Abstract
Legumes such as alfalfa (Medicago sativa L.) and white clover (Trifolium repens L.) increase forage productivity and quality in northern temperate pastures, but require re-establishment following broadleaf weed control using herbicides. To quantify the residual effects of two herbicides (aminocyclopyrachlor and aminopyralid) on potential legume re-establishment we examined alfalfa and clover recruitment at two field sites over two years. Sites were over-seeded with alfalfa and clover to populate the seed bank, and then sprayed with herbicide, after which seedling densities were monitored in late summer and fall of the current growing season. Defoliation (via mowing) effects were also assessed to evaluate the role of vegetation competition on legume establishment. Herbicides were applied at recommended rates (1.0), and 0.5, 0.25, 0.125, 0.0625, and 0 times recommended field rates, emulating exponential herbicide degradation (one through four half-lives). Alfalfa and white clover seedling densities were negatively impacted by all rates of herbicide, with modestly greater negative impacts from aminopyralid than aminocyclopyrachlor, although responses to herbicides remained site and legume specific. Reductions in alfalfa and clover were particularly evident through the 0.25 (i.e., two half-life) herbicide rate, with reductions in alfalfa ranging from 78% to 95%, and in clover from 73% to 88%. Legume densities at the 0.125 (three half-life) rate were 39%–68% lower than those in nonsprayed control plots. Our results suggest that at least three half-lives of degradation must occur, and likely four or more, before these legumes can re-establish at densities acceptable for pasture production. These findings have implications for producers seeking to promptly re-establish forage legumes within pastures sprayed for broadleaf weed control in northern temperate regions.
1. Introduction
Forage legumes such as alfalfa (Medicago sativa L.) and white clover (Trifolium repens L.) are important pasture and hayfield species in northern temperate pastures. Legumes increase forage productivity and quality [1,2,3,4], either directly through the complementary use of soil resources in diverse swards [5], or indirectly through the transfer of nitrogen fixed by legume plants to neighboring grass species [2]. A significant challenge in the maintenance of optimal pasture composition is the need for legume re-establishment following herbicide application intended for broadleaf weed control [6,7].
In many jurisdictions of western Canada, landowners are legally required to control noxious weeds such as Cirsium arvense L., which are known to cause forage yield losses [8], or otherwise take measures to prevent their spread [9]. Application of broadleaf herbicides increase forage production in the short term [10], but often do so at the expense of removing broadleaf legumes such as alfalfa and white clover. Foliar applications of broadleaf herbicide remove legumes along with weeds, and herbicide residues can further limit legume re-establishment from the existing seed bank or following reseeding [11], thereby extending the opportunity cost of legume absence. Producers therefore need to balance weed control efforts with the maintenance of desirable legumes.
Two auxinic herbicides, aminopyralid (AMP) or aminocyclopyrachlor (AMCP) may be used for providing residual control of broadleaf weeds in pasture [12], but are also known to negatively affect legume presence [11]. As little as 5.4 ppb aminocyclopyrachlor in soil has been estimated to cause 25% mortality of alfalfa seedlings [13]; however, the interval between herbicide application and safe re-establishment of legumes from seed has not been clearly established. Legume re-establishment requires herbicide dissipation from the soil and/or microbial degradation of the bioactive to levels below toxic levels, as well as favorable environmental conditions for germination, emergence, and growth of legume seedlings.
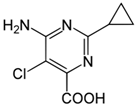
Herbicide dissipation and leaching can be modeled from quantifiable physio-chemical properties of soil and herbicides, such as water solubility, pKa and Kow—the coefficient of solubility in octane and water, respectively, and Koc—the coefficient of absorbance to organic carbon [14,15]. AMCP is more water soluble than AMP, and sorption of both bioactives increase with organic matter and clay content [16,17,18] (Table 1). While both herbicides are weak acids, indicating a change in solubility with pH, changes in pH do not affect adsorption in most soils. Relative herbicide microbial degradation is a quantified parameter, expressed as I50, or herbicide half-life, which is the time required for 50% of the herbicide present to degrade.

Table 1.
