Low Shrinkage Transparent UV-Cured 3D Printing Hard Silicone Resins
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of LMDT-AE
2.3. Preparation of PDMS-AE
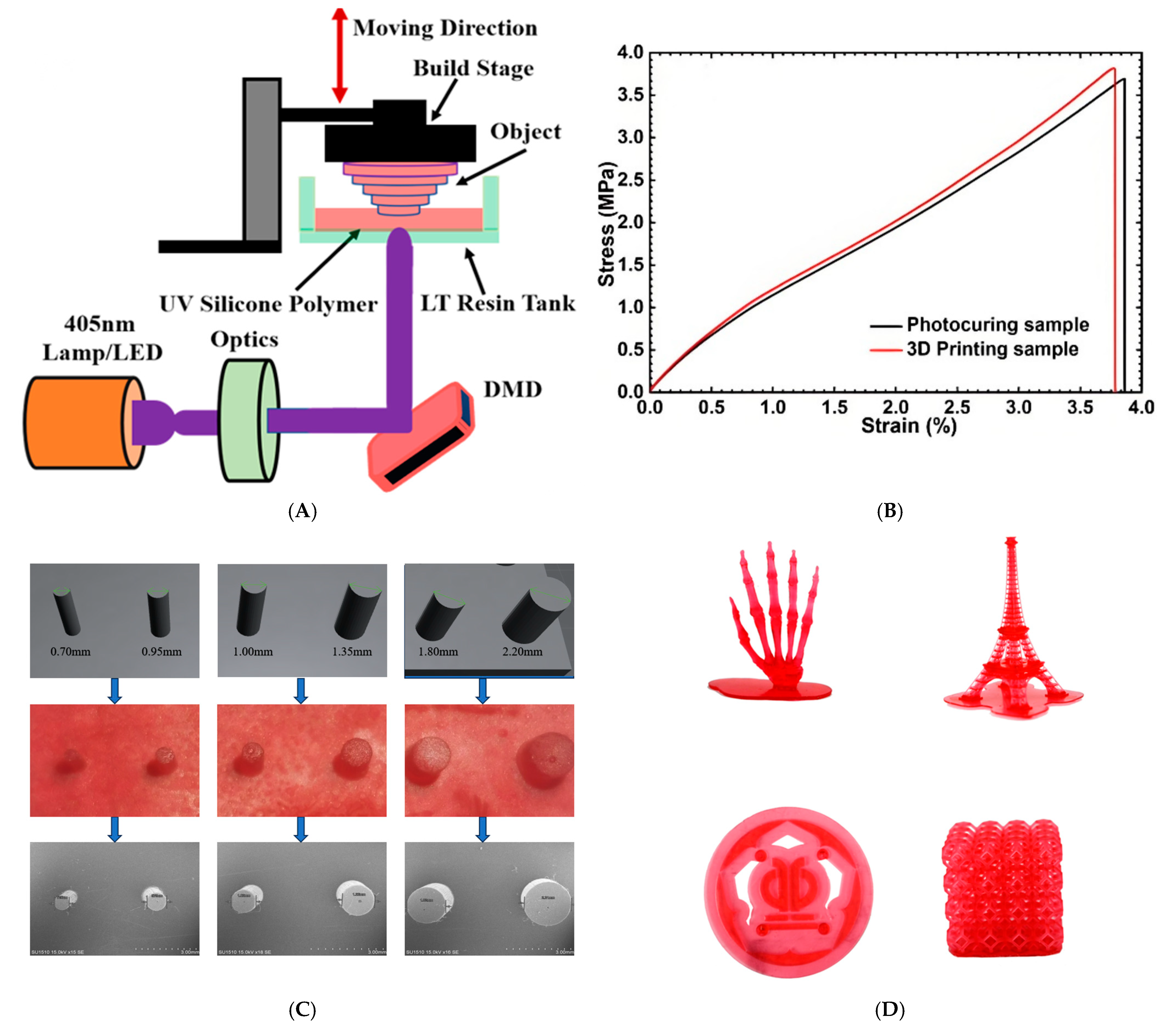
2.4. Preparation of 3D Printed Silicone Resin (3D-AE)
3. Results and Discussion
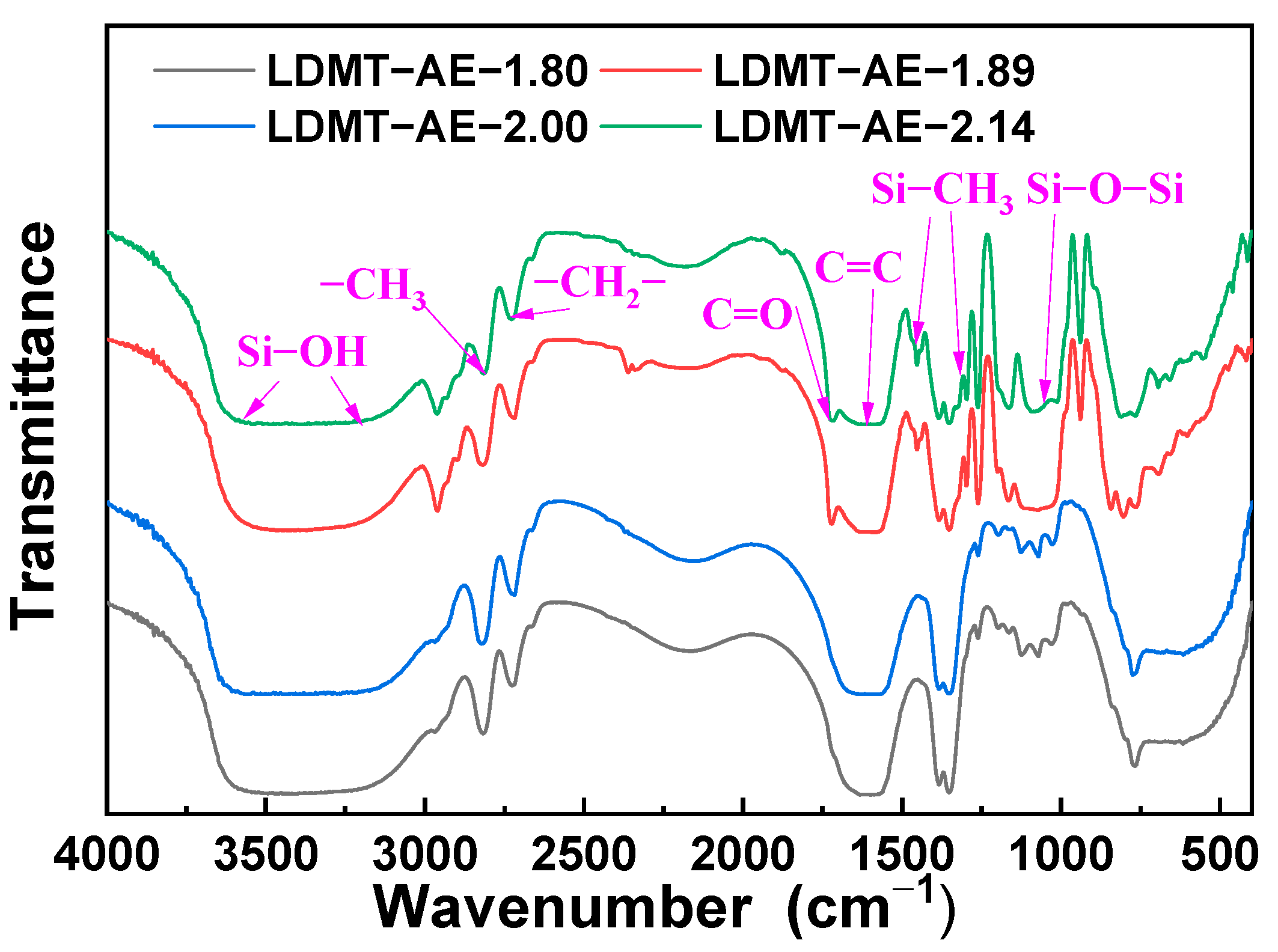
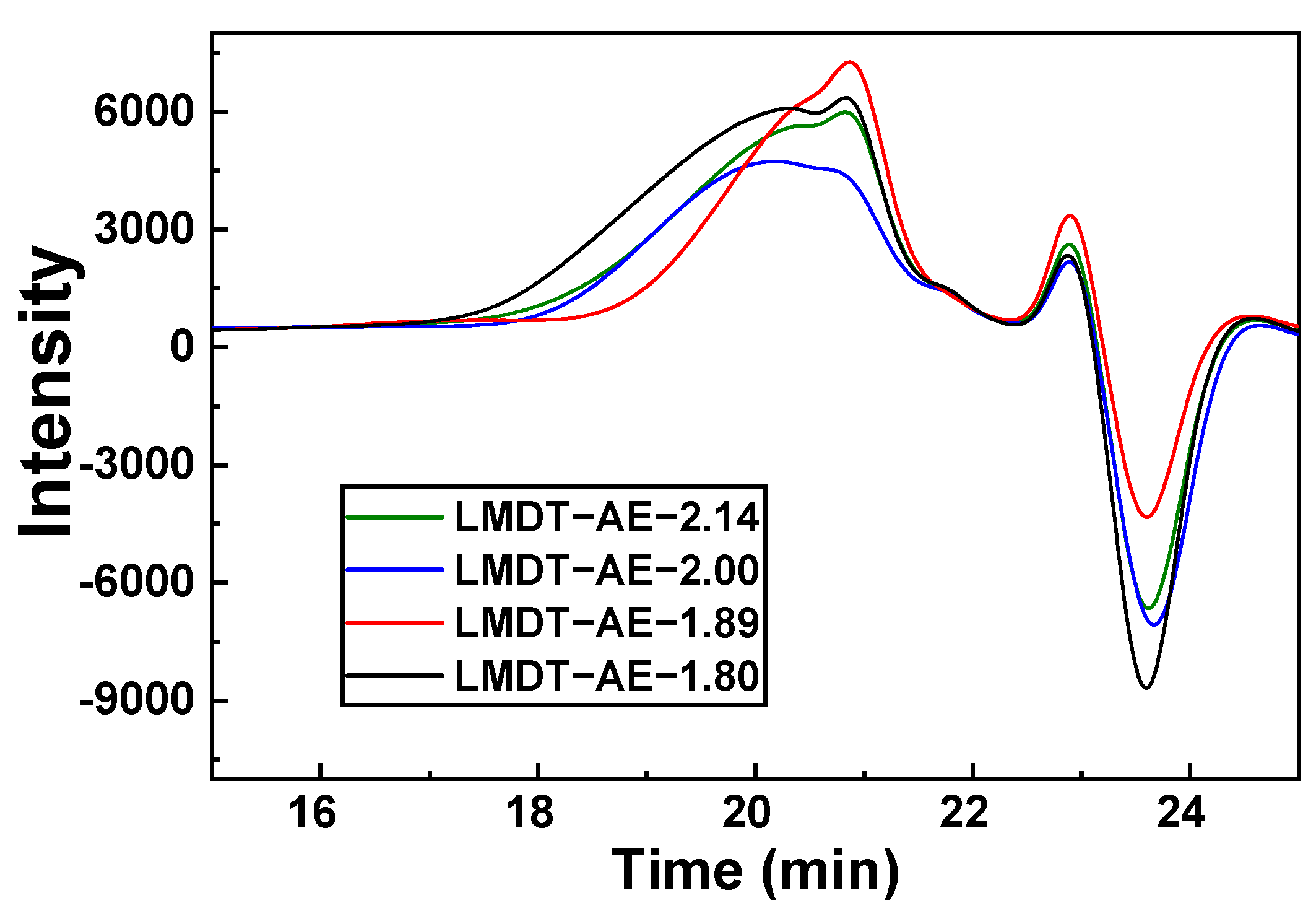
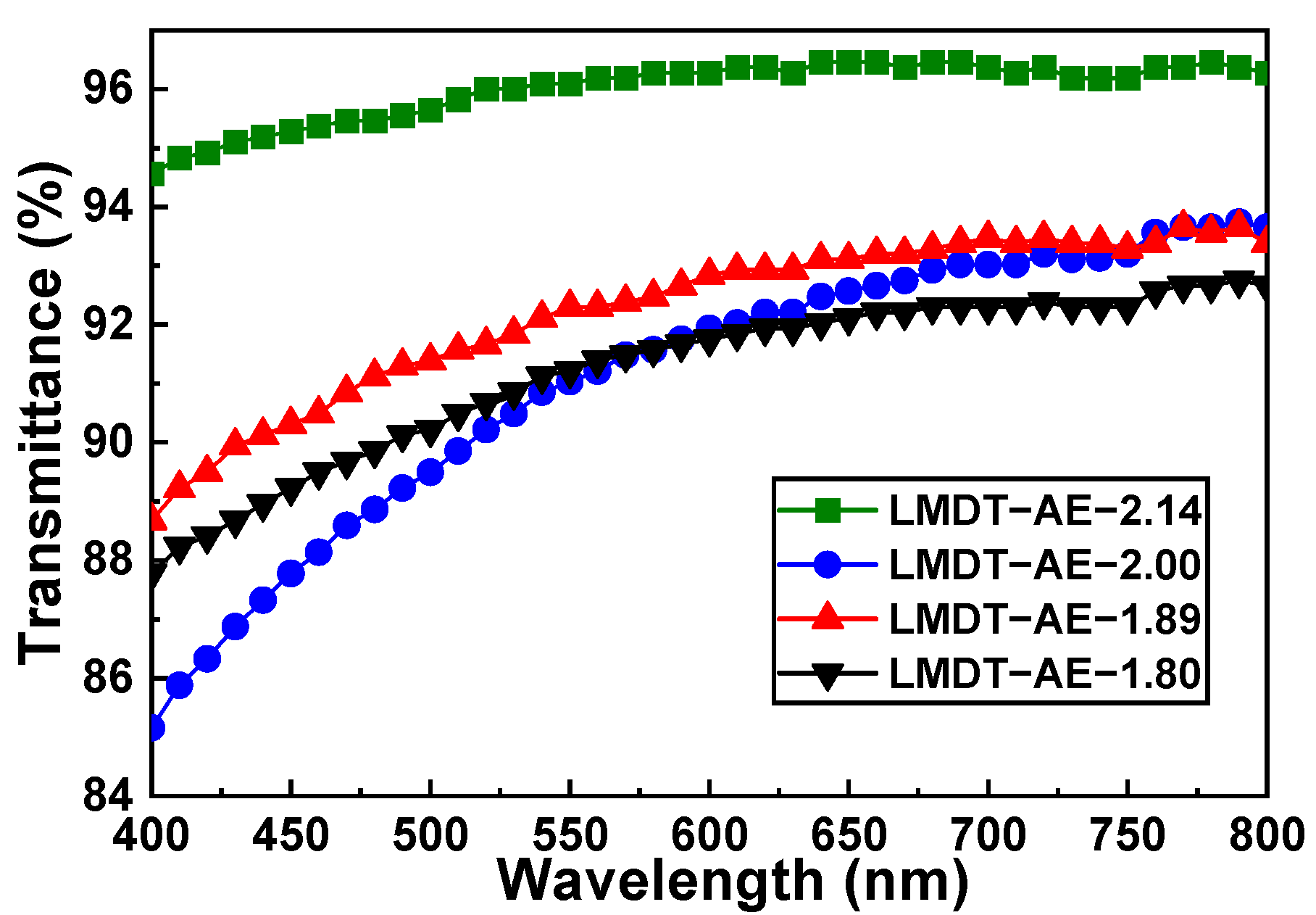
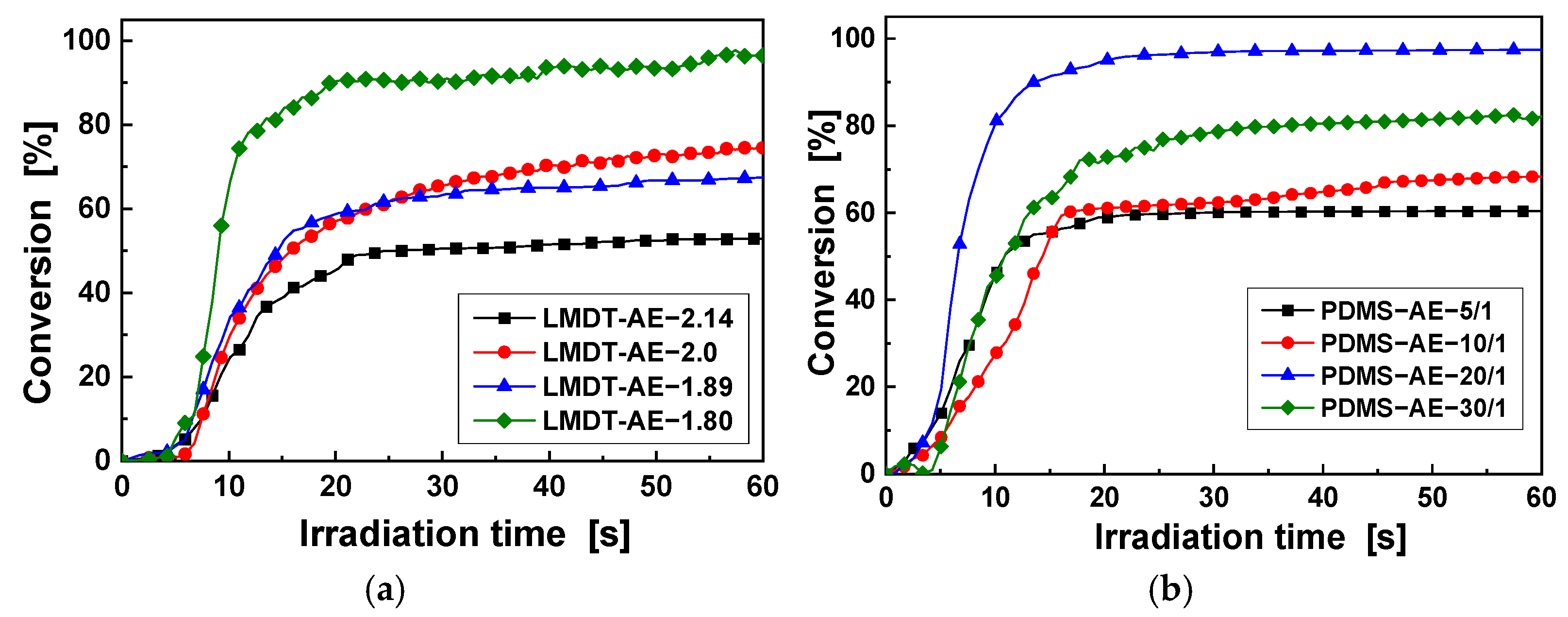
3.1. Characterization and Properties of LMDT-AE
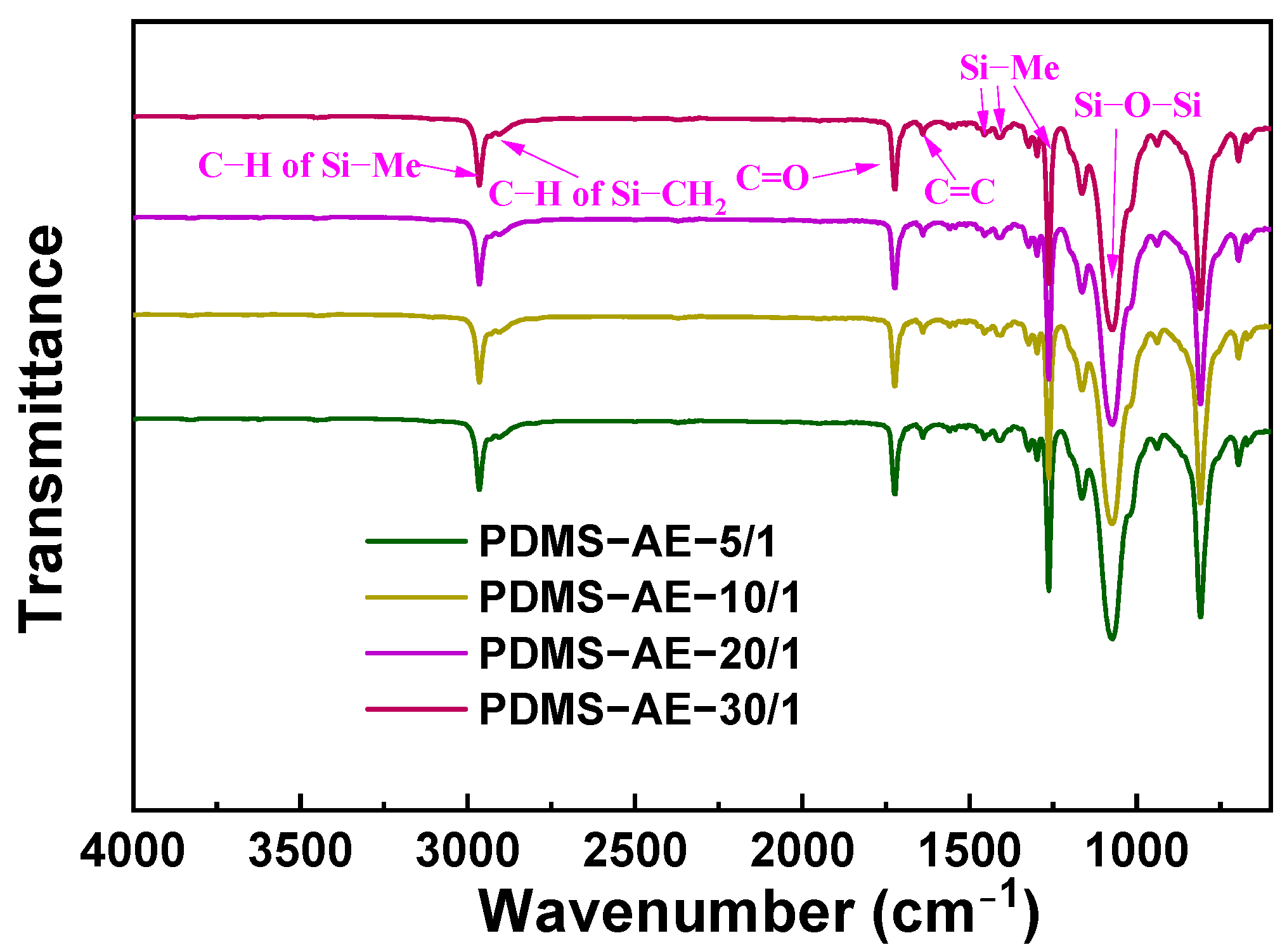
3.2. Characterization and Properties of PDMS-AE
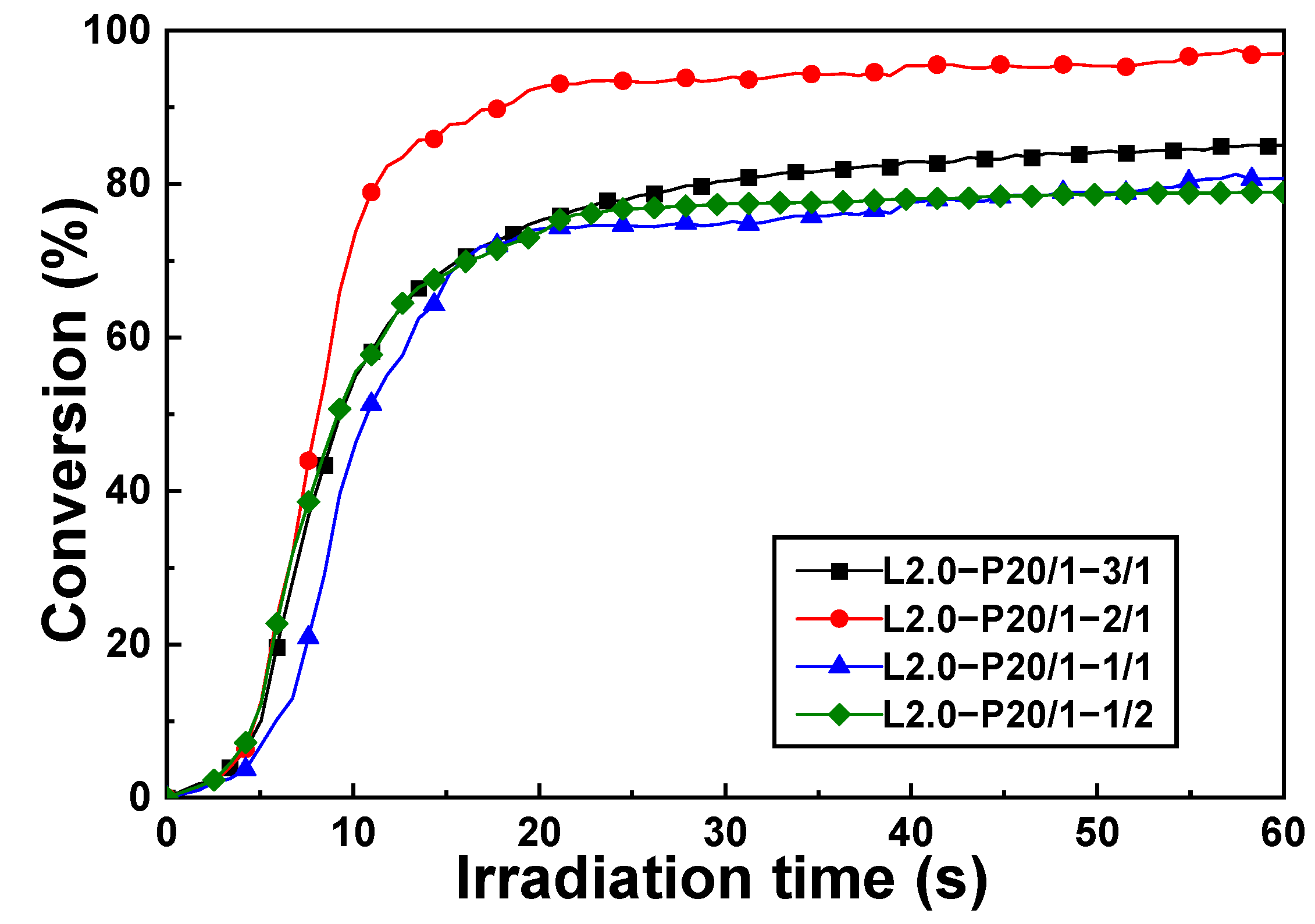
3.3. UV-Curing Kinetics of 3D-AE Elastomers
3.4. Mechanical Properties of 3D-AE Elastomers
3.5. Thermal Properties of 3D-AE Elastomers
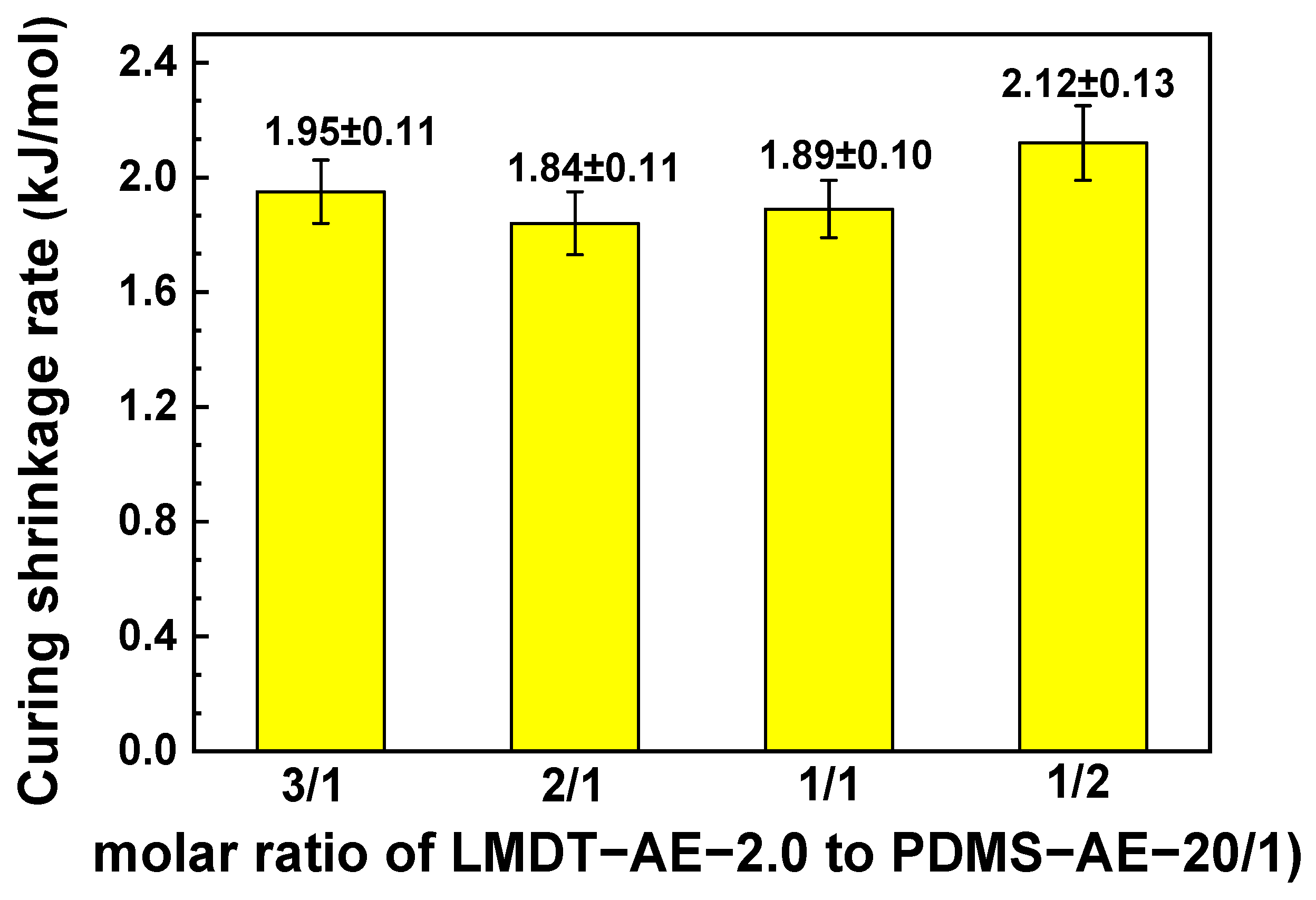
3.6. Curing Shrinkage Properties of 3D-AE Elastomer
3.7. Photo-Patterning and 3D Printing
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Jungst, T.; Smolan, W.; Schacht, K.; Scheibel, T.; Groll, J. Strategies and Molecular Design Criteria for 3D Printable Hydrogels. Chem. Rev. 2016, 116, 1496–1539. [Google Scholar] [CrossRef]
- Layani, M.; Wang, X.; Magdassi, S. Novel Materials for 3D Printing by Photopolymerization. Adv. Mater. 2018, 30, 1706344. [Google Scholar] [CrossRef]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D Printing and Customized Additive Manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef] [PubMed]
