Properties of Emulsion Co-Precipitated Collagen/Bambara Groundnut Protein-Based Film as Influenced by Basil Essential Oil and Soy Lecithin
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of Bambara Groundnut Protein Isolate (BGPI)
2.3. Extraction of Acid-Soluble Collagen (ASC) from Fish Skin
2.4. Co-Precipitated Protein (CPP) Preparation
2.5. Preparation of the Film-Forming Emulsion
2.6. Analysis of the Film-Forming Emulsion
2.6.1. Oil Droplet Size
2.6.2. Flocculation Factor (Ff) and Coalescence Index (Ci)
2.6.3. Confocal Laser Scanning Microscopy (CLSM)
2.7. Preparation of the Emulsion-Based Film
2.8. Analysis of the Films
2.8.1. Thickness
2.8.2. Mechanical Properties
2.8.3. Water Vapor Permeability (WVP)
2.8.4. Color and Light Transmission
2.8.5. Scanning Electron Microscopy (SEM)
2.8.6. Sealing Ability
2.8.7. FTIR
2.9. Antioxidant Activities of Films
2.9.1. DPPH Radical Scavenging Activity (DPPH-RSA)
2.9.2. Ferric Reducing Antioxidant Power (FRAP)
2.9.3. Oxygen Radical Absorbance Capacity (ORAC)
2.9.4. Metal Chelating Activity (MCA)
2.10. Statistical Analysis
3. Results and Discussion
3.1. Properties of Film-Forming Emulsion
3.1.1. Oil Droplet Size
3.1.2. Flocculation Factor (Ff) and Coalescence Index (Ci)
3.1.3. Confocal Laser Scanning Microscopy (CLSM) Images
3.2. Properties and Characteristics of the Films
3.2.1. Appearance and Thickness
3.2.2. Mechanical Properties
3.2.3. Water Vapor Permeability (WVP)
3.2.4. Color
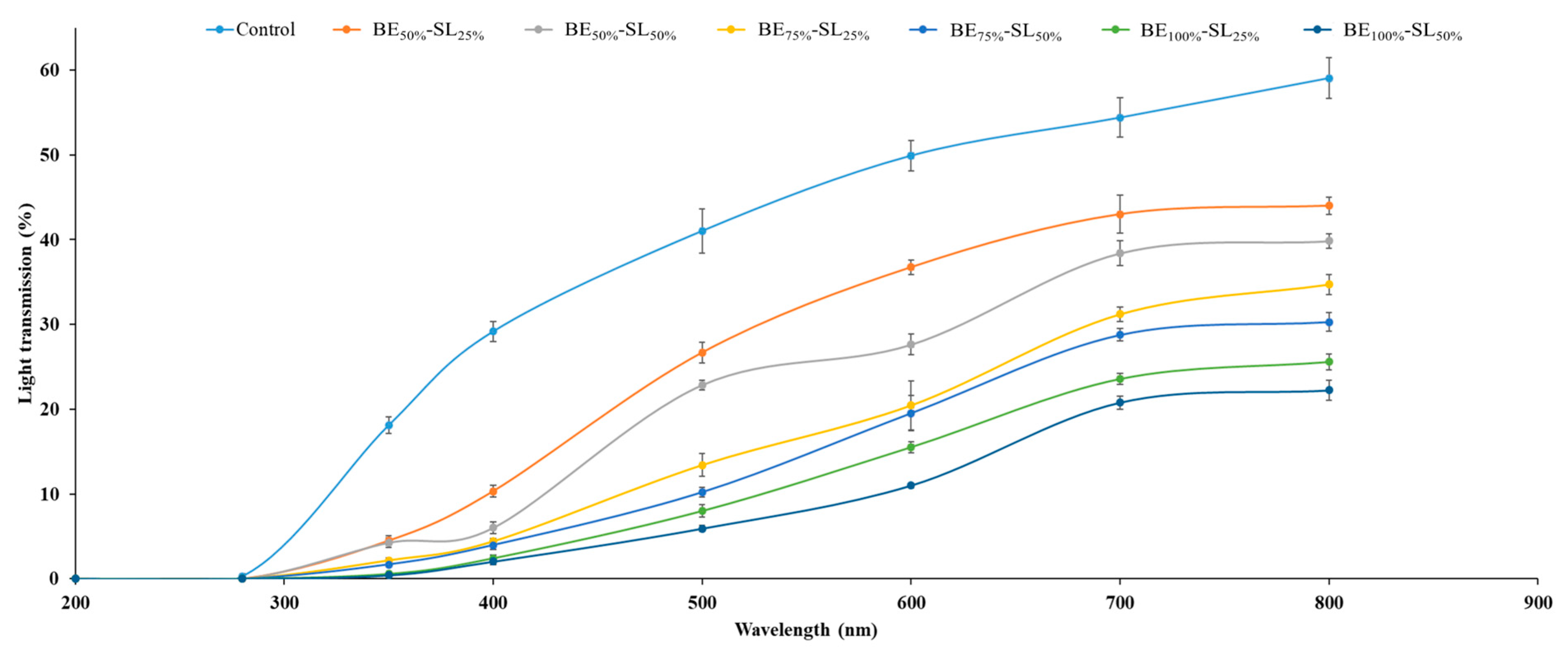
3.2.5. Light Transmission
3.2.6. Scanning Electron Microscopy (SEM)
3.2.7. Sealability
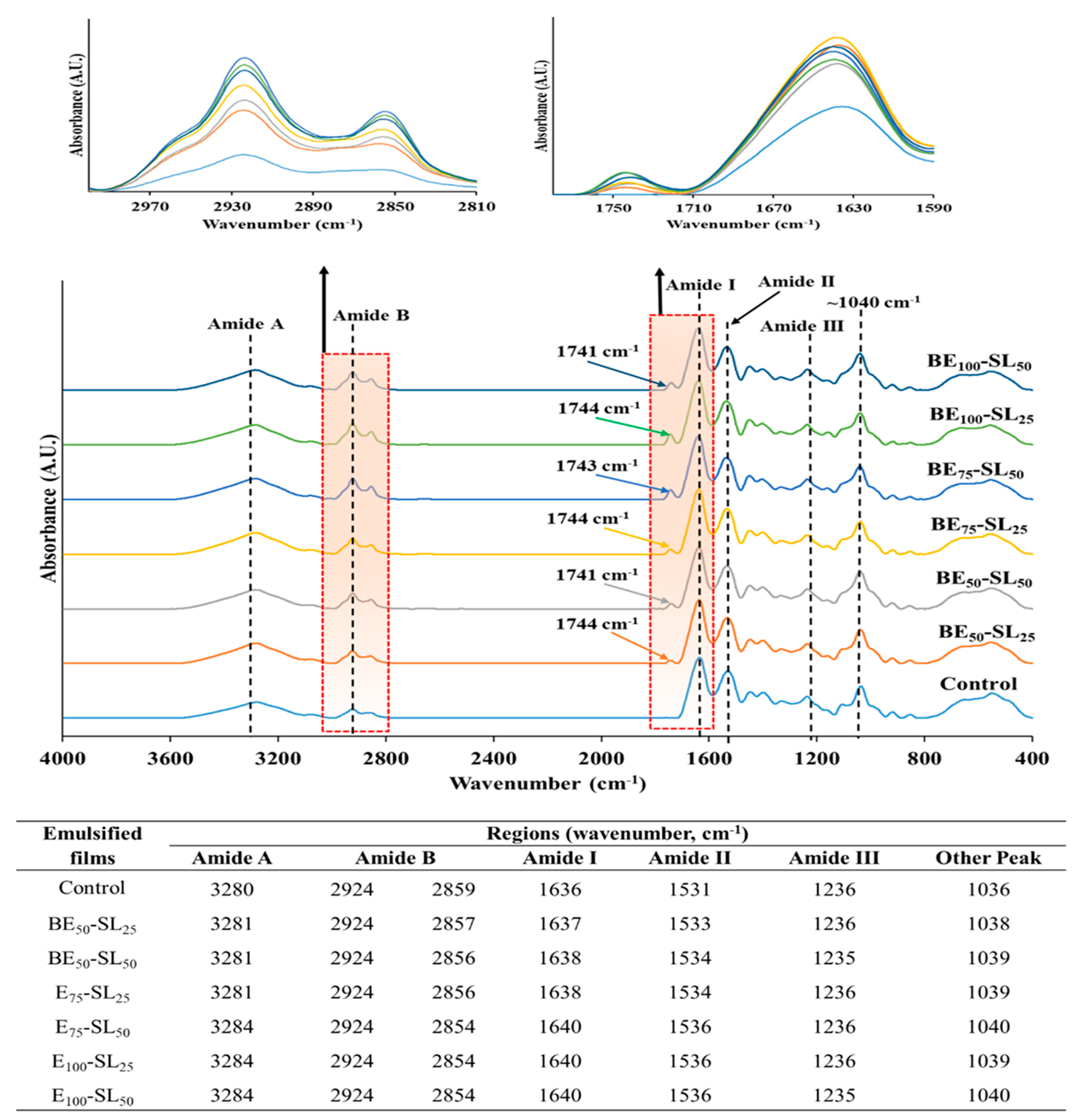
3.2.8. FTIR
3.3. Antioxidant Activities
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- He, Q.; Huang, Y.; Lin, B.; Wang, S. A nanocomposite film fabricated with simultaneously extracted protein-polysaccharide from a marine alga and TiO2 nanoparticles. J. Appl. Phycol. 2017, 29, 1541–1552. [Google Scholar] [CrossRef]
- Abdul Khalil, H.; Lai, T.K.; Tye, Y.Y.; Paridah, M.; Fazita, M.N.; Azniwati, A.; Dungani, R.; Rizal, S. Preparation and characterization of microcrystalline cellulose from sacred bali bamboo as reinforcing filler in seaweed-based composite film. Fibers Polym. 2018, 19, 423–434. [Google Scholar] [CrossRef]
