Health-Promoting Properties of Plant Products: The Role of Mycorrhizal Fungi and Associated Bacteria
Abstract
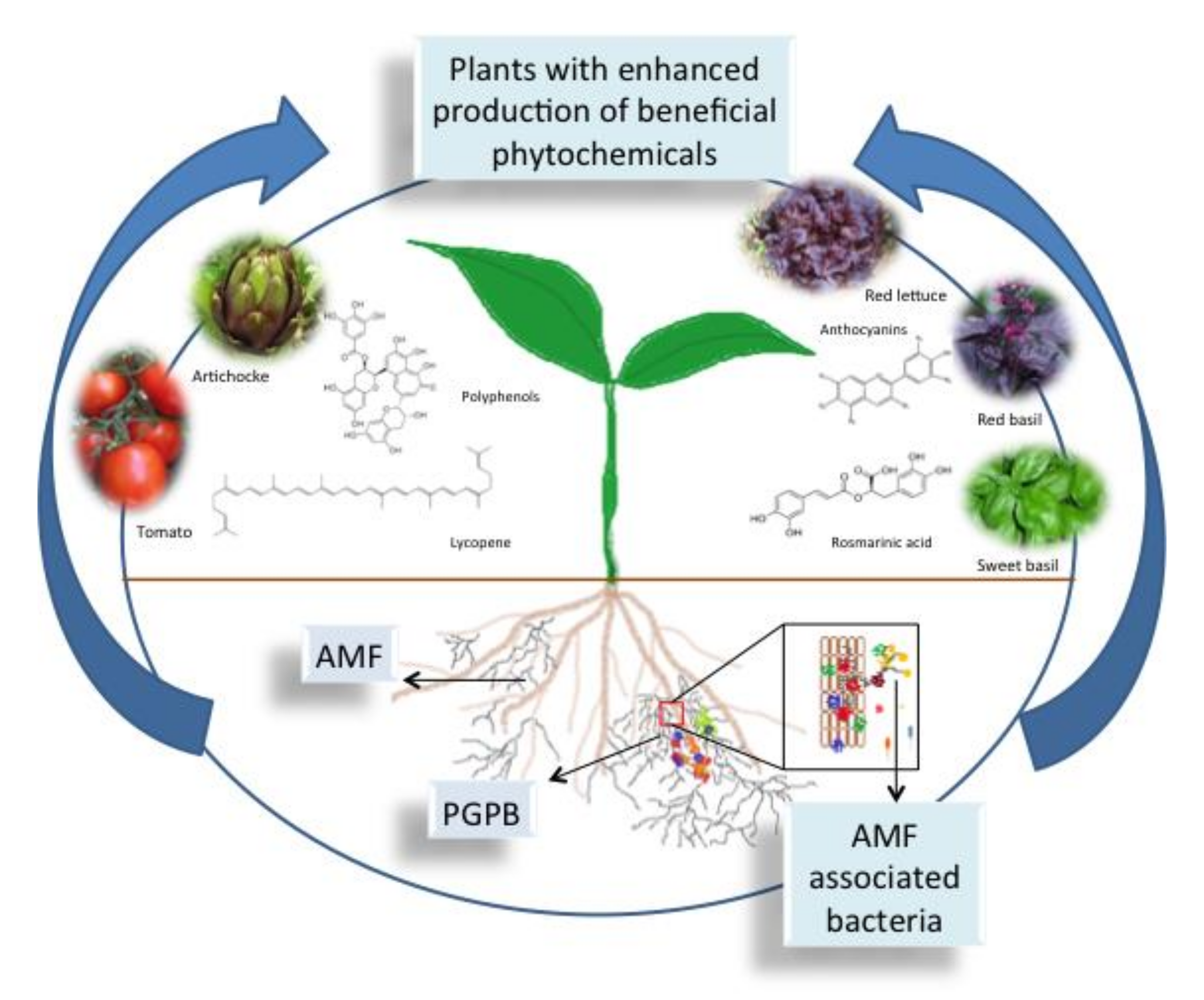
:1. Introduction
2. Arbuscular Mycorrhizal Fungi and Associated Bacteria
3. The Production of Beneficial Phytochemicals as Affected by AMF
4. The Production of Beneficial Phytochemicals as Affected by PGPB- and AMF-Associated Bacteria
5. Conclusions and Perspectives for Future Studies
Author Contributions
Funding
Conflicts of Interest
References
- Duthie, G.G.; Duthie, S.J.; Kyle, J.A.M. Plant polyphenols in cancer and heart disease: Implications as nutritional antioxidants. Nutr. Res. Rev. 2000, 13, 79–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, I.T. Phytochemicals and cancer. Proc. Nutr. Soc. 2007, 67, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Ahn-Jarvis, J.H.; Parihar, A.; Doseff, A.I. Dietary Flavonoids for Immunoregulation and Cancer: Food Design for Targeting Disease. Antioxidants 2019, 8, 202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, C.; Kapoor, H.C. Antioxidants in fruits and vegetables—The millennium’s health. Int. J. Food Sci. Technol. 2001, 36, 703–725. [Google Scholar] [CrossRef]
- Del Rio, D.; Rodriguez-Mateos, A.; Spencer, J.P.E.; Tognolini, M.; Borges, G.; Crozier, A. Dietary (poly)phenolics in human health: Structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid. Redox Signal. 2013, 18, 1818–1892. [Google Scholar] [CrossRef] [Green Version]
- Fairlie-Jones, L.; Davison, K.; Fromentin, E.; Hill, A.M. The Effect of Anthocyanin-Rich Foods or Extracts on Vascular Function in Adults: A Systematic Review and Meta-Analysis of Randomised Controlled Trials. Nutrients 2017, 9, 908. [Google Scholar] [CrossRef] [Green Version]
- Furuuchi, R.; Shimizu, I.; Yoshida, Y.; Hayashi, Y.; Ikegami, R.; Suda, M.; Katsuumi, G.; Wakasugi, T.; Nakao, M.; Minamino, T. Boysenberry polyphenol inhibits endothelial dysfunction and improves vascular health. PLoS ONE 2018, 13, e0202051. [Google Scholar] [CrossRef] [Green Version]
- Aune, D. Plant Foods, Antioxidant Biomarkers, and the Risk of Cardiovascular Disease, Cancer, and Mortality: A Review of the Evidence. Adv. Nutr. 2019, 10, 404–421. [Google Scholar] [CrossRef] [Green Version]
- Lund, E. Non-nutritive bioactive constituents of plants: Dietary sources and health benefits of glucosinolates. Int. J. Vitam. Nutr. Res. 2003, 73, 135–143. [Google Scholar] [CrossRef]
- Boudet, A.M. Evolution and current status of research in phenolic compounds. Phytochemistry 2007, 68, 2722–2735. [Google Scholar] [CrossRef]
- Seca, A.M.; Pinto, D.C. Plant secondary metabolites as anticancer agents: Successes in clinical trials and therapeutic application. Int. J. Mol. Sci. 2018, 19, 263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kashi, D.S.; Shabir, A.; Da Boit, M.; Bailey, S.J.; Higgins, M.F. The Efficacy of Administering Fruit-Derived Polyphenols to Improve Health Biomarkers, Exercise Performance and Related Physiological Responses. Nutrients 2019, 11, 2389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lutz, M.; Fuentes, E.; Ávila, F.; Alarcón, M.; Palomo, I. Roles of Phenolic Compounds in the Reduction of Risk Factors of Cardiovascular Diseases. Molecules 2019, 24, 366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitsiogianni, M.; Koutsidis, G.; Mavroudis, N.; Trafalis, D.T.; Botaitis, S.; Franco, R.; Zoumpourlis, V.; Amery, T.; Galanis, A.; Pappa, A.; et al. The Role of Isothiocyanates as Cancer Chemo-Preventive, Chemo-Therapeutic and Anti-Melanoma Agents. Antioxidants 2019, 8, 106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Beercher, G.R.; Holden, J.M.; Haytowitz, D.B.; Gebhardt, S.E.; Prior, R.L. Lipophilic and hydrophilic antioxidant capacities of common foods in the United States. J. Agric. Food Chem. 2004, 52, 4026–4037. [Google Scholar] [CrossRef]
- Barański, M.; Średnicka-Tober, D.; Volakakis, N.; Seal, C.; Sanderson, R.; Stewart, G.B.; Benbrook, C.; Biavati, B.; Markellou, E.; Giotis, C.; et al. Higher antioxidant and lower cadmium concentrations and lower incidence of pesticide residues in organically grown crops: A systematic literature review and meta-analyses. Br. J. Nutr. 2014, 112, 794–811. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis; Academic Press: Cambridge, UK, 2008. [Google Scholar]
- Turrini, A.; Avio, L.; Giovannetti, M.; Agnolucci, M. Functional complementarity of arbuscular mycorrhizal fungi and associated microbiota: The challenge of translational research. Front. Plant Sci. 2018, 9, 1407. [Google Scholar] [CrossRef]
- Struik, P.C.; Kuyper, T.W. Sustainable intensification in agriculture: The richer shade of green. A review. Agron. Sustain. Dev. 2017, 37, 39. [Google Scholar] [CrossRef]
- Avio, L.; Turrini, A.; Giovannetti, M.; Sbrana, C. Designing the ideotype mycorrhizal symbionts for the production of healthy food. Front. Plant Sci. 2018, 9, 1089. [Google Scholar] [CrossRef]
