Fabrication and Performance of PVAc-Incorporated Porous Self-Standing Zeolite-Based Geopolymer Membranes for Lead (Pb(II)) Removal in Water Treatment
Abstract
1. Introduction
| Source Material | Contaminant | Strength (MPa) | Curing Time and Temperature | Average Pore Size (nm) | Removal (mg/g) | Water Flux (kg/m2h) | Thickness (mm) | Adsorption Conditions | Removal Rate (%) |
|---|---|---|---|---|---|---|---|---|---|
| Metakaolin geopolymer [37] | Ni (II) | 18.67 | 60 °C–24 h | 10–1000 | 22.69–43.36 | 99.02 | 10 | pH 6 20–23 °C | ~95 |
| NaA zeolite geopolymer [27] | Na (I) | 57 | 90 °C–6 h | 3.77 | -- | 3.86 | <10 | -- | 99.55% |
| Metakaolin–Silica fume [22] | Cd (II) | -- | 50 °C–48 h | -- | -- | 26.12 | 10 | -- | 99.22% |
| Fly ash–Zeolite [22] | Cr (VI) | 12.4 | 80 °C–24 h | 15.99 | -- | 851 | 6 | -- | 85.45 |
| Silica fume Metakaolin [22] | Cr (III), Dye | -- | 80 °C–24 h/550 °C–4 h | -- | -- | 22 | 4 | -- | 100% |
2. Materials and Methods
2.1. Zeolite Geopolymer Membrane Preparation (ZGM)
2.2. Water Flux Experiments
2.3. Characterization Techniques
2.3.1. X-Ray Diffraction
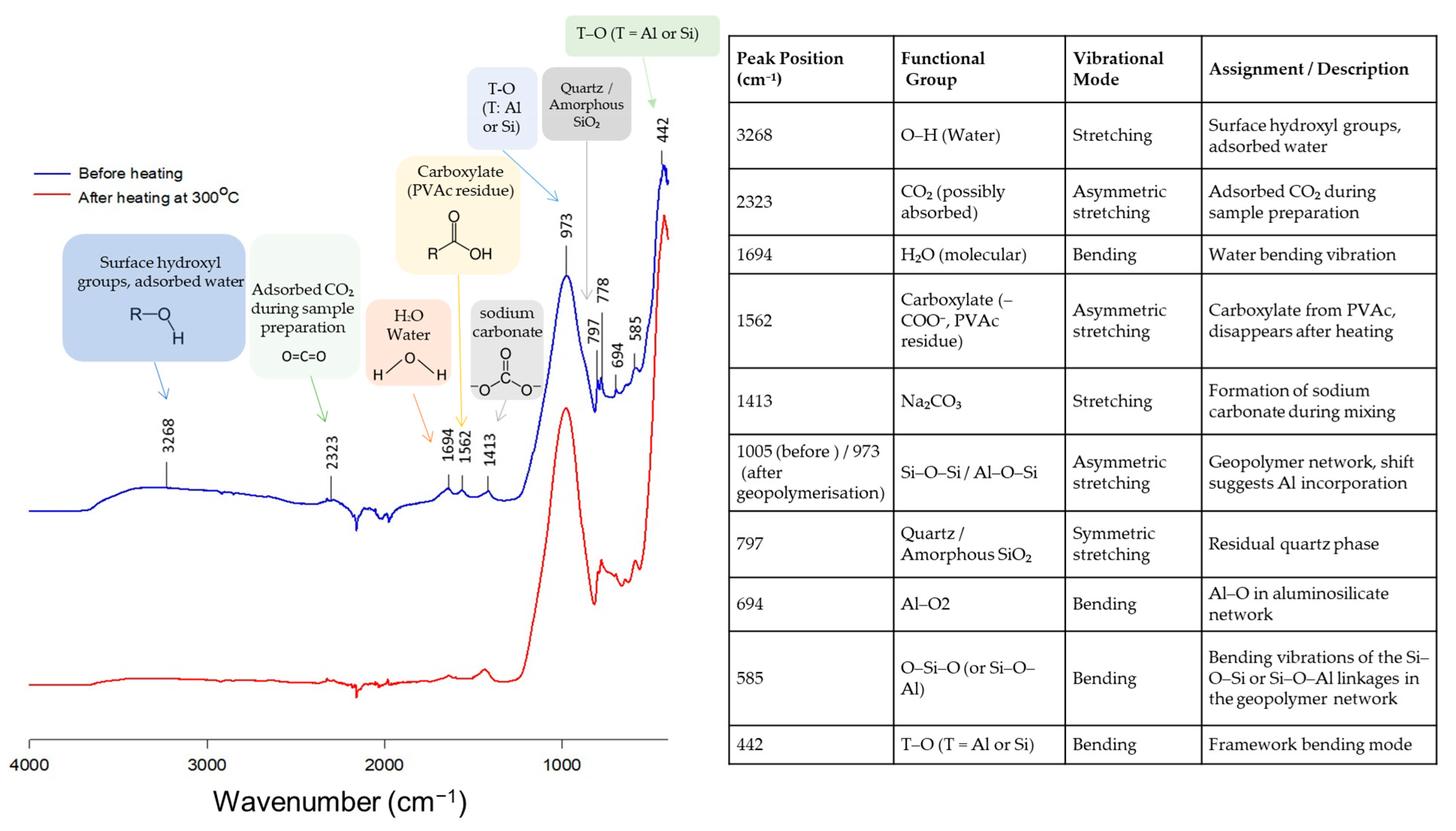
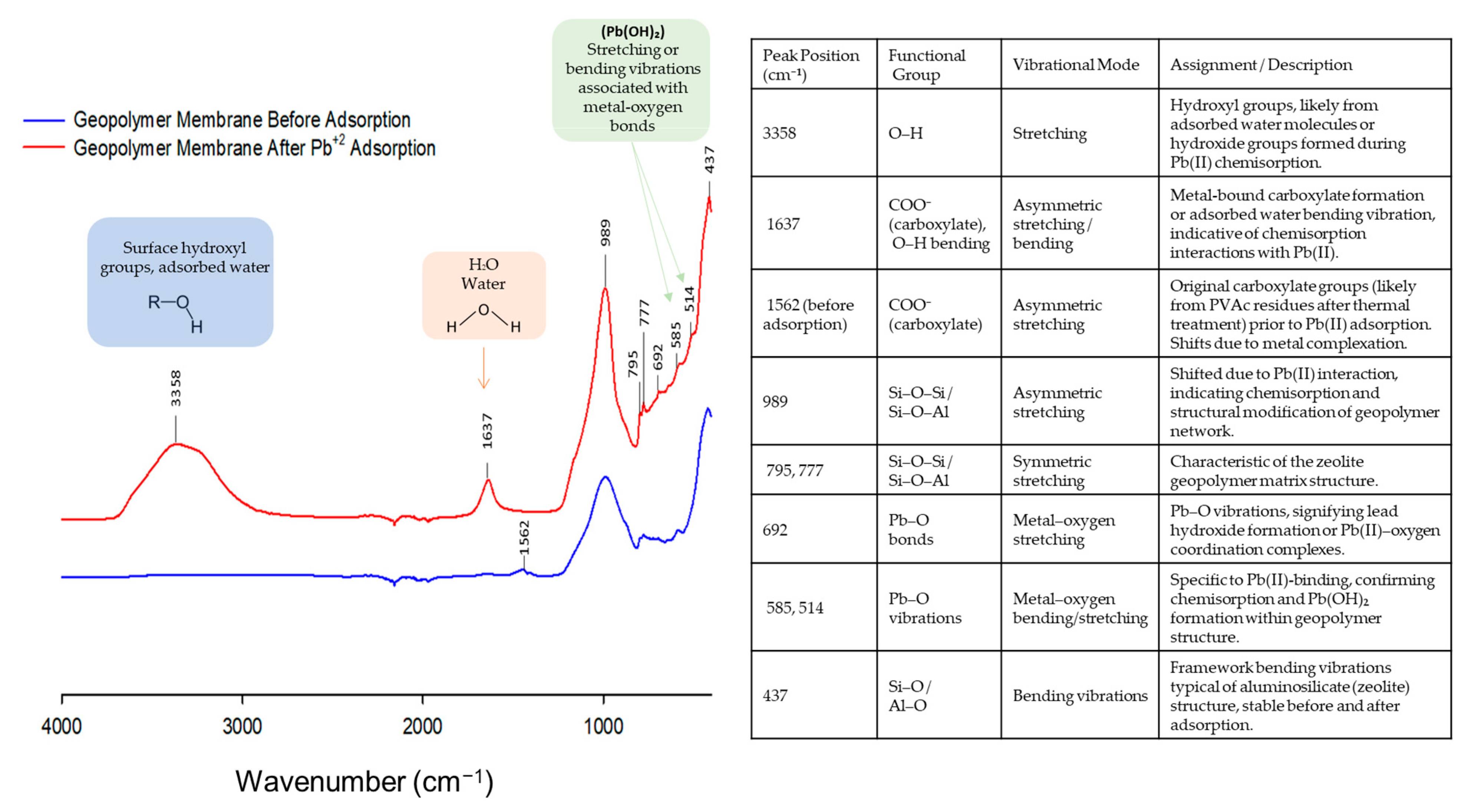
2.3.2. Fourier Transform Infrared Spectroscopy
2.3.3. Thermogravimetric/Differential Scanning Calorimetry
2.3.4. Inductively Coupled Plasma–Optical Emission Spectroscopy
2.3.5. Surface Area and Pore Size Distribution
2.3.6. Hardness and Elastic Modulus Analyses
2.3.7. Scanning Electron Microscopy/Energy-Dispersive Spectroscopy
3. Results and Discussion
3.1. Zeolite and ZGMs Characterization
3.2. Membrane Performance
3.2.1. Influence of PVAc Concentration on Membrane Porosity and Filtration Performance
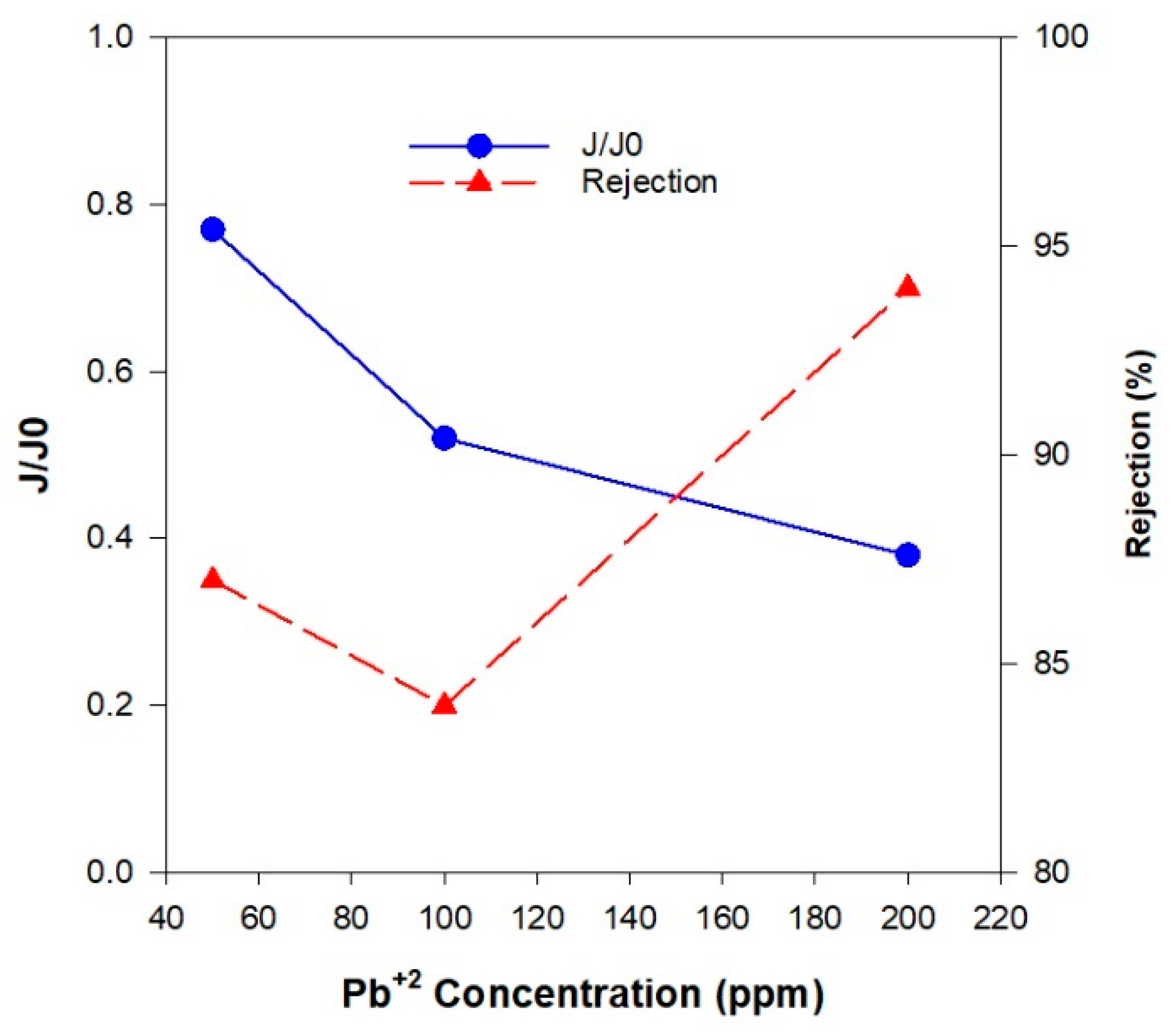
3.2.2. The Effect of Initial Pb(II) Ions Concentration on the Membrane Performance
3.2.3. Comparison of Zeolite-Based Geopolymer Membranes with Alternative Membrane Materials
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| A | Membrane surface area |
| BET | Brunauer–Emmett–Teller |
| BJH | Barrett–Joyner–Halenda |
| CARF | Central Analytical Research Facility |
| DSC | Differential Scanning Calorimetry |
| EDS | Energy-Dispersive Spectroscopy |
| FTIR | Fourier-Transform Infrared Spectroscopy |
| ICP-OES | Inductively Coupled Plasma Optical Emission Spectroscopy |
| IUPAC | International Union of Pure and Applied Chemistry |
| J | Water Flux |
| MDPI | Multidisciplinary Digital Publishing Institute |
| MPa | Megapascal |
| PVAC | Polyvinyl Acetate |
| QUT | Queensland University of Technology |
| R | Removal efficiency |
| SEM | Scanning Electron Microscopy |
| SiO2/Al2O3 | Silicon-to-Aluminum Ratio |
| TGA | Thermogravimetric Analysis |
| t | Time |
| XRD | X-ray Diffraction |
| ZGM | Zeolite Geopolymer Membrane |
References
- Ayuso-Álvarez, A.; Simón, L.; Nuñez, O.; Rodríguez-Blázquez, C.; Martín-Méndez, I.; Bel-lán, A.; López-Abente, G.; Merlo, J.; Fernandez-Navarro, P.; Galán, I. Association between Heavy Metals and Metalloids in Topsoil and Mental Health in the Adult Population of Spain. Environ. Res. 2019, 179, 108784. [Google Scholar] [CrossRef] [PubMed]
- Pyrzynska, K. Recent Applications of Carbon Nanotubes for Separation and Enrichment of Lead Ions. Separations 2023, 10, 152. [Google Scholar] [CrossRef]
- Amari, S. Innovative Natural Zeolite-Based Geopolymers for Water Treatment; Queensland University of Technology: Brisbane City, Australia, 2021. [Google Scholar]
