Static Magnetic Field Increases Polyhydroxyalkanoates Biosynthesis in Haloferax mediterranei: Parameter Optimization and Mechanistic Insights from Metabolomics
Abstract
:1. Introduction
2. Materials and Methods
2.1. Strain Cultivation and Fermentation Method
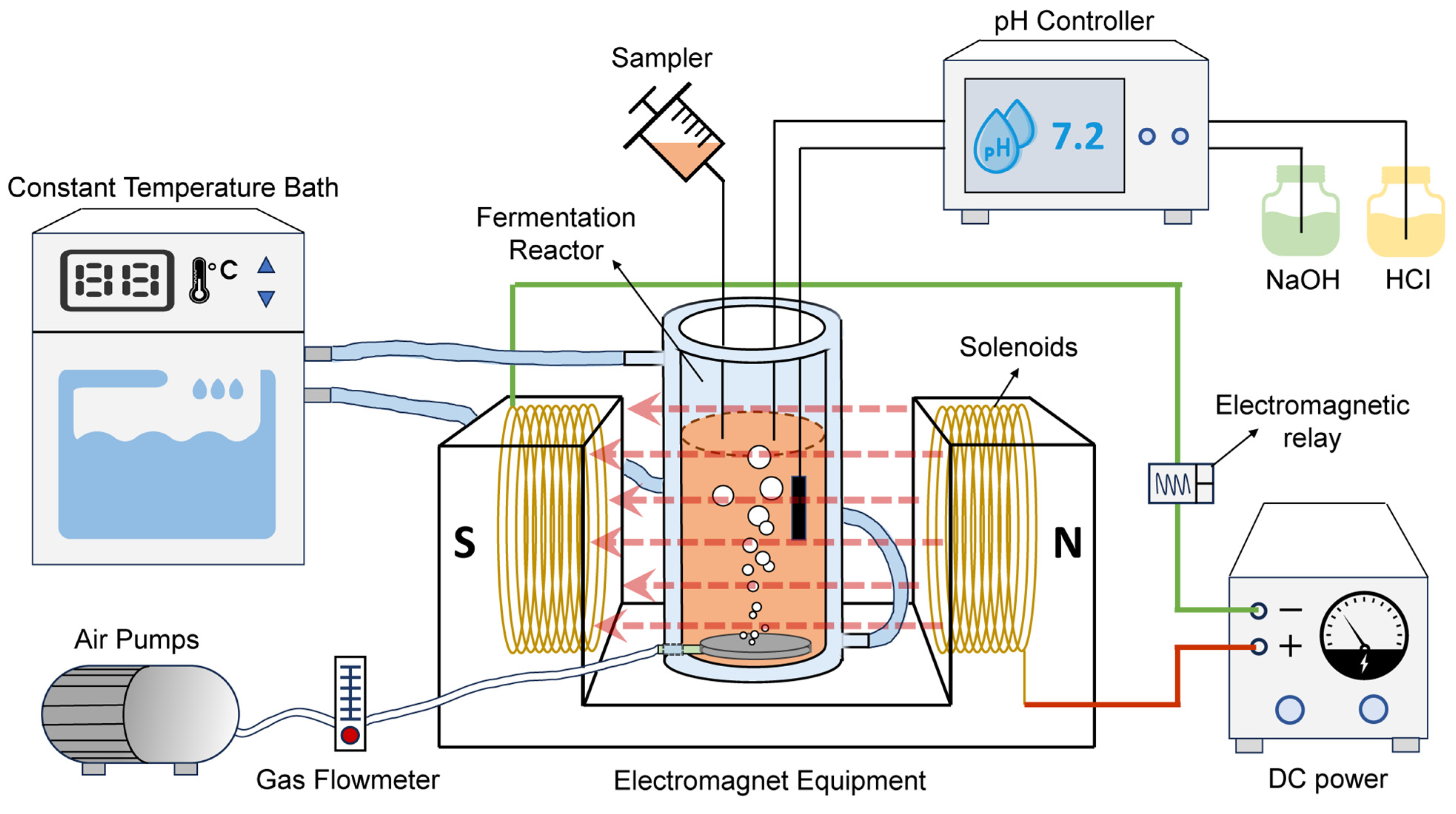
2.2. Reactors Set-Up and SMF Exposure
2.3. PHA Detection
2.4. Analytical Methods
2.5. Metabolomics Analysis
2.6. Statistical Analysis
3. Results and Discussion
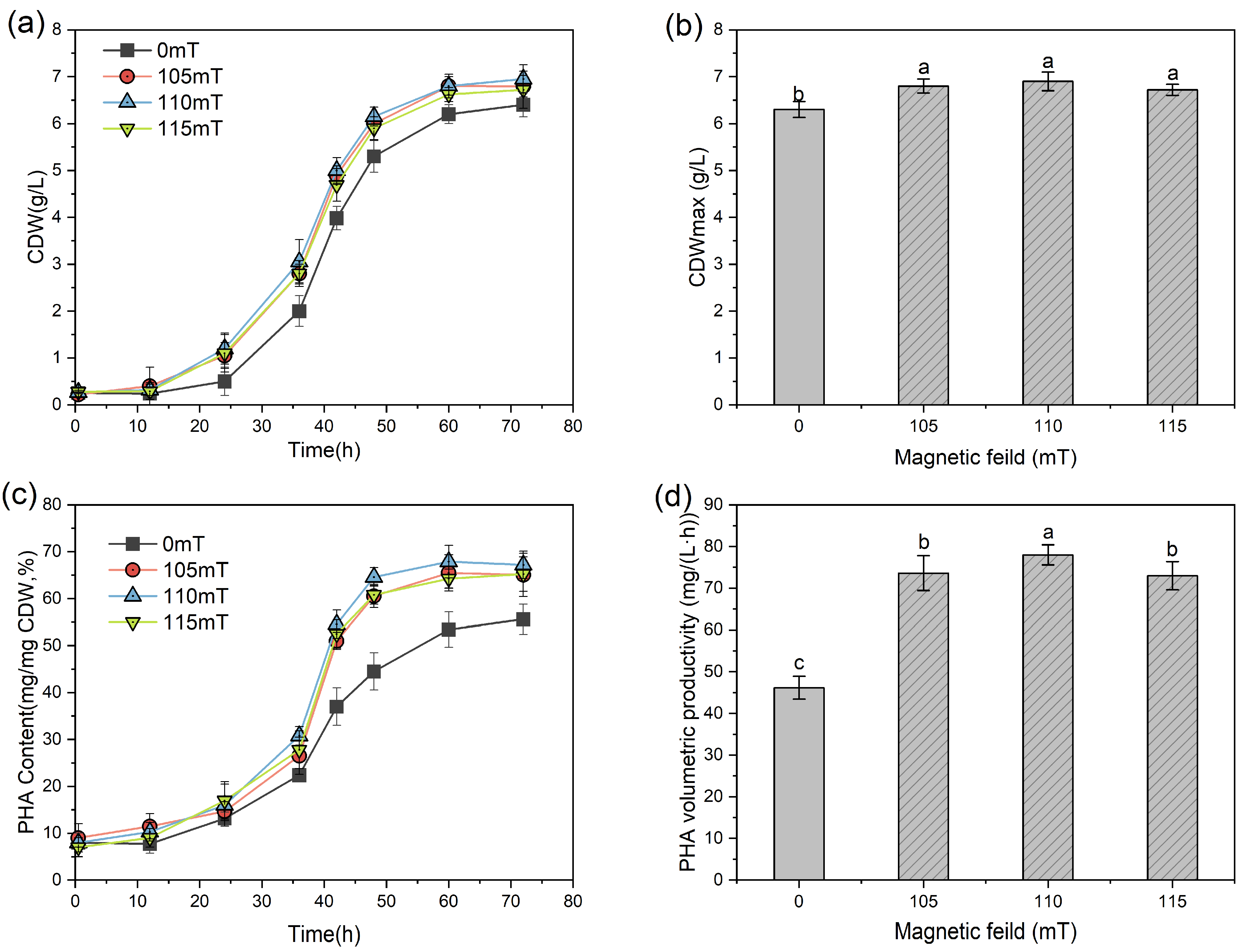
3.1. Optimization of MF Intensity for PHA Biosynthesis
3.2. Comparative Impacts of MF Types on PHA Biosynthesis
3.3. Effect of MF Exposure Durations on PHA Synthesis
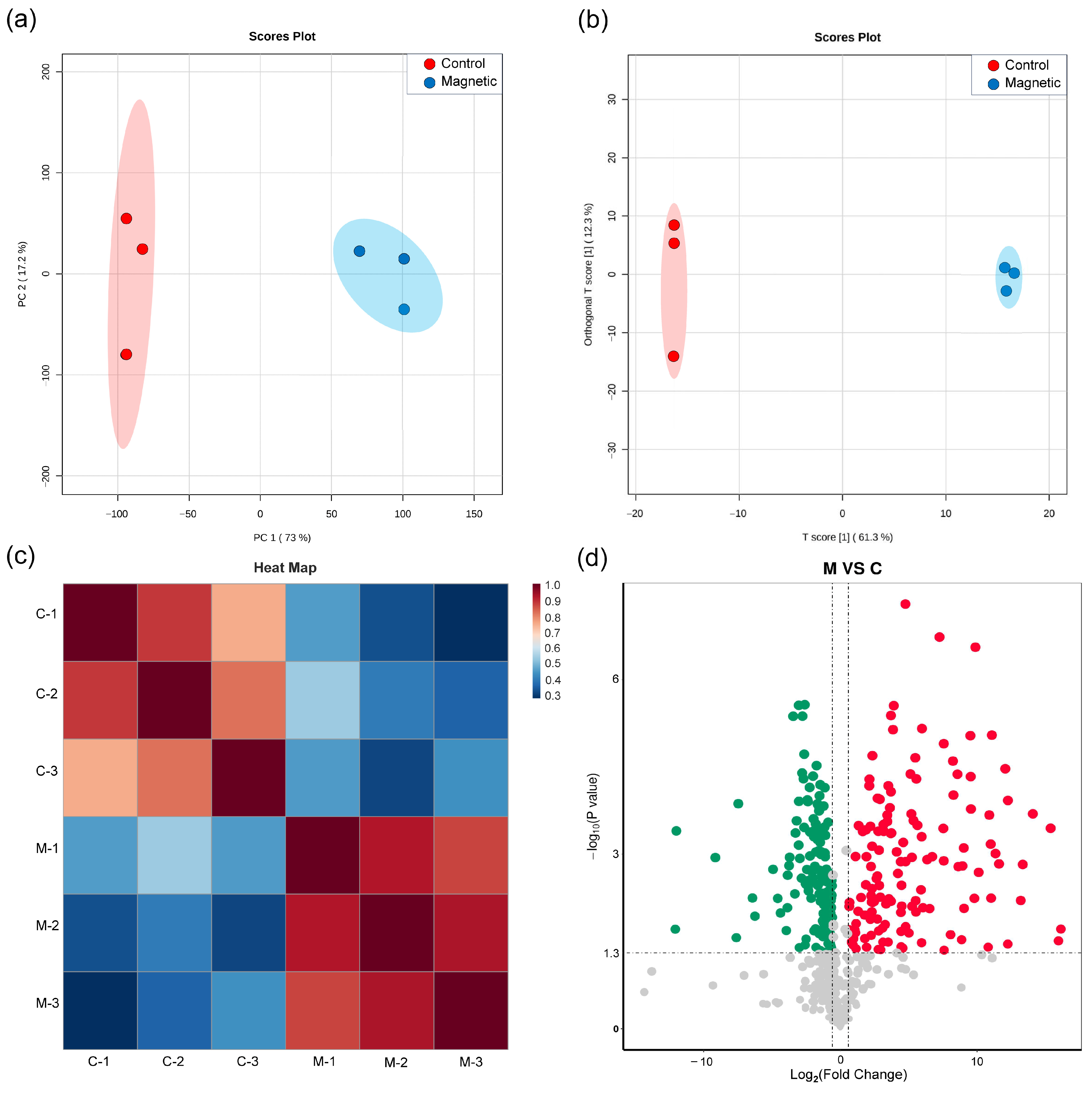
3.4. Metabolic Regulation Under SMF Exposure
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| H. mediterranei | Haloferax mediterranei |
| SMF | Static magnetic field |
| MF | Magnetic field |
| PMF | Pulsed magnetic field |
| PHA | Polyhydroxyalkanoates |
| PHB | Poly-β-hydroxybutyrate |
| PHV | Poly-β-hydroxyvalerate |
| COD | Chemical oxygen demand |
| CDW | Cell dry weight |
| SOD | Superoxide dismutase |
| MDA | Malondialdehyde |
| LDH | Lactate dehydrogenase |
| ROS | Reactive oxygen species |
| UHPLC | Ultra-high-performance liquid chromatography |
| DDA | Data-dependent acquisition |
| QC | Quality control |
| ANOVA | One-way analysis of variance |
| SD | Standard deviation |
| PCA | Principal component analysis |
| OPLS-DA | Orthogonal partial least squares discriminant analysis |
| OFAT | One-factor-at-a-time |
| VIP | Variable importance in projection |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
References