- Ronca, A.; Maiullari, F.; Milan, M.; Pace, V.; Gloria, A.; Rizzi, R.; De Santis, R.; Ambrosio, L. Surface functionalization of acrylic based photocrosslinkable resin for 3D printing applications. Bioact. Mater. 2017, 2, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, Y.; Sun, K.; Graff, B.; Xiao, P.; Dumur, F.; Lalevée, J. Design of photoinitiating systems based on the chalcone-anthracene scaffold for LED cationic photopolymerization and application in 3D printing. Eur. Polym. J. 2021, 147, 110300. [Google Scholar] [CrossRef]
- Xenikakis, I.; Tsongas, K.; Tzimtzimis, E.K.; Zacharis, C.K.; Theodoroula, N.; Kalogianni, E.P.; Demiri, E.; Vizirianakis, I.S.; Tzetzis, D.; Fatouros, D.G. Fabrication of hollow microneedles using liquid crystal display (LCD) vat polymerization 3D printing technology for transdermal macromolecular delivery. Int. J. Pharm. 2021, 597, 120303. [Google Scholar] [CrossRef]
- Wang, Z.; Martin, N.; Hini, D.; Mills, B.; Kim, K. Rapid fabrication of multilayer microfluidic devices using the liquid crystal display-based stereolithography 3D printing system. 3D Print. Addit. Manuf. 2017, 4, 156–164. [Google Scholar] [CrossRef]
- Huang, W.; Zu, Z.; Huang, Y.; Xiang, H.; Liu, X. UV-Curing 3D Printing of High-Performance, Recyclable, Biobased Photosensitive Resin Enabled by Dual-Crosslinking Networks. Addit. Manuf. 2024, 91, 104352. [Google Scholar] [CrossRef]
