Fabrication and Characterization of Poly(hydroxybutyrate)- and Poly(caprolactone)-Based Active Biodegradable Films Incorporating Allyl Isothiocyanate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Film Preparation
2.3. Thickness
2.4. Moisture Content
2.5. Optical Properties
2.6. Mechanical Properties
2.7. Barrier Properties
2.7.1. Water Vapor Permeability
2.7.2. Oxygen Permeability
2.8. Thermal Characteristics
2.9. SEM
2.10. FTIR
2.11. Film Biodegradability
2.12. Antimicrobial Activity
2.13. Antioxidant Activity
2.14. Statistical Analysis
3. Results and Discussion
3.1. Thickness
3.2. Moisture Content
3.3. Color
3.4. Mechanical Properties
3.5. Barrier Properties
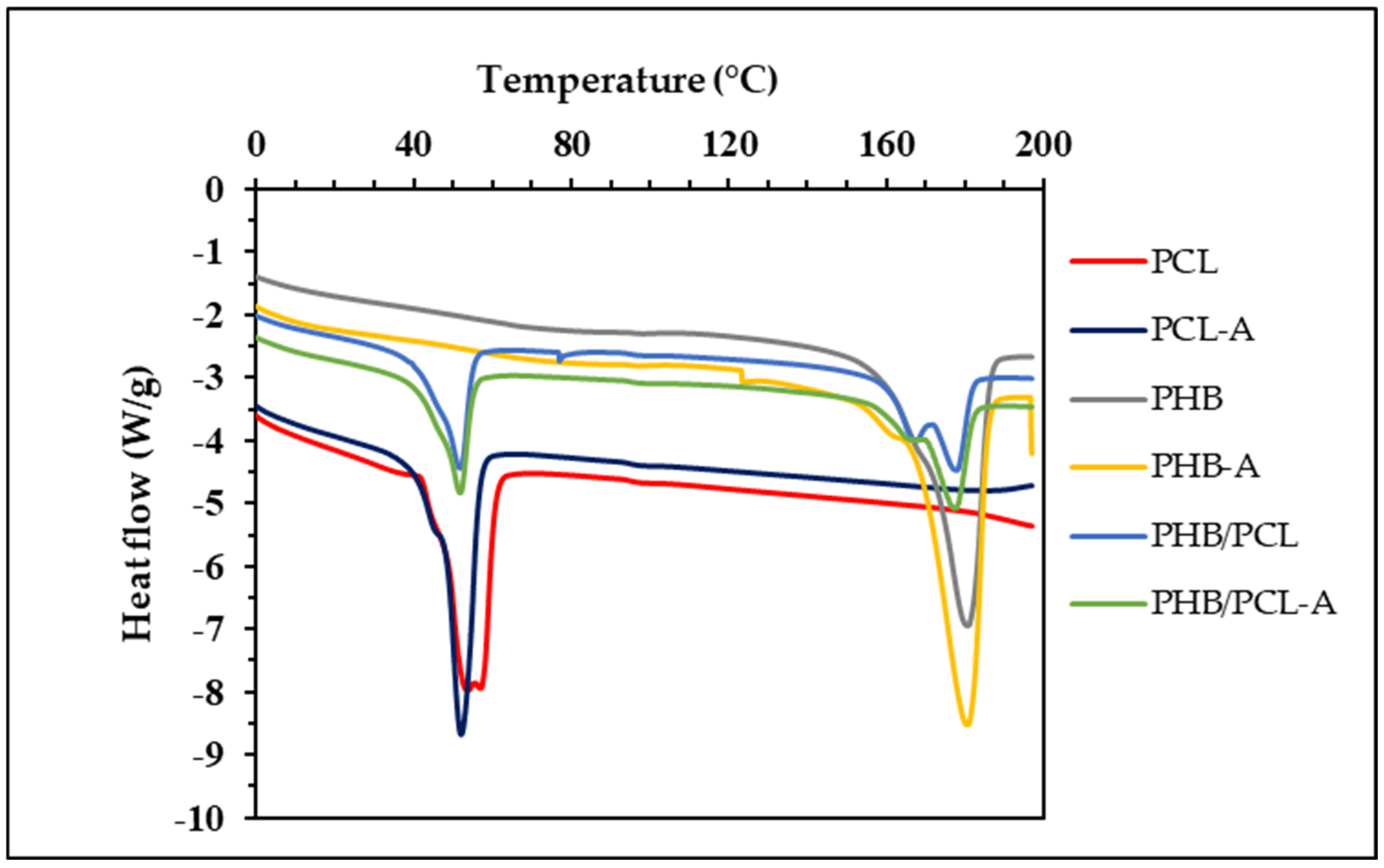
3.6. Thermal Characteristics
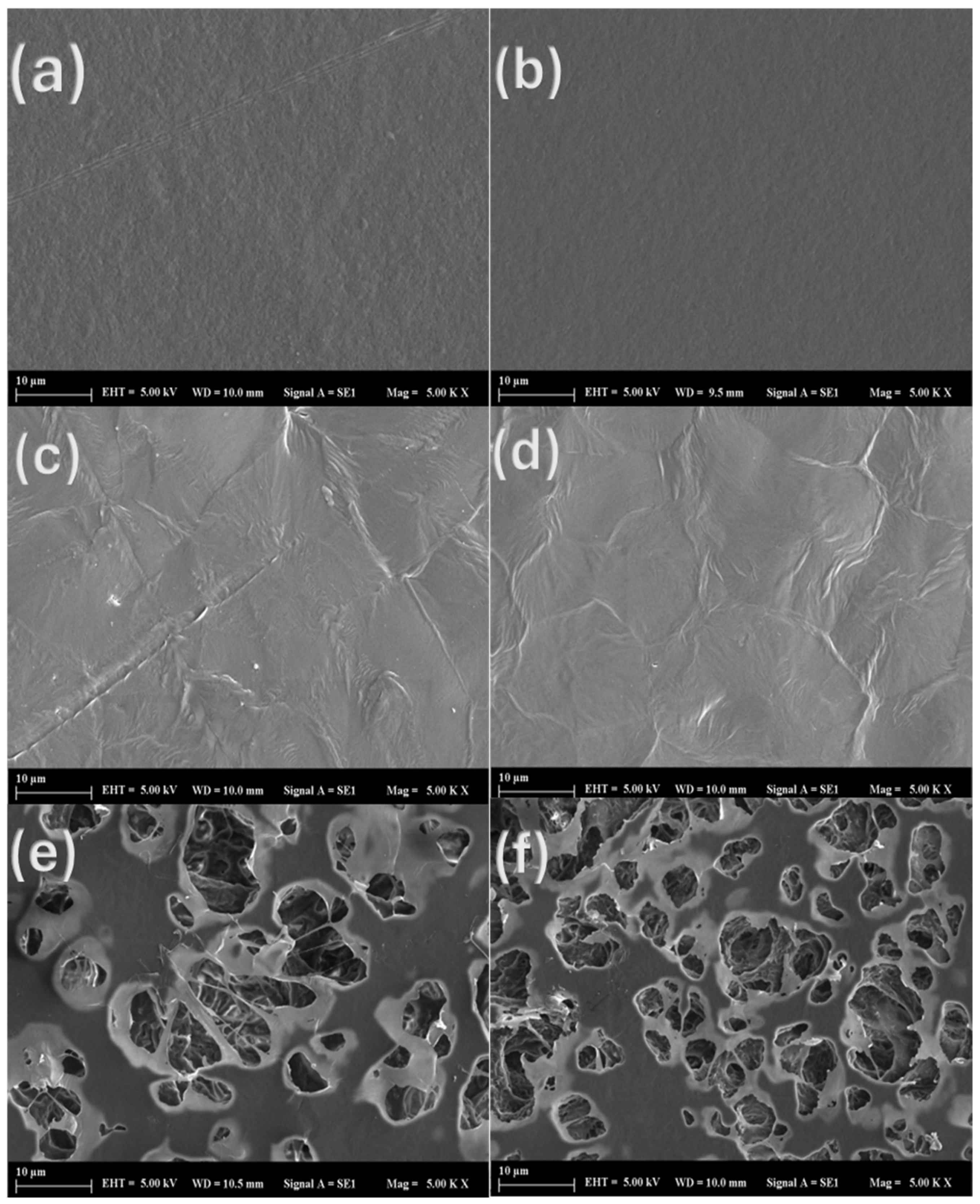
3.7. SEM
3.8. FTIR
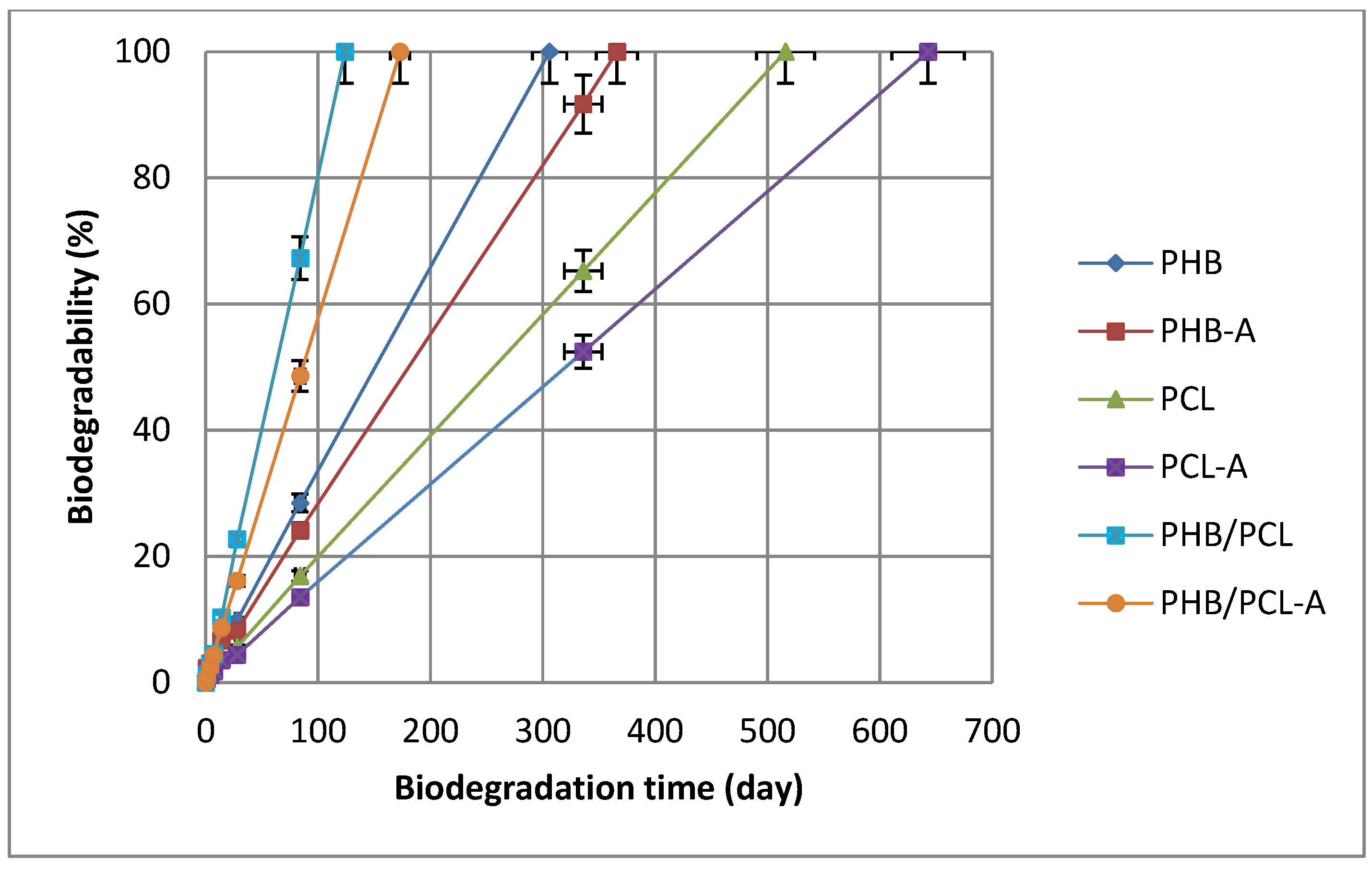
3.9. Film Biodegradability
3.10. Antimicrobial Activity
3.11. Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lamba, P.; Kaur, D.P.; Raj, S.; Sorout, J. Recycling/reuse of plastic waste as construction material for sustainable development: A review. Environ. Sci. Pollut. Res. 2022, 29, 86156–86179. [Google Scholar] [CrossRef] [PubMed]
- d’Ambrières, W. Plastics recycling worldwide: Current overview and desirable changes. Field Actions Sci. Rep. J. Field Actions 2019, 19, 12–21. Available online: https://journals.openedition.org/factsreports/5102 (accessed on 28 June 2024).
