Ecosystem Functions of Microbial Consortia in Sustainable Agriculture
Abstract
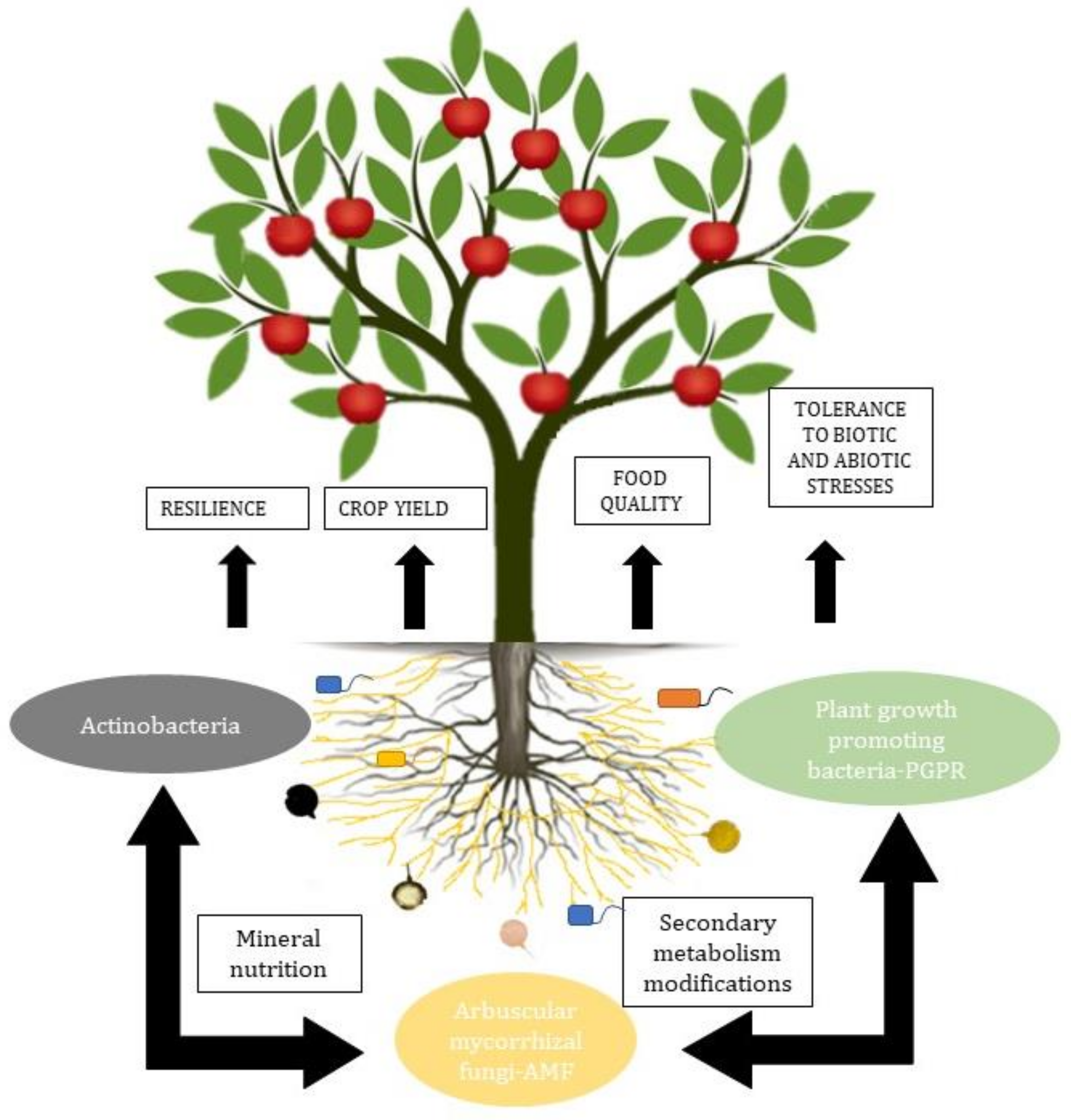
:1. Introduction
2. Discussion
2.1. Arbuscular Mycorrhizal Fungi (AMF)
2.2. Plant Growth-Promoting Rhizobacteria (PGPR)
2.3. Actinobacteria
2.4. The Microbial Consortia
3. Conclusive Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| GHG | greenhouse gases |
| AMF | arbuscular mycorrhizal fungi |
| PGPR | plant growth promoting bacteria |
| gibberellic acid, GA3 | gibberellins |
| indolacetic acid, AIA | auxins |
| ABA | abscisic acid |
| ACC | enzyme 1-aminocyclopropane 1-carboxylate deaminase |
References
- Porter, S.S.; Sachs, J.L. Agriculture and the Disruption of Plant–Microbial Symbiosis. Trends Ecol. Evol. 2020, 35, 426–439. [Google Scholar] [CrossRef]
- Hartman, K.; Van Der Heijden, M.G.A.; Wittwer, R.A.; Banerjee, S.; Walser, J.-C.; Schlaeppi, K. Cropping practices manipulate abundance patterns of root and soil microbiome members paving the way to smart farming. Microbiome 2018, 6, 1–14. [Google Scholar] [CrossRef]
- Springmann, M.; Clark, M.; Mason-D’Croz, D.; Wiebe, K.; Bodirsky, B.L.; Lassaletta, L.; De Vries, W.; Vermeulen, S.J.; Herrero, M.; Carlson, K.M.; et al. Options for keeping the food system within environmental limits. Nature 2018, 562, 519–525. [Google Scholar] [CrossRef] [PubMed]
- Stavi, I.; Lal, R. Achieving Zero Net Land Degradation: Challenges and opportunities. J. Arid. Environ. 2015, 112, 44–51. [Google Scholar] [CrossRef]
- Kughur, P.G.; Otene, V.; Audu, O.C. Effects of intensive agricultural production on the environment in benue state, Nigeria. IOSR J. Agric. Vet. Sci. (IOSR-JAVS) 2015, 8, 7–11. [Google Scholar] [CrossRef]
- Gomiero, T.; Pimentel, D.; Paoletti, M.G. Environmental Impact of Different Agricultural Management Practices: Conventional vs. Organic Agriculture. Crit. Rev. Plant Sci. 2011, 30, 95–124. [Google Scholar] [CrossRef]
- Devarinti, S.R. Natural Farming: Eco-Friendly and Sustainable? Agrotechnology 2016, 5, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Bonanomi, G.; D’Ascoli, R.; Antignani, V.; Capodilupo, M.; Cozzolino, L.; Marzaioli, R.; Puopolo, G.; Rutigliano, F.A.; Scelza, R.; Scotti, R.; et al. Assessing soil quality under intensive cultivation and tree orchards in Southern Italy. Appl. Soil Ecol. 2011, 47, 184–194. [Google Scholar] [CrossRef]
- Chen, Q.; Zhang, X.; Zhang, H.; Christie, P.; Li, X.; Horlacher, D.; Liebig, H.-P. Evaluation of current fertilizer practice and soil fertility in vegetable production in the Beijing region. Nutr. Cycl. Agroecosyst. 2004, 69, 51–58. [Google Scholar] [CrossRef] [Green Version]
- Purwanto, B.H.; Alam, S. Impact of intensive agricultural management on carbon and nitrogen dynamics in the humid tropics. Soil Sci. Plant Nutr. 2019, 66, 50–59. [Google Scholar] [CrossRef]
- Sá, J.C.D.M.; Lal, R.; Cerri, C.C.; Lorenz, K.; Hungria, M.; Carvalho, P.C.D.F. Low-carbon agriculture in South America to mitigate global climate change and advance food security. Environ. Int. 2017, 98, 102–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altieri, M.A. Agroecology the Science of Sustainable Agricultura, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2018; p. 448. [Google Scholar] [CrossRef]
- Bender, S.F.; Wagg, C.; Van Der Heijden, M.G.A. An Underground Revolution: Biodiversity and Soil Ecological Engineering for Agricultural Sustainability. Trends Ecol. Evol. 2016, 31, 440–452. [Google Scholar] [CrossRef] [PubMed]
- Eyhorn, F.; Muller, A.; Reganold, J.P.; Frison, E.; Herren, H.R.; Luttikholt, L.; Mueller, A.; Sanders, J.; Scialabba, N.E.-H.; Seufert, V.; et al. Sustainability in global agriculture driven by organic farming. Nat. Sustain. 2019, 2, 253–255. [Google Scholar] [CrossRef] [Green Version]
- Isbell, F.; Craven, D.; Connolly, J.; Loreau, M.; Schmid, B.; Beierkuhnlein, C.; Bezemer, T.M.; Bonin, C.L.; Bruelheide, H.; De Luca, E.; et al. Biodiversity increases the resistance of ecosystem productivity to climate extremes. Nat. Cell Biol. 2015, 526, 574–577. [Google Scholar] [CrossRef] [PubMed]
- Oldroyd, G.E.D. Speak, friend, and enter: Signalling systems that promote beneficial symbiotic associations in plants. Nat. Rev. Microbiol. 2013, 11, 252–263. [Google Scholar] [CrossRef]
- Bassler, B.L.; Losick, R. Bacterially Speaking. Cell 2006, 125, 237–246. [Google Scholar] [CrossRef] [Green Version]
- Brameyer, S.; Bode, H.B.; Heermann, R. Languages and dialects: Bacterial communication beyond homoserine lactones. Trends Microbiol. 2015, 23, 521–523. [Google Scholar] [CrossRef]
- Bardgett, R.D.; Van Der Putten, W.H. Belowground biodiversity and ecosystem functioning. Nat. Cell Biol. 2014, 515, 505–511. [Google Scholar] [CrossRef]
