A High-Temperature-Resistant and Conductive Flexible Silicone Rubber with High Phenyl Content Based on Silver-Coated Glass Fibers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
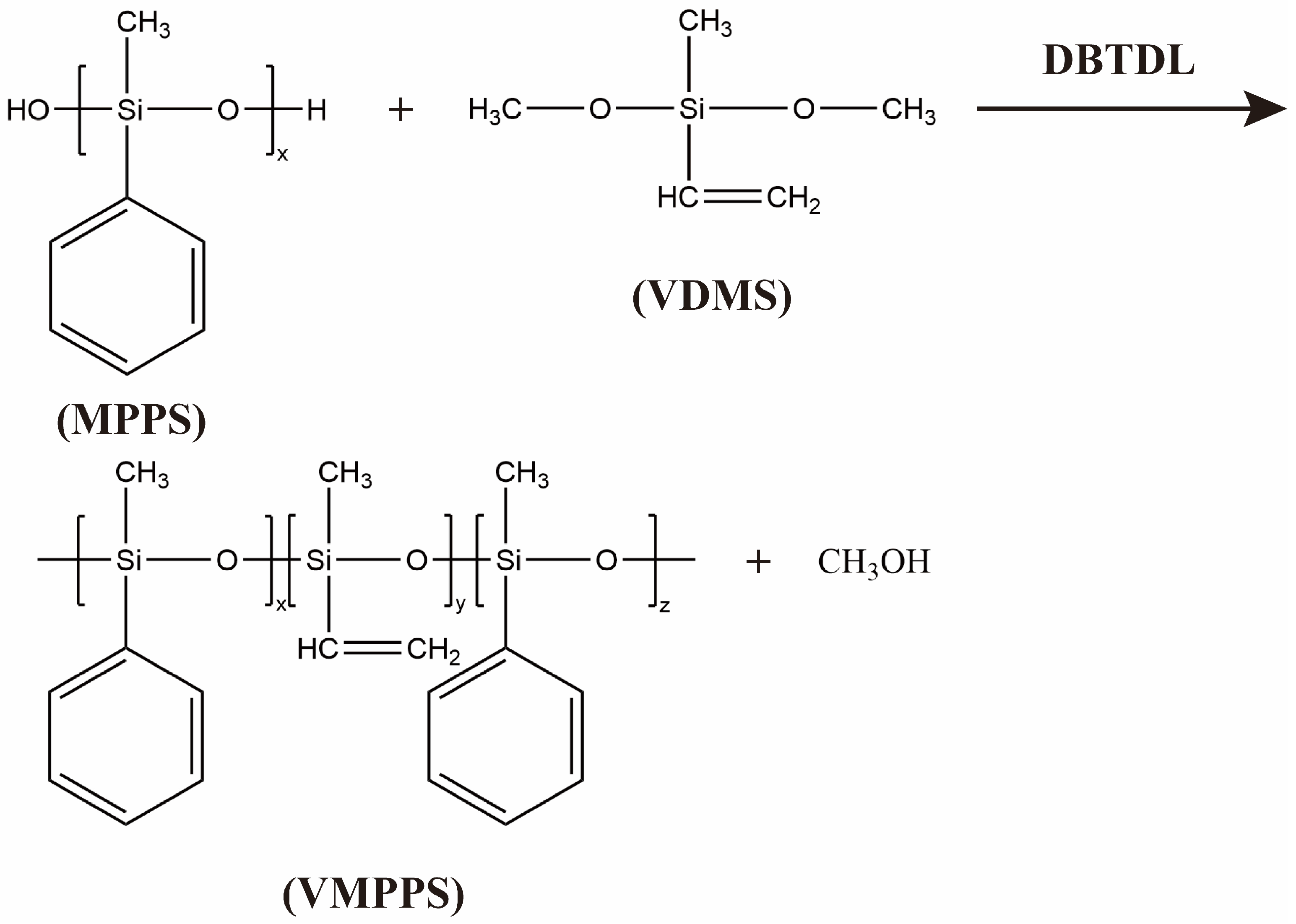
2.2. Preparation of Vinyl Methyl Phenyl Polysiloxane
2.3. Preparation of Chemically Plated Silver Glass Fibers
2.4. Preparation of Phenyl Silicone Rubber
2.5. Characterization
3. Results and Discussion
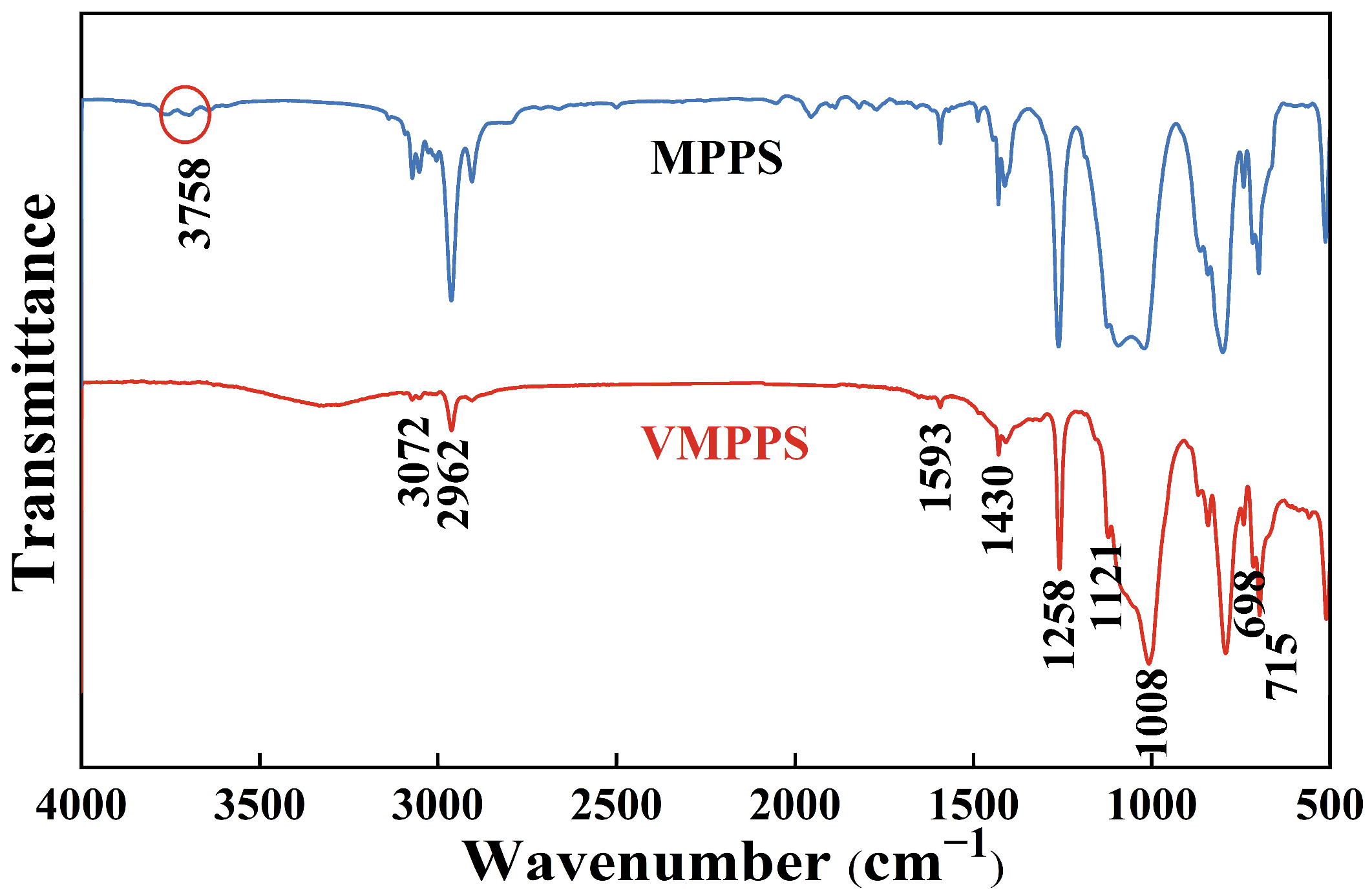
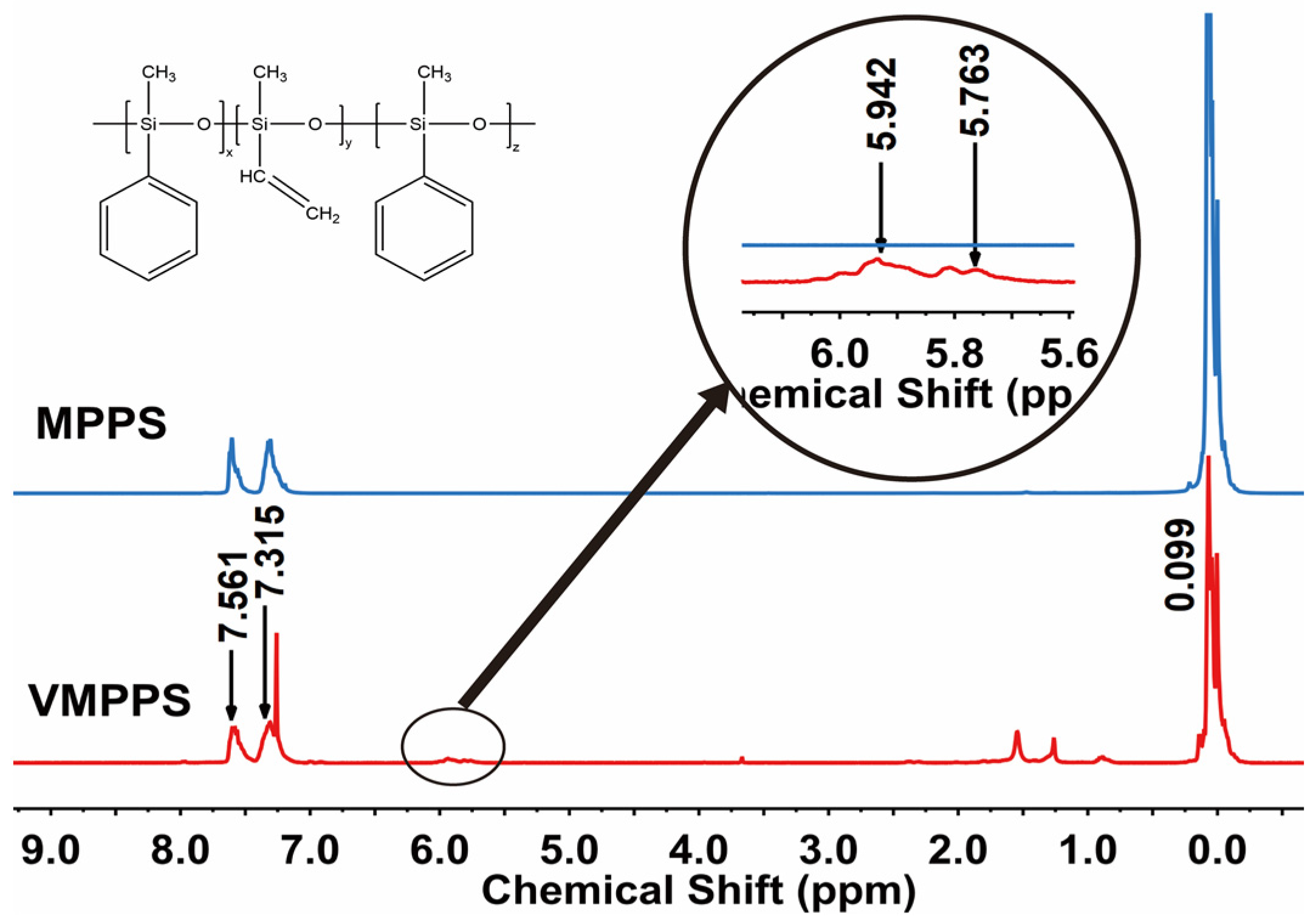
3.1. Preparation and Structural Characterization of Vinyl Methyl Phenyl Polysiloxane
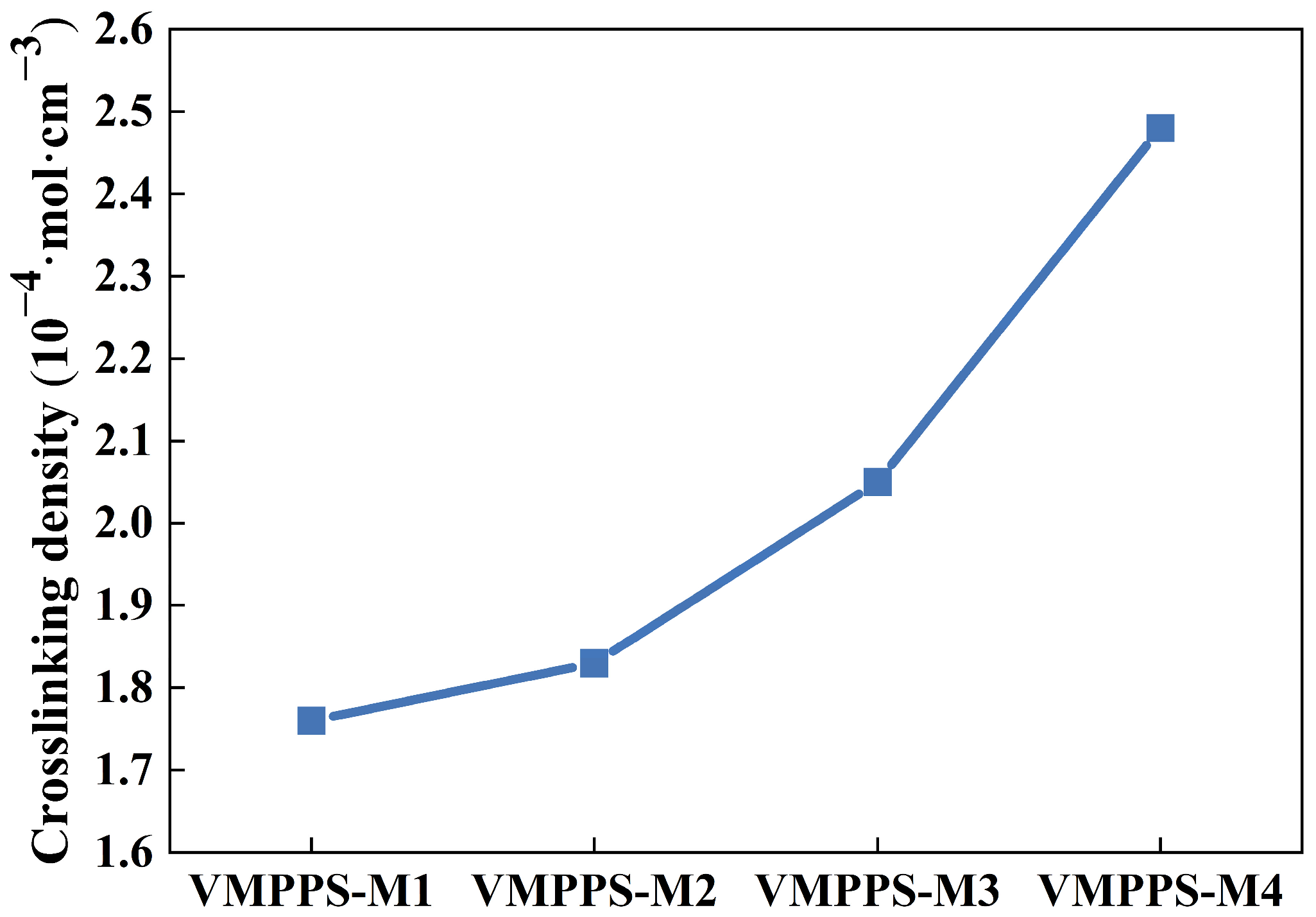
3.2. Curing Characteristics and Crosslinking Density of Phenyl Silicone Rubber with Different Molecular Weights and Vinyl Contents
3.3. The Processability of Phenyl Silicone Rubber with Different Molecular Weights and Vinyl Contents
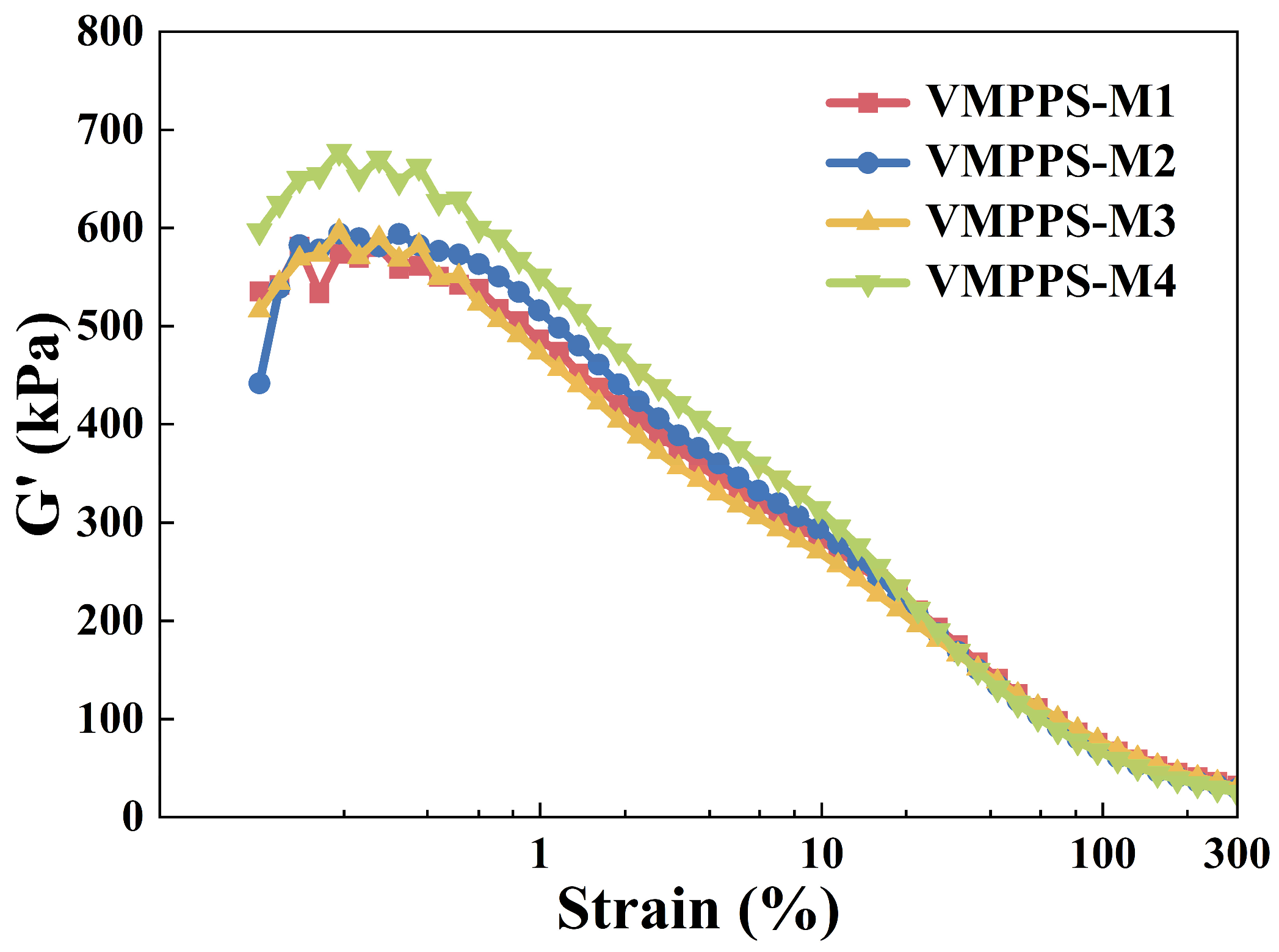
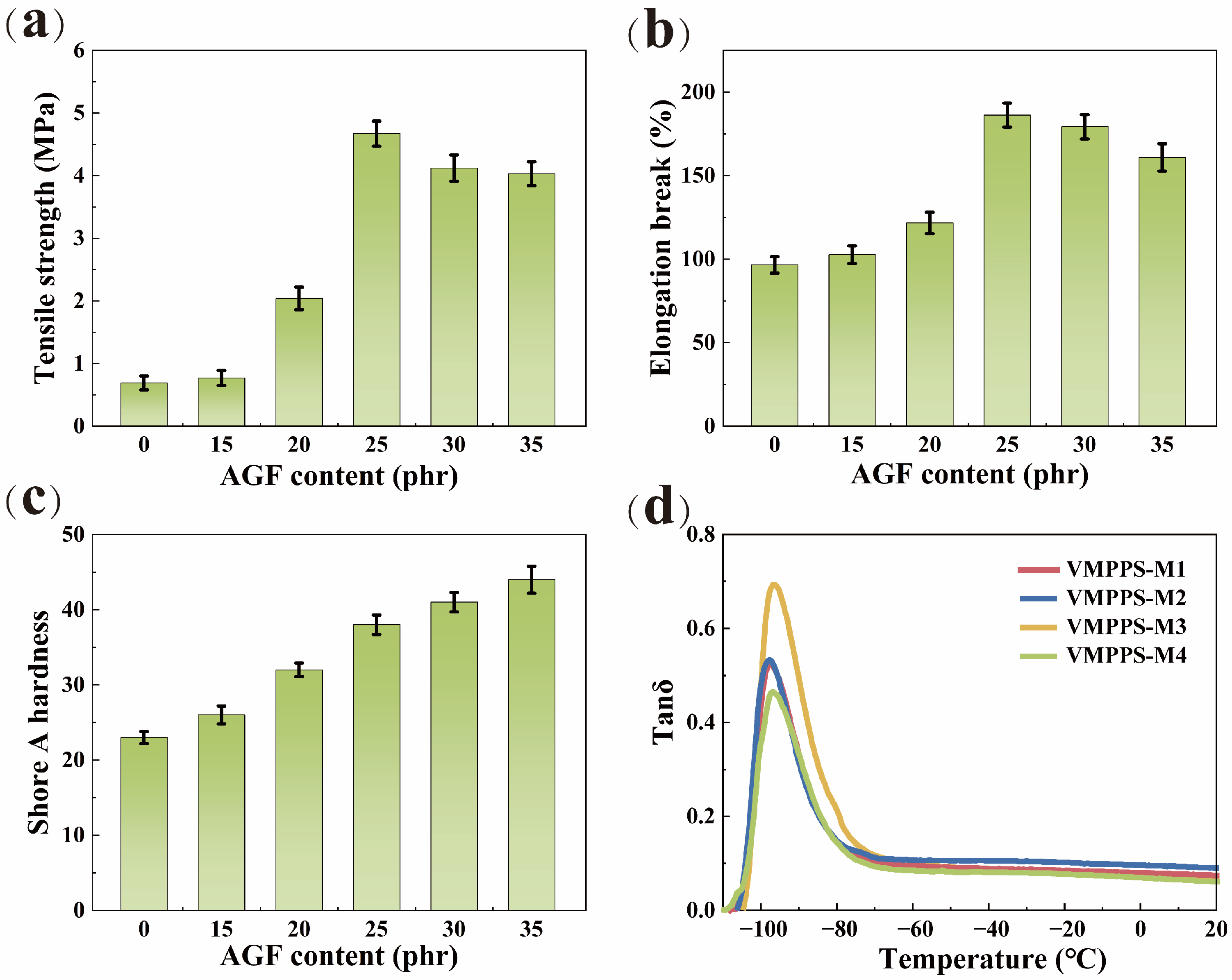
3.4. Mechanical Properties of VMPPS Silicone Rubber
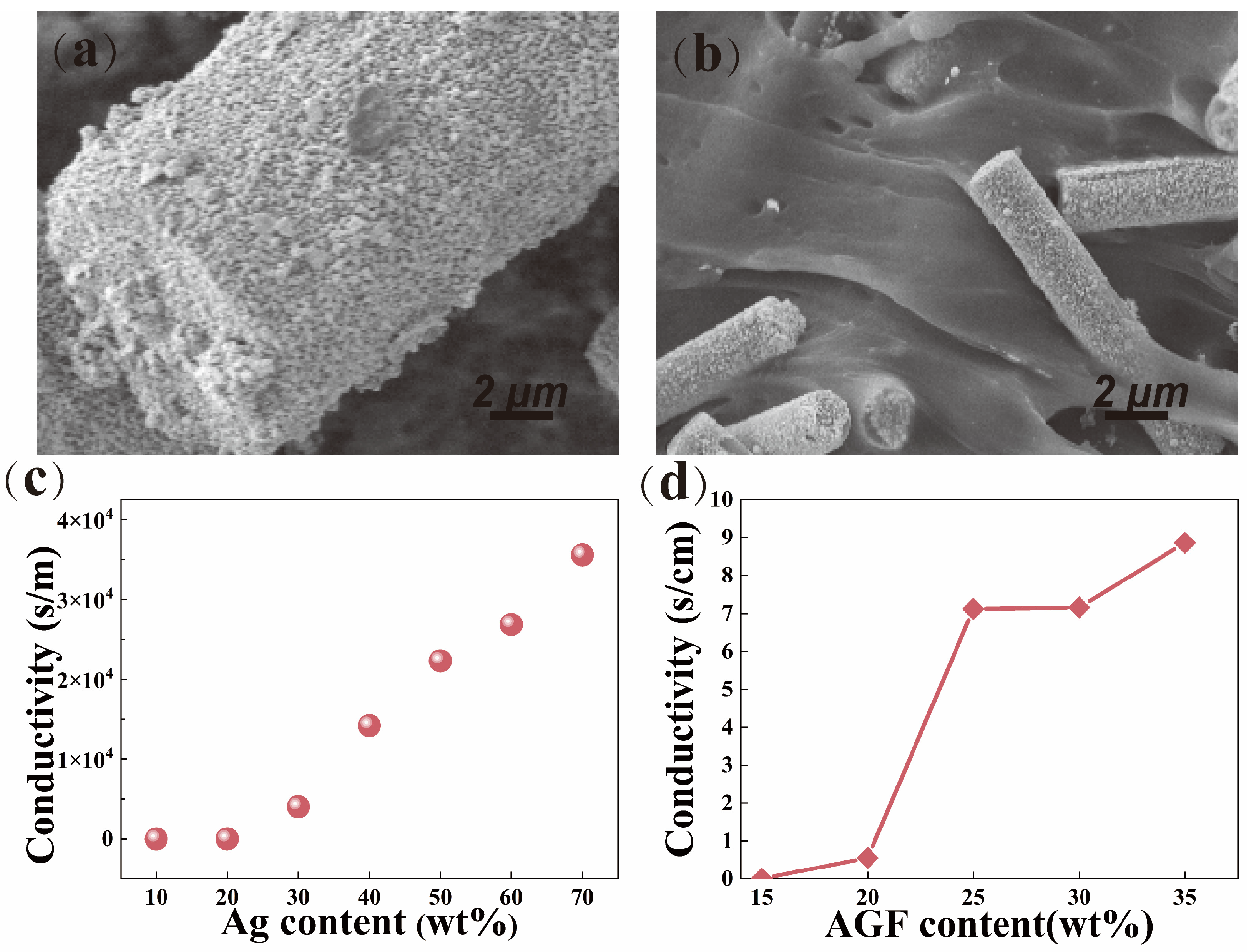
3.5. Conductivity of VMPPS Silicone Rubber
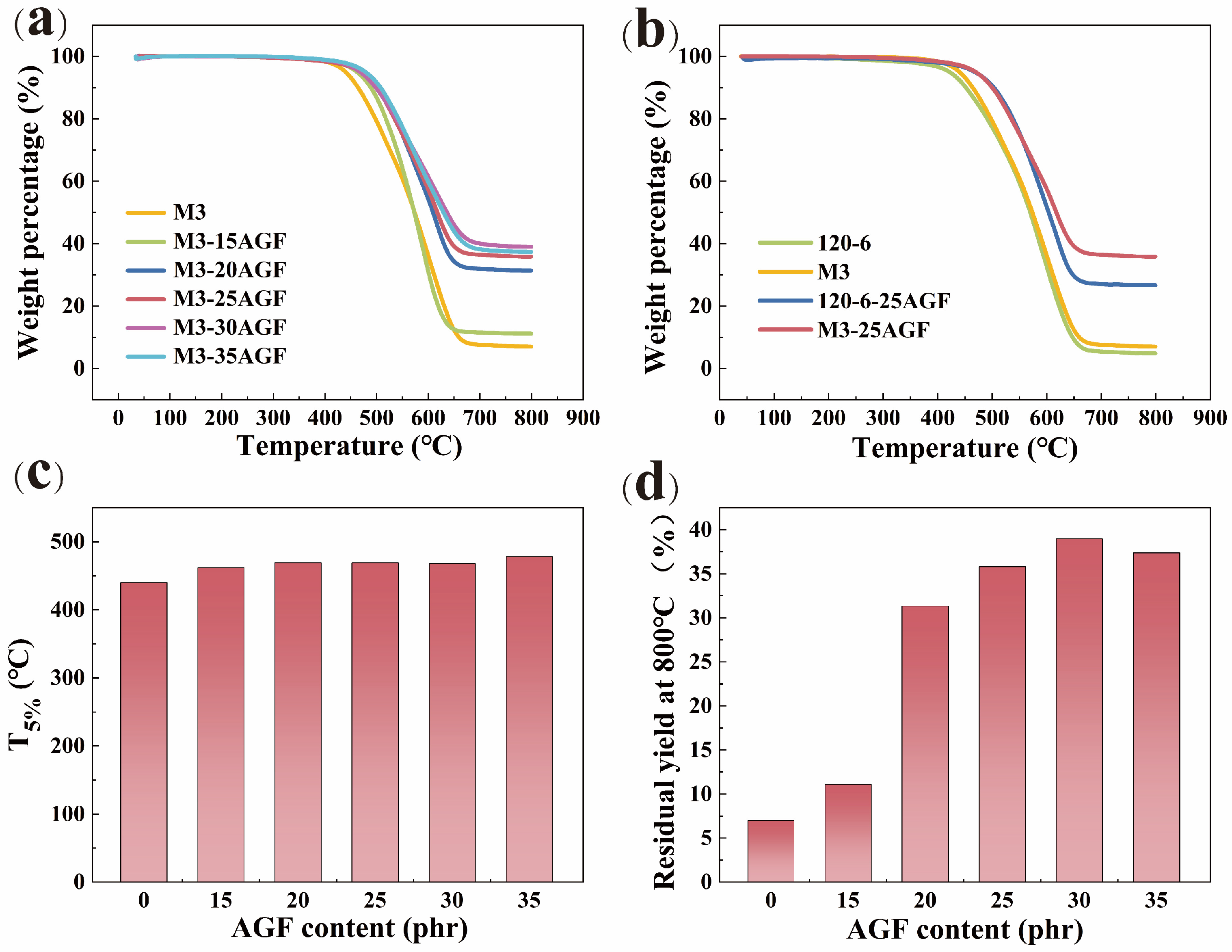
3.6. Heat Resistance of VMPPS Silicone Rubber
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gu, J.; Guo, Y.; Yang, X.; Liang, C.; Geng, W.; Tang, L.; Li, N.; Zhang, Q. Synergistic improvement of thermal conductivities of polyphenylene sulfide composites filled with boron nitride hybrid fillers. Compos. Part A Appl. Sci. Manuf. 2017, 95, 267–273. [Google Scholar] [CrossRef]
- Song, P.; Song, J.; Zhang, Y. Stretchable conductor based on carbon nanotube/carbon black silicone rubber nanocomposites with highly mechanical, electrical properties and strain sensitivity. Compos. Part B Eng. 2020, 191, 107979. [Google Scholar] [CrossRef]
