Synthesis and Electrochromic Properties of Triphenylamine-Based Aromatic Poly(amide-imide)s
Abstract
1. Introduction
2. Materials and Methods
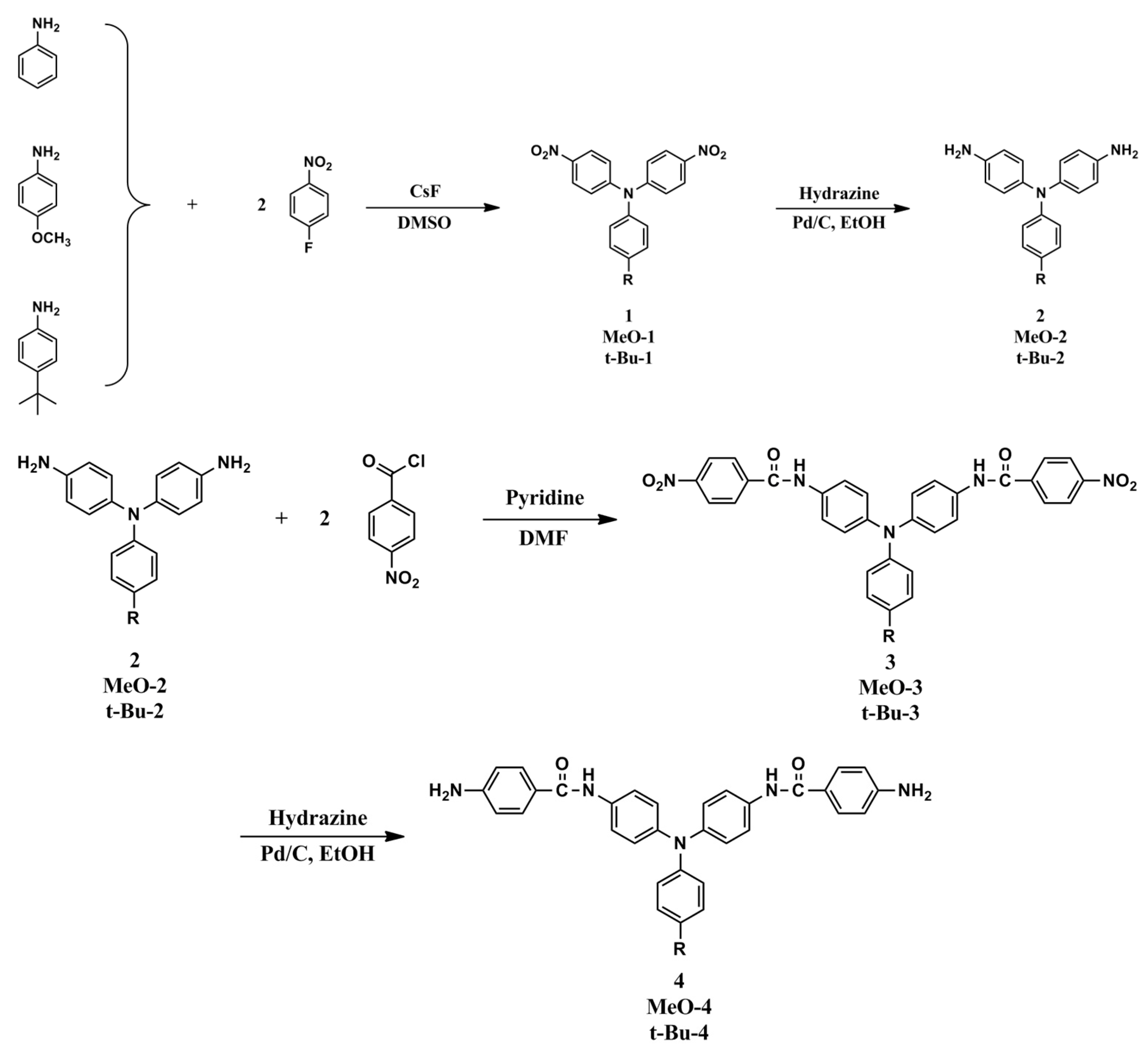
2.1. Synthesis of 4,4′-Bis(p-nitrobenzamido)triphenylamine (3), 4,4′-Bis(p-nitrobenzamido)-4″-methoxytriphenylamine (MeO-3), and 4,4′-Bis(p-nitrobenzamido)-4″-tert-butyltriphenylamine (t-Bu-3)


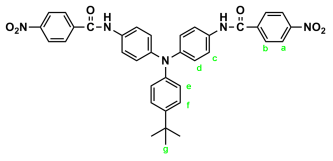
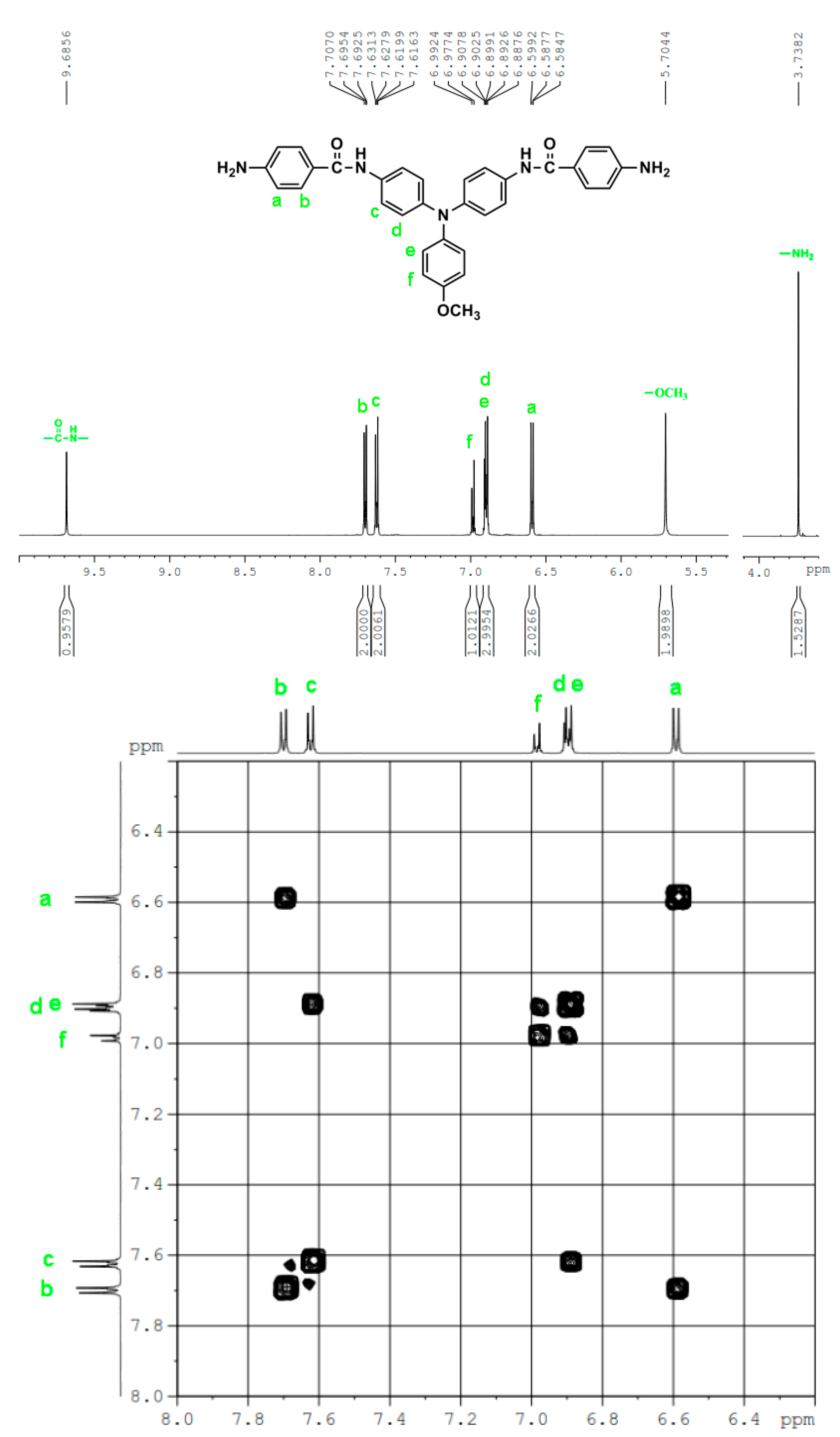
2.2. Synthesis of 4,4′-Bis(p-aminobenzamido)triphenylamine (4), 4,4′-Bis(p-aminobenzamido)-4″methoxytriphenylamine (MeO-4), and 4,4′-Bis(p-aminobenzamido)-4″-tert-butytriphenylamine (t-Bu-4)
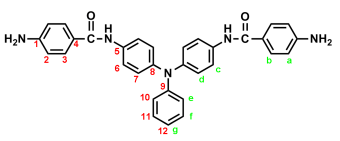
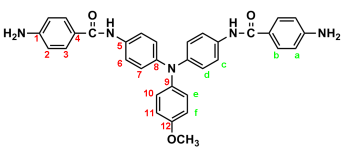
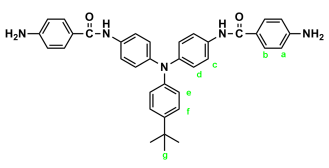
2.3. Synthesis of Poly(amide-imide)s
3. Results and Discussion
3.1. Monomer Synthesis
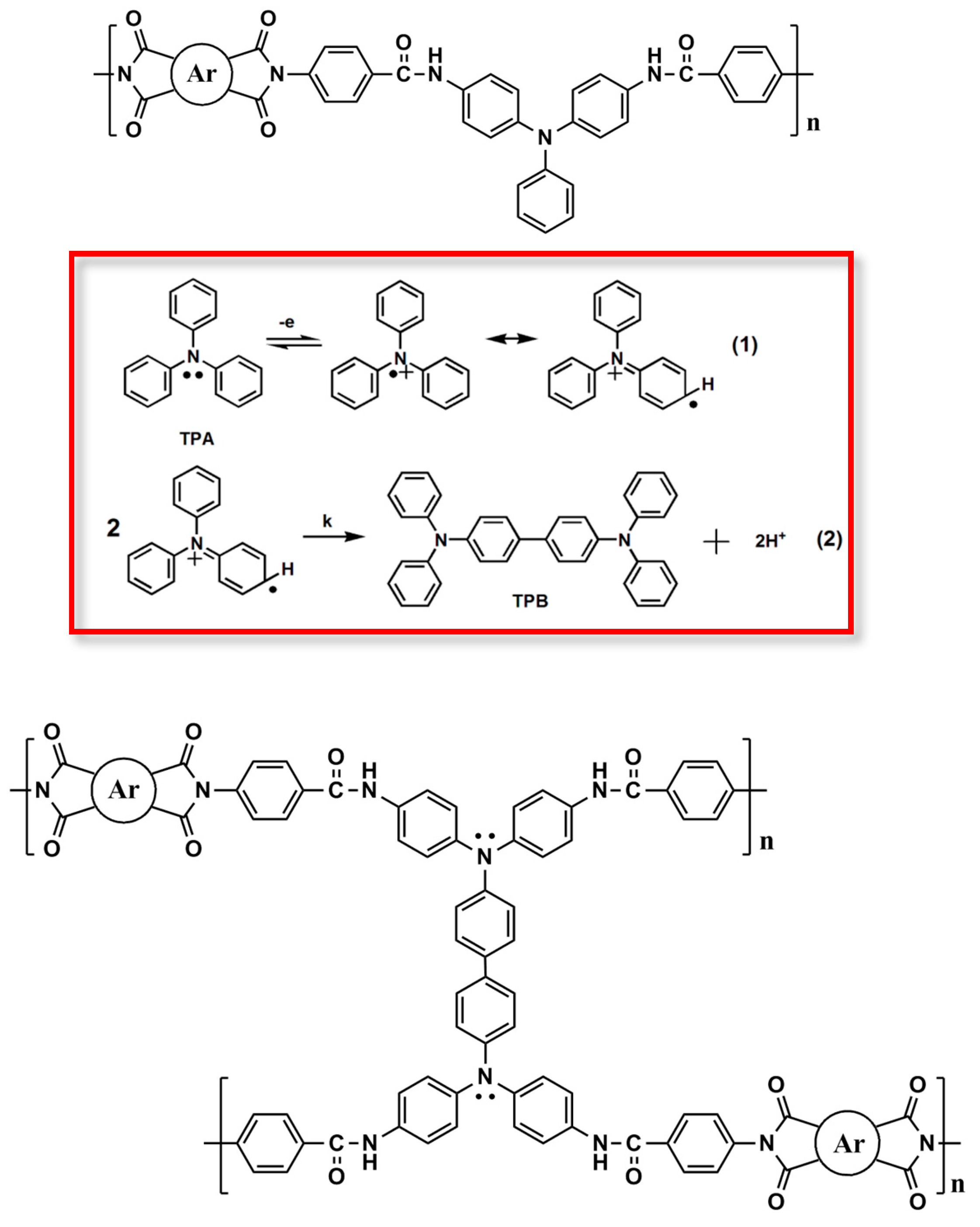
3.2. Synthesis of Poly(amide-imide)s (PAIs)
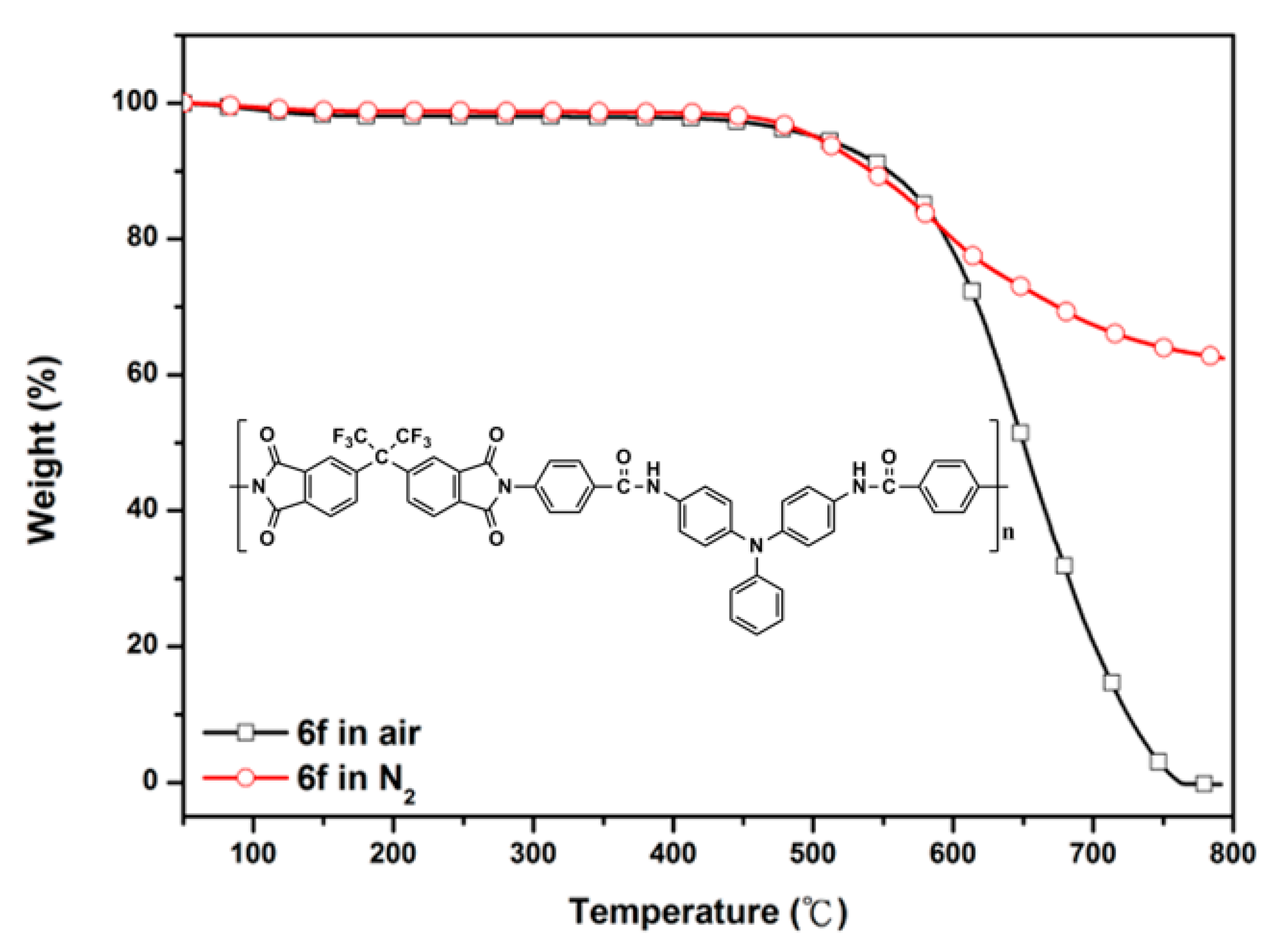
3.3. Solubility and Thermal Properties
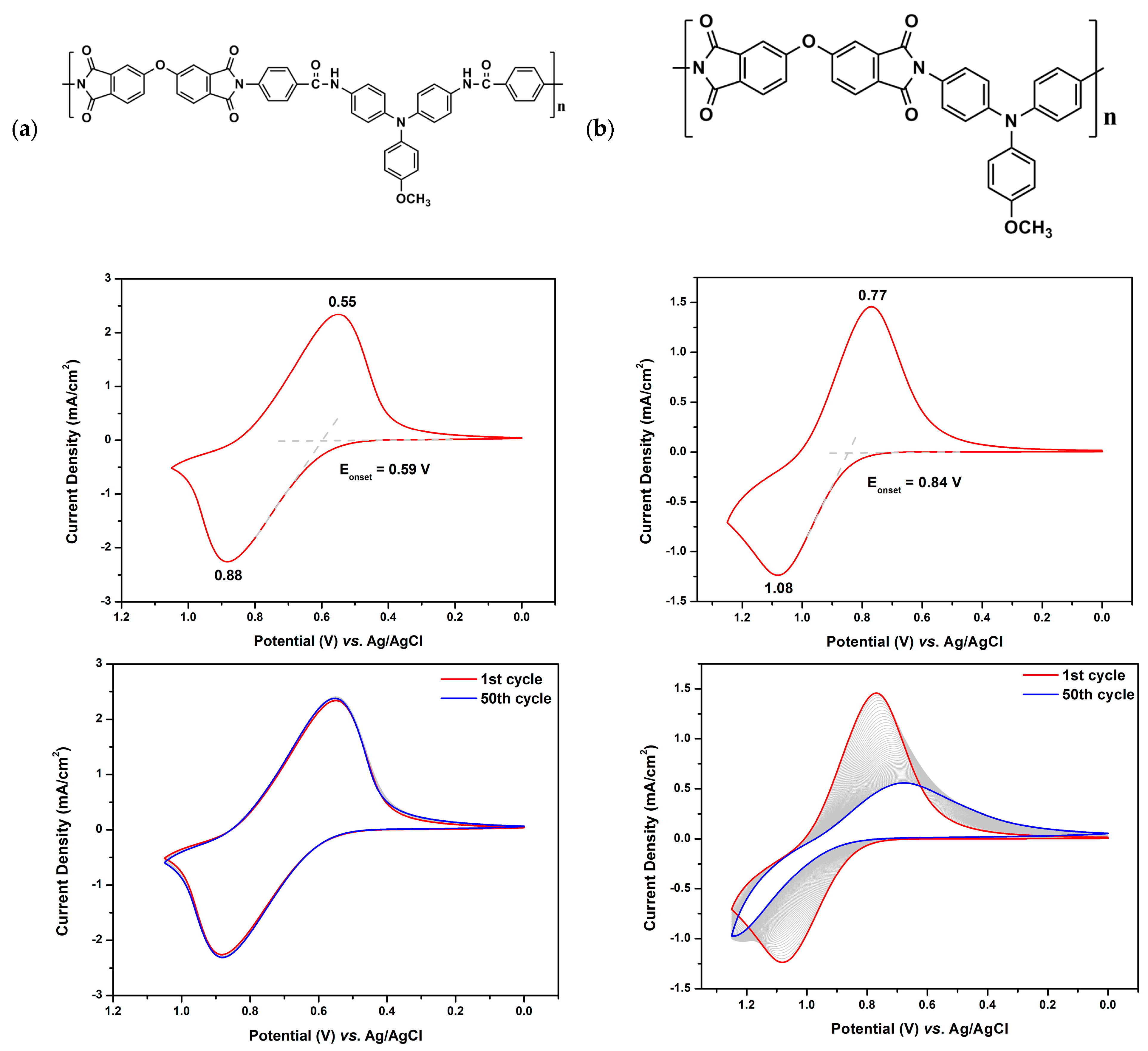
3.4. Electrochemical Properties
3.5. Spectroelectrochemical Properties
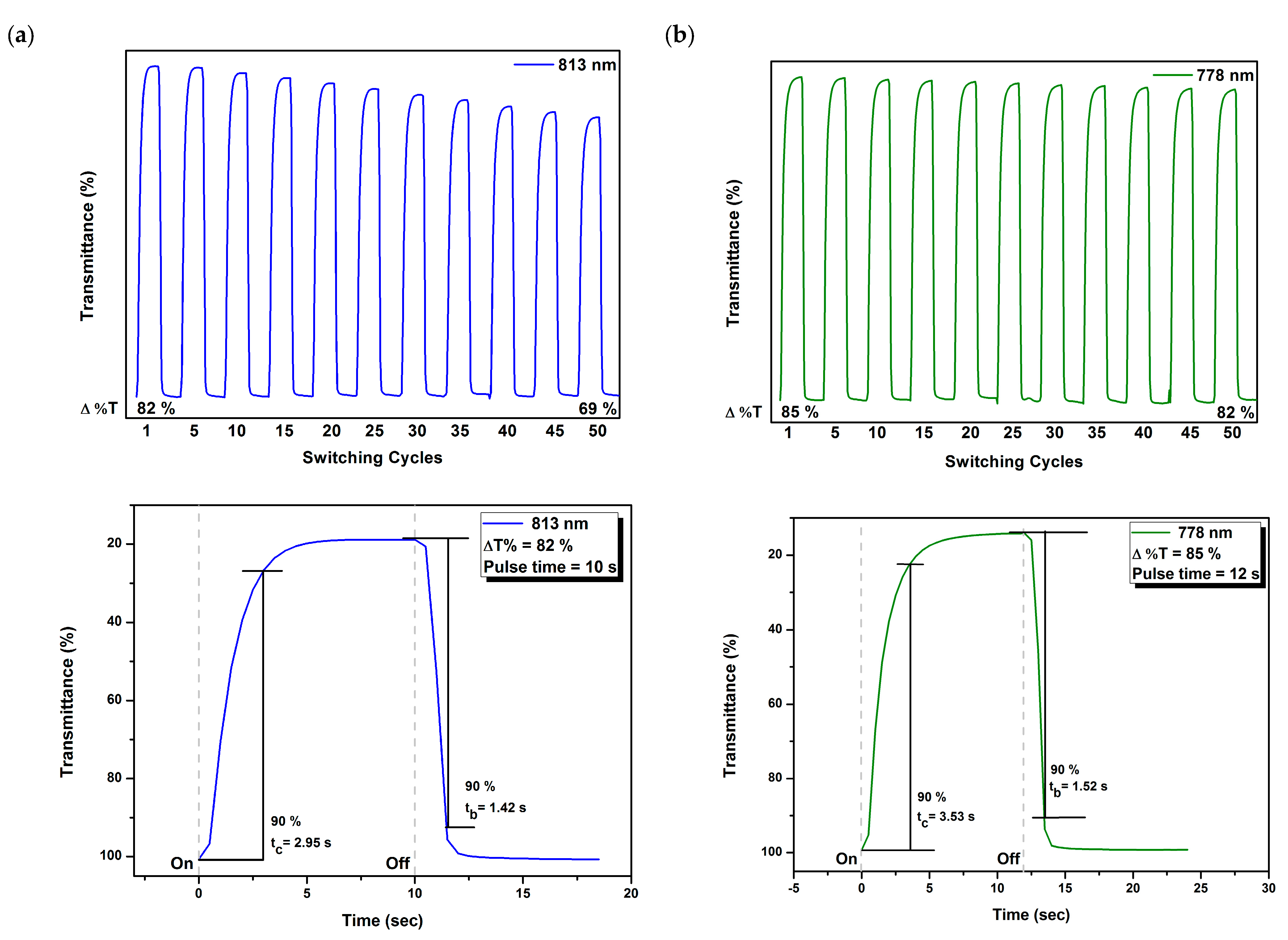
3.6. Electrochromic Switching Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rosseinsky, D.R.; Mortimer, R.J. Electrochromic Systems and the Prospects for Devices. Adv. Mater. 2001, 13, 783–793. [Google Scholar] [CrossRef]
- Monk, P.M.S.; Mortimer, R.J.; Rosseinsky, D.R. Electrochromism and Electrochromic Devices; Cambridge University Press: Cambridge, UK, 2007. [Google Scholar]
- Beaujuge, P.M.; Reynolds, J.R. Color Control in π-Conjugated Organic Polymers for Use in Electrochromic Devices. Chem. Rev. 2010, 110, 268–320. [Google Scholar] [CrossRef] [PubMed]
