Alginate Edible Films Containing Essential Oils: Characterization and Bioactive Potential
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Alginate Films Incorporated with EOs
2.3. Characterization of the Films
2.3.1. FTIR Spectroscopy
2.3.2. Film Thickness
2.3.3. Moisture Content and Solubility in Water
2.3.4. Water Vapor Transmission Rate
2.3.5. Water Contact Angle (WCA) Measurements
2.4. Bioactivities
2.4.1. Antibacterial Activity
2.4.2. Antifungal Activity
2.4.3. Antioxidant Activity
2.5. Statistical Analysis
3. Results
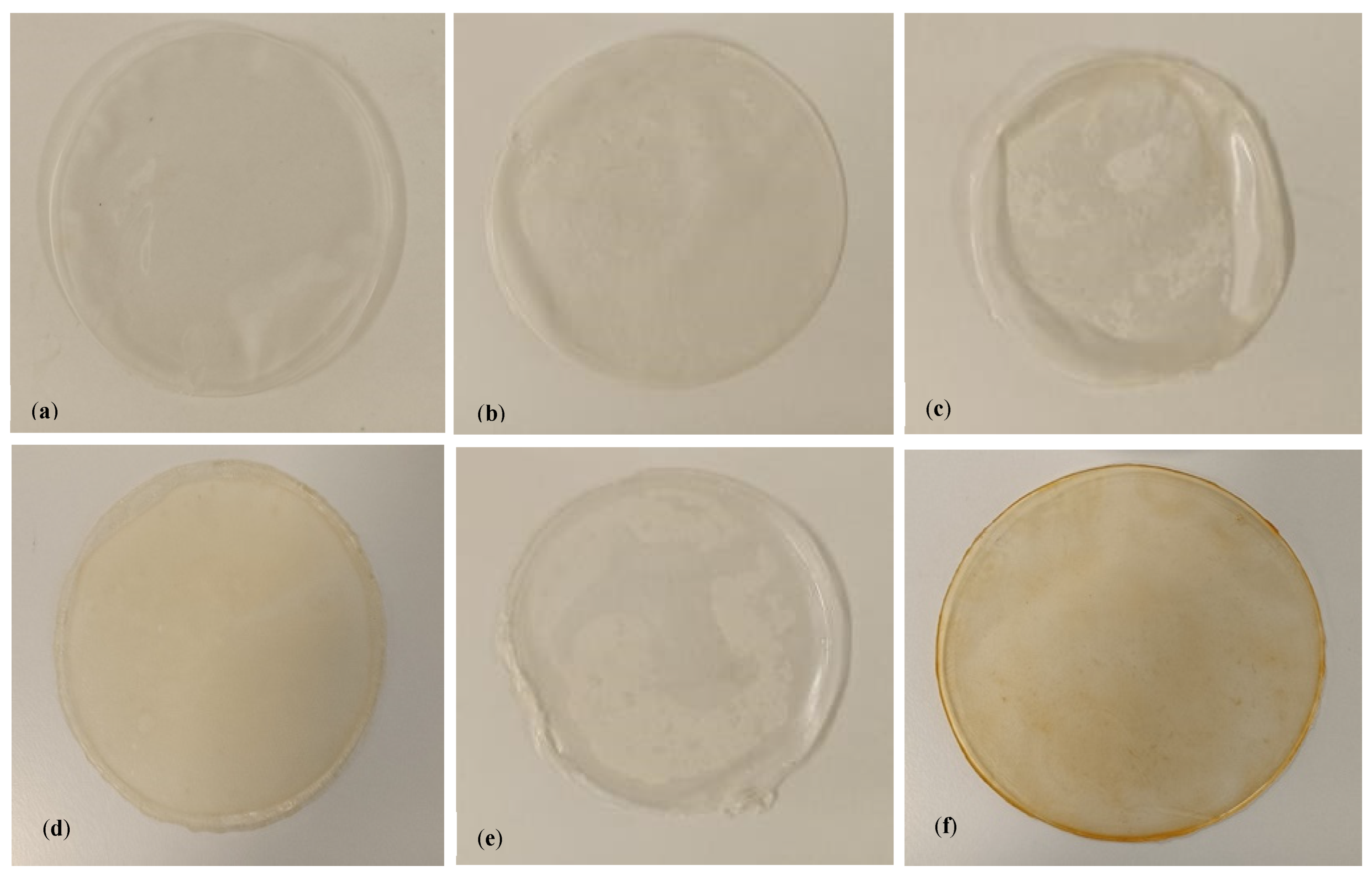
3.1. Alginate Films with EOs
3.2. Characterization of the Films
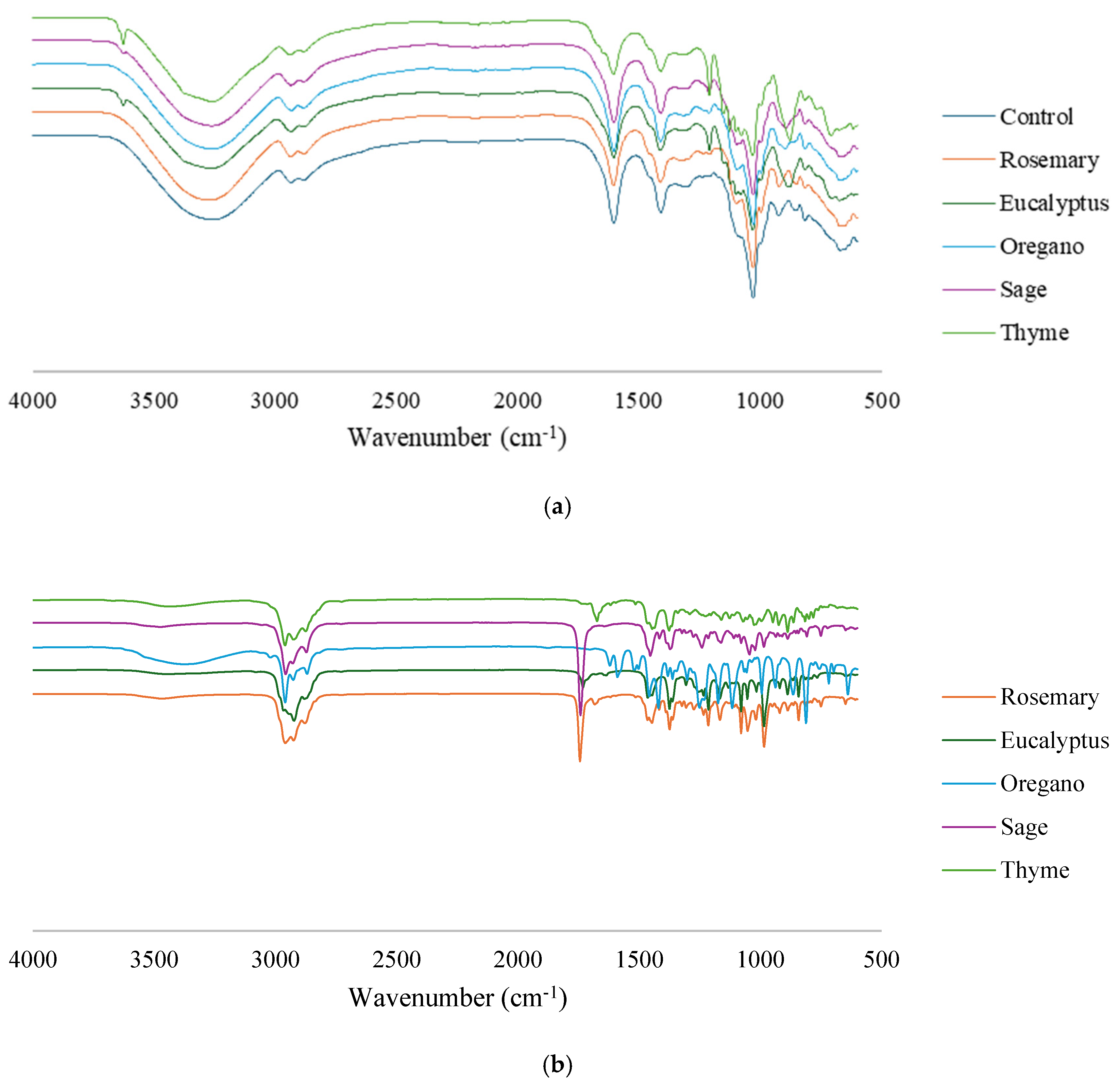
3.2.1. FTIR Spectroscopy Analysis
3.2.2. Thickness, Moisture Content, and Solubility in Water
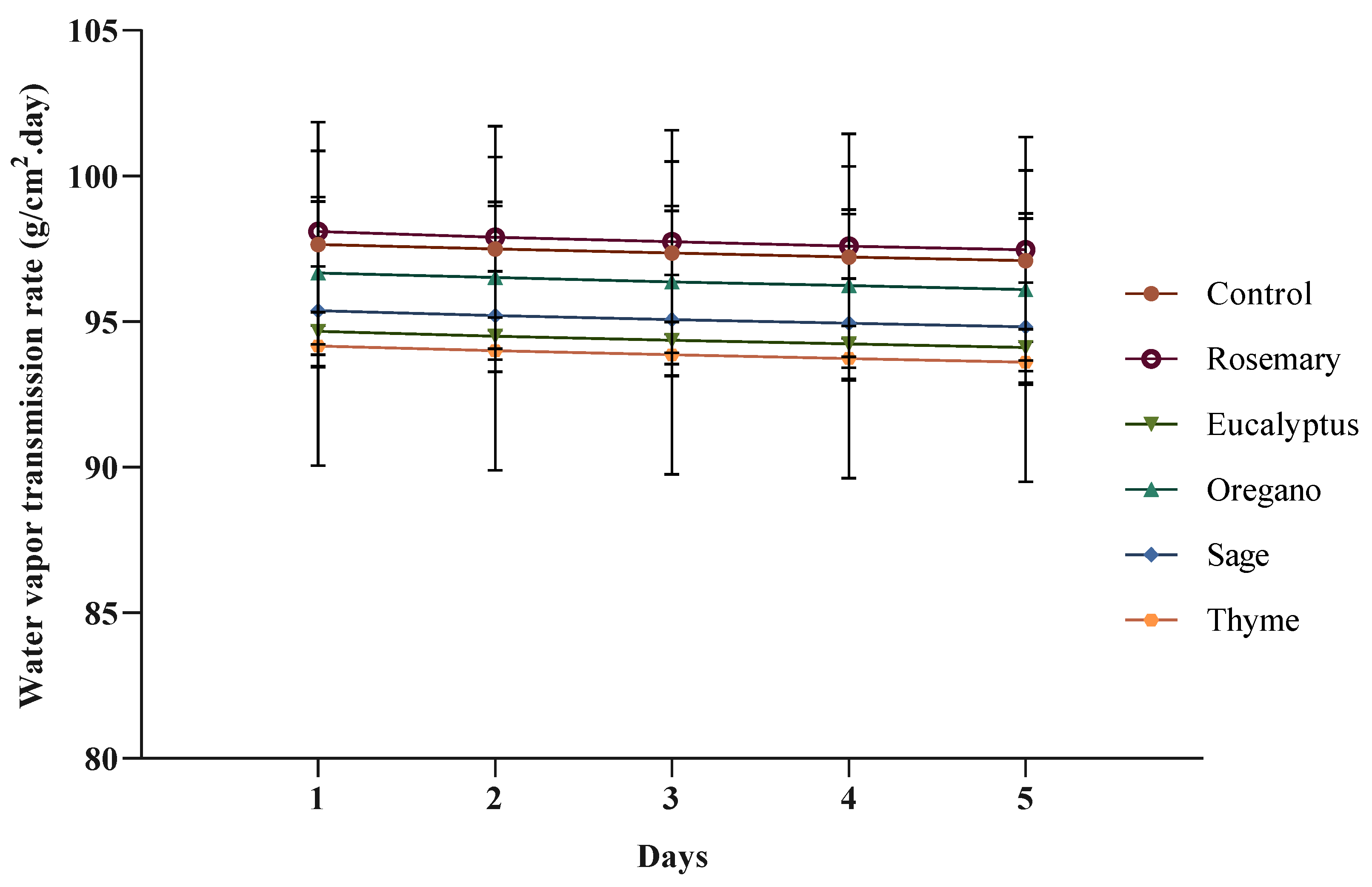
3.2.3. Water Vapor Transmission Rate
3.2.4. Water Contact Angle
3.3. Bioactivities
3.3.1. Antimicrobial Activity
Antibacterial Activity
Antifungal Activity
3.3.2. Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luo, Y.; Liu, H.; Yang, S.; Zeng, J.; Wu, Z. Sodium Alginate-Based Green Packaging Films Functionalized by Guava Leaf Extracts and Their Bioactivities. Materials 2019, 12, 2923. [Google Scholar] [CrossRef]
