Synthesis and Physical–Chemical Characterization of a Biopolymer Derived from Cassava Starch, Cashew Nutshell Liquid, and Diammonium Phosphate
Abstract
1. Introduction
2. Materials and Methods
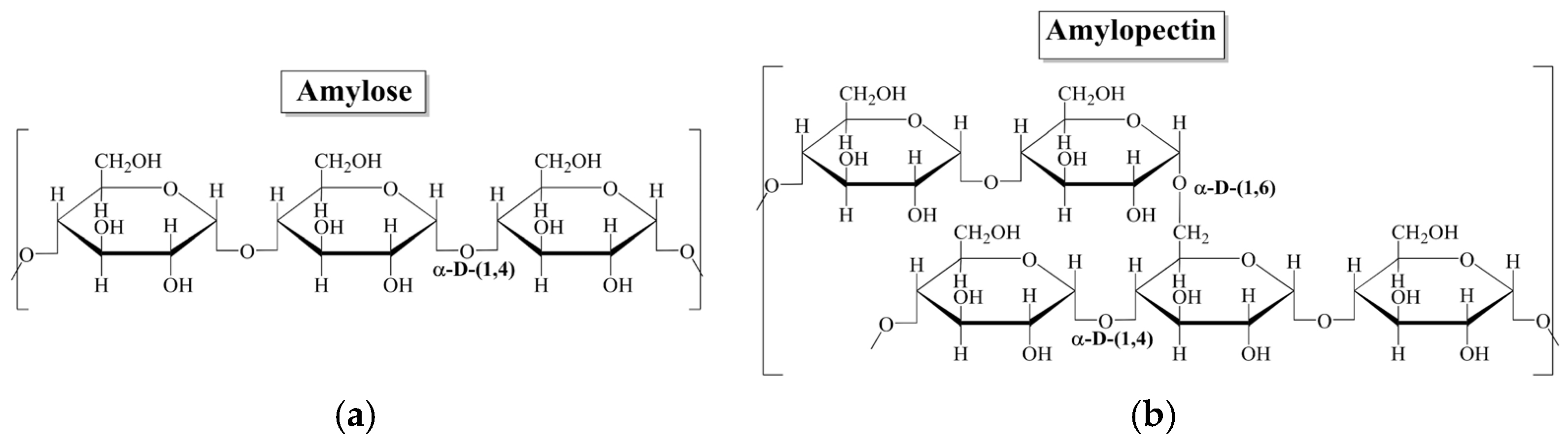
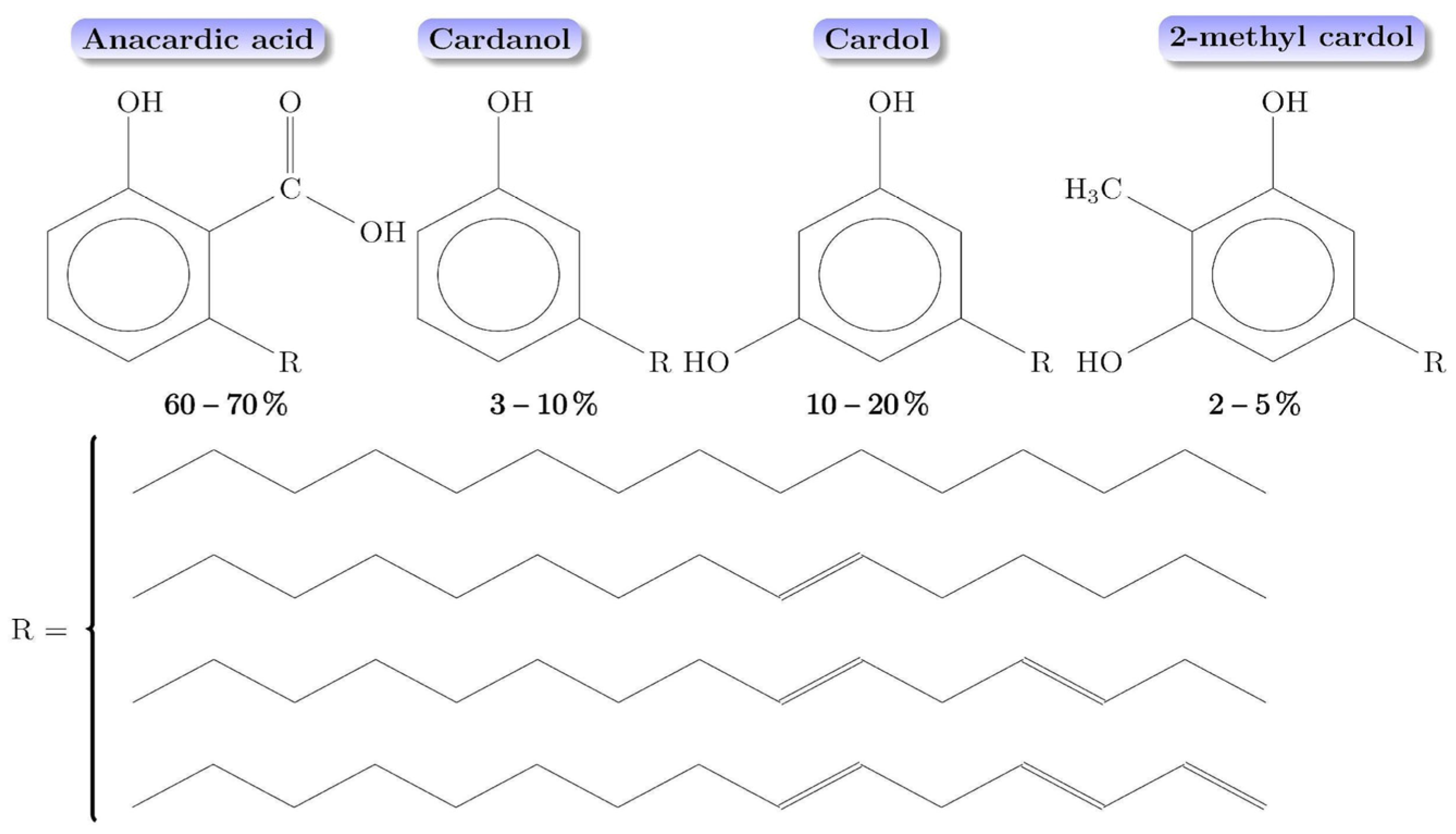
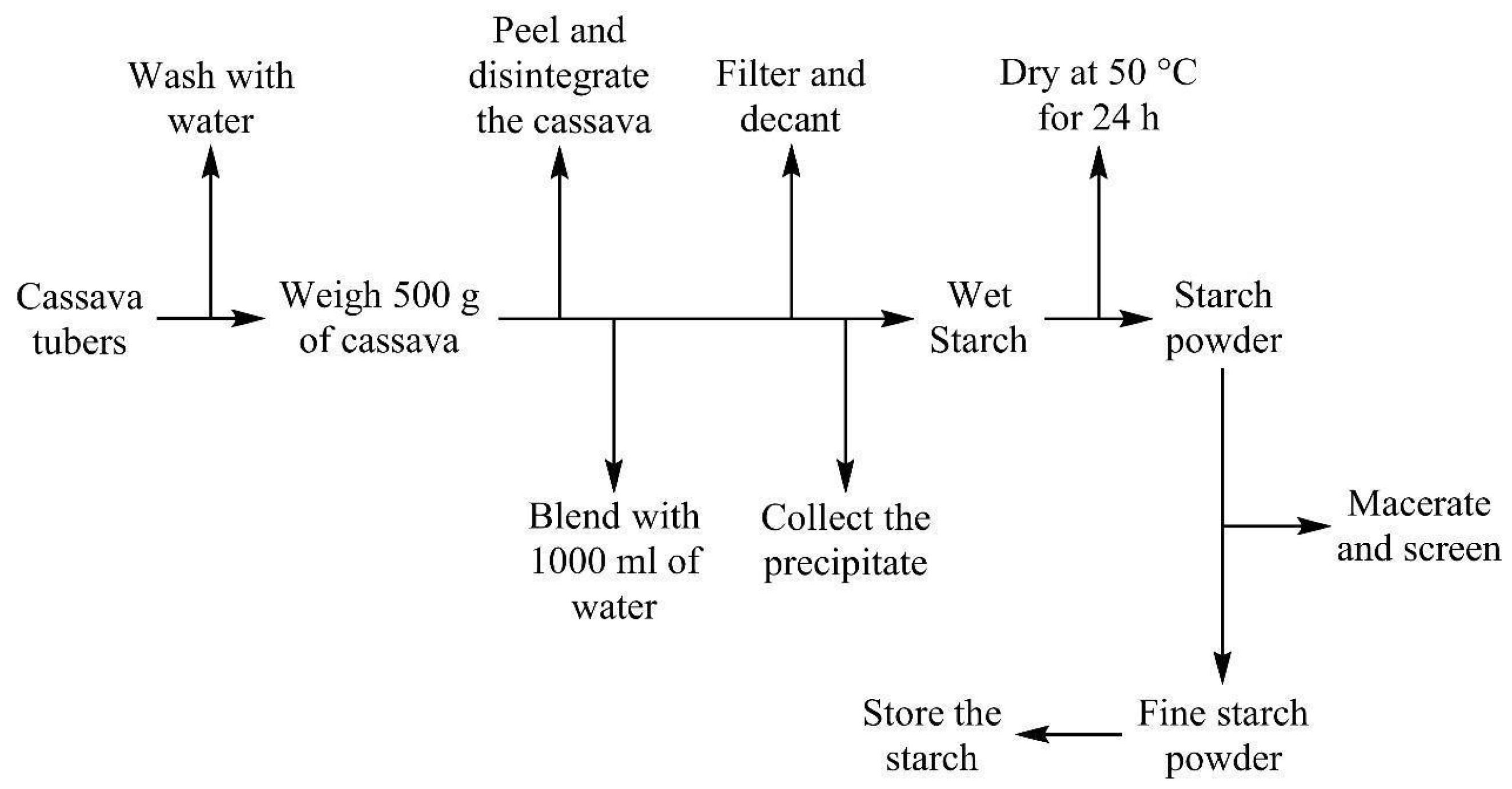
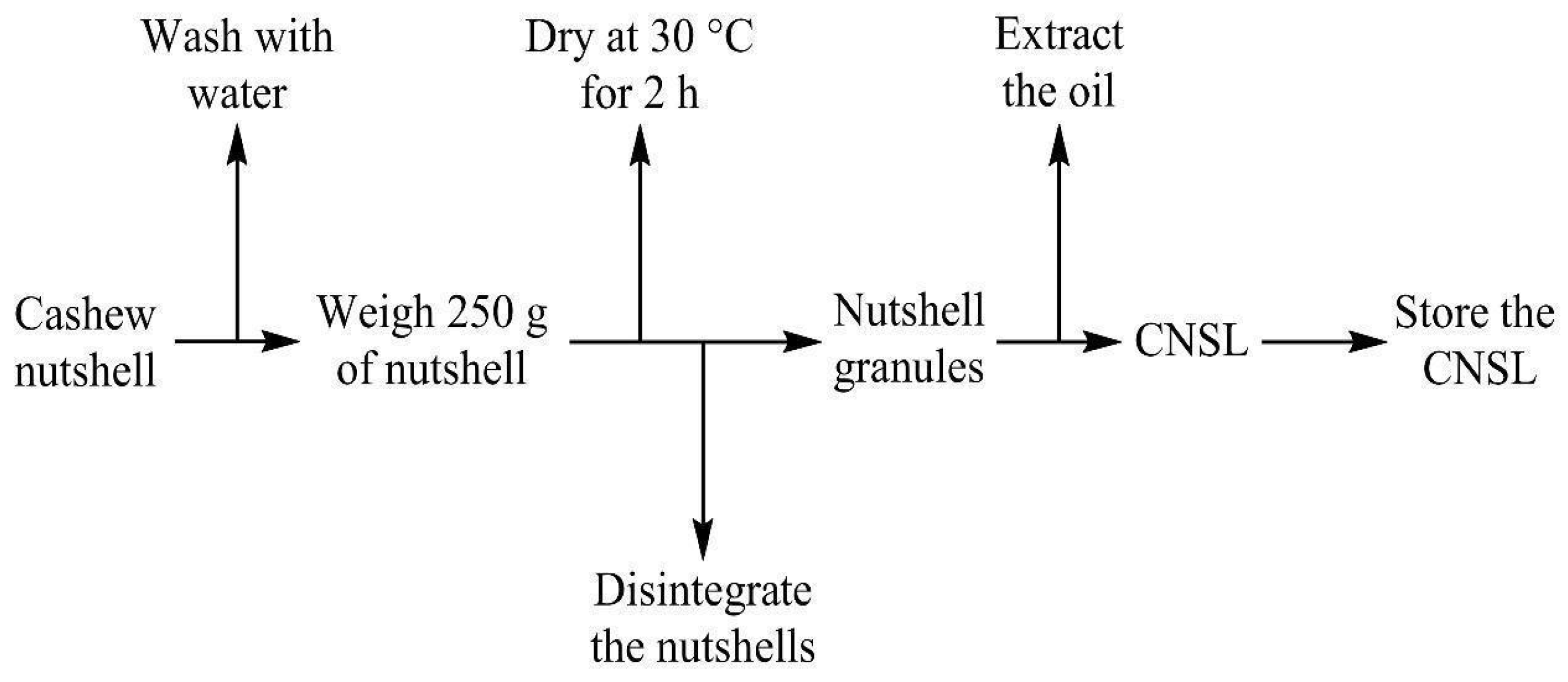
2.1. Cassava Starch, Cashew Nutshell Oil Extractions, and DAP Fertilizer
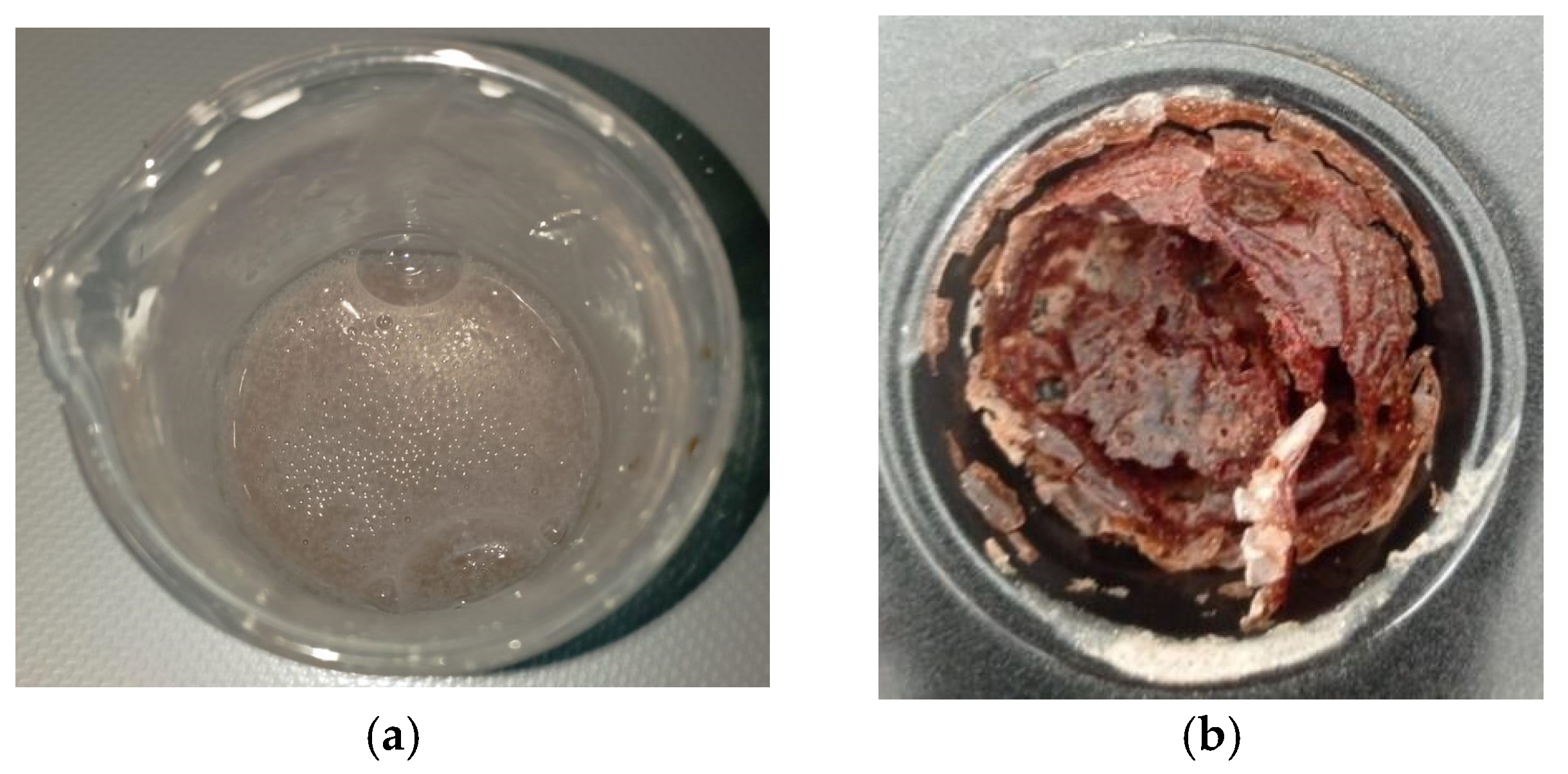
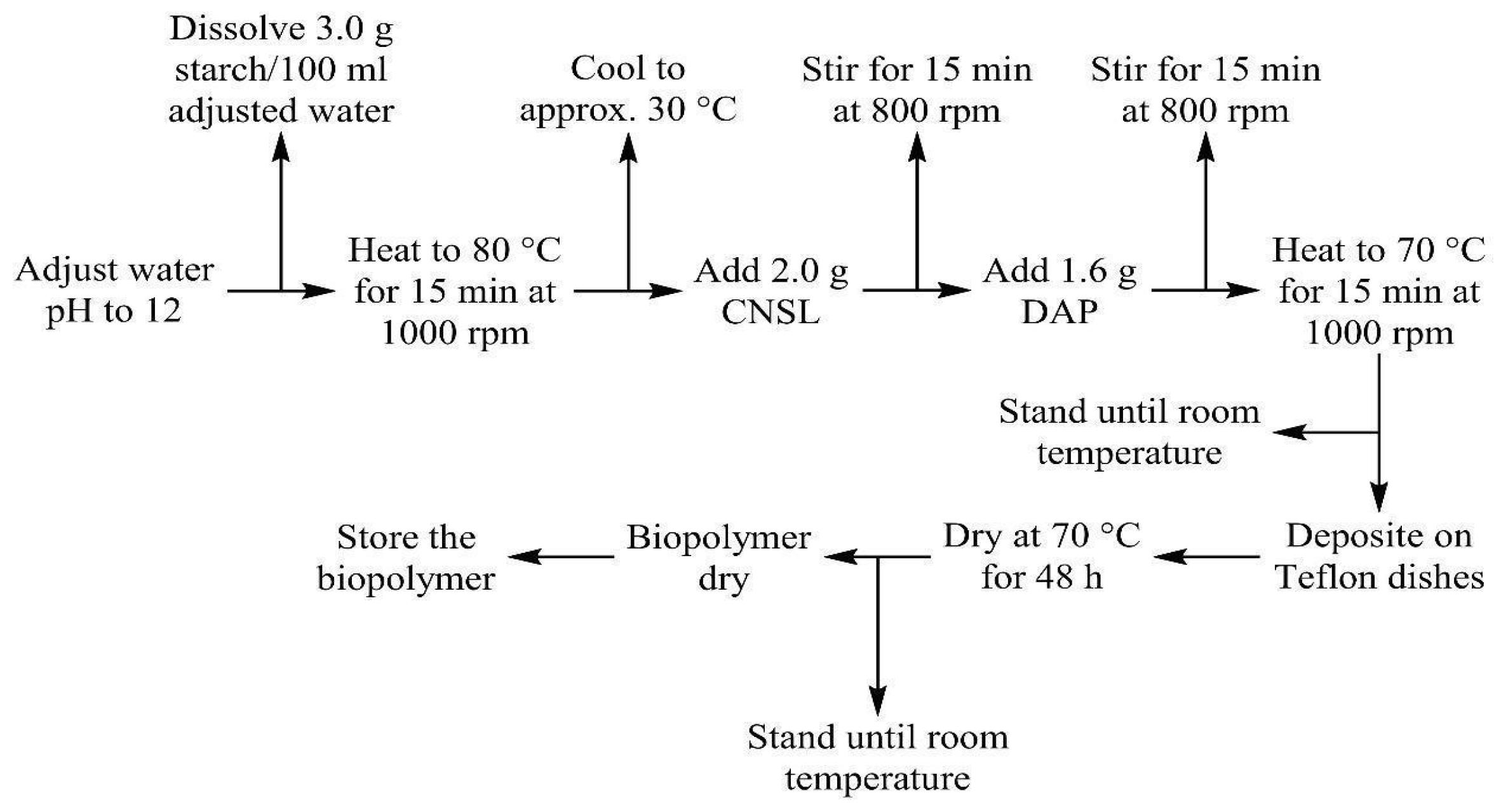
2.2. Starch–CNSL–DAP Biopolymer Synthesis
2.3. Characterization Techniques
3. Results and Discussion
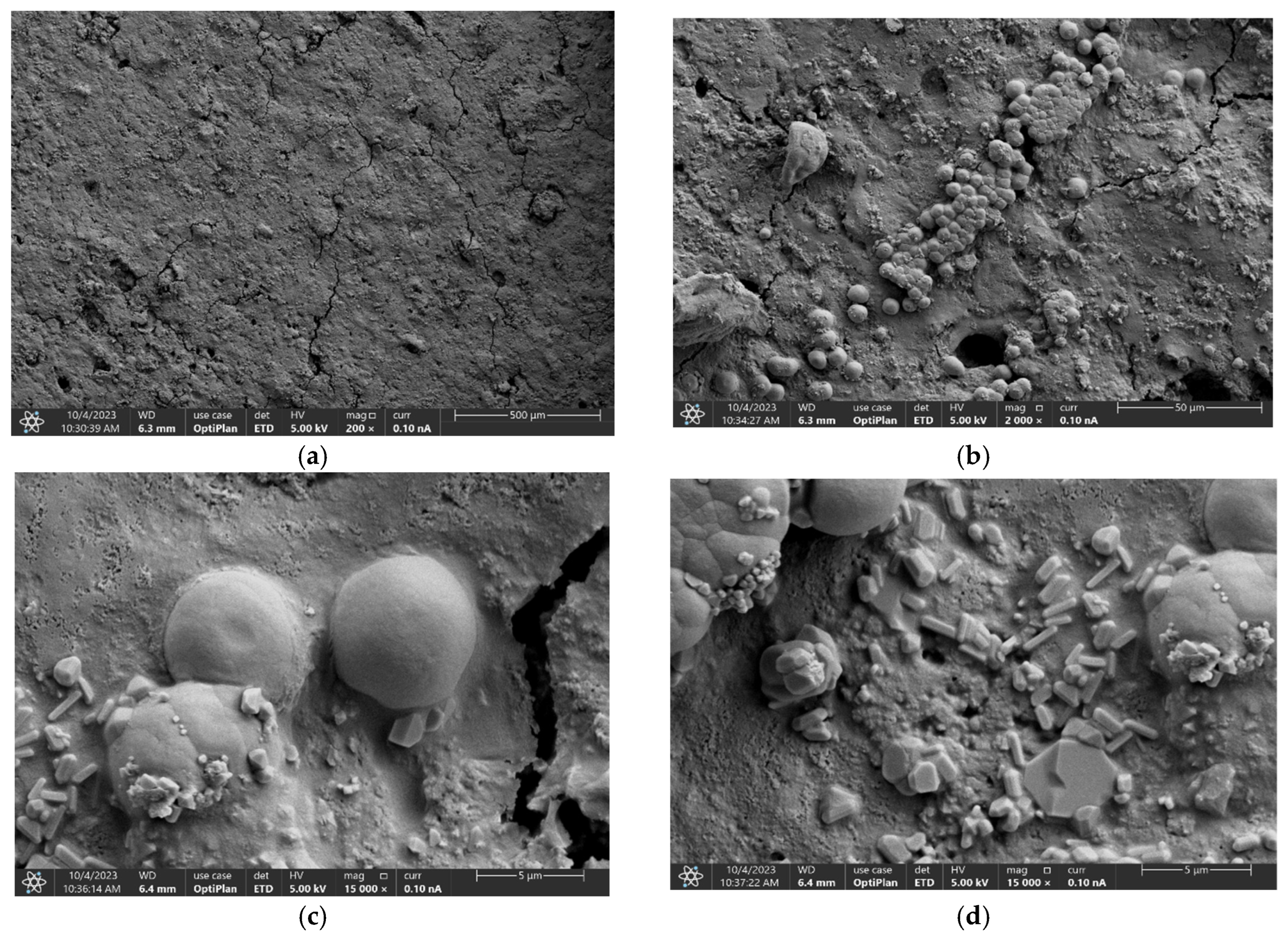
3.1. Scanning Electron Microscopy Analysis
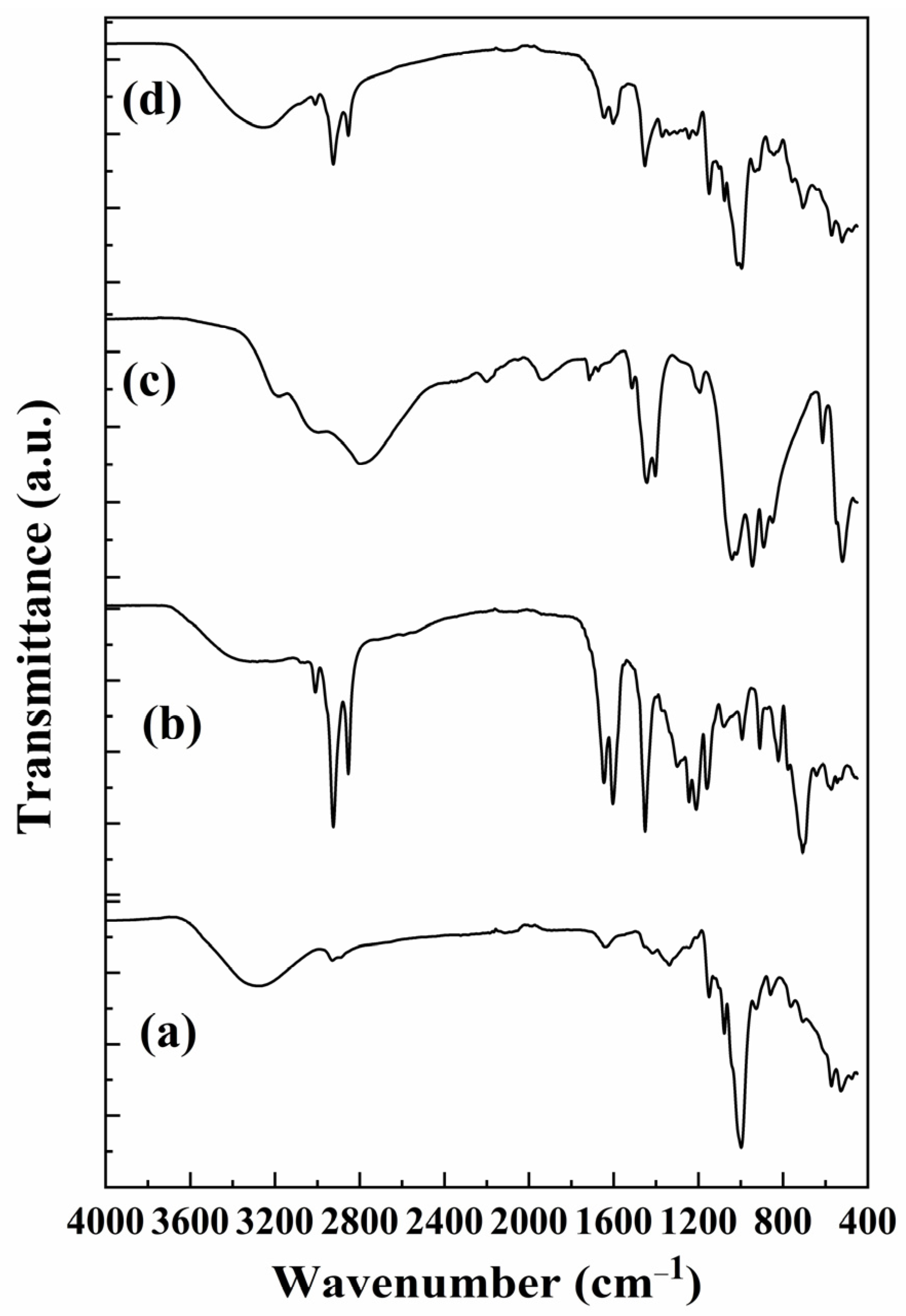
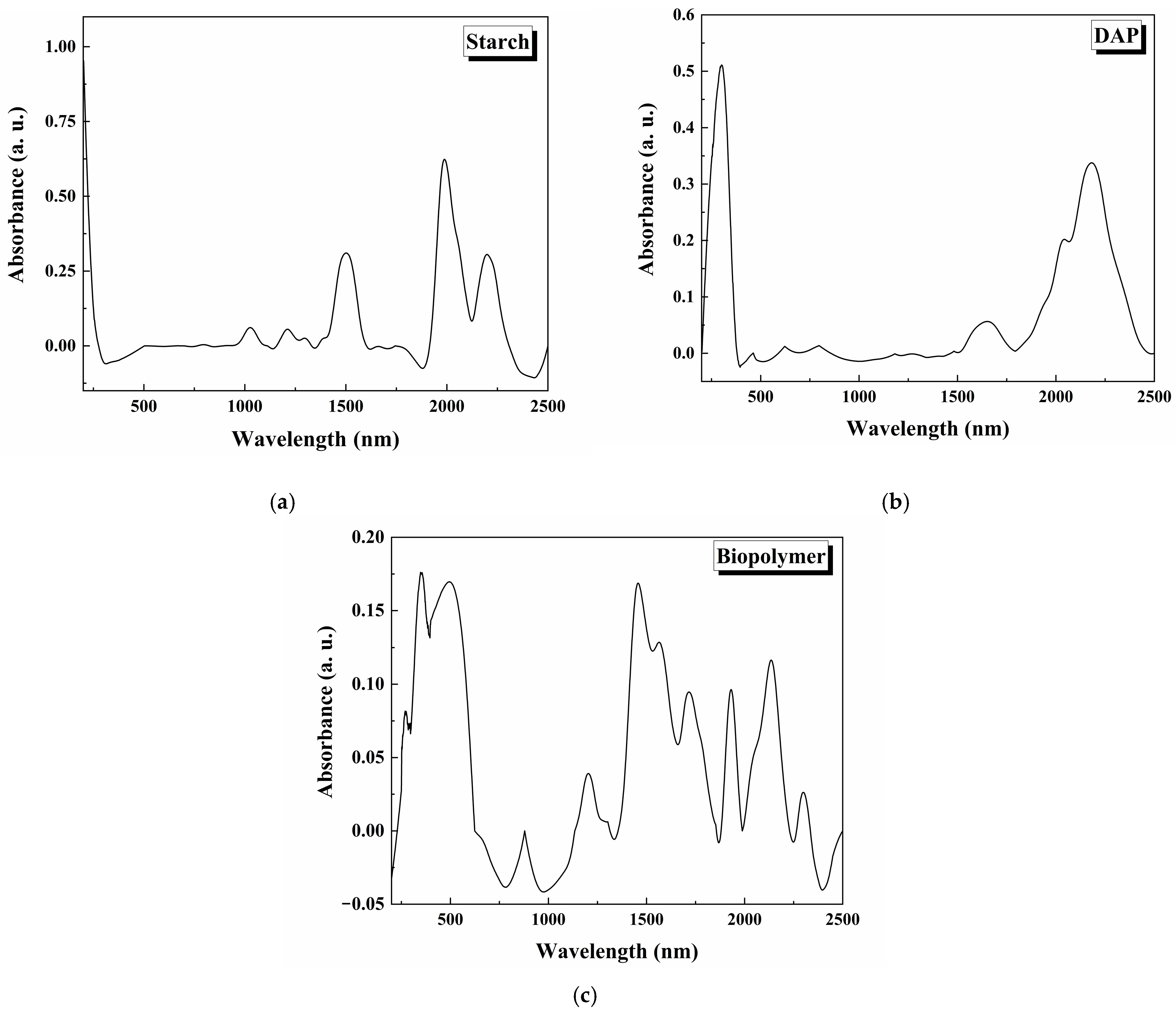
3.2. FTIR Analysis
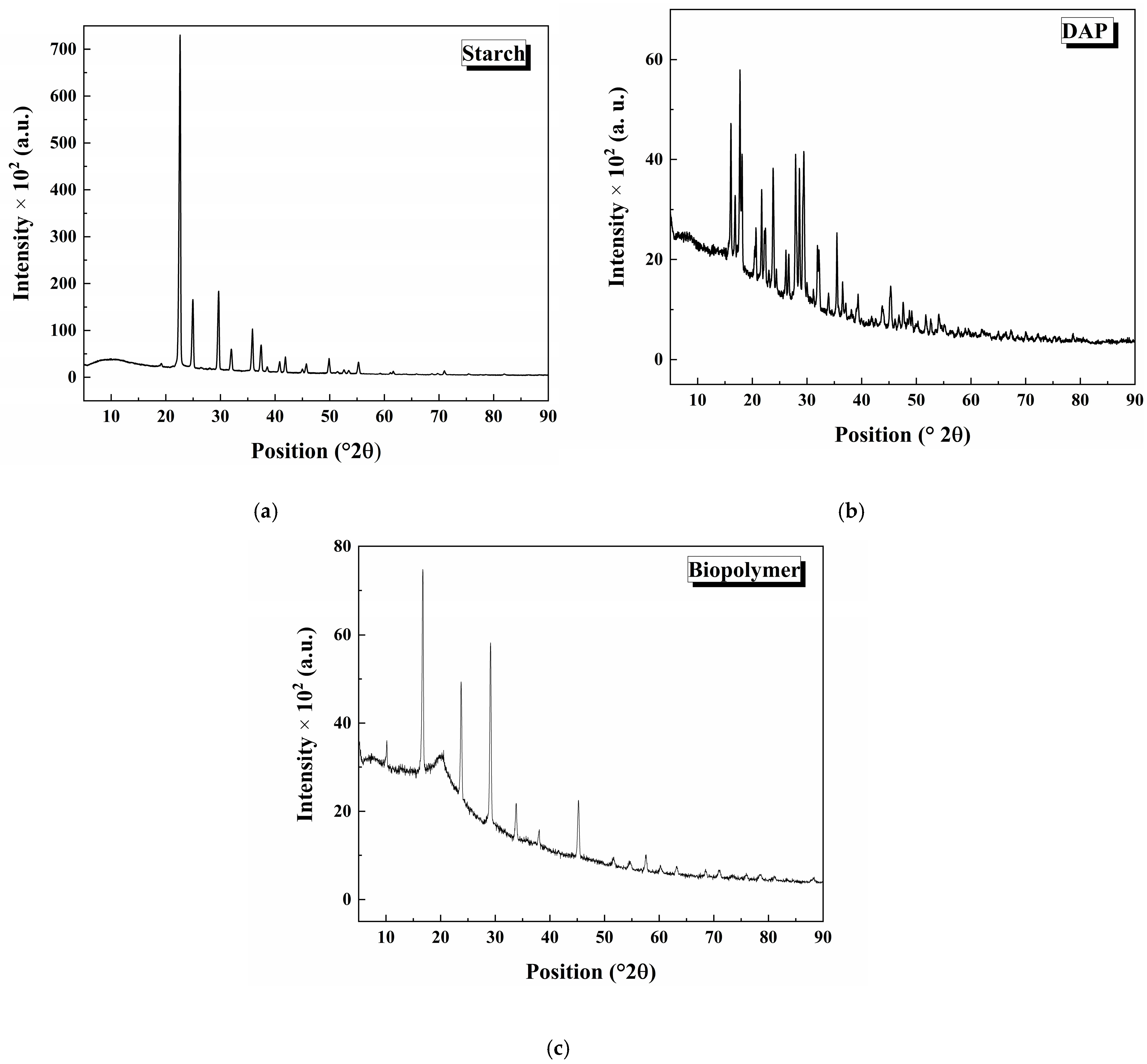
3.3. X-Ray Diffraction
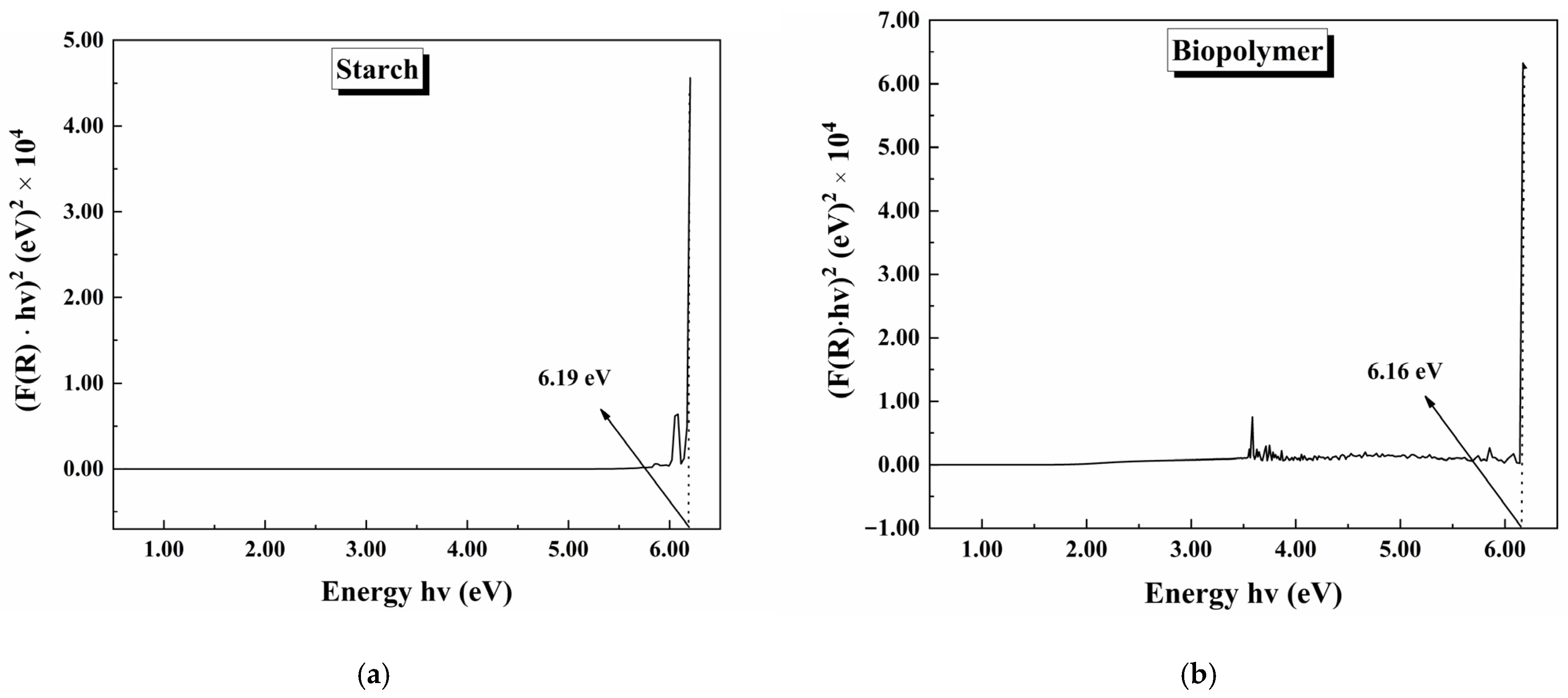
3.4. UV-Vis DRS Spectroscopy
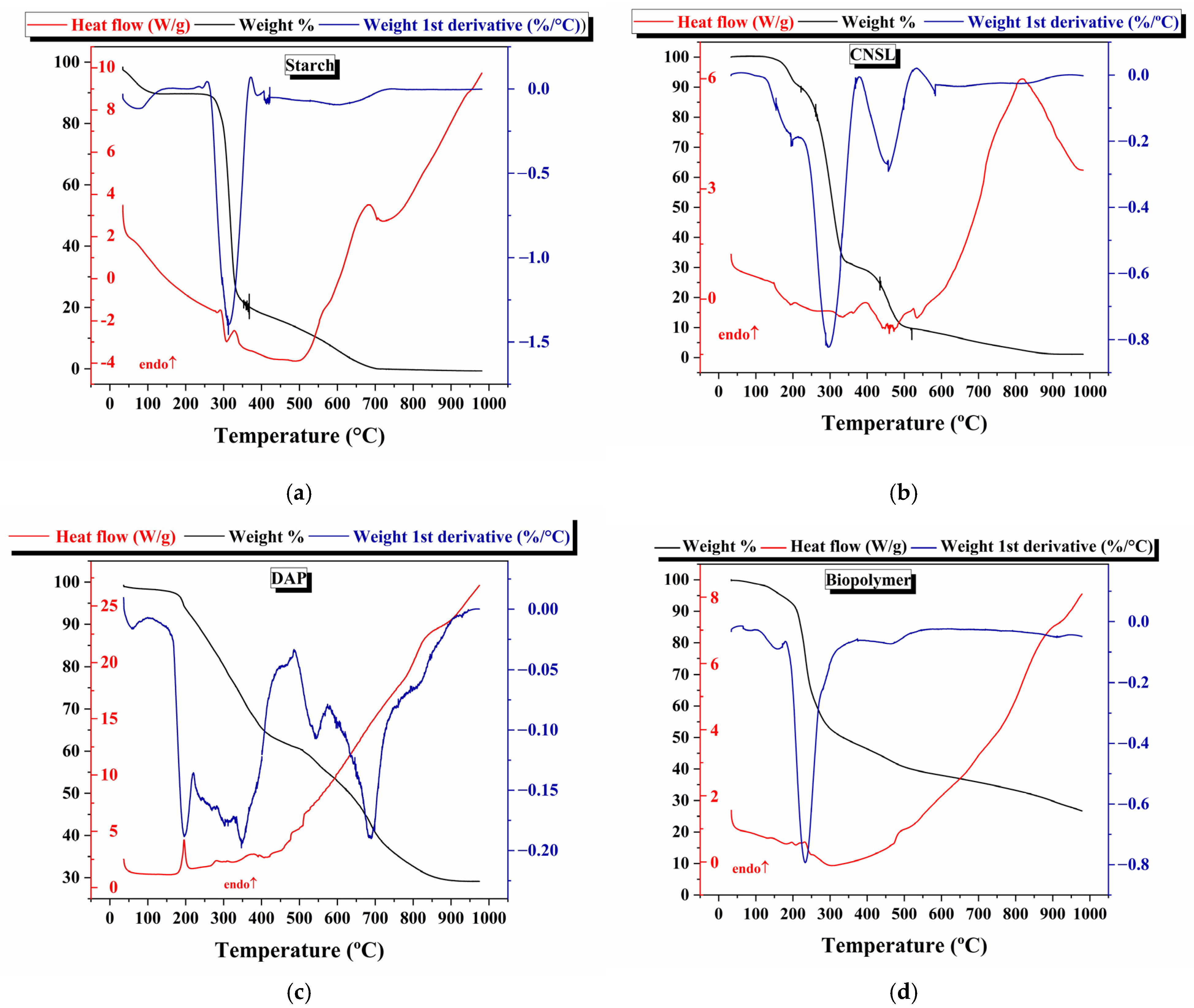
3.5. Thermal Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salimi, M.; Channab, B.; El Idrissi, A.; Zahouily, M.; Motamedi, E. A Comprehensive Review on Starch: Structure, Modification, and Applications in Slow/Controlled-Release Fertilizers in Agriculture. Carbohydr. Polym. 2023, 322, 121326. [Google Scholar] [CrossRef] [PubMed]
- Sim, D.H.H.; Tan, I.A.W.; Lim, L.L.P.; Hameed, B.H. Encapsulated Biochar-Based Sustained Release Fertilizer for Precision Agriculture: A Review. J. Clean. Prod. 2021, 303, 127018. [Google Scholar] [CrossRef]
- Xu, D.; Zhong, B.; Wang, X.; Li, X.; Zhong, Y.; Yan, Z.; Yang, J.; Li, X.; Wang, Y.; Zhou, X. The Development Road of Ammonium Phosphate Fertilizer in China. Chin. J. Chem. Eng. 2022, 41, 170–175. [Google Scholar] [CrossRef]
