Environmental Impact of Textile Materials: Challenges in Fiber–Dye Chemistry and Implication of Microbial Biodegradation
Abstract
1. Introduction
1.1. Fiber Composition and Its Significance
- (a)
- Stretchability or elasticity, which is an ability of a fiber material to return to the original shape (dimensions) after stretching. Spandex is highly elastic, making it ideal for sportswear and stretchable fabrics; the unique properties of different fibers make them suitable for various applications. Self-healing fibers or shape-reforming fibers are the most valuable features in this category, and most synthetic fibers are known to possess this property.
- (b)
- Water retention or moisture absorbency: The properties of water retention or moisture within the fiber can be associated with natural fibers such as cotton and wool, which exhibit high moisture retention, thereby making them comfortable for clothing. Unlike cotton or wool, which do have functional groups in their structure that can make an H-bond with moisture or water molecules and thereby can retain moisture/water within the fiber, synthetic fibers are generally devoid of such functional groups, therefore, tend to exhibit lower moisture retention.
- (c)
- Strength (tensile strength vs. mechanical strength): The ability of fiber material to withstand tension without breaking. Nylon and polyester are known for their high tensile strength, while natural fibers typically exhibit lower ability in such properties.
- (d)
- Insulation or thermal properties: The ability of fiber to retain or dissipate heat. Wool is an excellent insulator, while polyester is often used in thermal wear due to its low thermal conductivity.
- (e)
- Resistance towards chemical penetration or average reactivity: This means the ability to withstand exposure to chemicals. Polyester and nylon are resistant to many chemicals, making them suitable for industrial applications. In contrast, due to the presence of an H-bond network in the structure of natural fibers, they tend to show lower reactivity towards certain chemicals, as further discussed in Section 2.
- (f)
- Molecular arrangement of fibers: The properties of fibers are largely determined by their molecular structure. Most fibers are polymers, which are long chains of repeating molecular units called monomers. The arrangement and chemical composition of these monomers affects their fiber’s characteristics, determining the suitability of fibers for specific applications.
1.2. Natural Fibers and Their Challenges
1.3. Synthetic Fibers and Their Challenges
2. Specifics of Color or Dyeing Chemistry Towards Fibers
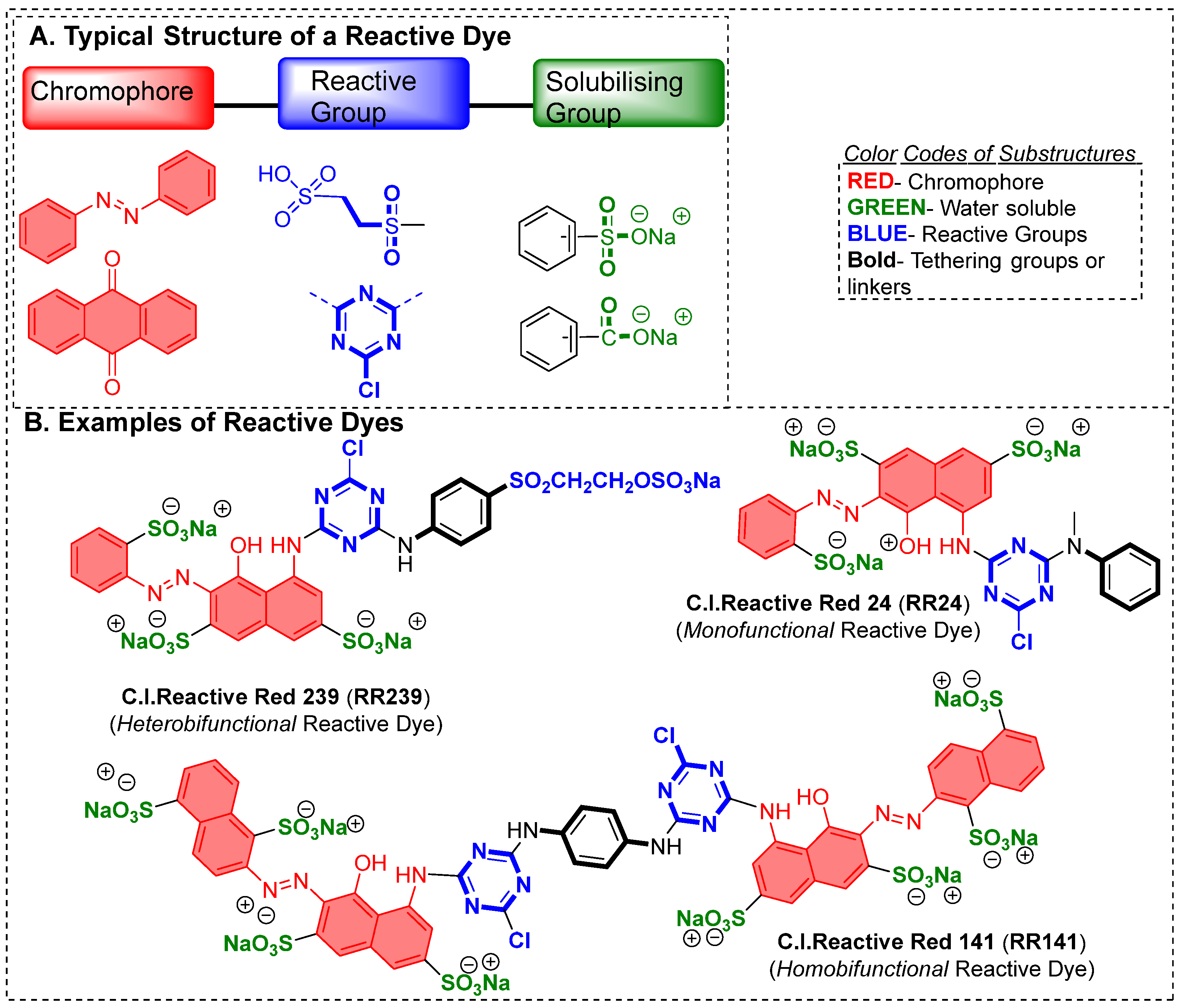
2.1. Dye: Structure, Origin, and Classification
2.2. Dyeing Chemistry and Challenges
2.2.1. Dyeing of Natural Fibers
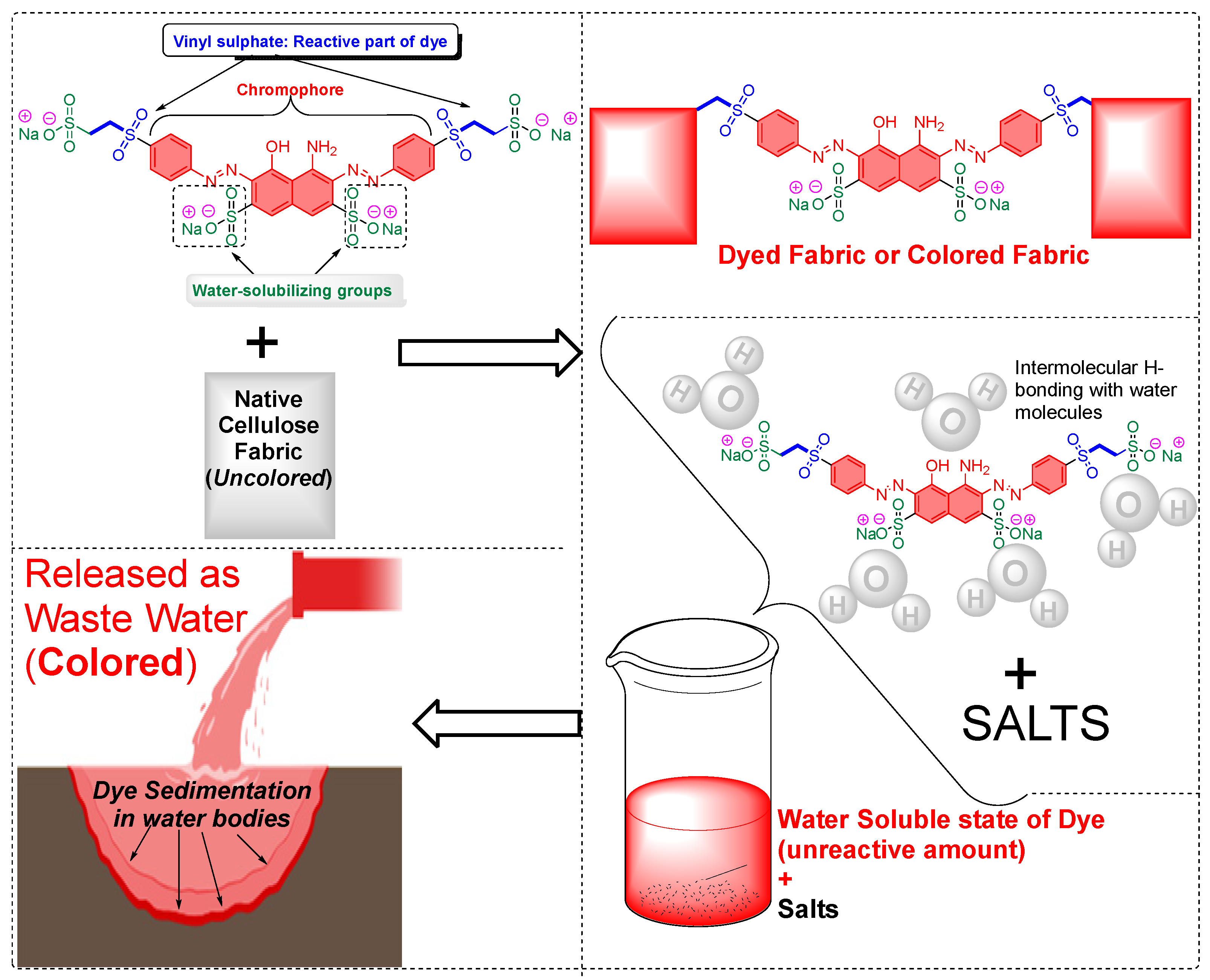
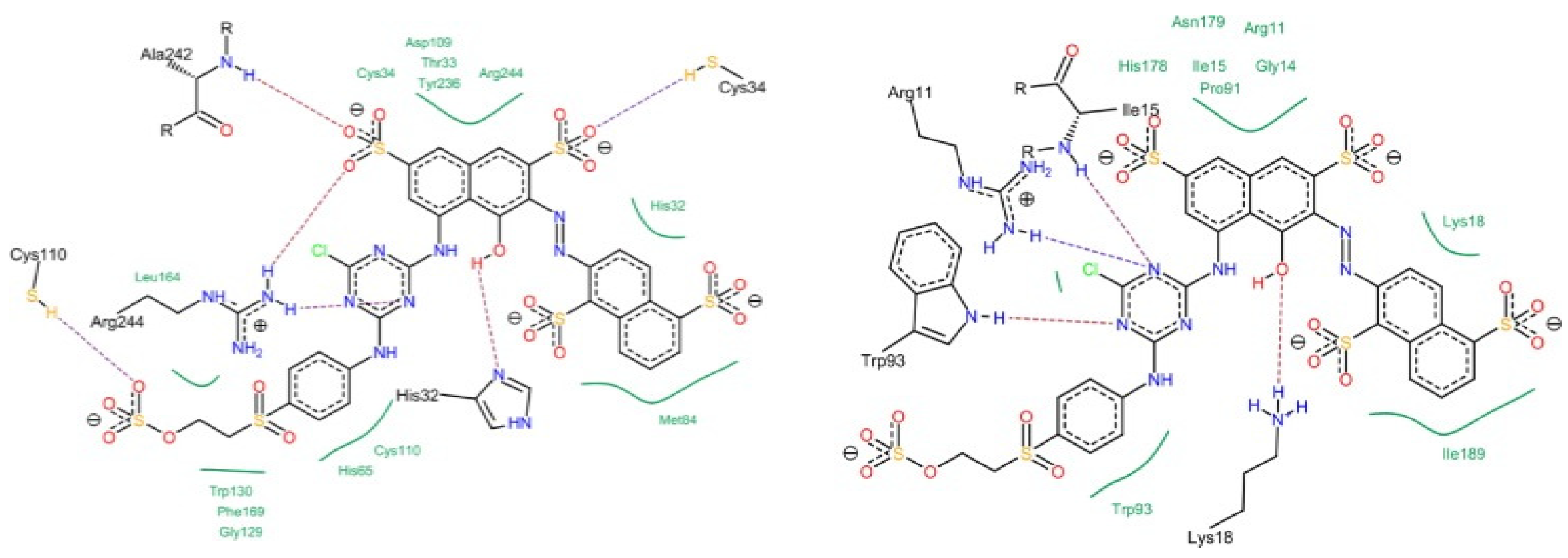
Cellulose Fiber Dyeing: High Usage of Alkali, Salts and Reactive Dyes
Protein Fiber Dyeing: Application of Mordants for Wool Dyeing
2.3. Strategies to Improve Dyeing or Coloration Processes
2.3.1. Blending Fiber or Composites
- (a)
- Synthesizing reactive dyes that have two or more reactive groups (as can be called bifunctional or polyfunctional reactive dyes) offers more opportunity for hydroxy groups of cellulose to react with these dyes and, therefore, improve the overall dyeing process; however, such innovations have limited commercial success as this will add burden on synthetic chemist to design a molecule using multistep synthesis, and also increase overall cost. Therefore, only a handful of bifunctional reactive dyes are known to date.