- Rahman, M.M.; Shaikh, M.A.A.; Yeasmin, M.S.; Gafur, M.A.; Hossain, M.I.; Alam, M.A.; Khan, M.S.; Paul, T.; Quddus, M.S. Simultaneous Removal of Ni2+ and Congo Red from Wastewater by Crystalline Nanocellulose—Modified Coal Bionanocomposites: Continuous Adsorption Study with Mathematical Modeling. Groundw. Sustain. Dev. 2024, 26, 101244. [Google Scholar] [CrossRef]
- Mahmudur Rahman, M.; Ismail Hossain, M.; Chandra Ghos, B.; Abdul Gafur, M.; Alam, M.A.; Jasim Uddin, M.; Sarmina Yeasmin, M.; Hasan, M.; Akter Chowdhury, T.; Masud Rana, G.M.; et al. Fabrication of CNC-AC Bionanosorbents from the Residual Mass of Magnolia Champaca l. Bark after Methanol Extraction for Wastewater Treatment: Continuous Column Adsorption Study. Environ. Nanotechnol. Monit. Manag. 2024, 22, 101015. [Google Scholar] [CrossRef]
- Jiao, Y.; Okada, M.; Nutan, B.; Nagaoka, N.; Bikharudin, A.; Musa, R.; Matsumoto, T. Fabrication of a Fish-Bone-Inspired Inorganic–Organic Composite Membrane. Polymers 2023, 15, 4190. [Google Scholar] [CrossRef]
- Carta, M.; Antonangelo, A.R.; Jansen, J.C.; Longo, M. The Difference in Performance and Compatibility between Crystalline and Amorphous Fillers in Mixed Matrix Membranes for Gas Separation (MMMs). Polymers 2023, 15, 2951. [Google Scholar] [CrossRef]
- Song, X.; Song, X.; Zhang, Y.; Fan, J. Improving the Pervaporation Performance of PDMS Membranes for Trichloroethylene by Incorporating Silane-Modified ZSM-5 Zeolite. Polymers 2023, 15, 3777. [Google Scholar] [CrossRef]
- Zhang, Z.; Huang, G.; Li, Y.; Chen, X.; Yao, Y.; Ren, S.; Li, M.; Wu, Y.; An, C. Electrically Conductive Inorganic Membranes: A Review on Principles, Characteristics and Applications. Chem. Eng. J. 2022, 427, 131987. [Google Scholar] [CrossRef]
- Goh, P.S.; Ismail, A.F. A Review on Inorganic Membranes for Desalination and Wastewater Treatment. Desalination 2018, 434, 60–80. [Google Scholar] [CrossRef]
- Goncharuk, V.V.; Dul’neva, T.Y.; Kucheruk, D.D. Water Purification of Hydroxocomlexes of Heavy Metals by Electromicrofiltration Using Inorganic Membranes. J. Water Chem. Technol. 2010, 32, 95–100. [Google Scholar] [CrossRef]
- Sprynskyy, M.; Buszewski, B.; Terzyk, A.P.; Namieśnik, J. Study of the Selection Mechanism of Heavy Metal (Pb2+, Cu2+, Ni2+, and Cd2+) Adsorption on Clinoptilolite. J. Colloid Interface Sci. 2006, 304, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Terdkiatburana, T.; Tadé, M.O. Adsorption of Cu(II), Pb(II) and Humic Acid on Natural Zeolite Tuff in Single and Binary Systems. Sep. Purif. Technol. 2008, 62, 64–70. [Google Scholar] [CrossRef]
- Velarde, L.; Nabavi, M.S.; Escalera, E.; Antti, M.-L.; Akhtar, F. Adsorption of Heavy Metals on Natural Zeolites: A Review. Chemosphere 2023, 328, 138508. [Google Scholar] [CrossRef]
- Shariatinia, Z.; Bagherpour, A. Synthesis of Zeolite NaY and Its Nanocomposites with Chitosan as Adsorbents for Lead(II) Removal from Aqueous Solution. Powder Technol. 2018, 338, 744–763. [Google Scholar] [CrossRef]
- Hong, M.; Yu, L.; Wang, Y.; Zhang, J.; Chen, Z.; Dong, L.; Zan, Q.; Li, R. Heavy Metal Adsorption with Zeolites: The Role of Hierarchical Pore Architecture. Chem. Eng. J. 2019, 359, 363–372. [Google Scholar] [CrossRef]
- Panayotova, M.; Velikov, B. Kinetics of heavy metal ions removal by use of natural zeolite. J. Environ. Sci. Health Part A 2002, 37, 139–147. [Google Scholar] [CrossRef]
- El-Eswed, B.I.; Yousef, R.I.; Alshaaer, M.; Hamadneh, I.; Al-Gharabli, S.I.; Khalili, F. Stabilization/Solidification of Heavy Metals in Kaolin/Zeolite Based Geopolymers. Int. J. Miner. Process. 2015, 137, 34–42. [Google Scholar] [CrossRef]
