Preparation of Polypyrrole/Montmorillonite/Polypropylene Composite Membranes and Investigation of Their Adsorption Performance for Methyl Orange and Pb2+
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.2.1. Purification of Ca-MMT
2.2.2. Preparation of Polypyrrole/Montmorillonite
2.2.3. Preparation of Polypyrrole/Montmorillonite/Polypropylene Composite Membrane
2.2.4. Preparation of Adsorption Solution
2.3. Performance Testing and Characterization
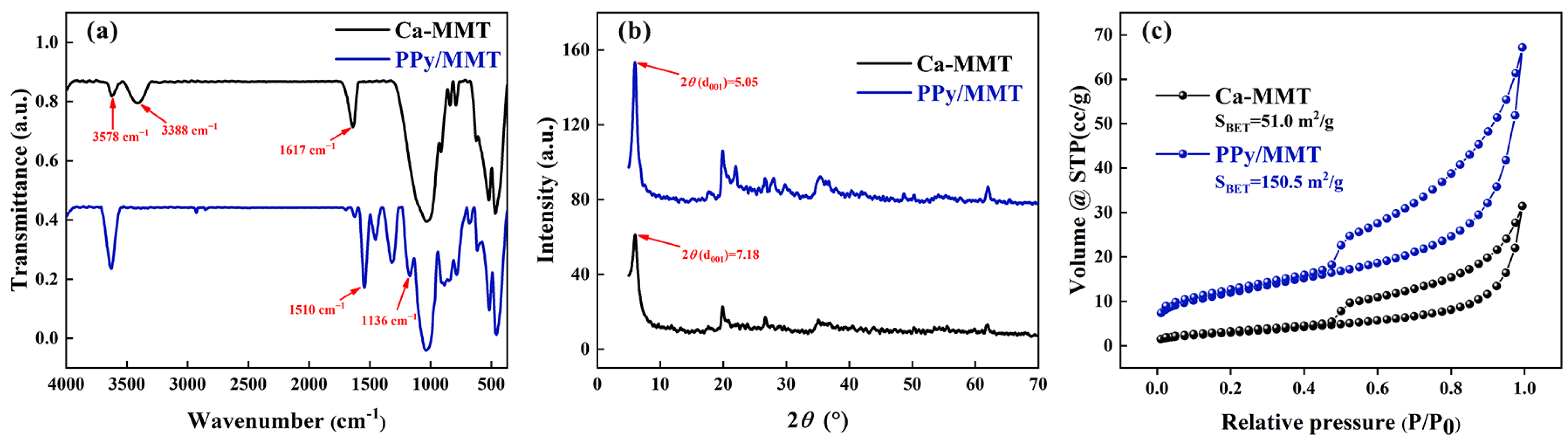
2.3.1. Fourier Transform Infrared Spectroscopy
2.3.2. X-Ray Diffraction
2.3.3. Specific Surface Area Measurement
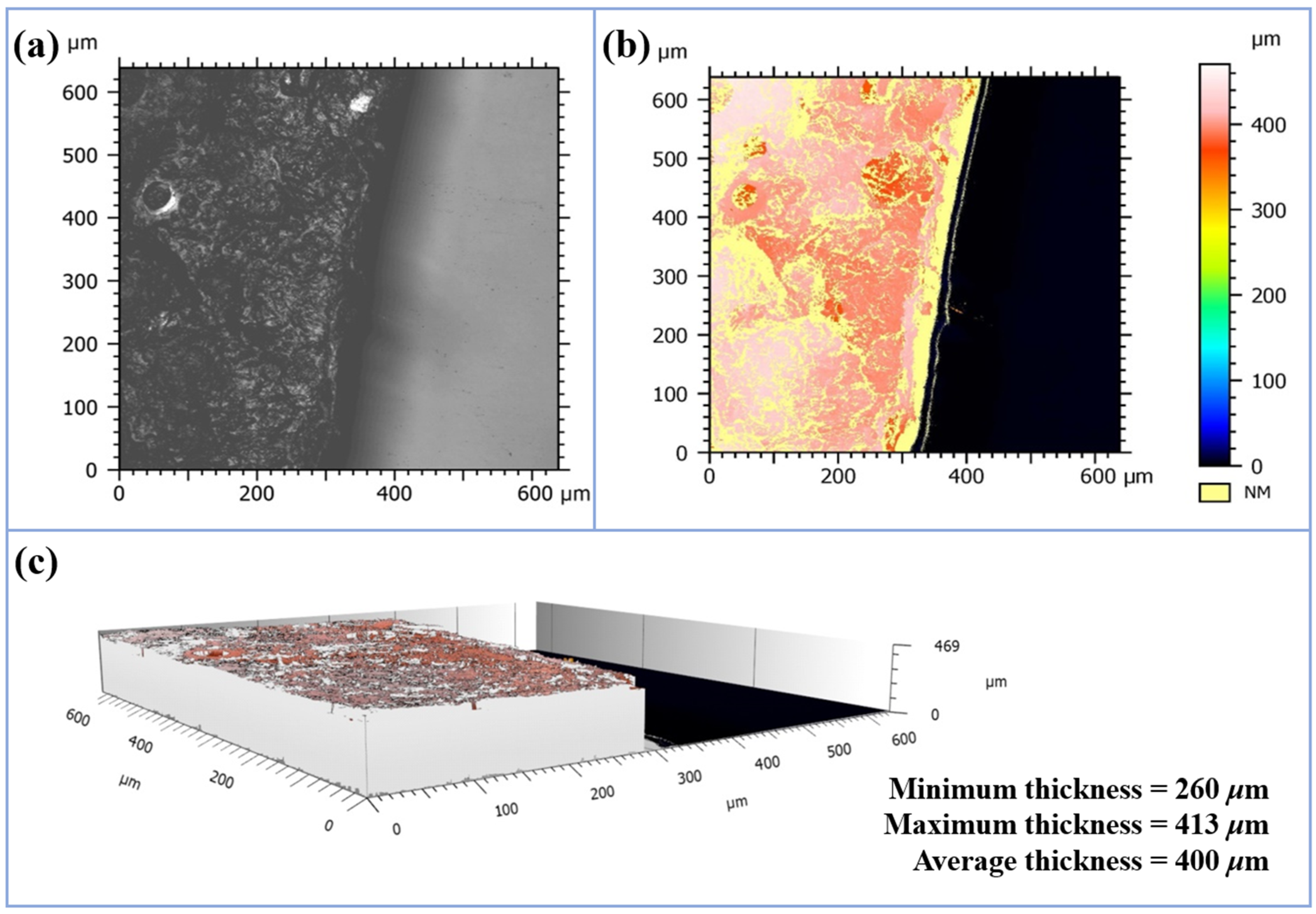
2.3.4. Laser Confocal Microscopy
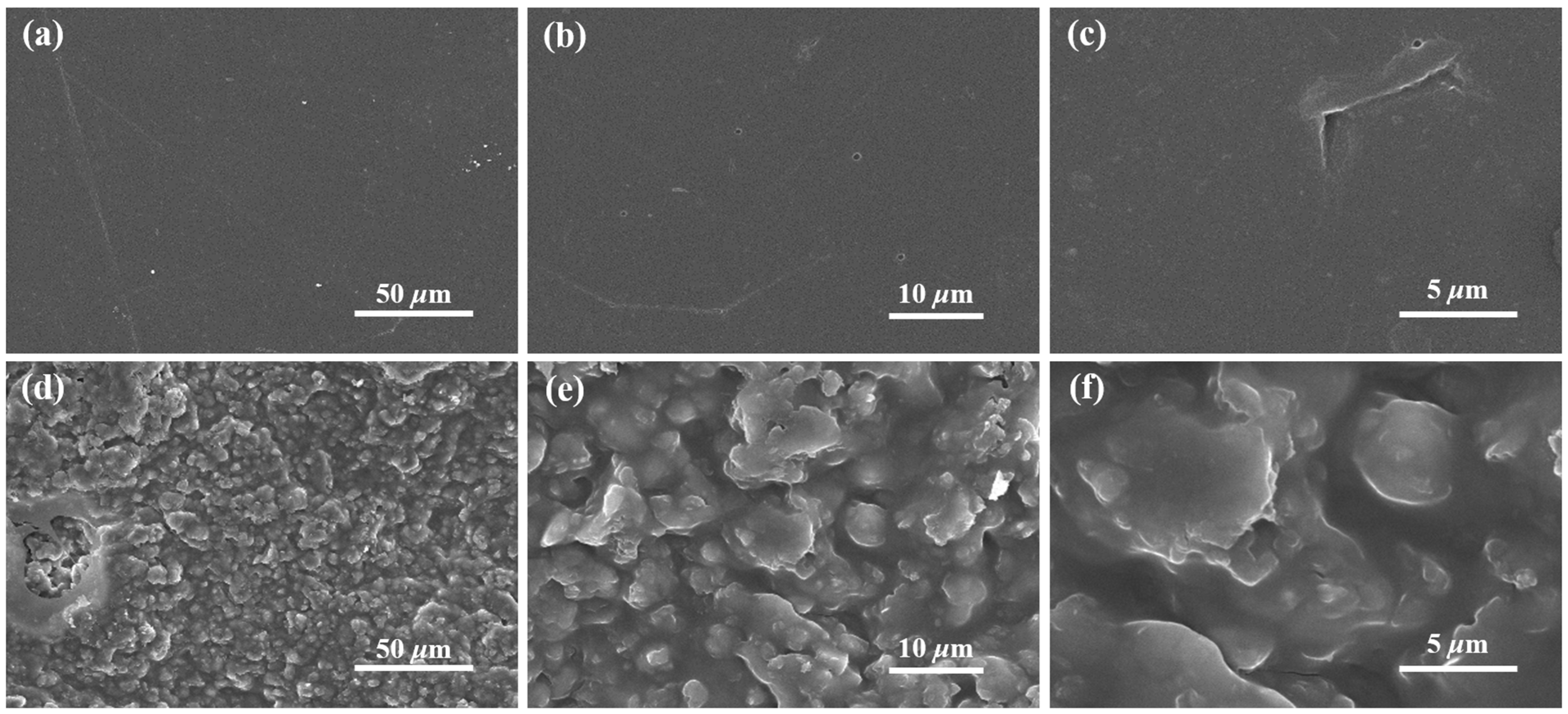
2.3.5. Scanning Electron Microscopy
2.3.6. X-Ray Photoelectron Spectroscopy
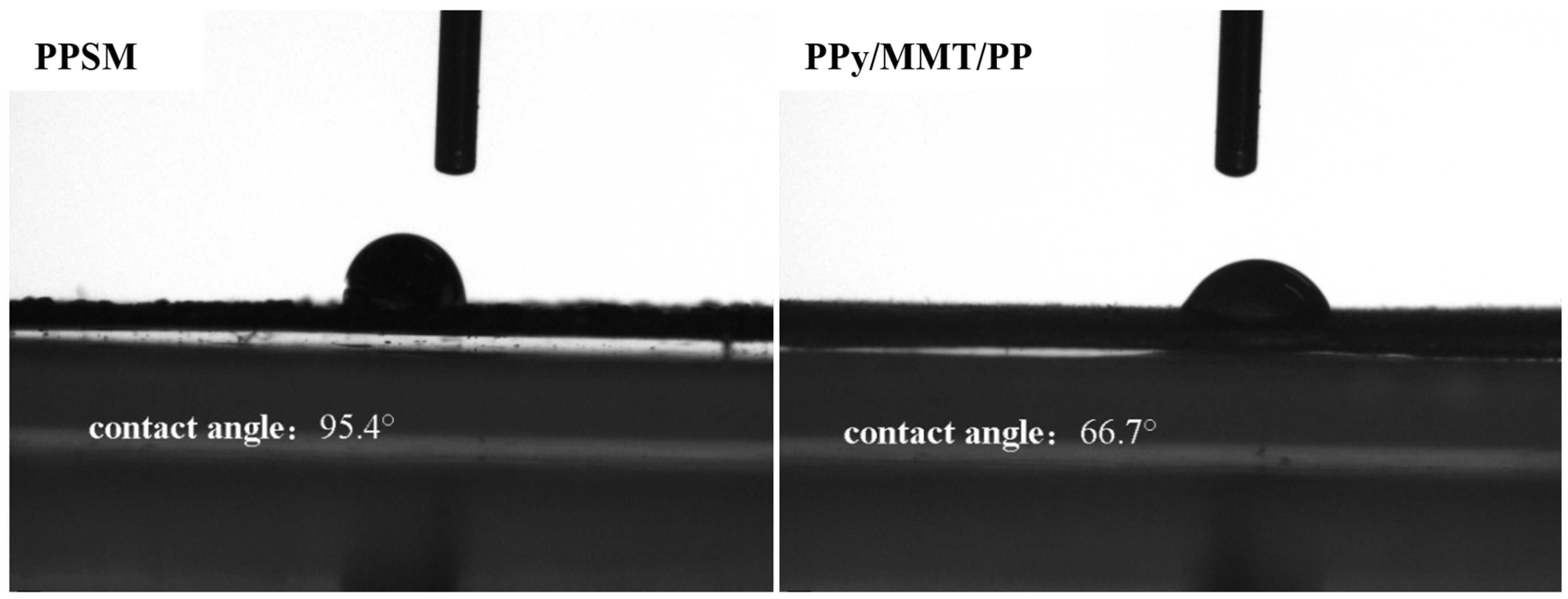
2.3.7. Contact Angle Measurement
2.3.8. UV-Visible Spectrophotometry
2.3.9. Flame Atomic Absorption Spectroscopy
2.3.10. Adsorption Kinetic Model
2.3.11. Thermodynamic Isothermal Adsorption Model
3. Results
3.1. Characterization of the Properties of Polypyrrole/Montmorillonite
3.2. Characterization and Analysis of the Polypyrrole/Montmorillonite/Polypropylene Composite Membrane
3.2.1. Thickness Analysis
3.2.2. Microscopic Morphological Analysis
3.2.3. Microscopic Structure Analysis
3.2.4. X-Ray Photoelectron Spectroscopy (XPS) Analysis
3.2.5. Contact Angle Analysis
3.3. Study on the Adsorption Performance of Polypyrrole/Montmorillonite/Polypropylene Composite Membrane
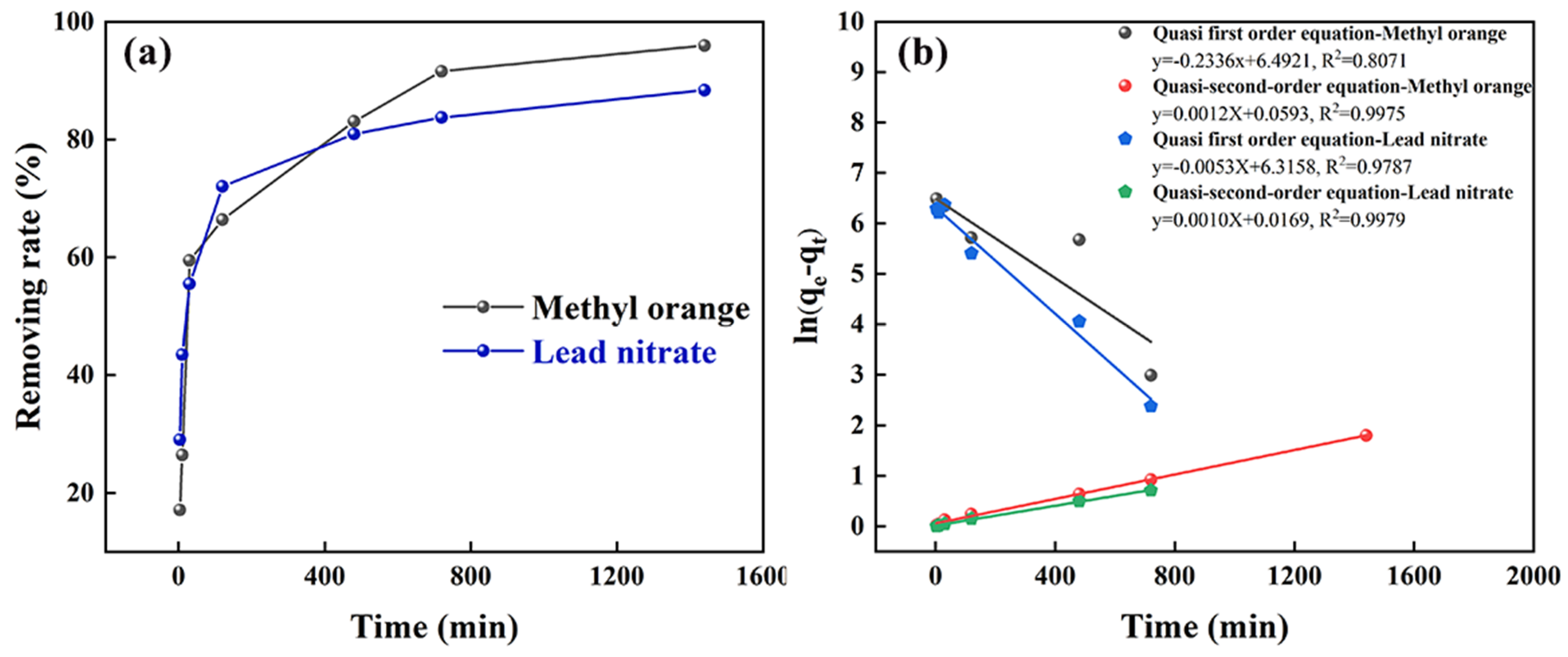
3.3.1. Adsorption Kinetics Study
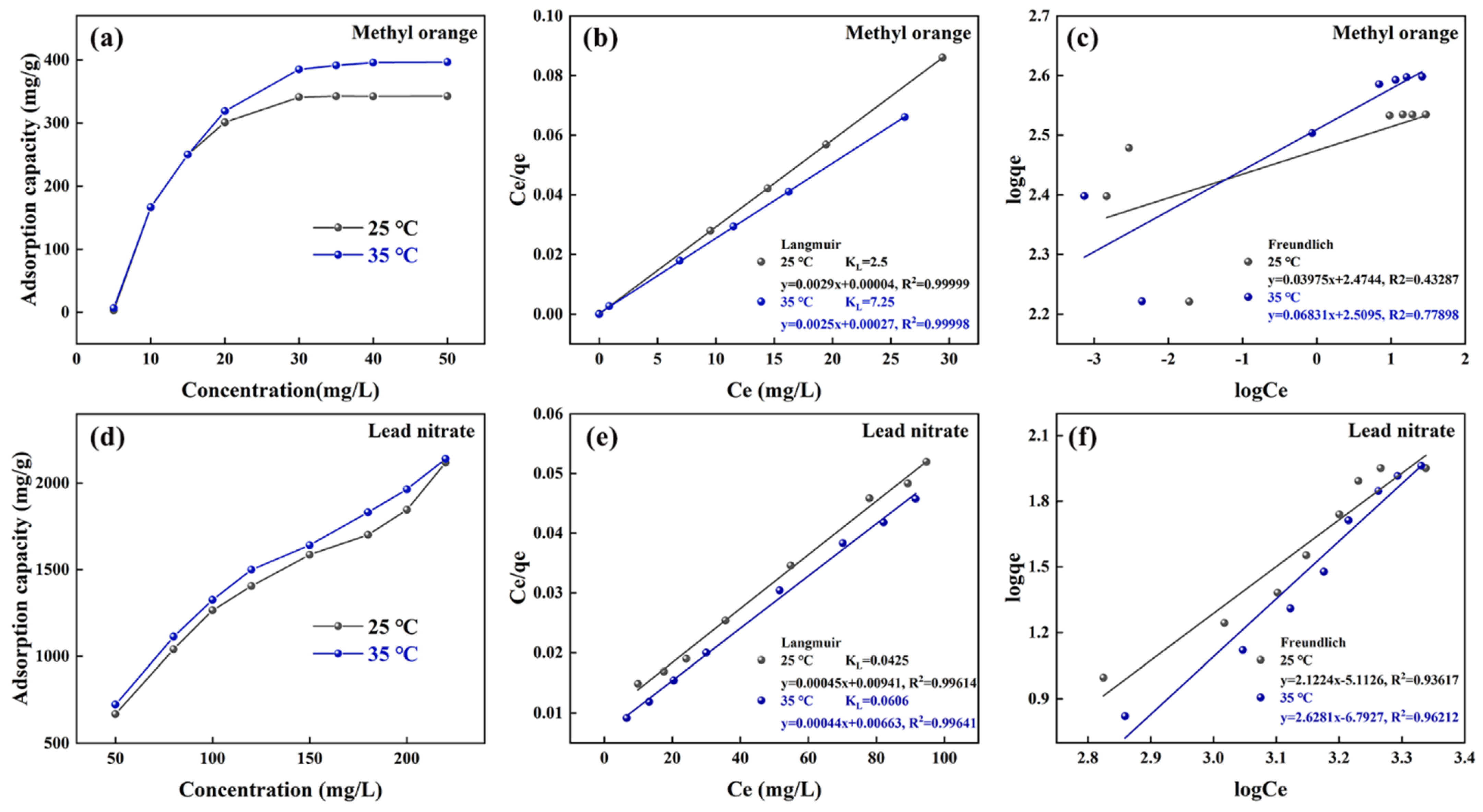