- (b)
- Using greener or more environment-friendly solvents, such as alcohols to decrease dye hydrolysis as it is well known that the alkaline solution (where water can act as a nucleophile to complete with the hydroxy group of cellulose) leads to the hydrolysis of the reactive dye. Therefore, incorporating greener solvents could lower the rate of hydrolysis of reactive dye during their commercial processing. However, in comparison, it is commercially not feasible for textile industries to use solvents as a replacement for water.
- (c)
- The inertness of cellulose fibers and the use of highly reactive dyes and chemical auxiliaries, ultimately increase the chemical waste in the wastewater produced from the textile industries, necessitating the development of cellulose blends with other fibers. Blending of fibers allows the dyeing of the blended fibers rather than just cellulose. Polycotton, as an example, is a blend of polyester and cotton and has been commercially successful. Although the dyeing of polycotton has been optimized and shows improved dye absorption, the presence of polyester can complicate the dyeing chemistry. This complication arises because polyester can inhibit the reactivity of cellulose fibers, thereby reducing their effectiveness in dye applications. One argument for the decreased societal and economic importance of dyeing the cellulose part of polyester fibers is the low reactivity of cellulose toward specialized dyes. As a result, achieving broader commercial success in this area is currently limited. However, in my opinion, the process will mature over time, and, therefore, the cost of industrial processing will also decrease. However, the use of blended cellulose fibers was intended not for dyeing the cellulose part but rather for dyeing the polyester fibers. This intention may pose challenges for the industry, as it can lead to a perceived value loss of one of the components of the material.
2.3.2. Sustainable Fiber Technologies
- (a)
- Renewable and Bio-based fibers
- (b)
- Waterless Dyeing Technologies
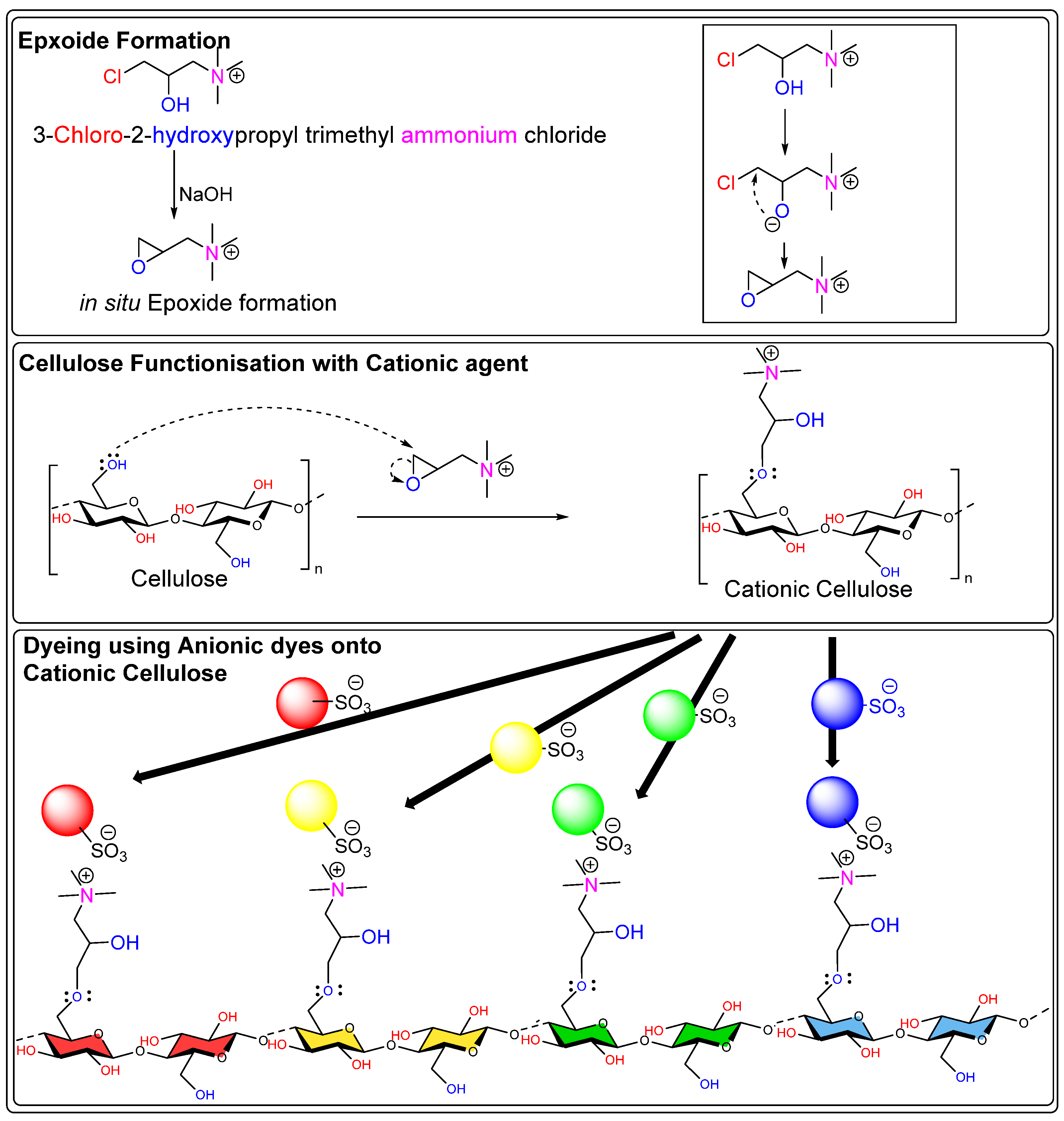
2.3.3. Cationization of Fibers
2.3.4. Salting-In/Salting-Out
2.3.5. Thermolinkable and Photolinkable Dyes
3. Environmental Impact of Textile Fibers and Dyeing Process
3.1. Microplastics from Textiles: Environmental Toxicity and Ecological Consequences
- It is estimated that over 14 million tons of microplastics have accumulated on the ocean floor, damaging ecosystems and impacting wildlife and humans.
- Synthetic textiles contribute nearly 8% of the microplastics released into oceans in Europe. However, worldwide, this figure varies for textiles, ranging from 16% to 35%. This results in nearly 200,000 tons of microplastics entering marine ecosystems annually.
- Most microplastics from textiles are released during their initial washings. Fast fashion materials, which are used for only short periods, tend to wear out quickly due to their inferior quality, leading to particularly high levels of microplastic release.
- The release of microplastics from textile materials could potentially be reduced through sustainable manufacturing practices and improved end-of-life processing and disposal management.