- Aitboulahsen, M.; El Galiou, O.; Laglaoui, A.; Bakkali, M.; Hassani Zerrouk, M. Effect of Plasticizer Type and Essential Oils on Mechanical, Physicochemical, and Antimicrobial Characteristics of Gelatin, Starch, and Pectin-Based Films. J. Food Process Preserv. 2020, 44, e14480. [Google Scholar] [CrossRef]
- Lim, L.I.; Tan, H.L.; Pui, L.P. Development and Characterization of Alginate-Based Edible Film Incorporated with Hawthorn Berry (Crataegus pinnatifida) Extract. J. Food Meas. Charact. 2021, 15, 2540–2548. [Google Scholar] [CrossRef]
- Acevedo-Fani, A.; Salvia-Trujillo, L.; Rojas-Graü, M.A.; Martín-Belloso, O. Edible Films from Essential-Oil-Loaded Nanoemulsions: Physicochemical Characterization and Antimicrobial Properties. Food Hydrocoll. 2015, 47, 168–177. [Google Scholar] [CrossRef]
- Siburian, P.W.; Falah, M.A.F.; Mangunwikarta, J. Alginate-Based Edible Coatings Enriched with Cinnamon Essential Oil Extend Storability and Maintain the Quality of Strawberries under Tropical Condition. Planta Trop. 2021, 9, 58–70. [Google Scholar] [CrossRef]
- Engin, M.S.; Zamahay, F.; Kalkan, S.; Otağ, M.R. Physical, Mechanical, and Bioactive Properties of Edible Film Based on Sodium Alginate Enriched with Lythrum salicaria L. Extract. J. Food Process Preserv. 2022, 46, e16620. [Google Scholar] [CrossRef]
- Ghasemlou, M.; Aliheidari, N.; Fahmi, R.; Shojaee-Aliabadi, S.; Keshavarz, B.; Cran, M.J.; Khaksar, R. Physical, Mechanical and Barrier Properties of Corn Starch Films Incorporated with Plant Essential Oils. Carbohydr. Polym. 2013, 98, 1117–1126. [Google Scholar] [CrossRef]