- Mendes, R.; Garbeva, P.; Raaijmakers, J.M. The rhizosphere microbiome: Significance of plant beneficial, plant pathogenic, and human pathogenic microorganisms. FEMS Microbiol. Rev. 2013, 37, 634–663. [Google Scholar] [CrossRef]
- Philippot, L.; Raaijmakers, J.M.; Lemanceau, P.; Van Der Putten, W.H. Going back to the roots: The microbial ecology of the rhizosphere. Nat. Rev. Mircobiol. 2013, 11, 789–799. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Maestre, F.T.; Reich, P.B.; Jeffries, T.C.; Gaitan, J.J.; Encinar, D.; Berdugo, M.; Campbell, C.D.; Singh, B.K. Microbial diversity drives multifunctionality in terrestrial ecosystems. Nat. Commun. 2016, 7, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chu, H.; Gao, G.-F.; Ma, Y.; Fan, K.; Delgado-Baquerizo, M. Soil Microbial Biogeography in a Changing World: Recent Advances and Future Perspectives. mSystems 2020, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kuzyakov, Y.; Blagodatskaya, E. Microbial hotspots and hot moments in soil: Concept & review. Soil Biol. Biochem. 2015, 83, 184–199. [Google Scholar] [CrossRef]
- Wagg, C.; Bender, S.F.; Widmer, F.; Van Der Heijden, M.G.A. Soil biodiversity and soil community composition determine ecosystem multifunctionality. Proc. Natl. Acad. Sci. USA 2014, 111, 5266–5270. [Google Scholar] [CrossRef] [Green Version]
- Delgado-Baquerizo, M.; Reich, P.B.; Trivedi, C.; Eldridge, D.J.; Abades, S.; Alfaro, F.D.; Bastida, F.; Berhe, A.A.; Cutler, N.A.; Gallardo, A.; et al. Multiple elements of soil biodiversity drive ecosystem functions across biomes. Nat. Ecol. Evol. 2020, 4, 210–220. [Google Scholar] [CrossRef]
- Das, S.; Ho, A.; Kim, P.J. Editorial: Role of Microbes in Climate Smart Agriculture. Front. Microbiol. 2019, 10, 2756. [Google Scholar] [CrossRef]
- Rillig, M.C.; Lehmann, A.; Lehmann, J.; Camenzind, T.; Rauh, C. Soil Biodiversity Effects from Field to Fork. Trends Plant Sci. 2018, 23, 17–24. [Google Scholar] [CrossRef]
- Ochoa-Hueso, R. Global Change and the Soil Microbiome: A Human-Health Perspective. Front. Ecol. Evol. 2017, 5, 71. [Google Scholar] [CrossRef] [Green Version]
- Wall, D.H.; Nielsen, U.N.; Six, J. Soil biodiversity and human health. Nat. Cell Biol. 2015, 528, 69–76. [Google Scholar] [CrossRef]
- Berruti, A.; Lumini, E.; Balestrini, R.; Bianciotto, V. Arbuscular Mycorrhizal Fungi as Natural Biofertilizers: Let’s Benefit from Past Successes. Front. Microbiol. 2016, 6, 1559. [Google Scholar] [CrossRef] [Green Version]
- Kong, Z.; Hart, M.; Liu, H. Paving the Way From the Lab to the Field: Using Synthetic Microbial Consortia to Produce High-Quality Crops. Front. Plant Sci. 2018, 9, 1467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Der Heijden, M.G.A.; Martin, F.M.; Selosse, M.-A.; Sanders, I.R. Mycorrhizal ecology and evolution: The past, the present, and the future. New Phytol. 2015, 205, 1406–1423. [Google Scholar] [CrossRef] [PubMed]
- Thirkell, T.J.; Charters, M.D.; Elliott, A.J.; Sait, S.M.; Field, K.J. Are mycorrhizal fungi our sustainable saviours? Considerations for achieving food security. J. Ecol. 2017, 105, 921–929. [Google Scholar] [CrossRef] [Green Version]
- Brundrett, M.C.; Tedersoo, L. Evolutionary history of mycorrhizal symbioses and global host plant diversity. New Phytol. 2018, 220, 1108–1115. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Read, D.J. Mycorrhizal Symbiosis, 2nd ed.; Academic Press: Cambridge, MA, USA; Elsevier: London, UK, 2008; p. 800. [Google Scholar] [CrossRef]
- Spatafora, J.W.; Chang, Y.; Benny, G.L.; Lazarus, K.; Smith, M.E.; Berbee, M.L.; Bonito, G.; Corradi, N.; Grigoriev, I.; Gryganskyi, A.; et al. A phylum-level phylogenetic classification of zygomycete fungi based on genome-scale data. Mycologia 2016, 108, 1028–1046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rich, M.K.; Nouri, E.; Courty, P.-E.; Reinhardt, D. Diet of Arbuscular Mycorrhizal Fungi: Bread and Butter? Trends Plant Sci. 2017, 22, 652–660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parniske, M. Arbuscular mycorrhiza: The mother of plant root endosymbioses. Nat. Rev. Microbiol. 2008, 6, 763–775. [Google Scholar] [CrossRef]
- Smith, S.E.; Jakobsen, I.; Grønlund, M.; Smith, F.A. Roles of Arbuscular Mycorrhizas in Plant Phosphorus Nutrition: Interactions between Pathways of Phosphorus Uptake in Arbuscular Mycorrhizal Roots Have Important Implications for Understanding and Manipulating Plant Phosphorus Acquisition. Plant Physiol. 2011, 156, 1050–1057. [Google Scholar] [CrossRef] [Green Version]
