D.; Zhang, Q. Review on development of ionic liquids in lignocellulosic biomass refining. J. Mol. Liq. 2022, 359, 119326. [Google Scholar] [CrossRef]
- Lobato-Rodríguez, A.; Gullón, B.; Romaní, A.; Ferreira-Santos, P.; Garrote, G.; Del-Río, P.G. Recent advances in biorefineries based on lignin extraction using deep eutectic solvents: A review. Bioresour. Technol. 2023, 388, 129744. [Google Scholar] [CrossRef]
- Tan, J.; Yu, D.; Yuan, J.; Wu, H.; Luo, H.; Zhang, H.; Li, X.; Li, H.; Yang, S. Efficient delignification of wheat straw for microbial lipid production enabled by a novel ternary deep eutectic solvent containing ethylene glycol. Fuel 2023, 347, 128485. [Google Scholar] [CrossRef]
- Wardani, N.I.; Samkumpim, T.; Alahmad, W.; King, A.W.; Varanusupakul, P.; Shishov, A.; Yahaya, N.; Zain, N.N.M. Recent cutting–edge approaches to the integration of solid-liquid extraction with deep eutectic solvents: Toward a greener procedure for biomass valorization. Adv. Sample Prep. 2024, 10, 100113. [Google Scholar] [CrossRef]
- Li, W.; Ma, S.; Luo, L.; Li, Z.; He, A.; Wang, C.; Lin, L.; Zeng, X. Pretreatment of biomass with ethanol/deep eutectic solvent towards higher component recovery and obtaining lignin with high β-O-4 content. Int. J. Biol. Macromol. 2024, 276, 133751. [Google Scholar] [CrossRef]
- Arriaga, S.; Aizpuru, A. Innovative non-aqueous phases and partitioning bioreactor configurations. In Advances and Applications of Partitioning Bioreactors; Elsevier: Amsterdam, The Netherlands, 2019; pp. 299–348. [Google Scholar] [CrossRef]
- Okonkwo, C.E.; Hussain, S.Z.; Manzoor, S.; Naseer, B.; Taiwo, A.E.; Ayyash, M.; Al-Marzouqi, A.H.; Kamal-Eldin, A. A comprehensive review on the use of deep eutectic solvents for biomass processing, and the synergistic coupling with physical technology and biological method. Bioresour. Technol. Rep. 2023, 23, 101577. [Google Scholar] [CrossRef]
- Yang, F.; Zheng, Q.; Tan, H.; Wang, X. Insight into the role of hydrogen bond donor in deep eutectic solvents. J. Mol. Liq. 2024, 399, 124332. [Google Scholar] [CrossRef]
- Zhou, M.; Fakayode, O.A.; Ahmed Yagoub, A.E.; Ji, Q.; Zhou, C. Lignin fractionation from lignocellulosic biomass using deep eutectic solvents and its valorization. Renew. Sustain. Energy Rev. 2022, 156, 111986. [Google Scholar] [CrossRef]
- Nkosi, N.; Tumba, K.; Ramsuroop, S. Tetramethylammonium chloride + glycerol deep eutectic solvent as separation agent for organic liquid mixtures: Assessment from experimental limiting activity coefficients. Fluid Phase Equilibria 2018, 473, 98–105. [Google Scholar] [CrossRef]
- Wang, J.; Lan, D.; Zhuang, J.; Wang, Y. Pretreatment of Camellia Oleifera Shell Ethanolamine-Based Solvents Sel. Delignification Enhanc. Enzym. Saccharification. Ind. Crop. Prod. 2024, 222, 119523. [Google Scholar] [CrossRef]
- Provost, V.; Dumarcay, S.; Ziegler-Devin, I.; Boltoeva, M.; Trébouet, D.; Villain-Gambier, M. Deep eutectic solvent pretreatment of biomass: Influence of hydrogen bond donor and temperature on lignin extraction with high β-O-4 content. Bioresour. Technol. 2022, 349, 126837. [Google Scholar] [CrossRef]
- Fan, Y.; Ji, H.; Ji, X.; Tian, Z.; Chen, J. A deep eutectic solvent with a lignin stabilization and functionalization for lignocellulosic biomass pretreatment. Chem. Eng. J. 2024, 499, 156482. [Google Scholar] [CrossRef]
- Sharma, V.; Tsai, M.L.; Chen, C.W.; Sun, P.P.; Patel, A.K.; Singhania, R.R.; Nargotra, P.; Dong, C.D. Deep eutectic solvents as promising pretreatment agents for sustainable lignocellulosic biorefineries: A review. Bioresour. Technol. 2022, 360, 127631. [Google Scholar] [CrossRef]
- Abbasi, N.M.; Farooq, M.Q.; Anderson, J.L. Investigating the effect of systematically modifying the molar ratio of hydrogen bond donor and acceptor on solvation characteristics of deep eutectic solvents formed using choline chloride salt and polyalcohols. J. Chromatogr. A 2022, 1667, 462871. [Google Scholar] [CrossRef]
- Bao, Y.; Wang, Y.; Yan, C.; Xue, Z. Deep eutectic solvents for fractionation and valorization of lignocellulose. Green Chem. Eng. 2025, 6, 21–35. [Google Scholar] [CrossRef]
- He, Y.; Shen, R.; Shao, L.; Wang, C.; Yang, G.; Xu, F. Efficient binary diol-based deep eutectic solvent pretreatment for enhancing enzymatic saccharification and lignin fractionation from eucalyptus. Bioresour. Technol. 2024, 412, 131423. [Google Scholar] [CrossRef] [PubMed]
- Yanak, S.; Buyukkileci, A.O. Delignification of corncob by choline chloride-urea deep eutectic solvent for enzymatic production of xylooligosaccharides. Ind. Crop. Prod. 2024, 217, 118894. [Google Scholar] [CrossRef]
- Procentese, A.; Johnson, E.; Orr, V.; Garruto Campanile, A.; Wood, J.A.; Marzocchella, A.; Rehmann, L. Deep eutectic solvent pretreatment and subsequent saccharification of corncob. Bioresour. Technol. 2015, 192, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Duan, W.; An, X.; Qiao, Y.; Tian, Y.; Zhou, H. Novel betaine-amino acid based natural deep eutectic solvents for enhancing the enzymatic hydrolysis of corncob. Bioresour. Technol. 2020, 310, 123389. [Google Scholar] [CrossRef]
- Liang, X.; Zhu, Y.; Qi, B.; Li, S.; Luo, J.; Wan, Y. Structure-property-performance relationships of lactic acid-based deep eutectic solvents with different hydrogen bond acceptors for corn stover pretreatment. Bioresour. Technol. 2021, 336, 125312. [Google Scholar] [CrossRef]
- Liu, X.; Li, T.; Wu, S.; Ma, H.; Yin, Y. Structural characterization and comparison of enzymatic and deep eutectic solvents isolated lignin from various green processes: Toward lignin valorization. Bioresour. Technol. 2020, 310, 123460. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Din, S.Z.U.; Mao, J.; Guo, Z.; Xu, F. Sustainable production of fermentable sugars and antioxidant lignin from industrial xylose residue with facile deep eutectic solvents. Ind. Crop. Prod. 2024, 219, 119079. [Google Scholar] [CrossRef]
- Tan, Y.T.; Ngoh, G.C.; Chua, A.S.M. Effect of functional groups in acid constituent of deep eutectic solvent for extraction of reactive lignin. Bioresour. Technol. 2019, 281, 359–366. [Google Scholar] [CrossRef]
- Yaakob, M.N.A.; Salim, N.; Mustapha, S.N.H.; Misnon, I.I.; Rahim, M.H.A.; Roslan, R. Efficient lignin extraction from oil palm empty fruit bunches using guanidine-based deep eutectic solvents under microwave assistance. Ind. Crop. Prod. 2024, 218, 118968. [Google Scholar] [CrossRef]
- Zhang, Z.; Xu, J.; Xie, J.; Zhu, S.; Wang, B.; Li, J.; Chen, K. Physicochemical transformation and enzymatic hydrolysis promotion of reed straw after pretreatment with a new deep eutectic solvent. Carbohydr. Polym. 2022, 290, 119472. [Google Scholar] [CrossRef] [PubMed]
- Popa-Tudor, I.; De?liu-Avram, M.; Vladulescu, L.; Constantinescu-Aruxandei, D.; Oancea, F. The recovery of lignin from reed using solid-DES Extraction (SDESE). In Proceedings of the NeXT-Chem 2023, Bucharest, Romania, 22–23 May 2023; p. 9. [Google Scholar] [CrossRef]
- Hou, X.D.; Feng, G.J.; Ye, M.; Huang, C.M.; Zhang, Y. Significantly enhanced enzymatic hydrolysis of rice straw via a high-performance two-stage deep eutectic solvents synergistic pretreatment. Bioresour. Technol. 2017, 238, 139–146. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.A.; Rahaman, M.S.; Yelle, D.; Shang, H.; Sun, Z.; Renneckar, S.; Dong, J.; Tulaphol, S.; Sathitsuksanoh, N. Effects of polyol-based deep eutectic solvents on the efficiency of rice straw enzymatic hydrolysis. Ind. Crop. Prod. 2021, 167, 113480. [Google Scholar] [CrossRef]
- Morán-Aguilar, M.G.; Calderón-Santoyo, M.; de Souza Oliveira, R.P.; Aguilar-Uscanga, M.G.; Domínguez, J.M. Deconstructing sugarcane bagasse lignocellulose by acid-based deep eutectic solvents to enhance enzymatic digestibility. Carbohydr. Polym. 2022, 298, 120097. [Google Scholar] [CrossRef] [PubMed]
- Sunar, S.L.; Oruganti, R.K.; Bhattacharyya, D.; Shee, D.; Panda, T.K. Deep eutectic solvent pretreatment of sugarcane bagasse for efficient lignin recovery and enhanced enzymatic hydrolysis. J. Ind. Eng. Chem. 2024, 139, 539–553. [Google Scholar] [CrossRef]
- Raj, T.; Dien, B.S.; Singh, V. Process strategies for recovery of sugars, lipids, and lignin from oilcane bagasse using natural deep eutectic solvents (NADES). Chem. Eng. J. 2024, 493, 152657. [Google Scholar] [CrossRef]
- Yue, X.; Suopajarvi, T.; Sun, S.; Mankinen, O.; Mikkelson, A.; Huttunen, H.; Komulainen, S.; Romakkaniemi, I.; Ahola, J.; Telkki, V.V.; et al. High-purity lignin fractions and nanospheres rich in phenolic hydroxyl and carboxyl groups isolated with alkaline deep eutectic solvent from wheat straw. Bioresour. Technol. 2022, 360, 127570. [Google Scholar] [CrossRef]