- Gunbas, G.; Toppare, L. Electrochromic Conjugated Polyheterocycles and Derivatives—Highlights from the Last Decade towards Realization of Long Lived Aspirations. Chem. Commun. 2012, 48, 1083–1101. [Google Scholar] [CrossRef] [PubMed]
- Neo, W.; Ye, Q.; Chua, S.-J.; Xu, J. Conjugated Polymer-Based Electrochromics: Materials, Device Fabrication and Application Prospects. J. Mater. Chem. C 2016, 4, 7364–7376. [Google Scholar] [CrossRef]
- Lv, X.; Li, W.; Ouyang, M.; Zhang, Y.; Wright, D.S.; Zhang, C. Polymeric Electrochromic Materials with Donor-Acceptor Structures. J. Mater. Chem. C 2017, 5, 12–28. [Google Scholar] [CrossRef]
- Granqvist, C.G. Electrochromics for Smart Windows: Oxide-based Thin Films and Devices. Thin Solid Films 2014, 564, 1–38. [Google Scholar]
- Osterholm, A.M.; Shen, D.E.; Kerszulis, J.A.; Bulloch, R.H.; Kuepfert, M.; Dyer, A.L.; Reynolds, J.R. Four Shades of Brown: Tuning of Electrochromic Polymer Blends toward High-contrast Eyewear. ACS Appl. Mater. Interfaces 2015, 7, 1413–1421. [Google Scholar] [CrossRef]
- Yang, P.; Sun, P.; Mai, W. Electrochromic Energy Storage Devices. Mater. Today 2016, 19, 394–402. [Google Scholar] [CrossRef]
- Fu, H.; Zhang, L.; Dong, Y.; Zhang, C.; Weijun Li, W. Recent Advances in Electrochromic Materials and Devices for Camouflage Applications. Mater. Chem. Front. 2023, 7, 2337–2358. [Google Scholar]
- Shirota, Y. Organic Materials for Electronic and Optoelectronic Devices. J. Mater. Chem. 2000, 10, 1–25. [Google Scholar] [CrossRef]
- Thelakkat, M. Star-Shaped, Dendrimeric and Polymeric Triarylamines as Photoconductors and Hole Transport Materials for Electro-optical Applications. Macromol. Mater. Eng. 2002, 287, 442–461. [Google Scholar] [CrossRef]
- Shirota, Y. Photo- and Electroactive Amorphous Molecular Materials—Molecular Design, Syntheses, Reactions, Properties, and Applications. J. Mater. Chem. 2005, 15, 75–93. [Google Scholar] [CrossRef]
