Extraction Process Research and Characterization of Microcrystalline Cellulose Derived from Bamboo (Phyllostachys edulis (Carrière) J. Houz.) Fibers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Isolation of Cellulose
2.2.2. Preparation of Microcrystalline Cellulose
2.2.3. Characterization
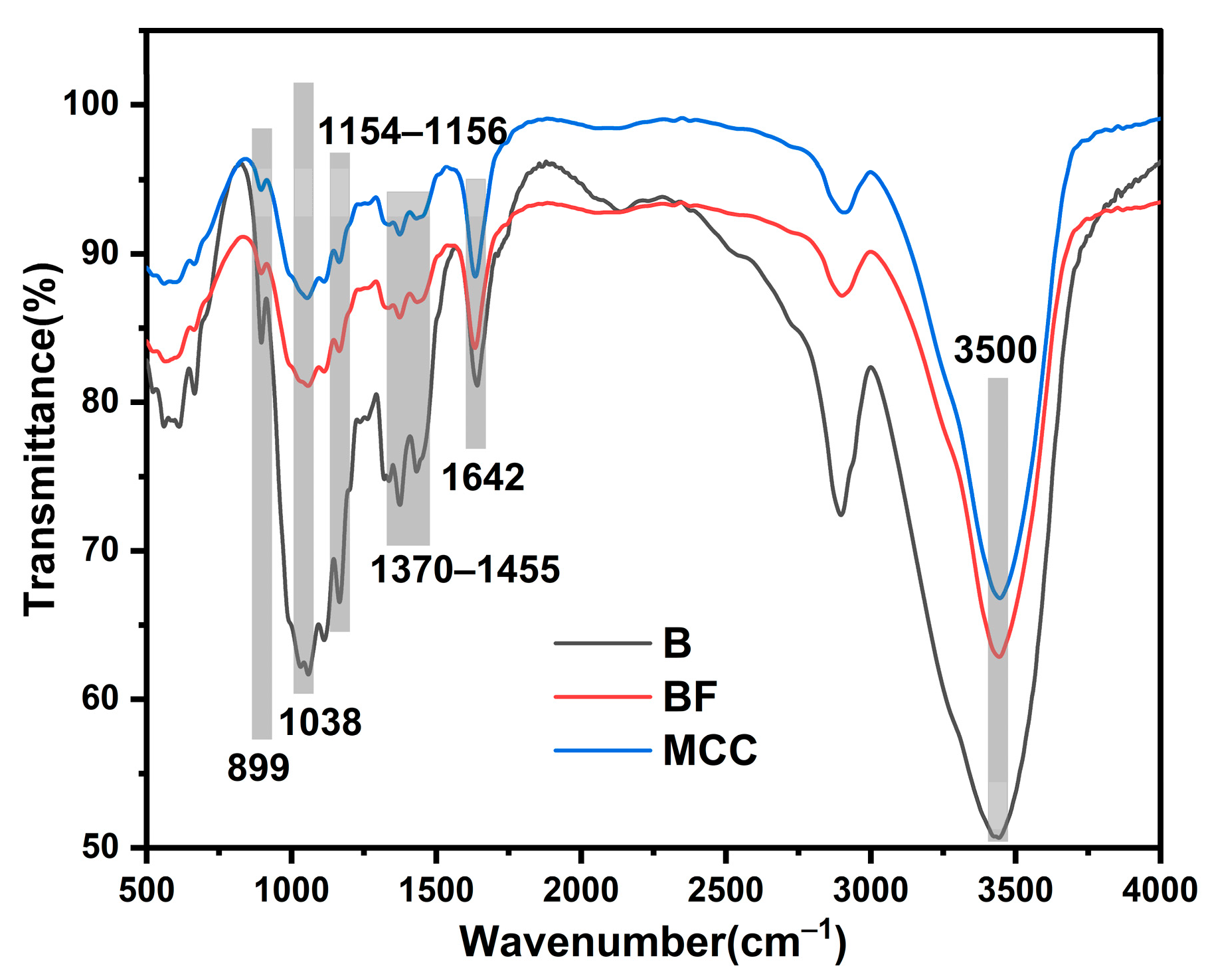
FTIR Analysis
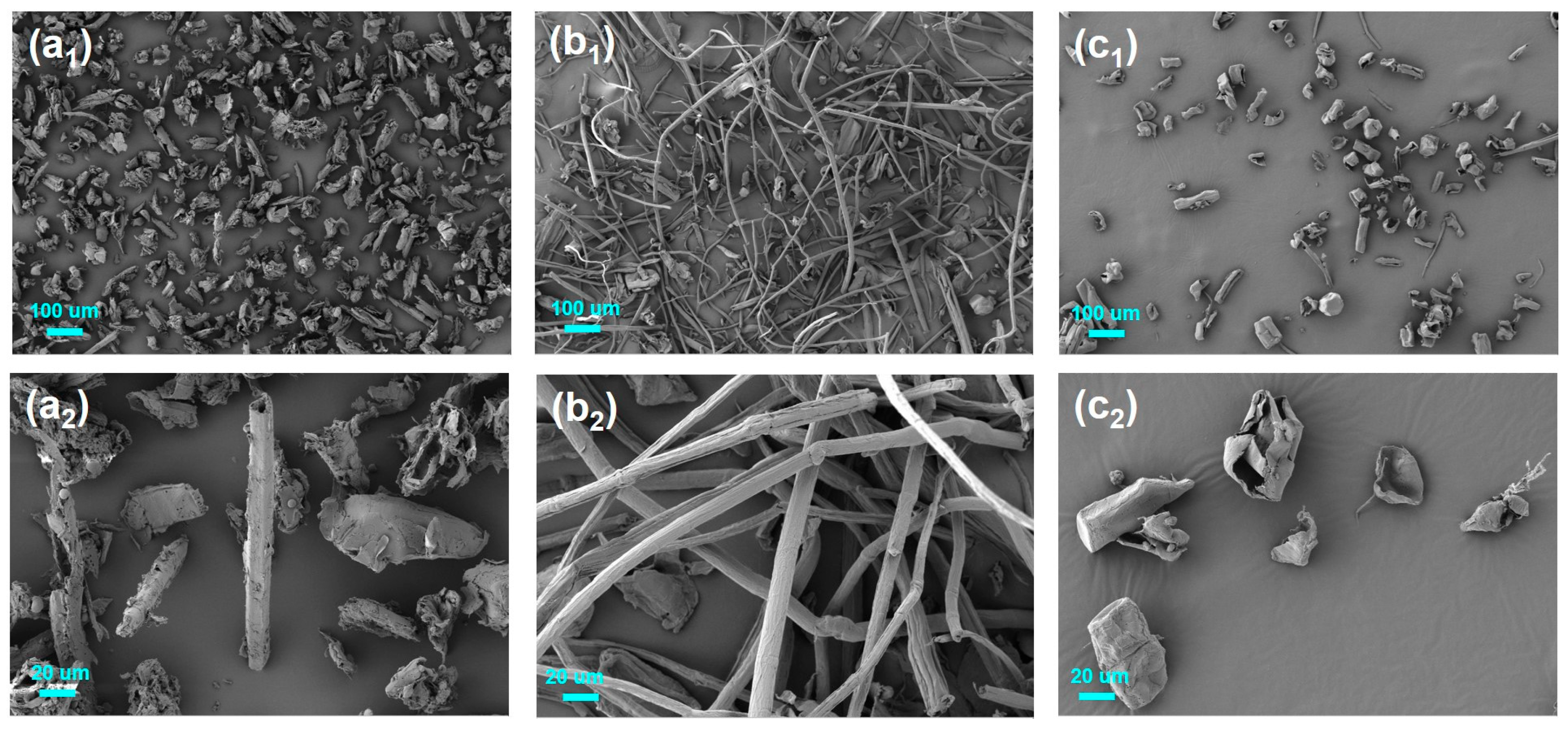
SEM Analysis
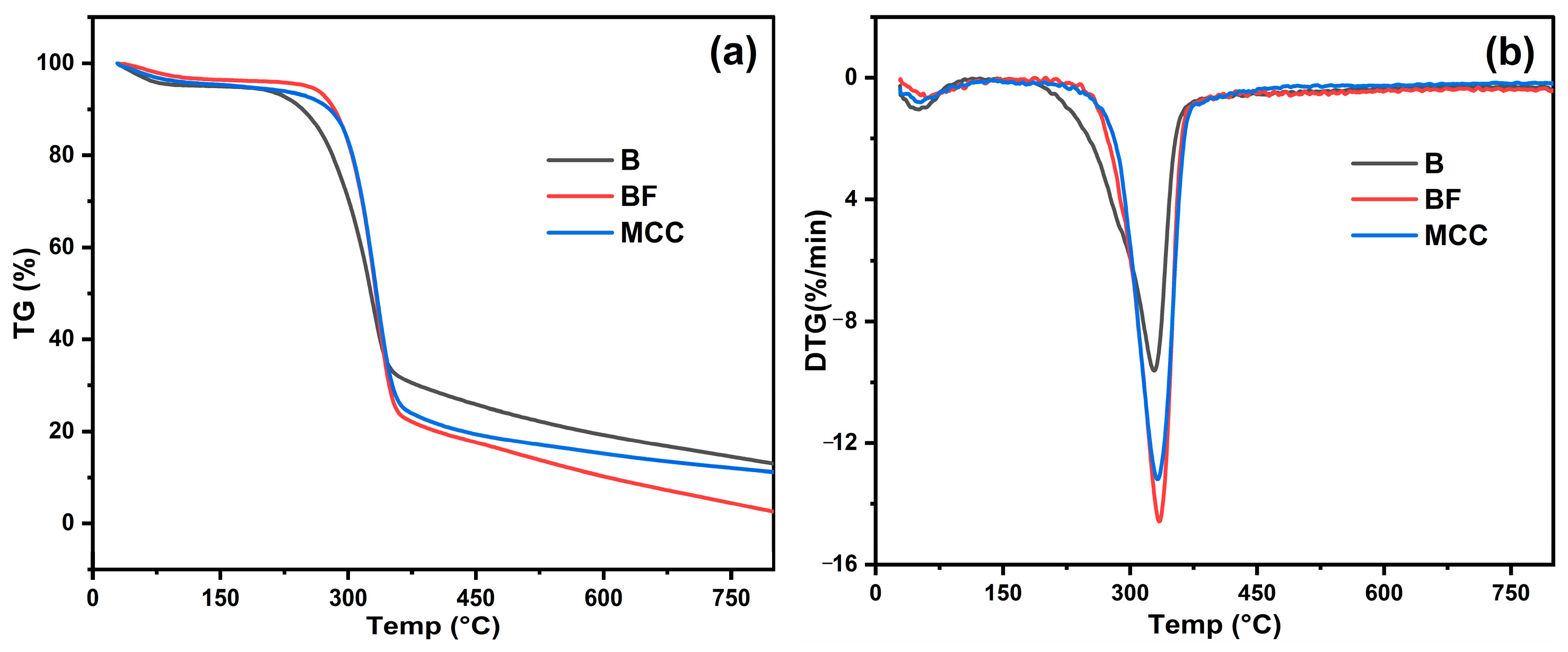
TGA Analysis
XRD Analysis
Cellulose, Hemicellulose and Lignin Content Analysis
Gel Permeation Chromatography (GPC) Measurements
3. Results and Discussion
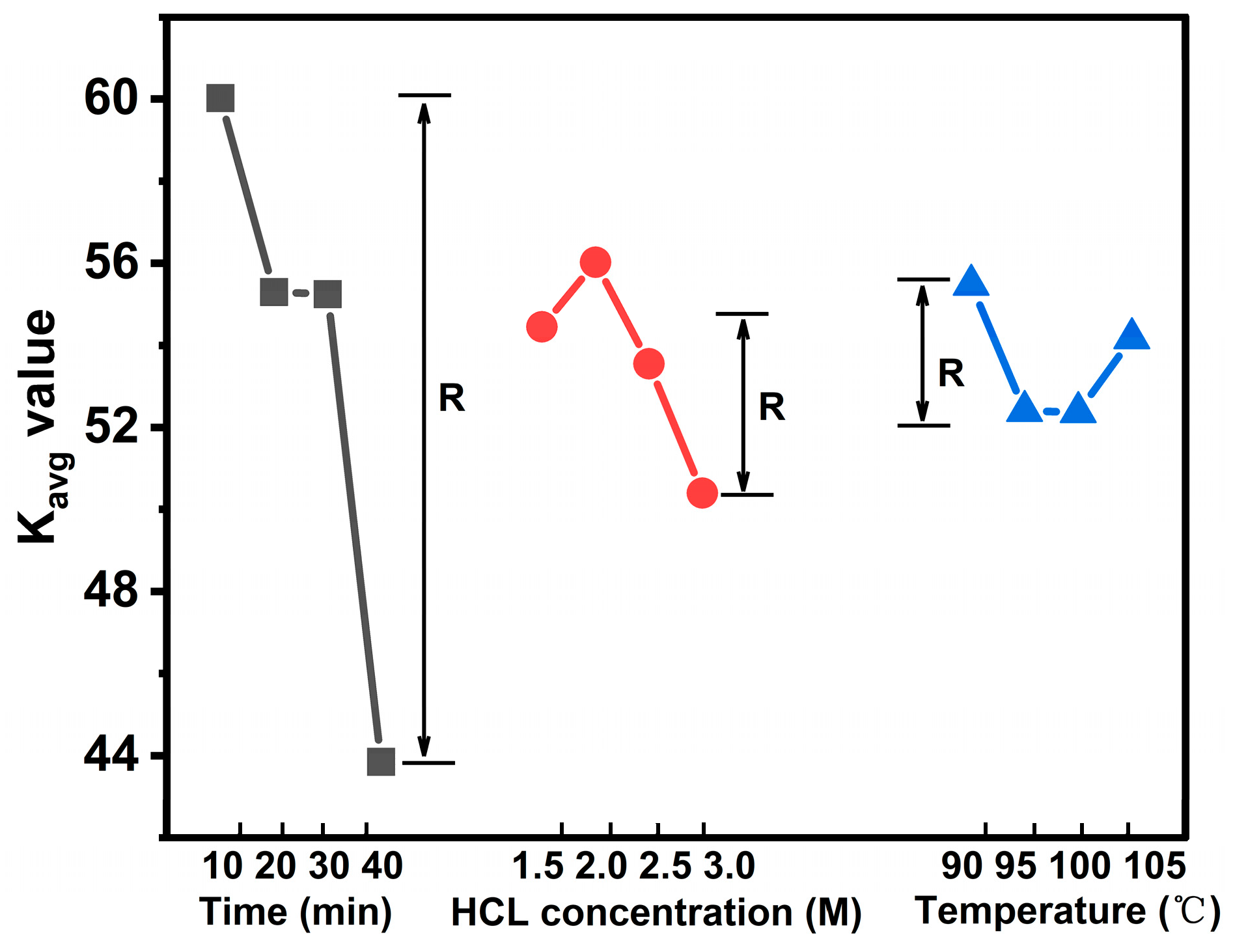
3.1. Effects of Process Variables on MCC
3.2. FTIR Spectroscopy
3.3. XRD Analysis
3.4. Thermal Analysis
3.5. SEM Analysis
3.6. Compositional Analysis
| Sample | Mn | Mw | DPI | DPn | DPw | Cellulose | Hemicellulose | Lignin |
|---|---|---|---|---|---|---|---|---|
| MCC | 27,819 | 153,004 | 5.50 | 54 | 295 | 69.3% | 12.2% | 6.3% |
| BF | 63,336 | 380,020 | 6.00 | 122 | 732 | 57.4% | 16.7% | 8.0% |
| Raw bamboo | - | - | - | - | - | 39.3% | 20.5% | 32.4% |
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Trache, D.; Hussin, M.; Chuin, C.; Sabar, S.; Fazita, M.R.; Taiwo, O.; Hassan, T.M.; Haafiz, M.K. Microcrystalline cellulose: Isolation, characterization and bio-composites application—A review. Int. J. Biol. Macromol. 2016, 93, 789–804. [Google Scholar] [CrossRef] [PubMed]
- Taiwo, O.; Ghazali, A.; Khalil, H.P.S.A.; Hassan, A.; Arjmandi, R.; Fazita, M.; Haafiz, M. Isolation and characterization of microcrystalline cellulose from oil palm fronds using chemomechanical process. Wood Fiber Sci. 2016, 48, 260–270. [Google Scholar]