- Chen, Q.; Qu, Z.; Li, Z.; Zhang, Z.; Ma, G.; Liu, Z.; Wang, Y.; Wu, L.; Fang, F.; Wei, Z.; et al. Coated Diammonium Phosphate Combined With Humic Acid Improves Soil Phosphorus Availability and Photosynthesis and the Yield of Maize. Front. Plant Sci. 2021, 12, 759929. [Google Scholar] [CrossRef]
- Abd El-Aziz, M.E.; Salama, D.M.; Morsi, S.M.M.; Youssef, A.M.; El-Sakhawy, M. Development of Polymer Composites and Encapsulation Technology for Slow-Release Fertilizers. Rev. Chem. Eng. 2022, 38, 603–616. [Google Scholar] [CrossRef]
- Song, Y.; Ma, L.; Duan, Q.; Xie, H.; Dong, X.; Zhang, H.; Yu, L. Development of Slow-Release Fertilizers with Function of Water Retention Using Eco-Friendly Starch Hydrogels. Molecules 2024, 29, 4835. [Google Scholar] [CrossRef]
- Zhang, M.; Yang, J. Preparation and Characterization of Multifunctional Slow Release Fertilizer Coated with Cellulose Derivatives. Int. J. Polym. Mater. Polym. Biomater. 2021, 70, 774–781. [Google Scholar] [CrossRef]
- Pimsen, R.; Porrawatkul, P.; Nuengmatcha, P.; Ramasoot, S.; Chanthai, S. Efficiency Enhancement of Slow Release of Fertilizer Using Nanozeolite–Chitosan/Sago Starch-Based Biopolymer Composite. J. Coatings Technol. Res. 2021, 18, 1321–1332. [Google Scholar] [CrossRef]
- Lawrencia, D.; Wong, S.K.; Low, D.Y.S.; Goh, B.H.; Goh, J.K.; Ruktanonchai, U.R.; Soottitantawat, A.; Lee, L.H.; Tang, S.Y. Controlled Release Fertilizers: A Review on Coating Materials and Mechanism of Release. Plants 2021, 10, 238. [Google Scholar] [CrossRef]