3.3.2. Thermodynamic Study of Adsorption
4. Discussion
4.1. Cyclic Stability Test
4.2. Cycle Test
4.3. Adsorption Mechanism Analysis
4.4. Performance Comparison
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MMT | Montmorillonite |
| Ca-MMT | Calcium-based montmorillonite |
| Py | Pyrrole |
| PPy/MMT | Polypyrrole/montmorillonite |
| PPSM | Polypropylene substrate membrane |
| PPy/MMT/PP | Polypyrrole/montmorillonite/polypropylene composite membrane |
| FTIR | Fourier transform infrared spectroscopy |
| XRD | X-ray diffraction |
| BET | Brunau-er-Emmett-Teller surface area analysis |
| CLSM | Laser confocal scanning microscopy |
| SEM | scanning electron microscopy |
| XPS | X-ray photoelectron spectroscopy |
| CTAB | Hexadecyltrimethylammonium bromide |
References
- Sharghi, E.A.; Farzin, M.; Earaqi, M.T.; Ghazale, F. Gaining comprehensive insight into the effect of electrocoagulation integrated in a membrane bioreactor on the detergent manufacturing plant wastewater treatment and membrane fouling. Chemosphere 2025, 370, 144007. [Google Scholar] [CrossRef] [PubMed]
- Wang, E.; Li, J.; Liu, S.; Wu, W.; Zhang, X.; Su, B. Constructing positively charged hollow fiber nanofiltration membranes with high performance for the treatment of wastewater containing heavy metal ions. Desalination 2025, 597, 118395. [Google Scholar] [CrossRef]
- Naddaf, M. The world faces a water crisis-4 powerful charts show how. Nature 2023, 615, 774–775. [Google Scholar] [CrossRef]
- Kumar, A.; Thakur, A.; Panesar, P.S. A review on the industrial wastewater with the efficient treatment techniques. Chem. Pap. 2023, 77, 4131–4163. [Google Scholar] [CrossRef]