- Xian, X.; Fang, L.; Zhou, Y.; Li, B.; Zheng, X.; Liu, Y.; Lin, X. Integrated bioprocess for cellulosic ethanol production from wheat straw: New ternary deep-eutectic-solvent pretreatment, enzymatic saccharification, and fermentation. Fermentation 2022, 8, 371. [Google Scholar] [CrossRef]
- Zhan, Y.; Liu, X.; Huang, C.; Zhou, X.; Lyu, Y.; Lin, Y.; Huang, C.; Ma, W.; Xie, Z.; Fang, G.; et al. Investigation of the alkaline hydrogen peroxide pretreatment: From cellulose saccharification to lignin isolation. Ind. Crop. Prod. 2024, 214, 118533. [Google Scholar] [CrossRef]
- Olatunji, K.O.; Ahmed, N.A.; Ogunkunle, O. Optimization of biogas yield from lignocellulosic materials with different pretreatment methods: A review. Biotechnol. Biofuels 2021, 14, 159. [Google Scholar] [CrossRef]
- Toghiani, J.; Malekzadeh, S.; Jamali, N.; Afsham, N.; Fallah, N.; Mahboubi, A.; Nasernejad, B.; Taherzadeh, M.J.; Oladzad, S. Novel advanced oxidation processes (AOPs) as lignocellulosic biomass pretreatment approaches and their sustainability assessment: A review. Curr. Pollut. Rep. 2024, 10, 207–246. [Google Scholar] [CrossRef]
- Ribeiro-Sanches, M.A.; Stochi, V.A.L.; Borges-Machado, A.L.; Augusto, P.E.D.; Polachini, T.C.; Telis-Romero, J. Valorization of brewer spent grains (BSG) through alkaline hydrogen peroxide processing: Effect on composition, structure and rheological properties. Food Bioprod. Process. 2024, 147, 239–250. [Google Scholar] [CrossRef]
- Wang, Z.K.; Liu, Y.; Zhong, J.; Huan, W.; Sheng, J.; Xu, C.; Chen, L.; Shen, X. A waste-free biorefinery pathway to the valorisation of Chinese hickory shell through alkaline hydrogen peroxide pretreatment. Chem. Eng. J. 2023, 476, 146657. [Google Scholar] [CrossRef]
- Bazargan, A.; Wang, Z.; Barford, J.P.; Saleem, J.; McKay, G. Optimization of the removal of lignin and silica from rice husks with alkaline peroxide. J. Clean. Prod. 2020, 260, 120848. [Google Scholar] [CrossRef]
- Cao, J.; Yang, J.; Yang, Y.; Wang, Z. Enhanced enzymatic hydrolysis of sisal waste by sequential pretreatment with UV-catalyzed alkaline hydrogen peroxide and ionic liquid. Renew. Energy 2021, 169, 1157–1165. [Google Scholar] [CrossRef]
- Lin, J.; Wen, P.; Ying, W.; Yu, J.; Zhang, J. Comparison of lactic and propionic acid hydrolysis for production of xylo-oligosaccharides and ethanol from polysaccharides in Toona sinensis branch. Int. J. Biol. Macromol. 2024, 270, 132339. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, H.; Lin, Q.; Zhu, R.; Zhao, L.; Wang, X.; Ren, J.; Meng, L. Fractionation of lignin and fermentable sugars from wheat straw using an alkaline hydrogen peroxide/pentanol biphasic pretreatment. J. Biotechnol. 2024, 396, 62–71. [Google Scholar] [CrossRef]
- Carvalheiro, F.; Duarte, L.; Gírio, F.; Moniz, P. Hydrothermal Liquid Hot Water Pretreatment (Autohydrolysis). In Biomass Fractionation Technologies for a Lignocellulosic Feedstock Based Biorefinery; Elsevier: Amsterdam, The Netherlands, 2016; pp. 315–347. [Google Scholar] [CrossRef]
- Zhuang, X.; Wang, W.; Yu, Q.; Qi, W.; Wang, Q.; Tan, X.; Zhou, G.; Yuan, Z. Liquid hot water pretreatment of lignocellulosic biomass for bioethanol production accompanying with high valuable products. Bioresour. Technol. 2016, 199, 68–75. [Google Scholar] [CrossRef]
- Sarker, T.R.; Pattnaik, F.; Nanda, S.; Dalai, A.K.; Meda, V.; Naik, S. Hydrothermal pretreatment technologies for lignocellulosic biomass: A review of steam explosion and subcritical water hydrolysis. Chemosphere 2021, 284, 131372. [Google Scholar] [CrossRef]
- Gomez-Contreras, P.A.; Obando, C.; Freitas, P.A.V.d.; Martin-Perez, L.; Chiralt, A.; Gonzalez-Martinez, C. Applying Subcritical Water Extraction to Obtain Bioactive Compounds and Cellulose Fibers from Brewer Spent Grains. Molecules 2024, 29, 4897. [Google Scholar] [CrossRef]
- Monteiro, C.R.; Ávila, P.F.; Pereira, M.A.F.; Pereira, G.N.; Bordignon, S.E.; Zanella, E.; Stambuk, B.U.; de Oliveira, D.; Goldbeck, R.; Poletto, P. Hydrothermal treatment on depolymerization of hemicellulose of mango seed shell for the production of xylooligosaccharides. Carbohydr. Polym. 2021, 253, 117274. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.M.; Strieder, M.M.; Saldaña, M.D.A.; Rostagno, M.A.; Forster-Carneiro, T. Recent advances in the processing of agri-food by-products by subcritical water. Food Bioprocess Technol. 2023, 16, 2705–2724. [Google Scholar] [CrossRef]
- Draszewski, C.P.; Bragato, C.A.; Lachos-Perez, D.; Celante, D.; Frizzo, C.P.; Castilhos, F.; Tres, M.V.; Zabot, G.L.; Abaide, E.R.; Mayer, F.D. Subcritical water hydrolysis of rice husks pretreated with deep eutectic solvent for enhance fermentable sugars production. J. Supercrit. Fluids 2021, 178, 105355. [Google Scholar] [CrossRef]
- Roy, R.; Hewetson, B.; Schooff, B.; Bandi, S.; LaTour, P.; Iverson, B.D.; Fry, A. Steam explosion treated biomass as a renewable fuel source: A review from collection to combustion. Fuel 2024, 378, 132883. [Google Scholar] [CrossRef]
- Yu, Y.; Wu, J.; Ren, X.; Lau, A.; Rezaei, H.; Takada, M.; Bi, X.; Sokhansanj, S. Steam explosion of lignocellulosic biomass for multiple advanced bioenergy processes: A review. Renew. Sustain. Energy Rev. 2022, 154, 111871. [Google Scholar] [CrossRef]
- Hoang, A.T.; Nguyen, X.P.; Duong, X.Q.; Agbulut, U.; Len, C.; Nguyen, P.Q.P.; Kchaou, M.; Chen, W.H. Steam explosion as sustainable biomass pretreatment technique for biofuel production: Characteristics and challenges. Bioresour. Technol. 2023, 385, 129398. [Google Scholar] [CrossRef] [PubMed]
- Warren-Walker, D.; Ravella, S.R.; Gallagher, J.; Winters, A.; Charlton, A.; Bryant, D.N. Optimising parameters for pilot scale steam explosion and continuous pressurised disc refining of Miscanthus and sugarcane bagasse for xylose and xylo-oligosaccharide release. Bioresour. Technol. 2024, 405, 130932. [Google Scholar] [CrossRef]
- Huerta, R.R.; Saldaña, M.D. Pressurized fluid treatment of barley and canola straws to obtain carbohydrates and phenolics. J. Supercrit. Fluids 2018, 141, 12–20. [Google Scholar] [CrossRef]
- Bianco, F.; Senol, H.; Papirio, S. Enhanced lignocellulosic component removal and biomethane potential from chestnut shell by a combined hydrothermal-alkaline pretreatment. Sci. Total Environ. 2021, 762, 144178. [Google Scholar] [CrossRef]
- Machmudah, S.; Wahyudiono, W.; Kanda, H.; Sasaki, M.; Goto, M. Hot compressed water extraction of lignin by using a flow-through reactor. Eng. J. 2015, 19, 25–44. [Google Scholar] [CrossRef]
- Silva, T.A.L.; Zamora, H.D.Z.; Varaão, L.H.R.; Prado, N.S.; Baffi, M.A.; Pasquini, D. Effect of steam explosion pretreatment catalysed by organic acid and alkali on chemical and structural properties and enzymatic hydrolysis of sugarcane bagasse. Waste Biomass Valorization 2017, 9, 2191–2201. [Google Scholar] [CrossRef]
- Gurgel, L.V.A.; Pimenta, M.T.B.; Curvelo, A.A.d.S. Ethanol–water organosolv delignification of liquid hot water (LHW) pretreated sugarcane bagasse enhanced by high pressure carbon dioxide (HP–CO2). Ind. Crop. Prod. 2016, 94, 942–950. [Google Scholar] [CrossRef]
- Brenelli, L.B.; Bhatia, R.; Djajadi, D.T.; Thygesen, L.G.; Rabelo, S.C.; Leak, D.J.; Franco, T.T.; Gallagher, J.A. Xylo-oligosaccharides, fermentable sugars, and bioenergy production from sugarcane straw using steam explosion pretreatment at pilot-scale. Bioresour. Technol. 2022, 357, 127093. [Google Scholar] [CrossRef]
- Mihiretu, G.T.; Chimphango, A.F.; Görgens, J.F. Steam explosion pre-treatment of alkali-impregnated lignocelluloses for hemicelluloses extraction and improved digestibility. Bioresour. Technol. 2019, 294, 122121. [Google Scholar] [CrossRef]
- Wu, X.; Huang, C.; Zhai, S.; Liang, C.; Huang, C.; Lai, C.; Yong, Q. Improving enzymatic hydrolysis efficiency of wheat straw through sequential autohydrolysis and alkaline post-extraction. Bioresour. Technol. 2018, 251, 374–380. [Google Scholar] [CrossRef] [PubMed]
- Serna-Loaiza, S.; Dias, M.; Daza-Serna, L.; de Carvalho, C.C.C.R.; Friedl, A. Integral analysis of liquid-hot-water pretreatment of wheat straw: Evaluation of the production of sugars, degradation products, and lignin. Sustainability 2021, 14, 362. [Google Scholar] [CrossRef]
- Escobar, E.L.N.; da Silva, T.A.; Pirich, C.L.; Corazza, M.L.; Pereira Ramos, L. Supercritical fluids: A promising technique for biomass pretreatment and fractionation. Front. Bioeng. Biotechnol. 2020, 8, 252. [Google Scholar] [CrossRef]
- Takada, M.; Minami, E.; Kawamoto, H. Topochemistry of the delignification of japanese beech (Fagus Crenata) Wood Supercrit. Methanol Treat. ACS Omega 2021, 6, 20924–20930. [Google Scholar] [CrossRef] [PubMed]
- Bertran-Llorens, S.; Perondi, F.; Slama de Freitas, A.L.; Chen, J.; van Erven, G.; Deuss, P.J. Supercritical CO2 as effective wheat straw pretreatment for subsequent mild fractionation strategies. Chem. Eng. J. 2024, 497, 154491. [Google Scholar] [CrossRef]