- Sarkar, A.; Biswas, D.R.; Datta, S.C.; Dwivedi, B.S.; Bhattacharyya, R.; Kumar, R.; Bandyopadhyay, K.K.; Saha, M.; Chawla, G.; Saha, J.K.; et al. Preparation of Novel Biodegradable Starch/Poly(Vinyl Alcohol)/Bentonite Grafted Polymeric Films for Fertilizer Encapsulation. Carbohydr. Polym. 2021, 259, 117679. [Google Scholar] [CrossRef]
- Zafar, N.; Niazi, M.B.K.; Sher, F.; Khalid, U.; Jahan, Z.; Shah, G.A.; Zia, M. Starch and Polyvinyl Alcohol Encapsulated Biodegradable Nanocomposites for Environment Friendly Slow Release of Urea Fertilizer. Chem. Eng. J. Adv. 2021, 7, 100123. [Google Scholar] [CrossRef]
- Wang, S.; Li, X.; Huang, R.; Shen, L.; Yang, H.; Lei, G.; Shen, L.; Zhan, Y.; Jiang, L. Preparation of Starch-Based Composite Hydrogel for Green Fertilizer System with Superior Controlled Release Performance. SRRN Preprint Service. 2022. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4207555 (accessed on 20 March 2025).
- Channab, B.E.; Tayi, F.; Aqlil, M.; Akil, A.; Essamlali, Y.; Chakir, A.; Zahouily, M. Graphene Oxide, Starch, and Kraft Lignin Bio-Nanocomposite Controlled-Release Phosphorus Fertilizer: Effect on P Management and Maize Growth. Int. J. Biol. Macromol. 2024, 282, 137190. [Google Scholar] [CrossRef] [PubMed]
- Araújo, R.G.; Rodríguez-Jasso, R.M.; Ruiz, H.A.; Govea-Salas, M.; Rosas-Flores, W.; Aguilar-González, M.A.; Pintado, M.E.; Lopez-Badillo, C.; Luevanos, C.; Aguilar, C.N. Hydrothermal-Microwave Processing for Starch Extraction from Mexican Avocado Seeds: Operational Conditions and Characterization. Processes 2020, 8, 759. [Google Scholar] [CrossRef]