- Andrejkovičová, S.; Sudagar, A.; Rocha, J.; Patinha, C.; Hajjaji, W.; da Silva, E.F.; Velosa, A.; Rocha, F. The Effect of Natural Zeolite on Microstructure, Mechanical and Heavy Metals Adsorption Properties of Metakaolin Based Geopolymers. Appl. Clay Sci. 2016, 126, 141–152. [Google Scholar] [CrossRef]
- Amari, S.; Darestani, M.; Millar, G.J.; Samali, B.; Strounina, E. Engineering and Life Cycle Assessment (LCA) of Sustainable Zeolite-Based Geopolymer Incorporating Blast Furnace Slag. Sustainability 2024, 16, 440. [Google Scholar] [CrossRef]
- Nurlina, N.; Pratama, J.H.; Pambudi, A.B.; Rahmawati, Z.; Subaer, S.; Abdullah, M.M.A.B.; Gusrizal, G.; Fansuri, H. A Review of Geopolymer Membrane for Water Treatment. Appl. Clay Sci. 2024, 251, 107301. [Google Scholar] [CrossRef]
- Li, C.J.; Zhang, Y.J.; Chen, H.; He, P.Y. High Value-Added Utilization of Silica Fume to Synthesize ZSM-35 Zeolite Membrane for Cd2+ Removal. Mater. Lett. 2020, 260, 126940. [Google Scholar] [CrossRef]
- He, P.Y.; Zhang, Y.J.; Chen, H.; Han, Z.C.; Liu, L.C. Low-Cost and Facile Synthesis of Geopolymer-Zeolite Composite Membrane for Chromium(VI) Separation from Aqueous Solution. J. Hazard. Mater. 2020, 392, 122359. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ge, Y.; Li, Z.; Wang, Y. Facile Fabrication of a Low-Cost and Environmentally Friendly Inorganic-Organic Composite Membrane for Aquatic Dye Removal. J. Environ. Manag. 2020, 256, 109969. [Google Scholar] [CrossRef]
- Song, Y.; Li, Z.; Zhang, J.; Tang, Y.; Ge, Y.; Cui, X. A Low-Cost Biomimetic Heterostructured Multilayer Membrane with Geopolymer Microparticles for Broad-Spectrum Water Purification. ACS Appl. Mater. Interfaces 2020, 12, 12133–12142. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zhang, Y.J.; He, P.Y.; Li, C.J.; Li, H. Coupling of Self-Supporting Geopolymer Membrane with Intercepted Cr(III) for Dye Wastewater Treatment by Hybrid Photocatalysis and Membrane Separation. Appl. Surf. Sci. 2020, 515, 146024. [Google Scholar] [CrossRef]
- He, Y.; Cui, X.; Liu, X.; Wang, Y.; Zhang, J.; Liu, K. Preparation of Self-Supporting NaA Zeolite Membranes Using Geopolymers. J. Memb. Sci. 2013, 447, 66–72. [Google Scholar] [CrossRef]
- Xu, M.; He, Y.; Wang, Y.; Cui, X. Preparation of a Non-Hydrothermal NaA Zeolite Membrane and Defect Elimination by Vacuum-Inhalation Repair Method. Chem. Eng. Sci. 2017, 158, 117–123. [Google Scholar] [CrossRef]
- Subaer; Haris, A.; Irhamsyah, A.; Permatasari, A.D.; Desa, S.S.; Irfanita, R.; Wahyuni, S. Pervaporation Membrane Based on Laterite Zeolite-Geopolymer for Ethanol-Water Separation. J. Clean. Prod. 2020, 249, 119413. [Google Scholar] [CrossRef]
- Shao, N.; Tang, S.; Li, S.; Chen, H.; Zhang, Z. Defective Analcime/Geopolymer Composite Membrane Derived from Fly Ash for Ultrafast and Highly Efficient Filtration of Organic Pollutants. J. Hazard. Mater. 2020, 388, 121736. [Google Scholar] [CrossRef]
- Wang, J.; Ge, Y.; He, Y.; Xu, M.; Cui, X. A Porous Gradient Geopolymer-Based Tube Membrane with High PM Removal Rate for Air Pollution. J. Clean. Prod. 2019, 217, 335–343. [Google Scholar] [CrossRef]
- AI, A.K.S.; Cindrella, L. Self-Humidifying Novel Chitosan-Geopolymer Hybrid Membrane for Fuel Cell Applications. Carbohydr. Polym. 2019, 223, 115073. [Google Scholar] [CrossRef] [PubMed]
- Amari, S.; Darestani, M.; Millar, G.J.; Rintoul, L.; Samali, B. Microchemistry and Microstructure of Sustainable Mined Zeolite-Geopolymer. J. Clean. Prod. 2019, 234, 1165–1177. [Google Scholar] [CrossRef]