- Ma, J.; Lu, J.; Liu, Y.; Fan, Z. Industrial potential of electro-oxidation and peroxymonosulfate coupling for efficient organic degradation in acidic dye wastewater. Process Saf. Environ. Prot. 2025, 194, 1572–1583. [Google Scholar] [CrossRef]
- Sharma, J.; Sharma, S.; Soni, V. Classification and impact of synthetic textile dyes on Aquatic Flora: A review. Reg. Stu. in Mar. Sci. 2021, 45, 101802. [Google Scholar] [CrossRef]
- Deng, J.; Gao, L.; Liu, W.; Yin, F.; Chen, C.; Jia, T.; He, Y.; Mao, T.; Wu, W. Distributions and transformation of polyhalogenated carbazoles in environmental matrices contaminated by printing and dyeing plants. Environ. Pollut. 2024, 357, 124451. [Google Scholar] [CrossRef]
- Cheng, H.; Peng, L.; Liu, J.; Ma, C.; Hao, F.; Zheng, B.; Yang, J. Adsorption separation of various polar dyes in water by oil sludge-based porous carbon. Appl. Sci. 2024, 14, 7283–7300. [Google Scholar] [CrossRef]
- Zhang, M.; Ruan, J.; Wang, X.; Shao, W.; Chen, Z.; Chen, Z.; Gu, C.; Qiao, W.; Li, J. Selective oxidation of organic pollutants based on reactive oxygen species and the molecular structure: Degradation behavior and mechanism analysis. Water Res. 2023, 246, 120697. [Google Scholar] [CrossRef]
- Cui, X.; Wu, D.; Wang, H.; Ding, S.; Fan, Y. Pore features and seepage characteristics of natural gap graded sand with two size distributions. Géotechnique 2022, 73, 1003–1014. [Google Scholar] [CrossRef]