- Wang, W.; Liu, Y.; Liu, A.; Zhao, Y.; Chen, X. Effect of in situ apatite on performance of collagen fiber film for food packaging applications. J. Appl. Polym. Sci. 2016, 133, 44155. [Google Scholar] [CrossRef]
- Nilsuwan, K.; Rajagukguk, Y.V.; Patil, U.; Prodpran, T.; Benjakul, S. Fish gelatin-based film containing maillard reaction products: Properties and its use as bag for packing chicken skin oil. J. Renew. Mater. 2024, 12, 1125–1143. [Google Scholar] [CrossRef]
- Kantakul, J.; Nilsuwan, K.; Kotcharat, C.; Chuecheen, K.; Saetang, J.; Prodpran, T.; Hong, H.; Zhang, B.; Benjakul, S. Properties of antioxidant film based on protein isolate and seed coat extract from Bambara groundnut. Foods 2024, 13, 3424. [Google Scholar] [CrossRef]
- Dong, M.; Tian, L.; Li, J.; Jia, J.; Dong, Y.; Tu, Y.; Liu, X.; Tan, C.; Duan, X. Improving physicochemical properties of edible wheat gluten protein films with proteins, polysaccharides and organic acid. LWT 2022, 154, 112868. [Google Scholar] [CrossRef]
- Awal, M.S.; Benjakul, S.; Prodpran, T.; Nilsuwan, K. Characteristics and properties of co-precipitated protein and film based on Bambara groundnut protein isolate and fish skin acid-soluble collagen. J. Agric. Food Res. 2024, 18, 101430. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S.; Prodpran, T. Comparative studies on properties and antioxidative activity of fish skin gelatin films incorporated with essential oils from various sources. Int. Aquat. Res. 2014, 6, 1–12. [Google Scholar] [CrossRef]
- Amin, U.; Khan, M.K.I.; Maan, A.A.; Nazir, A.; Riaz, S.; Khan, M.U.; Sultan, M.; Munekata, P.E.; Lorenzo, J.M. Biodegradable active, intelligent, and smart packaging materials for food applications. Food Packag. Shelf Life 2022, 33, 100903. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S.; Prodpran, T. Characteristics and antioxidant activity of leaf essential oil–incorporated fish gelatin films as affected by surfactants. Int. J. Food Sci. Technol. 2013, 48, 2143–2149. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S. Essential oils: Extraction, bioactivities, and their uses for food preservation. J. Food Sci. 2014, 79, R1231–R1249. [Google Scholar] [CrossRef]
- Ahmed, A.F.; Attia, F.A.; Liu, Z.; Li, C.; Wei, J.; Kang, W. Antioxidant activity and total phenolic content of essential oils and extracts of sweet basil (Ocimum basilicum L.) plants. Food Sci. Hum. Wellness 2019, 8, 299–305. [Google Scholar] [CrossRef]