- Oishi, Y.; Takado, H.; Yoneyama, M.; Kakimoto, M.; Imai, Y.; Kurosaki, T. Preparation and Properties of New Aromatic Polyamides from 4,4′-Diaminotriphenylamine and Aromatic Dicarboxylic Acids. J. Polym. Sci. Part A Polym. Chem. 1990, 30, 1763–1769. [Google Scholar] [CrossRef]
- Oishi, Y.; Ishida, M.; Kakimoto, M.; Imai, Y.; Kurosaki, T. Preparation and Properties of Novel Soluble Aromatic Polyimides from 4,4′-Diaminotriphenylamine and Aromatic Tetracarboxylic Dianhydrides. J. Polym. Sci. Part A Polym. Chem. 1992, 30, 1027–1035. [Google Scholar] [CrossRef]
- Cheng, S.-H.; Hsiao, S.-H.; Su, T.-H.; Liou, G.-S. Novel Aromatic Poly(amine-imide)s Bearing a Pendent Triphenylamine Group: Synthesis, Thermal, Photophysical, Electrochemical, and Electrochromic Characteristics. Macromolecules 2005, 38, 307–316. [Google Scholar] [CrossRef]
- Liou, G.-S.; Hsiao, S.-H.; Chen, H.-W. Novel High Tg Poly(amine-imide)s Bearing Pendent N-phenylcarbazole Units: Synthesis and Photophysical, Electrochemical and Electrochromic Properties. J. Mater. Chem. 2006, 16, 1831–1842. [Google Scholar] [CrossRef]
- Chang, C.-W.; Liou, G.-S.; Hsiao, S.-H. Highly Stable Anodic Green Electrochromic Aromatic Polyamides: Synthesis and Electrochromic Properties. J. Mater. Chem. 2007, 17, 1007–1015. [Google Scholar] [CrossRef]
- Li, S.; Liu, G.; Ju, X.; Zhang, Y.; Zhao, J. Synthesis, Characterization and Application of Four Novel Electrochromic Materials Employing Nitrotriphenylamine Unit as the Acceptor and Different Thiophene Derivatives as the Donor. Polymers 2017, 9, 173. [Google Scholar] [CrossRef]
- Gao, Z.; Zhao, F.; Ming, S.; Yan, Z.; Zhao, J. Donor-acceptor Type Conjugated Porous Polymers Based on Triphenylamine and Benzothiadiazole Units as Ambipolar Electrochromic Materials. Polymer 2023, 274, 125908. [Google Scholar] [CrossRef]