3.2. Water Pollution and Its Impact on Agriculture
3.3. Aquatic and Terrestrial Toxicity of Dyed Wastewater
4. Methods for the Degradation of Textile Dyes
4.1. Bioremediation Mechanism: Study-Supported Theories
4.2. Role of Bacteria in Bioremediation of Dye Solutions: Initial Challenges or Limitations
4.3. Anaerobic Bioremediation of Dye Solutions Using Microbial Bacteria
4.4. Aerobic Bioremediation of Dye Solutions Using Microbial Bacteria
4.5. Anaerobic/Aerobic Bioremediation of Dye Solutions Using Microbial Bacteria
5. Biodegradation of Fibers
5.1. Biodegradation of Natural Fibers
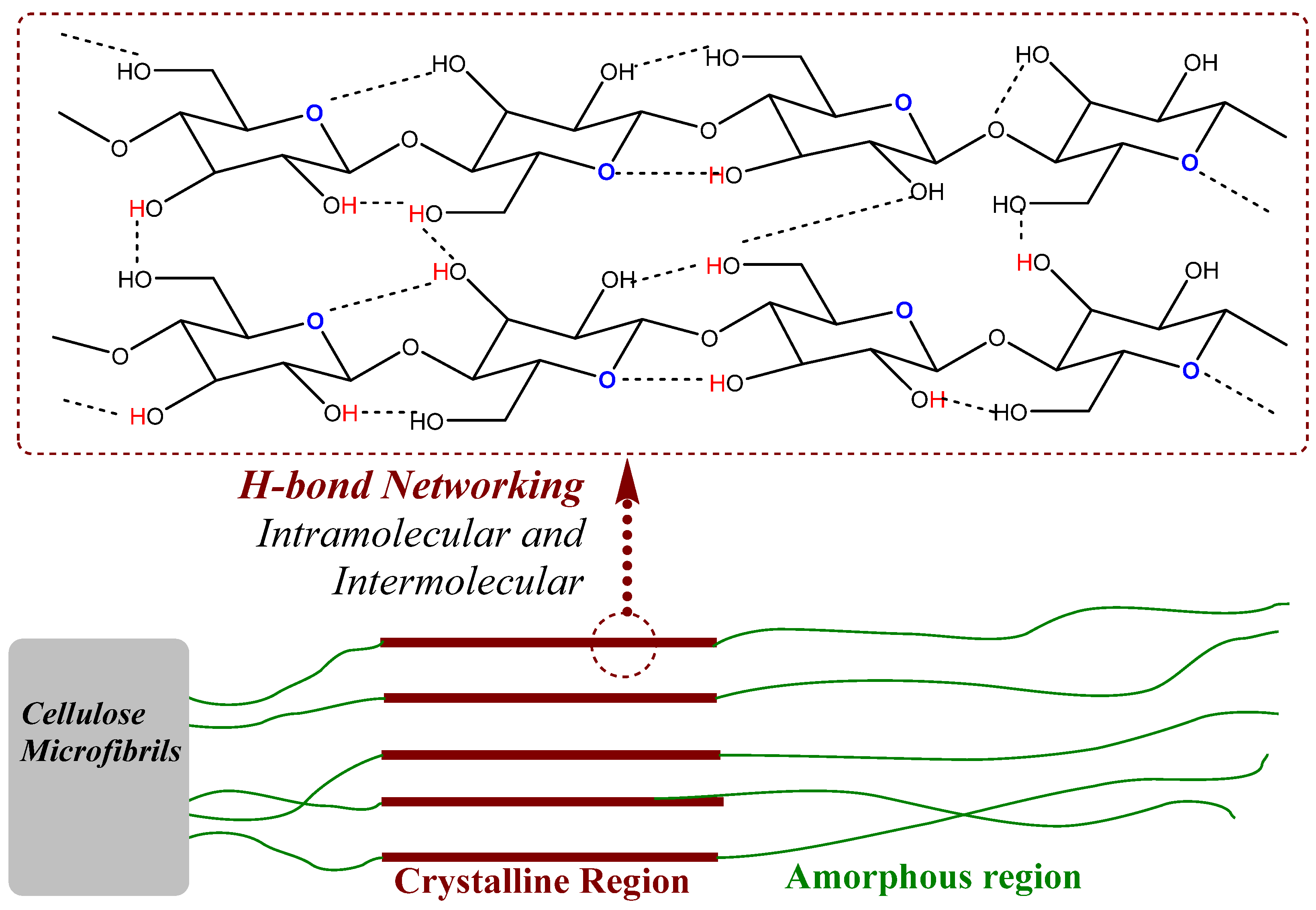
5.1.1. Biodegradation of Cellulose
5.1.2. Biodegradation of Silk and Wool
5.2. Biodegradation of Synthetic Fibers
5.2.1. Biodegradation of Cellulose Acetate
5.2.2. Biodegradation of Polyethylene Terephthalate (PET)
| Fiber/Polymer | Fiber-Type | Expected Depolymerization Products | Enzymes | Ref. |
|---|---|---|---|---|
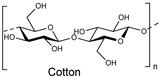 | Natural fiber | 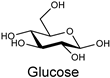 | Cellulase AP3 (Aspergillus) | [187] |
| Cellulase: cellulase cocktail | [188] | |||
 | Natural fiber | 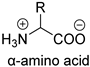 | Protease: Protease XIV, 1 for silk fibroin sheetcollagenase IA | [189] |
| Serine Protease: Ronozyme® | [190] | |||
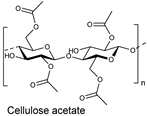 | Synthetic fiber |  | Esterase and cellulase: Synergism | [194] |
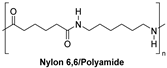 | Synthetic fiber | 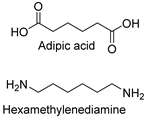 | Nylon hydrolase: 6-aminohexanoate oligomer hydrolase | [199] |
| Protease: Protex® modified subtilisin, Lipase: Lipex® serine hydrolase | [200] | |||
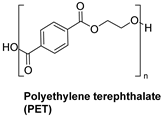 | Synthetic fiber | 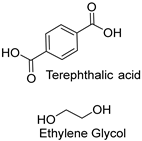 | Cutinase (Thermobifida fusca KW3) | [195] |
| Cutinase (Leaf–branch compost cutinase variant) | [196] | |||
| Cutinase (Humicola insolens) | [197] | |||
 | Synthetic fiber | 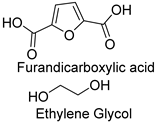 | Cutinase (Humicola insolens) | [201] |
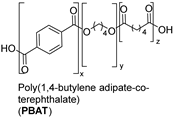 | Synthetic fiber |  | Lipase: Pelosinus fermentans | [202] |
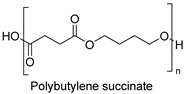 | Synthetic fiber |  | Cutinase (Fusarium solani) | [203] |
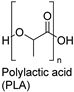 | Synthetic fiber |  | Protease: (Tritirachium album) | [204] |
| Protease (Proteinase K) | [205] | |||
| Protease (Bacillus subtilis) | [206] | |||
 | Synthetic fiber | 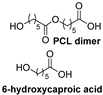 | Cutinase (Humicola insolens) | [207] |
 | Synthetic fiber |  | Esterases: (Fusarium solani pisi) | [208] |
| Nitrile hydrolyzing enzymes (Micrococcus luteus strain BST20) | [209] | |||
| Nitrilase: Commercial Cyanovacta Lyase | [210] |
6. Conclusions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Jabar, J.M.; Adedayo, T.E.; Odusote, Y.A. Green, eco-friendly and sustainable alternative in dyeing cotton fabric using aqueous extract Mucuna slonaei F dye: Effects of metal salts pre-mordanting on color strength and fastness properties. Curr. Res. Green Sustain. Chem. 2021, 4, 100151. [Google Scholar]
- Tegegne, W.; Haile, A.; Zeleke, Y.; Temesgen, Y.; Bantie, H.; Biyable, S. Natural dyeing and anti bacterial finishing of cotton fabric with extracts from Justicia schimperiana leaf extract: A step towards sustainable dyeing and finishing. Int. J. Sustain. Eng. 2024, 17, 52–61. [Google Scholar]
- Batool, F.; Iqbal, N.; Adeel, S.; Azeem, M.; Hussaan, M.; Mia, R. Sugar beet (Beta vulgaris L.) leaves as natural colorant for cotton dyeing using an ecofriendly approach toward industrial progress. Sci. Prog. 2024, 107, 00368504241271737. [Google Scholar]
- Periyasamy, A.P.; Negi, A. Alkoxide-based solvent dyeing: A feasible strategy for pollution minimization and sustainable approach for the reactive dyeing of cellulosic materials. Cellulose 2024, 31, 7765–7791. [Google Scholar]
- Negi, A.; Tehrani-Bagha, A.R. Cellulose Functionalization Using N-Heterocyclic-Based Leaving Group Chemistry. Polymers 2024, 16, 149. [Google Scholar] [CrossRef]
- Lange, N.; Szlasa, W.; Saczko, J.; Chwiłkowska, A. Potential of cyanine derived dyes in photodynamic therapy. Pharmaceutics 2021, 13, 818. [Google Scholar] [CrossRef]
- Rejinold, N.S.; Choi, G.; Choy, J.-H. Recent developments on semiconducting polymer nanoparticles as smart photo-therapeutic agents for cancer treatments—A review. Polymers 2021, 13, 981. [Google Scholar] [CrossRef]
- Luzardo-Ocampo, I.; Ramírez-Jiménez, A.K.; Yañez, J.; Mojica, L.; Luna-Vital, D.A. Technological applications of natural colorants in food systems: A review. Foods 2021, 10, 634. [Google Scholar] [CrossRef]
- Negi, A.; Kieffer, C.; Voisin-Chiret, A.S. Azobenzene photoswitches in proteolysis targeting chimeras: Photochemical control strategies and therapeutic benefits. ChemistrySelect 2022, 7, e202200981. [Google Scholar]
- Atmakuri, A.; Palevicius, A.; Vilkauskas, A.; Janusas, G. Review of hybrid fiber based composites with nano particles—Material properties and applications. Polymers 2020, 12, 2088. [Google Scholar] [CrossRef]
- Kumari, N.; Mohan, C.; Negi, A. An investigative study on the structural, thermal and mechanical properties of clay-based PVC polymer composite films. Polymers 2023, 15, 1922. [Google Scholar] [CrossRef]
- Sánchez-Safont, E.L.; Aldureid, A.; Lagarón, J.M.; Cabedo, L.; Gámez-Pérez, J. Study of the compatibilization effect of different reactive agents in PHB/natural fiber-based composites. Polymers 2020, 12, 1967. [Google Scholar] [CrossRef]
- da Luz, F.S.; Garcia Filho, F.d.C.; Del-Rio, M.T.G.; Nascimento, L.F.C.; Pinheiro, W.A.; Monteiro, S.N. Graphene-incorporated natural fiber polymer composites: A first overview. Polymers 2020, 12, 1601. [Google Scholar] [CrossRef] [PubMed]
- Raji, R.K.; Miao, X.; Boakye, A. Electrical conductivity in textile fibers and yarns. AATCC J. Res. 2017, 4, 8–21. [Google Scholar]
- Maity, S.; Chatterjee, A. Conductive polymer-based electro-conductive textile composites for electromagnetic interference shielding: A review. J. Ind. Text. 2018, 47, 2228–2252. [Google Scholar]
- Krifa, M. Electrically conductive textile materials—Application in flexible sensors and antennas. Textiles 2021, 1, 239–257. [Google Scholar] [CrossRef]
- Coetzee, D.; Venkataraman, M.; Militky, J.; Petru, M. Influence of nanoparticles on thermal and electrical conductivity of composites. Polymers 2020, 12, 742. [Google Scholar] [CrossRef]
- Ojstršek, A.; Jug, L.; Plohl, O. A review of electro conductive textiles utilizing the dip-coating technique: Their functionality, durability and sustainability. Polymers 2022, 14, 4713. [Google Scholar] [CrossRef]
- Micó-Vicent, B.; Viqueira, V.; Ramos, M.; Luzi, F.; Dominici, F.; Torre, L.; Jiménez, A.; Puglia, D.; Garrigós, M.C. Effect of lemon waste natural dye and essential oil loaded into laminar nanoclays on thermomechanical and color properties of polyester based bionanocomposites. Polymers 2020, 12, 1451. [Google Scholar] [CrossRef]
- Mohan, C.; Kumari, N.; Jeandet, P.; Kumari, P.; Negi, A. Synthesis of Nano Pigments Using Clay Minerals and Organic Dyes and Their Application as Colorants in Polymer Matrix. Micromachines 2023, 14, 1087. [Google Scholar] [CrossRef]
- Mohan, C.; Kumari, P.; Kumari, N.; Negi, A. Fabrication of colored polymeric membrane using clay-based nano pigments of safranin O (SO) dye. Membranes 2023, 13, 619. [Google Scholar] [CrossRef]
- Patti, A.; Cicala, G.; Acierno, D. Eco-sustainability of the textile production: Waste recovery and current recycling in the composites world. Polymers 2020, 13, 134. [Google Scholar] [CrossRef] [PubMed]
- Karimah, A.; Ridho, M.R.; Munawar, S.S.; Ismadi; Amin, Y.; Damayanti, R.; Lubis, M.A.R.; Wulandari, A.P.; Nurindah; Iswanto, A.H. A comprehensive review on natural fibers: Technological and socio-economical aspects. Polymers 2021, 13, 4280. [Google Scholar] [CrossRef] [PubMed]
- Aisyah, H.; Paridah, M.; Sapuan, S.; Ilyas, R.; Khalina, A.; Nurazzi, N.; Lee, S.; Lee, C. A comprehensive review on advanced sustainable woven natural fibre polymer composites. Polymers 2021, 13, 471. [Google Scholar] [CrossRef]
- Fazli, A.; Rodrigue, D. Sustainable Reuse of waste tire textile fibers (WTTF) as reinforcements. Polymers 2022, 14, 3933. [Google Scholar] [CrossRef] [PubMed]
- Aldalbahi, A.; El-Naggar, M.E.; El-Newehy, M.H.; Rahaman, M.; Hatshan, M.R.; Khattab, T.A. Effects of technical textiles and synthetic nanofibers on environmental pollution. Polymers 2021, 13, 155. [Google Scholar] [CrossRef]
- Patti, A.; Acierno, D. Towards the sustainability of the plastic industry through biopolymers: Properties and potential applications to the textiles world. Polymers 2022, 14, 692. [Google Scholar] [CrossRef]