Chemical properties and structures of the two bioactives tested, including aminopyralid (AMP) and aminocyclopyrachlor (AMCP).
In a comparative study, I50 values of 32.5 and 28.9 days have been reported for AMP and AMCP [19], respectively, in field trials in Colorado (Table 1). I50 values generally decrease in soils that are warmer and moist due to enhanced microbial activity [14]. Therefore, soils that are cooler, have higher organic matter, and are situated in regions where the growing season is short (such as in western Canada), may expect longer degradation periods. Greater growing season precipitation will also increase the dissipation of herbicides away from the soil surface. To guide forage growers following weed control on the safe withdrawal intervals necessary prior to legume re-establishment, a bioassay is recommended for AMP prior to seeding [20], while reseeding recommendations for AMCP have not been identified [21].
To relate legume re-establishment to herbicide degradation, plant species sensitivity to herbicides must be considered. Species vary in sensitivity to herbicides, and sensitivity varies with plant life stages and whether the herbicide is encountered as a soil residue or as a foliar application. Alfalfa seedlings were found to be injured eight and eleven months after the application of AMP, but not 20 or 23 months, following application, in North Dakota on a silt-clay soil [22]. Alfalfa was also reported to be sensitive to AMCP present at 5.4 ppb [13], though the interval following herbicide application at which legumes can be safely re-established has not been determined for sensitive legume species within northern temperate grasslands.
Together with herbicide residues, environmental factors and plant competition play important roles in legume re-establishment, and success can vary across different climatic and disturbance regimes [22,23]. Competition, specifically for available resources (light, water, and nutrients), is modified by defoliation in pastures and further alters legume establishment and persistence depending on plant species, as well as defoliation frequency and intensity [24,25,26]. Alfalfa generally does not persist under frequent grazing, and therefore its abundance decreases in grazed pastures over time [27]. In contrast, white clover maintains or increases under grazing, and can become dominant in heavily grazed pastures [25,28]. Effects of defoliation, herbicide use, and associated soil residues and species sensitivity may interact to effect legume establishment and persistence following herbicide application.
The objectives of this study were to build on a previous investigation evaluating the long-term recovery of legumes following herbicide application in northern temperate pastures [11] by quantifying the specific sensitivity of alfalfa and white clover to different levels of AMCP and AMP herbicide. In this trial, we examined the effect of different herbicide rates emulating progressive herbicide degradation on emergent live legume seedling density. These results were used to estimate the number of half-lives required for legume survival after herbicide application, and develop specific half-life re-establishment intervals for alfalfa and white clover following AMCP and AMP application in northern temperate grasslands.
2. Materials and Methods
2.1. Study Sites
Experiments were initiated in May 2010 and 2011 at two locations in the Central Parkland Natural Subregion of Alberta, Canada, including the University of Alberta Ellerslie (53°25′6.02ʺ N, 113°32′29.79ʺ W) and St. Albert (53°41′34.31ʺ N, 113°38′5.40ʺ W) Research Stations. Treatments were applied to mature hayfields that had been defoliated annually but not subject to grazing, were uniform in ecosite conditions (elevation, slope, aspect, drainage), and had internally homogenous plant composition with a legume component (alfalfa and clover combined) of 10%–30% by ground cover. Sites differed however, in their initial plant community composition. Ellerslie was dominated by reed canary grass (Phalaris arundinacea L.) while St. Albert was composed primarily of orchard grass (Dactylis glomerata L.) and meadow brome (Bromus biebersteinii Roem. and Schult.) Both sites also contained smooth brome (Bromus inermis Leyss), Kentucky bluegrass (Poa pratensis L), and quackgrass (Elytrigia repens (L.) Beauv.). Soil samples were collected in May of 2010 and 2011 at each site. A minimum of 10 cores (2 cm wide × 30 cm deep) were taken in a W-layout across each site and bulked to form a composite sample. Soil samples were analyzed for soil texture (% sand, silt, and clay), organic matter (%), pH, electrical conductivity (salinity) (µS cm−1), total carbon (%), total nitrogen (%), and available nitrogen (NH4 + NO3 mg kg−1). In addition, the soil profile and type were described (Table 2).