- Zuzarte, M.; Gonçalves, M.; Canhoto, J.; Salgueiro, L. Antidermatophytic Activity of Essential Oils. In Science Against Microbial Pathogens: Communicating Current Research and Technological Advances; Formatex Research Center: Norristown, PA, USA, 2011; pp. 1167–1178. [Google Scholar]
- Baptista-Silva, S.; Borges, S.; Ramos, O.L.; Pintado, M.; Sarmento, B. The Progress of Essential Oils as Potential Therapeutic Agents: A Review. J. Essent. Oil Res. 2020, 32, 279–295. [Google Scholar] [CrossRef]
- Benavides, S.; Villalobos-Carvajal, R.; Reyes, J.E. Physical, Mechanical and Antibacterial Properties of Alginate Film: Effect of the Crosslinking Degree and Oregano Essential Oil Concentration. J. Food Eng. 2012, 110, 232–239. [Google Scholar] [CrossRef]
- El Fawal, G.F.; Omer, A.M.; Tamer, T.M. Evaluation of Antimicrobial and Antioxidant Activities for Cellulose Acetate Films Incorporated with Rosemary and Aloe Vera Essential Oils. J. Food Sci. Technol. 2019, 56, 1510–1518. [Google Scholar] [CrossRef]
- Bal-Öztürk, A.; Özkahraman, B.; Özbaş, Z.; Yaşayan, G.; Tamahkar, E.; Alarçin, E. Advancements and Future Directions in the Antibacterial Wound Dressings—A Review. J. Biomed. Mater. Res. B Appl. Biomater. 2021, 109B, 703–716. [Google Scholar] [CrossRef]
