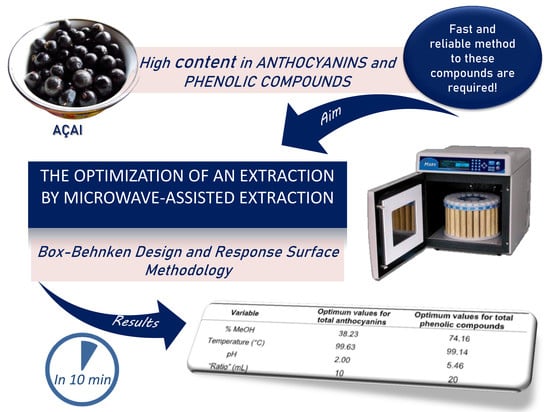
Extraction of Anthocyanins and Total Phenolic Compounds from Açai (Euterpe oleracea Mart.) Using an Experimental Design Methodology. Part 3: Microwave-Assisted Extraction
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Açai Samples
2.3. Microwave-Assisted Extraction
2.4. Experimental Design Methodology
2.5. Identification of Anthocyanins by UHPLC-QToF-MS
2.6. Separation and Quantification of Anthocyanins by UHPLC-UV-vis
2.7. Total Phenolic Content
3. Results
3.1. Box-Behnken Design
3.2. Anthocyanins Optimization
3.2.1. Optimization of the Extraction Method
3.2.2. Kinetics of the Extraction Process
3.2.3. Repeatability and Intermediate Precision of the Method
3.3. Phenolic Compounds Optimization
3.3.1. Optimization of the Extraction Method
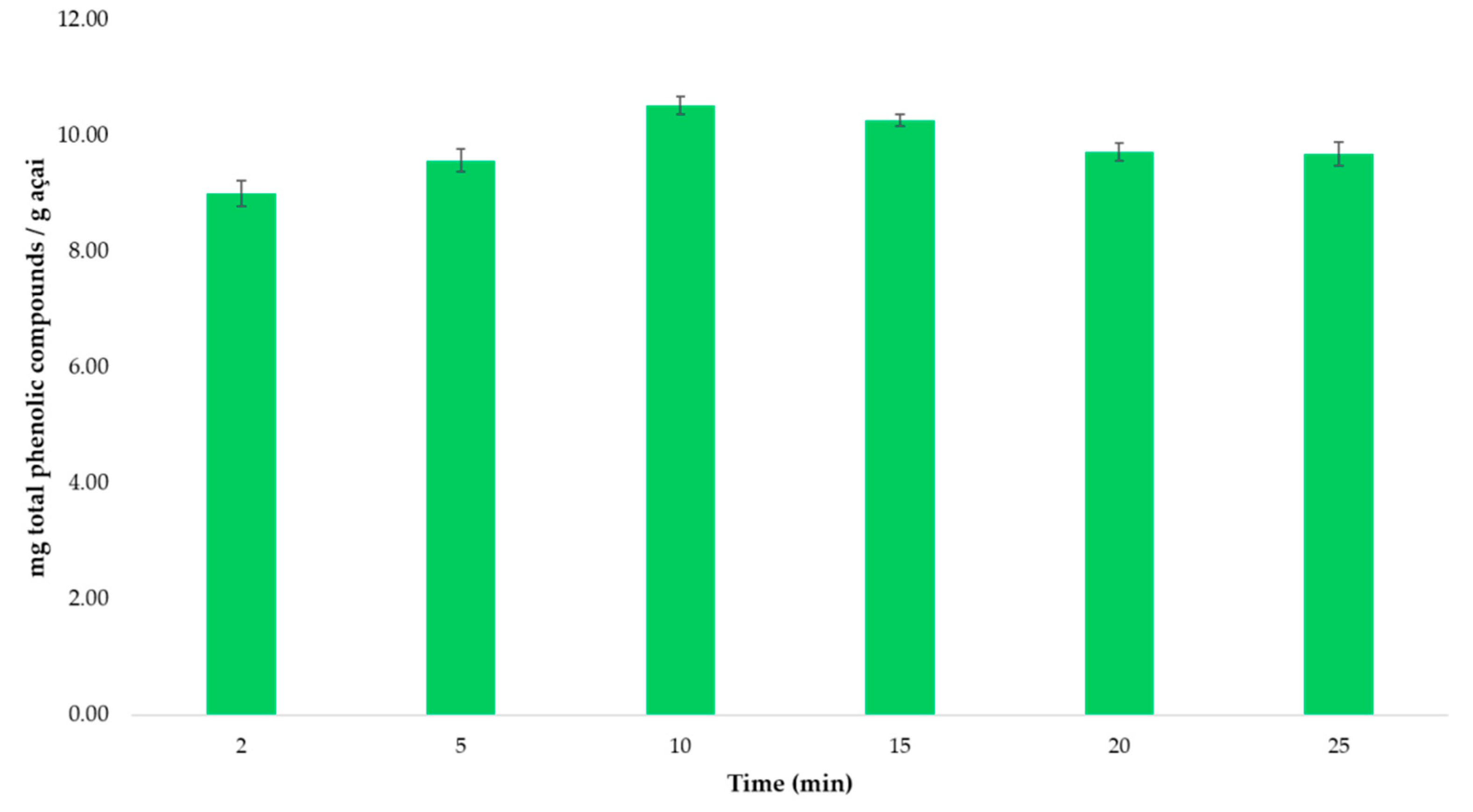
3.3.2. Kinetics of the Extraction Process
3.3.3. Repeatability and Intermediate Precision of the Method
3.4. Re-Extraction Experiments
3.5. Application of the Methods to Real Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ye, D.; Jiang, Z.; Zheng, F.; Wang, H.; Zhang, Y.; Gao, F.; Chen, P.; Chen, Y.; Shi, G. Optimized Extraction of Polysaccharides from Grateloupia livida (Harv.) Yamada and Biological Activities. Molecules 2015, 20, 16817–16832. [Google Scholar] [CrossRef] [PubMed]
- Munro, B.; Vuong, Q.; Chalmers, A.; Goldsmith, C.; Bowyer, M.; Scarlett, C. Phytochemical, Antioxidant and Anti-Cancer Properties of Euphorbia tirucalli Methanolic and Aqueous Extracts. Antioxidants 2015, 4, 647–661. [Google Scholar] [CrossRef] [PubMed]
- Velderrain-Rodríguez, G.; Torres-Moreno, H.; Villegas-Ochoa, M.; Ayala-Zavala, J.; Robles-Zepeda, R.; Wall-Medrano, A.; González-Aguilar, G. Gallic Acid Content and an Antioxidant Mechanism Are Responsible for the Antiproliferative Activity of ‘Ataulfo’ Mango Peel on LS180 Cells. Molecules 2018, 23, 695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bogusz, S.; Libardi, S.H.; Dias, F.F.; Coutinho, J.P.; Bochi, V.C.; Rodrigues, D.; Melo, A.M.; Godoy, H.T. Brazilian Capsicum peppers: Capsaicinoid content and antioxidant activity. J. Sci. Food Agric. 2018, 98, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Gironés-Vilaplana, A.; Valentão, P.; Moreno, D.A.; Ferreres, F.; Garcı́a-Viguera, C.; Andrade, P.B. New Beverages of Lemon Juice Enriched with the Exotic Berries Maqui, Açaı́, and Blackthorn: Bioactive Components and in Vitro Biological Properties. J. Agric. Food Chem. 2012, 60, 6571–6580. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Thakali, K.M.; Xie, C.; Kondo, M.; Tong, Y.; Ou, B.; Jensen, G.; Medina, M.B.; Schauss, A.G.; Wu, X. Bioactivities of açaí (Euterpe precatoria Mart.) fruit pulp, superior antioxidant and anti-inflammatory properties to Euterpe oleracea Mart. Food Chem. 2012, 133, 671–677. [Google Scholar] [CrossRef]