- Koroch, A.R.; Simon, J.E.; Juliani, H.R. Essential oil composition of purple basils, their reverted green varieties (Ocimum basilicum) and their associated biological activity. Ind. Crops Prod. 2017, 107, 526–530. [Google Scholar] [CrossRef]
- Flores, Z.; San-Martin, D.; Beldarraín-Iznaga, T.; Leiva-Vega, J.; Villalobos-Carvajal, R. Effect of homogenization method and carvacrol content on microstructural and physical properties of chitosan-based films. Foods 2021, 10, 141. [Google Scholar] [CrossRef]
- Cruz-Diaz, K.; Cobos, Á.; Fernández-Valle, M.E.; Díaz, O.; Cambero, M.I. Characterization of edible films from whey proteins treated with heat, ultrasounds and/or transglutaminase. Application in cheese slices packaging. Food Packag. Shelf Life 2019, 22, 100397. [Google Scholar] [CrossRef]
- Gul, O.; Saricaoglu, F.T.; Besir, A.; Atalar, I.; Yazici, F. Effect of ultrasound treatment on the properties of nano-emulsion films obtained from hazelnut meal protein and clove essential oil. Ultrason. Sonochem. 2018, 41, 466–474. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S.; Prodpran, T. Structural, morphological and thermal behaviour characterisations of fish gelatin film incorporated with basil and citronella essential oils as affected by surfactants. Food Hydrocoll. 2014, 41, 33–43. [Google Scholar] [CrossRef]
- Nilsuwan, K.; Patil, U.; Tu, C.; Zhang, B.; Benjakul, S. Salmon skin acid-soluble collagen produced by a simplified recovery process: Yield, compositions, and molecular characteristics. Fishes 2022, 7, 330. [Google Scholar] [CrossRef]
- Nilsuwan, K.; Benjakul, S.; Prodpran, T. Emulsion stability and properties of fish gelatin-based films as affected by palm oil and surfactants. J. Sci. Food Agric. 2016, 96, 2504–2513. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S.; Prodpran, T.; Pisuchpen, S.; Osako, K. Mechanical, thermal and heat sealing properties of fish skin gelatin film containing palm oil and basil essential oil with different surfactants. Food Hydrocoll. 2016, 56, 93–107. [Google Scholar] [CrossRef]
- ASTM F-88; Standard Test Method for Seal Strength of Flexible Barrier Materials. ASTM: West Conshohocken, PA, USA, 2016.