Table 2.
Physical site (i.e., soil) characteristics of short-term dose trials, as sampled in May 2010 and 2011. Values represent the average of 10 soil cores collected at 0–30 cm depth. All sites were level with negligible slope.
2.2. Experimental Design and Treatments
Field experiments were designed as a strip-split plot randomized complete block with four replicate blocks, which was further repeated in each of four site years. The main treatment (5 × 36 m) in each replicate was mowing, randomly assigned to half of the block, split by a randomly assigned herbicide sub-plot (3 × 10 m). Either AMCP or AMP were applied at six different rates: 1.0, 0.5, 0.25, 0.125, 0.0625, and 0 times the recommended field rate of AMCP (60 g a.i. ha−1) or AMP (120 g a.i. ha−1), respectively, further randomized in each subplot. Decreasing rates emulated the maximum amount of herbicide residue present after 0, 1, 2, 3, and 4 I50 (half-life) intervals.
Sites were mowed and raked prior to seeding to a height of 10 cm to facilitate seeding and ensure vegetation was at the same phenological stage at the start of each trial. Sites were broadcast seeded using a Valmar seeder (Salford Inc., Elie, Manitoba, Canada) with a 50:50 mix of white clover1 (Common #1) and alfalfa (cv. Algonquin) seed, at a rate of 16 kg ha−1. Plots were then raked twice with a hay rake to ensure the seed reached the soil surface in preparation for germination. A high seeding rate was used to ensure a hyper-abundant legume seed bank and maximize opportunities for establishment. Germination tests prior to seeding indicated alfalfa and clover germination rates were 87.2% and 91.7%, respectively.
Within a week following seeding, herbicide subplots were sprayed using a high clearance self-propelled Spider Trac sprayer (Spider Trac Sprayer, West Texas Lee Co., Idalou, TX, USA), on 8 June, 2010 and 4 July, 2011. Herbicide was applied to plots in a 2-m wide strip using TeeJet® XR110015 nozzles (Tee Jet Spraying Systems Co., Wheaton, IL, USA), spaced 50 cm apart on the 2-m boom, delivering 100 L ha -1 with CO2 for each herbicide. Permanently marked quadrats 1 m2 in size were established in each subplot for repeated quantification of legume density over the growing season. Mowed plots were cut every four weeks to a height of 10 cm to mimic repeated light grazing, but remained high enough above ground to avoid cutting of emergent legume seedlings.
Alfalfa and white clover seedling density was quantified within marked quadrats in late July and early September of the year of seeding and spraying. Light intensity (µmol m−2 sec−1) was recorded monthly for each quadrat using a 1-m long AccuPAR model LP-80 PAR/LAI Ceptometer light wand (Decagon Devices Inc., Pullman, WA, USA) just prior to each mowing treatment when light was likely to be at its lowest. Soil moisture was also measured monthly for each quadrat using an ML2X-ThetaProbe (DeltaT Devices, Cambridge, United Kingdom) soil moisture meter, at least seven days after rain.
2.3. Statistical Analysis
To assess the relationship between legume plant density and environmental factors (i.e., minimum light availability and mean soil moisture), correlations were performed between legume density counts in unsprayed plots and both soil moisture and minimum light intensity (p < 0.05), using a Kendall’s Tau rank correlation. All analyses were conducted separately for the two study sites due to inherent differences in soil conditions (see Table 2).
Legume seedling density data were tested for normality prior to analysis using a Kolmogorov–Smirnov test and examination of the residuals, and found to be non-normal (p < 0.10). Thus, densities were subject to a (log+1) transformation. Thereafter, a mixed model analysis of variance (ANOVA) was conducted to assess seedling density responses to the fixed effects of herbicide type, rate, and mowing, together with the season of sampling within the growing season (summer vs. fall). Replicate blocks and duplicate trial year were considered random in the analysis. Season was included as a repeated measure, and effectively formed a split-split plot in the analysis. For all significant main effects and their interactions (p < 0.05), a Tukey’s honest significance difference (HSD) test was performed among treatments to adjust for multiple comparisons among LSmeans (p < 0.05). All data analyses were performed in Statistical Analysis Software, version 9.2 [29].