- Huang, X.; Peng, S.; Zheng, L.; Zhuo, D.; Wu, L.; Weng, Z. 3D Printing of High Viscosity UV-curable Resin for Highly Stretchable and Resilient Elastomer. Adv. Mater. 2023, 35, 2304430. [Google Scholar] [CrossRef]
- Peng, S.; Wang, Z.; Lin, J.; Miao, J.; Zheng, L.; Yang, Z.; Weng, Z.; Wu, L. Tailored and Highly Stretchable Sensor Prepared by Crosslinking an Enhanced 3D Printed UV-curable Sacrificial Mold. Adv. Funct. Mater. 2021, 31, 2008729. [Google Scholar] [CrossRef]
- Yang, Z.; Peng, S.; Wang, Z.; Miao, J.-T.; Zheng, L.; Wu, L.; Weng, Z. UV-Curable, Low-Viscosity Resin with a High Silica Filler Content for Preparing Ultrastiff, 3D-Printed Molds. ACS Appl. Polym. Mater. 2022, 4, 2636–2647. [Google Scholar] [CrossRef]
- Shi, H.; Wang, K.; Liu, Y.; He, K.; Huo, P.; Dong, J.; Jiang, Z.; Zhang, D. Rapidly UV-Curable Resin for Soft Sensors of Embedded 3D Printing. Eur. Polym. J. 2022, 181, 111680. [Google Scholar] [CrossRef]
- Schlögl, S.; Reischl, M.; Ribitsch, V.; Kern, W. UV Induced Microcellular Foaming—A New Approach towards the Production of 3D Structures in Offset Printing Techniques. Prog. Org. Coat. 2012, 73, 54–61. [Google Scholar] [CrossRef]
- Oliveira, R.; Sousa, L.M.; Rocha, A.M.; Nogueira, R.; Bilro, L. UV Inscription and Pressure Induced Long-Period Gratings through 3D Printed Amplitude Masks. Sensors 2021, 21, 1977. [Google Scholar] [CrossRef]