- Gruber Chiaregato, C.; Faez, R. Micronutrients Encapsulation by Starch as an Enhanced Efficiency Fertilizer. Carbohydr. Polym. 2021, 271, 118419. [Google Scholar] [CrossRef] [PubMed]
- Supare, K.; Mahanwar, P.A. Starch-Derived Superabsorbent Polymers in Agriculture Applications: An Overview. Polym. Bull. 2022, 79, 5795–5824. [Google Scholar] [CrossRef]
- Qamruzzaman, M.; Ahmed, F.; Mondal, M.I.H. An Overview on Starch-Based Sustainable Hydrogels: Potential Applications and Aspects. J. Polym. Environ. 2022, 30, 19–50. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Y.; Liu, J.; Xu, X. Preparation, Characterization, Physicochemical Property and Potential Application of Porous Starch: A Review. Int. J. Biol. Macromol. 2020, 148, 1169–1181. [Google Scholar] [CrossRef]
- Gautam, N.; Garg, S.; Yadav, S. Underutilized Finger Millet Crop for Starch Extraction, Characterization, and Utilization in the Development of Flexible Thin Film. J. Food Sci. Technol. 2021, 58, 4411–4419. [Google Scholar] [CrossRef]
- de Souza Silva, G.M.; Martins Veloso, C.; Soares Santos, L.; Alves de Melo Neto, B.; Ilhéu Fontan, R.d.C.; Ferreira Bonomo, R.C. Extraction and Characterization of Native Starch Obtained from the Inhambu Tuber. J. Food Sci. Technol. 2020, 57, 1830–1839. [Google Scholar] [CrossRef]
- Preethi, R.; Moses, J.A.; Anandharamakrishnan, C. Development of Anacardic Acid Incorporated Biopolymeric Film for Active Packaging Applications. Food Packag. Shelf Life 2021, 28, 100656. [Google Scholar] [CrossRef]
- Kyei, S.K.; Darko, G.; Akaranta, O. Chemistry and Application of Emerging Ecofriendly Antifouling Paints: A Review. J. Coat. Technol. Res. 2020, 17, 315–332. [Google Scholar] [CrossRef]