- Lv, H.; Yan, L.; Zhang, M.; Geng, Z.; Ren, M.; Sun, Y. Influence of supercritical CO2 pretreatment of corn stover with ethanol–water as co–solvent on lignin degradation. Chem. Eng. Technol. 2013, 36, 1899–1906. [Google Scholar] [CrossRef]
- Pasquini, D.; Pimenta, M.T.B.; Ferreira, L.H.; Curvelo, A.A.d.S. Extraction of lignin from sugar cane bagasse and Pinus taeda wood chips using ethanol–water mixtures and carbon dioxide at high pressures. J. Supercrit. Fluids 2005, 36, 31–39. [Google Scholar] [CrossRef]
- Rosero-Henao, J.C.; Bueno, B.E.; de Souza, R.; Ribeiro, R.; Lopes de Oliveira, A.; Gomide, C.A.; Gomes, T.M.; Tommaso, G. Potential benefits of near critical and supercritical pretreatment of lignocellulosic biomass towards anaerobic digestion. Waste Manag. Res. J. A Sustain. Circ. Econ. 2018, 37, 74–82. [Google Scholar] [CrossRef]
- Ashokkumar, V.; Venkatkarthick, R.; Jayashree, S.; Chuetor, S.; Dharmaraj, S.; Kumar, G.; Chen, W.H.; Ngamcharussrivichai, C. Recent advances in lignocellulosic biomass for biofuels and value-added bioproducts. A critical review. Bioresour. Technol. 2022, 344, 126195. [Google Scholar] [CrossRef]
- Banu Jamaldheen, S.; Kurade, M.B.; Basak, B.; Yoo, C.G.; Oh, K.K.; Jeon, B.H.; Kim, T.H. A review on physico-chemical delignification as a pretreatment of lignocellulosic biomass for enhanced bioconversion. Bioresour. Technol. 2022, 346, 126591. [Google Scholar] [CrossRef]
- Li, C.; Cheng, G.; Balan, V.; Kent, M.S.; Ong, M.; Chundawat, S.P.; Sousa, L.d.; Melnichenko, Y.B.; Dale, B.E.; Simmons, B.A.; et al. Influence of physico–chemical changes on enzymatic digestibility of ionic liquid and AFEX pretreated corn stover. Bioresour. Technol. 2011, 102, 6928–6936. [Google Scholar] [CrossRef] [PubMed]
- Perez-Pimienta, J.A.; Flores-Gómez, C.A.; Ruiz, H.A.; Sathitsuksanoh, N.; Balan, V.; da Costa Sousa, L.; Dale, B.E.; Singh, S.; Simmons, B.A. Evaluation of agave bagasse recalcitrance using AFEX, autohydrolysis, and ionic liquid pretreatments. Bioresour. Technol. 2016, 211, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Garver, M.P.; Liu, S. Development of thermochemical and biochemical technologies for biorefineries. In Bioenergy Research: Advances and Applications; Elsevier: Amsterdam, The Netherlands, 2014; pp. 457–488. [Google Scholar] [CrossRef]
- Kumar, A.K.; Sharma, S. Recent updates on different methods of pretreatment of lignocellulosic feedstocks: A review. Bioresour. Bioprocess. 2017, 4, 7. [Google Scholar] [CrossRef]
- Zhao, C.; Ding, W.; Chen, F.; Cheng, C.; Shao, Q. Effects of compositional changes of AFEX-treated and H-AFEX-treated corn stover on enzymatic digestibility. Bioresour. Technol. 2014, 155, 34–40. [Google Scholar] [CrossRef]
- Hu, M.; Cai, Z.; Zhang, J.; Fu, Q.; Yuan, L.; Ji, D. Ammonia fiber expansion (AFEX) pretreatment combined with white rot fungi treatment to improve enzymatic hydrolysis of lignocellulose. SSRN Electron. J. 2022. [Google Scholar] [CrossRef]
- Hu, M.; Yuan, L.; Cai, Z.; Zhang, W.; Fu, Q.; Ji, D. Ammonia fiber expansion-assisted deep eutectic solvent treatment for wheat straw fraction separation and bioconversion. Bioresour. Technol. 2023, 367, 128242. [Google Scholar] [CrossRef]
- Sun, X.; Liu, S.; Zhang, X.; Tao, Y.; Boczkaj, G.; Yoon, J.Y.; Xuan, X. Recent advances in hydrodynamic cavitation-based pretreatments of lignocellulosic biomass for valorization. Bioresour. Technol. 2022, 345, 126251. [Google Scholar] [CrossRef] [PubMed]
- Terán Hilares, R.; Ienny, J.a.V.; Marcelino, P.F.; Ahmed, M.A.; Antunes, F.A.; da Silva, S.S.; Santos, J.C.d. Ethanol production in a simultaneous saccharification and fermentation process with interconnected reactors employing hydrodynamic cavitation–pretreated sugarcane bagasse as raw material. Bioresour. Technol. 2017, 243, 652–659. [Google Scholar] [CrossRef] [PubMed]
- Iskalieva, A.; Yimmou, B.M.; Gogate, P.R.; Horvath, M.; Horvath, P.G.; Csoka, L. Cavitation assisted delignification of wheat straw: A review. Ultrason. Sonochem. 2012, 19, 984–993. [Google Scholar] [CrossRef] [PubMed]
- Meoli, C.M.; Iervolino, G.; Procentese, A. Non–Thermal Plasma as a Biomass Pretreatment in Biorefining Processes. Processes 2023, 11, 536. [Google Scholar] [CrossRef]
- Pereira, G.N.; Cesca, K.; Cubas, A.L.V.; Bianchet, R.T.; Junior, S.E.B.; Zanella, E.; Stambuk, B.U.; Poletto, P.; de Oliveira, D. Non–thermal plasma as an innovative pretreatment technology in delignification of brewery by-product. Innov. Food Sci. Emerg. Technol. 2021, 74, 102827. [Google Scholar] [CrossRef]
- Margellou, A.G.; Pappa, C.P.; Psochia, E.A.; Petala, M.D.; Triantafyllidis, K.S. Mild isolation and characterization of surface lignin from hydrothermally pretreated lignocellulosic forestry and agro-industrial waste biomass. Sustain. Chem. Pharm. 2023, 33, 101056. [Google Scholar] [CrossRef]
- Latif, N.H.A.; Brosse, N.; Ziegler-Devin, I.; Chrusiel, L.; Hashim, R.; Hussin, M.H. Structural characterization of modified coconut husk lignin via steam explosion pretreatment as a renewable phenol substitutes. Int. J. Biol. Macromol. 2023, 253, 127210. [Google Scholar] [CrossRef]
- Grbić, J.Z.; Mladenović, D.D.; Veljković, M.B.; Lazarević, S.S.; Levic, S.M.; Lazovic, S.S.; Djukic-Vukovic, A.P. Cold plasma/alkaline pretreatment facilitates corn stalk fractionation and valorization towards zero-waste approach. Ind. Crop. Prod. 2024, 222, 119498. [Google Scholar] [CrossRef]
- Ntakirutimana, S.; Xu, T.; Ding, M.Z.; Liu, Z.H.; Li, B.Z.; Yuan, Y.J. Chemomechanical pretreatment for efficient delignification and saccharification of corn stover biomass. Chem. Eng. J. 2023, 471, 144588. [Google Scholar] [CrossRef]
- Maté, I.; Vargas, M.; Atarés, L.; Chiralt, A. Fractionation of winemaking grape stalks by subcritical water extraction to obtain added-value products. Foods 2024, 13, 3566. [Google Scholar] [CrossRef]
- Moya, A.J.; Peinado, S.; Mateo, S.; Fonseca, B.G.; Sánchez, S. Improving bioethanol production from olive pruning biomass by deacetylation step prior acid hydrolysis and fermentation processes. Bioresour. Technol. 2016, 220, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Mateo, S.; Fabbrizi, G.; Chapeta, M.R.; Moya, A.J. Optimization of microwave-assisted organosolv cellulose recovery from olive-tree pruning with three different solvents. Appl. Sci. 2024, 14, 10670. [Google Scholar] [CrossRef]
- Andhalkar, V.V.; Montané, D.; Medina, F.; Constantí, M. Biorefinery design with combined deacetylation and microwave pretreatment for enhanced production of polyhydroxyalkanoates and efficient carbon utilization from lignocellulose. Chem. Eng. J. 2024, 486, 149754. [Google Scholar] [CrossRef]
- Chakraborty, P.; Kumar, R.; Banerjee, A.; Chakrabortty, S.; Pal, M.; Upadhyaya, A.; Chowdhury, S.; Khan, M.A.; Jeon, B.H.; Tripathy, S.K.; et al. Advancing biorefineries with ultrasonically assisted ionic liquid-based delignification: Optimizing biomass processing for enhanced bio-based product yields. Biomass Bioenergy 2025, 192, 107495. [Google Scholar] [CrossRef]
- Kaur, A.; Kuhad, R.C. Valorization of rice straw for ethanol production and lignin recovery using combined acid-alkali pretreatment. BioEnergy Res. 2019, 12, 570–582. [Google Scholar] [CrossRef]
- Kim, J.W.; Kim, K.S.; Lee, J.S.; Park, S.M.; Cho, H.Y.; Park, J.C.; Kim, J.S. Two-stage pretreatment of rice straw using aqueous ammonia and dilute acid. Bioresour. Technol. 2011, 102, 8992–8999. [Google Scholar] [CrossRef] [PubMed]
- Cardoza, D.; Contreras, M.d.M.; Lara-Serrano, M.; Morales-delaRosa, S.; Campos-Martín, J.M.; Romero, I.; Castro, E. Sustainable vine shoots-to-ethanol valorisation by a sequential acid/organosolv pretreatment. Process Saf. Environ. Prot. 2024, 183, 1059–1070. [Google Scholar] [CrossRef]
- Aftab, M.N.; Iqbal, I.; Riaz, F.; Karadag, A.; Tabatabaei, M. Different pretreatment methods of lignocellulosic niomass for use in biofuel production. In Biomass for Bioenergy: Recent Trends and Future Challenges; InTech Open: Houston, TX, USA, 2019; pp. 15–38. [Google Scholar]
- Ullah, M.; Liu, P.; Xie, S.; Sun, S. Recent advancements and challenges in lignin valorization: Green routes towards sustainable bioproducts. Molecules 2022, 27, 6055. [Google Scholar] [CrossRef]
- Arapova, O.V.; Chistyakov, A.V.; Tsodikov, M.V.; Moiseev, I.I. Lignin as a renewable resource of hydrocarbon products and energy carriers (a review). Pet. Chem. 2020, 60, 227–243. [Google Scholar] [CrossRef]
- Yogalakshmi, K.N.; Poornima, D.T.; Sivashanmugam, P.; Kavitha, S.; Yukesh Kannah, R.; Varjani, S.; AdishKumar, S.; Kumar, G.; Rajesh, B.J. Lignocellulosic biomass-based pyrolysis: A comprehensive review. Chemosphere 2022, 286, 131824. [Google Scholar] [CrossRef]
- Pahnila, M.; Koskela, A.; Sulasalmi, P.; Fabritius, T. A review of pyrolysis technologies and the effect of process parameters on biocarbon properties. Energies 2023, 16, 6936. [Google Scholar] [CrossRef]