- Bhatia, S.; Al-Harrasi, A.; Shah, Y.A.; Jawad, M.; Al-Azri, M.S.; Ullah, S.; Anwer, M.K.; Aldawsari, M.F.; Koca, E.; Aydemir, L.Y. The Effect of Sage (Salvia sclarea) Essential Oil on the Physiochemical and Antioxidant Properties of Sodium Alginate and Casein-Based Composite Edible Films. Gels 2023, 9, 233. [Google Scholar] [CrossRef]
- Spréa, R.M.; Caleja, C.; Finimundy, T.C.; Calhelha, R.C.; Pires, T.C.S.P.; Amaral, J.S.; Prieto, M.A.; Ferreira, I.C.F.R.; Pereira, E.; Barros, L. Chemical and Bioactive Evaluation of Essential Oils from Edible and Aromatic Mediterranean Lamiaceae Plants. Molecules 2024, 29, 2827. [Google Scholar] [CrossRef]
- Lopes, A.I.; Melo, A.; Caleja, C.; Pereira, E.; Finimundy, T.C.; Afonso, T.B.; Silva, S.; Ivanov, M.; Soković, M.; Tavaria, F.K.; et al. Evaluation of Antimicrobial and Antioxidant Activities of Alginate Edible Coatings Incorporated with Plant Extracts. Coatings 2023, 13, 1487. [Google Scholar] [CrossRef]
- Pereira, J.O.; Soares, J.; Costa, E.; Silva, S.; Gomes, A.; Pintado, M. Characterization of Edible Films Based on Alginate or Whey Protein Incorporated with Bifidobacterium animalis Subsp. Lactis BB-12 and Prebiotics. Coatings 2019, 9, 493. [Google Scholar] [CrossRef]
- ASTM E96; ASTM Standard Test Methods for Water Vapor Transmission of Materials. American Society for Testing and Materials: Philadelphia, PA, USA, 2000.