- Papa, E.; Medri, V.; Amari, S.; Manaud, J.; Benito, P.; Vaccari, A.; Landi, E. Zeolite-Geopolymer Composite Materials: Production and Characterization. J. Clean. Prod. 2018, 171, 76–84. [Google Scholar] [CrossRef]
- Liang, K.; Wang, X.Q.; Chow, C.L.; Lau, D. A Review of Geopolymer and Its Adsorption Capacity with Molecular Insights: A Promising Adsorbent of Heavy Metal Ions. J. Environ. Manag. 2022, 322, 116066. [Google Scholar] [CrossRef]
- Ghandashtani, M.B.; Zokaee Ashtiani, F.; Karimi, M.; Fouladitajar, A. A Novel Approach to Fabricate High Performance Nano-SiO2 Embedded PES Membranes for Microfiltration of Oil-in-Water Emulsion. Appl. Surf. Sci. 2015, 349, 393–402. [Google Scholar] [CrossRef]
- Ge, Y.; Yuan, Y.; Wang, K.; He, Y.; Cui, X. Preparation of Geopolymer-Based Inorganic Membrane for Removing Ni2+ from Wastewater. J. Hazard. Mater. 2015, 299, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Shamim Sheikh, M.; Mahmudur Rahman, M.; Safiur Rahman, M.; Yildirim, K.; Maniruzzaman, M. Fabrication of Nano Composite Membrane Filter from Graphene Oxide(GO) and Banana Rachis Cellulose Nano Crystal(CNC) for Industrial Effluent Treatment. J. Ind. Eng. Chem. 2023, 128, 196–208. [Google Scholar] [CrossRef]
- Rahman, M.M.; Maniruzzaman, M.; Gafur, M.A.; Al-Ahmary, K.M.; Shawabkeh, A.; Alsharif, A.; Naznin, S.; Al-Otaibi, J.S. Fabrication of Chitosan Coated Bentonite Clay Multifunctional Nanosorbents from Waste Biomass for the Effective Elimination of Hazardous Pollutants from Waterbodies: A Fixed Bed Biosorption, Mechanism, and Mathematical Model Study. Int. J. Biol. Macromol. 2024, 282, 137439. [Google Scholar] [CrossRef]
- Boshrouyeh Ghandashtani, M.; Edraki, M.; Baumgartl, T.; Costine, A.; Amari, S. Investigation of the Attenuation and Release of Cu2+ Ions by Polymer-Treated Tailings. Minerals 2022, 12, 846. [Google Scholar] [CrossRef]
- Rahman, M.M.; Hossain, M.I.; Ghos, B.C.; Uddin, M.J.; Knani, S.; Waliullah, M. A State-of-the-Art Review Focusing on the Fabrication Technique of Activated Chitosan-Bitumin Coal Based Multifunctional Bionanocomposites for Industrial Wastewater Treatment: Production, Characterization, and Fixed Bed Column Adsorption Study. J. Environ. Chem. Eng. 2025, 13, 115908. [Google Scholar] [CrossRef]
- Yan, W.; Pun, C.L.; Simon, G.P. Conditions of Applying Oliver–Pharr Method to the Nanoindentation of Particles in Composites. Compos. Sci. Technol. 2012, 72, 1147–1152. [Google Scholar] [CrossRef]
- Elaiopoulos, K.; Perraki, T.; Grigoropoulou, E. Monitoring the Effect of Hydrothermal Treatments on the Structure of a Natural Zeolite through a Combined XRD, FTIR, XRF, SEM and N2-Porosimetry Analysis. Microporous Mesoporous Mater. 2010, 134, 29–43. [Google Scholar] [CrossRef]
- Ulloa, N.A.; Baykara, H.; Cornejo, M.H.; Rigail, A.; Paredes, C.; Villalba, J.L. Application-Oriented Mix Design Optimization and Characterization of Zeolite-Based Geopolymer Mortars. Constr. Build. Mater. 2018, 174, 138–149. [Google Scholar] [CrossRef]
- Liang, G.; Nguyen, A.V.; Chen, W.; Nguyen, T.A.H.; Biggs, S. Interaction Forces between Goethite and Polymeric Flocculants and Their Effect on the Flocculation of Fine Goethite Particles. Chem. Eng. J. 2018, 334, 1034–1045. [Google Scholar] [CrossRef]
- Asadullah, M.; Kabir, M.S.; Ahmed, M.B.; Razak, N.A.; Rasid, N.S.A.; Aezzira, A. Role of Microporosity and Surface Functionality of Activated Carbon in Methylene Blue Dye Removal from Water. Korean J. Chem. Eng. 2013, 30, 2228–2234. [Google Scholar] [CrossRef]