- Coccina, A.; Cavagnaro, T.R.; Pellegrino, E.; Ercoli, L.; McLaughlin, M.J.; Watts-Williams, S.J. The mycorrhizal pathway of zinc uptake contributes to zinc accumulation in barley and wheat grain. BMC Plant Biol. 2019, 19, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Azcón-Aguilar, C.; Barea, J. Nutrient cycling in the mycorrhizosphere. J. Soil Sci. Plant Nutr. 2015, 15, 372–396. [Google Scholar] [CrossRef] [Green Version]
- Cavagnaro, T.R.; Bender, S.F.; Asghari, H.R.; Van Der Heijden, M.G. The role of arbuscular mycorrhizas in reducing soil nutrient loss. Trends Plant Sci. 2015, 20, 283–290. [Google Scholar] [CrossRef] [Green Version]
- Augé, R.M. Arbuscular mycorrhizae and soil/plant water relations. Can. J. Soil Sci. 2004, 84, 373–381. [Google Scholar] [CrossRef] [Green Version]
- Cameron, D.D.; Neal, A.L.; Van Wees, S.C.; Ton, J. Mycorrhiza-induced resistance: More than the sum of its parts? Trends Plant Sci. 2013, 18, 539–545. [Google Scholar] [CrossRef] [Green Version]
- Oehl, F.; Sieverding, E.; Ineichen, K.; Mäder, P.; Boller, T.; Wiemken, A. Impact of Land Use Intensity on the Species Diversity of Arbuscular Mycorrhizal Fungi in Agroecosystems of Central Europe. Appl. Environ. Microbiol. 2003, 69, 2816–2824. [Google Scholar] [CrossRef] [Green Version]
- Oehl, F.; Sieverding, E.; Dubois, D.; Ineichen, K.; Boller, T.; Wiemken, A. Impact of long-term conventional and organic farming on the diversity of arbuscular mycorrhizal fungi. Oecologia 2004, 138, 574–583. [Google Scholar] [CrossRef]
- Turrini, A.; Avio, L.; Giovannetti, M.; Agnolucci, M. Functional Complementarity of Arbuscular Mycorrhizal Fungi and Associated Microbiota: The Challenge of Translational Research. Front. Plant Sci. 2018, 9, 1407. [Google Scholar] [CrossRef]
- Gianinazzi, S.; Gollotte, A.; Binet, M.-N.; Van Tuinen, D.; Redecker, D.; Wipf, D. Agroecology: The key role of arbuscular mycorrhizas in ecosystem services. Mycorrhiza 2010, 20, 519–530. [Google Scholar] [CrossRef]
- Silva-Flores, P.; Aguilar, A.; Dibán, M.J.; Mujica, M.I. Chapter 14 Mycorrhizas in the South American Mediterranean-Type Ecosystem: Chilean Matorral. In Mycorrhizal Fungi in South America, 1st ed.; Pagano, M., Lugo, M., Eds.; Springer: Cham, Switzerland, 2019; pp. 277–294. [Google Scholar] [CrossRef]
- Säle, V.; Aguilera, P.; Laczko, E.; Mäder, P.; Berner, A.; Zihlmann, U.; Van Der Heijden, M.G.A.; Oehl, F. Impact of conservation tillage and organic farming on the diversity of arbuscular mycorrhizal fungi. Soil Biol. Biochem. 2015, 84, 38–52. [Google Scholar] [CrossRef]
- Bedini, S.; Avio, L.; Sbrana, C.; Turrini, A.; Migliorini, P.; Vazzana, C.; Giovannetti, M. Mycorrhizal activity and diversity in a long-term organic Mediterranean agroecosystem. Biol. Fertil. Soils 2013, 49, 781–790. [Google Scholar] [CrossRef]
- Verbruggen, E.; Röling, W.F.M.; Gamper, H.A.; Kowalchuk, G.A.; Verhoef, H.A.; Van Der Heijden, M.G.A. Positive effects of organic farming on below-ground mutualists: Large-scale comparison of mycorrhizal fungal communities in agricultural soils. New Phytol. 2010, 186, 968–979. [Google Scholar] [CrossRef]
- Gosling, P.; Hodge, A.; Goodlass, G.; Bending, G. Arbuscular mycorrhizal fungi and organic farming. Agric. Ecosyst. Environ. 2006, 113, 17–35. [Google Scholar] [CrossRef]
- Pellegrino, E.; Bedini, S. Enhancing ecosystem services in sustainable agriculture: Biofertilization and biofortification of chickpea (Cicer arietinum L.) by arbuscular mycorrhizal fungi. Soil Biol. Biochem. 2014, 68, 429–439. [Google Scholar] [CrossRef]
- Rang-Jin, X.; Franken, P.; Schneider, C.; Schwarz, D.; Giovannetti, M.; Agnolucci, M.; De Pascale, S.; Bonini, P.; Colla, G. Arbuscular mycorrhizal fungi act as biostimulants in horticultural crops. Sci. Hortic. 2015, 196, 91–108. [Google Scholar] [CrossRef]
- Verbruggen, E.; Van Der Heijden, M.G.A.; Rillig, M.C.; Kiers, E.T. Mycorrhizal fungal establishment in agricultural soils: Factors determining inoculation success. New Phytol. 2013, 197, 1104–1109. [Google Scholar] [CrossRef] [Green Version]
- Crossay, T.; Majorel, C.; Redecker, D.; Gensous, S.; Medevielle, V.; Durrieu, G.; Cavaloc, Y.; Amir, H. Is a mixture of arbuscular mycorrhizal fungi better for plant growth than single-species inoculants? Mycorrhiza 2019, 29, 325–339. [Google Scholar] [CrossRef]
- Hart, M.M.; Antunes, P.M.; Chaudhary, V.B.; Abbott, L.K. Fungal inoculants in the field: Is the reward greater than the risk? Funct. Ecol. 2018, 32, 126–135. [Google Scholar] [CrossRef] [Green Version]
- Makus, D. Mycorrhizal inoculation of tomato and onion transplants improves earliness. Acta Hortic. 2004, 631, 275–281. [Google Scholar] [CrossRef] [Green Version]