- Mwakalesi, A.J.; Potter, I.D. Targeting of Cationic Organic Pesticide Residues Using Polymer Inclusion Membranes Containing Anacardic Acid from Cashew Nut Shell Liquid as a Green Carrier. J. Water Process Eng. 2021, 43, 102222. [Google Scholar] [CrossRef]
- Nambela, L.; Haule, L.V.; Mgani, Q.A. Anacardic Acid Isolated from Cashew Nut Shells Liquid: A Potential Precursor for the Synthesis of Anthraquinone Dyes. Clean. Chem. Eng. 2022, 3, 100056. [Google Scholar] [CrossRef]
- Sharma, P.; Gaur, V.K.; Sirohi, R.; Larroche, C.; Kim, S.H.; Pandey, A. Valorization of Cashew Nut Processing Residues for Industrial Applications. Ind. Crops Prod. 2020, 152, 112550. [Google Scholar] [CrossRef]
- Lomonaco, D.; Mele, G.; Mazzeto, S. Cashew Nutshell Liquid (CNSL): From an Agro-Industrial Waste to a Sustainable Alternative to Petrochemical Resources. In Cashew Nut Shell Liquid: A Goldfield for Functional Materials; Anilkumar, P., Ed.; Springer International Publishing AG: Cham, Switzerland, 2017; ISBN 9783319474557. [Google Scholar]
- Arrieta, A.A.; Ducuara, J.A.; Combatt, E.M. Bio-Based Electroactive Composite Biopolymer Films from Cassava Starch/Anacardic Acid. Rasayan J. Chem. 2022, 2022, 103–109. [Google Scholar] [CrossRef]
- Arrieta, A.; Barrera, I.; Mendoza, J. Impedantiometric Behavior of Solid Biopolymer Electrolyte Elaborated from Cassava Starch Synthesized in Different PH. Int. J. Technol. 2022, 13, 912–920. [Google Scholar] [CrossRef]
- Abdullah, A.H.D.; Chalimah, S.; Primadona, I.; Hanantyo, M.H.G. Physical and Chemical Properties of Corn, Cassava, and Potato Starchs. IOP Conf. Ser. Earth Environ. Sci. 2018, 160, 012003. [Google Scholar] [CrossRef]