- Zhu, L.; Chen, X.; Shi, R.; Zhang, H.; Han, R.; Cheng, X.; Zhou, C. Tetraphenylphenyl-modified damping additives for silicone rubber: Experimental and molecular simulation investigation. Mater. Des. 2021, 202, 109551. [Google Scholar] [CrossRef]
- Xue, Y.; Li, X.; Wang, H.; Zhao, F.; Zhang, D.; Chen, Y. Improvement in thermal conductivity of through-plane aligned boron nitride/silicone rubber composites. Mater. Des. 2019, 165, 107580. [Google Scholar] [CrossRef]
- Kareem, A.A.; Rasheed, H.K.; Nasir, E.M. Influence methods of preparation on the thermal stability of polyimide/silica dust. Polym. Bull. 2022, 79, 6617–6626. [Google Scholar] [CrossRef]
- Wu, W.; Cong, S. Silica- and diatomite-modified fluorine rubber nanocomposites. Bull. Mater. Sci. 2019, 42, 176. [Google Scholar] [CrossRef]
- Hao, Y.; Xie, J.; Xu, B.; Hu, B.; Zheng, Y.; Shen, Y. Tunnel elasticity enhancement effect of 3D submicron ceramics (Al2O3, TiO2, ZrO2) fiber on polydimethylsiloxane (PDMS). J. Adv. Ceram. 2021, 10, 502–508. [Google Scholar] [CrossRef]
- Zheng, R.; Chen, Y.; Chi, H.; Qiu, H.; Xue, H.; Bai, H. 3D Printing of a Polydimethylsiloxane/Polytetrafluoroethylene Composite Elastomer and its Application in a Triboelectric Nanogenerator. ACS Appl. Mater. Interfaces 2020, 12, 57441–57449. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, Q.; Li, Z.; Xu, X.; Liu, H.; Shang, S.; Song, Z. Preparation and Characterization of Room-Temperature-Vulcanized Silicone Rubber Using Acrylpimaric Acid-Modified Aminopropyltriethoxysilane as a Cross-Linking Agent. ACS Sustain. Chem. Eng. 2019, 7, 4964–4974. [Google Scholar] [CrossRef]
