Study on the Preparation and Modification of a Novel Bio-Based Cardanol-Furfurylamine Oxazine Resin
Abstract
1. Introduction
2. Materials and Methods
2.1. Material Preparation
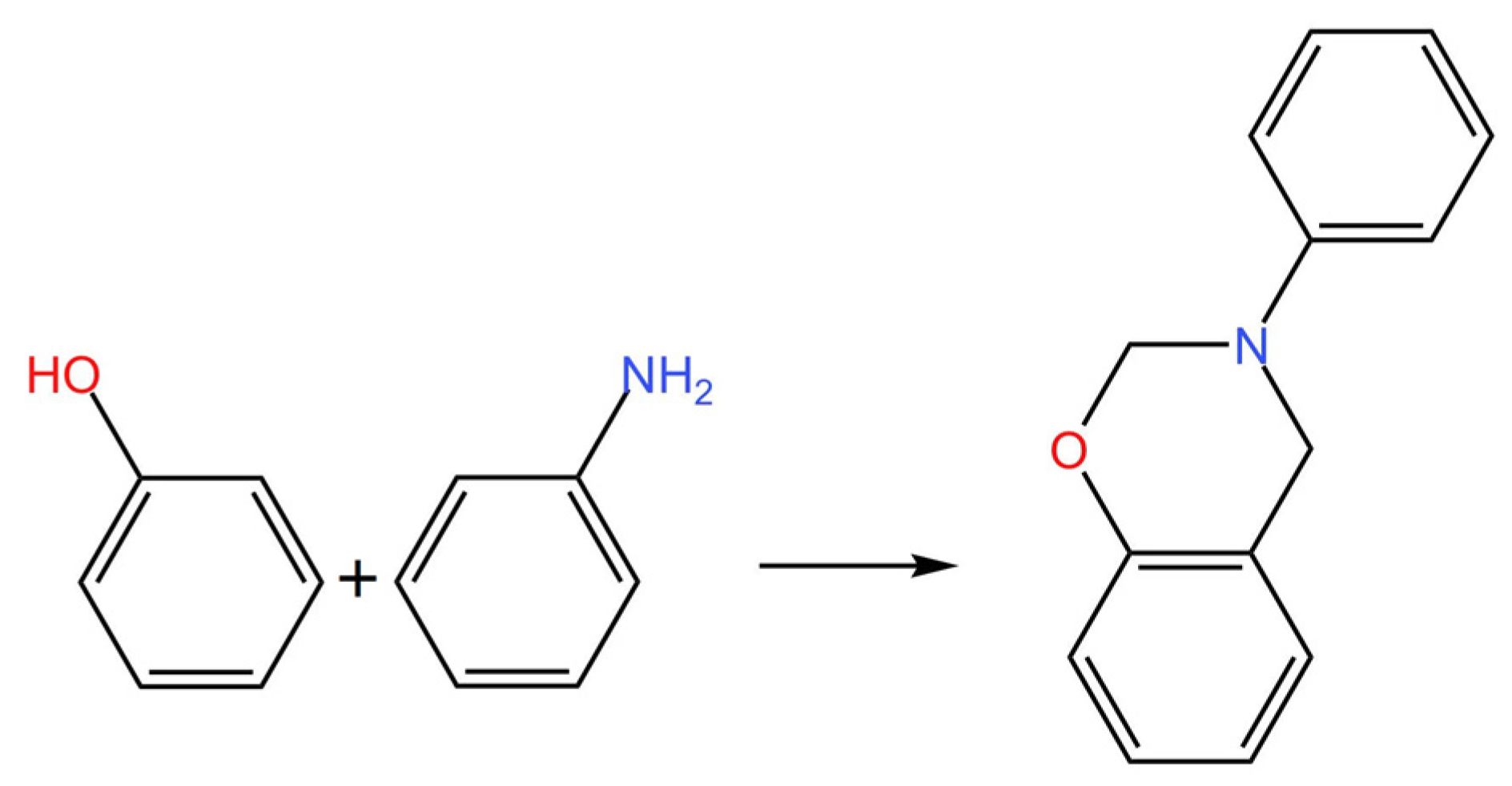
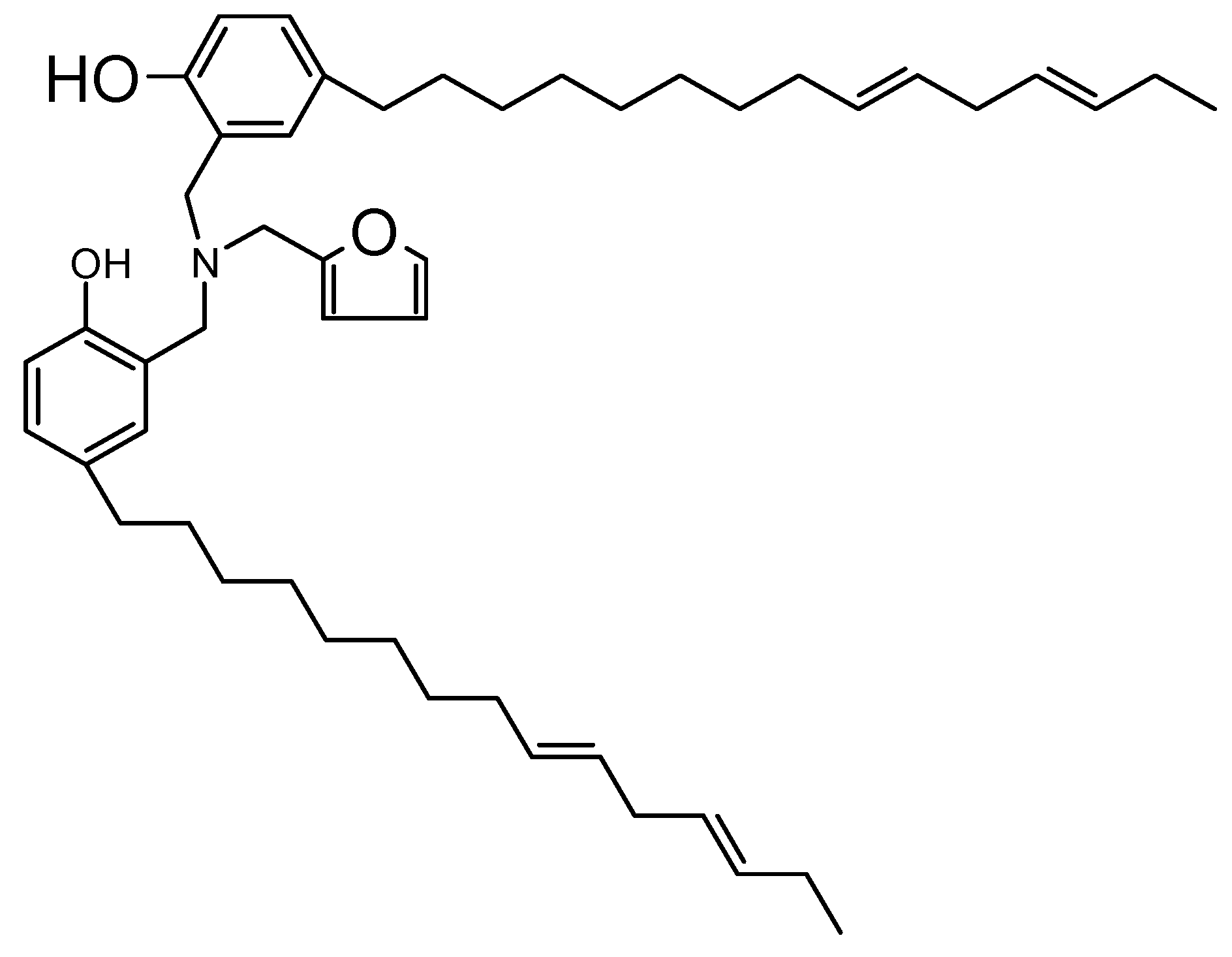
2.1.1. Preparation of Cardanol-Furfurylamine Oxazine (CFZ) Monomer
2.1.2. Preparation of Benzoxazine (BZ) Monomer
2.1.3. Preparation of CFZ-BZ Resin
2.2. Test and Characterization
3. Results and Discussion
3.1. Optimization of Synthetic Conditions
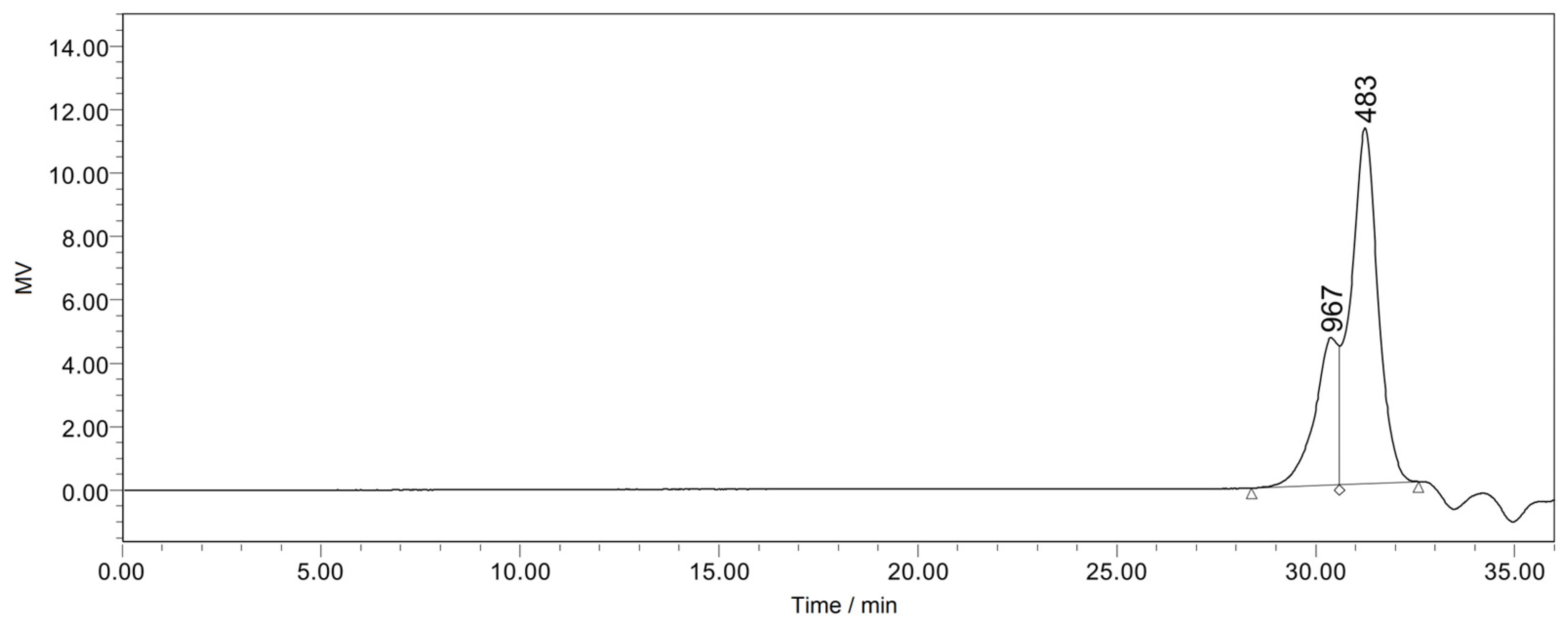
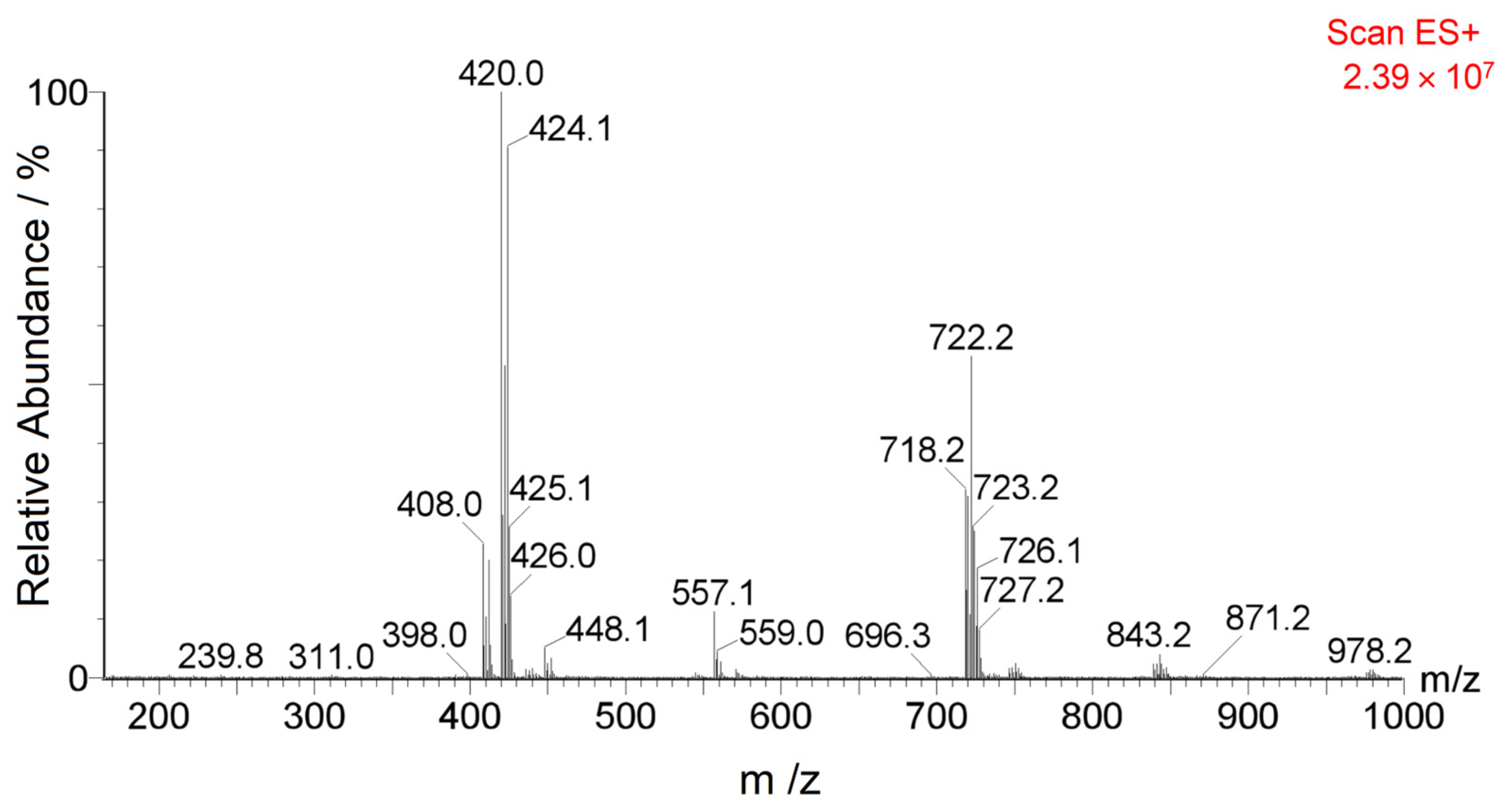
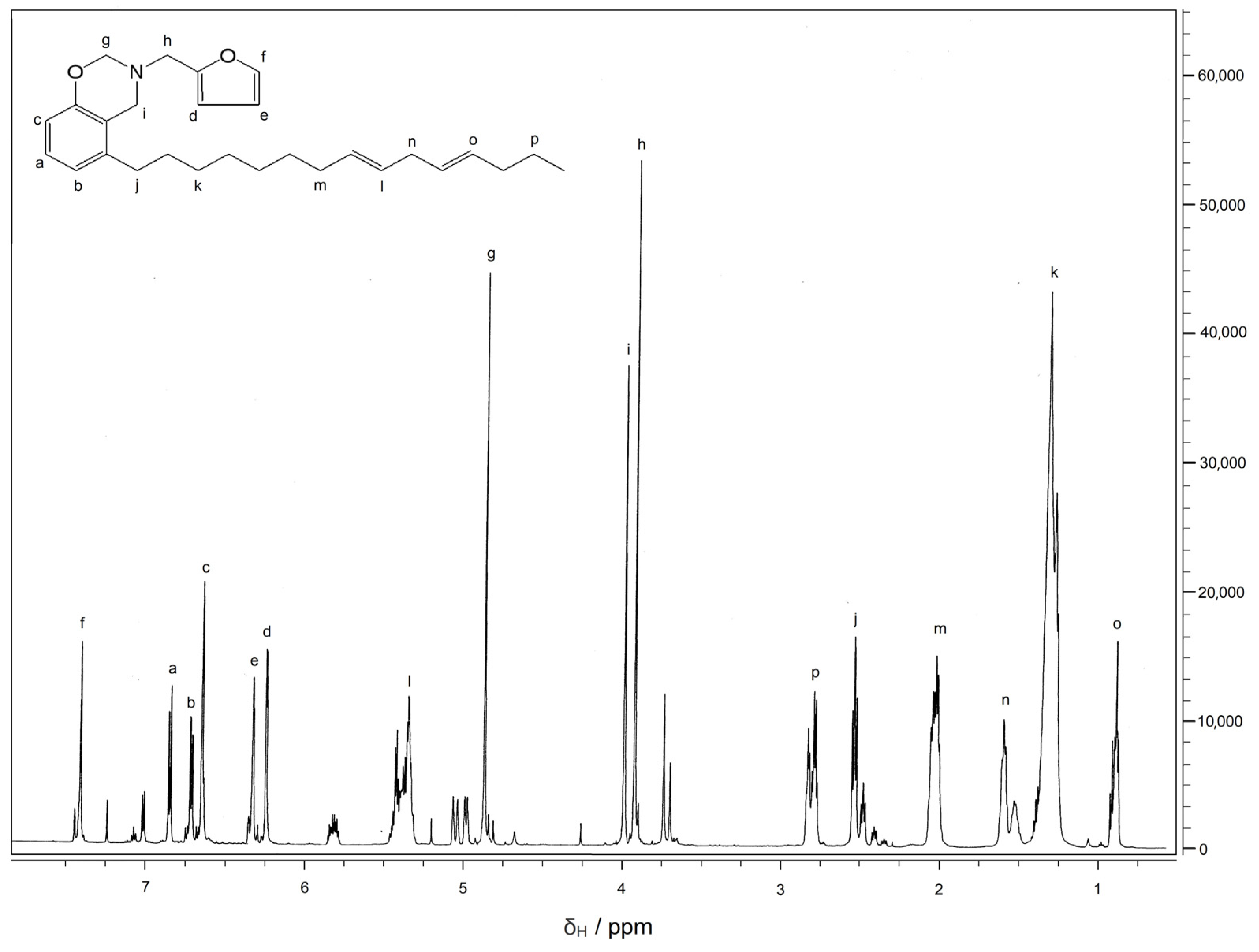
3.2. Characterization of CFZ
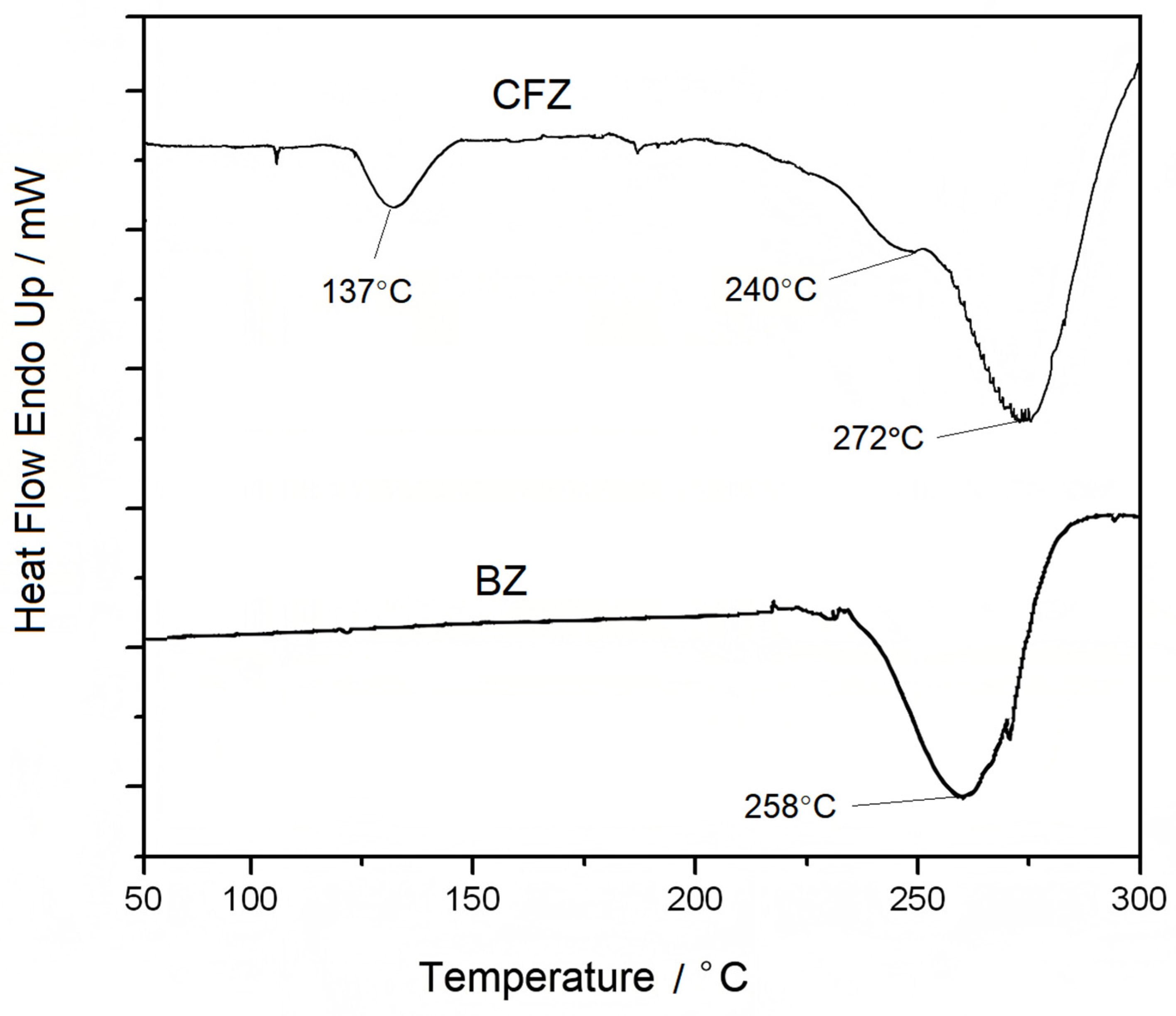
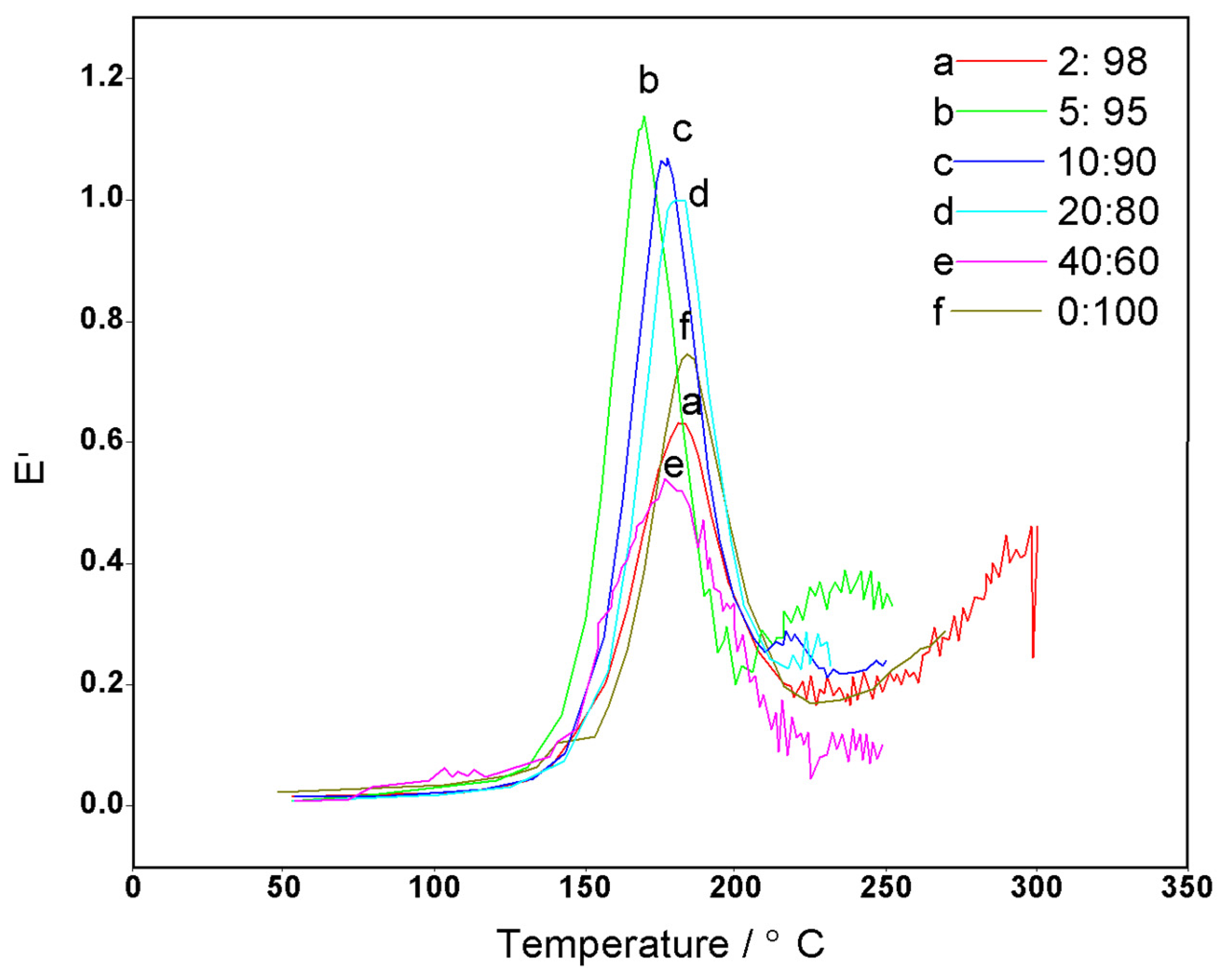