- Benjakul, S.; Kittiphattanabawon, P.; Sumpavapol, P.; Maqsood, S. Antioxidant activities of lead (Leucaena leucocephala) seed as affected by extraction solvent, prior dechlorophyllisation and drying methods. J. Food Sci. Technol. 2014, 51, 3026–3037. [Google Scholar] [CrossRef] [PubMed]
- Deng, L. Current progress in the utilization of soy-based emulsifiers in food applications—A Review. Foods 2021, 10, 1354. [Google Scholar] [CrossRef] [PubMed]
- Langevin, D. Recent advances on emulsion and foam stability. Langmuir 2023, 39, 3821–3828. [Google Scholar] [CrossRef]
- Nilsuwan, K.; Benjakul, S.; Prodpran, T. Effects of soy lecithin levels and microfluidization conditions on properties of fish gelatin-based film incorporated with palm oil. Int. J. Food Eng. 2016, 12, 647–660. [Google Scholar] [CrossRef]
- Tongnuanchan, P.; Benjakul, S.; Prodpran, T.; Nilsuwan, K. Emulsion film based on fish skin gelatin and palm oil: Physical, structural and thermal properties. Food Hydrocoll. 2015, 48, 248–259. [Google Scholar] [CrossRef]
- Ettoumi, Y.L.; Chibane, M.; Romero, A. Emulsifying properties of legume proteins at acidic conditions: Effect of protein concentration and ionic strength. LWT Food Sci. Technol. 2016, 66, 260–266. [Google Scholar] [CrossRef]
- Patil, U.; Benjakul, S. Physical and textural properties of mayonnaise prepared using virgin coconut oil/fish oil blend. Food Biophys. 2019, 14, 260–268. [Google Scholar] [CrossRef]
- Ribeiro-Santos, R.; Sanches-Silva, A.; Motta, J.F.G.; Andrade, M.; de Araújo Neves, I.; Teófilo, R.F.; de Carvalho, M.G.; de Melo, N.R. Combined use of essential oils applied to protein base active food packaging: Study in vitro and in a food simulant. Eur. Polym. J. 2017, 93, 75–86. [Google Scholar] [CrossRef]
- Zhao, Y.; Ren, Z.; Shi, L.; Weng, W. Effect of W/O pre-emulsion prepared with different emulsifiers on the physicochemical properties of soy protein isolate-based emulsion films. Food Hydrocoll. 2023, 139, 108440. [Google Scholar] [CrossRef]