- Thielemans, K.; De Bondt, Y.; Bosch, S.V.D.; Bautil, A.; Roye, C.; Deneyer, A.; Courtin, C.M.; Sels, B.F. Decreasing the degree of polymerization of microcrystalline cellulose by mechanical impact and acid hydrolysis. Carbohydr. Polym. 2022, 294, 119764. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wen, X.; Zhang, X.; Liu, C. Acetylation of microcrystalline cellulose by transesterification in AmimCl/DMSO cosolvent system. Molecules 2017, 22, 1419. [Google Scholar] [CrossRef]
- Krishnan, S.G.; Pua, F.-L.; Zhang, F. Oil palm empty fruit bunch derived microcrystalline cellulose supported magnetic acid catalyst for esterification reaction: An optimization study. Energy Convers. Manag. X 2022, 13, 100159. [Google Scholar] [CrossRef]
- Zulkifli, N.; Samat, N.; Anuar, H.; Zainuddin, N. Mechanical properties and failure modes of recycled polypropylene/microcrystalline cellulose composites. Mater. Des. 2015, 69, 114–123. [Google Scholar] [CrossRef]
- Sun, X.; Lu, C.; Liu, Y.; Zhang, W.; Zhang, X. Melt-processed poly(vinyl alcohol) composites filled with microcrystalline cellulose from waste cotton fabrics. Carbohydr. Polym. 2014, 101, 642–649. [Google Scholar] [CrossRef]
- Azizi Samir, M.A.S.; Alloin, F.; Dufresne, A. Review of recent research into cellulosic whiskers, their properties and their application in nanocomposite field. Biomacromolecules 2005, 6, 612–626. [Google Scholar] [CrossRef]
- Merci, A.; Urbano, A.; Grossmann, M.V.E.; Tischer, C.A.; Mali, S. Properties of microcrystalline cellulose extracted from soybean hulls by reactive extrusion. Food Res. Int. 2015, 73, 38–43. [Google Scholar] [CrossRef]
- Thoorens, G.; Krier, F.; Leclercq, B.; Carlin, B.; Evrard, B. Microcrystalline cellulose, a direct compression binder in a quality by design environment-a review. Int. J. Pharm. 2014, 473, 64–72. [Google Scholar] [CrossRef]