- Baldovino-Medrano, V.G.; Niño-Celis, V.; Isaacs Giraldo, R. Systematic Analysis of the Nitrogen Adsorption–Desorption Isotherms Recorded for a Series of Materials Based on Microporous–Mesoporous Amorphous Aluminosilicates Using Classical Methods. J. Chem. Eng. Data 2023, 68, 2512–2528. [Google Scholar] [CrossRef]
- Adam, M.R.; Matsuura, T.; Othman, M.H.D.; Puteh, M.H.; Pauzan, M.A.B.; Ismail, A.F.; Mustafa, A.; Rahman, M.A.; Jaafar, J.; Abdullah, M.S. Feasibility Study of the Hybrid Adsorptive Hollow Fibre Ceramic Membrane (HFCM) Derived from Natural Zeolite for the Removal of Ammonia in Wastewater. Process Saf. Environ. Prot. 2019, 122, 378–385. [Google Scholar] [CrossRef]
- Chen, S.; Wu, C.; Yan, D. Binder-Scale Creep Behavior of Metakaolin-Based Geopolymer. Cem. Concr. Res. 2019, 124, 105810. [Google Scholar] [CrossRef]
- Tan, X.; Liu, S.; Li, K. Preparation and Characterization of Inorganic Hollow Fiber Membranes. J. Memb. Sci. 2001, 188, 87–95. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts; John Wiley & Sons: Hoboken, NJ, USA, 2004; ISBN 0470093072. [Google Scholar]
- Mozgawa, W. The Influence of Some Heavy Metals Cations on the FTIR Spectra of Zeolites. J. Mol. Struct. 2000, 555, 299–304. [Google Scholar] [CrossRef]
- Rees, C.A.; Provis, J.L.; Lukey, G.C.; van Deventer, J.S.J. Attenuated Total Reflectance Fourier Transform Infrared Analysis of Fly Ash Geopolymer Gel Aging. Langmuir 2007, 23, 8170–8179. [Google Scholar] [CrossRef] [PubMed]
- Venegas-Sanchez, J.A.; Tagaya, M.; Kobayashi, T. Effect of Ultrasound on the Aqueous Viscosity of Several Water-Soluble Polymers. Polym. J. 2013, 45, 1224–1233. [Google Scholar] [CrossRef]
- Boshrouyeh Ghandashtani, M.; Tavangar, T.; Zokaee Ashtiani, F.; Karimi, M.; Fouladitajar, A. Experimental Investigation and Mathematical Modeling of Nano-composite Membrane Fabrication Process: Focus on the Role of Solvent Type. Asia-Pac. J. Chem. Eng. 2018, 13, e2260. [Google Scholar] [CrossRef]
- Rahmanian, O.; Amini, S.; Dinari, M. Preparation of Zinc/Iron Layered Double Hydroxide Intercalated by Citrate Anion for Capturing Lead (II) from Aqueous Solution. J. Mol. Liq. 2018, 256, 9–15. [Google Scholar] [CrossRef]
- Zhu, L.; Ji, J.; Wang, S.; Xu, C.; Yang, K.; Xu, M. Removal of Pb(II) from Wastewater Using Al2O3-NaA Zeolite Composite Hollow Fiber Membranes Synthesized from Solid Waste Coal Fly Ash. Chemosphere 2018, 206, 278–284. [Google Scholar] [CrossRef] [PubMed]
- Poolachira, S.; Velmurugan, S. Efficient Removal of Lead Ions from Aqueous Solution by Graphene Oxide Modified Polyethersulfone Adsorptive Mixed Matrix Membrane. Environ. Res. 2022, 210, 112924. [Google Scholar] [CrossRef]
- Zou, D.; Lee, Y.M. Design Strategy of Poly(Vinylidene Fluoride) Membranes for Water Treatment. Prog. Polym. Sci. 2022, 128, 101535. [Google Scholar] [CrossRef]
- Xu, S.; Liu, Y.; Yu, Y.; Zhang, X.; Zhang, J.; Li, Y. PAN/PVDF Chelating Membrane for Simultaneous Removal of Heavy Metal and Organic Pollutants from Mimic Industrial Wastewater. Sep. Purif. Technol. 2020, 235, 116185. [Google Scholar] [CrossRef]
- Chheang, M.; Hongprasith, N.; Ratanatawanate, C.; Lohwacharin, J. Effects of Chemical Cleaning on the Ageing of Polyvinylidene Fluoride Microfiltration and Ultrafiltration Membranes Fouled with Organic and Inorganic Matter. Membranes 2022, 12, 280. [Google Scholar] [CrossRef]
- Scientifi, T. Thermo Scientific Nalgene Rapid-Flow PES Filter Units and Bottle Tops Superior Flow Rates Reduce Downtime for Cell Culture Applications. 2017. Available online: https://assets.thermofisher.cn/TFS-Assets/LCD/Specification-Sheets/ThermoScientific-RapidFlow-SpecSheet-SSLSPRFPES%200217-EN.pdf (accessed on 1 January 2017).