- Wei, J.; Ma, Y.; Hu, Y.; Zhu, J.; Gou, H.; Qian, D.; Fan, H. Enhanced Mechanical Strength and Water Resistance in Waterborne Polyurethanes through Hydroxyl-Functionalized MQ Silicone Resin Modification. Prog. Org. Coat. 2024, 194, 108555. [Google Scholar] [CrossRef]
- Silvaroli, A.J.; Chang, V.; Heyl, T.R.; Beebe, J.M.; Ahn, D.; Mangold, S.; Clark, B.; Wang, M.; Shull, K.R. Effects of Composition and Cure Parameters on the Mechanical and Optical Properties of Silicone/Methacrylate Hybrid Elastomers. Macromolecules 2024, 57, 8588–8600. [Google Scholar] [CrossRef]
- Lai, X.; Peng, J.; Cheng, Q.; Tomsia, A.P.; Zhao, G.; Liu, L.; Zou, G.; Song, Y.; Jiang, L.; Li, M. Bioinspired Color Switchable Photonic Crystal Silicone Elastomer Kirigami. Angew. Chem. Int. Ed. 2021, 60, 14307–14312. [Google Scholar] [CrossRef]
- Mazurek, P.; Vudayagiri, S.; Skov, A.L. How to Tailor Flexible Silicone Elastomers with Mechanical Integrity: A Tutorial Review. Chem. Soc. Rev. 2019, 48, 1448–1464. [Google Scholar] [CrossRef]
- Wang, C.; Qiao, L.; Li, S.; Duan, P.; Fu, X.; Duan, Y.; Cheng, H.; Liu, J.; Zhang, L. Innovative Synthesis of Photo-responsive, Self-healing Silicone Elastomers with Enhanced Mechanical Properties and Thermal Stability. Small 2024, 20, 2403941. [Google Scholar] [CrossRef]
- Zhou, Z.; Yu, M.; Cao, S.; Huang, Y.; Dai, Z.; Shen, J.; Cao, X.; Wang, Y.; Han, X. Branched Silicone Acrylates Enhanced Photocurable Epoxy Resins with High Strength, Toughness, and Low Dielectric Constant for SLA 3D Printing. ACS Appl. Polym. Mater. 2024, 6, 2733–2740. [Google Scholar] [CrossRef]