- Glogar, M.; Petrak, S.; Mahnić Naglić, M. Digital Technologies in the Sustainable Design and Development of Textiles and Clothing—A Literature Review. Sustainability 2025, 17, 1371. [Google Scholar] [CrossRef]
- Sigaard, A.S.; Laitala, K. Natural and sustainable? Consumers’ textile fiber preferences. Fibers 2023, 11, 12. [Google Scholar] [CrossRef]
- Hassan, R.; Acerbi, F.; Rosa, P.; Terzi, S. The role of digital technologies in the circular transition of the textile sector. J. Text. Inst. 2024, 1–14. [Google Scholar] [CrossRef]
- Špiler, M.; Gostimirović, L.; Milosević, D.; Beslać, M.; Miškić, M.; Jevtić, B. Investments in digital technology advances in textiles. Ind. Textila 2023, 74, 90–97. [Google Scholar]
- Larsson, J.K.J. Digital innovation for sustainable apparel systems: Experiences based on projects in textile value chain development. Res. J. Text. Appar. 2018, 22, 370–389. [Google Scholar] [CrossRef]
- Dutta, S.; Bansal, P. Evolution of sustainable wearables: Integrating cutting-edge techniques for future textile innovation. Res. J. Text. Appar. 2024, ahead-of-print. [Google Scholar] [CrossRef]
- Palamutcu, S. Sustainable textile technologies. In Textiles and Clothing Sustainability; Springer: Singapore, 2017; pp. 1–22. [Google Scholar] [CrossRef]
- Islam, M.S.; Ahmed, S.; Azady, M.A.R. Sustainable technologies for textile production. In Fundamentals of Natural Fibres and Textiles; Elsevier: Amsterdam, The Netherlands, 2021; pp. 625–655. [Google Scholar]
- Choudhury, A.K.R. Sustainable chemical technologies for textile production. In Sustainable Fibres and Textiles; Elsevier: Amsterdam, The Netherlands, 2017; pp. 267–322. [Google Scholar]
- Sixta, H.; Michud, A.; Hauru, L.; Asaadi, S.; Ma, Y.; King, A.W.; Kilpeläinen, I.; Hummel, M. Ioncell-F: A high-strength regenerated cellulose fibre. Nord. Pulp Pap. Res. J. 2015, 30, 43–57. [Google Scholar]
- Lindman, B.; Medronho, B.; Alves, L.; Costa, C.; Edlund, H.; Norgren, M. The relevance of structural features of cellulose and its interactions to dissolution, regeneration, gelation and plasticization phenomena. Phys. Chem. Chem. Phys. 2017, 19, 23704–23718. [Google Scholar] [CrossRef] [PubMed]
- Berga, L.; Bruce, I.; Nicol, T.W.; Holding, A.J.; Isobe, N.; Shimizu, S.; Walker, A.J.; Reid, J.E. Cellulose dissolution and regeneration using a non-aqueous, non-stoichiometric protic ionic liquid system. Cellulose 2020, 27, 9593–9603. [Google Scholar]
- Medronho, B.; Romano, A.; Miguel, M.G.; Stigsson, L.; Lindman, B. Rationalizing cellulose (in) solubility: Reviewing basic physicochemical aspects and role of hydrophobic interactions. Cellulose 2012, 19, 581–587. [Google Scholar]
- Xue, Y.; Li, W.; Yang, G.; Lin, Z.; Qi, L.; Zhu, P.; Yu, J.; Chen, J. Strength enhancement of regenerated cellulose fibers by adjustment of hydrogen bond distribution in ionic liquid. Polymers 2022, 14, 2030. [Google Scholar] [CrossRef]
- Anceschi, A.; Riccardi, C.; Patrucco, A. The Role of Ionic Liquids in Textile Processes: A Comprehensive Review. Molecules 2025, 30, 353. [Google Scholar] [CrossRef]
- Al Aiti, M.; Das, A.; Kanerva, M.; Järventausta, M.; Johansson, P.; Scheffler, C.; Göbel, M.; Jehnichen, D.; Brünig, H.; Wulff, L. Dry-jet wet spinning of thermally stable lignin-textile grade polyacrylonitrile fibers regenerated from chloride-based ionic liquids compounds. Materials 2020, 13, 3687. [Google Scholar] [CrossRef]
- El Seoud, O.A.; Kostag, M.; Jedvert, K.; Malek, N.I. Cellulose in ionic liquids and alkaline solutions: Advances in the mechanisms of biopolymer dissolution and regeneration. Polymers 2019, 11, 1917. [Google Scholar] [CrossRef] [PubMed]
- Pei, Z.; Liu, X.; Chen, J.; Wang, H.; Li, H. Research progress on lignin depolymerization strategies: A review. Polymers 2024, 16, 2388. [Google Scholar] [CrossRef]
- Negi, A.; Kesari, K.K. Light-driven depolymerization of cellulosic biomass into hydrocarbons. Polymers 2023, 15, 3671. [Google Scholar] [CrossRef]
- Dedes, G.; Karnaouri, A.; Topakas, E. Novel routes in transformation of lignocellulosic biomass to furan platform chemicals: From pretreatment to enzyme catalysis. Catalysts 2020, 10, 743. [Google Scholar] [CrossRef]
- Fontecha-Cámara, M.Á.; Delgado-Blanca, I.; Mañas-Villar, M.; Orriach-Fernández, F.J.; Soriano-Cuadrado, B. Extraction and Depolymerization of lignin from different agricultural and forestry wastes to obtain building blocks in a circular economy framework. Polymers 2024, 16, 1981. [Google Scholar] [CrossRef]
- Franco, A.; Negi, A.; Luque, R.; Carrillo-Carrión, C. Selectivity Control in the Oxidative Ring-Opening of Dimethylfuran Mediated by Zeolitic-Imidazolate Framework-8 Nanoparticles. ACS Sustain. Chem. Eng. 2021, 9, 8090–8096. [Google Scholar] [CrossRef]
- Eren, S.; Özyurt, İ. Waterless Dyeing of Polyamide 6.6. Polymers 2024, 16, 1472. [Google Scholar] [CrossRef]
- Ferri, A.; Banchero, M.; Manna, L.; Sicardi, S. An experimental technique for measuring high solubilities of dyes in supercritical carbon dioxide. J. Supercrit. Fluids 2004, 30, 41–49. [Google Scholar] [CrossRef]
- Montero, G.; Hinks, D.; Hooker, J. Reducing problems of cyclic trimer deposits in supercritical carbon dioxide polyester dyeing machinery. J. Supercrit. Fluids 2003, 26, 47–54. [Google Scholar] [CrossRef]
- Abou Elmaaty, T.; Abd El-Aziz, E. Supercritical carbon dioxide as a green media in textile dyeing: A review. Text. Res. J. 2018, 88, 1184–1212. [Google Scholar] [CrossRef]
- Park, G.; Kwon, D.E.; Kong, W.; Park, J.; Lee, Y.-W. A dissolution kinetic study of disperse dye in supercritical carbon dioxide to design an efficient supercritical dyeing process. Processes 2021, 9, 977. [Google Scholar] [CrossRef]
- Abou Elmaaty, T.; Abd El-Aziz, E.; Ma, J.; El-Taweel, F.; Okubayashi, S. Eco-friendly disperse dyeing and functional finishing of nylon 6 using supercritical carbon dioxide. Fibers 2015, 3, 309–322. [Google Scholar] [CrossRef]
- Cheng, Y.-W.; Benas, J.-S.; Liang, F.-C.; Lin, S.-M.; Huang, Y.-H.; Chen, W.-W.; Chen, Y.-T.; Lee, C.-H.; Yu, Y.-Y.; Kuo, C.-C. Red disperse azo dye side chains influence on polyethylene terephthalate dyeing performances in supercritical carbon dioxide media. Polymers 2022, 14, 5487. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Du, S.; Du, H.; Zhang, H.; Jiao, A.; Li, H.; Du, B.; Gao, D.; Wang, K. Comparison of Four Density-Based Semi-Empirical Models for the Solubility of Azo Disperse Dyes in Supercritical Carbon Dioxide. Processes 2022, 10, 1960. [Google Scholar] [CrossRef]
- Cheng, Y.-W.; Benas, J.-S.; Liang, F.-C.; Lin, S.-M.; Sun, T.-W.; Liu, F.-C.; Yu, Y.-Y.; Kuo, C.-C. Synthesis of azo disperse dyes with high absorption for efficient polyethylene terephthalate dyeing performances in supercritical carbon dioxide. Polymers 2022, 14, 3020. [Google Scholar] [CrossRef]
- Haque, A.N.M.A.; Naebe, M. Zero-water discharge and rapid natural dyeing of wool by plasma-assisted spray-dyeing. J. Clean. Prod. 2023, 402, 136807. [Google Scholar]
- Mowafi, S.; Abou Taleb, M.; El-Sayed, H.E.-D. A review of plasma-assisted treatments of textiles for eco-friendlier water-less processing. Egypt. J. Chem. 2022, 65, 737–749. [Google Scholar]
- Bapat, K.S.; Kee, T.; Russell, S.; Lin, L. Improved coloration of hemp fabrics via low-pressure argon plasma assisted surface modification. Clean. Chem. Eng. 2024, 10, 100123. [Google Scholar]
- Naebe, M.; Haque, A.N.M.A.; Haji, A. Plasma-assisted antimicrobial finishing of textiles: A review. Engineering 2022, 12, 145–163. [Google Scholar]
- Serrano-Martínez, V.M.; Ruzafa-Silvestre, C.; Hernández-Fernández, C.; Bañón-Gil, E.; Arán-Ais, F.; Orgilés-Calpena, E. Improving Cotton Fabric Dyeability by Oxygen Plasma Surface Activation. Surfaces 2024, 7, 1079–1095. [Google Scholar] [CrossRef]
- Peran, J.; Ercegović Ražić, S. Application of atmospheric pressure plasma technology for textile surface modification. Text. Res. J. 2020, 90, 1174–1197. [Google Scholar]
- Mostafa, K.; Ameen, H.; Medhat, A. Harnessing of low-temperature nitrogen plasma technique as an eco-friendly approach for dye-ability of cotton fabric with acid dye. Pigment Resin Technol. 2024, 53, 425–433. [Google Scholar]
- Gorjanc, M.; Mozetič, M.; Vesel, A.; Zaplotnik, R. Natural dyeing and UV protection of plasma treated cotton. Eur. Phys. J. D 2018, 72, 41. [Google Scholar]
- Struszczyk, M.H.; Puszkarz, A.; Wilbik-Hałgas, B.; Cichecka, M.; Litwa, P.; Urbaniak-Domagała, W.; Krucinska, I. The surface modification of ballistic textiles using plasma-assisted chemical vapor deposition (PACVD). Text. Res. J. 2014, 84, 2085–2093. [Google Scholar]
- Granados, A.; Pleixats, R.; Vallribera, A. Recent advances on antimicrobial and anti-inflammatory cotton fabrics containing nanostructures. Molecules 2021, 26, 3008. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; Eid, B.M.; Abdel-Aziz, M.S. Effect of plasma superficial treatments on antibacterial functionalization and coloration of cellulosic fabrics. Appl. Surf. Sci. 2017, 392, 1126–1133. [Google Scholar]