- Mahcene, Z.; Khelil, A.; Hasni, S.; Akman, P.K.; Bozkurt, F.; Birech, K.; Goudjil, M.B.; Tornuk, F. Development and Characterization of Sodium Alginate Based Active Edible Films Incorporated with Essential Oils of Some Medicinal Plants. Int. J. Biol. Macromol. 2020, 145, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Kowalonek, J.; Stachowiak, N.; Bolczak, K.; Richert, A. Physicochemical and Antibacterial Properties of Alginate Films Containing Tansy (Tanacetum vulgare L.) Essential Oil. Polymers 2023, 15, 260. [Google Scholar] [CrossRef] [PubMed]
- Motelica, L.; Ficai, D.; Oprea, O.C.; Ficai, A.; Ene, V.L.; Vasile, B.S.; Andronescu, E.; Holban, A.M. Antibacterial Biodegradable Films Based on Alginate with Silver Nanoparticles and Lemongrass Essential Oil–Innovative Packaging for Cheese. Nanomaterials 2021, 11, 2377. [Google Scholar] [CrossRef]
- Abdel Aziz, M.S.; Salama, H.E.; Sabaa, M.W. Biobased Alginate/Castor Oil Edible Films for Active Food Packaging. LWT 2018, 96, 455–460. [Google Scholar] [CrossRef]
- Bhatia, S.; Al-Harrasi, A.; Shah, Y.A.; Altoubi, H.W.K.; Kotta, S.; Sharma, P.; Anwer, M.K.; Kaithavalappil, D.S.; Koca, E.; Aydemir, L.Y. Fabrication, Characterization, and Antioxidant Potential of Sodium Alginate/Acacia Gum Hydrogel-Based Films Loaded with Cinnamon Essential Oil. Gels 2023, 9, 337. [Google Scholar] [CrossRef]
- Bhatia, S.; Al-Harrasi, A.; Al-Azri, M.S.; Ullah, S.; Bekhit, A.E.D.A.; Pratap-Singh, A.; Chatli, M.K.; Anwer, M.K.; Aldawsari, M.F. Preparation and Physiochemical Characterization of Bitter Orange Oil Loaded Sodium Alginate and Casein Based Edible Films. Polymers 2022, 14, 3855. [Google Scholar] [CrossRef] [PubMed]
- Dhouibi, I.; Flamini, G.; Bouaziz, M. Comparative Study on the Essential Oils Extracted from Tunisian Rosemary and Myrtle: Chemical Profiles, Quality, and Antimicrobial Activities. ACS Omega 2023, 8, 6431–6438. [Google Scholar] [CrossRef]
- El Orche, A.; El Mrabet, A.; Said, A.A.H.; Mousannif, S.; Elhamdaoui, O.; Ansari, S.A.; Alkahtani, H.M.; Ansari, S.A.; Sbai El Otmani, I.; Bouatia, M. Integration of FTIR Spectroscopy, Volatile Compound Profiling, and Chemometric Techniques for Advanced Geographical and Varietal Analysis of Moroccan Eucalyptus Essential Oils. Sensors 2024, 24, 7337. [Google Scholar] [CrossRef]
- Valderrama, A.C.S.; Rojas De, G.C. Traceability of Active Compounds of Essential Oils in Antimicrobial Food Packaging Using a Chemometric Method by ATR-FTIR. Am. J. Anal. Chem. 2017, 08, 726–741. [Google Scholar] [CrossRef]
- Gudi, G.; Krähmer, A.; Krüger, H.; Schulz, H. Attenuated Total Reflectance–Fourier Transform Infrared Spectroscopy on Intact Dried Leaves of Sage (Salvia officinalis L.): Accelerated Chemotaxonomic Discrimination and Analysis of Essential Oil Composition. J. Agric. Food Chem. 2015, 63, 8743–8750. [Google Scholar] [CrossRef] [PubMed]