- Xue, Z.; Cheng, W.; Wang, L.; Hu, W. Effects of bacterial inoculation and calcium source on microbial-induced carbonate precipitation for lead remediation. J. Hazard. Mater. 2022, 426, 128090. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.J.; Chakraborty, A.; Sehgal, R. A systematic review of industrial wastewater management: Evaluating challenges and enablers. J. Environ. Manage. 2023, 348, 119230. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Xing, X.; Chu, Y.; Dai, Y.; Zhang, H. Modification of sodium montmorillonite by two quaternary ammonium surfactants with different chain lengths: Preparation, characterization and adsorption of acetaminophen. J. Mol. Struct. 2025, 1326, 141149. [Google Scholar] [CrossRef]
- Kumar, P.G.; Kanmani, S.; Kumar, P.S.; Vellingiri, K. Efficacy of simultaneous advanced oxidation and adsorption for treating municipal wastewater for indirect potable reuse. Chemosphere 2023, 321, 138115. [Google Scholar] [CrossRef]
- Li, Y.; Yang, D.; Cheng, F.; Li, Z.; Wu, D. Regulating interlayer and surface properties of montmorillonite by dodecyl dimethyl betaine for enhanced lead ion capture. Surf. Interfaces 2023, 42, 103348. [Google Scholar] [CrossRef]
- Luo, D.; Wei, J. Efficacy of functionalized sodium-montmorillonite in mitigating alkali-silica reaction. Appl. Clay Sci. 2023, 245, 107139. [Google Scholar] [CrossRef]
- Zhang, L.; Zaoui, A.; Sekkal, W.; Zheng, Y. Interlayer adsorption of cationic dye on cationic surfactant-modified and unmodified montmorillonite. J. Hazard. Mater. 2023, 442, 130107. [Google Scholar] [CrossRef]
- Zheng, X.; Rong, S.; Zhang, S.; Pan, C.; Guo, Y. Preparation of a MgAl-layered double oxide@polypyrrole composite for efficient and selective Hg(Ⅱ) removal from aqueous solutions. Mater. Today Commun. 2025, 42, 111267. [Google Scholar] [CrossRef]