- Okhamafe, A.; Ejike, E.; Akinrinola, F.; Ubuane-Inedegbo, A. Aspect of tablet disintegrant properties of cellulose derived from bagasse and maize cob. West Afr. J. Pharm. 1995, 9, 8–13. [Google Scholar]
- Hanna, M.; Biby, G.; Miladinov, V. Production of Microcrystalline Cellulose by Reactive Extrusion. US Patent 6228213, 5 August 2001. [Google Scholar]
- Virtanen, T.; Svedström, K.; Andersson, S.; Tervala, L.; Torkkeli, M.; Knaapila, M.; Kotelnikova, N.; Maunu, S.L.; Serimaa, R. A physico-chemical characterisation of new raw materials for microcrystalline cellulose manufacturing. Cellulose 2012, 19, 219–235. [Google Scholar] [CrossRef]
- Jain, J.; Dixit, V.; Varma, K. Preparation of microcrystalline cellulose from cereal straw and its evaluation as a tablet excipient. Indian J. Pharm. Sci. 1983, 45, 83–85. [Google Scholar]
- Okhamafe, A.; Igboechi, A.; Obaseki, T. Celluloses extracted from groundnut shell and rice husk 1: Preliminary physicochemical characterization. Pharm. World J. 1991, 8, 120–130. [Google Scholar]
- Bangar, S.P.; Esua, O.J.; Nickhil, C.; Whiteside, W.S. Microcrystalline cellulose for active food packaging applications: A review. Food Packag. Shelf Life 2023, 36, 101048. [Google Scholar] [CrossRef]
- Wang, M.; Wu, Q.; Feng, Z.; Gu, X.; Li, H.; Sun, J.; Fei, B.; Zhang, S. Surface Modification of cellulose microcrystalline with aluminate coupling agent and its effects on flame retardant and mechanical properties of epoxy resin. Fibers Polym. 2020, 21, 2344–2352. [Google Scholar] [CrossRef]
- Ramle, S.F.M.; Krishnan, T.D.R.; Jusoh, N.H.; Rahim, A.A.; Hamid, Z.A.A.; Rawi, N.F.M. Morphological study and biodegradability of PLA/PBAT thin-film reinforced with microcrystalline cellulose from bamboo. In Proceedings of the International Conference on Bioengineering and Technology, Virtual, 24–25 May 2021. [Google Scholar]