- Lu, Q.; Cai, W.; Niu, H.; Wang, W.; Bai, X.; Hou, Y. Novel Polyamides with 5H-Dibenzo[b,f]azepin-5-yl-Substituted Triphenylamine: Synthesis and Visible-NIR Electrochromic Properties. Polymers 2017, 9, 543. [Google Scholar] [CrossRef]
- Li, D.; Shi, X.; Cai, W.; Niu, H. Fluorescence Switching of Triarylamine-based Polyamides with Pendant Pyrene Units and its Application in Electrochromic Smart Displays. Electrochim. Acta 2024, 508, 145277. [Google Scholar] [CrossRef]
- Wu, T.-Y.; Chung, H.-H. Applications of Tris(4-(thiophen-2-yl)phenyl)amine and Dithienylpyrrole-based Conjugated Copolymers in High-Contrast Electrochromic Devices. Polymers 2016, 8, 206. [Google Scholar] [CrossRef] [PubMed]
- Simon, P.; Strojsa, J.; Klikar, M.; Buresova, Z.; Ruzicka, A.; Zelenka, J.; Kulhanek, J.; Tydlitat, J. Deep-blue Emissive and Colourless Polyimides: Optical Property Tuning by Triphenylamino and Carbazole Chromophores. Mater. Adv. 2024, 5, 3198–3206. [Google Scholar] [CrossRef]
- Tu, M.-H.; Shao, Y.-J.; Li, H.-L.; Hu, C.-C.; Liou, G.-S. A Tröger’s Base-cored Triarylamine Polyamide for Electrochromic Response Capability Enhancement. ACS Appl. Polym. Mater. 2024, 6, 658–668. [Google Scholar] [CrossRef]
- Muras, K.; Rybak, P.; Napierała, S.; Hoffmann, M.; Kubicki, M.; Wałęsa-Chorab, M. Bis(triphenylamine) Derivatives: Structure-dependent Photophysical Properties and Electropolymerization Ability. Pigm. Dyes 2025, 240, 112811. [Google Scholar] [CrossRef]
- Yen, H.-J.; Liou, G.-S. Solution-processable Triarylamine-based Electroactive High Performance Polymers for Anodically Electrochromic Applications. Polym. Chem. 2012, 3, 255–264. [Google Scholar] [CrossRef]