- Kaur, S.; Suseela, V. Unraveling Arbuscular Mycorrhiza-Induced Changes in Plant Primary and Secondary Metabolome. Metabolites 2020, 10, 335. [Google Scholar] [CrossRef]
- Casieri, L.; Ait Lahmidi, N.; Doidy, J.; Veneault-Fourrey, C.; Migeon, A.; Bonneau, L.; Courty, P.-E.; Garcia, K.; Charbonnier, M.; Delteil, A.; et al. Biotrophic transportome in mutualistic plant-fungal interactions. Mycorrhiza 2013, 23, 597–625. [Google Scholar] [CrossRef] [PubMed]
- Pepe, A.; Sbrana, C.; Ferrol, N.; Giovannetti, M. An In Vivo whole-plant experimental system for the analysis of gene expression in extraradical mycorrhizal mycelium. Mycorrhiza 2017, 27, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Kameoka, H.; Maeda, T.; Okuma, N.; Kawaguchi, M. Structure-specific regulation of nutrient transport and metabolism in arbuscular mycorrhizal fungi. Plant Cell Physiol. 2019, 60, 2272–2281. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, L.; Palla, M.; Agnolucci, M.; Avio, L.; Sbrana, C.; Turrini, A.; Giovannetti, M. Arbuscular mycorrhizal fungi and associated microbiota as plant biostimulants: Research strategies for the selection of the best performing inocula. Agronomy 2020, 10, 106. [Google Scholar] [CrossRef] [Green Version]
- Walley, F.L.; Germida, J.J. Failure to decontaminate Glomus clarum NT4 spores is due to spore wall-associated bacteria. Mycorrhiza 1996, 6, 43–49. [Google Scholar] [CrossRef]
- Filippi, C.; Bagnoli, G.; Citernesi, A.S.; Giovannetti, M. Ultrastructural spatial distribution of bacteria associated with sporocarps of Glomus mosseae. Symbiosis 1998, 24, 1–12. [Google Scholar]
- Maia, L.C.; Kimbrough, J.W. Ultrastructural studies of spores and hypha of a Glomus species. Int. J. Plant Sci. 1998, 159, 581–589. [Google Scholar] [CrossRef]
- Roesti, D.; Ineichen, K.; Braissant, O.; Redecker, D.; Wiemken, A.; Aragno, M. Bacteria associated with spores of the arbuscular mycorrhizal fungi Glomus geosporum and Glomus constrictum. Appl. Environ. Microbiol. 2005, 71, 6673–6679. [Google Scholar] [CrossRef] [Green Version]
- Long, L.; Zhu, H.; Yao, Q.; Ai, Y. Analysis of bacterial communities associated with spores of Gigaspora margarita and Gigaspora rosea. Plant Soil 2008, 310, 1–9. [Google Scholar] [CrossRef]
- Agnolucci, M.; Battini, F.; Cristani, C.; Giovannetti, M. Diverse bacterial communities are recruited on spores of different arbuscular mycorrhizal fungal isolates. Biol. Fertil. Soils 2015, 51, 379–389. [Google Scholar] [CrossRef]
- Agnolucci, M.; Avio, L.; Pepe, A.; Turrini, A.; Cristani, C.; Bonini, P.; Cirino, V.; Colosimo, F.; Ruzzi, M.; Giovannetti, M. Bacteria associated with a commercial mycorrhizal inoculum: Community composition and multifunctional activity as assessed by Illumina sequencing and culture-dependent tools. Front. Plant Sci. 2019, 9, 1956. [Google Scholar] [CrossRef] [PubMed]
- Mayo, K.; Davis, R.E.; Motta, J. Stimulation of germination of spores of Glomus versiforme by spore-associated bacteria. Mycologia 1986, 78, 426–431. [Google Scholar] [CrossRef]
- Xavier, L.J.C.; Germida, J.J. Bacteria associated with Glomus clarum spores influence mycorrhizal activity. Soil Biol. Biochem. 2003, 35, 471–478. [Google Scholar] [CrossRef]
- Bharadwaj, D.P.; Lundquist, P.O.; Alström, S. Arbuscular mycorrhizal fungal spore-associated bacteria affect mycorrhizal colonization, plant growth and potato pathogens. Soil Biol. Biochem. 2008, 40, 2494–2501. [Google Scholar] [CrossRef]
- Li, B.; Ravnskov, S.; Xie, G.; Larsen, J. Biocontrol of Pythium damping-off in cucumber by arbuscular mycorrhiza-associated bacteria from the genus Paenibacillus. Biocontrol 2007, 52, 863–875. [Google Scholar] [CrossRef]
- Bharadwaj, D.P.; Lundquist, P.O.; Persson, P.; Alström, S. Evidence for specificity of cultivable bacteria associated with arbuscular mycorrhizal fungal spores. FEMS Microbiol. Ecol. 2008, 65, 310–322. [Google Scholar] [CrossRef] [Green Version]
- Cruz, A.F.; Ishii, T. Arbuscular mycorrhizal fungal spores host bacteria that affect nutrient biodynamics and biocontrol of soil-borne plant pathogens. Biol. Open 2011, 1, 52–57. [Google Scholar] [CrossRef] [Green Version]
- Battini, F.; Cristani, C.; Giovannetti, M.; Agnolucci, M. Multifunctionality and diversity of culturable bacterial communities strictly associated with spores of the plant beneficial symbiont Rhizophagus intraradices. Microbiol. Res. 2016, 183, 68–79. [Google Scholar] [CrossRef]
- Battini, F.; Grønlund, M.; Agnolucci, M.; Giovannetti, M.; Jakobsen, I. Facilitation of phosphorus uptake in maize plants by mycorrhizosphere bacteria. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef]
- Palla, M.; Battini, F.; Cristani, C.; Giovannetti, M.; Squartini, A.; Agnolucci, M. Quorum sensing in rhizobia isolated from the spores of the mycorrhizal symbiont Rhizophagus intraradices. Mycorrhiza 2018, 28, 773–778. [Google Scholar] [CrossRef]