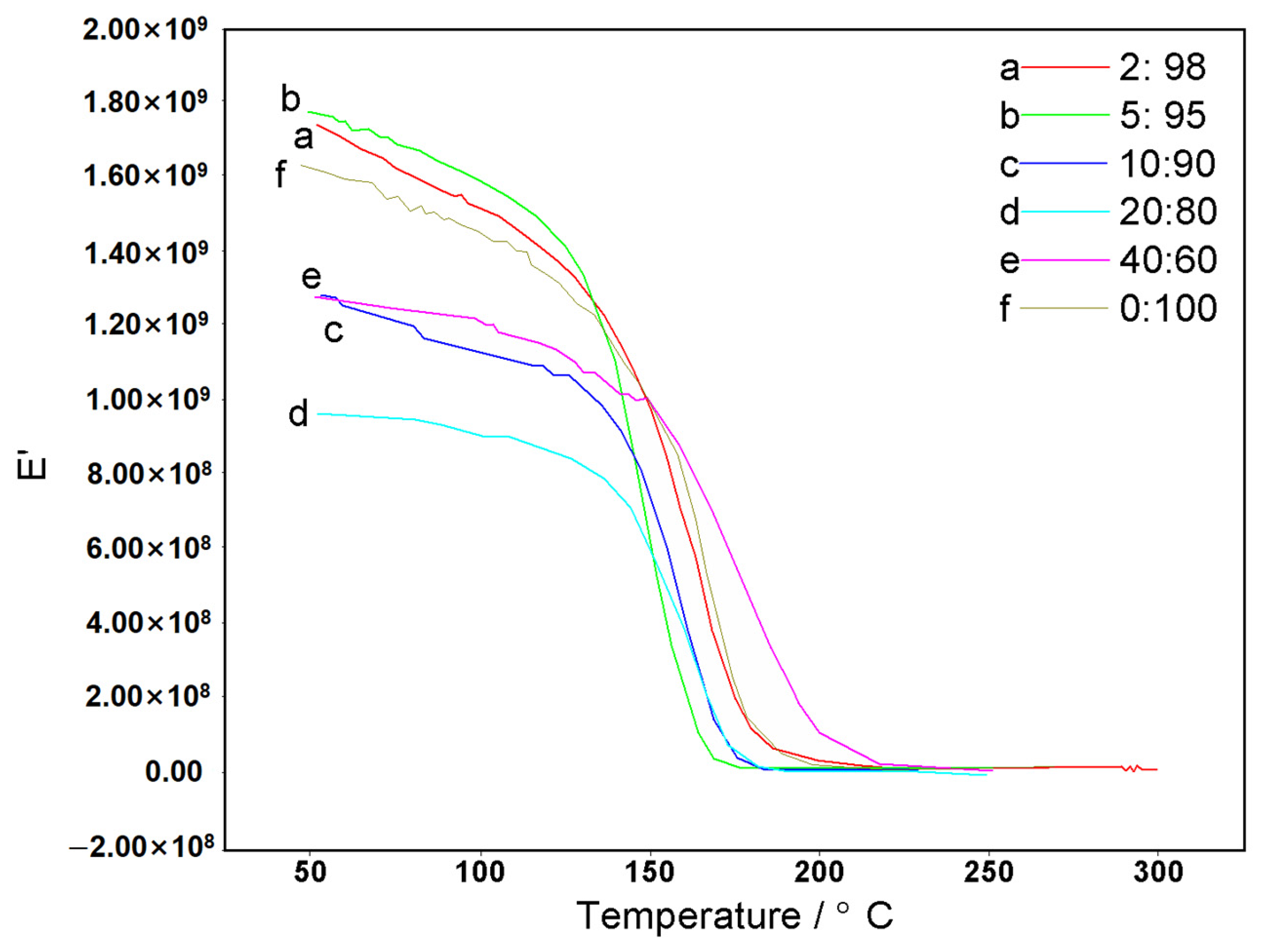
3.3. Performance Testing of CFZ-BZ Resin
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CFZ | Cardanol-furfurylamine oxazine |
| BZ | Benzoxazine |
| FT-IR | Fourier-transform Infrared Spectroscopy |
| GPC | Gel Permeation Chromatography |
| MS | Mass Spectrometry |
| NMR | Nuclear Magnetic Resonance |
| DSC | Differential Scanning Calorimetry |
| DMTA | Dynamic Mechanical Thermal Analysis |
Appendix A

References
- Lu, Y.; Yu, X.; Han, L.; Zhang, K. Recent Progress of High Performance Thermosets Based on Norbornene Functional Benzoxazine Resins. Polymers 2021, 13, 1417. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Gao, C.; Yuan, Y.; Wu, Z.; Zhou, D. Synthesis and application of a benzoxazine-type phosphorus-containing monomer on epoxy/benzoxazine copolymer: Thermal stability and compatibility with liquid oxygen. Polym. Degrad. Stab. 2018, 157, 131–142. [Google Scholar] [CrossRef]
- Zhang, K.; Liu, Y.; Hana, M.; Froimowicz, P. Smart and sustainable design of latent catalyst-containing benzoxazine-bio-resins and application studies. Green Chem. 2020, 22, 1209–1219. [Google Scholar] [CrossRef]
- Ohashi, S.; Kilbane, J.; Heyl, T.; Ishida, H. Synthesis and Characterization of Cyanate Ester Functional Benzoxazine and Its Polymer. Macromolecules 2015, 48, 8412–8417. [Google Scholar] [CrossRef]
- Naiker, V.E.; Patil, D.A.; More, A.P.; Mhaske, S.T. Synthesis of high-performance bio-based benzoxazine for flame retardant application. Polym. Adv. Technol. 2022, 5, 1481–1495. [Google Scholar] [CrossRef]
- Zhang, S.; Lan, T.; Ren, D.; Liu, X.; Ran, Q. Tuning the polymerization sequence of alkynyl-functionalized benzoxazine: Application as precursor for efficient magnetic EMI shielding materials. Polym. Biopolym. 2021, 56, 10691–10705. [Google Scholar] [CrossRef]
- Fan, X.; Li, S.; Wang, C.; Deng, Y.; Zhang, C.; Wang, Z. Research on fluoropyridine-based benzoxazine with high thermal stability and excellent flame retardancy for its application in coatings. Eur. Polym. J. 2023, 187, 111884. [Google Scholar] [CrossRef]