- Yen, H.-J.; Liou, G.-S. Recent Advances in Triphenylamine-based Electrochromic Derivatives and Polymers. Polym. Chem. 2018, 9, 3001–3018. [Google Scholar] [CrossRef]
- Yen, H.-J.; Liou, G.-S. Design and Preparation of Triphenylamine-based Polymeric Materials towards Emergent Optoelectronic Applications. Prog. Polym. Sci. 2019, 89, 250–287. [Google Scholar] [CrossRef]
- Wang, H.-M.; Hsiao, S.-H.; Liou, G.-S. Highly Stable Electrochromic Polyamides Based on N,N-Bis(4-aminophenyl)-N’,N’-bis(4-tert-butylphenyl)-1,4-phenylenediamine. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 2330–2343. [Google Scholar]
- Wang, H.-M.; Hsiao, S.-H. Electrochemically and Electrochromically Stable Polyimides Bearing tert-butyl-blocked N,N,N′,N′-Tetraphenyl-1,4-phenylenediamine units. Polymer 2009, 50, 1692–1699. [Google Scholar] [CrossRef]
- Hsiao, S.-H.; Wang, H.-M.; Liao, S.-H. Redox-stable and Visible/near-infrared Electrochromic Aramids with Main-chain Triphenylamine and Pendent 3,6-Di-tert-butylcarbazole Units. Polym. Chem. 2014, 5, 2473–2483. [Google Scholar] [CrossRef]
- Wang, H.-M.; Hsiao, S.-H. Enhancement of Redox Stability and Electrochromic Performance of Aromatic Polyamides by Incorporation of (3,6-Dimethoxycarbazol-9-yl)triphenylamine units. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 272–286. [Google Scholar] [CrossRef]
- Wang, H.-M.; Hsiao, S.-H. Substituent Effects on Electrochemical and Electrochromic Properties of Aromatic Polyimides with 4-(Carbazol-9-yl)triphenylamine Moieties. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 1172–1184. [Google Scholar] [CrossRef]
- Chern, Y.-T.; Yen, C.-C.; Wang, J.-M.; Lu, I.-S.; Huang, B.-W.; Hsiao, S.-H. Redox-Stable and Multicolor Electrochromic Polyamides with Four Triarylamine Cores in the Repeating Unit. Polymers 2024, 16, 1644. [Google Scholar] [CrossRef]