- Gordon, A.; Cruz, A.P.G.; Cabral, L.M.C.; de Freitas, S.C.; Taxi, C.M.A.D.; Donangelo, C.M.; de Andrade Mattietto, R.; Friedrich, M.; da Matta, V.M.; Marx, F. Chemical characterization and evaluation of antioxidant properties of Açaí fruits (Euterpe oleraceae Mart.) during ripening. Food Chem. 2012, 133, 256–263. [Google Scholar] [CrossRef]
- Kang, J.; Xie, C.; Li, Z.; Nagarajan, S.; Schauss, A.G.; Wu, T.; Wu, X. Flavonoids from acai (Euterpe oleracea Mart.) pulp and their antioxidant and anti-inflammatory activities. Food Chem. 2011, 128, 152–157. [Google Scholar] [CrossRef]
- Lucas, B.F.; Zambiazi, R.C.; Costa, J.A.V. Biocompounds and physical properties of açaí pulp dried by different methods. LWT-Food Sci. Technol. 2018, 98, 335–340. [Google Scholar] [CrossRef]
- Hogan, S.; Chung, H.; Zhang, L.; Li, J.; Lee, Y.; Dai, Y.; Zhou, K. Antiproliferative and antioxidant properties of Anthocyanin-rich extract from açai. Food Chem. 2010, 118, 208–214. [Google Scholar] [CrossRef]
- López de Dicastillo, C.; Piña, C.; Garrido, L.; Arancibia, C.; Galotto, M.J. Enhancing Thermal Stability and Bioaccesibility of Açaí Fruit Polyphenols through Electrohydrodynamic Encapsulation into Zein Electrosprayed Particles. Antioxidants 2019, 8, 464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Censi, R.; Vargas Peregrina, D.; Lacava, G.; Agas, D.; Lupidi, G.; Sabbieti, M.; Di Martino, P. Cosmetic Formulation Based on an Açai Extract. Cosmetics 2018, 5, 48. [Google Scholar] [CrossRef] [Green Version]
- Poulose, S.M.; Fisher, D.R.; Larson, J.; Bielinski, D.F.; Rimando, A.M.; Carey, A.N.; Schauss, A.G.; Shukitt-Hale, B. Anthocyanin-rich Açai (Euterpe oleracea Mart.) Fruit Pulp Fractions Attenuate Inflammatory Stress Signaling in Mouse Brain BV-2 Microglial Cells. J. Agric. Food Chem. 2012, 60, 1084–1093. [Google Scholar] [CrossRef] [PubMed]
- Martinez, R.; Guimarães, D.; Berniz, C.; Abreu, J.; Rocha, A.; Moura, R.; Resende, A.; Teodoro, A. Açai (Euterpe oleracea Mart.) Seed Extract Induces Cell Cycle Arrest and Apoptosis in Human Lung Carcinoma Cells. Foods 2018, 7, 178. [Google Scholar] [CrossRef] [Green Version]
- Pacheco-Palencia, L.A.; Mertens-Talcott, S.U.; Talcott, S.T. In vitro absorption and antiproliferative activities of monomeric and polymeric Anthocyanin fractions from açai fruit (Euterpe oleracea Mart.). Food Chem. 2010, 119, 1071–1078. [Google Scholar] [CrossRef]