- Ballav, N.; Maity, A.; Mishra, S.B. High efficient removal of chromium(VI) using glycine doped polypyrrole adsorbent from aqueous solution. Chem. Eng. J. 2012, 198–199, 536–546. [Google Scholar] [CrossRef]
- Ahsani, M.; Yegani, R. Study on the fouling behavior of silica nanocomposite modified polypropylene membrane in purification of collagen protein. Chem. Eng. Res. Des. 2015, 102, 261–273. [Google Scholar] [CrossRef]
- Jha, A.K.; Martinez, D.; Martinez, E.J.; Salinas, J.E.; Kent, M.S.; Davydovich, O. Discovery and adaptation of microbes that degrade oxidized low-density polyethylene films. J. Ind. Microbiol. Biotechnol. 2024, 51, kuae050. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Kim, D.; Park, J.Y. The function of adsorption, photo-oxidation, and humic acid using air backwashing in integrated water treatment of multichannel ceramic MF and PP particles. Membranes 2020, 10, 28. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Zheng, Y.; Zhou, Q.; Shuai, S.; Lü, Z.; Gao, C. Facile modification of polypropylene hollow fiber microfiltration membranes for nanofiltration. Desalination 2012, 298, 49–58. [Google Scholar] [CrossRef]
- Gao, J. Preparation of Multi-Morphology Polypyrrole and the Research on Theirmicrowave Absorbing Properties. Ph.D. Thesis, Donghua University, Shanghai, China, 2010. [Google Scholar]
- Dai, Y. Study of Sr2+, Co2+ Adsorption Onorgano-Montmorillonites. Master′s Thesis, South China University of Technology, Guangzhou, China, 2013. [Google Scholar]