- Alvarez-Chavez, B.J.; Godbout, S.; Palacios-Rios, J.H.; Le Roux, E.; Raghavan, V. Physical, chemical, thermal and biological pretreatment technologies in fast pyrolysis to maximize bio–oil quality: A critical review. Biomass Bioenergy 2019, 128, 105333. [Google Scholar] [CrossRef]
- Yiin, C.L.; Yusup, S.; Udomsap, P.; Yoosuk, B.; Sukkasi, S. Stabilization of empty fruit bunch (EFB) derived bio-oil using antioxidants. In 24th European Symposium on Computer Aided Process Engineering; Elsevier: Amsterdam, The Netherlands, 2014; pp. 223–228. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Leng, F.; Chen, J.; Zhu, L.; Luo, Z. Separation and characterization of pyrolytic lignins from the heavy fraction of bio-oil by molecular distillation. Sep. Purif. Technol. 2015, 152, 123–132. [Google Scholar] [CrossRef]
- Lu, X.; Gu, X. A review on lignin pyrolysis: Pyrolytic behavior, mechanism, and relevant upgrading for improving process efficiency. Biotechnol. Biofuels Bioprod. 2022, 15, 106. [Google Scholar] [CrossRef]
- Cao, L.; Zhang, C.; Chen, H.; Tsang, D.C.; Luo, G.; Zhang, S.; Chen, J. Hydrothermal liquefaction of agricultural and forestry wastes: State of the art review and future prospects. Bioresour. Technol. 2017, 245, 1184–1193. [Google Scholar] [CrossRef]
- Antonio, P.; Maurizio, V.; Fabio, C.L.; Antonio, M. Hydrothermal liquefaction of agro-waste biomass: Design of a novel experimental set up and preliminary results. Chem. Eng. Trans. 2022, 92, 145–150. [Google Scholar] [CrossRef]
- Silva, R.S.; da Silva, R.A.; de Andrade, F.M.; Acácio Neto, P.N.; do Nascimento, R.M.; Santos, J.M.; Stragevitch, L.; Pimentel, M.F.; Simoes, D.A.; Danielski, L. Hydrothermal liquefaction of sugarcane bagasse and straw: Effect of operational conditions on product fractionation and bio-oil zcomposition. Energies 2024, 17, 5439. [Google Scholar] [CrossRef]
- Dash, S.; Bhavanam, A.; Gera, P. Catalytic hydrothermal liquefaction of wheat straw black liquor: Product analysis and reaction pathways. Waste Biomass Valorization 2025, 1, 1–15. [Google Scholar] [CrossRef]
- Sewsynker-Sukai, Y.; Naomi David, A.; Gueguim Kana, E. Recent developments in the application of kraft pulping alkaline chemicals for lignocellulosic pretreatment: Potential beneficiation of green liquor dregs waste. Bioresour. Technol. 2020, 306, 123225. [Google Scholar] [CrossRef]
- Menezes, F.; Rocha, G.; Filho, R.M. Obtainment and characterization of lignin from enzymatic hydrolysis of sugarcane bagasse of 2G process in pilot scale. Chem. Eng. Trans. 2016, 50, 397–402. [Google Scholar] [CrossRef]
- Gu, X.; Fu, X.; Chen, S. Hydrothermal liquefaction conversion of lignocelluloses with enhanced fungal pretreatment. Ind. Crop. Prod. 2021, 162, 113268. [Google Scholar] [CrossRef]
- Andlar, M.; Rezic, T.; Mardetko, N.; Kracher, D.; Ludwig, R.; Santek, B. Lignocellulose degradation: An overview of fungi and fungal enzymes involved in lignocellulose degradation. Eng. Life Sci. 2018, 18, 768–778. [Google Scholar] [CrossRef] [PubMed]
- Saritha, M.; Arora, A.; Lata. Biological pretreatment of lignocellulosic substrates for enhanced delignification and enzymatic digestibility. Indian J. Microbiol. 2011, 52, 122–130. [Google Scholar] [CrossRef]
- Subramaniam, S.; Karunanandham, K.; ASM, R.; Uthandi, S. Delignification of the cotton stalk and ginning mill waste via EnZolv pretreatment and optimization of process parameters using response surface methodology (RSM). Bioresour. Technol. 2023, 387, 129655. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Zhou, W.; Zhang, Y.; Huang, Q.; Hu, Z.; Ouyang, L. Natural lignin as efficient catalyst for H2 production from NaBH4 methanolysis at low temperature. J. Environ. Chem. Eng. 2024, 12, 111615. [Google Scholar] [CrossRef]
- Tomani, P. The lignoboost process. Cellul. Chem. Technol. 2010, 44, 53–58. [Google Scholar]
- Suota, M.J.; da Silva, T.A.; Zawadzki, S.F.; Sassaki, G.L.; Hansel, F.A.; Paleologou, M.; Ramos, L.P. Chemical and structural characterization of hardwood and softwood LignoForce lignins. Ind. Crop. Prod. 2021, 173, 114138. [Google Scholar] [CrossRef]
- Rahman, M.S.; Roy, R.; Montoya, C.; Halim, M.A.; Raynie, D.E. Acidic and basic amino acid-based novel deep eutectic solvents and their role in depolymerization of lignin. J. Mol. Liq. 2022, 362, 119751. [Google Scholar] [CrossRef]
- Pei, Z.; Liu, X.; Chen, J.; Wang, H.; Li, H. Research progress on lignin depolymerization strategies: A review. Polymers 2024, 16, 2388. [Google Scholar] [CrossRef]
- Asawaworarit, P.; Daorattanachai, P.; Laosiripojana, W.; Sakdaronnarong, C.; Shotipruk, A.; Laosiripojana, N. Catalytic depolymerization of organosolv lignin from bagasse by carbonaceous solid acids derived from hydrothermal of lignocellulosic compounds. Chem. Eng. J. 2019, 356, 461–471. [Google Scholar] [CrossRef]
- Peng, M.; Nakabayashi, M.; Kim, K.; Kamiya, K.; Qian, E.W. Lignin depolymerization with alkaline ionic liquids and ethylene glycol in a continuous flow reactor. Fuel 2023, 335, 126960. [Google Scholar] [CrossRef]
- Dai, J.; Patti, A.F.; Longé, L.; Garnier, G.; Saito, K. Oxidized lignin depolymerization using formate ionic liquid as catalyst and solvent. ChemCatChem 2017, 9, 2684–2690. [Google Scholar] [CrossRef]
- Antunes, F.; Mota, I.F.; da Silva Burgal, J.a.; Pintado, M.; Costa, P.S. A review on the valorization of lignin from sugarcane by-products: From extraction to application. Biomass Bioenergy 2022, 166, 106603. [Google Scholar] [CrossRef]
- Solihat, N.N.; Pramasari, D.A.; Laksana, R.P.B.; Restu, W.K.; Ghozali, M.; Triwulandari, E.; Fatriasari, W.; Watanabe, T. Synthesis of lignin-based biosurfactant derived from kraft black liquor and its effect on enzymatic hydrolysis of pretreated biomass. Sustain. Chem. Pharm. 2023, 34, 101152. [Google Scholar] [CrossRef]
- Shen, F.; He, C.; Wang, Y.; Hu, J.; Huang, M.; Zhao, L.; Zhang, S.; Tian, D.; Shen, F. Fully upgrade lignocellulose to three nanomaterials by combinational pretreatment: Refining straw waste to pesticide nanocarrier. Chem. Eng. J. 2023, 467, 143376. [Google Scholar] [CrossRef]
- Adamovic, T.; Tarasov, D.; Demirkaya, E.; Balakshin, M.; Cocero, M.J. A feasibility study on green biorefinery of high lignin content agro-food industry waste through supercritical water treatment. J. Clean. Prod. 2021, 323, 129110. [Google Scholar] [CrossRef]
- Wang, N.; Xu, A.; Liu, K.; Zhao, Z.; Li, H.; Gao, X. Performance of green solvents in microwave-assisted pretreatment of lignocellulose. Chem. Eng. J. 2024, 482, 148786. [Google Scholar] [CrossRef]
- Mesquita, R.M.F.d.; Dal Prá, J.C.; Fontana, R.C.; Montipó, S.; Baudel, H.M.; Diebold, E.; Schneider, W.D.H.; Camassola, M. Processing of persimmon tree pruning waste through the circular economy: Lignin nanoparticles and cellulosic ethanol production. Renew. Energy 2025, 238, 121932. [Google Scholar] [CrossRef]
- Liu, H.; Chen, X.; Ni, S.; Li, Z.; Liu, N.; Fu, Y. Role of lignin removal on the properties of crude pulp fibers from corn stover via high-temperature formic acid pulping. Int. J. Biol. Macromol. 2025, 287, 138435. [Google Scholar] [CrossRef]
- Patel, R.; Dhar, P.; Babaei-Ghazvini, A.; Nikkhah Dafchahi, M.; Acharya, B. Transforming lignin into renewable fuels, chemicals, and materials: A review. Bioresour. Technol. Rep. 2023, 22, 101463. [Google Scholar] [CrossRef]
- Brienza, F.; Cannella, D.; Montesdeoca, D.; Cybulska, I.; Debecker, D.P. A guide to lignin valorization in biorefineries: Traditional, recent, and forthcoming approaches to convert raw lignocellulose into valuable materials and chemicals. RSC Sustain. 2024, 2, 37–90. [Google Scholar] [CrossRef]
- Sharma, S.; Moharana, S.; Sharma, A.K.; Srivastava, A. Development of lignin-based biodegradable polymer from Agro-waste. Mater. Chem. 2024. [Google Scholar] [CrossRef]
- Abdelaziz, O.Y.; Clemmensen, I.; Meier, S.; Costa, C.A.E.; Rodrigues, A.E.; Hulteberg, C.P.; Riisager, A. On the oxidative valorization of lignin to high-value chemicals: A critical review of opportunities and challenges. ChemSusChem 2022, 15, e202201232. [Google Scholar] [CrossRef] [PubMed]
- Lyu, G.; Yoo, C.G.; Pan, X. Alkaline oxidative cracking for effective depolymerization of biorefining lignin to mono-aromatic compounds and organic acids with molecular oxygen. Biomass Bioenergy 2018, 108, 7–14. [Google Scholar] [CrossRef]
- Ouyang, X.; Ruan, T.; Qiu, X. Effect of solvent on hydrothermal oxidation depolymerization of lignin for the production of monophenolic compounds. Fuel Process. Technol. 2016, 144, 181–185. [Google Scholar] [CrossRef]
- Wang, X.; Yao, B.; Su, X. Linking enzymatic oxidative degradation of lignin to organics detoxification. Int. J. Mol. Sci. 2018, 19, 3373. [Google Scholar] [CrossRef]