- Yu Wang, K.; Chung, T.-S.; Gryta, M. Hydrophobic PVDF Hollow Fiber Membranes with Narrow Pore Size Distribution and Ultra-Thin Skin for the Fresh Water Production through Membrane Distillation. Chem. Eng. Sci. 2008, 63, 2587–2594. [Google Scholar] [CrossRef]
- Bat-Amgalan, M.; Kano, N.; Miyamoto, N.; Kim, H.-J.; Yunden, G. Fabrication and Properties of Adsorptive Ceramic Membrane Made from Kaolin with Addition of Dolomite for Removal of Metal Ions in a Multielement Aqueous System. ACS Omega 2024, 9, 43068–43080. [Google Scholar] [CrossRef] [PubMed]
- Kunnakorn, D.; Rirksomboon, T.; Siemanond, K.; Aungkavattana, P.; Kuanchertchoo, N.; Chuntanalerg, P.; Hemra, K.; Kulprathipanja, S.; James, R.B.; Wongkasemjit (Dhumrongvaraporn), S. Techno-Economic Comparison of Energy Usage between Azeotropic Distillation and Hybrid System for Water–Ethanol Separation. Renew. Energy 2013, 51, 310–316. [Google Scholar] [CrossRef]



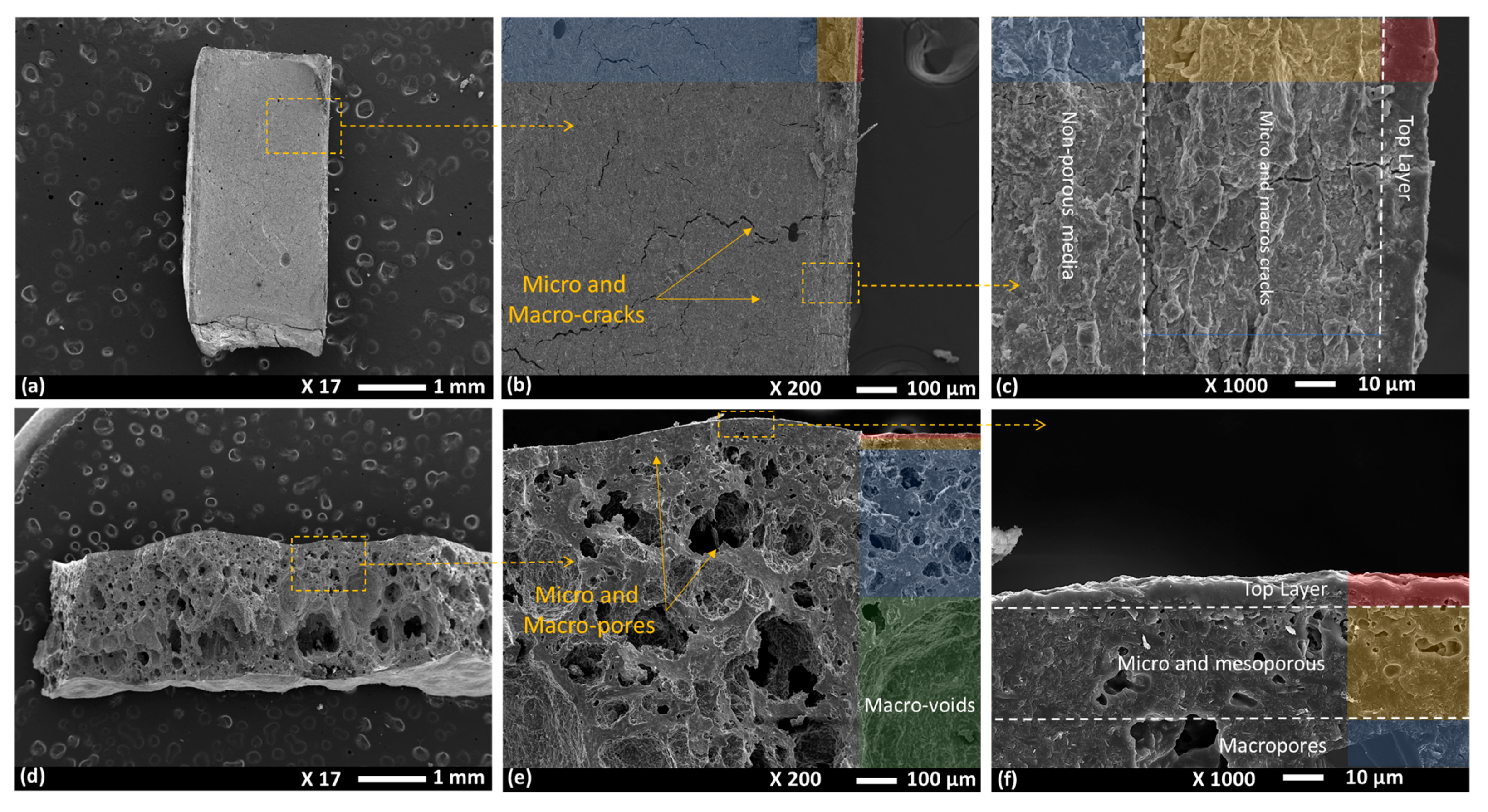
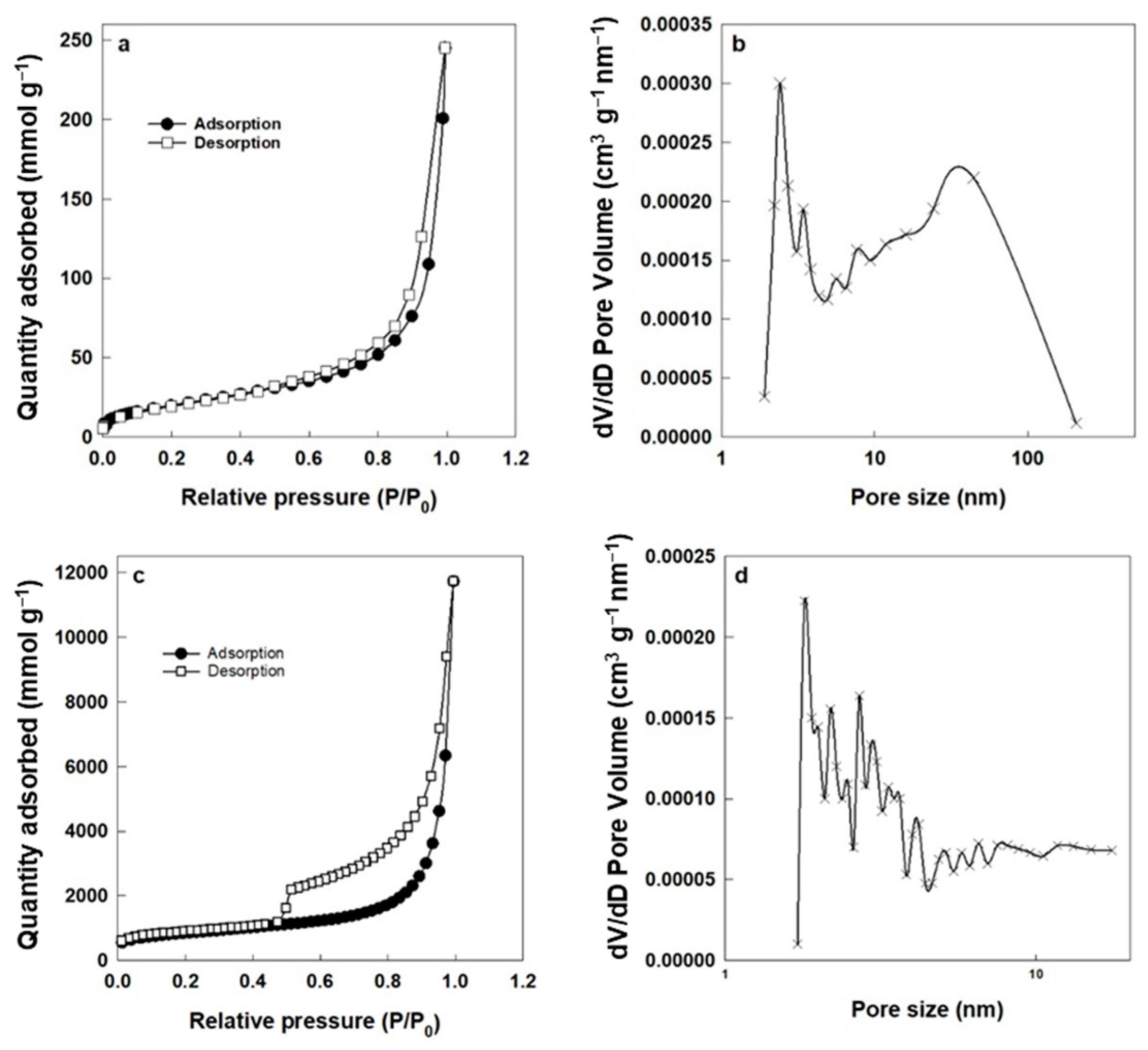
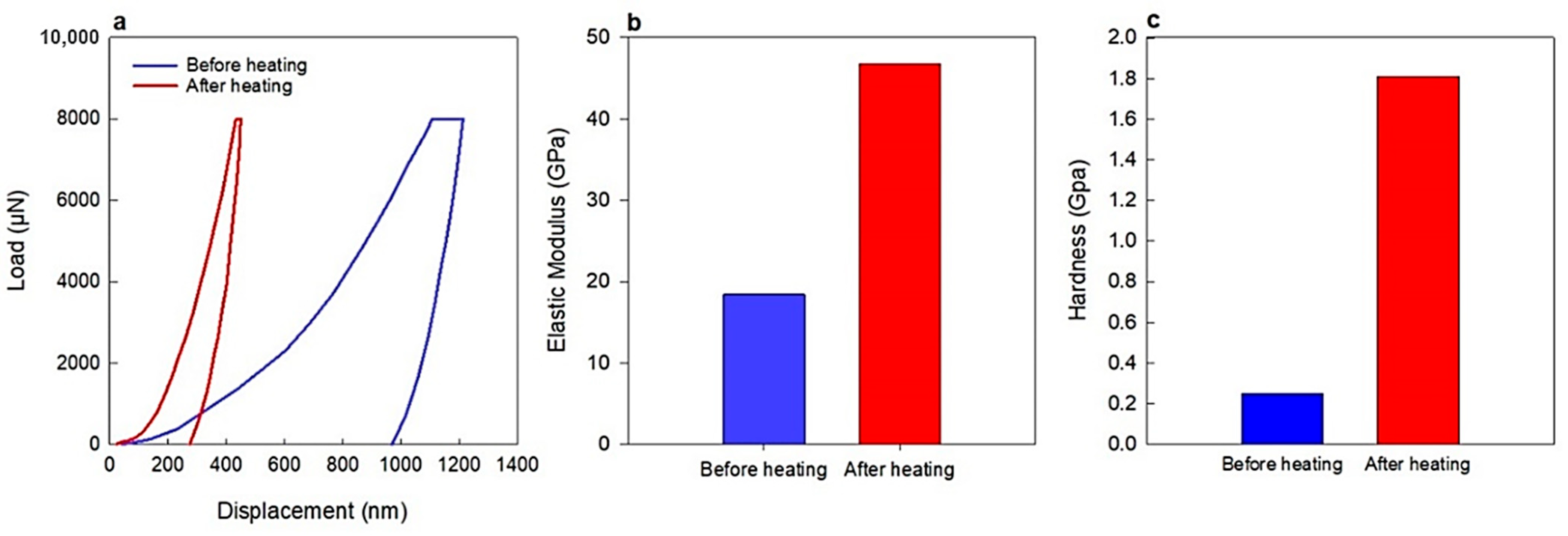
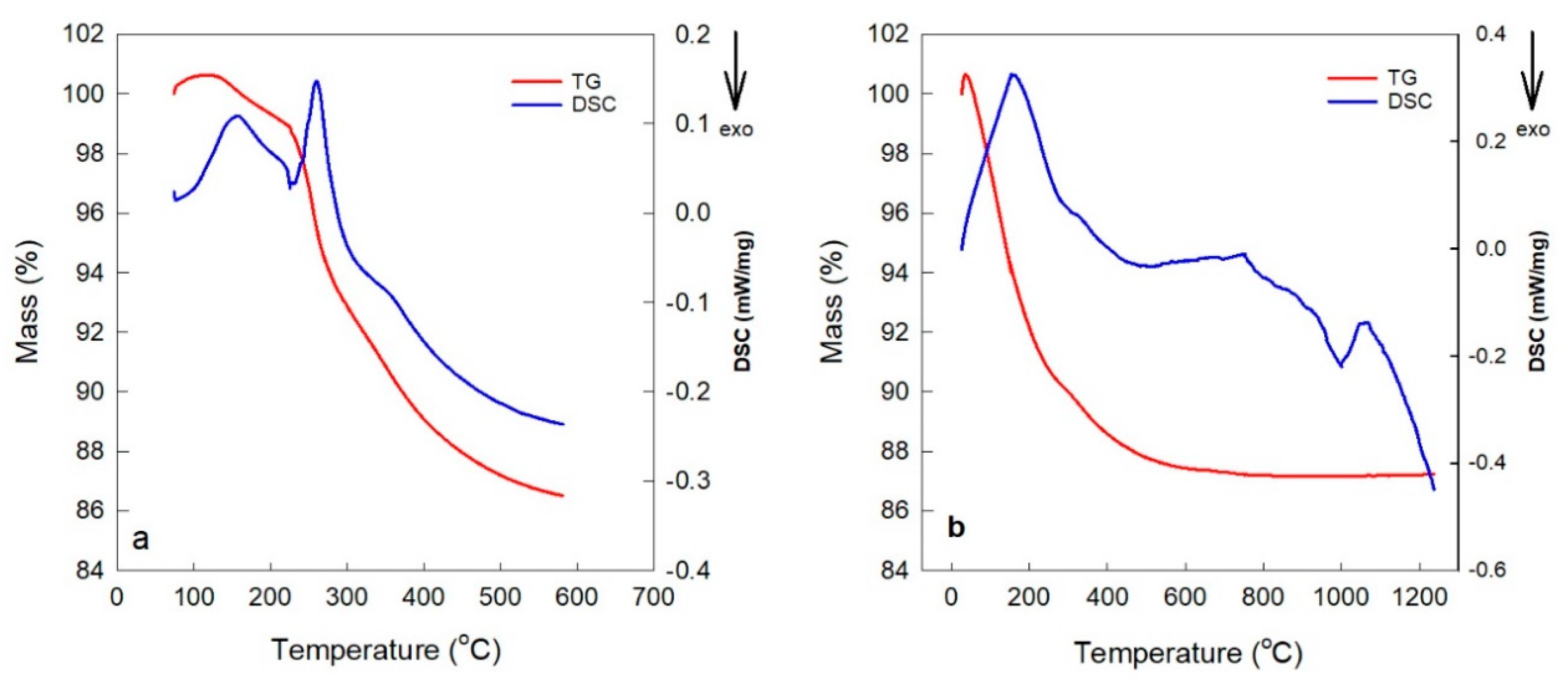




| Membrane Type | Advantages | Disadvantages | Key Applications |
|---|---|---|---|
| Metal Oxide Ceramic Membranes | High thermal and chemical stability, suitable for harsh environments, tunable pore sizes | High production costs, complex multi-step fabrication, high-temperature sintering, reproducibility challenges | Wastewater treatment, gas separation, pervaporation, nanofiltration |
| Zeolite Membranes | Molecular sieving properties, high salt rejection, chemical stability, eliminates costly pretreatment of polymeric RO membranes, tunable hydrophilicity, potential natural source | Fragile support layers, poor compatibility between the zeolite and substrate, difficult large-scale fabrication, presence of intercrystalline pores, microdefects | Water purification, desalination, gas separation, pervaporation |
| Metal Organic Framework (MOF) Membranes | High surface area, tunable porosity, low-temperature synthesis, potential for polymer support, mechanically robust, lower temperature activation than zeolites | High production costs, Limited industrial application, high synthesis complexity, may suffer from structural instability in aqueous environments | Gas separation, water purification, solvent recovery, catalysis |
| Carbon Nanotube (CNT) Membranes | High surface area, antimicrobial activity, ultra-fast water transport, antimicrobial properties, tunable pore size, compelling mechanical strength | Difficult large-scale production, high cost of synthesis, reproducibility issues | Desalination, wastewater treatment, antimicrobial filtration, molecular separation |