- Qiu, S.; Huo, Z.; Cao, K.; Da, W.; Wang, X.; Xu, H. Crystallographic behavior of nascent isotactic polypropylene produced under supercritical conditions. Chin. Ed. 2013, 64, 730–734. [Google Scholar]
- Bahrudin, N.N.; Nawi, M.A.; Jawad, A.H.; Sabar, S. Adsorption characteristics and mechanistic study of immobilized chitosan-montmorillonite composite for methyl orange removal. J. Polym. Environ. 2020, 28, 1901–1913. [Google Scholar] [CrossRef]
- Malek, A.; Faten, H.; Hatem, E. Growth of La-doped ZnO thin films on a porous silicon substrate for photocatalytic applications. ChemistrySelect. 2023, 8, e202300108. [Google Scholar] [CrossRef]
- Tahari, N.; de Hoyos-Martinez, P.L.; Izaguirre, N.; Houwaida, N.; Abderrabba, M.; Ayadi, S.; Labidi, J. Preparation of chitosan/tannin and montmorillonite films as adsorbents for Methyl Orange dye removal. Int. J. Biol. Macromol. 2022, 210, 94–106. [Google Scholar] [CrossRef]
- Thuy, M.T.T.; Van Anh, N.T.; Xuan, M.T.; Vinh, T.Q.; Binh, P.T. Study on the photoelectrocatalytic activity of reduced TiO2 nanotube films for removal of methyl orange. Green Process. Synth. 2023, 12, 20228159. [Google Scholar] [CrossRef]
- Sharanu, A.; Kompa, A.; Kekuda, D.; Rao, K.M. Novel dual-functional manganese stannate thin film for acetone gas sensing and photocatalytic methyl orange degradation. RSC Adv. 2025, 15, 10460–10472. [Google Scholar] [CrossRef]
- Ahmed, S.; Akram, M.Y.; Kumar, A.; Dhir, A.; Ali, Z.; Kansal, S.K.; Ratan, J.K. Development of magnesium oxide@carbon fiber paper composite film for the removal of methyl orange from aqueous phase. Nanotechnol. Environ. Eng. 2022, 7, 49–56. [Google Scholar] [CrossRef]
