The Effect of Organic and Conventional Farming Systems with Different Tillage on Soil Properties and Enzymatic Activity
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description and Soil Sampling
2.2. Soil Sample Treatment and Methods of Analysis
2.3. The Activity of Enzymes
2.4. EEGRSP Extraction and Determination
2.5. Statistical Analyses
3. Results
3.1. Basic Properties of Soil and Content of EEGRSP
3.2. The Activity of Enzymes in Soil
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Domagała-Świątkiewicz, I.; Gąstoł, M. Soil chemical properties under organic and conventional crop management systems in south Poland. Biol. Agric. Hortic. 2013, 29, 12–28. [Google Scholar] [CrossRef]
- Fess, T.L.; Benedito, V.A. Organic versus conventional cropping sustainability: A comparative system analysis. Sustainability 2018, 10, 272. [Google Scholar] [CrossRef] [Green Version]
- Medan, D.; Torretta, J.P.; Hodara, K.; de la Fuente, E.B.; Montaldo, N.H. Effects of agriculture expansion and intensification on the vertebrate and invertebrate diversity in the Pampas of Argentina. Biodivers Conserv. 2011, 20, 3077–3100. [Google Scholar] [CrossRef]
- Bobulská, L.; Fazekašová, D.; Angelovičová, L.; Kotorová, D. Impact of ecological and conventional farming systems on chemical and biological soil quality indices in a cold mountain climate in Slovakia. Biol. Agric. Hortic. 2015, 26, 2–17. [Google Scholar] [CrossRef]
- Council of the European Union. Council Regulation (EEC) No 2092/91 of 24 June 1991 on Organic Production of Agricultural Products and Indications Referring Thereto on Agricultural Products and Foodstuffs. EUR-Lex Home Page. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=CELEX%3A31991R2092 (accessed on 17 November 2020).
- Deen, W.; Kataki, P.K. Carbon sequestration in a long-term conventional versus conservation tillage experiment. Soil Tillage Res. 2003, 74, 143–150. [Google Scholar] [CrossRef]
- Skaalsveen, K.; Ingram, J.; Clarke, L.E. The effect of no-till farming on the soil functions of water purification and retention in north-western Europe: A literature review. Soil Tillage Res. 2019, 189, 98–109. [Google Scholar] [CrossRef]
- Holland, J.M. The environmental consequences of adopting conservation tillage in Europe: Reviewing the evidence. Agric. Ecosyst. Environ. 2004, 103, 1–25. [Google Scholar] [CrossRef]
- Mäder, P.; Berner, A. Development of reduced tillage systems in organic farming in Europe. Renew. Agric. Food Syst. 2012, 27, 7–11. [Google Scholar] [CrossRef] [Green Version]
- Hofmeijer, M.A.J.; Krauss, M.; Berner, A.; Peigné, J.; Mäder, P.; Armengot, L. Effects of reduced tillage on weed pressure, nitrogen availability and winter wheat yields under organic management. Agronomy 2019, 9, 180. [Google Scholar] [CrossRef] [Green Version]