- Zhang, S.; Xiao, J.; Wang, G.; Chen, G. Enzymatic hydrolysis of lignin by ligninolytic enzymes and analysis of the hydrolyzed lignin products. Bioresour. Technol. 2020, 304, 122975. [Google Scholar] [CrossRef]
- Kim, G.H.; Kim, Y.I.; Um, B.H. Base-catalyzed depolymerization of organosolv lignin into monoaromatic phenolic compounds. Waste Biomass Valorization 2024, 16, 257–270. [Google Scholar] [CrossRef]
- Li, J.; Zhang, J.; Zhang, S.; Gao, Q.; Li, J.; Zhang, W. Alkali lignin depolymerization under eco-friendly and cost-effective NaOH/urea aqueous solution for fast curing bio-based phenolic resin. Ind. Crop. Prod. 2018, 120, 25–33. [Google Scholar] [CrossRef]
- Hidajat, M.J.; Riaz, A.; Park, J.; Insyani, R.; Verma, D.; Kim, J. Depolymerization of concentrated sulfuric acid hydrolysis lignin to high-yield aromatic monomers in basic sub– and supercritical fluids. Chem. Eng. J. 2017, 317, 9–19. [Google Scholar] [CrossRef]
- Pérez, E.; Abad-Fernández, N.; Lourençon, T.; Balakshin, M.; Sixta, H.; Cocero, M.J. Base-catalysed depolymerization of lignins in supercritical water: Influence of lignin nature and valorisation of pulping and biorefinery by-products. Biomass Bioenergy 2022, 163, 106536. [Google Scholar] [CrossRef]
- Guo, D.; Liu, B.; Tang, Y.; Zhang, J.; Xia, X.; Tong, S. Catalytic depolymerization of alkali lignin in sub- and super-critical ethanol. BioResources 2017, 12, 5001–5016. [Google Scholar] [CrossRef]
- Luo, J.; Liu, T.L. Electrochemical valorization of lignin: Status, challenges, and prospects. J. Bioresour. Bioprod. 2023, 8, 1–14. [Google Scholar] [CrossRef]
- Lindenbeck, L.M.; Dahlhaus, S.; Wende, L.M.; Beele, B.B.; Frauscher, M.; Schebb, N.H.; Lehmann, C.W.; Bornhorst, J.; Slabon, A.; Rodrigues, B.V.M. Breaking down lignin in gamma-valerolactone: Advances into a bioelectrorefinery. Green Chem. Lett. Rev. 2024, 17, 2390867. [Google Scholar] [CrossRef]
- Dier, T.K.F.; Rauber, D.; Durneata, D.; Hempelmann, R.; Volmer, D.A. Sustainable electrochemical depolymerization of lignin in reusable ionic liquids. Sci. Rep. 2017, 7, 5041. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.S. Production, separation and applications of phenolic-rich bio-oil—A review. Bioresour. Technol. 2015, 178, 90–98. [Google Scholar] [CrossRef]
- Hu, X.; Gholizadeh, M. Progress of the applications of bio-oil. Renew. Sustain. Energy Rev. 2020, 134, 110124. [Google Scholar] [CrossRef]
- Bu, Q.; Lei, H.; Wang, L.; Wei, Y.; Zhu, L.; Zhang, X.; Liu, Y.; Yadavalli, G.; Tang, J. Bio-based phenols and fuel production from catalytic microwave pyrolysis of lignin by activated carbons. Bioresour. Technol. 2014, 162, 142–147. [Google Scholar] [CrossRef]
- Tang, D.; Huang, X.; Tang, W.; Jin, Y. Lignin-to-chemicals: Application of catalytic hydrogenolysis of lignin to produce phenols and terephthalic acid via metal-based catalysts. Int. J. Biol. Macromol. 2021, 190, 72–85. [Google Scholar] [CrossRef]
- Castro Garcia, A.; Cheng, S.; Cross, J.S. Lignin gasification: Current and future viability. Energies 2022, 15, 9062. [Google Scholar] [CrossRef]
- Biswas, B.; Kumar, A.; Krishna, B.B.; Bhaskar, T. Effects of solid base catalysts on depolymerization of alkali lignin for the production of phenolic monomer compounds. Renew. Energy 2021, 175, 270–280. [Google Scholar] [CrossRef]
- Yang, C.; Qin, J.; Sun, S.; Gao, D.; Fang, Y.; Chen, G.; Tian, C.; Bao, C.; Zhang, S. Progress in developing methods for lignin depolymerization and elucidating the associated mechanisms. Eur. Polym. J. 2024, 210, 112995. [Google Scholar] [CrossRef]
- Janusz, G.; Pawlik, A.; Sulej, J.; Swiderska-Burek, U.; Jarosz-Wilkolazka, A.; Paszczynski, A. Lignin degradation: Microorganisms, enzymes involved, genomes analysis and evolution. FEMS Microbiol. Rev. 2017, 41, 941–962. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.P.; Sridhar, M.; Rao, R.G. iological depolymerization of lignin using laccase harvested from the autochthonous fungus Schizophyllum commune employing various production methods and its efficacy in augmenting in vitro digestibility in ruminants. Sci. Rep. 2022, 12, 11170. [Google Scholar] [CrossRef]
- Lu, Y.; Lu, Y.C.; Hu, H.Q.; Xie, F.J.; Wei, X.Y.; Fan, X. Structural characterization of lignin and its degradation products with spectroscopic methods. J. Spectrosc. 2017, 2017, 8951658. [Google Scholar] [CrossRef]
- De Santi, A.; Monti, S.; Barcaro, G.; Zhang, Z.; Barta, K.; Deuss, P.J. New mechanistic insights into the lignin β-O-4 linkage acidolysis with ethylene glycol stabilization aided by multilevel computational chemistry. ACS Sustain. Chem. Eng. 2021, 9, 2388–2399. [Google Scholar] [CrossRef] [PubMed]
- Stark, N.M.; Yelle, D.J.; Agarwal, U.P. Techniques for characterizing lignin. In Lignin in Polymer Composites; Elsevier: Amsterdam, The Netherlands, 2016; pp. 49–66. [Google Scholar] [CrossRef]
- Ali, S.; Rani, A.; Dar, M.; Qaisrani, M.; Noman, M.; Yoganathan, K.; Asad, M.; Berhanu, A.; Barwant, M.; Zhu, D. Recent advances in characterization and valorization of lignin and its value-added products: Challenges and future perspectives. Biomass 2024, 4, 947–977. [Google Scholar] [CrossRef]
- Sun, R.C. Lignin source and structural characterization. ChemSusChem 2020, 13, 4385–4393. [Google Scholar] [CrossRef]
- Sen, S.; Patil, S.; Argyropoulos, D.S. Thermal properties of lignin in copolymers, blends, and composites: A review. Green Chem. 2015, 17, 4862–4887. [Google Scholar] [CrossRef]
- Hatakeyama, H.; Tsujimoto, Y.; Zarubin, M.J.; Krutov, S.M.; Hatakeyama, T. Thermal decomposition and glass transition of industrial hydrolysis lignin. J. Therm. Anal. Calorim. 2010, 101, 289–295. [Google Scholar] [CrossRef]
- Apaydin Varol, E.; Mutlu, U. TGA-FTIR Analysis of biomass samples based on the thermal decomposition behavior of hemicellulose, cellulose, and lignin. Energies 2023, 16, 3674. [Google Scholar] [CrossRef]
- Cavalaglio, G.; Fabbrizi, G.; Cardelli, F.; Lorenzi, L.; Angrisano, M.; Nicolini, A. Lignocellulosic residues from fruit trees: Availability, characterization, and energetic potential valorization. Energies 2024, 17, 2611. [Google Scholar] [CrossRef]
- Goudarzi, A.; Lin, L.T.; Ko, F.K. X-Ray diffraction analysis of Kraft lignins and lignin-derived carbon nanofibers. J. Nanotechnol. Eng. Med. 2014, 5, 021006. [Google Scholar] [CrossRef]
- Gomide, R.A.C.; de Oliveira, A.C.S.; Rodrigues, D.A.C.; de Oliveira, C.R.; de Assis, O.B.G.; Dias, M.V.; Borges, S.V. Development and characterization of lignin microparticles for physical and antioxidant enhancement of biodegradable polymers. J. Polym. Environ. 2020, 28, 1326–1334. [Google Scholar] [CrossRef]
- Gea, S.; Siregar, A.H.; Zaidar, E.; Harahap, M.; Indrawan, D.P.; Perangin-Angin, Y.A. Isolation and characterisation of cellulose nanofibre and lignin from oil palm empty fruit bunches. Materials 2020, 13, 2290. [Google Scholar] [CrossRef]
- Majira, A.; Godon, B.; Foulon, L.; van der Putten, J.C.; Cézard, L.; Thierry, M.; Pion, F.; Bado-Nilles, A.; Pandard, P.; Jayabalan, T.; et al. Enhancing the antioxidant activity of technical lignins by combining solvent fractionation and ionic-liquid treatment. ChemSusChem 2019, 12, 4799–4809. [Google Scholar] [CrossRef] [PubMed]
- Constant, S.; Wienk, H.L.J.; Frissen, A.E.; Peinder, P.D.; Boelens, R.; van Es, D.S.; Grisel, R.J.H.; Weckhuysen, B.M.; Huijgen, W.J.J.; Gosselink, R.J.A.; et al. New insights into the structure and composition of technical lignins: A comparative characterisation study. Green Chem. 2016, 18, 2651–2665. [Google Scholar] [CrossRef]
- Lu, Y.; Joosten, L.; Donkers, J.; Andriulo, F.; Slaghek, T.M.; Phillips-Jones, M.K.; Gosselink, R.J.A.; Harding, S.E. Characterisation of mass distributions of solvent-fractionated lignins using analytical ultracentrifugation and size exclusion chromatography methods. Sci. Rep. 2021, 11, 13937. [Google Scholar] [CrossRef]
- You, T.; Xu, F. Applications of Molecular Spectroscopic Methods to the Elucidation of Lignin Structure. In Applications of Molecular Spectroscopy to Current Research in the Chemical and Biological Sciences; InTech: Houston, TX, USA, 2016. [Google Scholar] [CrossRef]
- Popescu, C.M.; Popescu, M.C.; Singurel, G.; Vasile, C.; Argyropoulos, D.S.; Willfor, S. Spectral characterization of eucalyptus wood. Appl. Spectrosc. 2007, 61, 1168–1177. [Google Scholar] [CrossRef]
- Han, X.; Wang, Z.; Ding, L.; Chen, L.; Wang, F.; Pu, J.; Jiang, S. Water molecule-induced hydrogen bonding between cellulose nanofibers toward highly strong and tough materials from wood aerogel. Chin. Chem. Lett. 2021, 32, 3105–3108. [Google Scholar] [CrossRef]