- Kumar, V.; Parvin, N.; Park, S.-S.; Joo, S.W.; Mandal, T.K. Review on Cutting-Edge Innovations in Carbon Nanomaterials Reinforced Silicone Rubber Composites for Flexible Electronics and Healthcare Devices. ACS Appl. Polym. Mater. 2024, 6, 14235–14259. [Google Scholar] [CrossRef]
- He, S.; Hu, J.; Zhang, C.; Wang, J.; Chen, L.; Bian, X.; Lin, J.; Du, X. Performance improvement in nano-alumina filled silicone rubber composites by using vinyl tri-methoxysilane. Polym. Test. 2018, 67, 295–301. [Google Scholar] [CrossRef]
- Li, X.; Zhang, D.; Chen, Y. Silicone rubber/hollow silica spheres composites with enhanced mechanical and electrical insulating performances. Mater. Lett. 2017, 205, 240–244. [Google Scholar] [CrossRef]
- Ding, W.; Yan, L.; Huang, M.; Luo, Y.; Chen, Y.; Liang, M.; Zou, H.; Heng, Z. In-situ constructing SiC frame in silicone rubber to fabricate novel flexible thermal protective polymeric materials with excellent ablation resistance. Polymer 2023, 285, 126319. [Google Scholar] [CrossRef]
- Chen, X.; Wang, J.; Zhang, C.; Yang, W.; Lin, J.; Bian, X.; He, S. Performance of silicone rubber composites using boron nitride to replace alumina tri-hydrate. High Volt. 2021, 6, 480–486. [Google Scholar] [CrossRef]
- Zheng, J.; He, S.; Wang, J.; Fang, W.; Xue, Y.; Xie, L.; Lin, J. Performance of Silicone Rubber Composites Filled with Aluminum Nitride and Alumina Tri-Hydrate. Materials 2020, 13, 2489. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cao, R.; Wang, M.; Liu, X.; Zhao, X.; Lu, Y.; Feng, A.; Zhang, L. Design and synthesis of phenyl silicone rubber with functional epoxy groups through anionic copolymerization and subsequent epoxidation. Polymer 2020, 186, 122077. [Google Scholar] [CrossRef]