- Bisht, A.S.; Alam, M.S.; Bhatia, S.; Gupta, S.K. Studies on Development and Evaluation of Glycerol Incorporated Cellulose and Alginate Based Edible Films. Indian J. Agric. Biochem. 2017, 30, 67. [Google Scholar] [CrossRef]
- Jafari, R.; Zandi, M.; Ganjloo, A. Characterization of Alginate-Gelatin Edible Film Containing Anise (Pimpinella anisum L.) Essential Oil. J. Polym. Environ. 2023, 31, 1568–1583. [Google Scholar] [CrossRef]
- Baek, S.K.; Kim, S.; Song, K. Bin Characterization of Ecklonia Cava Alginate Films Containing Cinnamon Essential Oils. Int. J. Mol. Sci. 2018, 19, 3545. [Google Scholar] [CrossRef]
- Han, Y.; Yu, M.; Wang, L. Physical and Antimicrobial Properties of Sodium Alginate/Carboxymethyl Cellulose Films Incorporated with Cinnamon Essential Oil. Food Packag. Shelf Life 2018, 15, 35–42. [Google Scholar] [CrossRef]
- Atarés, L.; Chiralt, A. Essential Oils as Additives in Biodegradable Films and Coatings for Active Food Packaging. Trends Food Sci. Technol. 2016, 48, 51–62. [Google Scholar] [CrossRef]
- Cofelice, M.; Cuomo, F.; Chiralt, A. Alginate Films Encapsulating Lemongrass Essential Oil as Affected by Spray Calcium Application. Colloids Interfaces 2019, 3, 58. [Google Scholar] [CrossRef]
- Siracusa, V.; Romani, S.; Gigli, M.; Mannozzi, C.; Cecchini, J.P.; Tylewicz, U.; Lotti, N. Characterization of Active Edible Films Based on Citral Essential Oil, Alginate and Pectin. Materials 2018, 11, 1980. [Google Scholar] [CrossRef]
- Silva, N.C.; Castro, D.; Neto, C.; Madureira, A.R.; Pintado, M.; Moreira, P.R. Antimicrobial Chitosan/TPP-Based Coatings for the Prevention of Biodeterioration of Outdoor Stone Sculptures. Prog. Org. Coat. 2024, 189, 108246. [Google Scholar] [CrossRef]
- Anis, A.; Pal, K.; Al-Zahrani, S.M. Essential Oil-Containing Polysaccharide-Based Edible Films and Coatings for Food Security Applications. Polymers 2021, 13, 575. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Barkauskaite, S.; Duffy, B.; Jaiswal, A.K.; Swarna, J. Characterization and Antimicrobial Activity of Biodegradable Active Packaging Enriched with Clove and Thyme Essential Oil for Food Packaging Application. Foods 2020, 9, 1117. [Google Scholar] [CrossRef]
- Wang, L.; Liu, F.; Jiang, Y.; Chai, Z.; Li, P.; Cheng, Y.; Jing, H.; Leng, X. Synergistic Antimicrobial Activities of Natural Essential Oils with Chitosan Films. J. Agric. Food Chem. 2011, 59, 12411–12419. [Google Scholar] [CrossRef] [PubMed]
- Bustamante-Torres, M.; Arcentales-Vera, B.; Estrella-Nuñez, J.; Yánez-Vega, H.; Bucio, E. Antimicrobial Activity of Composites-Based on Biopolymers. Macromol 2022, 2, 258–283. [Google Scholar] [CrossRef]