- Hsu, S.L.; Patel, J.; Zhao, W. Vibrational Spectroscopy of Polymers. In Molecular Characterization of Polymers; Elsevier: Amsterdam, The Netherlands, 2021; pp. 369–407. [Google Scholar]
- Silverstein, R.; Webster, F.X.; Kiemle, D.J. Spectrometric Identificaction of Organic Compounds, 7th ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005; ISBN 978-0471393627. [Google Scholar]
- Awali, A.B.I.; Bachir, M.S.; Abdou-Rahimou, K.N.; Salam, M.A. Improvement of P2O5 Content in Tahoua Rock Phosphate Using Synthetic Diammonium Phosphate. Int. Res. J. Pure Appl. Chem. 2024, 25, 101–110. [Google Scholar] [CrossRef]
- Miller, F.A.; Wilkins, C.H. Infrared Spectra and Characteristic Frequencies of Inorganic Ions. Their Use in Qualitative Analysis. Anal. Chem. 1952, 24, 1253–1254. [Google Scholar] [CrossRef]
- Jastrzbski, W.; Sitarz, M.; Rokita, M.; Bułat, K. Infrared Spectroscopy of Different Phosphates Structures. Spectrochim. Acta—Part A Mol. Biomol. Spectrosc. 2011, 79, 722–727. [Google Scholar] [CrossRef]
- Marcilla, A.; Beltran, M.I.; Gómez-Siurana, A.; Martinez-Castellanos, I.; Berenguer, D.; Pastor, V.; García, A.N. TGA/FTIR Study of the Pyrolysis of Diammonium Hydrogen Phosphate-Tobacco Mixtures. J. Anal. Appl. Pyrolysis 2015, 112, 48–55. [Google Scholar] [CrossRef]
- Rodriguez-Garcia, M.E.; Hernandez-Landaverde, M.A.; Delgado, J.M.; Ramirez-Gutierrez, C.F.; Ramirez-Cardona, M.; Millan-Malo, B.M.; Londoño-Restrepo, S.M. Crystalline Structures of the Main Components of Starch. Curr. Opin. Food Sci. 2021, 37, 107–111. [Google Scholar] [CrossRef]