- Chern, Y.-T.; Zhang, S.-J.; Ho, S.-J.; Shao, Y.-J.; Wang, Y.-J.; Liou, G.-S. Substituents and Resonance Effects on the Electrochemical Stability of Polyelectrochromic Triarylamine-based Polymers. ACS Appl. Polym. Mater. 2024, 6, 5256–5267. [Google Scholar] [CrossRef]





| Polymer Code | ηinh (dL/g) a | Solvents b,c | ||||||
|---|---|---|---|---|---|---|---|---|
| PAA | PAI | NMP | DMAc | DMF | DMSO | m-Cresol | THF | |
| 6a | 0.69 | − | +− | +− | +− | +− | − | − |
| 6b | 0.91 | − | +− | +− | +− | +− | − | − |
| 6c | 0.67 | 0.66 | + | + | +− | + | +− | − |
| 6d | 0.50 | 0.49 | + | + | +− | + | + | − |
| 6e | 0.29 | 0.35 | + | + | + | + | +− | − |
| 6f | 0.68 | 0.64 | + | + | + | + | + | − |
| MeO-6a | 0.74 | 0.77 | + | + | + | + | +- | − |
| MeO-6b | 0.87 | 0.88 | + | + | +- | + | +- | − |
| MeO-6c | 0.58 | 0.58 | + | + | + | + | + | − |
| MeO-6d | 0.70 | 0.70 | + | + | +- | + | + | − |
| MeO-6e | 0.59 | 0.57 | ++ | ++ | ++ | ++ | + | − |
| MeO-6f | 0.92 | 0.56 | ++ | ++ | ++ | ++ | + | +− |
| t-Bu-6a | 0.40 | 0.80 | + | + | + | + | + | − |
| t-Bu-6b | 0.45 | 0.65 | + | + | + | + | + | − |
| t-Bu-6c | 0.65 | 0.64 | + | + | + | + | + | − |
| t-Bu-6d | 0.39 | 0.58 | + | + | + | + | + | − |
| t-Bu-6e | 0.46 | 0.43 | ++ | ++ | ++ | ++ | + | − |
| t-Bu-6f | 0.48 | 0.50 | ++ | ++ | ++ | ++ | + | +− |
| Polymer Code | Tg (°C) a | Td at 5% Weight Loss (°C) b | Td at 10% Weight Loss (°C) b | Char Yield (wt %) c | ||
|---|---|---|---|---|---|---|
| N2 | Air | N2 | Air | |||
| 6a | 355 | 525 | 537 | 553 | 575 | 58 |
| 6b | 326 | 545 | 550 | 578 | 600 | 60 |
| 6c | 304 | 532 | 539 | 576 | 589 | 55 |
| 6d | 296 | 504 | 488 | 541 | 549 | 58 |
| 6e | 331 | 496 | 514 | 523 | 559 | 58 |
| 6f | 326 | 549 | 535 | 585 | 576 | 62 |
| MeO-6a | 325 | 464 | 438 | 495 | 469 | 68 |
| MeO-6b | 323 | 483 | 482 | 519 | 520 | 66 |