- Han, X.; Wu, W.; Wang, J.; Tian, Z.; Jiang, S. Hydrogen-bonding-aided fabrication of wood derived cellulose scaffold/aramid nanofiber into high-performance bulk material. Materials 2021, 14, 5444. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, U.P.; McSweeny, J.D.; Ralph, S.A. FT-Raman investigation of milled-wood lignins: Softwood, hardwood, and chemically modified black spruce lignins. J. Wood Chem. Technol. 2011, 31, 324–344. [Google Scholar] [CrossRef]
- Huang, Y.; Wang, L.; Chao, Y.; Nawawi, D.S.; Akiyama, T.; Yokoyama, T.; Matsumoto, Y. Analysis of lignin aromatic Structure in wood based on the IR spectrum. J. Wood Chem. Technol. 2012, 32, 294–303. [Google Scholar] [CrossRef]
- Agarwal, U.P.; Ralph, S.A. Determination of ethylenic residues in wood and TMP of spruce by FT-Raman spectroscopy. hfsg 2008, 62, 667–675. [Google Scholar] [CrossRef]
- Constant, S.; Lancefield, C.S.; Weckhuysen, B.M.; Bruijnincx, P.C.A. Quantification and classification of carbonyls in industrial humins and lignins by 19F NMR. ACS Sustain. Chem. Eng. 2017, 5, 965–972. [Google Scholar] [CrossRef]
- Fernández-Costas, C.; Gouveia, S.; Sanromán, M.; Moldes, D. Structural characterization of Kraft lignins from different spent cooking liquors by 1D and 2D Nuclear Magnetic Resonance spectroscopy. Biomass Bioenergy 2014, 63, 156–166. [Google Scholar] [CrossRef]
- Costa, C.A.E.; Pinto, P.C.R.; Rodrigues, A.E. Evaluation of chemical processing impact on E. globuluswood lignin and comparison with bark lignin. Ind. Crop. Prod. 2014, 61, 479–491. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, S.; Yang, G.; Chen, J. Characterization of high-boiling-solvent lignin from hot-water-extracted bagasse. Energy Fuels 2014, 28, 3167–3171. [Google Scholar] [CrossRef]
- Ahvazi, B.; Cloutier, E.; Wojciechowicz, O.; Ngo, T.D. Lignin profiling: A guide for selecting appropriate lignins as precursors in biomaterials development. ACS Sustain. Chem. Eng. 2016, 4, 5090–5105. [Google Scholar] [CrossRef]
- Pu, Y.; Cao, S.; Ragauskas, A.J. Application of quantitative 31P NMR in biomass lignin and biofuel precursors characterization. Energy Environ. Sci. 2011, 4, 3154. [Google Scholar] [CrossRef]
- Zeng, J.; Helms, G.L.; Gao, X.; Chen, S. Quantification of wheat straw lignin structure by comprehensive NMR analysis. J. Agric. Food Chem. 2013, 61, 10848–10857. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Shard, A.G. Quantitative analysis of adsorbed proteins by X-ray photoelectron spectroscopy. Anal. Chem. 2011, 83, 8659–8666. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zheng, F.; Liu, X.; Tang, L.; Xue, G.; Du, G.; Yong, Q.; Chen, M.; Zhu, L. Glass transition of oxygen plasma treated enzymatic hydrolysis lignin. BioResources 2012, 7, 4776–4785. [Google Scholar] [CrossRef]
- Al, K.; Basakçilardan Kabakci, S. Oxygen-rich precursors via glycerol organosolv treatment: Preparation of activated carbon from hazelnut shell and its structural components for possible use in electrodes for supercapacitors. Int. J. Thermofluids 2024, 21, 100588. [Google Scholar] [CrossRef]
- Liu, H.; Xu, T.; Liu, K.; Zhang, M.; Liu, W.; Li, H.; Du, H.; Si, C. Lignin–based electrodes for energy storage application. Ind. Crop. Prod. 2021, 165, 113425. [Google Scholar] [CrossRef]
- Hasanov, I.; Raud, M.; Kikas, T. The role of ionic liquids in the lignin separation from lignocellulosic biomass. Energies 2020, 13, 4864. [Google Scholar] [CrossRef]
- Liu, S.; Cheng, G. Developments and perspectives on lignin-first biomass pretreatment for efficient enzymatic hydrolysis and isolation of lignin with minimized degradation. Ind. Crop. Prod. 2024, 208, 117926. [Google Scholar] [CrossRef]
- Jianfei, Y.; Zixing, F.; Liangmeng, N.; Qi, G.; Zhijia, L. Combustion characteristics of bamboo lignin from kraft pulping: Influence of washing process. Renew. Energy 2020, 162, 525–534. [Google Scholar] [CrossRef]
- Kocaturk, E.; Salan, T.; Ozcelik, O.; Alma, M.H.; Candan, Z. Recent advances in lignin–based biofuel production. Energies 2023, 16, 3382. [Google Scholar] [CrossRef]
- Bi, P.; Wang, J.; Zhang, Y.; Jiang, P.; Wu, X.; Liu, J.; Xue, H.; Wang, T.; Li, Q. From lignin to cycloparaffins and aromatics: Directional synthesis of jet and diesel fuel range biofuels using biomass. Bioresour. Technol. 2015, 183, 10–17. [Google Scholar] [CrossRef]
- Liakakou, E.; Vreugdenhil, B.; Cerone, N.; Zimbardi, F.; Pinto, F.; André, R.; Marques, P.; Mata, R.; Girio, F. Gasification of lignin-rich residues for the production of biofuels via syngas fermentation: Comparison of gasification technologies. Fuel 2019, 251, 580–592. [Google Scholar] [CrossRef]
- Argyropoulos, D.D.S.; Crestini, C.; Dahlstrand, C.; Furusjö, E.; Gioia, C.; Jedvert, K.; Henriksson, G.; Hulteberg, C.; Lawoko, M.; Pierrou, C.; et al. Kraft lignin: A valuable, sustainable resource, opportunities and challenges. ChemSusChem 2023, 16, e202300492. [Google Scholar] [CrossRef] [PubMed]
- Jedrzejczak, P.; Collins, M.N.; Jesionowski, T.; Klapiszewski, L. The role of lignin and lignin-based materials in sustainable construction—A comprehensive review. Int. J. Biol. Macromol. 2021, 187, 624–650. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.; Ji, X.; Wang, Q.; Tian, Z.; Fatehi, P. Production of nanocellulose using acidic deep eutectic solvents based on choline chloride and carboxylic acids: A review. Int. J. Biol. Macromol. 2023, 245, 125227. [Google Scholar] [CrossRef]
- Li, H.; Liang, Y.; Li, P.; He, C. Conversion of biomass lignin to high-value polyurethane: A review. J. Bioresour. Bioprod. 2020, 5, 163–179. [Google Scholar] [CrossRef]
- Yao, H.; Wang, Y.; Liu, J.; Xu, M.; Ma, P.; Ji, J.; You, Z. Review on applications of lignin in pavement engineering: A recent survey. Front. Mater. 2022, 8, 803524. [Google Scholar] [CrossRef]
- Kaya, C.; Stegmaier, T.; Gresser, G.T. Investigation of the protective function of a lignin coating of natural fiber geotextiles against biodegradation. Materials 2023, 16, 4849. [Google Scholar] [CrossRef]
- Vinod, A.; Pulikkalparambil, H.; Jagadeesh, P.; Rangappa, S.M.; Siengchin, S. Recent advancements in lignocellulose biomass-based carbon fiber: Synthesis, properties, and applications. Heliyon 2023, 9, e13614. [Google Scholar] [CrossRef]
- Nadányi, R.; Ház, A.; Lisy, A.; Jablonsky, M.; Surina, I.; Majová, V.; Baco, A. Lignin modifications, applications, and possible market prices. Energies 2022, 15, 6520. [Google Scholar] [CrossRef]
- Ren, Q.; Xue, Y.; Cui, Z.; Lu, Y.; Li, W.; Zhang, W.; Ye, T. Preparation of lignin based phenolic resin microspheres with controllable particle size and its application in capacitors. Diam. Relat. Mater. 2022, 125, 109000. [Google Scholar] [CrossRef]
- Muddasar, M.; Beaucamp, A.; Culebras, M.; Collins, M.N. High performance all lignin derived supercapacitors for energy storage applications. Mater. Today Sustain. 2024, 26, 100767. [Google Scholar] [CrossRef]
- Chen, W.; Wang, X.; Feizbakhshan, M.; Liu, C.; Hong, S.; Yang, P.; Zhou, X. Preparation of lignin-based porous carbon with hierarchical oxygen-enriched structure for high-performance supercapacitors. J. Colloid Interface Sci. 2019, 540, 524–534. [Google Scholar] [CrossRef] [PubMed]
- Rowlandson, J.L.; Edler, K.J.; Tian, M.; Ting, V.P. Toward process-resilient lignin-derived activated carbons for hydrogen storage applications. ACS Sustain. Chem. Eng. 2020, 8, 2186–2195. [Google Scholar] [CrossRef]
- Multhaupt, H.; Bottke, P.; Wark, M. Enhanced breaking of lignin and mesopore formation in zinc chloride assisted hydrothermal carbonization of waste biomasses. C 2021, 7, 77. [Google Scholar] [CrossRef]
- Su, W.; Li, P.; Wang, M.; Yi, D.; Jiang, B.; Wu, W. Simple preparation of lignin–based phenolic resin carbon and its efficient adsorption of Congo Red. Water 2023, 15, 2777. [Google Scholar] [CrossRef]
- Roa, K.; Oyarce, E.; Boulett, A.; ALSamman, M.; Oyarzún, D.; Pizarro, G.D.C.; Sánchez, J. Lignocellulose-based materials and their application in the removal of dyes from water: A review. Sustain. Mater. Technol. 2021, 29, e00320. [Google Scholar] [CrossRef]
- Ge, Y.; Li, Z. Application of lignin and its derivatives in adsorption of heavy metal ions in water: A review. ACS Sustain. Chem. Eng. 2018, 6, 7181–7192. [Google Scholar] [CrossRef]
- Cui, J.; Sun, H.; Wang, X.; Sun, J.; Niu, M.; Wen, Z. Preparation of siliceous lignin microparticles from wheat husks with a facile method. Ind. Crop. Prod. 2015, 74, 689–696. [Google Scholar] [CrossRef]
- Witzler, M.; Alzagameem, A.; Bergs, M.; Khaldi-Hansen, B.E.; Klein, S.E.; Hielscher, D.; Kamm, B.; Kreyenschmidt, J.; Tobiasch, E.; Schulze, M. Lignin-derived biomaterials for drug release and tissue engineering. Molecules 2018, 23, 1885. [Google Scholar] [CrossRef]
- Yang, W.; Ding, H.; Qi, G.; Guo, J.; Xu, F.; Li, C.; Puglia, D.; Kenny, J.; Ma, P. Enhancing the radical scavenging Activity and UV resistance of lignin nanoparticles via surface mannich amination toward a biobased antioxidant. Biomacromolecules 2021, 22, 2693–2701. [Google Scholar] [CrossRef]