- Myllärinen, P.; Buleon, A.; Lahtinen, R.; Forssell, P. The Crystallinity of Amylose and Amylopectin Films. Carbohydr. Polym. 2002, 48, 41–48. [Google Scholar] [CrossRef]
- Saraiva Rodrigues, S.C.; da Silva, A.S.; de Carvalho, L.H.; Alves, T.S.; Barbosa, R. Morphological, Structural, Thermal Properties of a Native Starch Obtained from Babassu Mesocarp for Food Packaging Application. J. Mater. Res. Technol. 2020, 9, 15670–15678. [Google Scholar] [CrossRef]
- Nara, S.; Komiya, T. Studies on the Relationship Between Water-satured State and Crystallinity by the Diffraction Method for Moistened Potato Starch. Starch—Stärke 1983, 35, 407–410. [Google Scholar] [CrossRef]
- El-Taher, A.; Abdelhalim, M.A.K. Elemental Analysis of Phosphate Fertilizer Consumed in Saudi Arabia. Life Sci. J. 2013, 10, 701–708. [Google Scholar]
- Pankove, J.I. Optical Process in Semiconductors; Dover Publications: Garden City, NY, USA, 1975; ISBN 0486602753. [Google Scholar]
- Kortüm, G. Reflectance Spectroscopy. Principles, Methods, Applications; Springer: Berlin/Heidelberg, Germany, 1969; ISBN 978-3-642-88073-5. [Google Scholar]
- Henning, F.G.; Schnitzler, E.; Demiate, I.M.; Lacerda, L.G.; Ito, V.C.; Malucelli, L.C.; da Silva Carvalho Filho, M.A. Fortified Rice Starches: The Role of Hydrothermal Treatments in Zinc Entrapment. Starch/Staerke 2019, 71, 1800130. [Google Scholar] [CrossRef]
- Pigłowska, M.; Kurc, B.; Rymaniak, Ł.; Lijewski, P.; Fuć, P. Kinetics and Thermodynamics of Thermal Degradation of Different Starches and Estimation the OH Group and H2O Content on the Surface by TG/DTG-DTA. Polymers 2020, 12, 357. [Google Scholar] [CrossRef]