- Lax, P.; Becerra, A.G.; Soteras, F.; Cabello, M.; Doucet, M.E. Effect of the arbuscular mycorrhizal fungus Glomus intraradices on the false root-knot nematode Nacobbus aberrans in tomato plants. Biol. Fertil. Soils 2011, 47, 591–597. [Google Scholar] [CrossRef] [Green Version]
- Berta, G.; Copetta, A.; Gamalero, E.; Bona, E.; Cesaro, P.; Scarafoni, A.; D’Agostino, G. Maize development and grain quality are differentially affected by mycorrhizal fungi and a growth-promoting pseudomonad in the field. Mycorrhiza 2013, 24, 161–170. [Google Scholar] [CrossRef]
- Bedini, S.; Pellegrino, E.; Avio, L.; Pellegrini, S.; Bazzoffi, P.; Argese, E.; Giovannetti, M. Changes in soil aggregation and glomalin-related soil protein content as affected by the arbuscular mycorrhizal fungal species Glomus mosseae and Glomus intraradices. Soil Biol. Biochem. 2009, 41, 1491–1496. [Google Scholar] [CrossRef]
- Liu, A.; Hamel, C.; Elmi, A.; Costa, C.A.V.; Ma, B.; Smith, D.L. Concentrations of K, Ca and Mg in maize colonized by arbuscular mycorrhizal fungi under field conditions. Can. J. Soil Sci. 2002, 82, 272–278. [Google Scholar] [CrossRef] [Green Version]
- Regvar, M.; Vogel-Mikuš, K.; Ševerkar, T. Effect of AMF inoculum from field isolates on the yield of green pepper, parsley, carrot, and tomato. Folia Geobot. 2003, 38, 223–234. [Google Scholar] [CrossRef]
- Singh, R.K.; Gogoi, P. Augmented growth of long pepper in response to arbuscular mycorrhizal inoculation. J. For. Res. 2012, 23, 339–344. [Google Scholar] [CrossRef]
- Douds, D.D.; Nagahashi, G.; Reider, C.; Hepperly, P.R. Inoculation with Arbuscular Mycorrhizal Fungi Increases the Yield of Potatoes in a High P Soil. Biol. Agric. Hortic. 2007, 25, 67–78. [Google Scholar] [CrossRef]
- Stewart, L.I.; Hamel, C.; Hogue, R.; Moutoglis, P. Response of strawberry to inoculation with arbuscular mycorrhizal fungi under very high soil phosphorus conditions. Mycorrhiza 2005, 15, 612–619. [Google Scholar] [CrossRef]
- Gholamhoseini, M.; Ghalavand, A.; Dolatabadian, A.; Jamshidi, E.; Khodaei-Joghan, A. Effects of arbuscular mycorrhizal inoculation on growth, yield, nutrient uptake and irrigation water productivity of sunflowers grown under drought stress. Agric. Water Manag. 2013, 117, 106–114. [Google Scholar] [CrossRef]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant Growth-Promoting Rhizobacteria: Context, Mechanisms of Action, and Roadmap to Commercialization of Biostimulants for Sustainable Agriculture. Front. Plant Sci. 2018, 9, 1473. [Google Scholar] [CrossRef] [Green Version]
- Hassan, M.K.; McInroy, J.A.; Kloepper, J.W. The Interactions of Rhizodeposits with Plant Growth-Promoting Rhizobacteria in the Rhizosphere: A Review. Agriculture 2019, 9, 142. [Google Scholar] [CrossRef] [Green Version]
- Braga, L.F.; De Oliveira, F.A.; Couto, E.A.P.D.; Santos, K.F.D.N.; Ferreira, E.P.D.B.; Martin-Didonet, C.C.G. Polyphasic characterization of bacteria obtained from upland rice cultivated in Cerrado soil. Braz. J. Microbiol. 2018, 49, 20–28. [Google Scholar] [CrossRef]
- Abbas, R.; Rasul, S.; Aslam, K.; Baber, M.; Shahid, M.; Mubeen, F.; Naqqash, T. Halotolerant PGPR: A hope for cultivation of saline soils. J. King Saud Univ. Sci. 2019, 31, 1195–1201. [Google Scholar] [CrossRef]
- Akram, M.S.; Shahid, M.; Tariq, M.; Azeem, M.; Javed, M.T.; Saleem, S.; Riaz, S. Deciphering Staphylococcus sciuri SAT-17 Mediated Anti-oxidative Defense Mechanisms and Growth Modulations in Salt Stressed Maize (Zea mays L.). Front. Microbiol. 2016, 7, 867. [Google Scholar] [CrossRef]
- Rijavec, T.; Lapanje, A. Hydrogen Cyanide in the Rhizosphere: Not Suppressing Plant Pathogens, but Rather Regulating Availability of Phosphate. Front. Microbiol. 2016, 7, 1785. [Google Scholar] [CrossRef] [Green Version]
- Angulo, V.C.; Sanfuentes, E.A.; Rodríguez, F.; Sossa, K.E. Caracterización de rizobacterias promotoras de crecimiento en plántulas de Eucalyptus nitens. Rev. Argent. Microbiol. 2014, 46, 338–347. [Google Scholar] [CrossRef] [Green Version]
- Rehman, F.; Pervez, A.; Khattak, B.N.; Ahmad, R. Plant Growth Promoting Rhizobacteria Impact onTypha latifoliaandPhragmites australisGrowth and Dissolved Oxygen. CLEAN Soil Air Water 2018, 46. [Google Scholar] [CrossRef]
- Penrose, D.M.; Glick, B.R. Levels of ACC and related compounds in exudate and extracts of canola seeds treated with ACC deaminase-containing plant growth-promoting bacteria. Can. J. Microbiol. 2001, 47, 368–372. [Google Scholar] [CrossRef]
- Takishita, Y.; Charron, J.-B.; Smith, D.L. Biocontrol Rhizobacterium Pseudomonas sp. 23S Induces Systemic Resistance in Tomato (Solanum lycopersicum L.) Against Bacterial Canker Clavibacter michiganensis subsp. michiganensis. Front. Microbiol. 2018, 9, 2119. [Google Scholar] [CrossRef]