3. Results
St. Albert received less precipitation during the study years compared to the 30-yr average, whereas Ellerslie received more precipitation (Figure 1). Precipitation was also more variable during 2011 (second test year) when rainfall was low for most of the growing season except mid-summer (June and July). Temperatures were near normal for both study locations and years (Figure 1).

Figure 1.
Monthly precipitation and temperature for 2010 and 2011 in relation to the long-term normal for each of the (A) St. Albert and (B) Ellerslie, study sites, during the study period.
Available light levels increased by 48% with mowing, from an average of 358.6 µmol m−2 se−1 (± 29.8 SE) in nonmowed plots to 692.3 µmol m−2 sec−1 (± 19.9 SE). Legume density within nonsprayed plots however, exhibited no correlation with light availability at either site (p > 0.05). In the absence of herbicide, clover density at both study sites was positively correlated with soil moisture (r ≥ + 0.18; p < 0.05). In contrast, alfalfa density was negatively associated with soil moisture (r ≤ −0.18; p < 0.05) at both locations.
Summary statistical tests of the primary treatment effects on legume plant densities following spraying are shown in Table 3. Overall, mowing did not impact the density of either legume species at either location (p ≥ 0.38), alone or in combination with other fixed effects. In contrast, herbicide type impacted legume establishment for three of the four different herbicide × legume combinations (Figure 2). Application of AMP led to 49% and 37% lower white clover densities compared to AMCP at St. Albert and Ellerslie, respectively, and also decreased alfalfa by 21% at Ellerslie (Figure 2). At the St. Albert site, herbicide type also interacted with the season of measurement to influence alfalfa seedling densities (p = 0.01); closer examination of this response showed no difference in alfalfa density during either summer or fall (p > 0.05), with AMCP (but not AMP) leading to a decline in alfalfa densities from summer (9.6 ± 4.1 SE plants m−2) to fall (7.4 ± 4.1 plants m−2). An increase was also observed in alfalfa densities (p = 0.01) at the Ellerslie study location from the July (mid-summer) sampling period (11.7 ± 1.9 plants m−2) to September (16.3 ± 1.9 plants m−2).

Table 3.
Summary of F-statistic and significance values associated with clover and alfalfa plant densities from over-seeded grasslands at each of the St. Albert and Ellerslie study sites, following different types of herbicide application at six different rates, under mowed and nonmowed conditions. Data represent measurements taken in summer and fall after spring seeding, and within two duplicate trials in consecutive years. All analyses done on log transformed data to similar clover densities between the 0, 0.0625, and 0.125 rates.
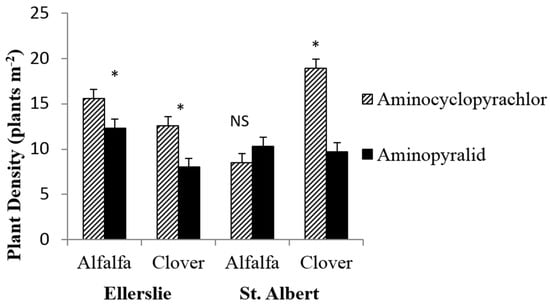
Figure 2.
Mean (± 1 SE) forage plant density for each of two legume species, alfalfa and white clover, at each of two study locations, following spraying with either aminocyclopyrachlor or aminopyralid herbicide. Means of herbicides within a pair denoted by a * differ, at p < 0.05. NS indicates not significant.
The most pronounced effect of the treatments tested was evident in the rate of herbicide application emulating different half-lives of herbicide, with significant effects for both species of legumes at both study locations (p < 0.0001; Table 3). In addition, herbicide rate interacted with season of measurement to influence legume densities (p ≤ 0.02), including alfalfa at Ellerslie, and white clover at St. Albert. Overall rate effects demonstrated strong residual effects of herbicide in reducing legume densities, including at rates that emulated half-lives associated with one through four cycles of degradation (Figure 3). Even small rates of 0.0625 × check (representing four half-lives of decay) led to decreases in alfalfa density of 54% to 66%, and declines of 39% to 63% for white clover. More moderate levels of herbicide (0.125 × check) led to larger declines in legume establishment in several instances, while rates of 0.5 (one half-life) to 0.25 (two half-lives) led to observed legume densities either similar to, or marginally different from, the full application rate of herbicide (Figure 3).