- Zhao, L.; Xu, X.; Huang, Y.; Bai, J.; Lv, D.; Zhong, R.; Yu, D.; Wu, X. 3D-Printable Hydrophobic Silicone Inks for Antiadhesion Tough Objects via Molecular Engineering. Chem. Eng. J. 2024, 480, 147972. [Google Scholar] [CrossRef]
- Pongwisuthiruchte, A.; Dubas, S.T.; Aumnate, C.; Potiyaraj, P. Mechanically Tunable Resins Based on Acrylate-Based Resin for Digital Light Processing (DLP) 3D Printing. Sci. Rep. 2022, 12, 20025. [Google Scholar] [CrossRef] [PubMed]
- Huo, M.; Hu, J.G.; Clarke, D.R. Covalent Adaptable Networks with Rapid UV Response Based on Reversible Thiol–Ene Reactions in Silicone Elastomers. Macromolecules 2023, 56, 9107–9116. [Google Scholar] [CrossRef]
- Nguyen, K.D.Q.; Megone, W.V.; Kong, D.; Gautrot, J.E. Ultrafast Diffusion-Controlled Thiol–Ene Based Crosslinking of Silicone Elastomers with Tailored Mechanical Properties for Biomedical Applications. Polym. Chem. 2016, 7, 5281–5293. [Google Scholar] [CrossRef]
- Bertlein, S.; Brown, G.; Lim, K.S.; Jungst, T.; Boeck, T.; Blunk, T.; Tessmar, J.; Hooper, G.J.; Woodfield, T.B.F.; Groll, J. Thiol–Ene Clickable Gelatin: A Platform Bioink for Multiple 3D Biofabrication Technologies. Adv. Mater. 2017, 29, 1703404. [Google Scholar] [CrossRef]
- Xiang, H.; Wang, X.; Ou, Z.; Lin, G.; Yin, J.; Liu, Z.; Zhang, L.; Liu, X. UV-Curable, 3D Printable and Biocompatible Silicone Elastomers. Prog. Org. Coat. 2019, 137, 105372. [Google Scholar] [CrossRef]