- Ren, Y.; Fan, T.; Wang, X.; Guan, Y.; Zhou, L.; Cui, L.; Li, M.; Zhang, G. In Situ Reduction of Silver Nanoparticles on the Plasma-Induced Chitosan Grafted Polylactic Acid Nonwoven Fabrics for Improvement of Antibacterial Activity. Coatings 2021, 11, 1517. [Google Scholar] [CrossRef]
- Khan, M.R.; Kim, H.G.; Park, J.S.; Shin, J.W.; Nguyen, C.T.; Lee, H.-B.-R. Tunable color coating of e-textiles by atomic layer deposition of multilayer TiO2/Al2O3 films. Langmuir 2020, 36, 2794–2801. [Google Scholar]
- Akyildiz, H.I.; Diler, S.; Islam, S. Evaluation of TiO2 and ZnO atomic layer deposition coated polyamide 66 fabrics for photocatalytic activity and antibacterial applications. J. Vac. Sci. Technol. A 2021, 39, 022405. [Google Scholar] [CrossRef]
- Chiappim, W.; Neto, B.B.; Shiotani, M.; Karnopp, J.; Gonçalves, L.; Chaves, J.P.; Sobrinho, A.d.S.; Leitão, J.P.; Fraga, M.; Pessoa, R. Plasma-Assisted Nanofabrication: The Potential and Challenges in Atomic Layer Deposition and Etching. Nanomaterials 2022, 12, 3497. [Google Scholar] [CrossRef]
- Chen, J.; Pei, L.; Shi, W.; Yi, J.; Wang, J. Effect of Dispersant on Disperse Dyeing in Silicone Waterless Dyeing System. Polymers 2023, 15, 1046. [Google Scholar] [CrossRef]
- Tang, A.Y.; Wang, Y.M.; Lee, C.H.; Kan, C.-W. Computer color matching and levelness of PEG-based reverse micellar decamethyl cyclopentasiloxane (D5) solvent-assisted reactive dyeing on cotton fiber. Appl. Sci. 2017, 7, 682. [Google Scholar] [CrossRef]
- Tang, A.Y.L.; Lee, C.H.; Wang, Y.; Kan, C.W. Dyeing properties of cotton with reactive dye in nonane nonaqueous reverse micelle system. ACS Omega 2018, 3, 2812–2819. [Google Scholar] [CrossRef] [PubMed]
- Derbyshire, A.; Harvey, E.; Parr, D. Solvent-assisted Dyeing of Nylon 6.6 and Polyester Fibres. J. Soc. Dye. Colour. 1975, 91, 106–111. [Google Scholar] [CrossRef]
- Beal, W.; Dickinson, K.; Bellhouse, E. The Dyeing of Wool by Solvent-assisted Processes. J. Soc. Dye. Colour. 1960, 76, 333–341. [Google Scholar] [CrossRef]
- Tang, A.Y.; Kan, C.W. Non-aqueous dyeing of cotton fibre with reactive dyes: A review. Color. Technol. 2020, 136, 214–223. [Google Scholar] [CrossRef]
- Beal, W.; Corbishley, G. Dyeing and printing by solvent-assisted processes. J. Soc. Dye. Colour. 1971, 87, 329–334. [Google Scholar] [CrossRef]
- Banchero, M. Recent advances in supercritical fluid dyeing. Color. Technol. 2020, 136, 317–335. [Google Scholar] [CrossRef]
- Petkevičiūtė, J.; Sankauskaitė, A.; Jasulaitienė, V.; Varnaitė-Žuravliova, S.; Abraitienė, A. Impact of Low-Pressure Plasma Treatment of Wool Fabric for Dyeing with PEDOT: PSS. Materials 2022, 15, 4797. [Google Scholar] [CrossRef]
- Pillai, R.R.; Thomas, V. Plasma surface engineering of natural and sustainable polymeric derivatives and their potential applications. Polymers 2023, 15, 400. [Google Scholar] [CrossRef]
- Zheng, M.; Sun, Y.; Li, C.; Lu, Y.; Dai, Y.; Wang, Z. A novel and eco-friendly approach for dyeing polyester fabrics by liquid disperse dyes treated with deep eutectic solvent. Color. Technol. 2023, 139, 552–564. [Google Scholar]
- Mouro, C.; Gomes, A.P.; Costa, R.V.; Moghtader, F.; Gouveia, I.C. The sustainable bioactive dyeing of textiles: A novel strategy using bacterial pigments, natural antibacterial ingredients, and deep eutectic solvents. Gels 2023, 9, 800. [Google Scholar] [CrossRef]
- Liu, Y.; Song, K. Study on the Smart Dyeing and Performance of Poplar Veneers Modified by Deep Eutectic Solvents. Forests 2024, 15, 2120. [Google Scholar] [CrossRef]
- Martins, R.; Mouro, C.; Pontes, R.; Nunes, J.; Gouveia, I. Natural deep eutectic solvent extraction of bioactive pigments from spirulina platensis and electrospinning ability assessment. Polymers 2023, 15, 1574. [Google Scholar] [CrossRef] [PubMed]
- Hussain, N.; Hussain, S.; Mehdi, M.; Khatri, M.; Ullah, S.; Khatri, Z.; Van Langenhove, L.; Kim, I.S. Introducing deep eutectic solvents as a water-free dyeing medium for poly(1,4-cYclohexane dimethylene isosorbide terephthalate) PICT nanofibers. Polymers 2021, 13, 2594. [Google Scholar] [CrossRef]
- Negi, A. Cationized Cellulose Materials: Enhancing Surface Adsorption Properties Towards Synthetic and Natural Dyes. Polymers 2024, 17, 36. [Google Scholar] [CrossRef]
- Mosaad, R.M.; Alhalafi, M.H.; Emam, E.-A.M.; Ibrahim, M.A.; Ibrahim, H. Enhancement of antimicrobial and dyeing properties of cellulosic fabrics via chitosan nanoparticles. Polymers 2022, 14, 4211. [Google Scholar] [CrossRef]
- Fabiano, A.; Beconcini, D.; Migone, C.; Piras, A.M.; Zambito, Y. Quaternary ammonium chitosans: The importance of the positive fixed charge of the drug delivery systems. Int. J. Mol. Sci. 2020, 21, 6617. [Google Scholar] [CrossRef]
- Freitas, E.D.; Moura, C.F., Jr.; Kerwald, J.; Beppu, M.M. An overview of current knowledge on the properties, synthesis and applications of quaternary chitosan derivatives. Polymers 2020, 12, 2878. [Google Scholar] [CrossRef]
- Pathak, K.; Misra, S.K.; Sehgal, A.; Singh, S.; Bungau, S.; Najda, A.; Gruszecki, R.; Behl, T. Biomedical applications of quaternized chitosan. Polymers 2021, 13, 2514. [Google Scholar] [CrossRef]
- Negi, A.; Kesari, K.K. Chitosan nanoparticle encapsulation of antibacterial essential oils. Micromachines 2022, 13, 1265. [Google Scholar] [CrossRef] [PubMed]
- Sachdeva, B.; Sachdeva, P.; Negi, A.; Ghosh, S.; Han, S.; Dewanjee, S.; Jha, S.K.; Bhaskar, R.; Sinha, J.K.; Paiva-Santos, A.C. Chitosan nanoparticles-based cancer drug delivery: Application and challenges. Mar. Drugs 2023, 21, 211. [Google Scholar] [CrossRef]
- Bhattacharjee, R.; Negi, A.; Bhattacharya, B.; Dey, T.; Mitra, P.; Preetam, S.; Kumar, L.; Kar, S.; Das, S.S.; Iqbal, D. Nanotheranostics to target antibiotic-resistant bacteria: Strategies and applications. OpenNano 2023, 11, 100138. [Google Scholar] [CrossRef]
- Chowdhury, M.A.; Joshi, M.; Butola, B. Photochromic and thermochromic colorants in textile applications. J. Eng. Fibers Fabr. 2014, 9, 155892501400900113. [Google Scholar]
- Ibrahim, W. An Investigation into Textile Applications of Thermochromic Pigments; Heriot-Watt University: Edinburg, UK, 2012. [Google Scholar]
- Mohamed, M.; El-AAty, A.; Moawaed, S.; Hashad, A.; Abdel-Aziz, E.; Othman, H.; Hassabo, A.G. Smart textiles via photochromic and thermochromic colorant. J. Text. Color. Polym. Sci. 2022, 19, 235–243. [Google Scholar] [CrossRef]
- Cabral, I.; Santiago, D.; Steffens, F. Chromic textiles: Colour fastness properties and irreversible colour change behaviour of textiles screen printed with thermochromic, photochromic and hydrochromic colourants. Color. Technol. 2023, 139, 200–208. [Google Scholar] [CrossRef]
- Sahebkar, K.; Indrakar, S.; Srinivasan, S.; Thomas, S.; Stefanakos, E. Electrospun microfibers with embedded leuco dye-based thermochromic material for textile applications. J. Ind. Text. 2022, 51, 3188S–3200S. [Google Scholar] [CrossRef]
- Ramlow, H.; Andrade, K.L.; Immich, A.P.S. Smart textiles: An overview of recent progress on chromic textiles. J. Text. Inst. 2021, 112, 152–171. [Google Scholar] [CrossRef]
- Negi, A.; Mirallai, S.I.; Konda, S.; Murphy, P.V. An improved method for synthesis of non-symmetric triarylpyridines. Tetrahedron 2022, 121, 132930. [Google Scholar] [CrossRef]
- Kotha, S.; Meshram, M.; Panguluri, N.R.; Shah, V.R.; Todeti, S.; Shirbhate, M.E. Synthetic Approaches to Star-Shaped Molecules with 1, 3, 5-Trisubstituted Aromatic Cores. Chem.-Asian J. 2019, 14, 1356–1403. [Google Scholar] [CrossRef]
- Partal, R.; Basturk, I.; Hocaoglu, S.M.; Baban, A.; Yilmaz, E. Recovery of water and reusable salt solution from reverse osmosis brine in textile industry: A case study. Water Resour. Ind. 2022, 27, 100174. [Google Scholar] [CrossRef]
- Wakankar, D. Regulations relating to the use of textile dyes and chemicals. In Advances in the Dyeing and Finishing of Technical Textiles; Elsevier: Amsterdam, The Netherlands, 2013; pp. 105–132. [Google Scholar]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.-G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A critical review on the treatment of dye-containing wastewater: Ecotoxicological and health concerns of textile dyes and possible remediation approaches for environmental safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef]
- Ellafi, A.; Dali, A.; Mnif, S.; Ben Younes, S. Microbial Enzymatic Degradation, Spectral Analysis and Phytotoxicity Assessment of Congo Red Removal by Bacillus spp. Catal. Lett. 2023, 153, 3620–3633. [Google Scholar]
- Slama, H.B.; Chenari Bouket, A.; Pourhassan, Z.; Alenezi, F.N.; Silini, A.; Cherif-Silini, H.; Oszako, T.; Luptakova, L.; Golińska, P.; Belbahri, L. Diversity of synthetic dyes from textile industries, discharge impacts and treatment methods. Appl. Sci. 2021, 11, 6255. [Google Scholar] [CrossRef]