- Del Pozo-Insfran, D.; Percival, S.S.; Talcott, S.T. Açai ( Euterpe oleracea Mart.) Polyphenolics in Their Glycoside and Aglycone Forms Induce Apoptosis of HL-60 Leukemia Cells. J. Agric. Food Chem. 2006, 54, 1222–1229. [Google Scholar] [CrossRef]
- Brunschwig, C.; Leba, L.-J.; Saout, M.; Martial, K.; Bereau, D.; Robinson, J.-C. Chemical Composition and Antioxidant Activity of Euterpe oleracea Roots and Leaflets. Int. J. Mol. Sci. 2016, 18, 61. [Google Scholar] [CrossRef] [Green Version]
- de Moura, R.S.; Ferreira, T.S.; Lopes, A.A.; Pires, K.M.P.; Nesi, R.T.; Resende, A.C.; Souza, P.J.C.; da Silva, A.J.R.; Borges, R.M.; Porto, L.C.; et al. Effects of Euterpe oleracea Mart. (AÇAÍ) extract in acute lung inflammation induced by cigarette smoke in the mouse. Phytomedicine 2012, 19, 262–269. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.-S.; Stoner, G.D. Anthocyanins and their role in cancer prevention. Cancer Lett. 2008, 269, 281–290. [Google Scholar] [CrossRef] [Green Version]
- V. González de Peredo, A.; Vázquez-Espinosa, M.; Espada-Bellido, E.; Ferreiro-González, M.; Amores-Arrocha, A.; Palma, M.; Barbero, G.F.; Jiménez-Cantizano, A. Alternative Ultrasound-Assisted Method for the Extraction of the Bioactive Compounds Present in Myrtle (Myrtus communis L.). Molecules 2019, 24, 882. [Google Scholar] [CrossRef] [Green Version]
- Vázquez-Espinosa, M.; V. González-de-Peredo, A.; Espada-Bellido, E.; Ferreiro-González, M.; Toledo-Domínguez, J.J.; Carrera, C.; Palma, M.; Barbero, G.F. Ultrasound-Assisted Extraction of Two Types of Antioxidant Compounds (TPC and TA) from Black Chokeberry (Aronia melanocarpa L.): Optimization of the Individual and Simultaneous Extraction Methods. Agronomy 2019, 9, 456. [Google Scholar] [CrossRef] [Green Version]
- Gallori, S.; Bilia, A.R.; Bergonzi, M.C.; Barbosa, W.L.R.; Vincieri, F.F. Polyphenolic Constituents of Fruit Pulp of Euterpe oleracea Mart. (Açai palm). Chromatographia 2004, 59. [Google Scholar] [CrossRef]
- Pacheco-Palencia, L.A.; Talcott, S.T. Chemical stability of açai fruit (Euterpe oleracea Mart.) Anthocyanins as influenced by naturally occurring and externally added polyphenolic cofactors in model systems. Food Chem. 2010, 118, 17–25. [Google Scholar] [CrossRef]
- Mazewski, C.; Liang, K.; Gonzalez de Mejia, E. Inhibitory potential of Anthocyanin-rich purple and red corn extracts on human colorectal cancer cell proliferation in vitro. J. Funct. Foods 2017, 34, 254–265. [Google Scholar] [CrossRef]
- Long, H.-L.; Zhang, F.-F.; Wang, H.-L.; Yang, W.-S.; Hou, H.-T.; Yu, J.-K.; Liu, B. Mulberry Anthocyanins improves thyroid cancer progression mainly by inducing apoptosis and autophagy cell death. Kaohsiung J. Med. Sci. 2018, 34, 255–262. [Google Scholar] [CrossRef] [Green Version]