- Zhang, Y.; Xu, Y.; Yue, X.; Dai, L.; Ni, Y. Isolation and characterization of microcrystalline cellulose from bamboo pulp through extremely low acid hydrolysis. J. Wood Chem. Technol. 2019, 39, 242–254. [Google Scholar] [CrossRef]
- Rasheed, M.; Jawaid, M.; Karim, Z.; Abdullah, L.C. Morphological, physiochemical and thermal properties of microcrystalline cellulose (MCC) extracted from bamboo fiber. Molecules 2020, 25, 2824. [Google Scholar] [CrossRef]
- Hasan, M.; Lai, T.K.; Gopakumar, D.A.; Jawaid, M.; Owolabi, F.A.T.; Mistar, E.M.; Alfatah, T.; Noriman, N.Z.; Haafiz, M.K.M.; Khalil, H.P.S.A. Micro crystalline bamboo cellulose based seaweed biodegradable composite films for sustainable packaging material. J. Polym. Environ. 2019, 27, 1602–1612. [Google Scholar] [CrossRef]
- Pachuau, L.; Vanlalfakawma, D.; Tripathi, S.; Lalhlenmawia, H. Muli bamboo (Melocanna baccifera) as a new source of microcrystalline cellulose. J. Appl. Pharm. Sci. 2014, 4, 087–094. [Google Scholar]
- Hermawan, D.; Lai, T.K.; Jafarzadeh, S.J.; Gopakumar, D.A.; Hasan, M.; Owolabi, F.A.T.; Sri Aprilia, N.A.; Rizal, S.; Abdul Khalil, H.P.S. Development of seaweed-based bamboo microcrystalline cellulose films intended for sustainable food packaging applications. Bioresources 2019, 14, 3389–3410. [Google Scholar] [CrossRef]
- Zhu, T.; Guo, J.; Fei, B.; Feng, Z.; Gu, X.; Li, H.; Sun, J.; Zhang, S. Preparation of methacrylic acid modified microcrystalline cellulose and their applications in polylactic acid: Flame retardancy, mechanical properties, thermal stability and crystallization behavior. Cellulose 2020, 27, 2309–2323. [Google Scholar] [CrossRef]
- Rasheed, M.; Jawaid, M.; Parveez, B.; Bhat, A.; Alamery, S. Morphology, structural, thermal, and tensile properties of bamboo microcrystalline cellulose/poly (Lactic acid)/poly(Butylene succinate) composites. Polymers 2021, 13, 465. [Google Scholar] [CrossRef] [PubMed]