- Castro, A.M.; Nogueira, V.; Lopes, I.; Rocha-Santos, T.; Pereira, R. Evaluation of the potential toxicity of effluents from the textile industry before and after treatment. Appl. Sci. 2019, 9, 3804. [Google Scholar] [CrossRef]
- Bailey, K.; Basu, A.; Sharma, S. The environmental impacts of fast fashion on water quality: A systematic review. Water 2022, 14, 1073. [Google Scholar] [CrossRef]
- Magalhães, S.; Paciência, D.; Rodrigues, J.M.; Lindman, B.; Alves, L.; Medronho, B.; Rasteiro, M.d.G. Insights on Microplastic Contamination from Municipal and Textile Industry Effluents and Their Removal Using a Cellulose-Based Approach. Polymers 2024, 16, 2803. [Google Scholar] [CrossRef]
- Klemola, K. Textile Toxicity: Cytotoxicity and Spermatozoa Motility Inhibition Resulting from Reactive Dyes and Dyed Fabrics (Tekstiilien Toksisuus; Reaktiiviväreistä ja Värjätyistä Kankaista Aiheutuva Sytotoksisuus ja Siittiöiden Heikko Liikkuvuus); Kuopion Yliopisto: Kuopio, Finland, 2008. [Google Scholar]
- Leme, D.M.; Primo, F.L.; Gobo, G.G.; da Costa, C.R.V.; Tedesco, A.C.; de Oliveira, D.P. Genotoxicity assessment of reactive and disperse textile dyes using human dermal equivalent (3D cell culture system). J. Toxicol. Environ. Health Part A 2015, 78, 466–480. [Google Scholar]
- Hemashenpagam, N.; Selvajeyanthi, S. Textile dyes and their effect on human beings. In Nanohybrid Materials for Treatment of Textiles Dyes; Springer: Berlin/Heidelberg, Germany, 2023; pp. 41–60. [Google Scholar]
- Schneider, K.; Hafner, C.; Jäger, I. Mutagenicity of textile dye products. J. Appl. Toxicol. Int. J. 2004, 24, 83–91. [Google Scholar]
- Fernandes, F.H.; Bustos-Obregon, E.; Salvadori, D.M.F. Disperse Red 1 (textile dye) induces cytotoxic and genotoxic effects in mouse germ cells. Reprod. Toxicol. 2015, 53, 75–81. [Google Scholar] [CrossRef]
- Ramamurthy, K.; Priya, P.S.; Murugan, R.; Arockiaraj, J. Hues of risk: Investigating genotoxicity and environmental impacts of azo textile dyes. Environ. Sci. Pollut. Res. 2024, 31, 33190–33211. [Google Scholar] [CrossRef] [PubMed]
- Chequer, F.D.; Dorta, D.J.; de Oliveira, D.P. Azo dyes and their metabolites: Does the discharge of the azo dye into water bodies represent human and ecological risks. Adv. Treat. Text. Effl. 2011, 48, 28–48. [Google Scholar]
- Islam, T.; Repon, M.R.; Islam, T.; Sarwar, Z.; Rahman, M.M. Impact of textile dyes on health and ecosystem: A review of structure, causes, and potential solutions. Environ. Sci. Pollut. Res. 2023, 30, 9207–9242. [Google Scholar] [CrossRef]
- Horobin, R.W. Where do dyes go inside living cells? Predicting uptake, intracellular localisation, and accumulation using QSAR models. Color. Technol. 2014, 130, 155–173. [Google Scholar] [CrossRef]
- Horobin, R.W.; Stockert, J.C.; Zhang, H. Reactive dyes for living cells: Applications, artefacts, and some comparisons with textile dyeing. Color. Technol. 2022, 138, 3–15. [Google Scholar] [CrossRef]
- Dönmez, G. Bioaccumulation of the reactive textile dyes by Candida tropicalis growing in molasses medium. Enzym. Microb. Technol. 2002, 30, 363–366. [Google Scholar] [CrossRef]
- Aksu, Z. Reactive dye bioaccumulation by Saccharomyces cerevisiae. Process Biochem. 2003, 38, 1437–1444. [Google Scholar] [CrossRef]
- Lellis, B.; Fávaro-Polonio, C.Z.; Pamphile, J.A.; Polonio, J.C. Effects of textile dyes on health and the environment and bioremediation potential of living organisms. Biotechnol. Res. Innov. 2019, 3, 275–290. [Google Scholar] [CrossRef]
- Mastrangelo, G.; Fedeli, U.; Fadda, E.; Milan, G.; Lange, J.H. Epidemiologic evidence of cancer risk in textile industry workers: A review and update. Toxicol. Ind. Health 2002, 18, 171–181. [Google Scholar] [CrossRef]
- Mathur, N.; Bhatnagar, P.; Sharma, P. Review of the mutagenicity of textile dye products. Univers. J. Environ. Res. Technol. 2012, 2, 1–18. [Google Scholar]
- Hagihara, R.; Katsuyama, Y.; Sugai, Y.; Onaka, H.; Ohnishi, Y. Novel desferrioxamine derivatives synthesized using the secondary metabolism-specific nitrous acid biosynthetic pathway in Streptomyces davawensis. J. Antibiot. 2018, 71, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.; Zeyad, M.T.; Malik, A. Genotoxicity assessment of textile waste contaminated soil and characterization of textile dye degradation by a novel indigenous bacterium Ochrobactrum intermedium BS39. Chemosphere 2022, 299, 134082. [Google Scholar] [CrossRef] [PubMed]
- Garcia, V.S.G.; de Freitas Tallarico, L.; Rosa, J.M.; Suzuki, C.F.; Roubicek, D.A.; Nakano, E.; Borrely, S.I. Multiple adverse effects of textile effluents and reactive Red 239 dye to aquatic organisms. Environ. Sci. Pollut. Res. 2021, 28, 63202–63214. [Google Scholar]
- Kusumlata; Ambade, B.; Kumar, A.; Gautam, S. Sustainable solutions: Reviewing the future of textile dye contaminant removal with emerging biological treatments. Limnol. Rev. 2024, 24, 126–149. [Google Scholar] [CrossRef]
- Ishak, S.A.; Murshed, M.F.; Md Akil, H.; Ismail, N.; Md Rasib, S.Z.; Al-Gheethi, A.A.S. The application of modified natural polymers in toxicant dye compounds wastewater: A review. Water 2020, 12, 2032. [Google Scholar] [CrossRef]
- Jabar, J.M.; Odusote, Y.A. Removal of cibacron blue 3G-A (CB) dye from aqueous solution using chemo-physically activated biochar from oil palm empty fruit bunch fiber. Arab. J. Chem. 2020, 13, 5417–5429. [Google Scholar]
- Guo, D.; Li, Y.; Cui, B.; Hu, M.; Luo, S.; Ji, B.; Liu, Y. Natural adsorption of methylene blue by waste fallen leaves of Magnoliaceae and its repeated thermal regeneration for reuse. J. Clean. Prod. 2020, 267, 121903. [Google Scholar]
- Cagide, C.; Marizcurrena, J.J.; Vallés, D.; Alvarez, B.; Castro-Sowinski, S. A bacterial cold-active dye-decolorizing peroxidase from an Antarctic Pseudomonas strain. Appl. Microbiol. Biotechnol. 2023, 107, 1707–1724. [Google Scholar]
- Solís, M.; Solís, A.; Pérez, H.I.; Manjarrez, N.; Flores, M. Microbial decolouration of azo dyes: A review. Process Biochem. 2012, 47, 1723–1748. [Google Scholar]
- Fernandes, C.D.; Nascimento, V.R.S.; Meneses, D.B.; Vilar, D.S.; Torres, N.H.; Leite, M.S.; Baudrit, J.R.V.; Bilal, M.; Iqbal, H.M.; Bharagava, R.N. Fungal biosynthesis of lignin-modifying enzymes from pulp wash and Luffa cylindrica for azo dye RB5 biodecolorization using modeling by response surface methodology and artificial neural network. J. Hazard. Mater. 2020, 399, 123094. [Google Scholar]
- Liu, Z.; Khan, T.A.; Islam, M.A.; Tabrez, U. A review on the treatment of dyes in printing and dyeing wastewater by plant biomass carbon. Bioresour. Technol. 2022, 354, 127168. [Google Scholar] [CrossRef] [PubMed]
- Sharma, M.; Sharma, S.; Alkhanjaf, A.A.M.; Arora, N.K.; Saxena, B.; Umar, A.; Ibrahim, A.A.; Akhtar, M.S.; Mahajan, A.; Negi, S. Microbial fuel cells for azo dye degradation: A perspective review. J. Ind. Eng. Chem. 2024, 142, 45–67. [Google Scholar]
- Urbaniak, M.; Przystaś, W.; Zabłocka-Godlewska, E.; Stępień, Ł.; Janusz, G. Decolorization of azo and triphenylmethane dyes by MW113 Beauveria bassiana strain. Desalination Water Treat. 2018, 136, 422–432. [Google Scholar] [CrossRef]
- Chen, K.; Huang, W.; Wu, J.; Houng, J. Microbial decolorization of azo dyes by Proteus mirabilis. J. Ind. Microbiol. Biotechnol. 1999, 23, 686–690. [Google Scholar] [CrossRef]
- Manogaran, M.; Yasid, N.A.; Othman, A.R.; Gunasekaran, B.; Halmi, M.I.E.; Shukor, M.Y.A. Biodecolourisation of Reactive Red 120 as a sole carbon source by a bacterial consortium—Toxicity assessment and statistical optimisation. Int. J. Environ. Res. Public Health 2021, 18, 2424. [Google Scholar] [CrossRef]
- Sarim, K.M.; Kukreja, K.; Shah, I.; Choudhary, C.K. Biosorption of direct textile dye Congo red by Bacillus subtilis HAU-KK01. Bioremediation J. 2019, 23, 185–195. [Google Scholar] [CrossRef]
- Danouche, M.; El Arroussi, H.; Bahafid, W.; El Ghachtouli, N. An overview of the biosorption mechanism for the bioremediation of synthetic dyes using yeast cells. Environ. Technol. Rev. 2021, 10, 58–76. [Google Scholar]
- Varjani, S.; Rakholiya, P.; Ng, H.Y.; You, S.; Teixeira, J.A. Microbial degradation of dyes: An overview. Bioresour. Technol. 2020, 314, 123728. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, J.; Yin, W.; Zhang, L.; Li, L.; Chen, T.; Ding, C.; Liu, W.; Wang, A.; Chen, F. In situ coupling of reduction and oxidation processes with alternating current-driven bioelectrodes for efficient mineralization of refractory pollutants. Engineering 2024, 43, 125–138. [Google Scholar] [CrossRef]
- Muliadi, F.N.A.; Halmi, M.I.E.; Wahid, S.B.A.; Gani, S.S.A.; Zaidan, U.H.; Mahmud, K.; Abd Shukor, M.Y. Biostimulation of microbial communities from Malaysian agricultural soil for detoxification of metanil yellow dye; a response surface methodological approach. Sustainability 2020, 13, 138. [Google Scholar] [CrossRef]