- Popa, M.S.; Frone, A.N.; Panaitescu, D.M. Polyhydroxybutyrate blends: A solution for biodegradable packaging? Int. J. Biol. Macromol. 2022, 207, 263–277. [Google Scholar] [CrossRef]
- Meereboer, K.W.; Misra, M.; Mohanty, A.K. Review of recent advances in the biodegradability of polyhydroxyalkanoate (PHA) bioplastics and their composites. Green Chem. 2020, 22, 5519–5558. [Google Scholar] [CrossRef]
- Bugnicourt, E.; Cinelli, P.; Lazzeri, A.; Alvarez, V. Polyhydroxyalkanoate (PHA): Review of synthesis, characteristics, processing and potential applications in packaging. Express Polym. Lett. 2014, 8, 791–808. [Google Scholar] [CrossRef]
- Li, J.; Li, D.; Su, Y.; Yan, X.; Wang, F.; Yu, L.; Ma, X. Efficient and economical production of polyhydroxyalkanoate from sustainable rubber wood hydrolysate and xylose as co-substrate by mixed microbial cultures. Bioresour. Technol. 2022, 355, 127238. [Google Scholar] [CrossRef] [PubMed]
- Urtuvia, V.; Villegas, P.; González, M.; Seeger, M. Bacterial production of the biodegradable plastics polyhydroxyalkanoates. Int. J. Biol. Macromol. 2014, 70, 208–213. [Google Scholar] [CrossRef]
- Longo, A.; Fanelli, F.; Villano, M.; Montemurro, M.; Rizzello, C.G. Bioplastic Production from Agri-Food Waste through the Use of Haloferax mediterranei: A Comprehensive Initial Overview. Microorganisms 2024, 12, 1038. [Google Scholar] [CrossRef]
- Alsafadi, D.; Alhesan, J.S.A.; Mansoura, A.; Oqdeha, S. Production of polyhydroxyalkanoate from sesame seed wastewater by sequencing batch reactor cultivation process of Haloferax mediterranei. Arab. J. Chem. 2023, 16, 104584. [Google Scholar] [CrossRef]