- Zhou, L.; Wang, H.; Yi, J.; Yang, B.; Li, M.; He, D.; Yang, W.; Zhang, Y.; Ni, H. Anti-tumor properties of Anthocyanins from Lonicera caerulea ‘Beilei’ fruit on human hepatocellular carcinoma: In vitro and in vivo study. Biomed. Pharmacother. 2018, 104, 520–529. [Google Scholar] [CrossRef]
- Phan, M.A.T.; Bucknall, M.; Arcot, J. Interactive effects of β-carotene and Anthocyanins on cellular uptake, antioxidant activity and anti-inflammatory activity in vitro and ex vivo. J. Funct. Foods 2018, 45, 129–137. [Google Scholar] [CrossRef]
- Rogez, H.; Pompeu, D.R.; Akwie, S.N.T.; Larondelle, Y. Sigmoidal kinetics of Anthocyanin accumulation during fruit ripening: A comparison between açai fruits (Euterpe oleracea) and other Anthocyanin-rich fruits. J. Food Compos. Anal. 2011, 24, 796–800. [Google Scholar] [CrossRef] [Green Version]
- Seeram, N.P.; Nair, M.G. Inhibition of Lipid Peroxidation and Structure−Activity-Related Studies of the Dietary Constituents Anthocyanins, Anthocyanidins, and Catechins. J. Agric. Food Chem. 2002, 50, 5308–5312. [Google Scholar] [CrossRef]
- Sandoval, V.; Femenias, A.; Martínez-Garza, Ú.; Sanz-Lamora, H.; Castagnini, J.; Quifer-Rada, P.; Lamuela-Raventós, R.; Marrero, P.; Haro, D.; Relat, J. Lyophilized Maqui (Aristotelia chilensis) Berry Induces Browning in the Subcutaneous White Adipose Tissue and Ameliorates the Insulin Resistance in High Fat Diet-Induced Obese Mice. Antioxidants 2019, 8, 360. [Google Scholar] [CrossRef] [Green Version]
- Parra-Galindo, M.-A.; Piñeros-Niño, C.; Soto-Sedano, J.C.; Mosquera-Vasquez, T. Chromosomes I and X Harbor Consistent Genetic Factors Associated with the Anthocyanin Variation in Potato. Agronomy 2019, 9, 366. [Google Scholar] [CrossRef] [Green Version]
- Kang, J.; Li, Z.; Wu, T.; Jensen, G.S.; Schauss, A.G.; Wu, X. Anti-oxidant capacities of flavonoid compounds isolated from acai pulp (Euterpe oleracea Mart.). Food Chem. 2010, 122, 610–617. [Google Scholar] [CrossRef]
- de Cássia Rodrigues Batista, C.; de Oliveira, M.S.; Araújo, M.E.; Rodrigues, A.M.C.; Botelho, J.R.S.; da Silva Souza Filho, A.P.; Machado, N.T.; Carvalho, R.N. Supercritical CO2 extraction of açaí (Euterpe oleracea) berry oil: Global yield, fatty acids, allelopathic activities, and determination of phenolic and Anthocyanins total compounds in the residual pulp. J. Supercrit. Fluids 2016, 107, 364–369. [Google Scholar] [CrossRef]