- Silva, N.d.S.; de Souza Farias, F.; dos Santos Freitas, M.M.; Hernández, E.J.G.P.; Dantas, V.V.; Oliveira, M.E.C.; Joele, M.R.S.P.; Lourenço, L.d.F.H. Artificial intelligence application for classification and selection of fish gelatin packaging film produced with incorporation of palm oil and plant essential oils. Food Packag. Shelf Life 2021, 27, 100611. [Google Scholar] [CrossRef]
- Hemalatha, T.; UmaMaheswari, T.; Senthil, R.; Krithiga, G.; Anbukkarasi, K. Efficacy of chitosan films with basil essential oil: Perspectives in food packaging. J. Food Meas. Charact. 2017, 11, 2160–2170. [Google Scholar] [CrossRef]
- Adjouman, Y.D.; Nindjin, C.; Tetchi, F.A.; Dalcq, A.-C.; Amani, N.G.; Sindic, M. Water vapor permeability of edible films based on improved Cassava (Manihot esculenta Crantz) native starches. J. Food Process. Technol. 2017, 8, 655. [Google Scholar]
- Arfat, Y.A.; Benjakul, S.; Prodpran, T.; Sumpavapol, P.; Songtipya, P. Properties and antimicrobial activity of fish protein isolate/fish skin gelatin film containing basil leaf essential oil and zinc oxide nanoparticles. Food Hydrocoll. 2014, 41, 265–273. [Google Scholar] [CrossRef]
- Rashid, A.; Qayum, A.; Liang, Q.; Kang, L.; Raza, H.; Chi, Z.; Chi, R.; Ren, X.; Ma, H. Preparation and characterization of ultrasound-assisted essential oil-loaded nanoemulsions stimulated pullulan-based bioactive film for strawberry fruit preservation. Food Chem. 2023, 422, 136254. [Google Scholar] [CrossRef]
- Cazón, P.; Antoniewska, A.; Rutkowska, J.; Vázquez, M. Evaluation of easy-removing antioxidant films of chitosan with Melaleuca alternifolia essential oil. Int. J. Biol. Macromol. 2021, 186, 365–376. [Google Scholar] [CrossRef]
- Sharma, S.; Barkauskaite, S.; Jaiswal, A.K.; Jaiswal, S. Essential oils as additives in active food packaging. Food Chem. 2021, 343, 128403. [Google Scholar] [CrossRef] [PubMed]
- Amjadi, S.; Almasi, H.; Ghadertaj, A.; Mehryar, L. Whey protein isolate-based films incorporated with nanoemulsions of orange peel (Citrus sinensis) essential oil: Preparation and characterization. J. Food Process. Preserv. 2021, 45, e15196. [Google Scholar] [CrossRef]