- Katagi, V.; Vytla, R.M.; Somashekara, D. Integrated production of microbial biopolymer (PHA) with other value-added bioproducts: An innovative approach for sustainable production. Green Chem. Lett. Rev. 2024, 17, 2289983. [Google Scholar] [CrossRef]
- Zadeh-Haghighi, H.; Simon, C. Magnetic field effects in biology from the perspective of the radical pair mechanism. J. R. Soc. Interface 2022, 19, 20220325. [Google Scholar] [CrossRef]
- Moore, R.L. Biological effects of magnetic fields: Studies with microorganisms. Can. J. Microbiol. 1979, 25, 1145–1151. [Google Scholar] [CrossRef]
- Beretta, G.; Mastorgio, A.F.; Pedrali, L.; Saponaro, S.; Sezenna, E. The effects of electric, magnetic and electromagnetic fields on microorganisms in the perspective of bioremediation. Rev. Environ. Sci. Bio/Technol. 2019, 18, 29–75. [Google Scholar] [CrossRef]
- Pospíšilová, D.; Schreiberová, O.; Jirků, V.; Lederer, T. Effects of magnetic field on phenol biodegradation and cell physiochemical properties of Rhodococcus erythropolis. Bioremediat. J. 2015, 19, 201–206. [Google Scholar] [CrossRef]
- Potenza, L.; Ubaldi, L.; De Sanctis, R.; De Bellis, R.; Cucchiarini, L.; Dachà, M. Effects of a static magnetic field on cell growth and gene expression in Escherichia coli. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2004, 561, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.Y.; Zhou, C.S.; Ma, H.L.; Li, S.J.; Yagoub, A.A.; Abdualrahman, M.A.Y. Biological Effect and Inactivation Mechanism of Bacillus subtilis Exposed to Pulsed Magnetic Field: Morphology, Membrane Permeability and Intracellular Contents. Food Biophys. 2016, 11, 429–435. [Google Scholar] [CrossRef]