- Lou, F.; Cheng, L.; Li, Q.; Wei, T.; Guan, X.; Guo, W. The combination of glass dust and glass fiber as fluxing agents for ceramifiable silicone rubber composites. RSC Adv. 2017, 7, 38805–38811. [Google Scholar] [CrossRef]
- Gan, T.-f.; Shentu, B.-q.; Weng, Z.-x. MODIFICATION OF CeO2 AND ITS EFFECT ON THE HEAT-RESISTANCE OF SILICONE RUBBER. Chin. J. Polym. Sci. 2008, 26, 489–494. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Z.; Lan, P.; Xu, H.; Lin, N. Hydrophobic and thermal-insulating aerogels based on rigid cellulose nanocrystal and elastic rubber. Carbohydr. Polym. 2022, 275, 118708. [Google Scholar] [CrossRef]
- Yang, X.; Li, Z.; Jiang, Z.; Wang, S.; Liu, H.; Xu, X.; Wang, D.; Miao, Y.; Shang, S.; Song, Z. Mechanical reinforcement of room-temperature-vulcanized silicone rubber using modified cellulose nanocrystals as cross-linker and nanofiller. Carbohydr. Polym. 2020, 229, 115509. [Google Scholar] [CrossRef]
- Grushevenko, E.A.; Borisov, I.L.; Bakhtin, D.S.; Bondarenko, G.N.; Levin, I.S.; Volkov, A.V. Silicone rubbers with alkyl side groups for C3+ hydrocarbon separation. React. Funct. Polym. 2019, 134, 156–165. [Google Scholar] [CrossRef]
- Zhu, C.; Wei, Y.; Zhang, J.; Geng, P.; Lu, Z. Preparation of polysiloxane oligomers bearing benzoxazine side groups and tunable properties of their thermosets. J. Appl. Polym. Sci. 2014, 131, 40960. [Google Scholar] [CrossRef]
- Yilgör, E.; Yilgör, I. Silicone containing copolymers: Synthesis, properties and applications. Prog. Polym. Sci. 2014, 39, 1165–1195. [Google Scholar] [CrossRef]