- Zheng, J.; Cai, Y.; Hu, Y.; Zhu, J.; Wei, J.; Ma, Y.; Wan, J.; Fan, H. Bio-Based Epoxy Functionalized MQ Silicone Resins: From Synthesis to Toughened Epoxy Composites with Good Mechanical Properties, Thermal Resistance and Transparency. Polym. Chem. 2022, 13, 5325–5336. [Google Scholar] [CrossRef]
- Huang, S.; Huang, H.; Liu, X.; Xiang, H. Free-Radical/Cationic Hybrid UV-Curable Silicone Resin for Age-Resistant Coatings. Ind. Eng. Chem. Res. 2024, 63, 14657–14667. [Google Scholar] [CrossRef]
- Pan, Z.; Chen, M.; Zeng, K.; Kang, Y. Synthesis of Epoxy-Modified Methyl Phenyl Silicone Resins for LED Encapsulation. Silicon 2022, 14, 1159–1167. [Google Scholar] [CrossRef]
- Du, D.; Chen, X.; Wu, Y.; Wu, C.; Qu, Z.; Song, Y.; Qin, D.; Li, Q.; Dong, H. The Preparation of Acryloxyl Group Functionalized Siloxane Polymers and the Study of Their Ultra Violet Curing Properties. Polymers 2024, 16, 465. [Google Scholar] [CrossRef]
- Goswami, A.D.; Singh, S.; Jadhav, A.J.; Pinjari, D.V. Development of Silicone-Polyether-Methacrylate Copolymer: A High-Performance UV-Cured Additive for Polyurethane Coatings. Polym.-Plast. Technol. Mater. 2025, 64, 876–890. [Google Scholar] [CrossRef]
- Friedrich, L.M.; Woodcock, J.W. Filament Disturbance and Fusion during Embedded 3D Printing of Silicones. ACS Biomater. Sci. Eng. 2024, 10, 6690–6710. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, R.; Chen, S.; Gao, J.; Cai, C.; Zheng, Y.; Liu, X.; Qu, B.; Chen, N.; Zhuo, D. Low Viscosity and Highly Flexible Stereolithographic 3D Printing Resins for Flexible Sensors. Mater. Design. 2024, 243, 113052. [Google Scholar] [CrossRef]