- Cappellari, L.D.R.; Chiappero, J.; Santoro, M.V.; Giordano, W.; Banchio, E. Inducing phenolic production and volatile organic compounds emission by inoculating Mentha piperita with plant growth-promoting rhizobacteria. Sci. Hortic. 2017, 220, 193–198. [Google Scholar] [CrossRef]
- Bhattacharyya, P.N.; Jha, D.K. Plant growth-promoting rhizobacteria (PGPR): Emergence in agriculture. World J. Microbiol. Biotechnol. 2012, 28, 1327–1350. [Google Scholar] [CrossRef]
- Zhao, L.; Xu, Y.; Lai, X. Antagonistic endophytic bacteria associated with nodules of soybean (Glycine max L.) and plant growth-promoting properties. Braz. J. Microbiol. 2018, 49, 269–278. [Google Scholar] [CrossRef]
- Sapre, S.; Gontia-Mishra, I.; Tiwari, S. Klebsiella sp. confers enhanced tolerance to salinity and plant growth promotion in oat seedlings (Avena sativa). Microbiol. Res. 2018, 206, 25–32. [Google Scholar] [CrossRef]
- Shahid, M.; Akram, M.; Khan, M.A.; Zubair, M.; Shah, S.M.; Ismail, M.; Shabir, G.; Basheer, S.; Aslam, K.; Tariq, M. A phytobeneficial strainPlanomicrobiumsp. MSSA-10 triggered oxidative stress responsive mechanisms and regulated the growth of pea plants under induced saline environment. J. Appl. Microbiol. 2018, 124, 1566–1579. [Google Scholar] [CrossRef]
- Shahid, M.; Shahid, M.; Hussain, S.; Shahzad, T.; Haider, M.Z.; Noman, M.; Mushtaq, A.; Fatima, Q.; Ahmed, T.; Mustafa, G. Enzymatic detoxification of azo dyes by a multifarious Bacillus sp. strain MR-1/2-bearing plant growth-promoting characteristics. 3 Biotech 2018, 8, 425. [Google Scholar] [CrossRef]
- Chatterjee, P.; Samaddar, S.; Anandham, R.; Kang, Y.; Kim, K.; Selvakumar, G.; Sa, T. Beneficial Soil Bacterium Pseudomonas frederiksbergensis OS261 Augments Salt Tolerance and Promotes Red Pepper Plant Growth. Front. Plant Sci. 2017, 8, 705. [Google Scholar] [CrossRef]
- Khan, M.Y.; Zahir, Z.A.; Asghar, H.N.; Waraich, E.A. Preliminary investigations on selection of synergistic halotolerant plant growth promoting rhizobacteria for inducing salinity tolerance in wheat. Pak. J. Bot. 2017, 49, 1541–1551. [Google Scholar]
- Salomon, M.V.; Purpora, R.; Bottini, R.; Piccoli, P.N. Rhizosphere associated bacteria trigger accumulation of terpenes in leaves of Vitis vinifera L. cv. Malbec that protect cells against reactive oxygen species. Plant Physiol. Biochem. 2016, 106, 295–304. [Google Scholar] [CrossRef]
- Liu, D.; Yang, Q.; Ge, K.; Hu, X.; Qi, G.; Du, B.; Liu, K.; Ding, Y. Promotion of iron nutrition and growth on peanut by Paenibacillus illinoisensis and Bacillus sp. strains in calcareous soil. Braz. J. Microbiol. 2017, 48, 656–670. [Google Scholar] [CrossRef]
- Naeem, M.; Aslam, Z.; Khaliq, A.; Ahmed, J.N.; Nawaz, A.; Hussain, M. Plant growth promoting rhizobacteria reduce aphid population and enhance the productivity of bread wheat. Braz. J. Microbiol. 2018, 49, 9–14. [Google Scholar] [CrossRef]
- Hussain, M.; Asgher, Z.; Tahir, M.; Ijaz, M.; Shahid, M.; Ali, H.; Sattar, A. Bacteria in Combination with Fertilizers Improve Growth, Productivity and Net Returns of Wheat (Triticum aestivum L.). Pak. J. Agric. Sci. 2016, 53, 633–645. [Google Scholar] [CrossRef]
- Turatto, M.F.; Dourado, F.D.S.; Zilli, J.E.; Botelho, G.R. Control potential of Meloidogyne javanica and Ditylenchus spp. using fluorescent Pseudomonas and Bacillus spp. Braz. J. Microbiol. 2017, 49, 54–58. [Google Scholar] [CrossRef]
- Rojas-Rojas, F.U.; López-Sánchez, D.; Meza-Radilla, G.; Méndez-Canarios, A.; Ibarra, J.A.; Santos, P.E.-D.L. El controvertido complejo Burkholderia cepacia, un grupo de especies promotoras del crecimiento vegetal y patógenas de plantas, animales y humanos. Rev. Argent. Microbiol. 2019, 51, 84–92. [Google Scholar] [CrossRef]
- Regolamento (UE) 2019/1009 del Parlamento Europeo e del Consiglio del 5 Giugno 2019 che Stabilisce Norme Relative Alla Messa a Disposizione sul Mercato di Prodotti Fertilizzanti dell’UE, Che Modifica i Regolamenti (CE) n. 1069/2009 e (CE) n. 1107/2009 e che Abroga Il Regolamento (CE) n. 2003/2003. Gazzetta Ufficiale dell’UE del 25.6.2019. Available online: https://eur-lex.europa.eu/legal-content/IT/TXT/PDF/?uri=CELEX:32019R1009&from=HU (accessed on 2 September 2020).
- Regolamento (CE) N. 1107/2009 del Parlamento Europeo e del Consig lio del 21 Ottobre 2009 Relativo All’immissione sul Mercato dei Prodotti Fitosanitari e che Abroga le Direttive del Consiglio 79/117/CEE e 91/414/CEE. Gazzetta Ufficiale dell’U.E. del 24.11.2009. Available online: https://eur-lex.europa.eu/eli/reg/2009/1107/oj (accessed on 2 September 2020).