- Klingner, A.; Hundeshagen, B.; Kernebeck, H.; Bothe, H. Localization of the yellow pigment formed in roots of gramineous plants colonized by arbuscular fungi. Protoplasma 1995, 185, 50–57. [Google Scholar] [CrossRef]
- Maier, W.; Peipp, H.; Schmidt, J.; Wray, V.; Strack, D. Levels of a Terpenoid Glycoside (Blumenin) and Cell Wall-Bound Phenolics in Some Cereal Mycorrhizas. Plant Physiol. 1995, 109, 465–470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, M.; Schäfer, M.; Li, D.; Halitschke, R.; Dong, C.; McGale, E.; Paetz, C.; Song, Y.; Li, S. Blumenols as shoot markers of root symbiosis with arbuscular mycorrhizal fungi. Elife 2018, 7, e37093. [Google Scholar] [CrossRef] [PubMed]
- Fester, T.; Strack, D.; Hause, B. Reorganization of tobacco root plastids during arbuscule development. Planta 2001, 213, 864–868. [Google Scholar] [CrossRef] [PubMed]
- Ponce, M.A.; Scervino, J.M.; Erra-Balsells, R.; Ocampo, J.A.; Godeas, A.M. Flavonoids from shoots and roots of Trifolium repens (white clover) grown in presence or absence of the arbuscular mycorrhizal fungus Glomus intraradices. Phytochemistry 2004, 65, 1925–1930. [Google Scholar] [CrossRef] [PubMed]
- Lohse, S.; Schliemann, W.; Ammer, C.; Kopka, J.; Strack, D.; Fester, T. Organization and Metabolism of Plastids and Mitochondria in Arbuscular Mycorrhizal Roots of Medicago truncatula. Plant Physiol. 2005, 139, 329–340. [Google Scholar] [CrossRef] [Green Version]
- Copetta, A.; Lingua, G.; Berta, G. Effects of three AM fungi on growth, distribution of glandular hairs, and essential oil production in Ocimum basilicum L. var. Genovese. Mycorrhiza 2006, 16, 485–494. [Google Scholar] [CrossRef]
- Schliemann, W.; Ammer, C.; Strack, D. Metabolite profiling of mycorrhizal roots of Medicago truncatula. Phytochemistry 2008, 69, 112–146. [Google Scholar] [CrossRef]
- Araim, G.; Saleem, A.; Arnason, J.T.; Charest, C. Root Colonization by an Arbuscular Mycorrhizal (AM) Fungus Increases Growth and Secondary Metabolism of Purple Coneflower, Echinacea purpurea (L.) Moench. J. Agric. Food Chem. 2009, 57, 2255–2258. [Google Scholar] [CrossRef]
- Caser, M.; Demasi, S.; Victorino, Í.M.M.; Donno, D.; Faccio, A.; Lumini, E.; Bianciotto, V.; Scariot, V. Arbuscular Mycorrhizal Fungi Modulate the Crop Performance and Metabolic Profile of Saffron in Soilless Cultivation. Agronomy 2019, 9, 232. [Google Scholar] [CrossRef] [Green Version]
- Saleh, A.M.; Abdel-Mawgoud, M.; Hassan, A.R.; Habeeb, T.H.; Yehia, R.S.; AbdElgawad, H. Global metabolic changes induced by arbuscular mycorrhizal fungi in oregano plants grown under ambient and elevated levels of atmospheric CO2. Plant Physiol. Biochem. 2020, 151, 255–263. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Lozano, J.M.; Azcon, R.; Palma, J.M. Superoxide dismutase activity in arbuscular mycorrhizal Lactuca sativa plants subjected to drought stress. N. Phytol. 1996, 134, 327–333. [Google Scholar] [CrossRef]
- Garmendia, I.; Goicoechea, N.; Aguirreolea, J. Antioxidant metabolism in asymptomatic leaves of Verticillium-infected pepper associated with an arbuscular mycorrhizal fungus. J. Phytopathol. 2004, 152, 593–599. [Google Scholar] [CrossRef] [Green Version]
- Lambais, M.R.; Rios-Ruiz, W.F.; Andrade, R.M. Antioxidant responses in bean (Phaseolus vulgaris) roots colonized by arbuscular mycorrhizal fungi. N. Phytol. 2003, 160, 421–428. [Google Scholar] [CrossRef]
- Marulanda, A.; Porcel, R.; Barea, J.M.; Azcon, R. Drought tolerance and antioxidant activities in lavender plants colonized by native drought-tolerant or drought-sensitive Glomus species. Microbial Ecol. 2007, 54, 543–552. [Google Scholar] [CrossRef]
- Begum, N.; Ahanger, M.A.; Zhang, L. AMF inoculation and phosphorus supplementation alleviates drought induced growth and photosynthetic decline in Nicotiana tabacum by up-regulating antioxidant metabolism and osmolyte accumulation. Environ. Exp. Bot. 2020, 176, 104088. [Google Scholar] [CrossRef]
- Harrison, M.J.; Dixon, R.A. Isoflavonoid accumulation and expression of defense gene transcripts during the establishment of vesicular-arbuscular mycorrhizal associations in roots of Medicago truncatula. Mol. Plant Microbe Interact. 1993, 6, 643–654. [Google Scholar] [CrossRef]
- Blilou, I.; Ocampo, J.A.; Garcia, G.J. Induction of Ltp (lipid transfer protein) and Pal (phenylalanine ammonia-lyase) gene expression in rice roots colonized by the arbuscular mycorrhizal fungus Glomus mosseae. J. Exp. Bot. 2000, 51, 1969–1977. [Google Scholar] [CrossRef] [Green Version]
- Bonanomi, A.; Oetiker, J.H.; Guggenheim, R.; Boller, T.; Wiemken, A.; Vögeli-Lange, R. Arbuscular mycorrhiza in mini-mycorrhizotrons: First contact of Medicago truncatula roots with Glomus intraradices induces chalcone synthase. N. Phytol. 2001, 150, 573–582. [Google Scholar] [CrossRef]