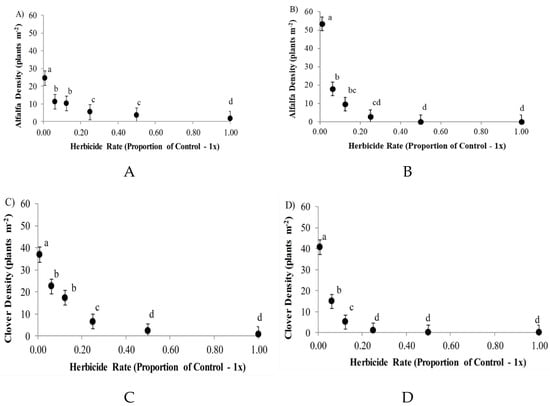
Figure 3.
Mean (± 1 SEM) forage plant density for each of two legume species at each of two study locations, following spraying with broadleaf herbicide at six rates representing 0, 1, 2, 3, or 4 progressive half-lives of degradation, as well as a nontreated check. Data include: (A) alfalfa at St. Albert, (B) alfalfa at Ellerslie, (C) white clover at St. Albert, and (D) white clover at Ellerslie. Y-axes are scaled the same for comparison among legume species and locations. Different letters differ based on a Tukey HSD test (p ≤ 0.05).
Interactions of herbicide application rate by season of sampling revealed limited new information. For alfalfa at the Ellerslie site, no differences were apparent in the rank comparison of treatments sprayed at different rates (Table 4), though alfalfa densities continued to increase (p < 0.05) from July through September at herbicide rates of 0.125 or less. For clover at the St. Albert site, statistical differences among rates were most apparent in summer; by fall (two months later), reductions in the density of clover within both nontreated check plots (0x) and the 0.0625x rate led to similar clover densities between the 0, 0.0625 and 0.125 rates.

Table 4.
Mean densities of alfalfa and clover (plants m−2) in response to varied herbicide application rates at either the Ellerslie or St. Albert study sites, in relation to different seasons of sampling. Results are combined across the AMCP and AMP treatments and over years (2010 and 2011). Rates emulate herbicide residues associated with sequential half-lives ranging from one (0.5 × check) to four (0.0625 × check).
4. Discussion
At both study sites, the density of white clover and alfalfa seedlings establishing decreased markedly with progressive increases in herbicide, with declines evident for both legumes at both study locations through rates as low as 0.0625 times the recommended rate of application. Considering that the herbicide rates tested emulated consecutive herbicide half-lives, these findings indicate that relatively long intervals are needed to facilitate legume re-establishment within these northern temperate pastures. White clover and alfalfa re-establishment have previously been reported to differ under varying intervals after AMP application, with improved re-establishment detected as little as 11 months after fall herbicide application [30]. In South Dakota, USA, no negative effects were found from AMP 20 months after treatment [22]. While our results are similar in that even modest reductions in herbicide presence, as represented by two half-lives or more, led to significant increases in legume density, it is important to note that the plant densities documented within the sprayed treatments (≤22 plants m−2; Figure 3) remained well below those likely to provide a productive forage sward for the region. For instance, current establishment guidelines for the black soil zone of Alberta suggest legume plant densities during the first year of establishment should be 80–100 plants m−2 [31]. This would create a mixed grass-legume sward able to produce sufficient forage capable of being stocked at 2–3 animal-unit-months (AUM) ha−1, where one AUM is the forage necessary to support a mature cow, with or without a calf, for 30.5 days.