- Kandel, S.L.; Joubert, P.M.; Doty, S.L. Bacterial Endophyte Colonization and Distribution within Plants. Microorganisms 2017, 5, 77. [Google Scholar] [CrossRef] [Green Version]
- Bhatti, A.A.; Haq, S.; Bhat, R.A. Actinomycetes benefaction role in soil and plant health. Microb. Pathog. 2017, 111, 458–467. [Google Scholar] [CrossRef]
- Sousa, J.A.D.J.; Olivares, F.L. Plant growth promotion by streptomycetes: Ecophysiology, mechanisms and applications. Chem. Biol. Technol. Agric. 2016, 3, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Barka, E.A.; Vatsa, P.; Sanchez, L.; Gaveau-Vaillant, N.; Jacquard, C.; Klenk, H.-P.; Clément, C.; Ouhdouch, Y.; Van Wezel, G.P. Taxonomy, Physiology, and Natural Products of Actinobacteria. Microbiol. Mol. Biol. Rev. 2016, 80, 1–43. [Google Scholar] [CrossRef] [Green Version]
- Trujillo, M.E.; Bacigalupe, R.; Pujic, P.; Igarashi, Y.; Benito, P.; Riesco, R.; Médigue, C.; Normand, P. Genome Features of the Endophytic Actinobacterium Micromonospora lupini Strain Lupac 08: On the Process of Adaptation to an Endophytic Life Style? PLoS ONE 2014, 9, e108522. [Google Scholar] [CrossRef] [Green Version]
- Manivasagan, P.; Venkatesan, J.; Sivakumar, K.; Kim, S.-K. Pharmaceutically active secondary metabolites of marine actinobacteria. Microbiol. Res. 2014, 169, 262–278. [Google Scholar] [CrossRef]
- Girard, G.; Traag, B.A.; Sangal, V.; Mascini, N.; Hoskisson, P.A.; Goodfellow, M.; Van Wezel, G.P. A novel taxonomic marker that discriminates between morphologically complex actinomycetes. Open Biol. 2013, 3, 130073. [Google Scholar] [CrossRef] [Green Version]
- Verma, M.; Lal, D.; Kaur, J.; Saxena, A.; Kaur, J.; Anand, S.; Lal, R. Phylogenetic analyses of phylum Actinobacteria based on whole genome sequences. Res. Microbiol. 2013, 164, 718–728. [Google Scholar] [CrossRef]
- Salwan, R.; Sharma, V. Molecular and biotechnological aspects of secondary metabolites in actinobacteria. Microbiol. Res. 2020, 231, 126374. [Google Scholar] [CrossRef]
- Gomes, K.M.; Duarte, R.S.; Bastos, M.D.C.D.F. Lantibiotics produced by Actinobacteria and their potential applications (a review). Microbiology 2017, 163, 109–121. [Google Scholar] [CrossRef]
- Bouizgarne, B.; Aouamar, A.A.B. Diversity of Plant Associated Actinobacteria. In Bacterial Diversity in Sustainable Agriculture, 1st ed.; Maheswari, D.K., Ed.; Springer: Cham, Switzerland, 2014; pp. 41–99. [Google Scholar]
- Lewin, G.R.; Carlos, C.; Chevrette, M.G.; Horn, H.A.; McDonald, B.R.; Stankey, R.J.; Fox, B.G.; Currie, C.R. Evolution and Ecology of Actinobacteria and Their Bioenergy Applications. Annu. Rev. Microbiol. 2016, 70, 235–254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, Y.; Wu, D.; Wei, D.; Zhao, Y.; Wu, J.; Xie, X.; Zhang, R.; Wei, Z. Improved lignocellulose-degrading performance during straw composting from diverse sources with actinomycetes inoculation by regulating the key enzyme activities. Bioresour. Technol. 2019, 271, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, S.; Zaheer, A.; Aiysha, D.; Malik, K.A.; Mehnaz, S. Actinomycetes: A Source of Industrially Important Enzymes. J. Proteom. Bioinform. 2017, 10, 316–319. [Google Scholar] [CrossRef]
- Martínez-Hidalgo, P.; Galindo-Villardón, P.; Trujillo, M.E.; Igual, J.M.; Martínez-Molina, E. Micromonospora from nitrogen fixing nodules of alfalfa (Medicago sativa L.). A new promising Plant Probiotic Bacteria. Sci. Rep. 2014, 4, 6389. [Google Scholar] [CrossRef] [Green Version]
- Vurukonda, S.S.K.P.; Giovanardi, D.; Stefani, E. Plant Growth Promoting and Biocontrol Activity of Streptomyces spp. as Endophytes. Int. J. Mol. Sci. 2018, 19, 952. [Google Scholar] [CrossRef] [Green Version]
- Fierer, N. Embracing the unknown: Disentangling the complexities of the soil microbiome. Nat. Rev. Genet. 2017, 15, 579–590. [Google Scholar] [CrossRef]
- Wagg, C.; Schlaeppi, K.; Banerjee, S.; Kuramae, E.E.; Van Der Heijden, M.G.A. Fungal-bacterial diversity and microbiome complexity predict ecosystem functioning. Nat. Commun. 2019, 10, 1–10. [Google Scholar] [CrossRef]
- Richardson, A.E.; Barea, J.-M.; McNeill, A.M.; Prigent-Combaret, C. Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil 2009, 321, 305–339. [Google Scholar] [CrossRef]
- Barea, J.M.; Pozo, M.J.; Azcón, R.; Azcón-Aguilar, C. Microbial co-operation in the rhizosphere. J. Exp. Bot. 2005, 56, 1761–1778. [Google Scholar] [CrossRef] [Green Version]
- Avio, L.; Turrini, A.; Giovannetti, M.; Sbrana, C. Designing the Ideotype Mycorrhizal Symbionts for the Production of Healthy Food. Front. Plant Sci. 2018, 9, 1–19. [Google Scholar] [CrossRef]
- Bonfante, P.; Anca, I.-A. Plants, Mycorrhizal Fungi, and Bacteria: A Network of Interactions. Annu. Rev. Microbiol. 2009, 63, 363–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miransari, M. Contribution of arbuscular mycorrhizal symbiosis to plant growth under different types of soil stress. Plant Biol. 2010, 12, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Johansson, J.F.; Paul, L.R.; Finlay, R.D. Microbial interactions in the mycorrhizosphere and their significance for sustainable agriculture. FEMS Microbiol. Ecol. 2004, 48, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Rillig, M.C.; Mummey, D.L. Mycorrhizas and soil structure. New Phytol. 2006, 171, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Piazza, G.; Pellegrino, E.; Moscatelli, M.C.; Ercoli, L. Long-term conservation tillage and nitrogen fertilization effects on soil aggregate distribution, nutrient stocks and enzymatic activities in bulk soil and occluded microaggregates. Soil Tillage Res. 2020, 196, 104482. [Google Scholar] [CrossRef]