- Arrieta, M.P.; Castro-Lopez, M.D.M.; Rayón, E.; Barral-Losada, L.F.; López-Vilariño, J.M.; López, J.; González-Rodríguez, M.V. Plasticized Poly(lactic acid)–Poly(hydroxybutyrate) (PLA–PHB) Blends Incorporated with Catechin Intended for Active Food-Packaging Applications. J. Agric. Food Chem. 2014, 62, 10170–10180. [Google Scholar] [CrossRef] [PubMed]
- Cavalcante, M.P.; Toledo, A.L.M.M.; Rodrigues, E.J.R.; Neto, R.P.C.; Tavares, M.I.B. Correlation between traditional techniques and TD-NMR to determine the morphology of PHB/PCL blends. Polym. Test. 2017, 58, 159–165. [Google Scholar] [CrossRef]
- Correa, J.P.; Molina, V.; Sanchez, M.; Kainz, C.; Eisenberg, P.; Massani, M.B. Improving ham shelf life with a polyhydroxybutyrate/polycaprolactone biodegradable film activated with nisin. Food Packag. Shelf Life 2017, 11, 31–39. [Google Scholar] [CrossRef]
- Garcia-Garcia, D.; Lopez-Martinez, J.; Balart, R.; Strömberg, E.; Moriana, R. Reinforcing capability of cellulose nanocrystals obtained from pine cones in a biodegradable poly(3-hydroxybutyrate)/poly(ε-caprolactone) (PHB/PCL) thermoplastic blend. Eur. Polym. J. 2018, 104, 10–18. [Google Scholar] [CrossRef]
- Borisova, I.; Stoilova, O.; Manolova, N.; Rashkov, I. Modulating the Mechanical Properties of Electrospun PHB/PCL Materials by Using Different Types of Collectors and Heat Sealing. Polymers 2020, 12, 693. [Google Scholar] [CrossRef]
- Garcia-Garcia, D.; Ferri, J.M.; Boronat, T.; Lopez-Martinez, J.; Balart, R. Processing and characterization of binary poly(hydroxybutyrate) (PHB) and poly(caprolactone) (PCL) blends with improved impact properties. Polym. Bull. 2016, 73, 3333–3350. [Google Scholar] [CrossRef]
- Lovera, D.; Márquez, L.; Balsamo, V.; Taddei, A.; Castelli, C.; Müller, A.J. Crystallization, Morphology, and Enzymatic Degradation of Polyhydroxybutyrate/Polycaprolactone (PHB/PCL) Blends. Macromol. Chem. Phys. 2007, 208, 924–937. [Google Scholar] [CrossRef]
- Sharma, P.; Ahuja, A.; Dilsad Izrayeel, A.M.; Samyn, P.; Rastogi, V.K. Physicochemical and thermal characterization of poly (3-hydroxybutyrate-co-4-hydroxybutyrate) films incorporating thyme essential oil for active packaging of white bread. Food Control 2022, 133, 108688. [Google Scholar] [CrossRef]
- Bahmid, N.A.; Dekker, M.; Fogliano, V.; Heising, J. Development of a moisture-activated antimicrobial film containing ground mustard seeds and its application on meat in active packaging system. Food Packag. Shelf Life 2021, 30, 100753. [Google Scholar] [CrossRef]
- Kara, H.H.; Xiao, F.; Sarker, M.; Jin, T.Z.; Sousa, A.M.M.; Liu, C.; Tomasula, P.M.; Liu, L. Antibacterial poly(lactic acid) (PLA) films grafted with electrospun PLA/allyl isothiocyanate fibers for food packaging. J. Appl. Polym. Sci. 2016, 133, 42475. [Google Scholar] [CrossRef]
- Plackett, D.; Ghanbari-Siahkali, A.; Szente, L. Behavior of α- and β-cyclodextrin-encapsulated allyl isothiocyanate as slow-release additives in polylactide-co-polycaprolactone films. J. Appl. Polym. Sci. 2007, 105, 2850–2857. [Google Scholar] [CrossRef]