In our study, all observed legume densities remained below the 80-plant threshold, including the nonsprayed controls, although it is notable that our treatments were intended to assess legume recovery potential from the soil seedbank within a perennial grassland, rather than a newly establishing forage sward on fallow ground. Legume re-establishment within an existing grassland is likely to lead to lower legume densities due to the competitive pressure of grasses, which is known to suppress both white clover [32] and alfalfa [4]. This trend was evidenced here by the decrease in white clover density at the St. Albert location between the summer and fall sampling times, which was attributed to a highly competitive grassland community (largely orchardgrass and meadow brome) at that location, which may have impacted legume seedlings to a greater extent as the growing season progressed. Clover would have been particularly susceptible to competition at St. Albert given the reduced precipitation at this location and the documented positive relationship between clover and soil moisture (i.e., current study, and [33]). In contrast, the negative correlation of alfalfa density with moisture is consistent with previous reports that alfalfa can be negatively associated with soil moisture [34], presumably due to alfalfa’s high drought tolerance and increased susceptibility to competition with neighboring forage grasses under high moisture [1]. Competing grasses take advantage of abundant surface soil moisture, while alfalfa plants have a taproot morphology that allows them to access deeper subsurface moisture. This result may explain why alfalfa densities at St. Albert remained particularly low in the current study, even within nonsprayed control plots (see Figure 3A).
Although we included a mowing treatment to suppress competition from grasses and facilitate legume recovery, mowing was not found to be a factor regulating legume densities, either alone or in conjunction with the herbicide treatments. This was surprising as white clover is well-known for being dependent on defoliation to maintain its presence [4,34], and mowing was found to increase light availability. This result suggests that factors other than light availability, such as moisture or nutrient supply, may have been a greater constraint to the overall establishment of legumes from the seedbank of these grasslands.
Legume establishment demonstrated similar overall patterns to the two bioactives tested using the simulated half-life rates, suggesting these two herbicides are likely to have a similar impact in restricting legume recovery over time. However, between the two herbicides, AMP led to lower legume densities than the AMCP treatment in all situations except alfalfa at St. Albert. Previous estimates of half-lives vary widely for these two bioactives in the literature (Table 1) [14,20,21]. Herbicide dissipation in soil under field conditions in Colorado was reported to be 32.5 and 28.9 d for AMCP and AMP, respectively [19]. A slightly faster dissipation rate of AMCP could account for why we observed greater legume densities within this treatment relative to areas treated with AMP, and suggest that legume recovery may occur more quickly in the long-term following application of AMCP.
Although no definitive guidelines exist for legume re-establishment in northern temperate pastures following spraying of these herbicides, the lower soil temperatures, shorter growing seasons, and high soil organic matter therein are likely to restrict herbicide breakdown in this environment. We therefore postulate that a longer dissipation interval is probable for the half-life of AMCP and AMP in central Alberta relative to that found in warmer climates with longer growing seasons, including that in Colorado [19]. While the data collected in this study do not allow us to calculate a specific I50 for the legumes tested due to the I50 being below our minimum herbicide application rate (i.e., 50% survival levels were below the 0.0625 × check rate) and therefore outside our effective treatment range, these results can nevertheless be combined with previous information on long-term legume responses to spraying in pasture [11] to provide insight on the expected residual impacts from these herbicides. Reductions in legume biomass in our study regions persisted through at least 26 months in the field (i.e., nearly 800 days, including well into the third growing season) after spraying with AMCP and AMP [11], with no differences between herbicides. Combined with the data here showing that legumes were negatively impacted by herbicide rates representative of at least four half-lives, it appears that much longer withdrawal periods are needed for these herbicides within northern temperate pastures relative to those reported in the adjacent agro-climatic regions of the United States (e.g., 20 months for AMP, [22]).
Further variation in legume responses to herbicide are likely based on soil conditions, and may explain some of the variation in legume recovery observed between the St. Albert and Ellerslie study locations. While soils at both sites were moderate in clay content and relatively basic in pH (8.1 and 8.4), they differed markedly in organic matter (Table 2). Ellerslie soils were in a lowland and contained much greater organic matter. Given that herbicide adsorption increases with organic matter, AMP and AMCP are likely to be bound and have reduced dissipation at this location. Ultimately, herbicide residues and its effect on legume re-establishment will be influenced by microbial mediated degradation and a host of factors that influence microbial activity, including temperature and moisture [35,36,37]. As a result, the expected period of withdrawal following herbicide application is likely to vary locally among grazing operations based on factors controlling microbial activity, including soil texture, pH, and organic matter, all of which are known to vary among farms in the study area [38].