| Graphene-Based Membranes | High water flux, ultra-thin structure, strong chemical resistance, potential for molecular sieving | Challenges in creating uniform nanopores, reproducibility, limited large-scale availability | Desalination, reverse osmosis, molecular sieving, gas separation |
| Source Material | Contaminant | Removal (mg/g) | Adsorbent Dosage/L | Adsorption Conditions |
|---|---|---|---|---|
| Rice husk ash-based geopolymer | Cu(II) | 126.26 | 0.1 g | pH 8, 25 °C |
| Metakaolin-based geopolymer | Cu(II) | 40.9 | 0.1 g | pH 8, 25 °C |
| Blast furnace slag-based geopolymer | As(III) | 0.52 | 25 g | pH 6, 20–23 °C |
| Metakaolin-based geopolymer | Mn(II) | 72.34 | 0.08 g | pH 6, 30 °C |
| Rice husk ash-based porous geopolymer | Hg(II) | 232.60 | 0.08 g | pH 6, 25 °C |
| Lateritic clay-based geopolymer | Co(II) | 500 | 0.5 g | pH 7, 60 °C |
| Metakaolin-based geopolymer | Pb(II) | 86.2 | 0.1 g | pH 5, 45 °C |
| Rice husk ash-based porous geopolymer | Pb(II) | 312.5 | 0.08 g | pH 6, 25 °C |
| Blast furnace slag-based geopolymer | Pb(II) | 137.49 | 2 g | pH 6.5, 25 °C |
| Natural zeolite-based geopolymer [3] | Pb(II) | 45.32 | 2 g | pH 7.1, 25 °C |
| Membrane Code | Sodium Hydroxide Solution (40% w/w) | Sodium Silicate (55.4–56.4% w/w) | Natural Zeolite Powder | PVAc Emulsion Concentration | PVAc Emulsion Volume |
|---|---|---|---|---|---|
| ZGM1 | 40 g | 50 g | 100 g | 10 wt.% | 10 mL |
| ZGM2 | 40 g | 50 g | 100 g | 20 wt.% | 10 mL |
| ZGM3 | 40 g | 50 g | 100 g | 30 wt.% | 10 mL |
| Phase | PDF # | Formula | SiO2/Al2O3 Ratio | Zeolite (wt.%) | Geopolymer (wt.%) |
|---|---|---|---|---|---|
| Quartz | 98-000-0369 | SiO2 | -- | 20 | 10.3 |
| Plagioclase | |||||
| -Albite | 01-075-1142 | NaAlSi3O8 | 3.4 | 3.5 | 5.3 |
| -Andesine | 05-001-0801 | Na0.51Ca0.49(Si2.56Al1.44O8) | 2 | ||
| -Anorthite | 00-012-0301 | CaAl2Si2O8 | 1.13 | ||
| K–Feldspar | |||||
| -Orthoclase | 00-019-0931 | KAlSi3O8 | 3.4 | 5.5 | 2.1 |
| -Sanidine | 00-010-0353 | KAlSi3O8 | 3.4 | ||
| Chabazite | 01-088-1263 | Ca1.76Al3.60Si8.40O24·(H2O)9.87 | 2.6 | 0.2 | 0.3 |
| Clinoptilolite–Heulandite | 00-039-1383 | KNa2Ca2(Si29Al7)O72·24H2O | 4.1 | 28.1 | 7.2 |
| Mordenite | 00-029-1257 | (Ca,Na2,K2)Al2Si10O24·7H2O | 5.7 | 2.2 | 1 |
| Stilbite–Na | 00-039-0223 | NaCa4(Si27Al9)O72·28(H2O) | 4.6 | 19.1 | 1 |
| Unidentified Amorphous | -- | -- | -- | 21.4 | 72.8 |
| Membrane Code | ZGM2 | ZGM3 | ZGM4 |
| Pb(II) concentration (ppm) | Flux(L/m2.h) | ||