- Dias, M.V.; Soares, N.d.F.F.; Borges, S.V.; de Sousa, M.M.; Nunes, C.A.; de Oliveira, I.R.N.; Medeiros, E.A.A. Use of allyl isothiocyanate and carbon nanotubes in an antimicrobial film to package shredded, cooked chicken meat. Food Chem. 2013, 141, 3160–3166. [Google Scholar] [CrossRef]
- İlaslan, K.; Tornuk, F.; Durak, M.Z. Development of polycaprolactone biodegradable films reinforced with silver-doped organoclay and effect on the microbiological quality of ground beef meat. J. Food Process Preserv. 2022, 46, e16862. [Google Scholar] [CrossRef]
- Mutlu, N. Physicochemical and antimicrobial properties of biodegradable films based on gelatin/guar gum incorporated with grape seed oil. J. Food Meas. Charact. 2023, 17, 1515–1525. [Google Scholar] [CrossRef]
- Akman, P.K.; Kutlu, G.; Tornuk, F. Development and characterization of a novel sodium alginate based active film supplemented with Lactiplantibacillus plantarum postbiotic. Int. J. Biol. Macromol. 2023, 244, 125240. [Google Scholar] [CrossRef]
- Kumari, S.V.G.; Pakshirajan, K.; Pugazhenthi, G. Facile fabrication and characterization of novel antimicrobial and antioxidant poly (3-hydroxybutyrate)/essential oil composites for potential use in active food packaging applications. Int. J. Biol. Macromol. 2023, 252, 126566. [Google Scholar] [CrossRef]
- Memiş, S.; Tornuk, F.; Bozkurt, F.; Durak, M.Z. Production and characterization of a new biodegradable fenugreek seed gum based active nanocomposite film reinforced with nanoclays. Int. J. Biol. Macromol. 2017, 103, 669–675. [Google Scholar] [CrossRef]
- Kurt, A.; Kahyaoglu, T. Characterization of a new biodegradable edible film made from salep glucomannan. Carbohydr. Polym. 2014, 104, 50–58. [Google Scholar] [CrossRef]
- Mahcene, Z.; Khelil, A.; Hasni, S.; Akman, P.K.; Bozkurt, F.; Birech, K.; Goudjil, M.B.; Tornuk, F. Development and characterization of sodium alginate based active edible films incorporated with essential oils of some medicinal plants. Int. J. Biol. Macromol. 2020, 145, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Atlar, G.C.; Kutlu, G.; Tornuk, F. Design and characterization of chitosan-based films incorporated with summer savory (Satureja hortensis L.) essential oil for active packaging. Int. J. Biol. Macromol. 2024, 254, 127732. [Google Scholar] [CrossRef] [PubMed]
- Apak, R.; Güçlü, K.; Ozyürek, M.; Karademir, S.E. Novel total antioxidant capacity index for dietary polyphenols and vitamins C and E, using their cupric ion reducing capability in the presence of neocuproine: CUPRAC method. J. Agric. Food Chem. 2004, 52, 7970–7981. [Google Scholar] [CrossRef]
- Sharma, S.; Barkauskaite, S.; Duffy, B.; Jaiswal, A.K.; Jaiswal, S. Characterization and Antimicrobial Activity of Biodegradable Active Packaging Enriched with Clove and Thyme Essential Oil for Food Packaging Application. Foods 2020, 9, 1117. [Google Scholar] [CrossRef]
- Nisar, T.; Wang, Z.C.; Yang, X.; Tian, Y.; Iqbal, M.; Guo, Y. Characterization of citrus pectin films integrated with clove bud essential oil: Physical, thermal, barrier, antioxidant and antibacterial properties. Int. J. Biol. Macromol. 2018, 106, 670–680. [Google Scholar] [CrossRef]