- Ogueira, G.F.; de Oliveira, R.A.; Velasco, J.I.; Fakhouri, F.M. Methods of Incorporating Plant-Derived Bioactive Compounds into Films Made with Agro-Based Polymers for Application as Food Packaging: A Brief Review. Polymers 2020, 12, 2518. [Google Scholar] [CrossRef]
- Jamróz, E.; Kopel, P. Polysaccharide and Protein Films with Antimicrobial/Antioxidant Activity in the Food Industry: A Review. Polymers 2020, 12, 1289. [Google Scholar] [CrossRef]
- Zivanovic, S.; Chi, S.; Draughon, A.F. Antimicrobial Activity of Chitosan Films Enriched with Essential Oils. J. Food Sci. 2005, 70, M45–M51. [Google Scholar] [CrossRef]
- Abdollahzadeh, E.; Nematollahi, A.; Hosseini, H. Composition of Antimicrobial Edible Films and Methods for Assessing Their Antimicrobial Activity: A Review. Trends Food Sci. Technol. 2021, 110, 291–303. [Google Scholar] [CrossRef]
- Guimarães, A.; Ramos, O.; Cerqueira, M.; Venâncio, A.; Abrunhosa, L. Active Whey Protein Edible Films and Coatings Incorporating Lactobacillus buchneri for Penicillium nordicum Control in Cheese. Food Bioproc. Technol. 2020, 13, 1074–1086. [Google Scholar] [CrossRef]
- Acosta, S.; Chiralt, A.; Santamarina Siurana, M.; Roselló, J.; Gonzalez-Martinez, C.; Cháfer, M. Antifungal Films Based on Starch-Gelatin Blend, Containing Essential Oils. Food Hydrocoll. 2016, 61, 233–240. [Google Scholar] [CrossRef]
- Scartazzini, L.; Tosati, J.V.; Cortez, D.H.C.; Rossi, M.J.; Flôres, S.H.; Hubinger, M.D.; Di Luccio, M.; Monteiro, A.R. Gelatin Edible Coatings with Mint Essential Oil (Mentha arvensis): Film Characterization and Antifungal Properties. J. Food Sci. Technol. 2019, 56, 4045–4056. [Google Scholar] [CrossRef] [PubMed]
- Yahyaoui, M.; Gordobil, O.; Herrera Díaz, R.; Abderrabba, M.; Labidi, J. Development of Novel Antimicrobial Films Based on Poly(Lactic Acid) and Essential Oils. React. Funct. Polym. 2016, 109, 1–8. [Google Scholar] [CrossRef]
- Muriel-Galet, V.; Cran, M.J.; Bigger, S.W.; Hernández-Muñoz, P.; Gavara, R. Antioxidant and Antimicrobial Properties of Ethylene Vinyl Alcohol Copolymer Films Based on the Release of Oregano Essential Oil and Green Tea Extract Components. J. Food Eng. 2015, 149, 9–16. [Google Scholar] [CrossRef]
- Sellimi, S.; Younes, I.; Ayed, H.B.; Maalej, H.; Montero, V.; Rinaudo, M.; Dahia, M.; Mechichi, T.; Hajji, M.; Nasri, M. Structural, Physicochemical and Antioxidant Properties of Sodium Alginate Isolated from a Tunisian Brown Seaweed. Int. J. Biol. Macromol. 2015, 72, 1358–1367. [Google Scholar] [CrossRef]
- Khan, S.; Abdo, A.A.A.; Shu, Y.; Zhang, Z.; Liang, T. The Extraction and Impact of Essential Oils on Bioactive Films and Food Preservation, with Emphasis on Antioxidant and Antibacterial Activities—A Review. Foods 2023, 12, 4169. [Google Scholar] [CrossRef]