- Meng, Y.; Wei, Z.; Liu, L.; Liu, L.; Zhang, L.; Nishi, T.; Ito, K. Significantly improving the thermal stability and dispersion morphology of polyhedral oligomeric silsesquioxane/polysiloxane composites by in-situ grafting reaction. Polymer 2013, 54, 3055–3064. [Google Scholar] [CrossRef]
- Ryu, H.-S.; Kim, D.-G.; Lee, J.-C. Polysiloxanes containing polyhedral oligomeric silsesquioxane groups in the side chains; synthesis and properties. Polymer 2010, 51, 2296–2304. [Google Scholar] [CrossRef]
- He, C.; Li, B.; Ren, Y.; Lu, W.; Zeng, Y.; He, W.; Feng, A. How the Crosslinking Agent Influences the Thermal Stability of RTV Phenyl Silicone Rubber. Materials 2019, 12, 88. [Google Scholar] [CrossRef] [PubMed]
- Pouget, E.; Tonnar, J.; Lucas, P.; Lacroix-Desmazes, P.; Ganachaud, F.; Boutevin, B. Well-Architectured Poly(dimethylsiloxane)-Containing Copolymers Obtained by Radical Chemistry. Chem. Rev. 2010, 110, 1233–1277. [Google Scholar] [CrossRef]
- Eduok, U.; Faye, O.; Szpunar, J. Recent developments and applications of protective silicone coatings: A review of PDMS functional materials. Prog. Org. Coat. 2017, 111, 124–163. [Google Scholar] [CrossRef]
- Shit, S.C.; Shah, P. A Review on Silicone Rubber. Natl. Acad. Sci. Lett. 2013, 36, 355–365. [Google Scholar] [CrossRef]
- Hamdani, S.; Longuet, C.; Perrin, D.; Lopez-cuesta, J.-M.; Ganachaud, F. Flame retardancy of silicone-based materials. Polym. Degrad. Stab. 2009, 94, 465–495. [Google Scholar] [CrossRef]
- Yang, D.; Wei, Q.; Li, B.; Yu, L.; Ni, Y.; Zhang, L. High thermal conductive silicone rubber composites constructed by strawberry-structured Al2O3-PCPA-Ag hybrids. Compos. Part A Appl. Sci. Manuf. 2021, 142, 106260. [Google Scholar] [CrossRef]