- Chen, Y.; Ye, P.; Huang, L.; Zhao, S.; Zhang, H.; Wang, Z.; Liu, Y.; Liu, H. Photopolymerized 3D Printing Materials for Optical Elements. Adv. Opt. Mater. 2024, 12, 2302685. [Google Scholar] [CrossRef]
- Liu, Z.; Hong, P.; Huang, Z.; Zhang, T.; Xu, R.; Chen, L.; Xiang, H.; Liu, X. Self-Healing, Reprocessing and 3D Printing of Transparent and Hydrolysis-Resistant Silicone Elastomers. Chem. Eng. J. 2020, 387, 124142. [Google Scholar] [CrossRef]
- Liu, Z.; Xiao, D.; Liu, G.; Xiang, H.; Rong, M.; Zhang, M. Self-Healing and Reprocessing of Transparent UV-Cured Polysiloxane Elastomer. Prog. Org. Coat. 2021, 159, 106450. [Google Scholar] [CrossRef]
- Zhai, Q.; Wu, X.; Zhao, S.; Zhou, C. Curing kinetics study by FTIR spectroscopy and properties analysis of methyl silicone resin membrane. Silicon 2020, 12, 2761–2768. [Google Scholar] [CrossRef]








| Name | KH570 | DMC | MM | TFSA 1 | H2O 2 | R |
|---|---|---|---|---|---|---|
| LMDT‒AE-2.14 | 24.84 g (0.10 mol) | 29.60 g (0.40 mol) | 16.2 g (0.10 mol) | 0.35 g | 4.32 g | 2.14 |
| LMDT‒AE-2.00 | 49.68 g (0.20 mol) | 29.60 g (0.40 mol) | 16.2 g (0.10 mol) | 0.48 g | 8.64 g | 2.00 |
| LMDT‒AE-1.89 | 74.52 g (0.30 mol | 29.60 g (0.40 mol) | 16.2 g (0.10 mol) | 0.60 g | 12.96 g | 1.89 |