- Wezel, A.; Casagrande, M.; Celette, F.; Vian, J.-F.; Ferrer, A.; Peigné, J. Agroecological practices for sustainable agriculture. A review. Agron. Sustain. Dev. 2014, 34, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Peigné, J.; Ball, B.C.; Roger-Estrade, J.; David, C. Is conservation tillage suitable for organic farming? A review. Soil Use Manag. 2007, 23, 129–144. [Google Scholar] [CrossRef]
- Bianchi, F.J.J.A.; Ives, A.R.; Schellhorn, N.A. Interactions between conventional and organic farming for biocontrol services across the landscape. Ecol. Appl. 2013, 23, 1531–1543. [Google Scholar] [CrossRef] [PubMed]
- Deguine, J.P.; Penvern, S. Agroecological crop protection in organic farming: Relevance and limits. In Organic Farming, Prototype for Sustainable Agricultures; Bellon, S., Penvern, S., Eds.; Springer: Dordrecht, The Netherlands, 2014; pp. 107–130. [Google Scholar] [CrossRef]
- Lundkvist, A.; Verwijst, T. Weed biology and weed management in organic farming. Res. Org. Farming 2011, 157–186. [Google Scholar] [CrossRef] [Green Version]
- Bastiaans, L.; Paolini, R.; Baumann, D.T. Focus on ecological weed management: What is hindering adoption? Weed Res. 2008, 48, 481–491. [Google Scholar] [CrossRef]
- McLachlan, K.K.; Hobbie, S.E. Comparison of soil organic matter fractionation technique. Soil Sci. Soc. Am. J. 2004, 68, 1616–1625. [Google Scholar] [CrossRef]
- Garbuio, F.J.; Jones, D.L.; Allioni, L.R.F.; Murphy, D.V.; Caires, E.F. Carbon and nitrogen dynamics in an Axisol as affected by liming and crop residue under no-till. Soil Sci. Soc. Am. J. 2011, 75, 1723–1730. [Google Scholar] [CrossRef]
- Kalbitz, K.; Solinger, S.; Park, J.-H.; Michalzik, B.; Matzner, E. Controls on the dynamics of organic matter in soils: A review. Soil Sci. 2000, 165, 277–304. [Google Scholar] [CrossRef]
- Nielsen, S.; Minchin, T.; Kimber, S.; Zwieten, L.; Gilbert, J.; Munroe, P.; Joseph, S.; Thomas, T. Comparative analysis of the microbial communities in agricultural soil amended with enhanced biochars or traditional fertilizers. Agric. Ecosyst. Environ. 2014, 191, 73–82. [Google Scholar] [CrossRef]
- Gianfreda, L.; Ruggiero, P. Enzyme Activities in Soil. In Nucleic Acids and Proteins in Soil; Soil Biology; Nannipieri, P., Smalla, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2006; Volume 8. [Google Scholar]
- Piotrowska-Długosz, A. Significance of enzymes and their application in agriculture. In Biocatalysis; Husain, Q., Ullah, M., Eds.; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Lemanowicz, J.; Haddad, S.A.; Bartkowiak, A.; Lamparski, R.; Wojewódzki, P. The role of an urban park’s tree stand in shaping the enzymatic activity, glomalin content and physicochemical properties of soil. Sci. Total Environ. 2020, 741, 140446. [Google Scholar] [CrossRef]