- Abraham, B.; Syamnath, V.; Arun, K.; Fathima Zahra, P.; Anjusha, P.; Kothakotta, A.; Chen, Y.H.; Ponnusamy, V.K.; Nisha, P. Lignin-based nanomaterials for food and pharmaceutical applications: Recent trends and future outlook. Sci. Total Environ. 2023, 881, 163316. [Google Scholar] [CrossRef]
- Karoki, P.K.; Zhang, S.; Cai, C.M.; Dim, P.E.; Ragauskas, A.J. Thermally stable and self-healable lignin-based polyester. Polym. Test. 2024, 137, 108515. [Google Scholar] [CrossRef]
- Esakkimuthu, E.S.; DeVallance, D.; Pylypchuk, I.; Moreno, A.; Sipponen, M.H. Multifunctional lignin–poly (lactic acid) biocomposites for packaging applications. Front. Bioeng. Biotechnol. 2022, 10, 1025076. [Google Scholar] [CrossRef]
- Boarino, A.; Klok, H.A. Opportunities and challenges for lignin valorization in food packaging, antimicrobial, and agricultural applications. Biomacromolecules 2023, 24, 1065–1077. [Google Scholar] [CrossRef] [PubMed]
- Nisar, S.; Raza, Z.A. Corn straw lignin—A sustainable bioinspired finish for superhydrophobic and UV-protective cellulose fabric. Int. J. Biol. Macromol. 2024, 257, 128393. [Google Scholar] [CrossRef]
- GilakHakimabadi, S.; Ehsani, M.; Khonakdar, H.A.; Ghaffari, M.; Jafari, S.H. Controlled-release of ferulic acid from active packaging based on LDPE/EVA blend: Experimental and modeling. Food Packag. Shelf Life 2019, 22, 100392. [Google Scholar] [CrossRef]
- Sun, X.; Li, Q.; Wu, H.; Zhou, Z.; Feng, S.; Deng, P.; Zou, H.; Tian, D.; Lu, C. Sustainable starch/lignin nanoparticle composites biofilms for food packaging applications. Polymers 2023, 15, 1959. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, Y.; Zhang, C.; Yuan, F.; Gao, H.; Li, Q. Lignin-based cmposite film and its application for agricultural mulching. Polymers 2024, 16, 2488. [Google Scholar] [CrossRef]
- Ahmad, U.M.; Ji, N.; Li, H.; Wu, Q.; Song, C.; Liu, Q.; Ma, D.; Lu, X. Can lignin be transformed into agrochemicals? Recent advances in the agricultural applications of lignin. Ind. Crop. Prod. 2021, 170, 113646. [Google Scholar] [CrossRef]
- Kemala, P.; Prianto, A.H.; Martino, A.D.; Idroes, R.; Javaid, S.; Hua, L.S.; Fatriasari, W. Valorization of lignin as an active agent in sustainable agriculture: A review. J. Ind. Eng. Chem. 2025. [Google Scholar] [CrossRef]
- Alwadani, N.; Fatehi, P. Synthetic and lignin-based surfactants: Challenges and opportunities. Carbon Resour. Convers. 2018, 1, 126–138. [Google Scholar] [CrossRef]
- Yuliestyan, A.; Partal, P.; Navarro, F.J.; Martín-Sampedro, R.; Ibarra, D.; Eugenio, M.E. Emulsion stabilization by cationic lignin surfactants derived from bioethanol production and kraft pulping processes. Polymers 2022, 14, 2879. [Google Scholar] [CrossRef] [PubMed]
- Martínková, L.; Grulich, M.; Pátek, M.; Kristková, B.; Winkler, M. Bio-based valorization of lignin-derived phenolic compounds: A review. Biomolecules 2023, 13, 717. [Google Scholar] [CrossRef] [PubMed]
- Gomes, E.; Rodrigues, A. Lignin biorefinery: Separation of vanillin, vanillic acid and acetovanillone by adsorption. Sep. Purif. Technol. 2019, 216, 92–101. [Google Scholar] [CrossRef]
- Robinson, A.J.; Giuliano, A.; Abdelaziz, O.Y.; Hulteberg, C.P.; Koutinas, A.; Triantafyllidis, K.S.; Barletta, D.; De Bari, I. Techno-economic optimization of a process superstructure for lignin valorization. Bioresour. Technol. 2022, 364, 128004. [Google Scholar] [CrossRef]
- Gordobil, O.; Olaizola, P.; Banales, J.M.; Labidi, J. Lignins from agroindustrial by–products as natural ingredients for cosmetics: Chemical structure and in vitro sunscreen and cytotoxic activities. Molecules 2020, 25, 1131. [Google Scholar] [CrossRef] [PubMed]
- Duy, N.V.; Tsygankov, P.Y.; Menshutina, N.V. Facile lignin extraction and application as natural UV blockers in cosmetic formulations. ChemEngineering 2024, 8, 69. [Google Scholar] [CrossRef]
- Market Research Intellect. The Rise of Lignin in Cosmetics. 2024. Available online: https://www.marketresearchintellect.com/blog/nature-s-beauty-secret-the-rise-of-lignin-in-cosmetics/ (accessed on 8 February 2025).
- Seçkin, M.; Üçgül, I. Investigation of lignin as a textile fiber. Celal Bayar Üniv. Fen Bilim. Derg. 2020, 16, 183–190. [Google Scholar]
- Ali, M.A.S.; Abdel-Moein, N.M.; Owis, A.S.; Ahmed, S.E.; Hanafy, E.A. Eco-friendly lignin nanoparticles as antioxidant and antimicrobial material for enhanced textile production. Sci. Rep. 2024, 14, 17470. [Google Scholar] [CrossRef]
- Gaynor, J.G.; Szlek, D.B.; Kwon, S.; Tiller, P.S.; Byington, M.S.; Argyropoulos, D.S. Lignin use in nonwovens: A review. BioResources 2022, 17, 3445–3488. [Google Scholar] [CrossRef]
- Dinari, A.; Abdollahi, M.; Sadeghizadeh, M. Design and fabrication of dual responsive lignin-based nanogel via “grafting from” atom transfer radical polymerization for curcumin loading and release. Sci. Rep. 2021, 11, 1962. [Google Scholar] [CrossRef] [PubMed]
- Khodadadi, F.; Nikzad, M.; Hamedi, S. Lignin nanoparticles as a promising nanomaterial for encapsulation of Rose damascene essential oil: Physicochemical, structural, antimicrobial and in-vitro release properties. Colloids Surf. A Physicochem. Eng. Asp. 2024, 687, 133580. [Google Scholar] [CrossRef]
- Kowalska, E.; Ziarno, M.; Ekielski, A.; Zelazinski, T. Materials used for the microencapsulation of probiotic bacteria in the food industry. Molecules 2022, 27, 3321. [Google Scholar] [CrossRef] [PubMed]
- Karagöz, P.; Özkan, M. Ethanol production from wheat straw by Saccharomyces Cerevisiae Scheffersomyces Stipitis Co-Cult. Batch Contin. Syst. Bioresour. Technol. 2014, 158, 286–293. [Google Scholar] [CrossRef] [PubMed]
- Karagoz, P.; Khiawjan, S.; Marques, M.P.C.; Santzouk, S.; Bugg, T.D.H.; Lye, G.J. Pharmaceutical applications of lignin-derived chemicals and lignin-based materials: Linking lignin source and processing with clinical indication. Biomass Convers. Biorefinery 2023, 14, 26553–26574. [Google Scholar] [CrossRef]
- Bartczak, P.; Wysokowski, M.; Szylinczuk, K.; Odalanowska, M.; Jesionowski, T.; Borysiak, S. Green synthesis of chitin/lignin based-polyurethane composites. Ind. Crop. Prod. 2023, 204, 117237. [Google Scholar] [CrossRef]
- Moreno, A.; Sipponen, M.H. Lignin-based smart materials: A roadmap to processing and synthesis for current and future applications. Mater. Horizons 2020, 7, 2237–2257. [Google Scholar] [CrossRef]
- Goh, S.W.; Ng, Q.H.; Rahim, S.K.E.A.; Low, S.C.; Hoo, P.Y.; Yeo, R.Y.Z.; Chew, T.L.; Jawad, Z.A. Recent advancements in smart materials for the removal of organic, inorganic and microbial pollutants in water treatment: A review. J. Water Process Eng. 2025, 70, 106993. [Google Scholar] [CrossRef]
- Bhowmik, S.; Agyei, D.; Ali, A. Smart chitosan films as intelligent food packaging: An approach to monitoring food freshness and biomarkers. Food Packag. Shelf Life 2024, 46, 101370. [Google Scholar] [CrossRef]
- Pillai, V.V.; Ramasubramanian, B.; Sequerth, O.; Pilla, S.; Wang, T.; Mohanty, A.K.; Govindaraj, P.; Alhassan, S.M.; Salim, N.; Kingshott, P.; et al. Nanomaterial advanced smart coatings: Emerging trends shaping the future. Appl. Mater. Today 2025, 42, 102574. [Google Scholar] [CrossRef]
- Pang, T.; Wang, G.; Sui, W.; Xu, T.; Wang, D.; Si, C. Lignin-based support for metal catalysts: Synthetic strategies, performance boost, and application advances. Coord. Chem. Rev. 2025, 528, 216426. [Google Scholar] [CrossRef]
- Jamrozik, A.; Strzemiecka, B.; Jakubowska, P.; Koltsov, I.; Klapiszewski, L.; Voelkel, A.; Jesionowski, T. The effect of lignin–alumina hybrid additive on the properties of composition used in abrasive tools. Int. J. Biol. Macromol. 2020, 161, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Klapiszewski, L.; Jamrozik, A.; Strzemiecka, B.; Matykiewicz, D.; Voelkel, A.; Jesionowski, T. Activation of magnesium lignosulfonate and kraft lignin: Influence on the properties of phenolic resin–based composites for potential applications in abrasive materials. Int. J. Mol. Sci. 2017, 18, 1224. [Google Scholar] [CrossRef] [PubMed]
- Bellinetto, E.; Fumagalli, N.; Astorri, M.; Turri, S.; Griffini, G. Elucidating the role of lignin type and functionality in the development of high–performance biobased phenolic thermoset resins. ACS Appl. Polym. Mater. 2024, 6, 1191–1203. [Google Scholar] [CrossRef] [PubMed]
- Clavijo, L.; Zlatanovic, S.; Braun, G.; Bongards, M.; Dieste, A.; Barbe, S. Eucalyptus Kraft lignin as an additive strongly enhances the mechanical resistance of tree-leaf pellets. Processes 2020, 8, 376. [Google Scholar] [CrossRef]
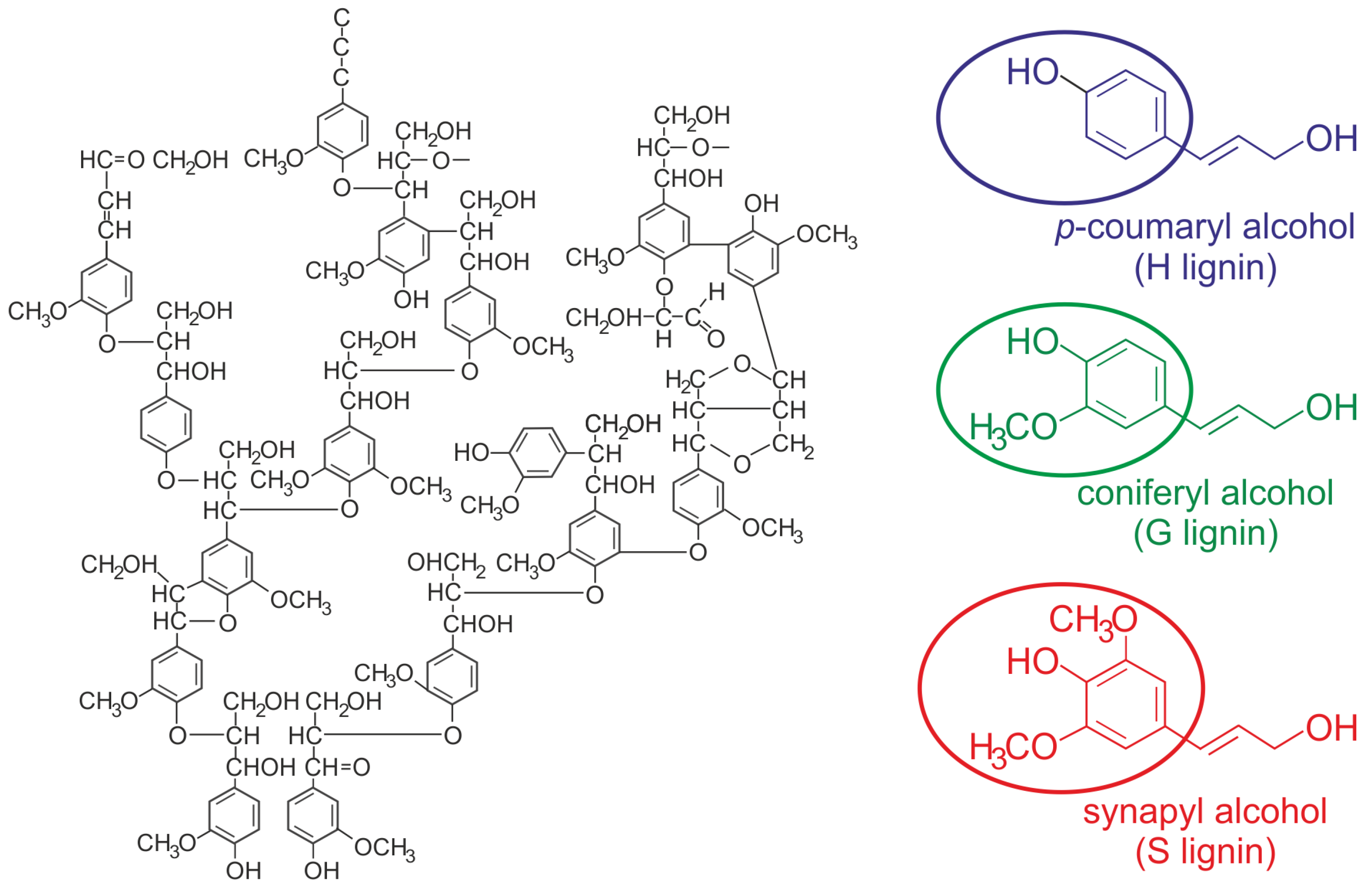
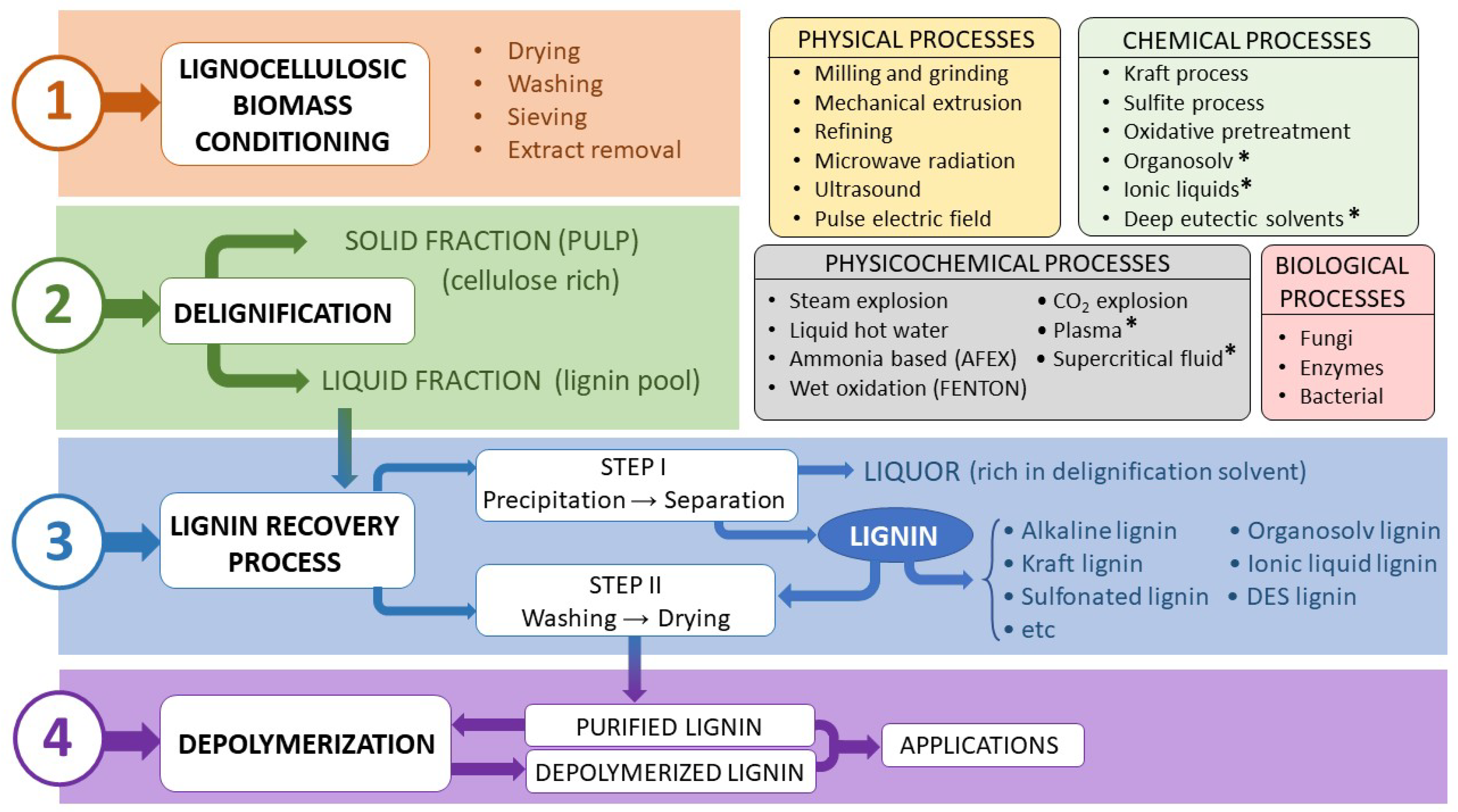
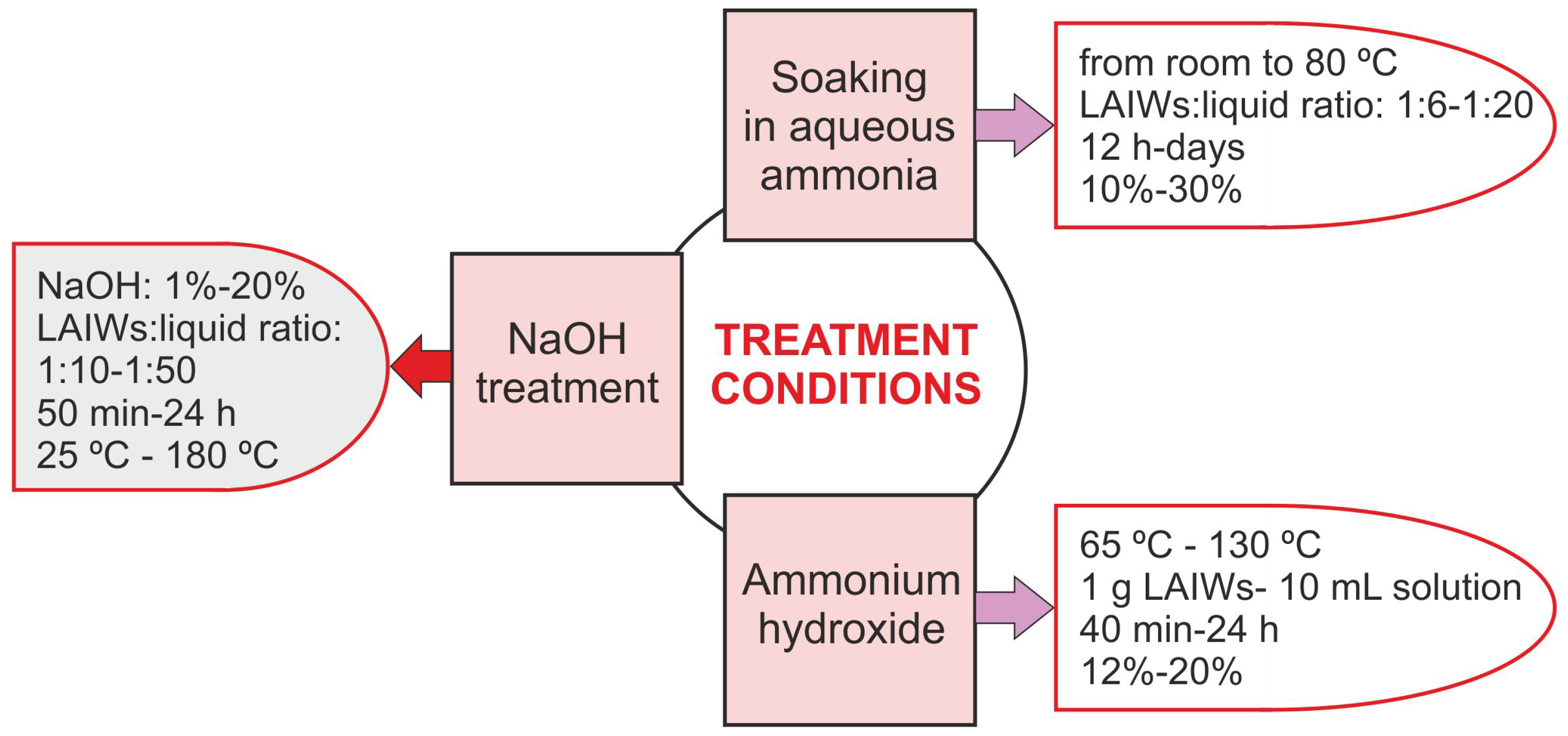
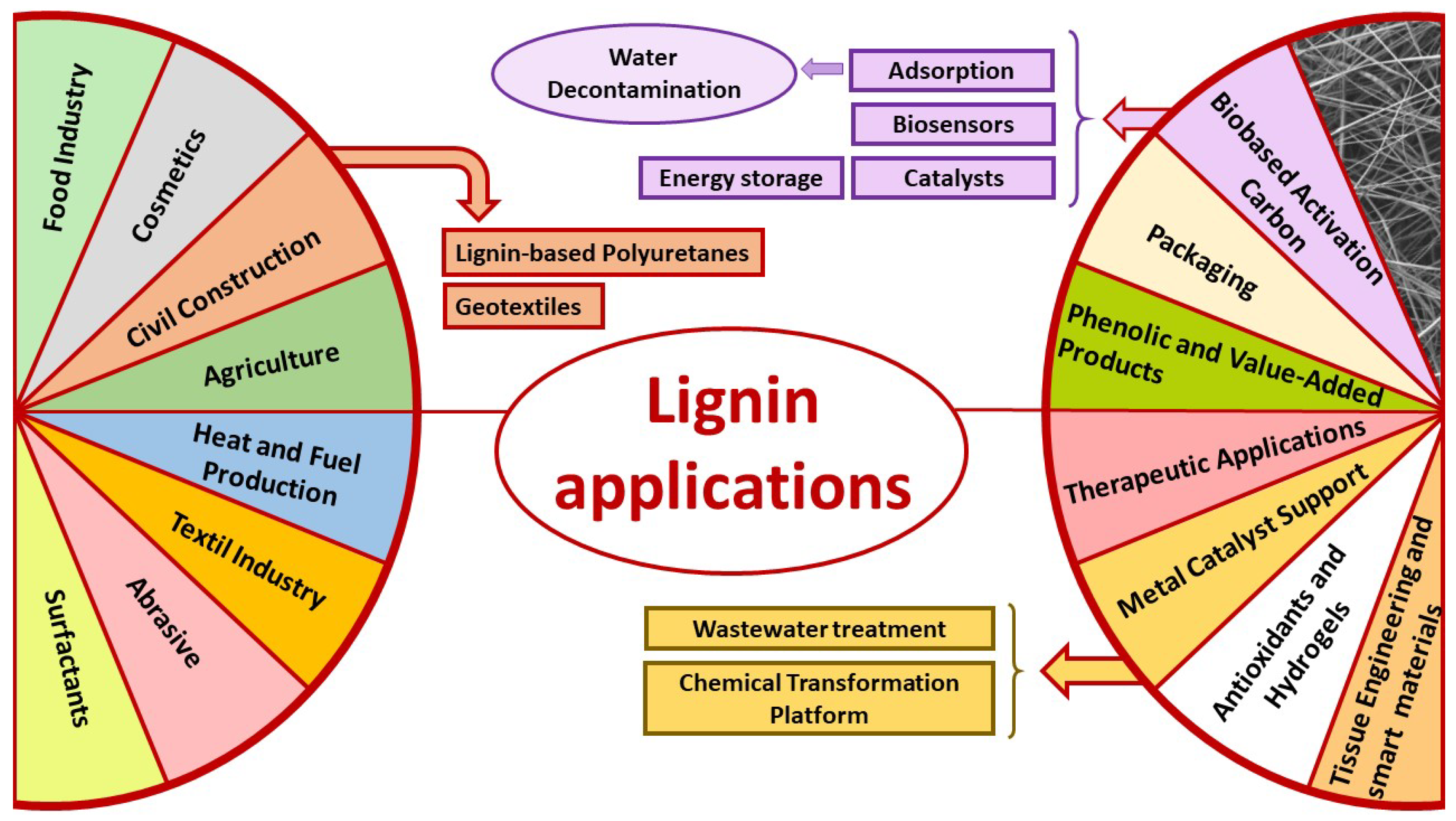
| LAIWs | Production | Ref. (1) | CEL | HEM | LIG | Ref. (2) |