- Khalil, H.P.S.A.; Lai, T.K.; Tye, Y.Y.; Paridah, M.T.; Fazita, M.R.N.; Azniwati, A.A.; Dungani, R.; Rizal, S. Preparation and characterization of microcrystalline cellulose from sacred bali bamboo as reinforcing filler in seaweed-based composite film. Fibers Polym. 2018, 19, 423–434. [Google Scholar] [CrossRef]
- Du, J.; Yang, S.; Zhu, Q.; Wu, Y.; Guo, J.; Jiang, J. Preparation and characterization of thermoplastic starch/bamboo shoot processing by-product microcrystalline cellulose composites. Biomass Convers. Biorefin. 2023, 13, 12105–12114. [Google Scholar] [CrossRef]
- Abe, K.; Yano, H. Comparison of the characteristics of cellulose microfibril aggregates of wood, rice straw and potato tuber. Cellulose 2009, 16, 1017–1023. [Google Scholar] [CrossRef]
- Wise, L.; Murphy, M.; D’Addieco, A. Chlorite holocellulose, its fractionation and bearing on summative wood analysis and on studies on the hemicelluloses. Tech. Assoc. Pap. 1946, 122, 35–43. [Google Scholar]
- Ejikeme, P.M. Investigation of the physicochemical properties of microcrystalline cellulose from agricultural wastes I: Orange mesocarp. Cellulose 2008, 15, 141–147. [Google Scholar] [CrossRef]
- Wang, S.; Liao, Y.; Liu, Q.; Luo, Z.; Cen, K. Experimental study of the influence of acid wash on cellulose pyrolysis. Front. Chem. Eng. China 2007, 1, 35–39. [Google Scholar] [CrossRef]
- Adela, A.; Wahab, Z.; Ibrahima, A.; Shemy, M. Characterization of microcrystalline cellulose prepared from lignocellulosic materials. Part I. Acid catalyzed hydrolysis. Bioresour. Technol. 2010, 101, 4446–4455. [Google Scholar] [CrossRef]
- Li, M.; He, B.; Li, J.; Zhao, L. Physico-chemical characterization and comparison of microcrystalline cellulose from several lignocellulosic sources. BioResources 2019, 14, 7886–7900. [Google Scholar] [CrossRef]