- Kobierski, M.; Kondratowicz-Maciejewska, K.; Banach-Szott, M.; Penas Castejon, J.M. Humic substances and aggregate stability in rhizospheric and non-rhizospheric soil. J. Soils Sediments 2018, 18, 2777–2789. [Google Scholar] [CrossRef] [Green Version]
- Chantigny, M.H. Dissolved and water-extractable organic matter in soils: A review on the influence of land use and management practice. Geoderma 2003, 113, 357–380. [Google Scholar] [CrossRef]
- Balík, J.; Černý, J.; Kulhánek, M.; Sedlář, O. Soil carbon transformation in long-term field experiments with different fertilization treatments. Plant Soil Environ. 2018, 64, 578–586. [Google Scholar] [CrossRef] [Green Version]
- Si, P.; Liu, E.; He, W.; Sun, Z.; Dong, W.; Yan, C.; Zhang, Y. Effect of no-tillage with straw mulch and conventional tillage on soil organic carbon pools in Northern China. Arch. Agron. Soil Sci. 2018, 64, 398–408. [Google Scholar] [CrossRef]
- Busari, A.M.; Kukal, S.S.; Kaur, A.; Bhatt, R.; Dulazi, A.A. Conservation tillage impacts on soil, crop and the environment. J. Soil Water Conserv. 2015, 3, 119–129. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Chavez, M.C.; Carrillo-Gonzalez, R.; Wright, S.F.; Nichols, K.A. The role of glomalin, a protein produced by arbuscular mycorrhizal fungi, in sequestering potentially toxic elements. Environ. Pollut. 2004, 130, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Wright, S.F.; Starr, J.L.; Paltineanu, I.C. Changes in aggregate stability and concentration of glomalin during tillage management transition. Soil Sci. Soc. Am. J. 1999, 63, 1825–1829. [Google Scholar] [CrossRef]
- Avio, L.; Castaldini, M.; Fabiani, A.; Bedini, S.; Sbrana, C.; Turrini, A.; Giovannetti, M. Impact of nitrogen fertilization and soil tillage on arbuscular mycorrhizal fungal communities in a Mediterranean agroecosystem. Soil Biol. Biochem. 2013, 67, 285–294. [Google Scholar] [CrossRef]
- Wright, S.F.; Anderson, R.L. Aggregate stability and glomalin in alternative crop rotations for the central Great Plains. Biol. Fertil. Soils. 2000, 31, 249–253. [Google Scholar] [CrossRef]
- Borie, F.; Rubio, R.; Rouanet, J.L.; Morales, A.; Borie, G.; Rojas, C. Effects of tillage systems on soil characteristics, glomalin and mycorrhizal propagules in a Chilean Ultisol. Soil Tillage Res. 2006, 88, 253–261. [Google Scholar] [CrossRef]
- Schindler, F.V.; Mercer, E.J.; Rice, J.A. Chemical characteristics of glomalin-related soil protein (GRSP) extracted from soils of varying organic matter content. Soil Biol. Biochem. 2007, 39, 320–329. [Google Scholar] [CrossRef]