- Roșu, C.M.; Vochița, G.; Mihășan, M.; Avădanei, M.; Mihai, C.T.; Gherghel, D. Performances of Pichia kudriavzevii in decolorization, biodegradation, and detoxification of CI Basic Blue 41 under optimized cultural conditions. Environ. Sci. Pollut. Res. 2019, 26, 431–445. [Google Scholar] [CrossRef] [PubMed]
- Senthilkumar, S.; Perumalsamy, M.; Prabhuy, H.J.; AhmedBasha, C.; Anantharaman, N. Response surface optimization for efficient dye removal by isolated strain Pseudomonas sp. Cent. Eur. J. Eng. 2012, 2, 425–434. [Google Scholar]
- Simelane, L.P.; Fosso-Kankeu, E.; Njobeh, P.; Pandey, S. Response of bacterial biosorbents to chemical treatment as influenced by cell membrane structure and impact on the adsorption behaviour of dyes. Curr. Sci. 2018, 114, 826–834. [Google Scholar]
- Mohan, C.; Robinson, J.; Negi, A. Ion-Selective Electrode (ISE) based on polyvinyl chloride membrane formed from heterocyclic quinazoline compounds as ionophore material. Eng. Proc. 2023, 48, 10. [Google Scholar] [CrossRef]
- Biswas, S.; Basak, P. Biosorption of the industrial dye remazol brilliant blue R by Bacillus rigiliprofundi. Microbiology 2021, 90, 816–828. [Google Scholar]
- Aracagök, Y.D. Biosorption of Remazol Brilliant Blue R dye onto chemically modified and unmodified Yarrowia lipolytica biomass. Arch. Microbiol. 2022, 204, 128. [Google Scholar] [CrossRef]
- Al-Ansari, M.M.; Li, Z.; Masood, A.; Rajaselvam, J. Decolourization of azo dye using a batch bioreactor by an indigenous bacterium Enterobacter aerogenes ES014 from the waste water dye effluent and toxicity analysis. Environ. Res. 2022, 205, 112189. [Google Scholar]
- Velayutham, K.; Madhava, A.K.; Pushparaj, M.; Thanarasu, A.; Devaraj, T.; Periyasamy, K.; Subramanian, S. Biodegradation of Remazol Brilliant Blue R using isolated bacterial culture (Staphylococcus sp. K2204). Environ. Technol. 2018, 39, 2900–2907. [Google Scholar] [CrossRef]
- Akansha, K.; Chakraborty, D.; Sachan, S.G. Decolorization and degradation of methyl orange by Bacillus stratosphericus SCA1007. Biocatal. Agric. Biotechnol. 2019, 18, 101044. [Google Scholar]
- Singh, G.; Dwivedi, S. Decolorization and degradation of Direct Blue-1 (Azo dye) by newly isolated fungus Aspergillus terreus GS28, from sludge of carpet industry. Environ. Technol. Innov. 2020, 18, 100751. [Google Scholar]
- Srinivasan, S.; Sadasivam, S.K. Biodegradation of textile azo dyes by textile effluent non-adapted and adapted Aeromonas hydrophila. Environ. Res. 2021, 194, 110643. [Google Scholar] [CrossRef]
- Phan, K.-H.; Le, L.-T.; Tran, T.-D.; Nguyen, T.-T.; Tra, V.-T.; Tran, C.-S.; Mai, T.-P.; Bui, X.-T. Anaerobic biodegradation of mixed azo dyes in thermophilic and mesophilic conditions. Case Stud. Chem. Environ. Eng. 2024, 9, 100667. [Google Scholar] [CrossRef]
- Li, Q.; Feng, X.-L.; Li, T.-T.; Lu, X.-R.; Liu, Q.-Y.; Han, X.; Feng, Y.-J.; Liu, Z.-Y.; Zhang, X.-J.; Xiao, X. Anaerobic decolorization and detoxification of cationic red X-GRL by Shewanella oneidensis MR-1. Environ. Technol. 2018, 39, 2382–2389. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Watari, T.; Vo, T.T.; Hatamoto, M.; Setiadi, T.; Yamaguchi, T. Enhancement of azo dye anaerobic bio-treatment performance with ferroferric oxide supplement. J. Environ. Chem. Eng. 2022, 10, 108350. [Google Scholar] [CrossRef]
- Zafar, S.; Bukhari, D.A.; Rehman, A. Azo dyes degradation by microorganisms—An efficient and sustainable approach. Saudi J. Biol. Sci. 2022, 29, 103437. [Google Scholar] [CrossRef] [PubMed]
- Das, A.; Mishra, S. Complete biodegradation of azo dye in an integrated microbial fuel cell-aerobic system using novel bacterial consortium. Int. J. Environ. Sci. Technol. 2019, 16, 1069–1078. [Google Scholar] [CrossRef]
- Seyedi, Z.S.; Zahraei, Z.; Jookar Kashi, F. Decolorization of reactive black 5 and reactive red 152 Azo dyes by new haloalkaliphilic bacteria isolated from the textile wastewater. Curr. Microbiol. 2020, 77, 2084–2092. [Google Scholar] [CrossRef] [PubMed]
- Fareed, A.; Zaffar, H.; Bilal, M.; Hussain, J.; Jackson, C.; Naqvi, T.A. Decolorization of azo dyes by a novel aerobic bacterial strain Bacillus cereus strain ROC. PLoS ONE 2022, 17, e0269559. [Google Scholar] [CrossRef]
- Jayapal, M.; Jagadeesan, H.; Shanmugam, M.; Murugesan, S. Sequential anaerobic-aerobic treatment using plant microbe integrated system for degradation of azo dyes and their aromatic amines by-products. J. Hazard. Mater. 2018, 354, 231–243. [Google Scholar] [CrossRef]
- Franca, R.D.G.; Vieira, A.; Carvalho, G.; Oehmen, A.; Pinheiro, H.M.; Crespo, M.T.B.; Lourenco, N.D. Oerskovia paurometabola can efficiently decolorize azo dye Acid Red 14 and remove its recalcitrant metabolite. Ecotoxicol. Environ. Saf. 2020, 191, 110007. [Google Scholar] [CrossRef]
- Tizazu, S.; Tesfaye, G.; Andualem, B.; Wang, A.; Guadie, A. Evaluating the potential of thermo-alkaliphilic microbial consortia for azo dye biodegradation under anaerobic-aerobic conditions: Optimization and microbial diversity analysis. J. Environ. Manag. 2022, 323, 116235. [Google Scholar]
- Kaur, J.; Mudgal, G.; Negi, A.; Tamang, J.; Singh, S.; Singh, G.B.; Bose, K.J.C.; Debnath, S.; Wadaan, M.A.; Farooq Khan, M. Reactive black-5, Congo red and methyl orange: Chemical degradation of azo-dyes by agrobacterium. Water 2023, 15, 1664. [Google Scholar] [CrossRef]
- Mandic, M.; Djokic, L.; Nikolaivits, E.; Prodanovic, R.; O’Connor, K.; Jeremic, S.; Topakas, E.; Nikodinovic-Runic, J. Identification and characterization of new laccase biocatalysts from Pseudomonas species suitable for degradation of synthetic textile dyes. Catalysts 2019, 9, 629. [Google Scholar] [CrossRef]
- Bu, T.; Yang, R.; Zhang, Y.; Cai, Y.; Tang, Z.; Li, C.; Wu, Q.; Chen, H. Improving decolorization of dyes by laccase from Bacillus licheniformis by random and site-directed mutagenesis. PeerJ 2020, 8, e10267. [Google Scholar]
- Arregui, L.; Ayala, M.; Gómez-Gil, X.; Gutiérrez-Soto, G.; Hernández-Luna, C.E.; Herrera De Los Santos, M.; Levin, L.; Rojo-Domínguez, A.; Romero-Martínez, D.; Saparrat, M.C. Laccases: Structure, function, and potential application in water bioremediation. Microb. Cell Factories 2019, 18, 200. [Google Scholar]
- Polak, J.; Wlizło, K.; Pogni, R.; Petricci, E.; Grąz, M.; Szałapata, K.; Osińska-Jaroszuk, M.; Kapral-Piotrowska, J.; Pawlikowska-Pawlęga, B.; Jarosz-Wilkołazka, A. Structure and bioactive properties of novel textile dyes synthesised by fungal laccase. Int. J. Mol. Sci. 2020, 21, 2052. [Google Scholar] [CrossRef]
- Gałązka, A.; Jankiewicz, U.; Szczepkowski, A. Biochemical characteristics of laccases and their practical application in the removal of xenobiotics from water. Appl. Sci. 2023, 13, 4394. [Google Scholar] [CrossRef]
- Janusz, G.; Skwarek, E.; Pawlik, A. Potential of laccase as a tool for biodegradation of wastewater micropollutants. Water 2023, 15, 3770. [Google Scholar] [CrossRef]
- Bento, R.M.; Almeida, M.R.; Bharmoria, P.; Freire, M.G.; Tavares, A.P. Improvements in the enzymatic degradation of textile dyes using ionic-liquid-based surfactants. Sep. Purif. Technol. 2020, 235, 116191. [Google Scholar]
- Rodríguez, O.; Cristóvão, R.O.; Tavares, A.P.; Macedo, E.A. Study of the alkyl chain length on laccase stability and enzymatic kinetic with imidazolium ionic liquids. Appl. Biochem. Biotechnol. 2011, 164, 524–533. [Google Scholar]
- Pârvulescu, V.I.; Hardacre, C. Catalysis in ionic liquids. Chem. Rev. 2007, 107, 2615–2665. [Google Scholar] [CrossRef] [PubMed]
- Savelli, G.; Spreti, N.; Di Profio, P. Enzyme activity and stability control by amphiphilic self-organizing systems in aqueous solutions. Curr. Opin. Colloid Interface Sci. 2000, 5, 111–117. [Google Scholar]
- Liu, H.; Wu, X.; Sun, J.; Chen, S. Stimulation of laccase biocatalysis in ionic liquids: A review on recent progress. Curr. Protein Pept. Sci. 2018, 19, 100–111. [Google Scholar] [PubMed]
- Iark, D.; dos Reis Buzzo, A.J.; Garcia, J.A.A.; Côrrea, V.G.; Helm, C.V.; Corrêa, R.C.G.; Peralta, R.A.; Moreira, R.d.F.P.M.; Bracht, A.; Peralta, R.M. Enzymatic degradation and detoxification of azo dye Congo red by a new laccase from Oudemansiella canarii. Bioresour. Technol. 2019, 289, 121655. [Google Scholar]
- Srinivasan, S.; Sadasivam, S.K. Exploring docking and aerobic-microaerophilic biodegradation of textile azo dye by bacterial systems. J. Water Process Eng. 2018, 22, 180–191. [Google Scholar] [CrossRef]
- Saxena, A.; Garg, S.; Verma, J. Simultaneous saccharification and fermentation of waste newspaper to ethanol. Bioresour. Technol. 1992, 42, 13–15. [Google Scholar]
- Andren, R.K.; Erickson, R.; Medeiros, J. Cellulosic substrates for enzymatic saccharification. Biotechnol. Bioeng. Symp. 1976, 6. Available online: https://www.osti.gov/biblio/5134813 (accessed on 1 January 1976).