- Harini, U.; Harish, S.; Harishankar, A.; Buvaneswaran, M.; Sinija, V. Extraction and characterization of microcrystalline cellulose from wine waste. Food Bioprod. Process. 2024, 144, 92–101. [Google Scholar] [CrossRef]
- Meraj, A.; Jawaid, M.; Karim, Z.; Awayssa, O.; Fouad, H.; Singh, B. Characterization of carboxymethyl microcrystalline cellulose derived from sustainable kenaf fiber. Carbohydr. Polym. Technol. Appl. 2025, 10, 100745. [Google Scholar] [CrossRef]
- Techawinyutham, L.; Sundaram, R.S.; Suyambulingam, I.; Mo-on, S.; Srisuk, R.; Divakaran, D.; Rangappa, S.M.; Siengchin, S. Rice husk biowaste derived microcrystalline cellulose reinforced sustainable green composites: A comprehensive characterization for lightweight applications. Int. J. Biol. Macromol. 2025, 299, 140153. [Google Scholar] [CrossRef]
- Xin, W.; Zhou, Y.; Xiong, W.; Yao, Y.; Zhang, J.; Wang, L. Characterisation of microcrystalline cellulose derived from wheat bran and evaluation of its ice recrystallisation inhibiting activity. Food Res. Int. 2025, 208, 116212. [Google Scholar] [CrossRef]
- Jantarat, C.; Kaewpradit, S.; Chingunpitak, J.; Srivaro, S. Characteristics of microcrystalline cellulose derived from oil palm trunk slabs and its potential for use as tablet diluent. Heliyon 2025, 11, e42902. [Google Scholar] [CrossRef]
- Trache, D.; Donnot, A.; Khimeche, K.; Benelmir, R.; Brosse, N. Physico-chemical properties and thermal stability of microcrystalline cellulose isolated from Alfa fibres. Carbohydr. Polym. 2014, 104, 223–230. [Google Scholar] [CrossRef]
- Schirmer, M.C.; Bertoni, F.A.; Giordano, E.D. One-pot synthesis of quaternized microcrystalline cellulose obtained from soybean hulls. Next Mater. 2023, 1, 100029. [Google Scholar] [CrossRef]