- Wright, S.F.; Green, V.S.; Cavigelli, M.A. Glomalin in aggregate size classes from three different farming systems. Soil Tillage Res. 2007, 94, 546–549. [Google Scholar] [CrossRef]
- Ghosh, A.; Bhattacharyya, R.; Meena, M.C.; Dwivedi, B.S.; Singh, G.; Agnihotri, R.; Sharma, C. Long-term fertilization effects on soil organic carbon sequestration in an Inceptisol. Soil Tillage Res. 2018, 177, 134–144. [Google Scholar] [CrossRef]
- Singh, G.; Bhattacharyya, R.; Das, T.K.; Sharma, A.R.; Ghosh, A.; Das, S.; Jha, P. Crop rotation and residue management effects on soil enzyme activities, glomalin and aggregate stability under zero tillage in the Indo-Gangetic Plains. Soil Tillage Res. 2018, 184, 291–300. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, Update 2015. International for Soil Classification System for Naming Soil and Creating Legends for Soil Maps; World Soil Resources Reports No 106; FAO: Rome, Italy, 2015. [Google Scholar]
- Thalmann, A. Zur methodic derestimung der Dehydrogenaseaktivität und Boden mittels Triphenyltetrazoliumchlorid (TTC). Landwirtsch. Forsch. 1968, 21, 249–258. [Google Scholar]
- Johnson, J.I.; Temple, K.I. Some variables affecting the measurements of catalase activity in soil. Soil Sci. Soci. Am. 1964, 28, 207–209. [Google Scholar] [CrossRef]
- Tabatabai, M.A.; Bremner, J.M. Use of p–nitrophenol phosphate for assay of soil phosphatase activity. Soil Biol. Biochem. 1969, 1, 301–307. [Google Scholar] [CrossRef]
- Dick, W.A.; Cheng, L.; Wang, P. Soil acid alkaline phosphatase activity as pH adjustment indicators. Soil Biol. Biochem. 2000, 32, 1915–1919. [Google Scholar] [CrossRef]
- Stefanic, F.; Ellade, G.; Chirnageanu, J. Researches concerning a biological index of soil fertility. In Proceeding of the Fifth Symposium of Soil Biology; Nemes, M.P., Kiss, S., Papacostea, P., Stefanic, C., Rusan, M., Eds.; Romanian National Society of Soil Science: Bucharest, Romania, 1984; pp. 35–45. [Google Scholar]
- Wyszkowska, J.; Borowik, A.; Kucharski, M.; Kucharski, J. Applicability of biochemical indices to quality assessment of soil polluted with heavy metals. J. Elemen. 2013, 18, 733–756. [Google Scholar] [CrossRef]
- Hinojosa, M.B.; Garcia-Ruiz, R.; Viñegla, B.; Carreira, J.A. Microbiological rates and enzyme activities as indicators of functionality in soils affected by the Aznalcóllar toxic spill. Soil Biol. Biochem. 2004, 36, 1637–1644. [Google Scholar] [CrossRef]
- Gillespie, A.W.; Farrell, R.E.; Walley, F.L.; Ross, A.R.S.; Leinweber, P.; Eckhardt, K.U.; Regier, T.Z.; Blyth, R.I.R. Glomalin-related soil protein contains non-mycorrhizal-related heat-stable proteins, lipids and humic materials. Soil Biol. Biochem. 2011, 43, 766–777. [Google Scholar] [CrossRef]