| MeO-6c | 299 | 500 | 476 | 532 | 522 | 50 |
| MeO-6d | 286 | 501 | 473 | 539 | 509 | 60 |
| MeO-6e | 300 | 444 | 447 | 562 | 478 | 52 |
| MeO-6f | 326 | 480 | 473 | 522 | 511 | 55 |
| t-Bu-6a | 350 | 527 | 515 | 560 | 554 | 58 |
| t-Bu-6b | 304 | 513 | 522 | 549 | 556 | 58 |
| t-Bu-6c | 309 | 479 | 473 | 512 | 522 | 55 |
| t-Bu-6d | 289 | 501 | 509 | 532 | 554 | 56 |
| t-Bu-6e | 313 | 423 | 437 | 448 | 471 | 50 |
| t-Bu-6f | 322 | 511 | 486 | 552 | 525 | 58 |
| Polymer Code | Thin Film (nm) | Oxidation Potential (V) a | Egopt(eV) b | HOMO (eV) c | LUMO (eV) d | ||
|---|---|---|---|---|---|---|---|
| λmax | λonset | Eonset | E1/2Ox | ||||
| 6a | 330 | 420 | 0.70 | 0.84 | 2.95 | 5.20 | 2.25 |
| 6b | 338 | 407 | 0.71 | 0.83 | 3.05 | 5.19 | 2.14 |
| 6c | 346 | 418 | 0.70 | 0.83 | 2.97 | 5.19 | 2.22 |
| 6d | 341 | 411 | 0.70 | 0.82 | 3.02 | 5.18 | 2.16 |
| 6e | 344 | 409 | 0.70 | 0.82 | 3.03 | 5.18 | 2.15 |
| 6f | 342 | 406 | 0.72 | 0.83 | 3.05 | 5.19 | 2.14 |
| MeO-6a | 343 | 425 | 0.56 | 0.71 | 2.92 | 5.07 | 2.15 |
| MeO-6b | 344 | 413 | 0.59 | 0.71 | 3.00 | 5.07 | 2.07 |
| MeO-6c | 347 | 422 | 0.59 | 0.72 | 2.94 | 5.08 | 2.14 |
| MeO-6d | 341 | 425 | 0.59 | 0.72 | 2.92 | 5.08 | 2.16 |
| MeO-6e | 348 | 425 | 0.59 | 0.71 | 2.92 | 5.07 | 2.15 |
| MeO-6f | 346 | 419 | 0.59 | 0.72 | 2.96 | 5.08 | 2.12 |
| t-Bu-6a | 333 | 421 | 0.67 | 0.82 | 2.94 | 5.18 | 2.24 |
| t-Bu-6b | 336 | 406 | 0.64 | 0.78 | 3.05 | 5.14 | 2.09 |
| t-Bu-6c | 332 | 421 | 0.63 | 0.77 | 2.95 | 5.13 | 2.18 |
| t-Bu-6d | 339 | 414 | 0.68 | 0.80 | 3.00 | 5.16 | 2.16 |
| t-Bu-6e | 341 | 421 | 0.65 | 0.78 | 2.95 | 5.14 | 2.19 |
| t-Bu-6f | 345 | 413 | 0.66 | 0.79 | 3.00 | 5.15 | 2.15 |
| Polymer Code | λmax a (nm) | Δ%T | Response Time b | ΔOD c | Qd d (mC/cm2) | CE e (cm2/C) | |
|---|---|---|---|---|---|---|---|