- Oliveira, A.F.A.; Mar, J.M.; Santos, S.F.; da Silva Júnior, J.L.; Kluczkovski, A.M.; Bakry, A.M.; de Bezerra, J.A.; de Nunomura, R.C.S.; Sanches, E.A.; Campelo, P.H. Non-thermal combined treatments in the processing of açai (Euterpe oleracea) juice. Food Chem. 2018, 265, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Vieira, G.S.; Cavalcanti, R.N.; Meireles, M.A.A.; Hubinger, M.D. Chemical and economic evaluation of natural antioxidant extracts obtained by ultrasound-assisted and agitated bed extraction from jussara pulp (Euterpe edulis). J. Food Eng. 2013, 119, 196–204. [Google Scholar] [CrossRef] [Green Version]
- Einaga, H.; Nasu, Y.; Oda, M.; Saito, H. Catalytic performances of perovskite oxides for CO oxidation under microwave irradiation. Chem. Eng. J. 2016, 283, 97–104. [Google Scholar] [CrossRef]
- Li, Y.; Han, L.; Ma, R.; Xu, X.; Zhao, C.; Wang, Z.; Chen, F.; Hu, X. Effect of energy density and citric acid concentration on Anthocyanins yield and solution temperature of grape peel in microwave-assisted extraction process. J. Food Eng. 2012, 109, 274–280. [Google Scholar] [CrossRef]
- Kapoore, R.; Butler, T.; Pandhal, J.; Vaidyanathan, S. Microwave-Assisted Extraction for Microalgae: From Biofuels to Biorefinery. Biology 2018, 7, 18. [Google Scholar] [CrossRef] [Green Version]
- Moret, S.; Conchione, C.; Srbinovska, A.; Lucci, P. Microwave-Based Technique for Fast and Reliable Extraction of Organic Contaminants from Food, with a Special Focus on Hydrocarbon Contaminants. Foods 2019, 8, 503. [Google Scholar] [CrossRef] [Green Version]
- Garcia-Salas, P.; Morales-Soto, A.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Phenolic-Compound-Extraction Systems for Fruit and Vegetable Samples. Molecules 2010, 15, 8813–8826. [Google Scholar] [CrossRef]
- Vázquez-Espinosa, M.; V. González de Peredo, A.; Ferreiro-González, M.; Carrera, C.; Palma, M.; Barbero, G.F.; Espada-Bellido, E. Assessment of Ultrasound Assisted Extraction as an Alternative Method for the Extraction of Anthocyanins and Total Phenolic Compounds from Maqui Berries (Aristotelia chilensis (Mol.) Stuntz). Agronomy 2019, 9, 148. [Google Scholar] [CrossRef] [Green Version]
- V. González de Peredo, A.; Vázquez-Espinosa, M.; Espada-Bellido, E.; Jiménez-Cantizano, A.; Ferreiro-González, M.; Amores-Arrocha, A.; Palma, M.; Barroso, C.G.; Barbero, G.F. Development of New Analytical Microwave-Assisted Extraction Methods for Bioactive Compounds from Myrtle (Myrtus communis L.). Molecules 2018, 23, 2992. [Google Scholar] [CrossRef] [Green Version]
- Espada-Bellido, E.; Ferreiro-González, M.; Carrera, C.; Palma, M.; Álvarez, J.A.; Barbero, G.F.; Ayuso, J. Extraction of Antioxidants from Blackberry (Rubus ulmifolius L.): Comparison between Ultrasound- and Microwave-Assisted Extraction Techniques. Agronomy 2019, 9, 745. [Google Scholar] [CrossRef] [Green Version]