- Hariharan, A.; Srinivasan, K.; Murthy, C.; Alagar, M. A Novel Imidazole-Core-Based Benzoxazine and Its Blends for High-Performance Applications. Ind. Eng. Chem. Res. 2017, 56, 9347–9354. [Google Scholar] [CrossRef]
- Huo, S.; Wang, J.; Yang, S.; Zhang, B.; Tang, Y. A phosphorus-containing phenolic derivative and its application in benzoxazine resins: Curing behavior, thermal, and flammability properties. J. Appl. Polym. Sci. 2016, 133, 43403. [Google Scholar] [CrossRef]
- Zhao, C.; Sun, Z.; Wei, J.; Li, Y.; Xiang, D.; Wu, Y.; Que, Y. A Phosphorous-Containing Bio-Based Furfurylamine Type Benzoxazine and Its Application in Bisphenol-A Type Benzoxazine Resins: Preparation, Thermal Properties and Flammability. Polymers 2022, 14, 1597. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Q.; Yu, J.; Xu, R.; Wang, C.; Xiong, J. Synthesis and Characterization of Benzoxazine Resin Based on Furfurylamine. Materials 2022, 15, 8364. [Google Scholar] [CrossRef]
- Wang, J.; Xu, R. Preparation and Characterization of Guaiacol-Furfuramine Benzoxazine and Its Modification of Bisphenol A-Aniline Oxazine Resin. Polymers 2024, 16, 783. [Google Scholar] [CrossRef] [PubMed]
- Higginson, C.J.; Malollari, K.G.; Xu, Y.; Kelleghan, A.V.; Ricapito, N.G.; Messersmith, P.B. Bioinspired Design Provides High-Strength Benzoxazine Structural Adhesives. Angew. Chem. 2019, 35, 12399–12407. [Google Scholar] [CrossRef]
- GB/T 2571-1995; Test Method for Impact Resistance of Resin Casting Body. State Administration of Technical Supervision: Beijing, China, 1995.