- Martino, C.F.; Perea, H.; Hopfner, U.; Ferguson, V.L.; Wintermantel, E. Effects of weak static magnetic fields on endothelial cells. Bioelectromagn. J. Bioelectromagn. Soc. Soc. Phys. Regul. Biol. Med. Eur. Bioelectromagn. Assoc. 2010, 31, 296–301. [Google Scholar] [CrossRef]
- Snoussi, S.; El May, A.; Coquet, L.; Chan, P.; Jouenne, T.; Dé, E.; Landoulsi, A. Unraveling the effects of static magnetic field stress on cytosolic proteins of Salmonella by using a proteomic approach. Can. J. Microbiol. 2016, 62, 338–348. [Google Scholar] [CrossRef]
- Hu, B.; Wang, Y.; Quan, J.; Huang, K.; Gu, X.; Zhu, J.; Yan, Y.; Wu, P.; Yang, L.; Zhao, J. Effects of static magnetic field on the performances of anoxic/oxic sequencing batch reactor. Bioresour. Technol. 2020, 309, 123299. [Google Scholar] [CrossRef]
- Xu, D.; Ji, H.; Ren, H.; Geng, J.; Li, K.; Xu, K. Inhibition effect of magnetic field on nitrous oxide emission from sequencing batch reactor treating domestic wastewater at low temperature. J. Environ. Sci. 2020, 87, 205–212. [Google Scholar] [CrossRef]
- Zhu, Y.-M.; Ji, H.; Ren, H.; Geng, J.; Xu, K. Enhancement of static magnetic field on nitrogen removal at different ammonium concentrations in a sequencing batch reactor: Performance and biological mechanism. Chemosphere 2021, 268, 128794. [Google Scholar] [CrossRef]
- Chen, S.; Cui, Y.-W.; Huang, M.-Q. Coupling magnetic field and salinity upshock to improve polyhydroxyalkanoate productivity by Haloferax mediterranei feeding on molasses wastewater. Appl. Environ. Microbiol. 2022, 88, e00305–e00322. [Google Scholar] [CrossRef]