- Ghosh, P.; Pamment, N.; Martin, W. Simultaneous saccharification and fermentation of cellulose: Effect of β-D-glucosidase activity and ethanol inhibition of cellulases. Enzym. Microb. Technol. 1982, 4, 425–430. [Google Scholar]
- Ooshima, H.; Ishitani, Y.; Harano, Y. Simultaneous saccharification and fermentation of cellulose: Effect of ethanol on enzymatic saccharification of cellulose. Biotechnol. Bioeng. 1985, 27, 389–397. [Google Scholar]
- Kuo, C.-H.; Lin, P.-J.; Wu, Y.-Q.; Ye, L.-Y.; Yang, D.-J.; Shieh, C.-J.; Lee, C.-K. Simultaneous saccharification and fermentation of waste textiles for ethanol production. BioResources 2014, 9, 2866–2875. [Google Scholar]
- Quartinello, F.; Vecchiato, S.; Weinberger, S.; Kremenser, K.; Skopek, L.; Pellis, A.; Guebitz, G.M. Highly selective enzymatic recovery of building blocks from wool-cotton-polyester textile waste blends. Polymers 2018, 10, 1107. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Ogiso, M.; Minoura, N. Enzymatic degradation behavior of porous silk fibroin sheets. Biomaterials 2003, 24, 357–365. [Google Scholar]
- Navone, L.; Moffitt, K.; Hansen, K.-A.; Blinco, J.; Payne, A.; Speight, R. Closing the textile loop: Enzymatic fibre separation and recycling of wool/polyester fabric blends. Waste Manag. 2020, 102, 149–160. [Google Scholar]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Ragaert, K.; Delva, L.; Van Geem, K. Mechanical and chemical recycling of solid plastic waste. Waste Manag. 2017, 69, 24–58. [Google Scholar] [CrossRef] [PubMed]
- Marten, E.; Müller, R.-J.; Deckwer, W.-D. Studies on the enzymatic hydrolysis of polyesters. II. Aliphatic–aromatic copolyesters. Polym. Degrad. Stab. 2005, 88, 371–381. [Google Scholar]
- Haske-Cornelius, O.; Pellis, A.; Tegl, G.; Wurz, S.; Saake, B.; Ludwig, R.; Sebastian, A.; Nyanhongo, G.S.; Guebitz, G.M. Enzymatic systems for cellulose acetate degradation. Catalysts 2017, 7, 287. [Google Scholar] [CrossRef]
- Wei, R.; Oeser, T.; Barth, M.; Weigl, N.; Lübs, A.; Schulz-Siegmund, M.; Hacker, M.C.; Zimmermann, W. Turbidimetric analysis of the enzymatic hydrolysis of polyethylene terephthalate nanoparticles. J. Mol. Catal. B Enzym. 2014, 103, 72–78. [Google Scholar]
- Tournier, V.; Topham, C.; Gilles, A.; David, B.; Folgoas, C.; Moya-Leclair, E.; Kamionka, E.; Desrousseaux, M.-L.; Texier, H.; Gavalda, S. An engineered PET depolymerase to break down and recycle plastic bottles. Nature 2020, 580, 216–219. [Google Scholar]
- Ronkvist, Å.M.; Xie, W.; Lu, W.; Gross, R.A. Cutinase-catalyzed hydrolysis of poly (ethylene terephthalate). Macromolecules 2009, 42, 5128–5138. [Google Scholar]
- Egan, J.; Salmon, S. Strategies and progress in synthetic textile fiber biodegradability. SN Appl. Sci. 2022, 4, 22. [Google Scholar]
- Nagai, K.; Iida, K.; Shimizu, K.; Kinugasa, R.; Izumi, M.; Kato, D.-I.; Takeo, M.; Mochiji, K.; Negoro, S. Enzymatic hydrolysis of nylons: Quantification of the reaction rate of nylon hydrolase for thin-layered nylons. Appl. Microbiol. Biotechnol. 2014, 98, 8751–8761. [Google Scholar] [PubMed]
- Gashti, M.P.; Assefipour, R.; Kiumarsi, A.; Gashti, M.P. Enzymatic surface hydrolysis of polyamide 6, 6 with mixtures of proteolytic and lipolytic enzymes. Prep. Biochem. Biotechnol. 2013, 43, 798–814. [Google Scholar]
- Weinberger, S.; Canadell, J.; Quartinello, F.; Yeniad, B.; Arias, A.; Pellis, A.; Guebitz, G.M. Enzymatic degradation of poly (ethylene 2, 5-furanoate) powders and amorphous films. Catalysts 2017, 7, 318. [Google Scholar]
- Biundo, A.; Hromic, A.; Pavkov-Keller, T.; Gruber, K.; Quartinello, F.; Haernvall, K.; Perz, V.; Arrell, M.S.; Zinn, M.; Ribitsch, D. Characterization of a poly (butylene adipate-co-terephthalate)-hydrolyzing lipase from Pelosinus fermentans. Appl. Microbiol. Biotechnol. 2016, 100, 1753–1764. [Google Scholar] [CrossRef] [PubMed]
- Mao, H.; Liu, H.; Gao, Z.; Su, T.; Wang, Z. Biodegradation of poly (butylene succinate) by Fusarium sp. FS1301 and purification and characterization of poly (butylene succinate) depolymerase. Polym. Degrad. Stab. 2015, 114, 1–7. [Google Scholar]
- Shirahama, H.; Mizuma, K.; Umemoto, K.; Yasuda, H. Synthesis of poly (lactide-ran-MOHEL) and its biodegradation with proteinase K. J. Polym. Sci. Part A Polym. Chem. 2001, 39, 1374–1381. [Google Scholar]
- Richert, A.; Olewnik-Kruszkowska, E.; Adamska, E.; Tarach, I. Enzymatic degradation of bacteriostatic polylactide composites. Int. Biodeterior. Biodegrad. 2019, 142, 103–108. [Google Scholar]
- Sawada, K.; Urakawa, H.; Ueda, M. Modification of polylactic acid fiber with enzymatic treatment. Text. Res. J. 2007, 77, 901–905. [Google Scholar]
- Baker, P.J.; Poultney, C.; Liu, Z.; Gross, R.; Montclare, J.K. Identification and comparison of cutinases for synthetic polyester degradation. Appl. Microbiol. Biotechnol. 2012, 93, 229–240. [Google Scholar]
- Matamá, T.; Vaz, F.; Gübitz, G.M.; Cavaco-Paulo, A. The effect of additives and mechanical agitation in surface modification of acrylic fibres by cutinase and esterase. Biotechnol. J. Healthc. Nutr. Technol. 2006, 1, 842–849. [Google Scholar]
- Fischer-Colbrie, G.; Matama, T.; Heumann, S.; Martinkova, L.; Paulo, A.C.; Guebitz, G. Surface hydrolysis of polyacrylonitrile with nitrile hydrolysing enzymes from Micrococcus luteus BST20. J. Biotechnol. 2007, 129, 62–68. [Google Scholar] [PubMed]
- Matamá, T.; Carneiro, F.; Caparrós, C.; Gübitz, G.M.; Cavaco-Paulo, A. Using a nitrilase for the surface modification of acrylic fibres. Biotechnol. J. Healthc. Nutr. Technol. 2007, 2, 353–360. [Google Scholar] [CrossRef] [PubMed]




| Textile Dyes | ||||||
|---|---|---|---|---|---|---|
| Nature | Non-Ionic | Cationic | Anionic | |||
| Disperse | Vat or Sulfur dyes | Acid | Direct | Reactive | ||
| Aqueous solubility | Insoluble | Insoluble | Soluble | Soluble | Soluble | Soluble |
| Fiber type | Synthetic fibers (polyester) | Cellulose | Wool Acrylic | Wool Silk Nylon | Cellulose | Cellulose |
| Color Spectrum * | Vibrant | Deep, Rich | Bright | Bright, vibrant | Dull | Bright |
| Chemical Auxiliaries | Dispersing agents, Leveling agents, Buffering agents | Reducing agents, Alkaline agents, Oxidising agents | Leveling agents, Acidic agents, Fixatives | Acidic agents, Leveling agents, Salts | Leveling agents, Salts pH adjusters | Salts, Alkaline agents, Fixatives |
| Fastness Properties * | Excellent fastness | Excellent wash fastness | Moderate-to-good fastness | Moderate-to-good fastness | Poor fastness | Excellent fastness |
| Examples | Disperse Yellow 3, Disperse Red 1, Disperse Blue 35, Disperse Blue 56, Disperse Violet 26, | Vat Blue 1, Vat Blue 4, Vat Green 1, Sulfur Black 1, Sulfur Yellow 3, | Cationic Blue 3 Cationic Yellow 2 Cationic Red 2 Cationic Violet 5 Cationic Green 1 | Acid Blue 113 Acid Red 27 Acid Yellow 17 Acid Green 25 | Direct Blue 1 Direct Red 28 Direct Yellow 86 Direct Green 6 | Reactive Blue 239 Reactive Red 180 Reactive Black 5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Negi, A. Environmental Impact of Textile Materials: Challenges in Fiber–Dye Chemistry and Implication of Microbial Biodegradation. Polymers 2025, 17, 871. https://doi.org/10.3390/polym17070871
Negi A. Environmental Impact of Textile Materials: Challenges in Fiber–Dye Chemistry and Implication of Microbial Biodegradation. Polymers. 2025; 17(7):871. https://doi.org/10.3390/polym17070871
Chicago/Turabian StyleNegi, Arvind. 2025. "Environmental Impact of Textile Materials: Challenges in Fiber–Dye Chemistry and Implication of Microbial Biodegradation" Polymers 17, no. 7: 871. https://doi.org/10.3390/polym17070871
APA StyleNegi, A. (2025). Environmental Impact of Textile Materials: Challenges in Fiber–Dye Chemistry and Implication of Microbial Biodegradation. Polymers, 17(7), 871. https://doi.org/10.3390/polym17070871