- Ali, A.M.M.; Prodpran, T.; Benjakul, S. Effect of Squalene as a Glycerol Substitute on Morphological and Barrier Properties of Golden Carp (Probarbus jullieni) Skin Gelatin Film. Food Hydrocoll. 2019, 97, 105201. [Google Scholar]
- dos Santos Paglione, I.; Galindo, M.V.; de Medeiros, J.A.S.; Yamashita, F.; Alvim, I.D.; Grosso, C.R.F.; Sakanaka, L.S.; Shirai, M.A. Comparative Study of the Properties of Soy Protein Concentrate Films Containing Free and Encapsulated Oregano Essential Oil. Food Packag. Shelf Life 2019, 22, 100419. [Google Scholar] [CrossRef]
- Rosenbloom, R.A.; Zhao, Y. Hydroxypropyl methylcellulose or soy protein isolate-based edible, water-soluble, and antioxidant films for safflower oil packaging. J. Food Sci. 2021, 86, 129–139. [Google Scholar] [CrossRef]
- Ilhan, I.; ten Klooster, R.; Gibson, I. Effects of process parameters and solid particle contaminants on the seal strength of low-density polyethylene-based flexible food packaging films. Packag. Technol. Sci. 2021, 34, 413–421. [Google Scholar] [CrossRef]
- Guerrero, P.; Hanani, Z.N.; Kerry, J.; De La Caba, K. Characterization of soy protein-based films prepared with acids and oils by compression. J. Food Eng. 2011, 107, 41–49. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, L.; Liu, Y.; Zeng, Z.; Li, C.; Fang, Z.; Hu, B.; Chen, H.; Wang, C.; Chen, S.; et al. Effect of hydroxypropyl-β-cyclodextrin and lecithin co-stabilized nanoemulsions on the konjac glucomannan/pullulan film. Int. J. Biol. Macromol. 2023, 235, 123802. [Google Scholar] [CrossRef] [PubMed]
- Ran, R.; Zheng, T.; Tang, P.; Xiong, Y.; Yang, C.; Gu, M.; Li, G. Antioxidant and antimicrobial collagen films incorporating Pickering emulsions of cinnamon essential oil for pork preservation. Food Chem. 2023, 420, 136108. [Google Scholar] [CrossRef]
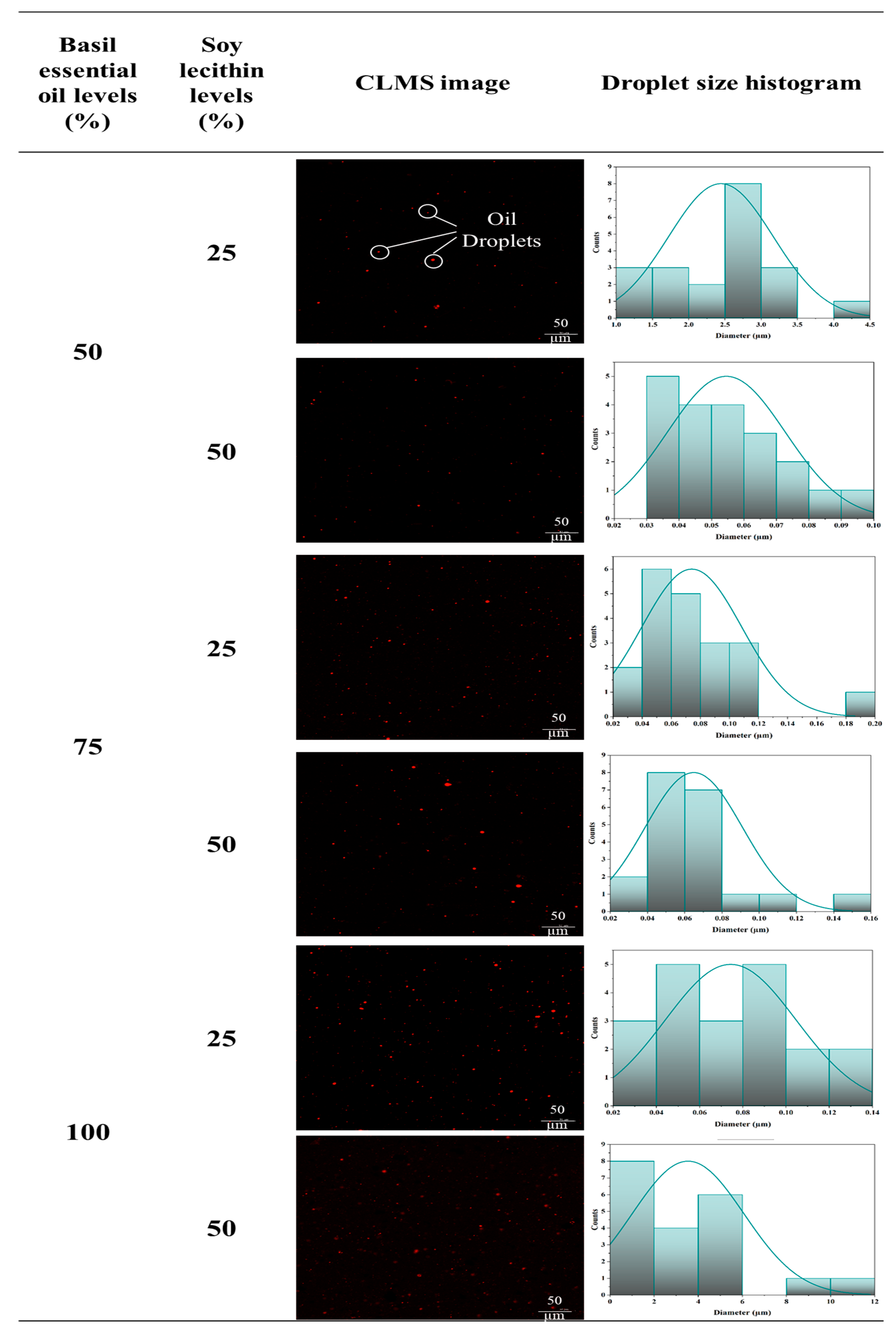
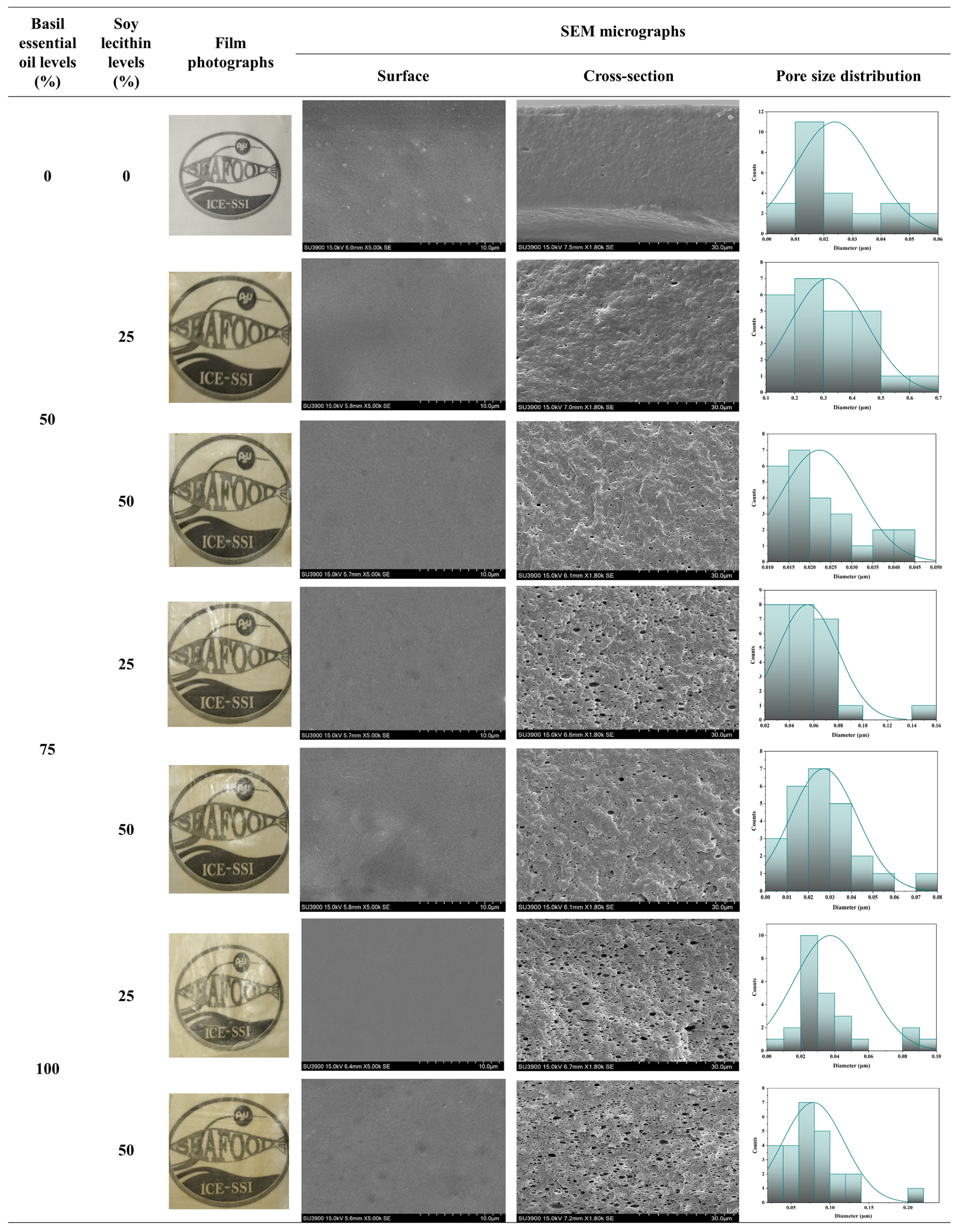
| Basil Essential Oil Levels (%) | Soy Lecithin Levels (%) | Storage Time (h) | d32 (µm) | d43 (µm) | Ff | Ci |
|---|---|---|---|---|---|---|
| 50 | 25 | 0 | 0.314 ± 0.01 *f | 0.568 ± 0.01 c | 4.348 ± 0.01 d | - |
| 24 | 0.402 ± 0.01 E | 0.623 ± 0.01 F | 10.844 ± 0.31 C | 8.754 ± 0.54 e | ||