- Gao, Z.-L.; Cui, Y.-W.; Sui, Y.; Yang, H.-J. Unveiling the mechanism of static magnetic field effects on carbon flux into polyhydroxyalkanoate synthesis by Haloferax mediterranei. Chem. Eng. J. 2023, 476, 146647. [Google Scholar] [CrossRef]
- Letuta, U.G.; Berdinskiy, V.L. Magnetosensitivity of bacteria E. coli: Magnetic isotope and magnetic field effects. Bioelectromagnetics 2017, 38, 581–591. [Google Scholar] [CrossRef] [PubMed]
- Rice, E.W.; Baird, R.B.; Eaton, A.D.; Clesceri, L.S. Standard Methods for the Examination of Water and Wastewater, 22nd ed.; American Public Health Association American Water Works Association Water Environment Federation: Washington, DC, USA, 2012; pp. 386–396. [Google Scholar]
- Zhou, B.; Xiao, J.F.; Tuli, L.; Ressom, H.W. LC-MS-based metabolomics. Mol. BioSyst. 2012, 8, 470–481. [Google Scholar] [CrossRef] [PubMed]
- Wen, B.; Mei, Z.; Zeng, C.; Liu, S. metaX: A flexible and comprehensive software for processing metabolomics data. BMC Bioinform. 2017, 18, 183. [Google Scholar] [CrossRef]
- Blasco, H.; Błaszczyński, J.; Billaut, J.-C.; Nadal-Desbarats, L.; Pradat, P.-F.; Devos, D.; Moreau, C.; Andres, C.R.; Emond, P.; Corcia, P. Comparative analysis of targeted metabolomics: Dominance-based rough set approach versus orthogonal partial least square-discriminant analysis. J. Biomed. Inform. 2015, 53, 291–299. [Google Scholar] [CrossRef]
- Dini, L.; Abbro, L. Bioeffects of moderate-intensity static magnetic fields on cell cultures. Micron 2005, 36, 195–217. [Google Scholar] [CrossRef]
- Zhang, Y.-T.; Wei, W.; Huang, Q.-S.; Wang, C.; Wang, Y.; Ni, B.-J. Insights into the microbial response of anaerobic granular sludge during long-term exposure to polyethylene terephthalate microplastics. Water Res. 2020, 179, 115898. [Google Scholar] [CrossRef]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef]