- Lynch, J.M.; Benedetti, A.; Insam, H.; Nuti, M.P.; Smalla, K.; Torsvik, V.; Nannipieri, P. Microbial diversity in soil: Ecological theories, the contribution of molecular techniques and the impact of transgenic plants and transgenic microorganisms. Biol. Fertil. Soils 2004, 40, 363–385. [Google Scholar] [CrossRef]
- Sosa-Hernández, M.A.; Leifheit, E.F.; Ingraffia, R.; Rillig, M.C. Subsoil Arbuscular Mycorrhizal Fungi for Sustainability and Climate-Smart Agriculture: A Solution Right Under Our Feet? Front. Microbiol. 2019, 10, 744. [Google Scholar] [CrossRef] [Green Version]
- Giovannini, L.; Palla, M.; Agnolucci, M.; Avio, L.; Sbrana, C.; Turrini, A.; Giovannetti, M. Arbuscular Mycorrhizal Fungi and Associated Microbiota as Plant Biostimulants: Research Strategies for the Selection of the Best Performing Inocula. Agronomy 2020, 10, 106. [Google Scholar] [CrossRef] [Green Version]
- Rouphael, Y.; Colla, G. Editorial: Biostimulants in Agriculture. Front. Plant Sci. 2020, 11, 40. [Google Scholar] [CrossRef] [Green Version]
- Migliorini, P.; Torri, L.; Whittaker, A.; Moschini, V.; Benedettelli, S.; Masoero, G. Old and new common wheat (Triticum aestivum L.) varieties in organic: Connecting agronomic, microorganism, phytochemical and bread sensory characteristics. J. Food Agric. Environ. 2018, 16, 22–27. [Google Scholar] [CrossRef]
- Cortivo, C.D.; Ferrari, M.; Visioli, G.; Lauro, M.; Fornasier, F.; Barion, G.; Panozzo, A.; Vamerali, T. Effects of Seed-Applied Biofertilizers on Rhizosphere Biodiversity and Growth of Common Wheat (Triticum aestivum L.) in the Field. Front. Plant Sci. 2020, 11, 72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raiola, A.; Tenore, G.C.; Petito, R.; Ciampaglia, R.; Ritieni, A. Improving of nutraceutical features of many important mediterranean vegetables by inoculation with a new commercial product. Curr. Pharm. Biotechnol. 2015, 16, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Rouphael, Y.; Cardarelli, M.; Bonini, P.; Colla, G. Synergistic Action of a Microbial-based Biostimulant and a Plant Derived-Protein Hydrolysate Enhances Lettuce Tolerance to Alkalinity and Salinity. Front. Plant Sci. 2017, 8, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colla, G.; Rouphael, Y.; Di Mattia, E.; El-Nakhel, C.; Cardarelli, M. Co-inoculation of Glomus intraradicesand Trichoderma atrovirideacts as a biostimulant to promote growth, yield and nutrient uptake of vegetable crops. J. Sci. Food Agric. 2015, 95, 1706–1715. [Google Scholar] [CrossRef] [PubMed]
- Battini, F.; Turrini, A.; Sgherri, C.; Malorgio, F.; Quartacci, M.F. Dual inoculation with AMF and associated bacteria improves nutraceutical value of sweet basil grown under commercial conditions. Agrochimica 2016, 60, 81–99. [Google Scholar] [CrossRef]
- Baldi, E.; Toselli, M.; Masoero, G.; Nuti, M. Organic and Symbiotic Fertilization of Tomato Plants Monitored by Litterbag-NIRS and Foliar-NIRS Rapid Spectroscopic Methods. J. Agron. Res. 2020, 3, 9–26. [Google Scholar] [CrossRef]
- Akhtar, N.; Naveed, M.; Khalid, M.; Ahmad, N.; Rizwan, M.; Siddique, S. Effect of bacterial consortia on growth and yield of maize grown in Fusarium infested soil. Soil Environ. 2018, 37, 35–44. [Google Scholar] [CrossRef]
- Cameron, E.K.; Martins, I.S.; Lavelle, P.; Mathieu, J.; Tedersoo, L.; Gottschall, F.; Guerra, C.A.; Hines, J.; Patoine, G.; Siebert, J.; et al. Global gaps in soil biodiversity data. Nat. Ecol. Evol. 2018, 2, 1042–1043. [Google Scholar] [CrossRef]
- Marín, C.; Bueno, C.G. A Systematic Review of South American and European Mycorrhizal Research: Is there a Need for Scientific Symbiosis? In Mycorrhizal Fungi in South America, 1st ed.; Pagano, M., Lugo, M., Eds.; Springer: Cham, Switzerland, 2019; pp. 97–110. [Google Scholar]
- Wall, L.G.; Gabbarini, L.A.; Ferrari, A.E.; Frene, J.P.; Covelli, J.; Reyna, D.; Robledo, N.B. Changes of paradigms in agriculture soil microbiology and new challenges in microbial ecology. Acta Oecol. 2019, 95, 68–73. [Google Scholar] [CrossRef]
- Piazza, G.; Ercoli, L.; Nuti, M.; Pellegrino, E. Interaction Between Conservation Tillage and Nitrogen Fertilization Shapes Prokaryotic and Fungal Diversity at Different Soil Depths: Evidence From a 23-Year Field Experiment in the Mediterranean Area. Front. Microbiol. 2019, 10, 2047. [Google Scholar] [CrossRef] [Green Version]
- Volpato, S.; Masoero, G.; Giovannetti, G.; Nuti, M. Arbuscular Mycorrhizal Biofertilizers Sources in the Potato (Solanum Tuberosum) Plant show Interactions with Cultivars on Yield and Litter-bags Spectral Features. J. Agron. Res. 2020, 2, 9–17. [Google Scholar] [CrossRef]
- Liu, H.; Brettell, L.E.; Qiu, Z.; Singh, B.K. Microbiome-Mediated Stress Resistance in Plants. Trends Plant Sci. 2020, 25, 733–743. [Google Scholar] [CrossRef]
- Ramirez, K.S.; Snoek, L.B.; Koorem, K.; Geisen, S.; Bloem, L.J.; Hooven, F.T.; Kostenko, O.; Krigas, N.; Manrubia, M.; Caković, D.; et al. Range-expansion effects on the belowground plant microbiome. Nat. Ecol. Evol. 2019, 3, 604–611. [Google Scholar] [CrossRef]
- Machado, A.A.D.S.; Valyi, K.; Rillig, M.C. Potential Environmental Impacts of an “Underground Revolution”: A Response to Bender et al. Trends Ecol. Evol. 2017, 32, 8–10. [Google Scholar] [CrossRef]
- Sarabia, M.; Cazares, S.; González-Rodríguez, A.; Mora, F.; Carreón-Abud, Y.; Larsen, J. Plant growth promotion traits of rhizosphere yeasts and their response to soil characteristics and crop cycle in maize agroecosystems. Rhizosphere 2018, 6, 67–73. [Google Scholar] [CrossRef]
- Nafady, N.A.; Hashem, M.; Hassan, E.A.; Ahmed, H.A.; Alamri, S.A. The combined effect of arbuscular mycorrhizae and plant-growth-promoting yeast improves sunflower defense against Macrophomina phaseolina diseases. Biol. Control 2019, 138, 104049. [Google Scholar] [CrossRef]