- Rech, C.R.; Brabes, K.C.d.S.; e Silva, B.E.B.; Bittencourt, P.R.S.; Koschevic, M.T.; da Silveira, T.F.S.; Martines, M.A.U.; Caon, T.; Martelli, S.M. Biodegradation of eugenol-loaded polyhydroxybutyrate films in different soil types. Case Stud. Chem. Environ. Eng. 2020, 2, 100014. [Google Scholar] [CrossRef]
- Przybysz, M.; Marć, M.; Klein, M.; Saeb, M.R.; Formela, K. Structural, mechanical and thermal behavior assessments of PCL/PHB blends reactively compatibilized with organic peroxides. Polym. Test. 2018, 67, 513–521. [Google Scholar] [CrossRef]
- Engler, L.G.; Della Giustina, M.; Giovanela, M.; Roesch-Ely, M.; Gately, N.; Major, I.; Crespo, J.S.; Devine, D.M. Exploring the Synergy of Metallic Antimicrobial Agents in Ternary Blends of PHB/PLA/PCL. J. Biomed. Mater. Res. A 2025, 113, e37857. [Google Scholar] [CrossRef]
- Erceg, T.; Rackov, S.; Terek, P.; Pilić, B. Preparation and Characterization of PHBV/PCL-Diol Blend Films. Polymers 2023, 15, 4694. [Google Scholar] [CrossRef]
- Manikandan, N.A.; Pakshirajan, K.; Pugazhenthi, G. A closed-loop biorefinery approach for polyhydroxybutyrate (PHB) production using sugars from carob pods as the sole raw material and downstream processing using the co-product lignin. Bioresour. Technol. 2020, 307, 123247. [Google Scholar] [CrossRef]
- Melendez-Rodriguez, B.; Torres-Giner, S.; Angulo, I.; Pardo-Figuerez, M.; Hilliou, L.; Escuin, J.M.; Cabedo, L.; Nevo, Y.; Prieto, C.; Lagaron, J.M. High-Oxygen-Barrier Multilayer Films Based on Polyhydroxyalkanoates and Cellulose Nanocrystals. Nanomaterials 2021, 11, 1443. [Google Scholar] [CrossRef] [PubMed]
- Arrieta, M.P.; López, J.; Hernández, A.; Rayón, E. Ternary PLA–PHB–Limonene blends intended for biodegradable food packaging applications. Eur. Polym. J. 2014, 50, 255–270. [Google Scholar] [CrossRef]
- Othman, N.A.F.; Selambakkannu, S.; Seko, N. Biodegradable dual-layer Polyhydroxyalkanoate (pha)/Polycaprolactone (pcl) mulch film for agriculture: Preparation and characterization. Energy Nexus 2022, 8, 100137. [Google Scholar] [CrossRef]
- Rech, C.R.; Brabes, K.C.S.; Silva, B.E.B.; Martines, M.A.U.; Silveira, T.F.S.; Alberton, J.; Amadeu, C.A.A.; Caon, T.; Arruda, E.J.; Martelli, S.M. Antimicrobial and Physical–Mechanical Properties of Polyhydroxybutyrate Edible Films Containing Essential Oil Mixtures. J. Polym. Environ. 2021, 29, 1202–1211. [Google Scholar] [CrossRef]
- Holešová, S.; Barabaszová, K.Č.; Hundáková, M.; Kratošová, G.; Kaloč, V.; Joszko, K.; Gzik-Zroska, B. Comprehensive study of antimicrobial polycaprolactone/clay nanocomposite films: Preparation, characterization, properties and degradation in simulated body fluid. Polym. Compos. 2024, 45, 9280–9298. [Google Scholar] [CrossRef]
- Altaee, N.; El-Hiti, G.A.; Fahdil, A.; Sudesh, K.; Yousif, E. Biodegradation of different formulations of polyhydroxybutyrate films in soil. SpringerPlus 2016, 5, 762. [Google Scholar] [CrossRef]