- Ismail, A.M.; Mahmoud, K.R.; Abd-El Salam, M.H. Electrical conductivity and positron annihilation characteristics of ternary silicone rubber/carbon black/TiB2 nanocomposites. Polym. Test. 2015, 48, 37–43. [Google Scholar] [CrossRef]
- Chen, T.; Pan, L.; Lin, M.; Wang, B.; Liu, L.; Li, Y.; Qiu, J.; Zhu, K. Dielectric, mechanical and electro-stimulus response properties studies of polyurethane dielectric elastomer modified by carbon nanotube-graphene nanosheet hybrid fillers. Polym. Test. 2015, 47, 4–11. [Google Scholar] [CrossRef]
- Surya, I.; Ismail, H.; Azura, A.R. The comparison of alkanolamide and silane coupling agent on the properties of silica-filled natural rubber (SMR-L) compounds. Polym. Test. 2014, 40, 24–32. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, H.; Niu, H.; Gestos, A.; Wang, X.; Lin, T. Fluoroalkyl Silane Modified Silicone Rubber/Nanoparticle Composite: A Super Durable, Robust Superhydrophobic Fabric Coating. Adv. Mater. 2012, 24, 2409–2412. [Google Scholar] [CrossRef]
- Li, Z.-W. Thermoelectric properties of carbon nanotube/silicone rubber composites. J. Exp. Nanosci. 2017, 12, 188–196. [Google Scholar] [CrossRef]
- Dou, Y.; Gu, H.; Sun, S.; Yao, W.; Guan, D. Synthesis of a grape-like conductive carbon black/Ag hybrid as the conductive filler for soft silicone rubber. RSC Adv. 2022, 12, 1184–1193. [Google Scholar] [CrossRef] [PubMed]
- Sheng, M.; Yu, J.; Gong, H.; Zhang, Y.; Wang, Y. Enhancing the thermal stability and mechanical properties of phenyl silicone rubbers by controlling BN addition and phenyl content. Compos. Commun. 2022, 35, 101340. [Google Scholar] [CrossRef]
- Yang, J.; Xian, B.; Li, H.; Zhang, L.; Han, D. Preparation of silica/natural rubber masterbatch using solution compounding. Polymer 2022, 244, 124661. [Google Scholar] [CrossRef]