- João, F.A.F.; Bárbara, C.L.; Acácio, S.S.; Sergio, P.; Sandro, J.G. Multicomponent Mannich reactions: General aspects, methodologies and applications. Tetrahedron 2017, 73, 6977–7004. [Google Scholar]
- Huisgen, R. Kinetics and Mechanism of 1,3-Dipolar Cycloadditions. Angew. Chem. Int. Ed. 1963, 2, 633–645. [Google Scholar] [CrossRef]
- Teckpeng, L.; Sarah, B.K.W.L.; Keeleng, T.; Linli, W. Three Component Synthesis of β-Amino Carbonyl Compounds Using Indium Trichloride-Catalyzed One-pot Mannich-type Reaction in Water. Tetrahedron 2000, 56, 3227–3237. [Google Scholar]
- Wang, C.; Li, H.; Cao, Y.; Liu, H.; Hou, X.; Zhang, Y. Thermodynamic analysis on synthesis of 4,4-methylenedianiline by reaction of aniline and formaldehyde. CIESC J. 2012, 63, 2348–2355. [Google Scholar]
- Xiang, H.; Gu, Y. Research Developments of A New Class Phenolic Resin-benxozazine Resin. Polym. Mater. Sci. Eng. 2004, 20, 1–4. [Google Scholar]
- Takeichi, T.; Kawauchi, T.; Agag, T. High Performance Polybenzoxazines as a Novel Type of Phenolic Resin. Polym. J. 2008, 40, 1121–1131. [Google Scholar] [CrossRef]
- Barbosa, L.R.; Souza, D.S.; Queiroz, L.H.K.; Neto, A.C.; de Lima, D.P.; Beatriz, A.; Romão, W.; de Castro, E.V.R.; Lacerda, V. Unequivocal structural assignments of three cardanol derivatives: An experimental and theoretical approach. J. Mol. Struct. 2019, 1175, 357–366. [Google Scholar] [CrossRef]
- Lu, F.; Yan, B.; Hu, G.; Zhang, B. NMR Study of Benzoxazines. J. Instrum. Anal. 2004, 23, 103–106. [Google Scholar]
- Wang, B.; Bai, H.; Su, T.; Chen, Z.; Wang, C. Study on influence of different curing conditions on curing behavior of polybenzoxazine. China Adhes. 2009, 18, 10–13. [Google Scholar]
- Liao, D.; Yan, C.; Pan, L.; Ding, J.; Li, X.; Liu, J. Study on curing kinetics of benzoxazine resin using non-isothermal DSC method. Thermosetting Resin 2011, 26, 1–5. [Google Scholar]
- Montoya-Ospina, M.C.; Verhoogt, H.; Osswald, T.A. Processing and rheological behavior of cross-linked polyethylene containing disulfide bonds. SPE Polym. 2021, 3, 25–40. [Google Scholar] [CrossRef]