- Kruglova, A.; Gonzalez-Martinez, A.; Kråkström, M.; Mikola, A.; Vahala, R. Bacterial diversity and population shifts driven by spotlight wastewater micropollutants in low-temperature highly nitrifying activated sludge. Sci. Total Environ. 2017, 605–606, 291–299. [Google Scholar] [CrossRef]
- Castello, P.; Jimenez, P.; Martino, C.F. The role of pulsed electromagnetic fields on the radical pair mechanism. Bioelectromagnetics 2021, 42, 491–500. [Google Scholar] [CrossRef]
- Guo, L.; Azam, S.R.; Guo, Y.; Liu, D.; Ma, H. Germicidal efficacy of the pulsed magnetic field against pathogens and spoilage microorganisms in food processing: An overview. Food Control 2022, 136, 108496. [Google Scholar] [CrossRef]
- Tomska, A.; Wolny, L. Enhancement of biological wastewater treatment by magnetic field exposure. Desalination 2008, 222, 368–373. [Google Scholar] [CrossRef]
- Khokhlova, G.; Vainshtein, M. Application of static and impulse magnetic fields to bacteria Rhodospirillum rubrum VKM B-1621. AMB Express 2017, 7, 60. [Google Scholar] [CrossRef] [PubMed]
- Tao, Q.; Zhou, S. Effect of static magnetic field on electricity production and wastewater treatment in microbial fuel cells. Appl. Microbiol. Biotechnol. 2014, 98, 9879–9887. [Google Scholar] [CrossRef]
- Wiesenthal, A.A.; Müller, C.; Harder, K.; Hildebrandt, J.-P. Alanine, proline and urea are major organic osmolytes in the snail Theodoxus fluviatilis under hyperosmotic stress. J. Exp. Biol. 2019, 222, jeb193557. [Google Scholar] [CrossRef]