- Setyaningsih, W.; Saputro, I.E.; Palma, M.; Barroso, C.G. Optimisation and validation of the microwave-assisted extraction of phenolic compounds from rice grains. Food Chem. 2015, 169, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Krongyut, O.; Sutthanut, K. Phenolic Profile, Antioxidant Activity, and Anti-obesogenic Bioactivity of Mao Luang Fruits (Antidesma bunius L.). Molecules 2019, 24, 4109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tarapatskyy, M.; Kapusta, I.; Gumienna, A.; Puchalski, C. Assessment of the Bioactive Compounds in White and Red Wines Enriched with a Primula veris L. Molecules 2019, 24, 4074. [Google Scholar] [CrossRef] [Green Version]
- Jiménez-Aspee, F.; Theoduloz, C.; Pormetter, L.; Mettke, J.; Ávila, F.; Schmeda-Hirschmann, G. Andean Prumnopitys Andina (Podocarpacae) Fruit Extracts: Characterization of Secondary Metabolites and Potential Cytoprotective Effect. Molecules 2019, 24, 4028. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.; Huang, Y.; Huang, W.; Feng, X.; Yang, F.; Li, D. Separation, Identification, and Antioxidant Activity of Polyphenols from Lotus Seed Epicarp. Molecules 2019, 24, 4007. [Google Scholar] [CrossRef] [Green Version]
- Bataglion, G.A.; da Silva, F.M.A.; Eberlin, M.N.; Koolen, H.H.F. Determination of the phenolic composition from Brazilian tropical fruits by UHPLC–MS/MS. Food Chem. 2015, 180, 280–287. [Google Scholar] [CrossRef]
- Larit, F.; León, F.; Benyahia, S.; Cutler, S.J. Total Phenolic and Flavonoid Content and Biological Activities of Extracts and Isolated Compounds of Cytisus villosus Pourr. Biomolecules 2019, 9, 732. [Google Scholar] [CrossRef] [Green Version]
- Provenzano, F.; Sánchez, J.L.; Rao, E.; Santonocito, R.; Ditta, L.A.; Borrás Linares, I.; Passantino, R.; Campisi, P.; Dia, M.G.; Costa, M.A.; et al. Water Extract of Cryphaea heteromalla (Hedw.) D. Mohr Bryophyte as a Natural Powerful Source of Biologically Active Compounds. Int. J. Mol. Sci. 2019, 20, 5560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duymuş, H.G.; Göger, F.; Başer, K.H.C. In vitro antioxidant properties and Anthocyanin compositions of elderberry extracts. Food Chem. 2014, 155, 112–119. [Google Scholar] [CrossRef] [PubMed]
- Ti, H.; Li, Q.; Zhang, R.; Zhang, M.; Deng, Y.; Wei, Z.; Chi, J.; Zhang, Y. Free and bound phenolic profiles and antioxidant activity of milled fractions of different indica rice varieties cultivated in southern China. Food Chem. 2014, 159, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Liazid, A.; Guerrero, R.F.; Cantos, E.; Palma, M.; Barroso, C.G. Microwave assisted extraction of Anthocyanins from grape skins. Food Chem. 2011, 124, 1238–1243. [Google Scholar] [CrossRef]