- Xin, C.; Yang, X.; Yu, D. Dynamic mechanical behavior and network structure of bisphenol-apolybenzoxazine. J. Beijing Univ. Chem. Technol. (Nat. Sci. Ed.) 2005, 32, 43–52. [Google Scholar]


| Solvent | Dielectric Constant (20 °C) | Product Yield | Product Status |
|---|---|---|---|
| n-Hexan | 1.89 | ———— | ———— |
| Toluene | 2.24 | 58.56% | Yellow oily viscous liquid |
| Nitromethane | 4.33 | ———— | ———— |
| Aldehydes | Theoretical Output/g | Actual Output/g | Yield |
|---|---|---|---|
| Paraformaldehyde | 41.1 | 17.04 | 41.46% |
| Trioxane | 41.1 | ———— | ———— |
| Aqueous formaldehyde solution | 41.1 | 10.04 | 24.43% |
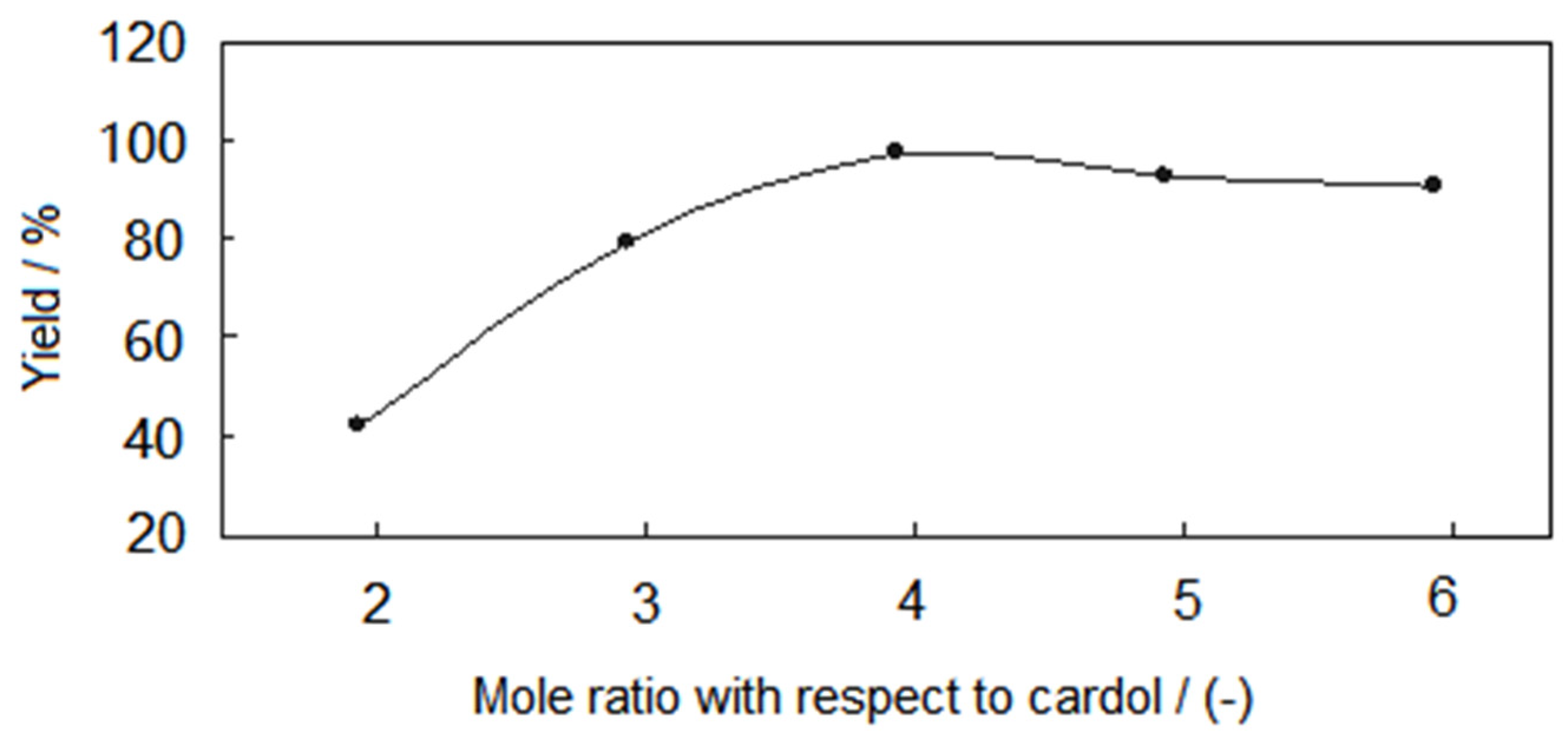
| Mole Ratio | Theoretical Output/g | Actual Output/g | Yield |
|---|---|---|---|
| 2:1:1 | 41.1 | 17.04 | 41.46% |
| 3:1:1 | 41.1 | 32.54 | 79.17% |
| 4:1:1 | 41.1 | 39.78 | 96.79% |
| 5:1:1 | 41.1 | 37.64 | 91.58% |
| 6:1:1 | 41.1 | 37.12 | 90.32% |
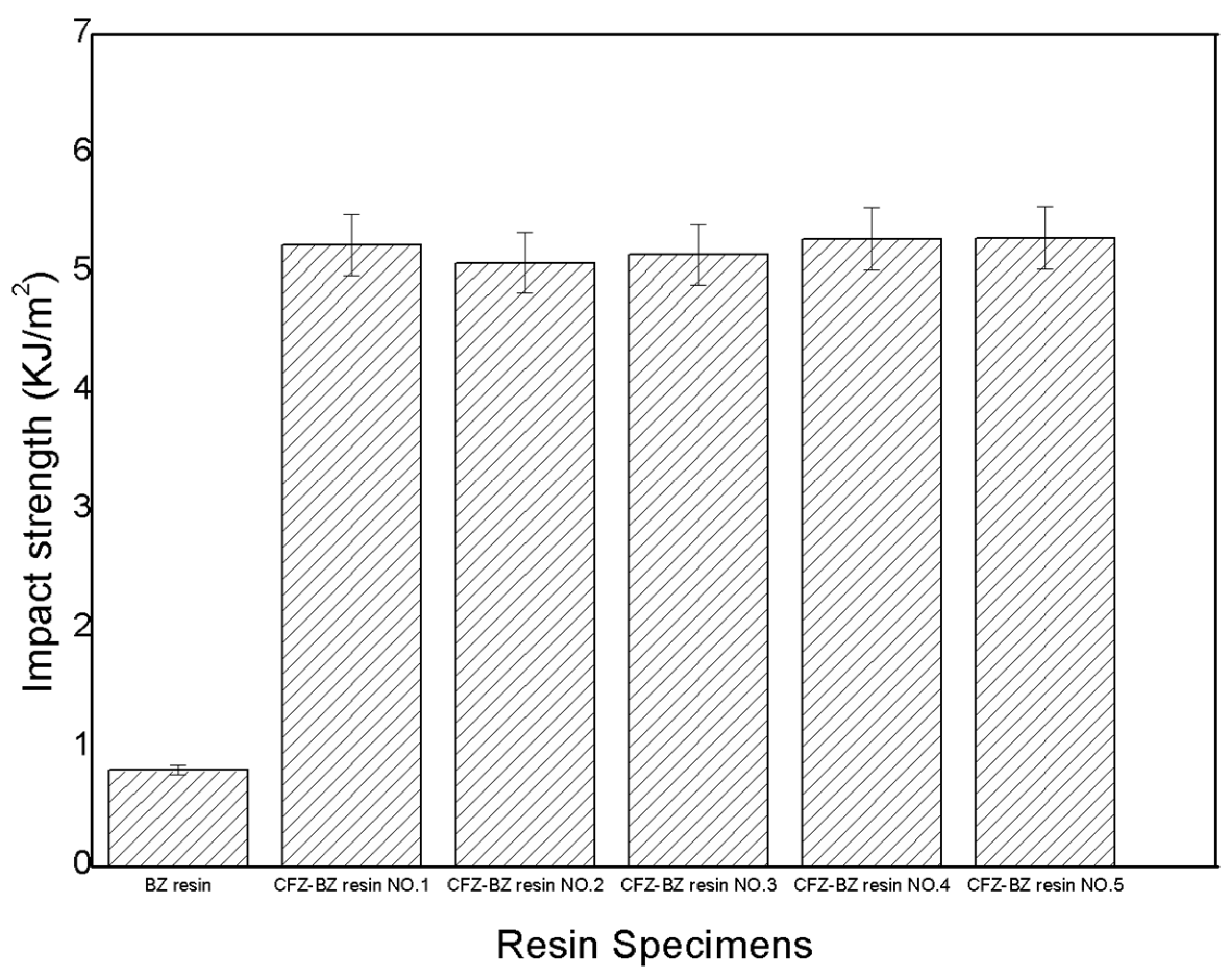
| Name of Resins | Impact Strength KJ/m2 | Impact Strength Ratio (CFZ-BZ/BZ) | Average Ratio |
|---|---|---|---|
| BZ resin | 0.81 | —— | —— |
| CFZ-BZ resin NO.1 | 5.23 | 6.45 | 6.42 |
| CFZ-BZ resin NO.2 | 5.08 | 6.27 | |
| CFZ-BZ resin NO.3 | 5.15 | 6.35 | |
| CFZ-BZ resin NO.4 | 5.28 | 6.51 | |
| CFZ-BZ resin NO.5 | 5.29 | 6.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, J.; Xu, R. Study on the Preparation and Modification of a Novel Bio-Based Cardanol-Furfurylamine Oxazine Resin. Polymers 2025, 17, 1084. https://doi.org/10.3390/polym17081084
Wang J, Xu R. Study on the Preparation and Modification of a Novel Bio-Based Cardanol-Furfurylamine Oxazine Resin. Polymers. 2025; 17(8):1084. https://doi.org/10.3390/polym17081084
Chicago/Turabian StyleWang, Jing, and Riwei Xu. 2025. "Study on the Preparation and Modification of a Novel Bio-Based Cardanol-Furfurylamine Oxazine Resin" Polymers 17, no. 8: 1084. https://doi.org/10.3390/polym17081084
APA StyleWang, J., & Xu, R. (2025). Study on the Preparation and Modification of a Novel Bio-Based Cardanol-Furfurylamine Oxazine Resin. Polymers, 17(8), 1084. https://doi.org/10.3390/polym17081084