- Flora, S.J.; Chouhan, S.; Kannan, G.M.; Mittal, M.; Swarnkar, H. Combined administration of taurine and monoisoamyl DMSA protects arsenic induced oxidative injury in rats. Oxid. Med. Cell Longev. 2008, 1, 39–45. [Google Scholar] [CrossRef]
- Bandookwala, M.; Thakkar, D.; Sengupta, P. Advancements in the Analytical Quantification of Nitroxidative Stress Biomarker 3-Nitrotyrosine in Biological Matrices. Crit. Rev. Anal. Chem. 2020, 50, 265–289. [Google Scholar] [CrossRef]
- Xu, Y.; Freund, D.M.; Hegeman, A.D.; Cohen, J.D. Metabolic signatures of Arabidopsis thaliana abiotic stress responses elucidate patterns in stress priming, acclimation, and recovery. Stress Biol. 2022, 2, 11. [Google Scholar] [CrossRef]
- Li, Z.; Gu, J.; Ding, J.; Ren, N.; Xing, D. Molecular mechanism of ethanol-H2 co-production fermentation in anaerobic acidogenesis: Challenges and perspectives. Biotechnol. Adv. 2021, 46, 107679. [Google Scholar] [CrossRef]
- Palmieri, E.M.; Holewinski, R.; McGinity, C.L.; Pierri, C.L.; Maio, N.; Weiss, J.M.; Tragni, V.; Miranda, K.M.; Rouault, T.A.; Andresson, T. Pyruvate dehydrogenase operates as an intramolecular nitroxyl generator during macrophage metabolic reprogramming. Nat. Commun. 2023, 14, 5114. [Google Scholar] [CrossRef]
- Wang, P.; Qiu, Y.-Q.; Chen, X.-T.; Liang, X.-F.; Ren, L.-H. Metabolomic insights into polyhydroxyalkanoates production by halophilic bacteria with acetic acid as carbon source. Biosci. Biotechnol. Biochem. 2019, 83, 1955–1963. [Google Scholar] [CrossRef]
- Pacholak, A.; Gao, Z.-L.; Gong, X.-Y.; Kaczorek, E.; Cui, Y.-W. The metabolic pathways of polyhydroxyalkanoates and exopolysaccharides synthesized by Haloferax mediterranei in response to elevated salinity. J. Proteom. 2021, 232, 104065. [Google Scholar] [CrossRef]