- Robertson, C.G.; Roland, C.M. Glass Transition and Interfacial Segmental Dynamics in Polymer-Particle Composites. Rubber Chem. Technol. 2008, 81, 506–522. [Google Scholar] [CrossRef]
- Diao, S.; Jin, K.; Yang, Z.; Lu, H.; Feng, S.; Zhang, C. The effect of phenyl modified fumed silica on radiation resistance of silicone rubber. Mater. Chem. Phys. 2011, 129, 202–208. [Google Scholar] [CrossRef]
- Han, R.; Wang, Z.; Zhang, Y.; Niu, K. Thermal stability of CeO2/graphene/phenyl silicone rubber composites. Polym. Test. 2019, 75, 277–283. [Google Scholar] [CrossRef]
- Ding, J.; Qiao, Z.; Zhang, Y.; Wei, D.; Chen, S.; Tang, J.; Chen, L.; Wei, D.; Sun, J.; Fan, H. NIR-responsive multi-healing HMPAM/dextran/AgNWs hydrogel sensor with recoverable mechanics and conductivity for human-machine interaction. Carbohydr. Polym. 2020, 247, 116686. [Google Scholar] [CrossRef]
- Li, J.; Peng, W.-J.; Fu, Z.-J.; Tang, X.-H.; Wu, H.; Guo, S.; Wang, M. Achieving high electrical conductivity and excellent electromagnetic interference shielding in poly(lactic acid)/silver nanocomposites by constructing large-area silver nanoplates in polymer matrix. Compos. Part B Eng. 2019, 171, 204–213. [Google Scholar] [CrossRef]


| Samples | Precursors | Product | |||
|---|---|---|---|---|---|
| MPPS/VDMS | Vinyl Content (%) | Mn (g/mol) | Đ | State at Room Temperature | |
| VMPPS-M0 | 100/1 | 0.01 | 38433 | 3.41 | Viscous liquid |
| VMPPS-M1 | 100/4 | 0.11 | 131591 | 2.49 | Solid |
| VMPPS-M2 | 100/8 | 0.15 | 147178 | 2.58 | Solid |
| VMPPS-M3 | 100/12 | 0.17 | 181136 | 2.43 | Solid |
| VMPPS-M4 | 100/16 | 0.23 | 122031 | 2.88 | Solid |