- Wright, S.F.; Upadhyaya, A. Extraction of an abundant and unusual protein from soil and comparison with hyphal protein of arbuscular mycorrhizal fungi. Soil Sci. 1996, 161, 575–586. [Google Scholar] [CrossRef]
- Rillig, M.C. Arbuscular mycorrhizae, glomalin, and soil aggregation. Can. J. Soil Sci. 2004, 84, 355–363. [Google Scholar] [CrossRef] [Green Version]
- Bradford, M.M. A rapid and sensitive metod for the quantitation of microgram quantities of protein utilizing the principle of proteine dye-binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Leinweber, P.; Schulten, H.R.; Kalbitz, K.; Meisner, R.; Jancke, H. Fulvic acid composition in degraded fenlands. J. Plant Nutr. Soil Sci. 2001, 164, 371–379. [Google Scholar] [CrossRef]
- Andruschkewitsch, R.; Geisseler, D.; Koch, H.J.; Ludwig, B. Effects of tillage on contents of organic carbon, nitrogen, water-stable aggregates and light fraction four different long-term trials. Geoderma 2013, 192, 368–377. [Google Scholar] [CrossRef]
- Sosulski, T.; Szara, E.; Korc, M.; Stepień, W. Dissolved organic carbon in Luvisol under different fertilization and crop rotation. Soil Sci. Ann. 2013, 64, 114–118. [Google Scholar] [CrossRef]
- Halpern, M.T.; Whalen, J.K.; Madramootoo, C.A. Long-term tillage and residue management influences soil C and N dynamics. Soil Sci. Soc. Am. J. 2010, 74, 1211–1217. [Google Scholar] [CrossRef]
- Moreno, F.; Murillo, J.M.; Pelegrin, F.; Giron, I.F. Long-term impact of conservation tillage on stratification ratio of soil organic carbon and loss of total and active CaCO3. Soil Tillage Res. 2006, 85, 86–93. [Google Scholar] [CrossRef]
- Rosa, E.; Dębska, B. Seasonal changes in the content of dissolved organic matter in arable soils. J. Soil Sediments 2018, 18, 2703–2714. [Google Scholar] [CrossRef] [Green Version]
- Lalande, R.; Gagnon, B.; Simard, R.R.; Coté, D. Soil microbial biomass and enzyme activity following liquid hog manure application in a long-term field trial. Can. J. Soil Sci. 2000, 80, 263–269. [Google Scholar] [CrossRef]
- Schoenau, J.J.; Davis, J.G. Optimizing soil and plant responses to land applied manure nutrients in the Great Plains of North America. Can. J. Soil. Sci. 2006, 86, 587–595. [Google Scholar] [CrossRef]
- Benke, M.B.; Hao, X.; O’Donovan, J.T.; Clayton, G.W.; Lupwayi, N.Z.; Caffyn, P.; Hall, M. Livestock manure improves acid soil productivity under a cold northern Alberta climate. Can. J. Soil Sci. 2009, 90, 685–697. [Google Scholar] [CrossRef]
- Preger, A.C.; Rillig, M.C.; Johns, A.R.; Du Preez, C.C.; Lobe, I.; Amelung, W. Losses of glomalin-related soil protein under prolonged arable cropping: A chronosequence study in sandy soils of the South African Highveld. Soil Biol. Biochem. 2007, 39, 445–453. [Google Scholar] [CrossRef]