|---|---|---|---|---|---|---|
| Mt/year | % | % | % | |||
| Acai seed | 1. | [33] | 3.6 | 58.0 | 11.7 | [34] |
| 36.1 | 16.6 | 47.9 | [35] | |||
| Almond hull | 1.6 | [36] | 20–35 | 10–15 | 8–15 | [37] |
| Almond shell | 2.3 | [38] | 31–48 | 27–30 | 22–32 | [38] |
| Apple pomace | 5–7 | [39] | 47.5 | 27.8 | 24.7 | [40] |
| 15.4 | 12.5 | 15.5 | [41] | |||
| Banana peel | 36.0 | [42] | 16.1 | 10.5 | 36.3 | [43] |
| Banana leaf | 9.3 | [44] | 43.3 | 34.3 | 15.0 | [45] |
| Barley husk | 9.3–12.4 | [46] | 35.6 | 26.1 | 14.3 | [47] |
| 39.0 | 12.0 | 22.0 | [48] | |||
| 33.6 | 37.2 | 19.3 | [49] | |||
| Barley straw | 51.3 | [50] | 42.5 | 26.6 | 18.3 | [51] |
| 34.3 | 22.6 | 20.7 | [52] | |||
| 38.0 | 21.9 | 17.3 | [53] | |||
| Cashew shell | 2.4 | [38] | 25–35 | 23–32 | 14–40 | [38] |
| 40.3 | 24.8 | 18.4 | [54] | |||
| Cassava stalks | 32–38 | [55] | 35.0 | 21.0 | 28.0 | [56] |
| 36.3 | 32.3 | 19.3 | [57] | |||
| Cassava peel | 27.7–41.5 | [58] | 43.6 | 10.4 | 7.7 | [59] |
| 40.5 | 21.4 | 11.7 | [60] | |||
| Cocoa pod husks | 48 | [61] | 19–35 | 8–13 | 14–23 | [62] |
| 23–32 | 8–38 | 11–33 | [63] | |||
| 29–32 | 25–27 | 19–22 | [64] | |||
| Cocoa bean shell | 0.7–0.9 | [65] | 23.4 | 18.1 | 29.6 | [61] |
| Coconut husk | 7 | [9] | 35.0 | 25.8 | 16.0 | [66] |
| 38.9 | 30.0 | 35.0 | [67] | |||
| Coconut shell | 2.4 | [68] | 33.6 | 19.3 | 36.5 | [69] |
| 25.2 | 27.7 | 46.0 | [70] | |||
| Coffee husk | 10 | [71] | 30–35 | 18–21 | 10–22 | [72] |
| 39.2 | 12.6 | 23.3 | [73] | |||
| Corncob | 200 | [74] | 17.8 | 44.7 | 20.3 | [75] |
| 17.8 | 44.7 | 20.3 | [76] | |||
| Corn stalk | 750 | [77] | 34.0 | 17.4 | 16.9 | [78] |
| 38.7 | 33.6 | 18.9 | [79] | |||
| Corn stover | 1661 | [80] | 31.5 | 18.0 | 14.1 | [81] |
| 30–40 | 24–27 | 17–21 | [82] | |||
| Corn straw | 1.2 × | [83] | 49.7 | 29.5 | 12.0 | [84] |
| 35.0 | 20.0 | 12.0 | [85] | |||
| Cotton stalks | 90.3–129 | [86] | 39.4 | 19.2 | 23.2 | [87] |
| 40–50 | 20–30 | 10–15 | [88] | |||
| Grape pomace | 67.1 | [89] | 20.9 | 13.3 | 34.8 | [90] |
| 19.3 | 7.2 | 15.6 | [91] | |||
| Grape stalk | 0.2–0.6 | [92] | 16.4 | 18.3 | 27.8 | [37] |
| 21.7 | 15.3 | 29.7 | [90] | |||
| Hazelnut shell | 0.5 | [38] | 15.4 | 22.4 | 25.9 | [93] |
| 17.2 | 15–28 | 30–38 | [38] | |||
| Hemp stalk | 0.9 | 43–47 | 37–40 | 20–23 | [94] | |
| 34–60 | 18–37 | 19–30 | [95] | |||
| Maize straw | 300 | [96] | 49.7 | 29.5 | 12.0 | [84] |
| 1000 | [97] | 35.9 | 24.5 | 20.4 | [98] | |
| Melon seed | 1.5–2.9 | [99] | 22.5 | 27.7 | 29.9 | [100] |
| Mustard waste | 1. | [101] | 53.2 | 13.5 | 1.1 | [101] |
| Oat husk | 5.5 | [102] | 48.4 | 16.1 | 16.2 | [103] |
| Oat straw | 0. | [104] | 26–35 | 25–28 | 13–16 | [105] |
| 37.6 | 23.3 | 12.9 | [40] | |||
| Oil palm trunk | [106] | 39.4 | 26.0 | 6.6 | [106] | |
| [107] | 38–41 | 12–17 | 18–23 | [108] | ||
| Olive pomace | 6.0 | [109] | 9.7 | 10.9 | 21.8 | [110] |
| 1. | [111] | 10.4 | 11.5 | 13.7 | [112] | |
| Olive stone | 4.1–5.0 | [113] | 34.2 | 25.0 | 33.5 | [114] |
| 0. | [115] | 30.8 | 17.1 | 32.6 | [116] | |
| Olive-tree leaves | 0.8–1.5 | [117] | 12.4 | 37.0 | 17.0 | [118] |
| 0. | 6.5 | 12.3 | 25.9 | [119] | ||
| Olive-tree pruning | 34.8 | [120] | 36.5 | 21.3 | 24.1 | [120] |
| 33.9 | 18.6 | 23.1 | [121] | |||
| Orange peel | 16 | [122] | 36.0 | 10.0 | 17.0 | [123] |
| 33.3 | 30.6 | 12.0 | [40] | |||
| Orange-tree pruning | 18.4 | [124] | 40.5 | 29.3 | 19.0 | [124] |
| 5. | [125] | 49.1 | 26.9 | 20.8 | [126] | |
| Palm kernel shell | 4.6 | [127] | 41.5 | 12.4 | 34.0 | [128] |
| Paper and pulping industry | 15 | [129] | 34.3 | 20.3 | 19.3 | [130] |
| Passion fruit peel | 0. | [131] | 48.0 | 20.8 | 12.6 | [131] |
| Peanut shell | 13.0 | [38] | 34–43 | 7–29 | 22–35 | [38] |
| 36.4 | 15.6 | 25.0 | [132] | |||
| Pigeon pea stalk | 10.3 | [133] | 33.0 | 24.0 | 18.3 | [134] |
| 43.2 | 29.5 | 15.1 | [135] | |||
| Pineapple peel | 8.3–11.5 | [136] | 30.4 | 44.4 | 4.2 | [137] |
| 24.2 | 29.4 | 6.4 | [138] | |||
| Pine sawdust | 0.07 | [139] | 55.9 | 15.3 | 10.6 | [87] |
| 39.4 | 20.3 | 31.4 | [140] | |||
| Pistachio shell | 0.51 | [141] | 38–57 | 21–31 | 17–24 | [38] |
| 4–5 | [142] | 31.2 | 31.3 | 21.2 | [141] | |
| Rice husk | 120 | [27] | 33.0 | 24.0 | 18.0 | [143] |
| 31.3 | 24.3 | 14.3 | [40] | |||
| Rice straw | 740 | [144] | 32–47 | 19–27 | 5–12 | [145] |
| Rye straw | 8–20 | [146] | 37.9 | 36.9 | 17.6 | [103] |
| Sorghum bagasse | 1. | [147] | 43.6 | 26.8 | 26.2 | [148] |
| Soybean husk | 18.0–28.7 | [149] | 29–52 | 18–34 | 2–13 | [149] |
| 29–51 | 10–25 | 1–18 | [150] | |||
| Soybean straw | 200 | [151] | 34.0 | 29.7 | 17.5 | [152] |
| Spent coffee grounds | 6.0 | [153] | 13.0 | 42.0 | 25.0 | [40] |
| Sugarcane bagasse | 1.8 × | [27] | 46.2 | 27.0 | 23.7 | [154] |
| 30.9 | 28.2 | 9.6 | [40] | |||
| Sunflower stalk | 186–8000 | [155] | 30.8 | 12.4 | 19.3 | [156] |
| Tobacco waste | 5.8 | [157] | 30.6 | 15.0 | 18.6 | [157] |
| Triticale straw | 36.1 | [158] | 48.5 | 21.9 | 11.1 | [159] |
| Walnut shell | 2.3 | [38] | 20–37 | 15–31 | 30–52 | [38] |
| Wheat straw | 500 | [160] | 40.2 | 27.6 | 21.0 | [161] |
| 27–30 | 27–50 | 15–16 | [40] |
| LAIWs | Treatment 1 | Treatment 2 | Delignification, % | Ref. |
|---|---|---|---|---|
| Apricot kernel shell | Liquid hot water,
220 °C, 15 min 1:15 LAIW:H2O | Soxhlet extraction ethanol or acetone | 35–58 | [334] |
| Bagasse | Lactic acid
0.1%, 170 °C, 60 min
1 g LAIW: 10 mL | Lactic acid 50–80% 170 °C, 60 min 1 g LAIW: 10 g acid | 69.5–96.6 | [98] |
| Bamboo culms | Hydrothermal, 180 °C, 30 min 10% solid load | Organosolv
30:50:20 Formic acid/acetic acid/water | 62.2 | [237] |
| Banana stem | KOH 5%, 90 °C 1 g LAIW: 10 mL | Microwave assisted 25 min, 360 Hz | 44.9 | [205] |
| Chestnut shell | Liquid hot water, 100 °C, 24 h
1 g LAIW: 10 mL | Alkaline treatment
KOH or NaOH, 1–5% | 25-71 | [306] |
| Coconut husk | Steam explosion, 170 °C, 2.5 min
12–15 bar | NaOH 30%, 170 °C, 3 h, 12–15 bar | 45.6–55.8 | [335] |
| Corn stalk | 2%, 80 °C, 120 min 1 g LAIW: 20 mL | Cold plasma
50 °C, 120 min | 86 | [336] |
| 30%, 80 °C, 120 min 1 g LAIW: 10 mL | Acetic acid, 1:1 v/v | 89 | [190] | |
| Corn stover | HCl 1%, 120 °C, 40 min 1 g LAIW: 10 mL HCl | 5–26% OH, 120 °C, 40 min | 40–75 | [208] |
| Milling, 400 rpm, 1 h | Ethylenediamine (EDA)
120 °C, 60 min | 70–85 | [337] | |
| Grape stalk | Subcritical water,
170–180 °C
30 min, 1 g LAIW: 10 g | 4–8%, 40 °C, 1 h
1 g:30 mL | 75 | [338] |
| Olive pomace | NaOH 4%, 25°C, 4 h | Microwave
assisted 450 W, 10 min | 47.1 | [213] |
| Olive-tree pruning | NaOH 0.8%, 60 °C, 59 min | Oxalic acid 0.075 M 150 °C, 30 min, 1 g:10 g | ≃20 | [339] |
| Organosolv | Microwave assisted | [340] | ||
| Acetone 65% | 155 °C, 8.4 min | 97.3 | ||
| Ethanol 65% | 138 °C, 14.8 min | 95.5 | ||
| -valerolactone 60% | 149 °C, 5 min | 97.7 | ||
| Pistachio shell | NaOH 1 N, 70 min
1 g LAIW: 200 mL | Ultrasound assisted 70 min, 400 W | 78.9 | [212] |
| Rice husk | NaOH 4.8% + 3%
1 g LAIW: 12 mL | Microwave assisted 80 °C, 90 min, 400 W | 45.5 | [341] |
| Deep eutectic solvent 120 °C, 1–6 h 1 g LAIW: 10 g DES | Subcritical water 200–260 °C, 15 MPa, 10 min | 42–46 | [300] | |
| Rice straw | Ionic liquid 1 g LAIW: 20 mL | Sonication assisted 75–90 °C, 80–160 min | 22–61 | [342] |
| 3%, 121 °C,
30 min 1 g LAIW: 10 mL | NaOH 4%, 121 °C, 30 min
1 g LAIW:12.5 mL | 54.9 | [343] | |
| Liquid ammonia 15% 100–190 °C, 20 min | Acid treatment, 130 °C, 20 min | 69–84 | [344] | |
| Sisal waste |
ultraviolet cat.
1 g LAIW: 20 g IL | Ionic liquid, 25% TAH 20–100 °C, 20–120 min | 79.2 | [291] |
| Sugarcane bagasse | Liquid hot water, 180 °C, 20 min
1 g LAIW: 9 mL | Organosol assisted 140 °C, 45 min | 82.5 | [309] |
| Vine shoots | 15%,
150 °C | Organosolv 50% ethanol 180 °C, 15% load | 43 | [345] |
| Wheat bran | 0.05%,
218 °C, 8 min | Hydrotopic solvent 40% w/w, 1 h | 24.1–42.6 | [228] |
| Wheat straw | Autohydrolysis 140–180 °C, 40 min | NaOH 2–6% w/w 1 g LAIW: 25 g | 33.9–65.6 | [312] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mateo, S.; Fabbrizi, G.; Moya, A.J. Lignin from Plant-Based Agro-Industrial Biowastes: From Extraction to Sustainable Applications. Polymers 2025, 17, 952. https://doi.org/10.3390/polym17070952
Mateo S, Fabbrizi G, Moya AJ. Lignin from Plant-Based Agro-Industrial Biowastes: From Extraction to Sustainable Applications. Polymers. 2025; 17(7):952. https://doi.org/10.3390/polym17070952
Chicago/Turabian StyleMateo, Soledad, Giacomo Fabbrizi, and Alberto J. Moya. 2025. "Lignin from Plant-Based Agro-Industrial Biowastes: From Extraction to Sustainable Applications" Polymers 17, no. 7: 952. https://doi.org/10.3390/polym17070952
APA StyleMateo, S., Fabbrizi, G., & Moya, A. J. (2025). Lignin from Plant-Based Agro-Industrial Biowastes: From Extraction to Sustainable Applications. Polymers, 17(7), 952. https://doi.org/10.3390/polym17070952