- Ateia, E.E.; Ateia, M.A.; Arman, M.M. Assessing of channel structure and magnetic properties on heavy metal ions removal from water. J. Mater. Sci. Mater. Electron. 2022, 33, 8958–8969. [Google Scholar] [CrossRef]
- Khandanlou, R.; Ahmad, M.B.; Masoumi, H.R.F.; Shameli, K.; Basri, M.; Kalantari, K. Rapid adsorption of Copper(II) and Lead(II) by rice straw/Fe3O4 nanocomposite: Optimization, equilibrium isotherms, and adsorption kinetics study. PLoS ONE. 2015, 10, e0120264. [Google Scholar] [CrossRef] [PubMed]
- Kalantari, K.; Ahmad, M.B.; Masoumi, H.R.F.; Shameli, K.; Basri, M.; Khandanlou, R. Rapid adsorption of heavy metals by Fe3O4/Talc nanocomposite and optimization study using response surface methodology. Int. J. Mol. Sci. 2014, 15, 12913–12927. [Google Scholar] [CrossRef] [PubMed]
- Mandal, S.; Hwang, S.; Marpu, S.B.; Omary, M.A.; Prybutok, V.; Shi, S.Q. Bioinspired synthesis of silver nanoparticles for the remediation of toxic pollutants and enhanced antibacterial activity. Biomolecules. 2023, 13, 1054. [Google Scholar] [CrossRef]




| Recyclable Cycles | Times | Average/Times |
|---|---|---|
| 2 | 1 | 3.15 |
| 3 | 15 | |
| 4 | 4 |
| Composite Membrane Materials | Methyl Orange Removal Rate/% | Pb2+ Removal Rate/% | Refs. |
|---|---|---|---|
| Growth of La-Doped ZnO Thin Films on a Porous Silicon Substrate | 91.53 | / | [28] |
| chitosan/tannin and montmorillonite films | 95.61 | / | [29] |
| photoelectrocatalytic activity of reduced TiO2 nanotube films | 95.10 | / | [30] |
| Novel manganese stannate (MTO) composite thin films | 67.00 | / | [31] |