- Pratama, A.W.; Mahardika, M.; Widiastuti, N.; Piluharto, B.; Ilyas, R.; Sapuan, S.; Amelia, D.; Firmanda, A. Isolation and characterization of highly thermal stable microcrystalline cellulose derived from belulang grass (Eleusine indica). Case Stud. Chem. Environ. Eng. 2024, 9, 100743. [Google Scholar] [CrossRef]
- Susi, S.; Ainuri, M.; Wagiman, W.; Falah, M.A.F. Characterization and Selection of Microcrystalline Cellulose from Oil Palm Empty Fruit Bunches for Strengthening Hydrogel Films. J. Renew. Mater. 2024, 12, 513–537. [Google Scholar] [CrossRef]


| Level | Factors | ||
|---|---|---|---|
| HCl Concentration (M) | Temperature (°C) | Time (min) | |
| 1 | 1.5 | 90 | 10 |
| 2 | 2.0 | 95 | 20 |
| 3 | 2.5 | 100 | 30 |
| 4 | 3.0 | 105 | 40 |
| Experiment Number | HCl Concentration (M) | Temperature (°C) | Time (min) |
|---|---|---|---|
| (1) | 1 | 1 | 1 |
| (2) | 1 | 2 | 2 |
| (3) | 1 | 3 | 3 |
| (4) | 1 | 4 | 4 |
| (5) | 2 | 1 | 2 |
| (6) | 2 | 2 | 1 |
| (7) | 2 | 3 | 4 |
| (8) | 2 | 4 | 3 |
| (9) | 3 | 1 | 3 |
| (10) | 3 | 2 | 4 |
| (11) | 3 | 3 | 1 |
| (12) | 3 | 4 | 2 |
| (13) | 4 | 1 | 4 |
| (14) | 4 | 2 | 3 |
| (15) | 4 | 3 | 2 |
| (16) | 4 | 4 | 1 |
| Term | Level | Temperature (°C) | HCl Concentration (M) | Time (min) |
|---|---|---|---|---|
| K value | 1 | 221.9 | 217.8 | 240.1 |
| 2 | 209.6 | 224.1 | 221.2 | |
| 3 | 209.5 | 214.2 | 221 | |
| 4 | 216.7 | 201.6 | 175.4 | |
| Kavg value | 1 | 55.48 | 54.45 | 60.03 |
| 2 | 52.4 | 56.03 | 55.3 | |
| 3 | 52.38 | 53.55 | 55.25 | |
| 4 | 54.17 | 50.4 | 43.85 | |
| Optimal level | 1 | 2 | 1 | |
| R | 3.1 | 5.63 | 16.18 | |
| Sum of Squares | df | Mean Square | F | p | |
|---|---|---|---|---|---|
| Intercept | 45,978.081 | 1 | 45,978.081 | 5080.568 | 0.000 ** |
| HCl concentration | 67.382 | 3 | 22.461 | 2.482 | 0.158 |