- Teoh, R.W.; Ting, A.S.Y.; Thoo, Y.Y. Characterization and Modeling of Diffusion Kinetics of Rosemary Oleoresin Extract from Gellan Gum-Based Film. J. Food Sci. Technol. 2023, 60, 2978–2989. [Google Scholar] [CrossRef]
- Roy, S.; Rhim, J.W. Genipin-Crosslinked Gelatin/Chitosan-Based Functional Films Incorporated with Rosemary Essential Oil and Quercetin. Materials 2022, 15, 3769. [Google Scholar] [CrossRef]
- Li, Y.; Tang, C.; He, Q. Effect of Orange (Citrus sinensis L.) Peel Essential Oil on Characteristics of Blend Films Based on Chitosan and Fish Skin Gelatin. Food Biosci. 2021, 41, 100927. [Google Scholar] [CrossRef]
- López de Dicastillo, C.; Bustos, F.; Guarda, A.; Galotto, M.J. Cross-Linked Methyl Cellulose Films with Murta Fruit Extract for Antioxidant and Antimicrobial Active Food Packaging. Food Hydrocoll. 2016, 60, 335–344. [Google Scholar] [CrossRef]
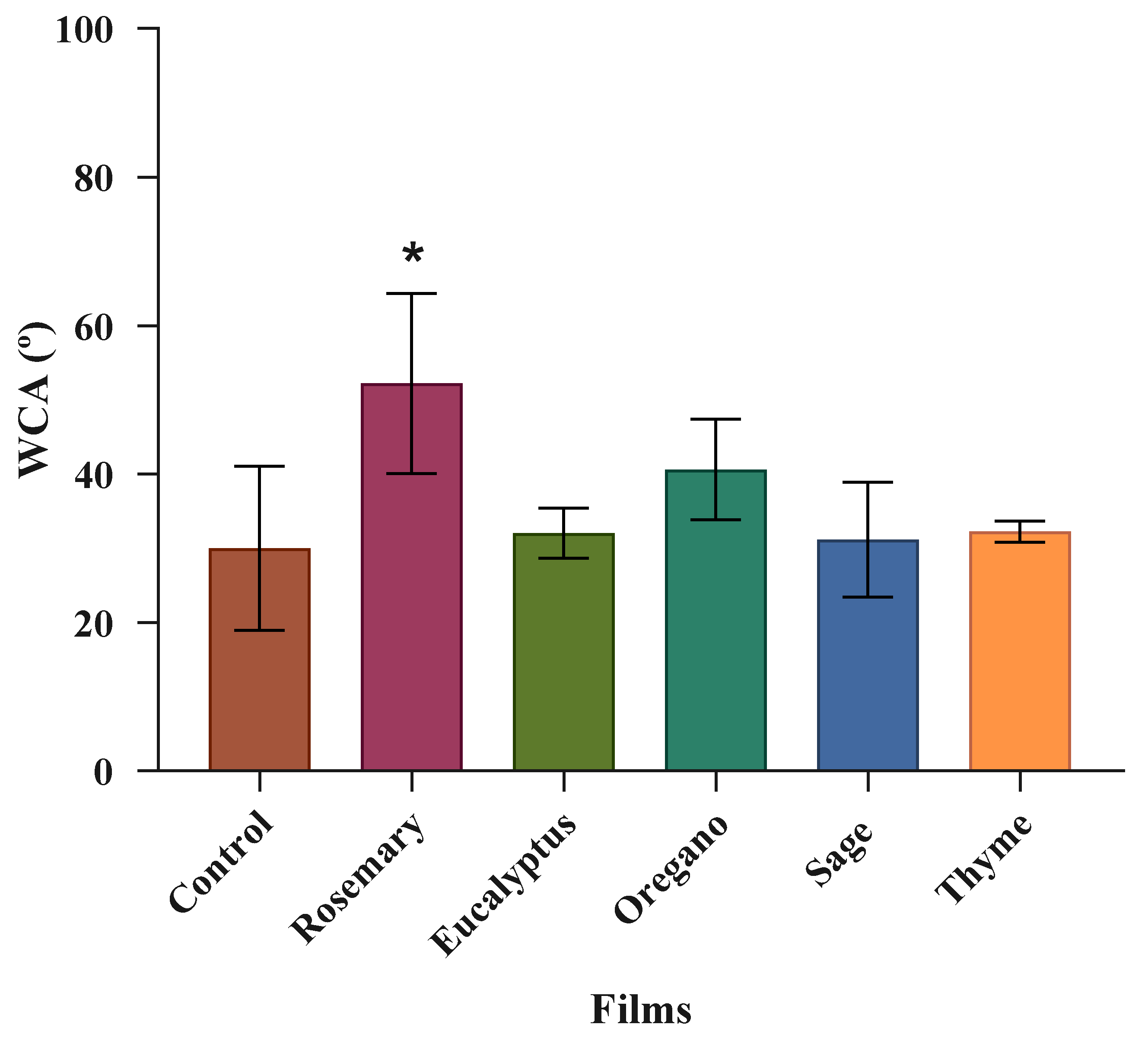


| Thickness (mm) | Moisture (%, Dry Basis) | * Solubility in Water (%) | |
|---|---|---|---|
| Control | 0.17 ± 0.01 A | 29.54 ± 0.03 A | 95.10 ± 2.38 A,a |
| Rosemary | 0.17 ± 0.01 A,a | 32.54 ± 0.38 A,a | 87.13 ± 4.54 A,a |
| Eucalyptus | 0.24 ± 0.02 B,b,e | 35.13 ± 1.27 B,a | 89.17 ± 13.85 A,a |
| Oregano | 0.12 ± 0.01 B,c,f | 35.24 ± 1.05 C,a,d | 83.72 ± 0.04 A,a |
| Sage | 0.29 ± 0.03 B,d | 37.79 ± 1.05 D,b,d | 94.29 ± 3.98 A,a |
| Thyme | 0.16 ± 0.01 A,a | 37.11 ± 0.39 E,c,d | 86.65 ± 1.52 A,a |
| Inhibition (%) | |||||
|---|---|---|---|---|---|
| Fungus | Rosemary | Eucalyptus | Oregano | Sage | Thyme |
| A. niger | NI | 11.9 ± 2.2 a | 100 ± 0 b | 11.5 ± 7.7 c | 36.7 ± 1.6 d |
| P. expansum | NI | NI | 100 ± 0 a | 14.5 ± 9.1 b | 30.4 ± 6.0 c |
| F. verticillioides | NI | NI | 100 ± 0 a | NI | 100 ± 0 a |
| Cladosporium sp. | NI | 100 ± 0 a | 100 ± 0 a | NI | 100 ± 0 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, A.I.; Melo, A.; Afonso, T.B.; Silva, S.; Barros, L.; Tavaria, F.K.; Pintado, M. Alginate Edible Films Containing Essential Oils: Characterization and Bioactive Potential. Polymers 2025, 17, 1188. https://doi.org/10.3390/polym17091188
Lopes AI, Melo A, Afonso TB, Silva S, Barros L, Tavaria FK, Pintado M. Alginate Edible Films Containing Essential Oils: Characterization and Bioactive Potential. Polymers. 2025; 17(9):1188. https://doi.org/10.3390/polym17091188
Chicago/Turabian StyleLopes, Ana I., Adma Melo, Tiago B. Afonso, Sara Silva, Lillian Barros, Freni K. Tavaria, and Manuela Pintado. 2025. "Alginate Edible Films Containing Essential Oils: Characterization and Bioactive Potential" Polymers 17, no. 9: 1188. https://doi.org/10.3390/polym17091188
APA StyleLopes, A. I., Melo, A., Afonso, T. B., Silva, S., Barros, L., Tavaria, F. K., & Pintado, M. (2025). Alginate Edible Films Containing Essential Oils: Characterization and Bioactive Potential. Polymers, 17(9), 1188. https://doi.org/10.3390/polym17091188