| tc (s) | tb (s) | ||||||
| 6a | 804 | 77 | 3.7 | 2.5 | 1.35 | 16.42 | 82 |
| 6b | 818 | 73 | 4.8 | 2.3 | 0.85 | 9.29 | 91 |
| 6c | 818 | 52 | 2.9 | 1.1 | 0.33 | 1.82 | 182 |
| 6d | 813 | 82 | 3.0 | 1.4 | 0.73 | 4.63 | 157 |
| 6e | 810 | 56 | 3.2 | 1.0 | 0.38 | 2.85 | 131 |
| 6f | 809 | 89 | 6.3 | 3.2 | 1.36 | 9.36 | 145 |
| MeO-6a | 779 | 74 | 4.4 | 2.0 | 0.88 | 6.71 | 131 |
| MeO-6b | 779 | 69 | 2.6 | 1.4 | 0.77 | 5.07 | 153 |
| MeO-6c | 780 | 80 | 5.4 | 3.1 | 0.63 | 4.55 | 140 |
| MeO-6d | 779 | 85 | 3.5 | 1.5 | 0.74 | 4.34 | 195 |
| MeO-6e | 780 | 86 | 3.2 | 1.7 | 1.06 | 7.17 | 148 |
| MeO-6f | 775 | 79 | 2.2 | 3.0 | 1.31 | 9.65 | 136 |
| t-Bu-6a | 791 | 97 | 2.7 | 2.0 | 1.63 | 10.39 | 157 |
| t-Bu-6b | 779 | 88 | 3.2 | 1.6 | 0.92 | 5.23 | 176 |
| t-Bu-6c | 799 | 77 | 3.1 | 1.4 | 0.63 | 4.63 | 136 |
| t-Bu-6d | 802 | 88 | 3.1 | 1.4 | 0.96 | 6.62 | 145 |
| t-Bu-6e | 795 | 72 | 2.9 | 1.4 | 0.57 | 4.42 | 128 |
| t-Bu-6f | 796 | 62 | 3.1 | 1.2 | 1.38 | 10.86 | 150 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsiao, S.-H.; Ni, Z.-D. Synthesis and Electrochromic Properties of Triphenylamine-Based Aromatic Poly(amide-imide)s. Polymers 2025, 17, 1152. https://doi.org/10.3390/polym17091152
Hsiao S-H, Ni Z-D. Synthesis and Electrochromic Properties of Triphenylamine-Based Aromatic Poly(amide-imide)s. Polymers. 2025; 17(9):1152. https://doi.org/10.3390/polym17091152
Chicago/Turabian StyleHsiao, Sheng-Huei, and Zong-De Ni. 2025. "Synthesis and Electrochromic Properties of Triphenylamine-Based Aromatic Poly(amide-imide)s" Polymers 17, no. 9: 1152. https://doi.org/10.3390/polym17091152
APA StyleHsiao, S.-H., & Ni, Z.-D. (2025). Synthesis and Electrochromic Properties of Triphenylamine-Based Aromatic Poly(amide-imide)s. Polymers, 17(9), 1152. https://doi.org/10.3390/polym17091152