| 50 | 61.9 | 78.5 | 41.2 |
| 100 | 38.6 | 42.5 | 40.7 |
| 200 | 33.7 | 35.8 | 35.1 |
| Pb(II) concentration (ppm) | Rejection (%) | ||
| 50 | 91 | 87 | 89 |
| 100 | 88 | 84 | 86 |
| 200 | 97 | 94 | 96 |
| Membrane Type | Contaminant | Flux (L/m2·h) | Rejection Rate (%) | Mechanical Strength (MPa) | Industrial-Scale Cost (USD/m2) | Life Span (Years) | Mean Pore Size |
|---|---|---|---|---|---|---|---|
| Polymeric Membranes | |||||||
| PVDF (/PAN *) [60] | Pb(II) | 186.14 | 52.11 | - | 10–40 | 1.8–5 [61] | 0.1–0.5 µm |
| Poly ether sulfone [36,58] | 18.69 | 38.9 | - | 50–65 | 2–5 [62] | 0.1–5 µm [63] | |
| Ceramic Membranes | |||||||
| Metal oxide Membranes | |||||||
| Kaolin–Dolomite [64] | Pb(II) | 246.78–1738.56 | 99.12 | 1–17 MPa | 15–19 nm | ||
| Zeolite Membranes | |||||||
| Al2O3-NaA zeolite [57] | Pb(II) | 727.5 | 99.9 | 85.8 ± 3.1 | 40–66 [65] | 5–8 | 0.41 nm |
| Metal–Organic Framework Membranes | |||||||
| ZIF-300 MOF membrane [64] | Cu(II) | 39.2 | 99.21 | - | 50–200 | - | 7.9 Å |
| Zeolite–Geopolymer Membrane (This Study) | Pb(II) | 78.5 | 87% | ~180 | ~20.6 | 5–8 | 2–5 nm & >5 nm |
| Carbon-Based Membranes | |||||||
| Graphene | - | - | |||||
| (PSF + GO + DMF) [65] | Pb(II) | 100 | >90 | 20 | 30–400 Å | ||
| Carbon Nanotubes [2] | Pb(II) | - | 99.99 | 50 | - | - | 18.8–23.7 μm |
| Hybrid Membranes | |||||||
| PES/PVP **/GO *** [58] | Pb(II) | 150.21 | 80.6 | - | 50–65 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amari, S.; Darestani, M.; Millar, G.; Boshrouyeh, B. Fabrication and Performance of PVAc-Incorporated Porous Self-Standing Zeolite-Based Geopolymer Membranes for Lead (Pb(II)) Removal in Water Treatment. Polymers 2025, 17, 1155. https://doi.org/10.3390/polym17091155
Amari S, Darestani M, Millar G, Boshrouyeh B. Fabrication and Performance of PVAc-Incorporated Porous Self-Standing Zeolite-Based Geopolymer Membranes for Lead (Pb(II)) Removal in Water Treatment. Polymers. 2025; 17(9):1155. https://doi.org/10.3390/polym17091155
Chicago/Turabian StyleAmari, Samar, Mariam Darestani, Graeme Millar, and Bob Boshrouyeh. 2025. "Fabrication and Performance of PVAc-Incorporated Porous Self-Standing Zeolite-Based Geopolymer Membranes for Lead (Pb(II)) Removal in Water Treatment" Polymers 17, no. 9: 1155. https://doi.org/10.3390/polym17091155
APA StyleAmari, S., Darestani, M., Millar, G., & Boshrouyeh, B. (2025). Fabrication and Performance of PVAc-Incorporated Porous Self-Standing Zeolite-Based Geopolymer Membranes for Lead (Pb(II)) Removal in Water Treatment. Polymers, 17(9), 1155. https://doi.org/10.3390/polym17091155