| VMPPS-M5 | 100/20 | 0.25 | 45827 | 2.83 | Viscous liquid |
| Samples | tc10/s | tc90/min | ML (dNm) | MH (dNm) | MH-ML (dNm) |
|---|---|---|---|---|---|
| VMPPS-M1 | 22 | 8.58 | 5.89 | 17.49 | 11.6 |
| VMPPS-M2 | 24 | 8.75 | 5.95 | 18.68 | 12.73 |
| VMPPS-M3 | 23 | 9.26 | 5.26 | 21.43 | 16.17 |
| VMPPS-M4 | 22 | 5.67 | 6.45 | 35.61 | 29.16 |
| Conductivity Loss Rate/% | 120-6-25AGF | M3-25AGF | M3-30AGF | M3-35AGF |
|---|---|---|---|---|
| 25 °C for 24 h | 1.82 | 1.24 | 1.12 | 1.35 |
| 25 °C for 72 h | 6.63 | 5.22 | 5.43 | 5.36 |
| 100 °C for 24 h | 18.12 | 14.65 | 15.86 | 15.21 |
| 100 °C for 72 h | 36.58 | 30.92 | 32.16 | 34.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, A.; Ouyang, L.; Gong, D.; Zhang, C. A High-Temperature-Resistant and Conductive Flexible Silicone Rubber with High Phenyl Content Based on Silver-Coated Glass Fibers. Polymers 2025, 17, 1187. https://doi.org/10.3390/polym17091187
Liu A, Ouyang L, Gong D, Zhang C. A High-Temperature-Resistant and Conductive Flexible Silicone Rubber with High Phenyl Content Based on Silver-Coated Glass Fibers. Polymers. 2025; 17(9):1187. https://doi.org/10.3390/polym17091187
Chicago/Turabian StyleLiu, Ao, Linlin Ouyang, Depeng Gong, and Chaocan Zhang. 2025. "A High-Temperature-Resistant and Conductive Flexible Silicone Rubber with High Phenyl Content Based on Silver-Coated Glass Fibers" Polymers 17, no. 9: 1187. https://doi.org/10.3390/polym17091187
APA StyleLiu, A., Ouyang, L., Gong, D., & Zhang, C. (2025). A High-Temperature-Resistant and Conductive Flexible Silicone Rubber with High Phenyl Content Based on Silver-Coated Glass Fibers. Polymers, 17(9), 1187. https://doi.org/10.3390/polym17091187