| 50 | 0 | 0.392 ± 0.03 e | 0.598 ± 0.01 c | 4.441 ± 0.05 d | - | |
| 24 | 0.609 ± 0.01 D | 0.692 ± 0.01 E | 10.998 ± 0.03 C | 13.596 ± 0.53 d | ||
| 75 | 25 | 0 | 0.514 ± 0.01 d | 0.780 ± 0.01 b | 4.770 ± 0.06 c | - |
| 24 | 0.674 ± 0.01 C | 0.925 ± 0.01 D | 11.368 ± 0.06 B | 15.684 ± 0.04 c | ||
| 50 | 0 | 0.575 ± 0.02 c | 0.792 ± 0.02 b | 4.859 ± 0.02 c | - | |
| 24 | 0.698 ± 0.01 B | 0.951 ± 0.01 C | 11.445 ± 0.12 B | 16.194 ± 0.22 c | ||
| 100 | 25 | 0 | 0.696 ± 0.02 b | 0.925 ± 0.02 a | 5.298 ± 0.09 b | - |
| 24 | 0.747 ± 0.01 A | 1.141 ± 0.01 B | 12.026 ± 0.02 A | 18.941 ± 0.73 b | ||
| 50 | 0 | 0.888 ± 0.04 a | 0.898 ± 0.06 a | 5.520 ± 0.06 a | - | |
| 24 | 0.750 ± 0.02 A | 1.198 ± 0.01 A | 12.287 ± 0.01 A | 20.908 ± 0.93 a |
| Basil Essential Oil Levels (%) | Soy Lecithin Levels (%) | Thickness (mm) | TS (MPa) | EAB (%) | WVP (×10−11 g m/m2 s Pa) | L* | a* | b* | ΔE* |
|---|---|---|---|---|---|---|---|---|---|
| Without BE | Without SL | 0.052 ± 0.001 *f | 5.78 ± 0.45 a | 93.96 ± 9.46 e | 5.12 ± 0.24 a | 84.90 ± 0.20 a | −0.77 ± 0.07 a | 11.33 ± 0.63 g | 13.40 ± 0.50 g |
| 50 | 25 | 0.085 ± 0.004 e | 4.44 ± 0.46 b | 105.41 ± 11.36 de | 4.76 ± 0.12 b | 81.63 ± 0.77 b | −1.42 ± 0.08 b | 24.74 ± 0.24 f | 26.68 ± 0.17 f |
| 50 | 0.093 ± 0.008 d | 3.60 ± 0.58 c | 115.63 ± 12.97 d | 4.74 ± 0.31 b | 81.26 ± 0.34 b | −1.54 ± 0.05 c | 27.47 ± 0.34 e | 29.32 ± 0.34 e | |
| 75 | 25 | 0.104 ± 0.004 c | 2.72 ± 0.3 d | 151.16 ± 19.08 c | 3.56 ± 0.16 c | 80.14 ± 0.21 c | −1.72 ± 0.08 d | 29.92 ± 0.40 d | 32.0 ± 0.34 d |
| 50 | 0.106 ± 0.002 c | 2.03 ± 0.39 e | 178.58 ± 23.75 b | 3.50 ± 0.05 c | 79.80 ± 0.63 cd | −1.83 ± 0.04 e | 31.24 ± 0.73 c | 33.36 ± 0.56 c | |
| 100 | 25 | 0.113 ± 0.003 b | 1.23 ± 0.18 f | 208.52 ± 17.53 a | 2.54 ± 0.09 d | 78.92 ± 1.05 de | −1.91 ± 0.03 ef | 34.03 ± 0.39 b | 36.28 ± 0.57 b |
| 50 | 0.118 ± 0.002 a | 0.95 ± 0.12 f | 221.9 ± 18.12 a | 2.51 ± 0.1 d | 78.66 ± 0.95 e | −1.96 ± 0.03 f | 35.30 ± 0.58 a | 37.55 ± 0.73 a |
| Basil Essential Oil Levels (%) | Soy Lecithin Levels (%) | Sealed Films | Seal Strength (N/m) * | Seal Efficiency (%) | Mode of Failure | DPPH-RSA ** | FRAP ** | MCA *** | ORAC ** |