- La Cara, F.; Immirzi, B.; Ionata, E.; Mazzella, A.; Portofino, S.; Orsello, G.; De Prisco, P. Biodegradation of poly-ε-caprolactone/poly-β-hydroxybutyrate blend. Polym. Degrad. Stab. 2003, 79, 37–43. [Google Scholar] [CrossRef]
- Sousa, F.M.; Cavalcanti, F.B.; Marinho, V.A.D.; Morais, D.D.S.; Almeida, T.G.; Carvalho, L.H. Effect of composition on permeability, mechanical properties and biodegradation of PBAT/PCL blends films. Polym. Bull. 2022, 79, 5327–5338. [Google Scholar] [CrossRef]
- Giaquinto, C.D.M.; Souza, G.K.M.D.; Caetano, V.F.; Vinhas, G.M. Evaluation of the mechanical and thermal properties of PHB/canola oil films. Polímeros 2017, 27, 201–207. [Google Scholar] [CrossRef]
- Martínez-Abad, A.; Sánchez, G.; Fuster, V.; Lagaron, J.M.; Ocio, M.J. Antibacterial performance of solvent cast polycaprolactone (PCL) films containing essential oils. Food Control 2013, 34, 214–220. [Google Scholar] [CrossRef]
- Wang, S.Y.; Chen, C.T.; Yin, J.J. Effect of allyl isothiocyanate on antioxidants and fruit decay of blueberries. Food Chem. 2010, 120, 199–204. [Google Scholar] [CrossRef]
- Lin, C.M.; Preston, J.F.; Wei, C.I. Antibacterial Mechanism of Allyl Isothiocyanate †. J. Food Prot. 2000, 63, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Alibrahem, W.; Nguyen, D.H.H.; Helu, N.K.; Tóth, F.; Nagy, P.T.; Posta, J.; Prokisch, J.; Oláh, C. Health Benefits, Applications, and Analytical Methods of Freshly Produced Allyl Isothiocyanate. Foods 2025, 14, 579. [Google Scholar] [CrossRef] [PubMed]
- Rajakumar, T.; Pugalendhi, P. Allyl isothiocyanate regulates oxidative stress, inflammation, cell proliferation, cell cycle arrest, apoptosis, angiogenesis, invasion and metastasis via interaction with multiple cell signaling pathways. Histochem. Cell Biol. 2024, 161, 211–221. [Google Scholar] [CrossRef]
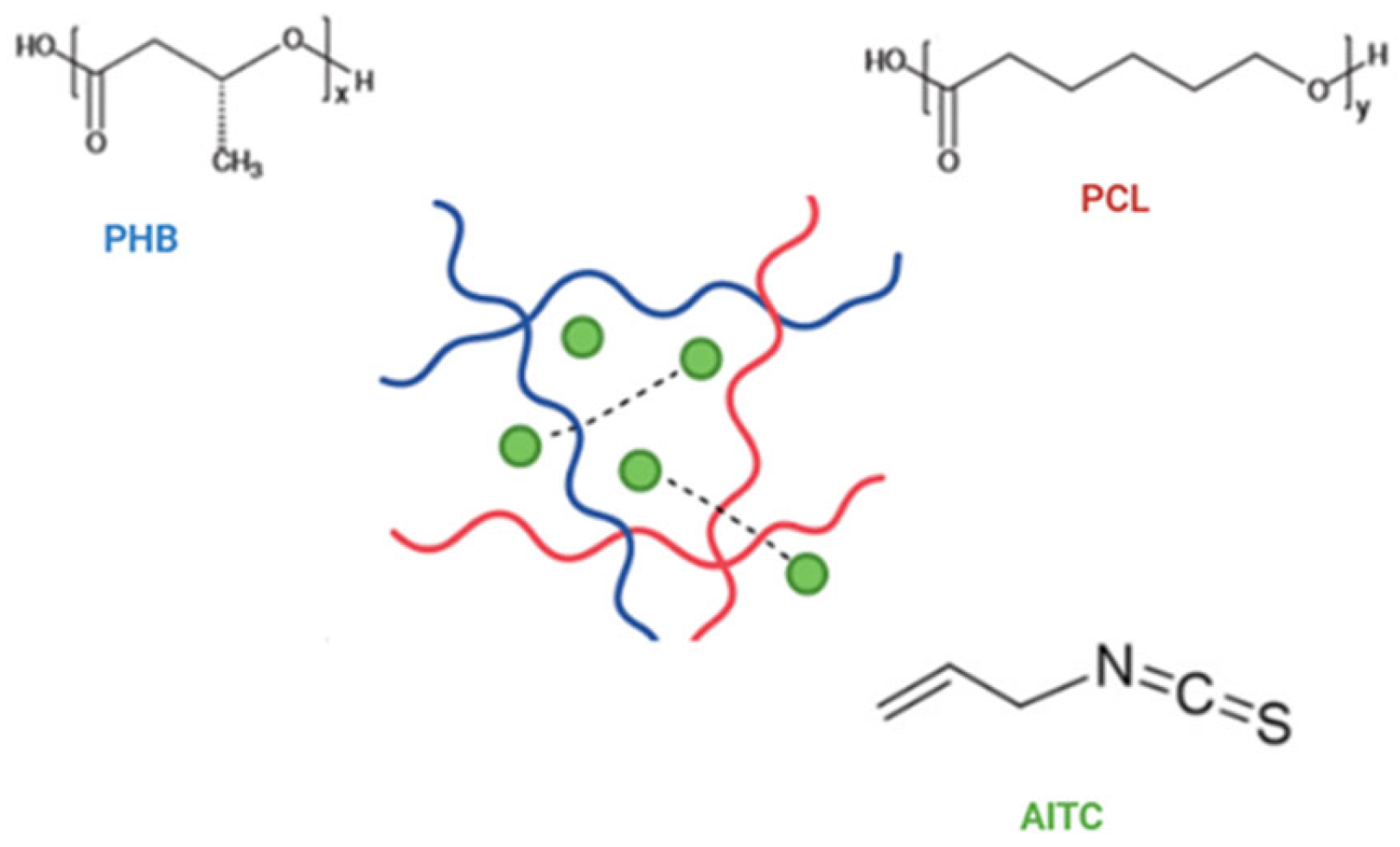

| Film Code | Polymer Composition | Polymer (g) | AITC (mL) | Key Process Parameters |
|---|---|---|---|---|
| PHB | PHB | 2.00 | 0 | 55 °C, 7–8 h stirring |
| PHB-A | PHB + AITC | 2.00 | 1 | 55 °C, 7–8 h + 10 min AITC |
| PCL | PCL | 4.00 | 0 | RT, 20 min stirring |
| PCL-A | PCL + AITC | 4.00 | 1 | RT, 20 min + 10 min AITC |
| PHB/PCL | PHB:PCL (1:2) | 1.00 PHB + 2.00 PCL | 0 | 55 °C (PHB) + RT (PCL) mix |
| PHB/PCL-A | PHB:PCL (1:2) + AITC | 1.00 PHB + 2.00 PCL | 1 | 55 °C (PHB) + RT (PCL) + 10 min AITC |
| Film Samples | Thickness (mm) | Moisture Content (%) | L* | a* | b* |
|---|---|---|---|---|---|
| PHB | 0.183 ± 0.007 d | 0.254 ± 0.017 ab | 90.50 ± 0.33 b | 0.14 ± 0.02 b | 1.60 ± 0.29 d |
| PHB-A | 0.171 ± 0.009 d | 0.215 ± 0.008 b | 89.12 ± 0.20 c | 0.20 ± 0.08 b | 2.13 ± 0.38 c |
| PCL | 0.210 ± 0.014 c | 0.270 ± 0.027 a | 90.18 ± 0.32 b | 0.41 ± 0.03 a | 0.23 ± 0.05 e |
| PCL-A | 0.222 ± 0.010 c | 0.218 ± 0.003 b | 90.24 ± 0.25 b | 0.17 ± 0.04 c | 2.52 ± 0.09 b |
| PHB-PCL | 0.267 ± 0.025 b | 0.256 ± 0.010 ab | 95.16 ± 0.46 a | 0.52 ± 0.02 d | 2.12 ± 0.04 c |
| PHB-PCL-A | 0.299 ± 0.016 a | 0.232 ± 0.020 ab | 95.83 ± 0.64 a | 0.81 ± 0.04 e | 3.48 ± 0.15 a |
| Film Samples | Tensile Strength (MPa) | Elongation at Break (%) | WVP (g mm h−1 m−2 kPa−1) | PV (meq/kg) |