- Liazid, A.; Palma, M.; Brigui, J.; Barroso, C.G. Investigation on phenolic compounds stability during microwave-assisted extraction. J. Chromatogr. A 2007, 1140, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Rossi, J.A. Colorimetry of Total Phenolics with Phosphomolybdic-Phosphotungstic Acid Reagents. Am. J. Enol. Vitic. 1965, 13, 144–158. [Google Scholar]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Machado, A.P.D.F.; Pasquel-Reátegui, J.L.; Barbero, G.F.; Martínez, J. Pressurized liquid extraction of bioactive compounds from blackberry (Rubus fruticosus L.) residues: A comparison with conventional methods. Food Res. Int. 2015, 77, 675–683. [Google Scholar] [CrossRef]
- Shang, Y.F.; Kim, S.M.; Um, B.-H. Optimisation of pressurised liquid extraction of antioxidants from black bamboo leaves. Food Chem. 2014, 154, 164–170. [Google Scholar] [CrossRef]
- Kumar, M.; Dahuja, A.; Sachdev, A.; Kaur, C.; Varghese, E.; Saha, S.; Sairam, K.V.S.S. Valorisation of black carrot pomace: Microwave assisted extraction of bioactive phytoceuticals and antioxidant activity using Box–Behnken design. J. Food Sci. Technol. 2019, 56, 995–1007. [Google Scholar] [CrossRef]
- Paucar-Menacho, L.M.; Martínez-Villaluenga, C.; Dueñas, M.; Frias, J.; Peñas, E. Response surface optimisation of germination conditions to improve the accumulation of bioactive compounds and the antioxidant activity in quinoa. Int. J. Food Sci. Technol. 2018, 53, 516–524. [Google Scholar] [CrossRef]
- Verbeyst, L.; Oey, I.; Van der Plancken, I.; Hendrickx, M.; Van Loey, A. Kinetic study on the thermal and pressure degradation of Anthocyanins in strawberries. Food Chem. 2010, 123, 269–274. [Google Scholar] [CrossRef]

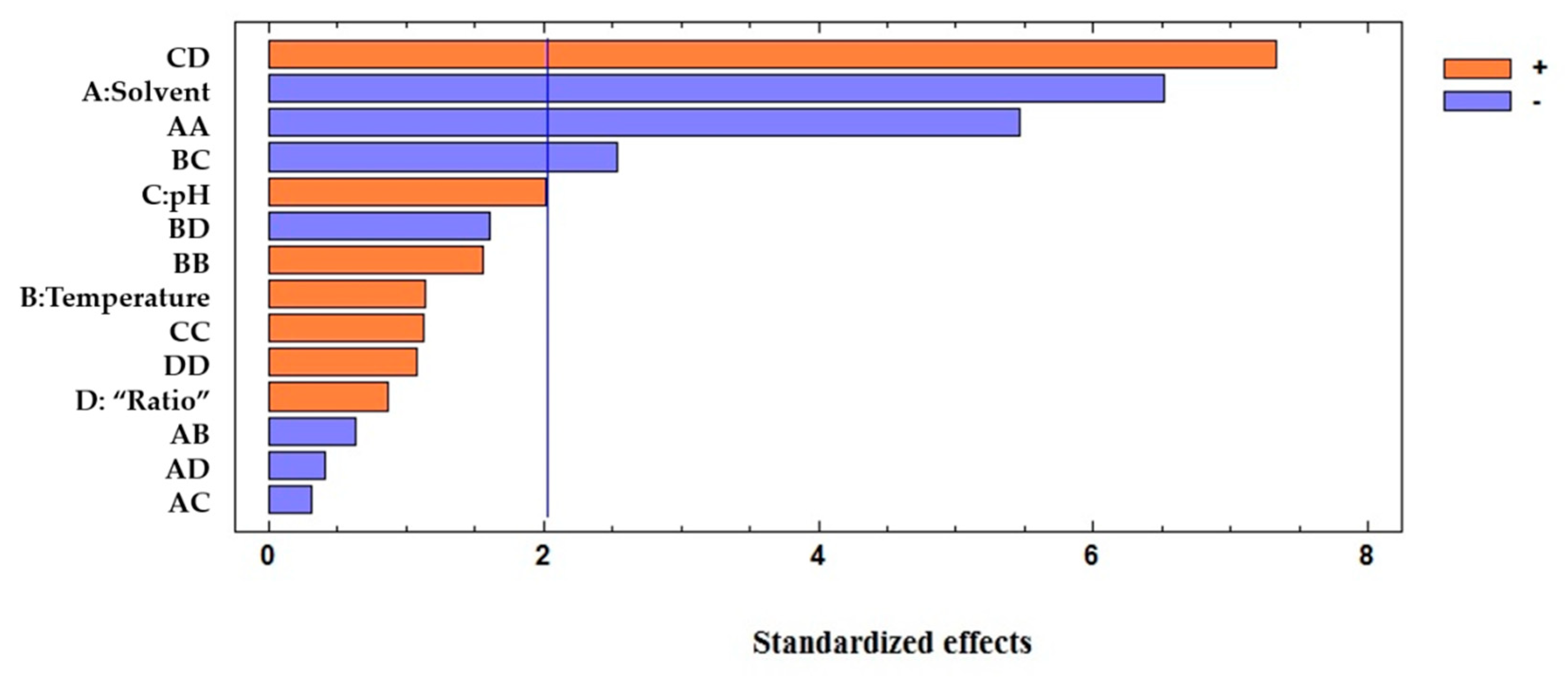

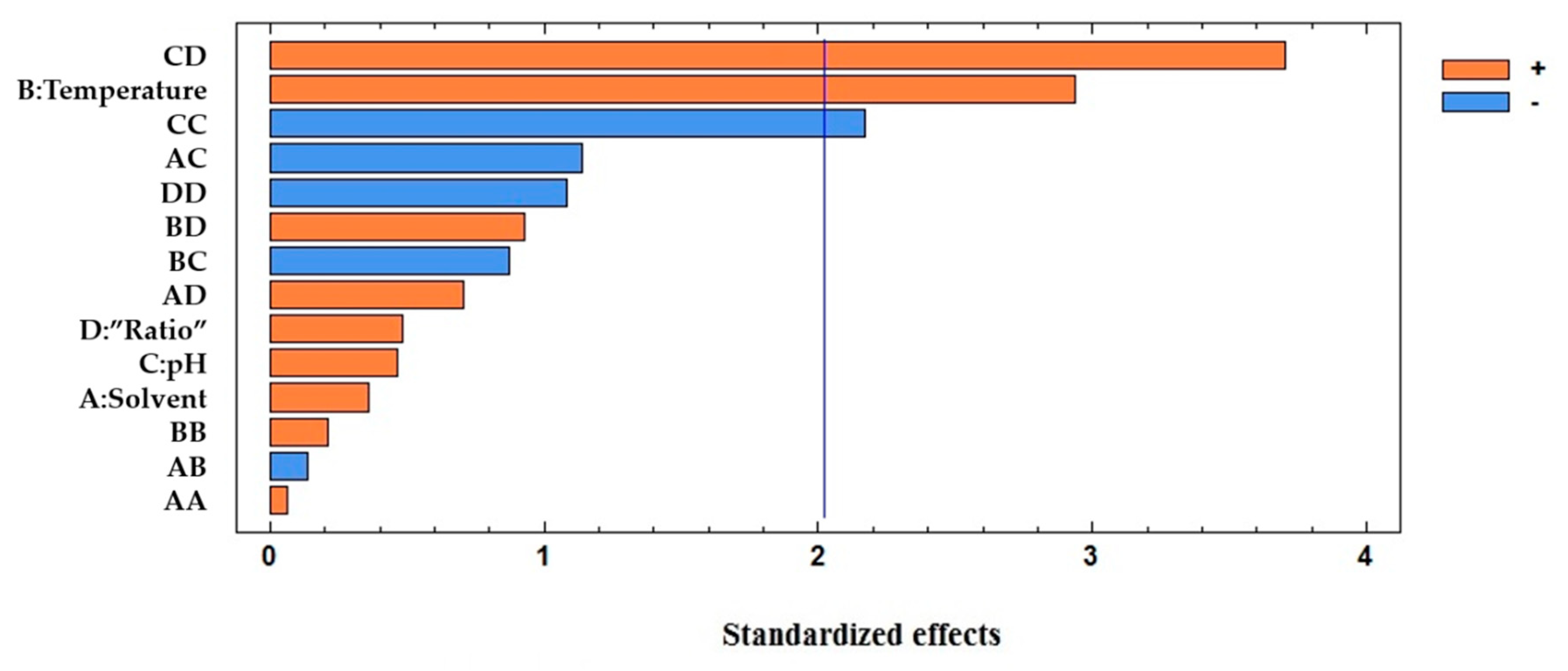
| Total Anthocyanins Coefficients | Total Anthocyanins p-Values | Total Phenolic Compounds Coefficients | Total Phenolic Compounds p-Values | |
|---|---|---|---|---|
| β0 | 1.1677 | 12.1491 | ||
| Solvent | −0.2796 | 0.0000 | 0.0910 | 0.7219 |
| Temperature | 0.0366 | 0.2743 | 0.5627 | 0.0055 |
| pH | 0.0863 | 0.0513 | 0.1178 | 0.6452 |
| “Ratio” | 0.0373 | 0.3895 | 0.1233 | 0.6298 |
| Solvent:Solvent | −0.2944 | 0.0000 | 0.0197 | 0.9511 |
| Solvent:Temperature | −0.0297 | 0.5310 | −0.0372 | 0.8942 |
| Solvent:pH | −0.0195 | 0.7560 | −0.4179 | 0.2630 |
| Solvent:”Ratio” | −0.0254 | 0.6849 | 0.2586 | 0.4863 |
| Temperature:Temperature | 0.0937 | 0.1276 | 0.0745 | 0.8355 |
| Temperature:pH | −0.1190 | 0.0154 | −0.2429 | 0.3878 |
| Temperature:”Ratio” | −0.0753 | 0.1170 | 0.2577 | 0.3598 |
| pH:pH | 0.0606 | 0.2671 | −0.6920 | 0.0360 |
| pH:”Ratio” | 0.4560 | 0.0000 | 1.3633 | 0.0007 |
| “Ratio”:”Ratio” | 0.0580 | 0.2879 | −0.3455 | 0.2849 |