| Temperature | 27.147 | 3 | 9.049 | 1 | 0.455 |
| Time | 567.822 | 3 | 189.274 | 20.915 | 0.001 ** |
| Residual | 54.299 | 6 | 9.05 |
| Microcrystalline Cellulose | CI (%) | Ref. |
|---|---|---|
| Bamboo | 78.0 | [26] |
| Cotton stalk | 80.29 | [33] |
| Hardwood | 80.33 | |
| Softwood | 81.56 | |
| Bamboo | 78.60 | |
| Sisal | 81.13 | |
| Bamboo | 71.82 | [21] |
| Bamboo Pulp | 75.64 | [19] |
| Wine waste | 32.45 | [34] |
| 22.60 | ||
| 34.76 | ||
| 28.21 | ||
| Douglas fir | 71 | [28] |
| Rice straw | 68.2 | |
| Potato tuber | 66.1 | |
| Kenaf fiber | 92.2 | [35] |
| Rice husk | 77.8 | [36] |
| Wheat bran | 43.93 | [37] |
| Oil palm trunk slabs | 60.81 | [38] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Wang, Z.; Yang, S.; Ji, N.; Li, D. Extraction Process Research and Characterization of Microcrystalline Cellulose Derived from Bamboo (Phyllostachys edulis (Carrière) J. Houz.) Fibers. Polymers 2025, 17, 1143. https://doi.org/10.3390/polym17091143
Liu Z, Wang Z, Yang S, Ji N, Li D. Extraction Process Research and Characterization of Microcrystalline Cellulose Derived from Bamboo (Phyllostachys edulis (Carrière) J. Houz.) Fibers. Polymers. 2025; 17(9):1143. https://doi.org/10.3390/polym17091143
Chicago/Turabian StyleLiu, Zhu, Zhongwei Wang, Shoulu Yang, Ning Ji, and Dan Li. 2025. "Extraction Process Research and Characterization of Microcrystalline Cellulose Derived from Bamboo (Phyllostachys edulis (Carrière) J. Houz.) Fibers" Polymers 17, no. 9: 1143. https://doi.org/10.3390/polym17091143
APA StyleLiu, Z., Wang, Z., Yang, S., Ji, N., & Li, D. (2025). Extraction Process Research and Characterization of Microcrystalline Cellulose Derived from Bamboo (Phyllostachys edulis (Carrière) J. Houz.) Fibers. Polymers, 17(9), 1143. https://doi.org/10.3390/polym17091143