- Wojewódzki, P.; Cieścińska, B. Effect of crop rotation and long term fertilization on the carbon and glomalin content in the soil. J. Cent. Eur. Agric. 2012, 13, 814–821. [Google Scholar] [CrossRef]
- Tamilselvi, S.M.; Chinnadurai, C.; Hamuruga, K.; Arulmozhiselvan, K.; Balachandran, D. Effect of long-term nutrient management on biological and biochemical properties of semi-arid tropical Alfisol during maize crop development stages. Ecol. Indic. 2015, 48, 76–87. [Google Scholar] [CrossRef]
- Piotrowska-Długosz, A.; Wilczewski, E. Soil phosphatase activity and phosphorus content as influenced by catch crops cultivated as green manure. Pol. J. Environ. Stud. 2014, 23, 157–165. [Google Scholar]
- Furtak, K.; Gajda, A.M. Activity of dehydrogenases as an indicator of soil environment quality. Pol. J. Soil Sci. 2017, 50, 33–40. [Google Scholar] [CrossRef] [Green Version]
- Sheoran, H.S.; Phogat, V.K.; Dahiya, R.; Gera, R. Long-term effect of organic and conventional farming practices on microbial biomass carbon. enzyme activities and microbial populations in different textured soils of haryana state (India). Appl. Ecol. Env. Res. 2018, 16, 3669–3689. [Google Scholar] [CrossRef]
- Gałązka, A.; Gawryjołek, K.; Grządziel, J.; Księżak, J. Effect of different agricultural management practices on soil biological parameters including glomalin fraction. Plant Soil Environ. 2017, 63. [Google Scholar] [CrossRef] [Green Version]
- Qiao, L.; Li, Y.; Song, Y.; Zhai, J.; Wu, Y.; Chen, W.; Liu, G.; Xue, S. Effects of vegetation restoration on the distribution of nutrients, glomalin-related soil protein, and enzyme activity in soil aggregates on the loess plateau, China. Forests 2019, 10, 796. [Google Scholar] [CrossRef] [Green Version]
- Yao, X.H.; Min, H.; Lü, Z.H.; Yuan, H.P. Influence of acetamiprid on soil enzymatic activities and respiration. Eur. J. Soil Biol. 2006, 42, 120–126. [Google Scholar] [CrossRef]
- Curyło, K.; Telesiński, A. Use of phosphatase and dehydrogenase activities in the assessment of calcium peroxide and citric acid effects in soil contaminated with petrol. Open Life Sci. 2020, 15, 12–20. [Google Scholar] [CrossRef] [Green Version]
- Nannipieri, P.; Giagnoni, L.; Landi, L.; Renella, G. Role of phosphatase enzymes in soil. In Phosphorus in Action; Soil Biology; Bünemann, E.K., Oberson, A., Frossard, E., Eds.; Springer: Berlin/Heidelberg, Germany, 2011; Volume 26, pp. 215–243. [Google Scholar] [CrossRef]
- Tian, L.; Dell, E.; Shi, W. Chemical composition of dissolved organic matter in agroecosystems: Correlations with soil enzyme activity and carbon and nitrogen mineralization. Appl. Soil Ecol. 2010, 46, 426–435. [Google Scholar] [CrossRef]