| Variable | Optimum Values for Total Anthocyanins | Optimum Values for Total Phenolic Compounds |
|---|---|---|
| Solvent (% MeOH) | 38.23 | 74.16 |
| Temperature (°C) | 99.63 | 99.14 |
| pH | 2.00 | 5.46 |
| “Ratio” (mL) | 10 | 20 |
| Repeatability 1 | Intermediate Precision 2 | |
|---|---|---|
| Average (mg total anthocyanins/g açai) | 4.377 | 4.346 |
| Standard deviation | 0.073 | 0.140 |
| Relative standard deviation (%) | 1.66 | 3.23 |
| Average (mg total phenolics/g açai) | 10.523 | 10.574 |
| Standard deviation | 0.228 | 0.256 |
| Relative standard deviation (%) | 2.17 | 2.42 |
| Samples | mg Total Anthocyanins/g sample | mg Total Phenolic Compounds/g sample |
|---|---|---|
| Freeze-dried açai (1) | 5.56 ± 0.34 | 12.37 ± 0.49 |
| Freeze-dried açai (2) | 4.47 ± 0.41 | 10.61 ± 0.37 |
| Freeze-dried açai (3) | 4.05 ± 0.25 | 9.67 ± 0.50 |
| Açai juice | 0.04 ± 0.00 | 2.48 ± 0.22 |
| Açai juice food supplement | 0.05 ± 0.00 | 2.53 ± 0.18 |
| Concentrated juice açai-banana | 0.01 ± 0.00 | 5.15 ± 0.29 |
| Açai tablet | 0.04 ± 0.00 | 2.17 ± 0.25 |
| Açai jam | 0.14 ± 0.01 | 1.42 ± 0.08 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aliaño-González, M.J.; Ferreiro-González, M.; Espada-Bellido, E.; Carrera, C.; Palma, M.; Ayuso, J.; Barbero, G.F.; Álvarez, J.Á. Extraction of Anthocyanins and Total Phenolic Compounds from Açai (Euterpe oleracea Mart.) Using an Experimental Design Methodology. Part 3: Microwave-Assisted Extraction. Agronomy 2020, 10, 179. https://doi.org/10.3390/agronomy10020179
Aliaño-González MJ, Ferreiro-González M, Espada-Bellido E, Carrera C, Palma M, Ayuso J, Barbero GF, Álvarez JÁ. Extraction of Anthocyanins and Total Phenolic Compounds from Açai (Euterpe oleracea Mart.) Using an Experimental Design Methodology. Part 3: Microwave-Assisted Extraction. Agronomy. 2020; 10(2):179. https://doi.org/10.3390/agronomy10020179
Chicago/Turabian StyleAliaño-González, María José, Marta Ferreiro-González, Estrella Espada-Bellido, Ceferino Carrera, Miguel Palma, Jesús Ayuso, Gerardo F. Barbero, and José Á. Álvarez. 2020. "Extraction of Anthocyanins and Total Phenolic Compounds from Açai (Euterpe oleracea Mart.) Using an Experimental Design Methodology. Part 3: Microwave-Assisted Extraction" Agronomy 10, no. 2: 179. https://doi.org/10.3390/agronomy10020179
APA StyleAliaño-González, M. J., Ferreiro-González, M., Espada-Bellido, E., Carrera, C., Palma, M., Ayuso, J., Barbero, G. F., & Álvarez, J. Á. (2020). Extraction of Anthocyanins and Total Phenolic Compounds from Açai (Euterpe oleracea Mart.) Using an Experimental Design Methodology. Part 3: Microwave-Assisted Extraction. Agronomy, 10(2), 179. https://doi.org/10.3390/agronomy10020179