- Harrison, M.J.; Dixon, R.A. Spatial patterns of expression of flavonoid/isoflavonoid pathway genes during interactions between roots of Medicago truncatula and the mycorrhizal fungus Glomus versiforme. Plant J. 1994, 6, 9–20. [Google Scholar] [CrossRef]
- Liu, J.; Maldonado-Mendoza, I.; Lopez-Meyer, M.; Cheung, F.; Town, C.D.; Harrison, M.J. Arbuscular mycorrhizal symbiosis is accompanied by local and systemic alterations in gene expression and an increase in disease resistance in the shoots. Plant J. 2007, 50, 529–544. [Google Scholar] [CrossRef] [PubMed]
- Handa, Y.; Nishide, H.; Takeda, N.; Suzuki, Y.; Kawaguchi, M.; Saito, K. RNA-seq transcriptional profiling of an arbuscular mycorrhiza provides insights into regulated and coordinated gene expression in Lotus japonicus and Rhizophagus irregularis. Plant Cell Physiol. 2015, 56, 1490–1511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shu, B.; Li, W.; Liu, L.; Wei, Y.; Shi, S. Transcriptomes of arbuscular mycorrhizal fungi and litchi host interaction after tree girdling. Front. Microbiol. 2016, 7, 408. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Q.; Bai, L.; Sun, H.B.; Yang, C.; Cai, B.Y. Transcriptomic and Proteomic Analysis Revealed the Effect of Funneliformis mosseae in Soybean Roots Differential Expression Genes and Proteins. J. Proteome Res. 2020, 19, 3631–3643. [Google Scholar] [CrossRef]
- Zeng, Y.; Guo, L.P.; Chen, B.D.; Hao, Z.P.; Wang, J.Y.; Huang, L.Q.; Yang, G.; Cui, X.M.; Yang, L.; Wu, Z.-X.; et al. Arbuscular mycorrhizal symbiosis and active ingredients of medicinal plants: Current research status and prospectives. Mycorrhiza 2013, 23, 253–265. [Google Scholar] [CrossRef]
- Toussaint, J.P.; Smith, F.A.; Smith, S.E. Arbuscular mycorrhizal fungi can induce the production of phytochemicals in sweet basil irrespective of phosphorus nutrition. Mycorrhiza 2007, 17, 291–297. [Google Scholar] [CrossRef]
- Toussaint, J.P.; Kraml, M.; Nell, M.; Smith, S.E.; Smith, F.A.; Steinkellner, S.; Schmiderer, C.; Vierheilig, H.; Novak, J. Effect of Glomus mosseae on concentrations of rosmarinic and caffeic acids and essential oil compounds in basil inoculated with Fusarium oxysporum f. sp. basilici. Plant Pathol. 2008, 57, 1109–1116. [Google Scholar] [CrossRef]
- Copetta, A.; Lingua, G.; Bardi, L.; Masoero, G.; Berta, G. Influence of arbuscular mycorrhizal fungi on growth and essential oil composition in Ocimum basilicum var. Genovese. Caryologia G. Citol. Citosistematica Citogenet. 2007, 60, 106–110. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Scagel, C.F. Chicoric acid found in basil (Ocimum basilicum L.) leaves. Food Chem. 2009, 115, 650–656. [Google Scholar] [CrossRef]
- Rasouli-Sadaghiani, M.; Hassani, A.; Barin, M.; Rezaee Danesh, Y.; Sefidkon, F. Effects of AM fungi on growth, essential oil production and nutrients uptake in basil. J. Med. Plant Res. 2010, 4, 2222–2228. [Google Scholar] [CrossRef]
- Battini, F.; Bernardi, R.; Turrini, A.; Agnolucci, M.; Giovannetti, M. Rhizophagus intraradices or its associated bacteria affect gene expression of key enzymes involved in the rosmarinic acid biosynthetic pathway of basil. Mycorrhiza 2016, 26, 699–707. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.; Simmonds, M.S.J. Rosmarinic acid. Phytochemistry 2003, 62, 121–125. [Google Scholar] [CrossRef]
- Kapoor, R.; Giri, B.; Mukerji, K.G. Glomus macrocarpum: A potential bioinoculant to improve essential oil quality and concentration in Dill (Anethum graveolens L.) and Carum (Trachyspermum ammi (Linn.) Sprague). World J. Microbiol. Biotechnol. 2002, 18, 459–463. [Google Scholar] [CrossRef]
- Kapoor, R.; Giri, B.; Mukerji, K.G. Mycorrhization of coriander (Coriandrum sativum L) to enhance the concentration and quality of essential oil. J. Sci. Food Agric. 2002, 82, 339–342. [Google Scholar] [CrossRef]
- Chaudhary, V.; Kapoor, R.; Bhatnagar, A.K. Effectiveness of two arbuscular mycorrhizal fungi on concentrations of essential oil and artemisinin in three accessions of Artemisia annua L. Appl. Soil Ecol. 2008, 40, 174–181. [Google Scholar] [CrossRef]
- Karagiannidis, N.; Thomidis, T.; Lazari, D.; Panou-Filotheou, E.; Karagiannidou, C. Effect of three Greek arbuscular mycorrhizal fungi in improving the growth, nutrient concentration, and production of essential oils of oregano and mint plants. Sci. Hortic. 2011, 129, 329–334. [Google Scholar] [CrossRef]
- Dai, Y.-F.; Zhou, W.-W.; Meng, J.; Du, X.-L.; Sui, Y.-S.; Dai, L.; Wang, P.-Q.; Huo, H.-R.; Sui, F. The pharmacological activities and mechanisms of artemisin and its derivatives: A systematic review. Med. Chem. Res. 2017, 26, 867–880. [Google Scholar] [CrossRef]
- Gualandi Jr, R.J. Fungal endophytes enhance growth and production of natural products in Echinacea purpurea (Moench.). Master’s Thesis, University of Tennessee, Knoxville, TN, USA, 2010. [Google Scholar]