- Singh, S.; Tripathi, A.; Maji, D.; Awasthi, A.; Vajpayee, P.; Kalra, A. Evaluating the potential of combined inoculation of Trichoderma harzianum and Brevibacterium halotolerans for increased growth and oil yield in Mentha arvensis under greenhouse and field conditions. Ind. Crop. Prod. 2019, 131, 173–181. [Google Scholar] [CrossRef]
| AMF Species | Crops | Beneficial Effects | Condition | Reference |
|---|---|---|---|---|
| Funneliformis mosseae Rhizophagus irregularis | Chickpea (Cicer arietinum L.) | Increases plant biomass, production and grain quality | Field | [55] |
| Glomus intraradices | Tomato (Solanum Lycopersicum L.) | Improve the yield | Field | [60] |
| Glomus intraradices | Tomato (Solanum Lycopersicum L.) | Root knot nematode suppression | Greenhouse | [61] |
| Rhizophagusintraradices, Glomus aggregatum, Glomus viscosum, Glomus etunicatum, and Glomus claroideum | Corn (Zea mays L.) | Improves crop growth, yield and grain quality | Field | [62] |
| Glomus mosseae, Glomus intraradices | Alfalfa (Medicago sativa L.) | Increase in glomalin-related soil protein (GRSP) and stability of soil aggregate | Greenhouse | [63] |
| AMF natives | Corn (Zea mays L.) | Increased absorption of K, Ca and Mg | Field | [64] |
| AMF native consortia: Glomus mosseae, Glomus fasciculatum, Glomus etunicatum, Glomus intraradices, Scutellospora sp. | Green pepper (Capsicum annuum L.), parsley (Petroselinum crispum (Mill.) Fuss), carrot (Daucus carota L.), and tomato (Solanum lycopersicum L.) | Increased plant and root biomass and yield quality | Greenhouse and field | [65] |
| Glomus fasciculatum, Glomus clarum, Glomusetunicatum, Glomus versiforme | Long pepper (Piper longum L.) | Improve growth | Greenhouse and field | [66] |
| Glomus intraradices | Potato (Solanum tuberosum L.) | Improve biomass yield | Field | [67] |
| Glomus intraradices, Glomus mosseae, Glomusetunicatum | Strawberry (Fragaria ananassa Duch.) | Improve productivity | Field | [68] |
| Glomus mosseae, Glomus hoi | Sunflower (Helianthus annuus L.) | Improve biomass yield | Greenhouse | [69] |
| PGPR Species | Crops | Beneficial Effects | Reference |
|---|---|---|---|
| Azospirillum spp., Pseudomonas spp. | Rice (Oryza sativa L.) | Increase growth and yield | [72] |
| Bacillus spp., Pseudomonas spp. and Azospirillum spp. | Seedlings and cuttings | Increase the germination and the rooting of cuttings, biocontrol of bacterial wilt and the survival of plants after transplanting | [76] |
| Pantoea spp., Serratia spp., Acinetobacter spp., Bacillus spp., Agrobacterium spp., Burkholderia spp., Pseudomonas spp., Ochrobactrum spp. | Soybean (Glycine max L.) | Production of nodules with high capacity to fix nitrogen. Important inhibitory activity against pathogens | [82] |
| Pseudomonas frederiksbergensis | Red pepper (Capsicum annuum L.) | Biostimulant under water and salt stress conditions | [86] |
| Paenibacillus illinoisensis, Bacillus spp. | Peanut (Arachis hypogaea L.) | Increase growth and yield | [89] |
| Pseudomonas spp., Paenibacillus spp. | Calcareous soil | High capacity to make Fe3+ available to plants | [89] |
| Bacillus spp., Pseudomonas spp. | Wheat (Triticum aestivum L.) | Excellent biofertilizer inoculants, have direct and indirect effects on insect pests | [90,91] |
| Bacillus spp., Pseudomonas spp. | Soil | Control potential of Meloidogyne javanica and Ditylenchus spp. nematodes | [92] |
| Actinobacteria Species | Crops | Beneficial Effects | Reference |
|---|---|---|---|
| Micromonospora spp. | Alfalfa (Medicago sativa L.) | Plant probiotic bacteria | [110] |
| Streptomyces spp. | Grow on cellulose, hemicellulose and potentially lignin | Biomass degradation | [107] |
| Streptomyces davawensis | Soil | Antibacterial antibiotic roseoflavin | [97] |
| Micromonospora rifamycinica | Mangrove sediment | Antibacterial antibiotic rifamycin | [97] |
| Streptomyces lydicus WYEC 108 | Grass, ornamentals, vegetables, and forest species | Biocontrol by soil-borne plant pathogens and foliar diseases | [98] |
| Streptomyces avermitilis | Ornamentals, vegetables, and forest species | Biocontrol of nematodes and insects | [98] |
| Actinomadura spp. | Vegetables grain | Bioherbicide/biopesticide producers 2,4-Dihydro-4-(β-d-ribofuranosyl)-1,2,4 (3H)-triazol-3-one (herbicide) | [99] |
| Streptomyces violaceusniger YCED-9 | Soil | Antifungal agent producing guanidylfungin | [99] |
| Micromonospora lupini Lupac 08 | Nitrogen-fixing nodule of the legume Lupinus angustifolius L. | Plant growth-promoting bacterium | [100] |
| Corynebacterium spp., Pseudonocardia dioxanivorans, Streptomyces spp., Micromonospora spp., Streptomyces sp. Strain MBCN152-1, S. lydicus WYEC 108 | Vegetables, fruits, and grains | Plant growth-promoting bacterium and nitrogen fixation. Biocontrol agent and biofungicide | [104] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguilar-Paredes, A.; Valdés, G.; Nuti, M. Ecosystem Functions of Microbial Consortia in Sustainable Agriculture. Agronomy 2020, 10, 1902. https://doi.org/10.3390/agronomy10121902
Aguilar-Paredes A, Valdés G, Nuti M. Ecosystem Functions of Microbial Consortia in Sustainable Agriculture. Agronomy. 2020; 10(12):1902. https://doi.org/10.3390/agronomy10121902
Chicago/Turabian StyleAguilar-Paredes, Ana, Gabriela Valdés, and Marco Nuti. 2020. "Ecosystem Functions of Microbial Consortia in Sustainable Agriculture" Agronomy 10, no. 12: 1902. https://doi.org/10.3390/agronomy10121902
APA StyleAguilar-Paredes, A., Valdés, G., & Nuti, M. (2020). Ecosystem Functions of Microbial Consortia in Sustainable Agriculture. Agronomy, 10(12), 1902. https://doi.org/10.3390/agronomy10121902