| LMDT‒AE-1.80 | 99.36 g (0.40 mol) | 29.60 g (0.40 mol) | 16.2 g (0.10 mol) | 0.73 g | 17.28 g | 1.80 |
| Name | KH571 | DMC | MM | TMAH 1 | H2O 2 |
|---|---|---|---|---|---|
| PDMS-AE-5/1 | 83.64 g (0.36 mol) | 133.49 g (1.8 mol) | 0.81 g (0.005 mol) | 0.44 g | 15.55 g |
| PDMS-AE-10/1 | 41.82 g (0.18 mol) | 133.49 g (1.8 mol) | 0.81 g (0.005 mol) | 0.35 g | 6.48 g |
| PDMS-AE-20/1 | 20.91 g (0.09 mol) | 133.49 g (1.8 mol) | 0.81 g (0.005 mol) | 0.31 g | 3.24 g |
| PDMS-AE-30/1 | 13.94 g (0.06 mol) | 133.49 g (1.8 mol) | 0.81 g (0.005 mol) | 0.30 g | 2.60 g |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, H.; Shen, Q.; Liu, Z.; Zhou, X.; Fang, Y.; Xiang, H.; Liu, X. Low Shrinkage Transparent UV-Cured 3D Printing Hard Silicone Resins. Polymers 2025, 17, 1123. https://doi.org/10.3390/polym17081123
Wu H, Shen Q, Liu Z, Zhou X, Fang Y, Xiang H, Liu X. Low Shrinkage Transparent UV-Cured 3D Printing Hard Silicone Resins. Polymers. 2025; 17(8):1123. https://doi.org/10.3390/polym17081123
Chicago/Turabian StyleWu, Haibo, Qili Shen, Zhu Liu, Xiantai Zhou, Yanxiong Fang, Hongping Xiang, and Xiaoxuan Liu. 2025. "Low Shrinkage Transparent UV-Cured 3D Printing Hard Silicone Resins" Polymers 17, no. 8: 1123. https://doi.org/10.3390/polym17081123
APA StyleWu, H., Shen, Q., Liu, Z., Zhou, X., Fang, Y., Xiang, H., & Liu, X. (2025). Low Shrinkage Transparent UV-Cured 3D Printing Hard Silicone Resins. Polymers, 17(8), 1123. https://doi.org/10.3390/polym17081123