| magnesium oxide@carbon fiber paper composite film | 91.00 | / | [32] |
| hannel structure and magnetic properties | / | 53.88 | [33] |
| Rice Straw/Fe3O4 Nanocomposite | / | 82.65 | [34] |
| Fe3O4/Talc Nanocomposite | / | 80.67 | [35] |
| Silver Nanoparticles | / | 72.30 | [36] |
| This work | 95.98 | 88.48 | / |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, B.; Xu, B.; Chen, G.; Wang, C.; Liu, Y.; Bai, Y.; Li, M.; Peng, L. Preparation of Polypyrrole/Montmorillonite/Polypropylene Composite Membranes and Investigation of Their Adsorption Performance for Methyl Orange and Pb2+. Polymers 2025, 17, 1158. https://doi.org/10.3390/polym17091158
Wang B, Xu B, Chen G, Wang C, Liu Y, Bai Y, Li M, Peng L. Preparation of Polypyrrole/Montmorillonite/Polypropylene Composite Membranes and Investigation of Their Adsorption Performance for Methyl Orange and Pb2+. Polymers. 2025; 17(9):1158. https://doi.org/10.3390/polym17091158
Chicago/Turabian StyleWang, Baoxin, Binbin Xu, Gaofeng Chen, Chaozhong Wang, Yang Liu, Yang Bai, Mengge Li, and Longgui Peng. 2025. "Preparation of Polypyrrole/Montmorillonite/Polypropylene Composite Membranes and Investigation of Their Adsorption Performance for Methyl Orange and Pb2+" Polymers 17, no. 9: 1158. https://doi.org/10.3390/polym17091158
APA StyleWang, B., Xu, B., Chen, G., Wang, C., Liu, Y., Bai, Y., Li, M., & Peng, L. (2025). Preparation of Polypyrrole/Montmorillonite/Polypropylene Composite Membranes and Investigation of Their Adsorption Performance for Methyl Orange and Pb2+. Polymers, 17(9), 1158. https://doi.org/10.3390/polym17091158