|---|---|---|---|---|
| PHB | 19.82 ± 2.86 a | 1.13 ± 0.37 c | 0.041 ± 0.009 b | 23.05 ± 3.38 ab |
| PHB-A | 17.92 ± 0.18 ab | 1.27 ± 0.64 c | 0.027 ± 0.004 b | 27.08 ± 1.71 a |
| PCL | 12.21 ± 2.17 bc | 53.00 ± 7.55 a | 0.056 ± 0.010 b | 18.53 ± 1.73 b |
| PCL-A | 10.38 ± 2.32 c | 55.67 ± 8.02 a | 0.192 ± 0.010 a | 18.48 ± 1.45 b |
| PHB-PCL | 15.34 ± 2.25 abc | 21.33 ± 7.51 b | 0.212 ± 0.046 a | 22.70 ± 1.75 ab |
| PHB-PCL-A | 13.88 ± 1.86 bc | 21.67 ± 7.09 b | 0.174 ± 0.013 a | 21.69 ± 1.10 ab |
| Film Samples | T1onset (°C) | T1peak (°C) | T1end (°C) | ΔH1 (J/g) | T2onset (°C) | T2peak (°C) | T2end (°C) | ΔH2 (J/g) |
|---|---|---|---|---|---|---|---|---|
| PHB | - | - | - | - | 142.29 | 180.27 | 192.35 | 89.69 |
| PHB-A | - | - | - | - | 161.51 | 180.69 | 190.56 | 69.70 |
| PCL | 40.16 | 53.71 | 66.53 | 50.35 | - | - | - | - |
| PCL-A | 18.93 | 51.94 | 62.51 | 54.93 | - | - | - | - |
| PHB/PCL | 27.65 | 51.79 | 60.50 | 24.53 | 151.45 | 177.67 | 185.42 | 27.94 |
| PHB/PCL-A | 33.01 | 51.84 | 61.17 | 22.05 | 154.58 | 177.05 | 182.42 | 26.26 |
| Film Sample | S. aureus | B. cereus | L. monocytogenes | E. coli O157:H7 | S. Typhimurium | Antioxidant Capacity CUPRAC (mg TE/1 g FS) |
|---|---|---|---|---|---|---|
| PHB | 00.00 ± 0.00 Ba | 00.00 ± 0.00 Ca | 00.00 ± 0.00 Da | 00.00 ± 0.00 Ca | 00.00 ± 0.00 Ca | - |
| PHB-A | 30.75 ± 3.30 Ab | 30.50 ± 2.08 Ab | 20.75 ± 2.06 Bc | 37.25 ± 5.31 Aa | 31.50 ± 4.93 ABb | 286.41 ± 4.75 A |
| PCL | 00.00 ± 0.00 Ba | 00.00 ± 0.00 Ca | 00.00 ± 0.00 Da | 00.00 ± 0.00 Ca | 00.00 ± 0.00 Ca | - |
| PCL-A | 32.50 ± 3.00 Aab | 30.00 ± 4.24 Abc | 24.50 ± 2.88 Ac | 33.25 ± 3.86 Aab | 36.25 ± 4.03 Aa | 283.10 ± 6.22 A |
| PHB/PCL | 00.00 ± 0.00 Ba | 00.00 ± 0.00 Ca | 00.00 ± 0.00 Da | 00.00 ± 0.00 Ca | 00.00 ± 0.00 Ca | - |
| PHB/PCL-A | 29.50 ± 4.65 Aa | 18.25 ± 2.75 Bb | 16.25 ± 1.50 Cb | 26.00 ± 1.83 Ba | 25.50 ± 1.91 Ba | 281.85 ± 4.68 A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Memis Karabuga, S.; Akman, P.K.; Tornuk, F. Fabrication and Characterization of Poly(hydroxybutyrate)- and Poly(caprolactone)-Based Active Biodegradable Films Incorporating Allyl Isothiocyanate. Polymers 2025, 17, 1189. https://doi.org/10.3390/polym17091189
Memis Karabuga S, Akman PK, Tornuk F. Fabrication and Characterization of Poly(hydroxybutyrate)- and Poly(caprolactone)-Based Active Biodegradable Films Incorporating Allyl Isothiocyanate. Polymers. 2025; 17(9):1189. https://doi.org/10.3390/polym17091189
Chicago/Turabian StyleMemis Karabuga, Saliha, Perihan Kubra Akman, and Fatih Tornuk. 2025. "Fabrication and Characterization of Poly(hydroxybutyrate)- and Poly(caprolactone)-Based Active Biodegradable Films Incorporating Allyl Isothiocyanate" Polymers 17, no. 9: 1189. https://doi.org/10.3390/polym17091189
APA StyleMemis Karabuga, S., Akman, P. K., & Tornuk, F. (2025). Fabrication and Characterization of Poly(hydroxybutyrate)- and Poly(caprolactone)-Based Active Biodegradable Films Incorporating Allyl Isothiocyanate. Polymers, 17(9), 1189. https://doi.org/10.3390/polym17091189