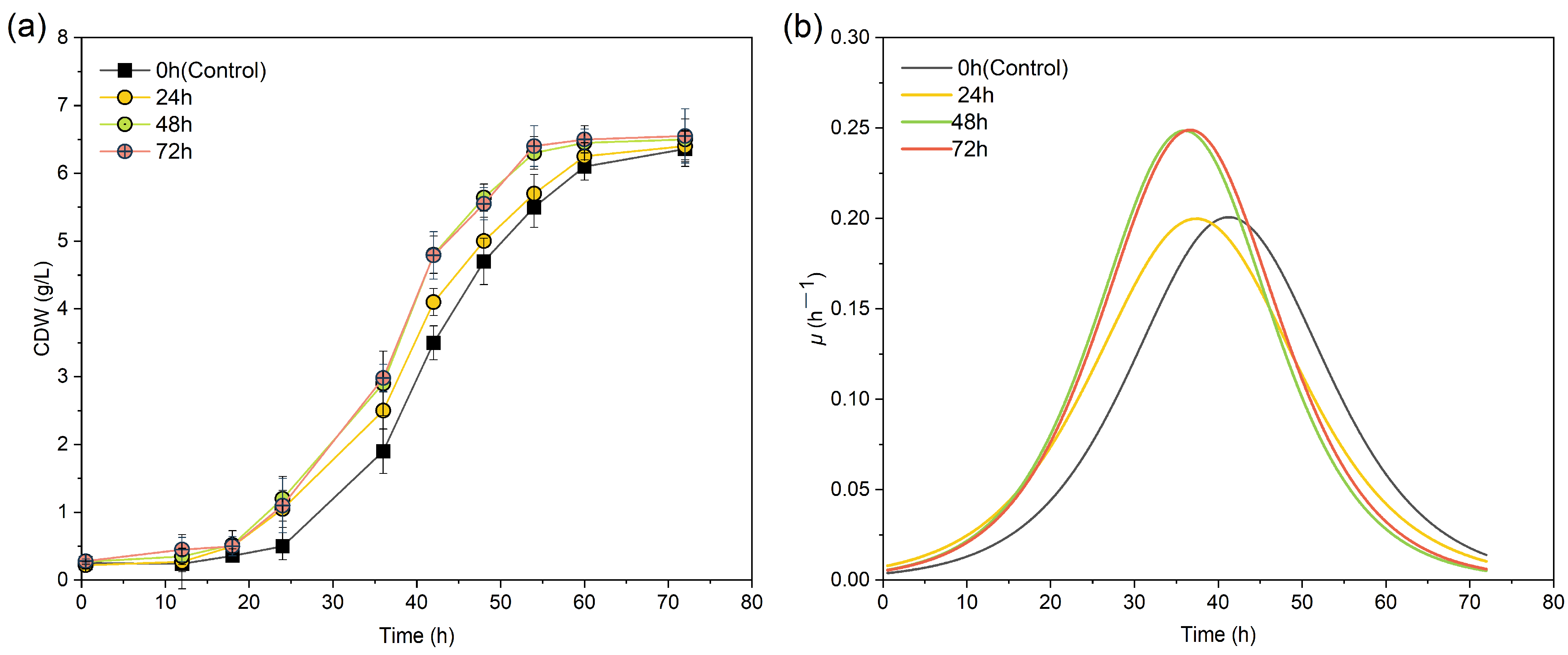

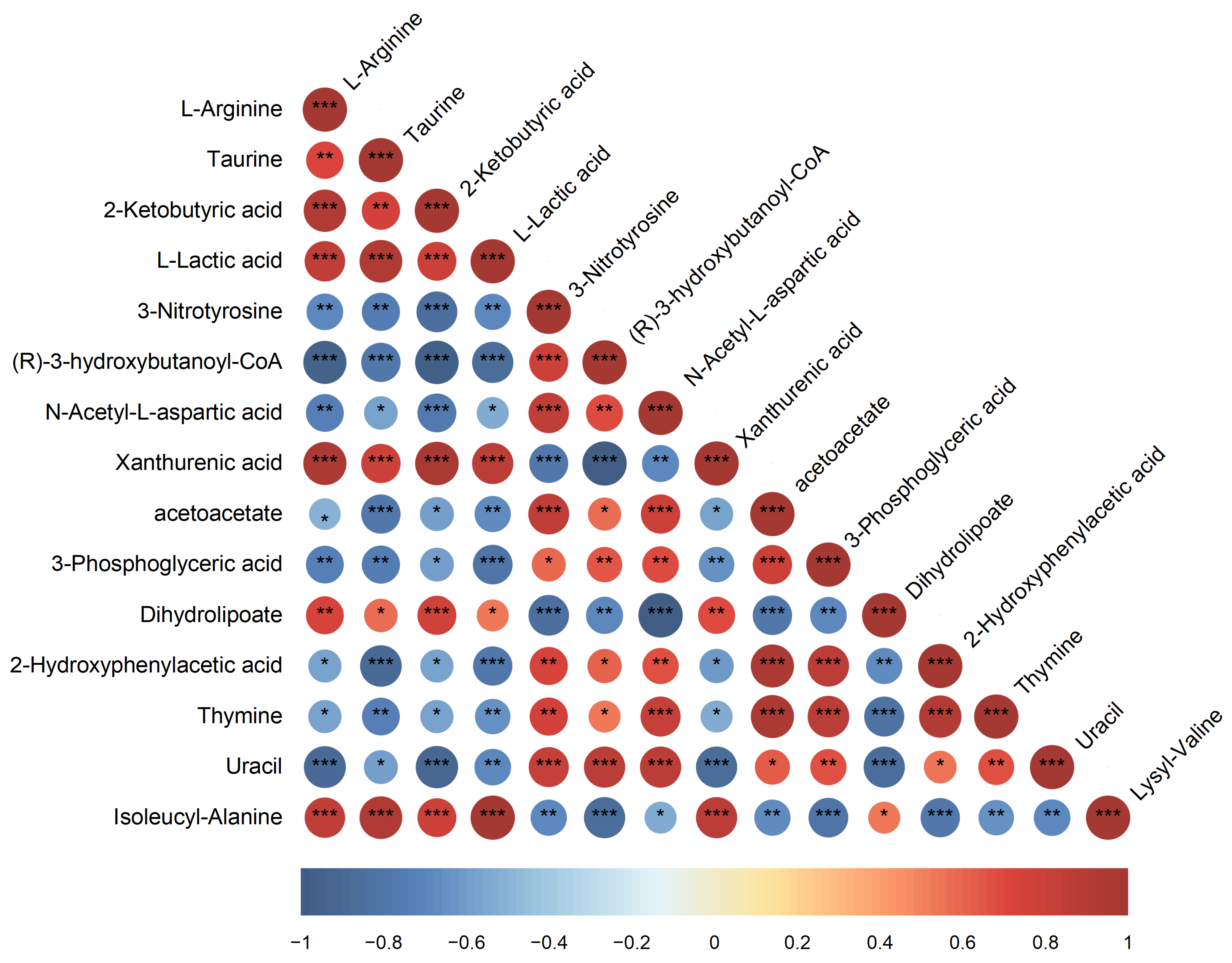
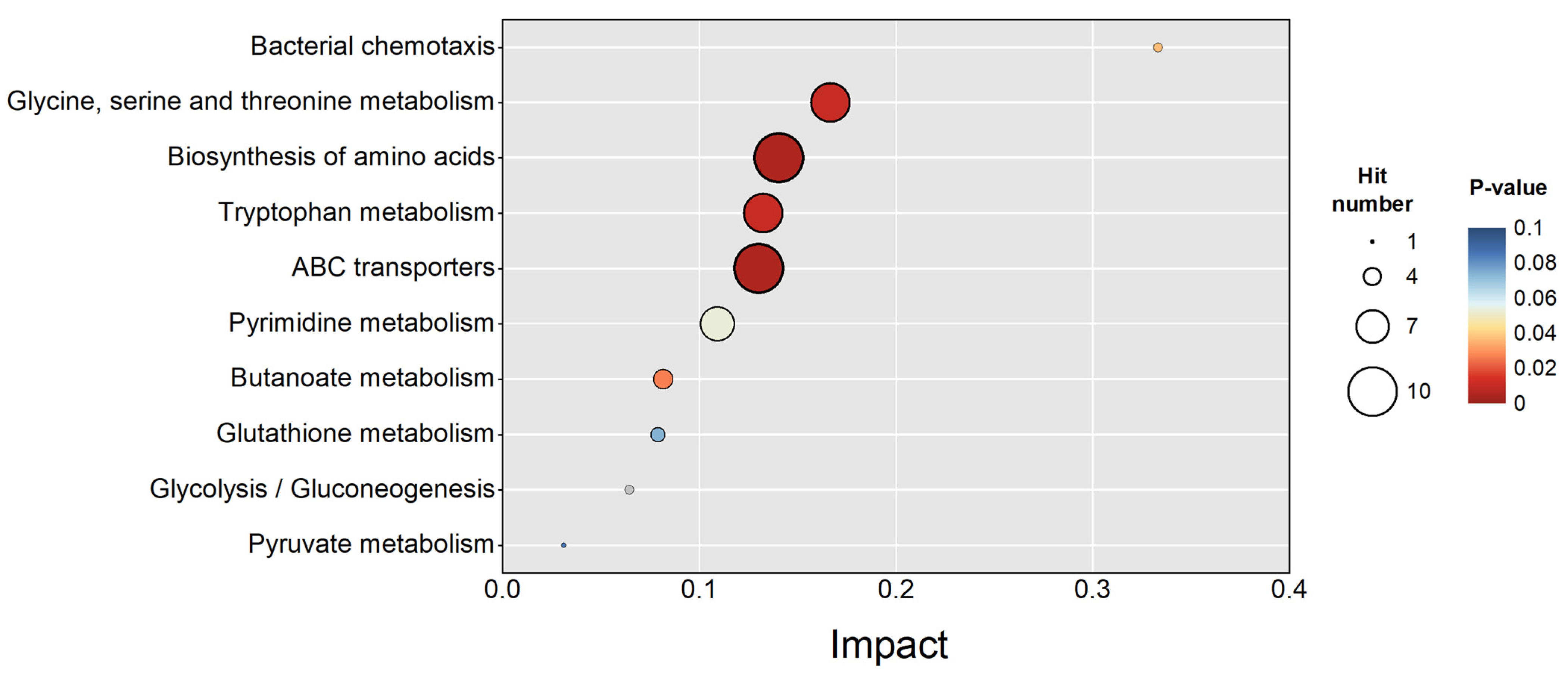
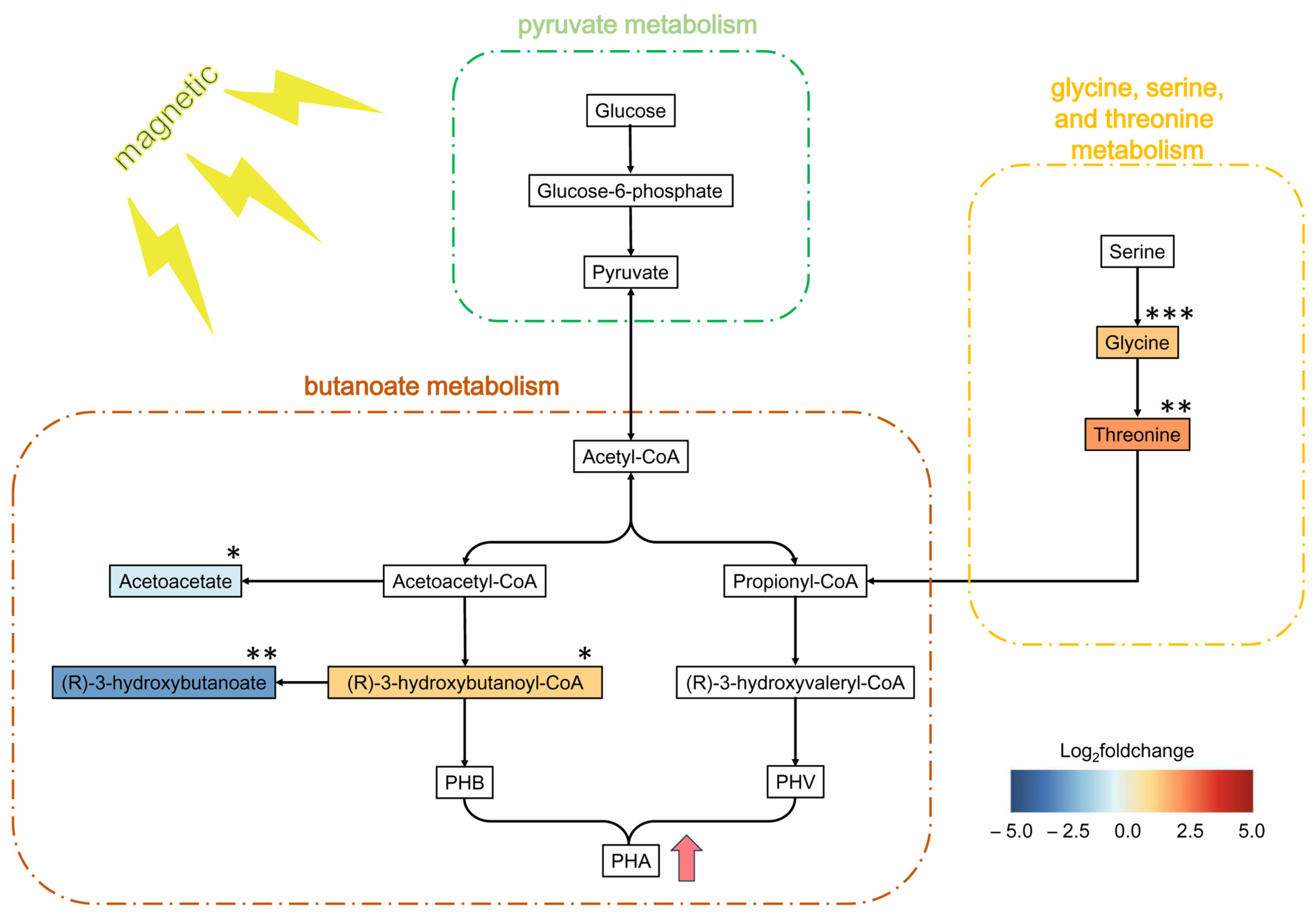
| Treatment Group | CDWmax (g/L) | Tμmax (h) | μmax (h−1) | R2 |
|---|---|---|---|---|
| Control | 6.36 ± 0.26 a | 42.011 ± 0.288 a | 0.201 ± 0.006 b | 0.996 |
| 24 h | 6.41 ± 0.71 a | 37.645 ± 0.421 b | 0.199 ± 0.010 b | 0.990 |
| 48 h | 6.58 ± 0.32 a | 36.000 ± 0.326 b | 0.248 ± 0.013 a | 0.993 |
| 72 h | 6.61 ± 0.46 a | 37.645 ± 0.423 b | 0.249 ± 0.015 a | 0.989 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Z.-L.; Cui, Y.-W. Static Magnetic Field Increases Polyhydroxyalkanoates Biosynthesis in Haloferax mediterranei: Parameter Optimization and Mechanistic Insights from Metabolomics. Polymers 2025, 17, 1190. https://doi.org/10.3390/polym17091190
Gao Z-L, Cui Y-W. Static Magnetic Field Increases Polyhydroxyalkanoates Biosynthesis in Haloferax mediterranei: Parameter Optimization and Mechanistic Insights from Metabolomics. Polymers. 2025; 17(9):1190. https://doi.org/10.3390/polym17091190
Chicago/Turabian StyleGao, Ze-Liang, and You-Wei Cui. 2025. "Static Magnetic Field Increases Polyhydroxyalkanoates Biosynthesis in Haloferax mediterranei: Parameter Optimization and Mechanistic Insights from Metabolomics" Polymers 17, no. 9: 1190. https://doi.org/10.3390/polym17091190
APA StyleGao, Z.-L., & Cui, Y.-W. (2025). Static Magnetic Field Increases Polyhydroxyalkanoates Biosynthesis in Haloferax mediterranei: Parameter Optimization and Mechanistic Insights from Metabolomics. Polymers, 17(9), 1190. https://doi.org/10.3390/polym17091190