- Ososki, A.L.; Kennelly, E.J. Phytoestrogens: A review of the present state of research. Phytother. Res. 2003, 17, 845–869. [Google Scholar] [CrossRef]
- Khaosaad, T.; Krenn, L.; Medjakovic, S.; Ranner, A.; Lössl, A.; Nell, M.; Jungbauer, A.; Vierheilig, H. Effect of mycorrhization on the isoflavone content and the phytoestrogen activity of red clover. J. Plant Physiol. 2008, 165, 1161–1167. [Google Scholar] [CrossRef]
- Teiten, M.-H.; Gaascht, F.; Dicato, M.; Diederich, M. Anticancer bioactivity of compounds from medicinal plants used in European medieval traditions. Biochem. Pharmacol. 2013, 86, 1239–1247. [Google Scholar] [CrossRef]
- Jurkiewicz, A.; Ryszka, P.; Anielska, T.; Waligorski, P.; Białonska, D.; Goralska, K.; Tsimilli-Michael, M.; Turnau, K. Optimization of culture conditions of Arnica montana L.: Effects of mycorrhizal fungi and competing plants. Mycorrhiza 2010, 20, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Abu-Zeyad, R.; Khan, A.G.; Khoo, C. Occurrence of arbuscular mycorrhiza in Castanospermum australe A. Cunn. & C. Fraser and effects on growth and production of castanospermine. Mycorrhiza 1999, 9, 111–117. [Google Scholar]
- Maurich, T.; Iorio, M.; Chimenti, D.; Turchi, G. Erybraedin C and bitucarpin A, two structurally related pterocarpans purified from Bituminaria bituminosa, induced apoptosis in human colon adenocarcinoma cell lines MMR-and p53-proficient and-deficient in a dose-, time-, and structure-dependent fashion. Chem. Biol. Interact. 2006, 159, 104–116. [Google Scholar] [CrossRef]
- Pistelli, L.; Ulivieri, V.; Giovanelli, S.; Avio, L.; Giovannetti, M.; Pistelli, L. Arbuscular mycorrhizal fungi alter the content and composition of secondary metabolites in Bituminaria bituminosa L. Plant Biol. 2017, 19, 926–933. [Google Scholar] [CrossRef] [PubMed]
- Tavarini, S.; Passera, B.; Martini, A.; Avio, L.; Sbrana, C.; Giovannetti, M.; Angelini, L. Plant growth, steviol glycosides and nutrient uptake as affected by arbuscular mycorrhizal fungi and phosphorous fertilization in Stevia rebaudiana Bert. Ind. Crops Prod. 2018, 111, 899–907. [Google Scholar] [CrossRef]
- Zubek, S.; Stojakowska, A.; Anielska, T.; Turnau, K. Arbuscular mycorrhizal fungi alter thymol derivative contents of Inula ensifolia L. Mycorrhiza 2010, 20, 497–504. [Google Scholar] [CrossRef] [PubMed]
- Zubek, S.; Mielcarek, S.; Turnau, K. Hypericin and pseudohypericin concentrations of a valuable medicinal plant Hypericum perforatum L. are enhanced by arbuscular mycorrhizal fungi. Mycorrhiza 2012, 22, 149–156. [Google Scholar] [CrossRef] [Green Version]
- Sailo, G.L.; Bagyaraj, D.J. Influence of Glomus bagyarajii and PGPRs on the growth, nutrition and forskolin concentration of Coleus forskohlii. Biol. Agric. Hortic. 2006, 23, 371–382. [Google Scholar] [CrossRef]
- Perner, H.; Rohn, S.; Driemel, G.; Batt, N.; Schwarz, D.; Kroh, L.W.; George, E. Effect of nitrogen species supply and mycorrhizal colonization on organosulfur and phenolic compounds in onions. J. Agric. Food Chem. 2008, 56, 3538–3545. [Google Scholar] [CrossRef]
- Albrechtova, J.; Latr, A.; Nedorost, L.; Pokluda, R.; Posta, K.; Vosatka, M. Dual inoculation with mycorrhizal and saprotrophic fungi applicable in sustainable cultivation improves the yield and nutritive value of onion. Sci. World J. 2012, 2012. [Google Scholar] [CrossRef] [Green Version]
- Hart, M.; Ehret, D.L.; Krumbein, A.; Leung, C.; Murch, S.; Turi, C.; Franken, P. Inoculation with arbuscular mycorrhizal fungi improves the nutritional value of tomatoes. Mycorrhiza 2015, 25, 359–376. [Google Scholar] [CrossRef] [PubMed]
- Baslam, M.; Garmendia, I.; Goicoechea, N. Arbuscular mycorrhizal fungi (AMF) improved growth and nutritional quality of greenhouse-grown lettuce. J. Agric. Food Chem. 2011, 59, 5504–5515. [Google Scholar] [CrossRef] [PubMed]
- Baslam, M.; Garmendia, I.; Goicoechea, N. Enhanced accumulation of vitamins, nutraceuticals and minerals in lettuces associated with Arbuscular Mycorrhizal Fungi (AMF): A question of interest for both vegetables and humans. Agriculture 2013, 3, 188–209. [Google Scholar] [CrossRef] [Green Version]
- Avio, L.; Sbrana, C.; Giovannetti, M.; Frassinetti, S. Arbuscular mycorrhizal fungi affect total phenolics content and antioxidantactivity in leaves of oak leaf lettuce varieties. Sci. Hortic. 2017, 224, 265–271. [Google Scholar] [CrossRef]
- Ceccarelli, N.; Curadi, M.; Martelloni, L.; Sbrana, C.; Picciarelli, P.; Giovannetti, M. Mycorrhizal colonization impacts on phenolic content and antioxidant properties of artichoke leaves and flower heads two years after field transplant. Plant Soil 2010, 335, 311–323. [Google Scholar] [CrossRef]
- Gostin, A.-I.; Waisundara, V.Y. Edible flowers as functional food: A review on artichoke (Cynara cardunculus L.). Trends Food Sci. Technol. 2019, 86, 381–391. [Google Scholar] [CrossRef]