- Ábrego, J.; Plaza, D.; Luño, F.; Atienza-Martínez, M.; Gea, G. Pyrolysis of Cashew Nutshells: Characterization of Products and Energy Balance. Energy 2018, 158, 72–80. [Google Scholar] [CrossRef]
| Element | Atomic % | Atomic % Error |
|---|---|---|
| C | 38.5 | 0.2 |
| O | 52.0 | 0.4 |
| F | 4.0 | 0.3 |
| Na | 1.0 | 0.1 |
| P | 3.4 | 0.1 |
| K | 1.1 | 0.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Humánez, M.A.; Vega, Y.V.; Almario, A.A.; Palma Calabokis, O.; Baldovino, J.d.J.A. Synthesis and Physical–Chemical Characterization of a Biopolymer Derived from Cassava Starch, Cashew Nutshell Liquid, and Diammonium Phosphate. Polymers 2025, 17, 1184. https://doi.org/10.3390/polym17091184
Humánez MA, Vega YV, Almario AA, Palma Calabokis O, Baldovino JdJA. Synthesis and Physical–Chemical Characterization of a Biopolymer Derived from Cassava Starch, Cashew Nutshell Liquid, and Diammonium Phosphate. Polymers. 2025; 17(9):1184. https://doi.org/10.3390/polym17091184
Chicago/Turabian StyleHumánez, Manuel Acosta, Yair Vega Vega, Alvaro Arrieta Almario, Oriana Palma Calabokis, and Jair de Jesús Arrieta Baldovino. 2025. "Synthesis and Physical–Chemical Characterization of a Biopolymer Derived from Cassava Starch, Cashew Nutshell Liquid, and Diammonium Phosphate" Polymers 17, no. 9: 1184. https://doi.org/10.3390/polym17091184
APA StyleHumánez, M. A., Vega, Y. V., Almario, A. A., Palma Calabokis, O., & Baldovino, J. d. J. A. (2025). Synthesis and Physical–Chemical Characterization of a Biopolymer Derived from Cassava Starch, Cashew Nutshell Liquid, and Diammonium Phosphate. Polymers, 17(9), 1184. https://doi.org/10.3390/polym17091184