- Lemanowicz, J. Activity of selected enzymes as markers of ecotoxicity in technogenic salinization soils. Environ. Sci. Pollut. Res. 2019, 26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bastida, F.; Zsolnay, A.; Hernández, T.; García, C. Past, present and future of soil quality indices: A biological perspective. Geoderma 2008, 147, 159–171. [Google Scholar] [CrossRef]
- Piotrowska-Długosz, A.; Lemanowicz, J.; Długosz, J. The spatial pattern and seasonal changes in the soil phosphorus content in relation to the phosphatase activity: A case study of Luvisols. Arch. Agron. Soil Sci. 2020, 66, 1583–1597. [Google Scholar] [CrossRef]
- García-Ruiz, R.; Ochoa, V.; Hinojosa, M.B.; Carreira, J.A. Suitability of enzyme activities for the monitoring of soil quality improvement in organic agricultural systems. Soil Biol. Biochem. 2008, 40, 2137–2145. [Google Scholar] [CrossRef]
- Saviozzi, A.; Levi-Minzi, R.; Cardelli, R.; Riffaldi, R. A comparison of soil quality in adjacent cultivated, forest and native grassland soils. Plant Soil 2001, 233, 251–259. [Google Scholar] [CrossRef]
- Lemanowicz, J.; Bartkowiak, A.; Lamparski, R.; Wojewódzki, P.; Pobereżny, J.; Wszelaczyńska, E.; Szczepanek, M. Physicochemical and enzymatic soil properties influenced by cropping of primary wheat under organic and conventional farming systems. Agronomy 2020, 10, 1652. [Google Scholar] [CrossRef]
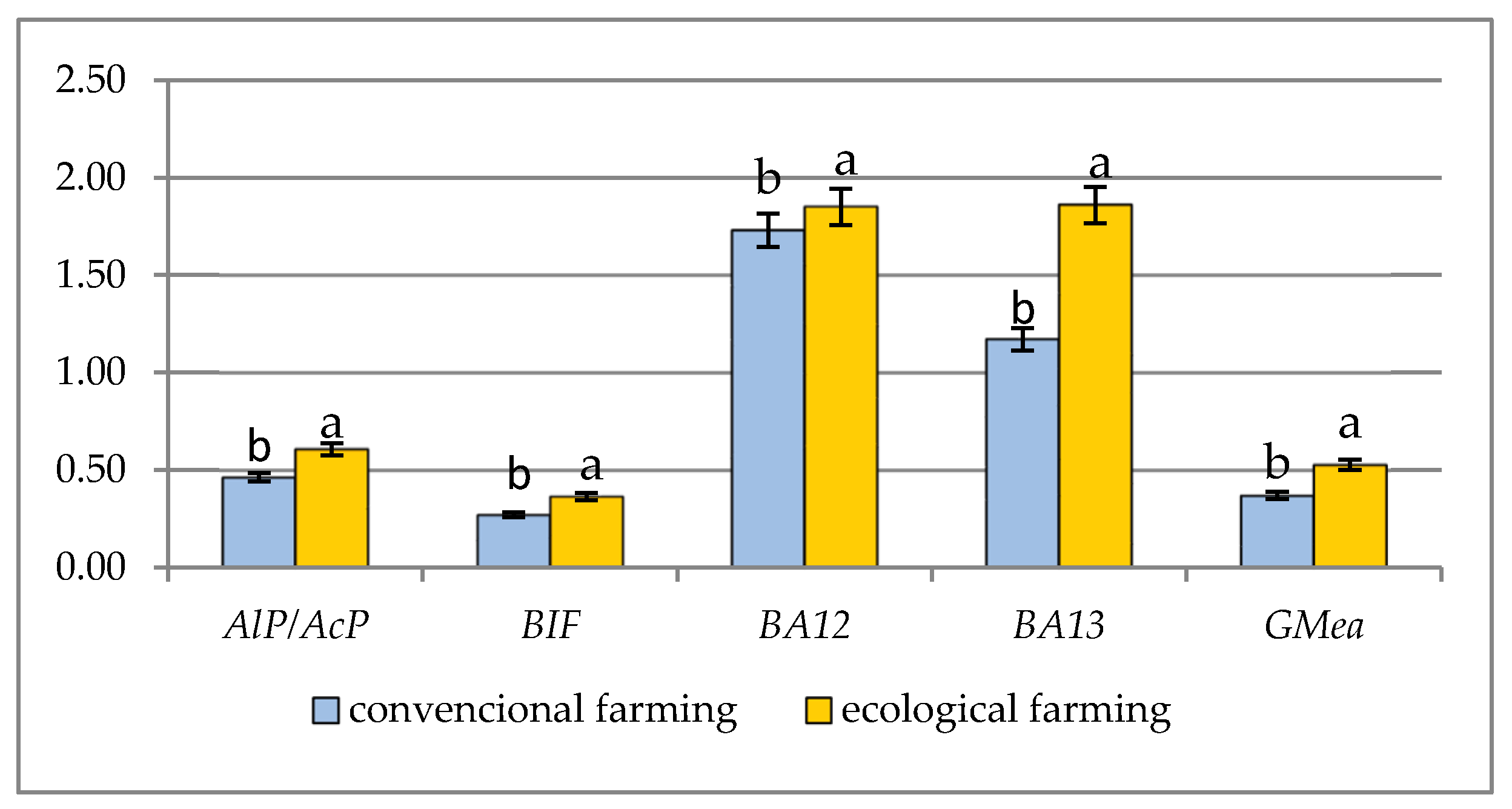
| OF—Reduced Tillage | CF—Conventional Tillage |
|---|---|
| Post-harvest cultivation: stubble cultivator to a depth of 6–8 cm and harrow | Post-harvest cultivation: disk harrow to a depth of 8–10 cm and fertilization |
| Basic preparation: cultivator to a depth of 15–20 cm | Basic preparation: ploughing to a depth of 30 cm |
| Pre-plant tillage: cultivating and sowing aggregate | Pre-plant tillage: cultivator followed by harrowing |
| Parameters | Organic Farming | Conventional Farming | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Min. | Max. | Med. | SD | CV | Min. | Max. | Med. | SD | CV | |
| Sand | 1.08 | 68.80 | 50.91 | 16.34 | 34.8 | 20.33 | 72.45 | 55.80 | 11.83 | 22.7 |
| Silt | 28.41 | 89.42 | 44.55 | 14.64 | 30.4 | 23.90 | 71.82 | 39.59 | 10.66 | 24.7 |
| Clay | 2.59 | 9.50 | 4.46 | 1.84 | 37.5 | 2.65 | 7.85 | 4.79 | 1.32 | 27.4 |
| pH (1 M KCl) | 4.52 | 6.82 | 6.06 | 0.53 | 8.80 | 2.65 | 7.85 | 4.79 | 1.32 | 27.4 |
| TOC | 8.80 | 36.2 | 11.4 | 6.92 | 48.7 | 6.50 | 24.0 | 9.70 | 3.73 | 36.2 |
| TN | 0.80 | 3.90 | 1.10 | 0.71 | 50.7 | 0.70 | 2.4 | 1.00 | 0.38 | 34.5 |
| DEH | 0.25 | 0.66 | 0.41 | 0.12 | 28.6 | 0.21 | 0.48 | 0.29 | 0.07 | 22.6 |
| CAT | 0.05 | 0.16 | 0.08 | 0.03 | 33.3 | 0.03 | 0.11 | 0.08 | 0.02 | 28.6 |
| AlP | 0.63 | 2.21 | 1.01 | 0.41 | 36.6 | 0.41 | 1.48 | 0.71 | 0.29 | 37.2 |