- Avio, L.; Maggini, R.; Ujvári, G.; Incrocci, L.; Giovannetti, M.; Turrini, A. Phenolics content nad antioxidant activity in the leaves of two artichoke cultivars are differentially affected by six mycorrhizal symbionts. Sci. Hortic. 2020, 264, 109153. [Google Scholar] [CrossRef]
- Lingua, G.; Bona, E.; Manassero, P.; Marsano, F.; Todeschini, V.; Cantamessa, S.; Copetta, A.; D’Agostino, G.; Gamalero, E.; Berta, G. Arbuscular mycorrhizal fungi and plant growth-promoting pseudomonads increases anthocyanin concentration in strawberry fruits (Fragaria × ananassa var. Selva) in conditions of reduced fertilization. Int. J. Mol. Sci. 2013, 14, 16207–16225. [Google Scholar] [CrossRef]
- Bona, E.; Lingua, G.; Manassero, P.; Cantamessa, S.; Marsano, F.; Todeschini, V.; Copetta, A.; D’Agostino, G.; Massa, N.; Avidano, L.; et al. AM fungi and PGP pseudomonads increase flowering, fruit production, and vitamin content in strawberry grown at low nitrogen and phosphorus levels. Mycorrhiza 2015, 25, 181–193. [Google Scholar] [CrossRef]
- Castellanos Morales, V.; Villegas, J.; Wendelin, S.; Vierheilig, H.; Eder, R.; Cárdenas Navarro, R. Root colonisation by the arbuscular mycorrhizal fungus Glomus intraradices alters the quality of strawberry fruits (Fragaria× ananassa Duch.) at different nitrogen levels. J. Sci. Food Agric. 2010, 90, 1774–1782. [Google Scholar] [CrossRef]
- Cecatto, A.P.; Ruiz, F.M.; Calvete, E.O.; Martínez, J.; Palencia, P. Mycorrhizal inoculation affects the phytochemical content in strawberry fruits. Acta Sci. Agron. 2016, 38, 227–237. [Google Scholar] [CrossRef] [Green Version]
- Krishna, H.; Singh, S.K.; Sharma, R.R.; Khawale, R.N.; Grover, M.; Patel, V.B. Biochemical changes in micropropagated grape (Vitis vinifera L.) plantlets due to arbuscular-mycorrhizal fungi (AMF) inoculation during ex vitro acclimatization. Sci. Hortic. 2005, 106, 554–567. [Google Scholar] [CrossRef]
- Dudareva, N.; Negre, F.; Nagegowda, D.A.; Orlova, I. Plant volatiles: Recent advances and future perspectives. Crit. Rev. Plant Sci. 2006, 25, 417–440. [Google Scholar] [CrossRef]
- Velásquez, A.; Valenzuela, M.; Carvajal, M.; Fiaschi, G.; Avio, L.; Giovannetti, M.; D’Onofrio, C.; Seeger, M. The arbuscular mycorrhizal fungus Funneliformis mosseae induces changes and increases the concentration of volatile organic compounds in Vitis vinifera cv. Sangiovese leaf tissue. Plant Physiol. Biochem. 2020, 155, 437–443. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; Leon-Reyes, A.; Van der Ent, S.; Van Wees, S.C.M. Networking by small-molecule hormones in plant immunity. Nat. Chem. Biol. 2009, 5, 308–316. [Google Scholar] [CrossRef] [Green Version]
- Glick, B.R.; Cheng, Z.; Czarny, J.; Duan, J. Promotion of plant growth by ACC deaminase-producing soil bacteria. Eur. J. Plant Pathol. 2007, 119, 329–339. [Google Scholar] [CrossRef]
- Bona, E.; Cantamessa, S.; Massa, N.; Manassero, P.; Marsano, F.; Copetta, A.; Lingua, G.; D’Agostino, G.; Gamalero, E.; Berta, G. Arbuscular mycorrhizal fungi and plant growth-promoting pseudomonads improve yield, quality and nutritional value of tomato: A field study. Mycorrhiza 2017, 27, 1–11. [Google Scholar] [CrossRef]
- Bona, E.; Todeschini, V.; Cantamessa, S.; Cesaro, P.; Copetta, A.; Lingua, G.; Gamalero, E.; Berta, G.; Massa, N. Combined bacterial and mycorrhizal inocula improve tomato quality at reduced fertilization. Sci. Hortic. 2018, 234, 160–165. [Google Scholar] [CrossRef]
- Todeschini, V.; AitLahmidi, N.; Mazzucco, E.; Marsano, F.; Gosetti, F.; Robotti, E.; Bona, E.; Massa, N.; Bonneau, L.; Marengo, E. Impact of beneficial microorganisms on strawberry growth, fruit production, nutritional quality and volatilome. Front. Plant Sci. 2018, 9, 1611. [Google Scholar] [CrossRef]
- Nosheen, A.; Bano, A.; Ullah, F. Nutritive value of canola (Brassica napus L.) as affected by plant growth-promoting rhizobacteria. Eur. J. Lipid Sci. Technol. 2011, 113, 1342–1346. [Google Scholar] [CrossRef]
- Akbari, P.; Ghalavand, A.; Modarres, S.A.; Alikhani, M.A. The effect of biofertilizers, nitrogen fertilizer and farmyard manure on grain yield and seed quality of sunflower (Helianthus annuus L.). J. Agric. Technol. 2011, 7, 173–184. [Google Scholar]
- Nautiyal, C.S.; Govindarajan, R.; Lavania, M.; Pushpangadan, P. Novel mechanism of modulating natural antioxidants in functional foods: Involvement of plant growth-promoting rhizobacteria NRRL B-30488. J. Agric. Food Chem. 2008, 56, 4474–4481. [Google Scholar] [CrossRef]
- Khalid, M.; Hassani, D.; Bilal, M.; Asad, F.; Huang, D. Influence of bio-fertilizer containing beneficial fungi and rhizospheric bacteria on health promoting compounds and antioxidant activity of Spinacia oleracea L. Bot. Stud. 2017, 58, 35. [Google Scholar] [CrossRef] [Green Version]
- Akca, Y.; Ercisli, S. Effect of plant growth promoting rhizobacteria (PGPR) inoculation on fruit quality in sweet cherry (Prunus avium L.) cv. 0900 Ziraat. J. Food Agric. Environ. 2010, 8, 769–771. [Google Scholar]