|---|---|---|---|---|---|---|---|---|---|
| Without BE | Without SL |  | 222.44± 12.61 *a | 79.82 ± 7.59 f | II, I | 2.69 ± 0.03 *f | 5.86 ± 0.74 g | 528.15 ± 16.97 e | 377.74 ± 4.45 d |
| 50 | 25 |  | 191.44 ± 10.81 b | 87.30 ± 5.56 ef | I | 18.66 ± 0.12 e | 13.48 ± 0.29 f | 568.89 ± 22.22 d | 855.90 ± 22.54 c |
| 50 |  | 177.88 ± 11.63 c | 93.71 ± 5.69 e | I | 19.86 ± 0.23 e | 15.78 ± 0.53 e | 597.04 ± 14.29 c | 891.51 ± 24.16 bc | |
| 75 | 25 |  | 159.38 ± 9.27 d | 114.97 ± 8.14 d | I | 28.31 ± 0.46 d | 20.84 ± 0.39 d | 619.63 ± 5.70 bc | 933.99 ± 31.89 b |
| 50 |  | 143.63 ± 7.08 e | 123.80 ± 10.24 c | I | 37.42 ± 5.17 c | 24.11 ± 0.35 c | 639.63 ± 11.98 b | 941.46 ± 5.81 b | |
| 100 | 25 |  | 121.81 ± 9.96 f | 136.76 ± 8.35 b | I, II | 51.97 ± 2.29 b | 30.18 ± 0.40 b | 669.26 ± 3.39 a | 1128.30 ± 45.95 a |
| 50 |  | 107.56 ± 5.66 g | 145.88 ± 10.17 a | I, II | 60.45 ± 2.73 a | 33.10 ± 0.55 a | 676.30 ± 6.41 a | 1140.22 ± 45.08 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Awal, M.S.; Benjakul, S.; Prodpran, T.; Nilsuwan, K. Properties of Emulsion Co-Precipitated Collagen/Bambara Groundnut Protein-Based Film as Influenced by Basil Essential Oil and Soy Lecithin. Polymers 2025, 17, 1139. https://doi.org/10.3390/polym17091139
Awal MS, Benjakul S, Prodpran T, Nilsuwan K. Properties of Emulsion Co-Precipitated Collagen/Bambara Groundnut Protein-Based Film as Influenced by Basil Essential Oil and Soy Lecithin. Polymers. 2025; 17(9):1139. https://doi.org/10.3390/polym17091139
Chicago/Turabian StyleAwal, Md. Shihabul, Soottawat Benjakul, Thummanoon Prodpran, and Krisana Nilsuwan. 2025. "Properties of Emulsion Co-Precipitated Collagen/Bambara Groundnut Protein-Based Film as Influenced by Basil Essential Oil and Soy Lecithin" Polymers 17, no. 9: 1139. https://doi.org/10.3390/polym17091139
APA StyleAwal, M. S., Benjakul, S., Prodpran, T., & Nilsuwan, K. (2025). Properties of Emulsion Co-Precipitated Collagen/Bambara Groundnut Protein-Based Film as Influenced by Basil Essential Oil and Soy Lecithin. Polymers, 17(9), 1139. https://doi.org/10.3390/polym17091139