| AcP | 1.08 | 3.46 | 1.90 | 0.59 | 29.9 | 0.79 | 3.22 | 1.62 | 0.52 | 30.8 |
| EEGRSP | 0.41 | 2.12 | 1.05 | 0.341 | 33.1 | 0.42 | 1.17 | 0.87 | 0.247 | 31.3 |
| DOC | 70.3 | 138 | 93.1 | 18.46 | 19.0 | 89.9 | 251 | 118 | 42.64 | 31.3 |
| DON | 19.9 | 47.1 | 30.3 | 7.82 | 25.1 | 17.7 | 46.9 | 22.0 | 7.93 | 30.5 |
| Parameters * | Organic Farming Mean (N = 24) | Conventional Farming Mean (N = 24) | Significant (p) |
|---|---|---|---|
| pH (1 M KCl) | 6.04 | 4.82 | 0.0002 |
| sand | 46.92 | 52.04 | 0.21 |
| silt | 48.17 | 43.14 | 0.18 |
| Clay | 4.91 | 4.82 | 0.84 |
| TOC | 14.2 | 10.3 | 0.019 |
| TN | 1.42 | 1.10 | 0.043 |
| DHA | 0.42 | 0.31 | 0.0005 |
| CAT | 0.09 | 0.07 | 0.048 |
| AlP | 1.12 | 0.78 | 0.002 |
| AcP | 1.97 | 1.69 | 0.094 |
| EEGRSP | 1.03 | 0.79 | 0.007 |
| DOC | 136 | 97.1 | 0.007 |
| DON | 31.2 | 26.0 | 0.026 |
| Parameters * | Organic Farming (N = 24) | |||||||
| Clay | TOC | TN | DOC | DEH | AlP | AcP | EEGRSP | |
| pH | −0.55 | |||||||
| TN | 0.99 | 0.53 | 0.64 | 0.80 | ||||
| DOC | 0.95 | 0.44 | 0.62 | 0.75 | ||||
| DON | 0.69 | 0.69 | 0.78 | 0.60 | ||||
| DEH | 0.58 | 0.54 | ||||||
| AcP | 0.65 | 0.65 | ||||||
| EEGRSP | −0.60 | 0.78 | ||||||
| Parameters * | Conventional Farming (N = 24) | |||||||
| Clay | TOC | TN | DOC | DEH | AlP | AcP | EEGRSP | |
| TN | 0.97 | 0.58 | 0.76 | 0.79 | 0.58 | |||
| DOC | 0.84 | 0.42 | 0.73 | 0.79 | 0.69 | |||
| DON | 0.80 | 0.87 | 0.82 | 0.48 | 0.57 | 0.63 | ||
| DEH | 0.54 | |||||||
| AlP | 0.81 | |||||||
| AcP | 0.81 | 0.51 | 0.73 | |||||
| EEGRSP | −0.47 | 0.65 | 0.81 | 0.59 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kobierski, M.; Lemanowicz, J.; Wojewódzki, P.; Kondratowicz-Maciejewska, K. The Effect of Organic and Conventional Farming Systems with Different Tillage on Soil Properties and Enzymatic Activity. Agronomy 2020, 10, 1809. https://doi.org/10.3390/agronomy10111809
Kobierski M, Lemanowicz J, Wojewódzki P, Kondratowicz-Maciejewska K. The Effect of Organic and Conventional Farming Systems with Different Tillage on Soil Properties and Enzymatic Activity. Agronomy. 2020; 10(11):1809. https://doi.org/10.3390/agronomy10111809
Chicago/Turabian StyleKobierski, Mirosław, Joanna Lemanowicz, Piotr Wojewódzki, and Krystyna Kondratowicz-Maciejewska. 2020. "The Effect of Organic and Conventional Farming Systems with Different Tillage on Soil Properties and Enzymatic Activity" Agronomy 10, no. 11: 1809. https://doi.org/10.3390/agronomy10111809
APA StyleKobierski, M., Lemanowicz, J., Wojewódzki, P., & Kondratowicz-Maciejewska, K. (2020). The Effect of Organic and Conventional Farming Systems with Different Tillage on Soil Properties and Enzymatic Activity. Agronomy, 10(11), 1809. https://doi.org/10.3390/agronomy10111809