- Berta, G.; Copetta, A.; Gamalero, E.; Bona, E.; Cesaro, P.; Scarafoni, A.; D’Agostino, G. Maize development and grain quality are differentially affected by mycorrhizal fungi and a growth-promoting pseudomonad in the field. Mycorrhiza 2014, 24, 161–170. [Google Scholar] [CrossRef]
- Awasthi, A.; Bharti, N.; Nair, P.; Singh, R.; Shukla, A.K.; Gupta, M.M.; Darokar, M.P.; Kalra, A. Synergistic effect of Glomus mosseae and nitrogen fixing Bacillus subtilis strain Daz26 on artemisinin content in Artemisia annua L. Appl. Soil Ecol. 2011, 49, 125–130. [Google Scholar] [CrossRef]
- Battini, F.; Turrini, A.; Quartacci, M.; Malorgio, F.; Sgherri, C.; Picciarelli, P.; Pardossi, A.; Giovannetti, M.; Agnolucci, M. Dual inoculation with AMF and associated bacteria improves nutraceutical value of sweet basil grown under commercial conditions. Agrochimica 2016, 60, 81–99. [Google Scholar] [CrossRef]
| Phytochemicals | Plant Species | Microorganisms | Effect on Phytochemical Concentration | Source | ||||
|---|---|---|---|---|---|---|---|---|
| AMF | Bacteria | |||||||
| Phenolics | Total phenolics | Lactuca sativa L. | Glomus fasciculatum | increased concentration or no effect depending on cultivar | [94] | |||
| Glomus intraradices+Glomus mosseae (Atens, Spain) | increased concentration or no effect depending on cultivar | [94] | ||||||
| Glomus intraradices+Glomus mosseae (Atens, Spain) | no effect | [95] | ||||||
| Rhizoglomus irregulare | increased concentration | [96] | ||||||
| Funneliformis mosseae | no effect | [96] | ||||||
| Bacillus lentimorbus B-30488 | increased concentration | [114] | ||||||
| Ocimum basilicum L. | G. intraradices NPI, USA | no effect | [70] | |||||
| G. mosseae BEG 12 | no effect | [68] | ||||||
| Rhizophagus intraradices | Sinorhizobium meliloti TSA41 * + Streptomyces sp. W43N * | no effect | With AMF With bacterial mix | [119] | ||||
| decreased concentration or no effect depending on the cultivar | With co-inoculation of AMF+bacterial mix | |||||||
| Fragaria x ananassa Duch. | G. intraradices (Premier Tech Biotechnologies, Canada) | no effect | [102] | |||||
| Glomus iranicum var. tenuihypharum (Mycogrowth®, Spain) | increased concentration | At early inoculation | [103] | |||||
| Cynara cardunculus L. var. scolymus | G. mosseae G. intraradices | increased concentration | With G. intraradices With AMF mix | [97] | ||||
| no effect | With G. mosseae | |||||||
| F. mosseae 2W3 F. mosseae IMA1 F. mosseae IN101C Claroideoglomus claroideum 22W3 R. irregulare IMA6 Glomus sp. 14W1 | increased concentration | With 2W3 | [99] | |||||
| no effect | With all the other AMF (single inoculation) | |||||||
| Allium cepa L. | Mix1 Plantworks, UK | no effect | [91] | |||||
| Hydroxycinnamic acids | Caffeic acid | Ocimum basilicum L. | G. intraradices BEG 159 Glomus caledonium BEG 162 G. mosseae NBR 1–2 | increased concentration | With G. caledonium | [67] | ||
| G. mosseae BEG 12 | no effect | [68] | ||||||
| Rosmarinic acid | G. intraradices BEG 159 G. caledonium BEG 162 G. mosseae NBR 1–2 | increased concentration | With G. caledonium With G. mosseae (single inoculation) | [67] | ||||
| G. mosseae BEG 12 | no effect | [68] | ||||||
| G. intraradices (NPI, USA) | no effect | [70] | ||||||
| R. intraradices | S. meliloti TSA41 * + Streptomyces sp. W43N * | increased or decreased concentration depending on the cultivar | With co-inoculation of AMF + bacterial mix | [119] | ||||
| decreased concentration or no effect depending on the cultivar | With bacterial mix | |||||||
| decreased concentration | With AMF | |||||||
| Chicoric acid | G. intraradices (NPI, USA) | no effect | [70] | |||||
| Flavonols | Quercetin | Fragaria x ananassa Duch. | G. intraradices (Premier Tech Biotechnologies Company, Canada) | increased concentration | [102] | |||
| Kaempferol | increased concentration | |||||||
| Catechin | no effect | |||||||
| Anthocyanidins | Pelargonidin 3-glucoside, pelargonidin 3-rutinoside | Fragaria x ananassa Duch. | Mix2 (Mybasol, Italy) | Pseudomonas fluorescens Pf4 Pseudomonas sp. 5Vm1K | increased concentration | With Mix With P5Vm1K With co-inoculation Mix + Pf4 | [100] | |
| no effect | With Mix With Pf4 With co-inoculation Mix + P5Vm1K | |||||||
| Pelargonidin 3-glucoside | F. mosseae BEG12 | P. fluorescens 19Fv1t P. fluorescens Pf 4Pseudomonas sp. 5Vm1K | no effect | With co-inoculation of AMF + any bacterial isolate | [111] | |||
| Septoglomus viscosum | P. fluorescens 19Fv1t P. fluorescens Pf4 Pseudomonas sp. 5Vm1K | increased concentration | With co-inoculation of AMF + P5Vm1K | |||||
| Rhizophagus irregularis DAOM197-200 | P. fluorescens 19Fv1t P. fluorescens Pf4 Pseudomonas sp. 5Vm1K | no effect | With co-inoculation of AMF + any bacterial isolate | |||||
| G. intraradices (Premier Tech Biotechnologies Company, Canada) | no effect | [102] | ||||||
| Pelargonidin malonyl glucoside | Mix2 (Mybasol, Italy) | P. fluorescens Pf4 Pseudomonas sp. 5Vm1K | increased concentration | With AMF Mix With co-inoculation Mix + Pf4 | [100] | |||
| no effect | With Pf4 With P5Vm1K With co-inoculation Mix + P5Vm1K | |||||||
| Pelargonidin acetyl glucoside | Mix2 (Mybasol, Italy) | P. fluorescens Pf4 Pseudomonas sp. 5Vm1K | no effect | With all combination | [100] | |||
| Cyanidin 3-glucoside | Mix2 (Mybasol, Italy) | P. fluorescens Pf4 Pseudomonas sp. 5Vm1K | increased concentration | With P5Vm1K With co-inoculation Mix + PV5m1K | [100] | |||
| no effect | With Pf4 With co-inoculation Mix + P5Vm1K | |||||||
| G. intraradices (Premier Tech Biotechnologies Company, Canada) | increased concentration | [102] | ||||||
| Total anthocyanins | Ocimum basilicum L. | R. intraradices | S. meliloti TSA41 * + Streptomyces sp. W43N * | increased concentration | With co-inoculation AMF + bacterial mix | [119] | ||
| no effect | With bacterial mix | |||||||
| decreased concentration | With AMF | |||||||
| G. intraradices (NPI, USA) | increased concentration | [70] | ||||||
| Terpenoids | Total carotenoids | Ocimum basilicum L. | R. intraradices | S. meliloti TSA41 * + Streptomyces sp. W43N * | decreased concentration | With AMF With bacterial mix | [119] | |
| decreased concentration or no effect depending on cultivar | With co-inoculation of AMF + bacterial mix | |||||||
| Solanum lycopersicum L. | F. mosseae BEG12 R. irregularis BB-E (Agrauxine, F) | increased concentration | With co-inoculation | [93] | ||||
| no effect | With BEG12 With BB-E (single inoculation) | |||||||
| R. irregularis (Premier Tech Inc., Canada) | increased concentration | [93] | ||||||
| Carotenes | Lycopene | Solanum lycopersicum L. | Mix2 (Mybasol, Italy) | P. fluorescens C7 Pseudomonas sp. 19Fv1T | no effect | With all combinations | [109] | |
| Mix2 (Mybasol, Italy) | P. fluorescens C7Pseudomonas sp. 19Fv1T | decreased concentration | With co-inoculation Mix + single bacterial isolate With triple inoculation | [110] | ||||
| F. mosseae BEG12 R. irregularis BB-E (Agrauxine, F) | increased concentration | With BEG12 With BB-E (single inoculation) | [93] | |||||
| no effect | With co-inoculation | |||||||
| β-carotene | Solanum lycopersicum L. | Mix2 (Mybasol, Italy) | P. fluorescens C7 Pseudomonas sp. 19Fv1T | decreased concentration | With AMF Mix With C7 (single inoculation) | [109] | ||
| no effect | With 19Fv1T With co-inoculation Mix + single bacterial isolate With triple inoculation | |||||||
| Mix2 (Mybasol, Italy) | P. fluorescens C7 Pseudomonas sp. 19Fv1T | decreased concentration | With co-inoculation Mix + single bacterial isolate | [110] | ||||
| no effect | With triple inoculation | |||||||
| F. mosseae BEG12 R. irregularis BB-E (Agrauxine, F) | increased concentration | With co-inoculation | [93] | |||||
| no effect | With BEG12 With BB-E (single inoculation) | |||||||
| Xanthophylls | Luteine | Solanum lycopersicum L. | Mix2 (Mybasol, Italy) | P. fluorescens C7 Pseudomonas sp. 19Fv1T | increased concentration | With co-inoculation Mix + single bacterial isolate | [110] | |
| no effect | With triple inoculation | |||||||
| Other | Ascorbic acid | Fragaria x ananassa Duch. | Mix2 (Mybasol, Italy) | P. fluorescens Pf4 Pseudomonas sp. 5Vm1K | increased concentration | With Mix With single bacterial isolates With co-inoculation Mix + single bacterial isolate | [101] | |
| Solanum lycopersicum L. | Mix2 (Mybasol, Italy) | P. fluorescens C7 Pseudomonas sp. 19Fv1T | increased concentration | With 19Fv1T | [109] | |||
| no effect | With co-inoculation AMF + 19Fv1T | |||||||
| decreased concentration | With Mix With C7 With co-inoculation Mix+C7 | |||||||
| Mix2 (Mybasol, Italy) | P. fluorescens C7 Pseudomonas sp. 19Fv1T | increased concentration | With triple inoculation | [110] | ||||
| no effect | With co-inoculation of AMF + 19Fv1T | |||||||
| decreased concentration | With co-inoculation of AMF + C7 | |||||||
| Mix3 (Symbivit, Czech Rep.) G. intraradices BEG140 | no effect | With AMF (single inoculation) | [92] | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agnolucci, M.; Avio, L.; Palla, M.; Sbrana, C.; Turrini, A.; Giovannetti, M. Health-Promoting Properties of Plant Products: The Role of Mycorrhizal Fungi and Associated Bacteria. Agronomy 2020, 10, 1864. https://doi.org/10.3390/agronomy10121864
Agnolucci M, Avio L, Palla M, Sbrana C, Turrini A, Giovannetti M. Health-Promoting Properties of Plant Products: The Role of Mycorrhizal Fungi and Associated Bacteria. Agronomy. 2020; 10(12):1864. https://doi.org/10.3390/agronomy10121864
Chicago/Turabian StyleAgnolucci, Monica, Luciano Avio, Michela Palla, Cristiana Sbrana, Alessandra Turrini, and Manuela Giovannetti. 2020. "Health-Promoting Properties of Plant Products: The Role of Mycorrhizal Fungi and Associated Bacteria" Agronomy 10, no. 12: 1864. https://doi.org/10.3390/agronomy10121864