5. Conclusions
Knowledge on legume re-establishment dynamics specific to northern temperate pastures could help producers to effectively manage both weed and legume communities in their pasture systems. From this study, it was apparent that the herbicide aminocyclopyrachlor (AMCP) had a less detrimental impact compared to aminopyralid (AMP) on legume re-establishment. Both alfalfa and white clover remained prone to decreases in plant density, even when exposed to relatively low herbicide rates (0.0625 × registered rates) emulating a herbicide residue level of about four half-lives with ongoing degradation from the full registered rates. In doing so, this study provides valuable insight into the legume re-establishment dynamics that may be expected following application of the broadleaf herbicides AMCP and AMP.
Author Contributions
E.W.B. and L.M.H. conceived and designed the study, and assisted with analysis and manuscript development; A.J.M. conducted the field sampling and data analysis, as well as the initial manuscript draft; V.M.L. assisted with data analysis and writing. All authors contributed to writing and editing of the manuscript, and have approved the final manuscript. All authors have read and agreed to the published version of the manuscript.
Acknowledgments
We thank Erin McLeod, Scott Dunn, Meaghan Dunn, Katelyn Céh, Pat Forsythe, Lisa Raatz, Jamie Crowe, Keith Topinka, Judy Irving, Carly Moore, and Tanner Broadbent, for technical support, and Laki Goonewardene for assistance with statistical analysis. Financial support for this project was provided by DuPont Canada, Dow AgroSciences Canada, the University of Alberta, and a Collaborative Research and Development grant from the Natural Sciences and Engineering Research Council (NSERC) of Canada to E.W.B. and L.M.H.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Groya, F.L.; Sheaffer, C.C. Establishment of sod-seeded alfalfa at various levels of soil moisture and grass competition. Agron. J. 1981, 73, 560–565. [Google Scholar] [CrossRef]
- Kunelius, H.T.; Campbell, A.J.; McRae, K.B.; Ivany, J.A. Effects of vegetation suppression and drilling techniques on the establishment and growth of sod-seeded alfalfa and bird’s-foot trefoil in grass dominant swards. Can. J. Plant Sci. 1982, 62, 667–675. [Google Scholar] [CrossRef]
- Papadopoulos, Y.A.; McIlroy, M.S.; Fillmore, S.A.E.; McRae, K.B.; Duyinsveld, J.L.; Fredeen, A.H. Sward complexity and grass species composition affect the performance of grass-white clover pasture mixtures. Can. J. Plant Sci. 2012, 92, 1199–1205. [Google Scholar] [CrossRef]
- Bork, E.W.; Gabruck, D.T.; McLeod, E.M.; Hall, L.M. Five-year forage dynamics arising from four legume-grass seed mixes. Agron. J. 2017, 109, 2789–2799. [Google Scholar] [CrossRef]
- Popp, J.D.; McCaughey, W.P.; Cohen, R.D.H.; McAllister, T.A.; Majak, W. Enhancing pasture productivity with alfalfa: A review. Can. J. Plant Sci. 2000, 80, 513–519. [Google Scholar] [CrossRef]
- Grekul, C.W.; Cole, D.E.; Bork, E.W. Canada thistle (Cirsium arvense) and pasture forage responses to wiping with various herbicides. Weed Technol. 2005, 19, 298–306. [Google Scholar] [CrossRef]
- Enloe, S.F.; Lym, R.G.; Wilson, R.; Westra, P.; Nissen, S.; Beck, G.; Moechnig, M.; Peterson, V.; Masters, R.A.; Halstvedt, M. Canada thistle (Cirsium arvense) control with aminopyralid in range, pasture, and noncrop areas. Weed Technol. 2007, 21, 890–894. [Google Scholar] [CrossRef]
- Grekul, C.W.; Bork, E.W. Herbage yield losses in perennial pasture due to Canada thistle (Cirsium arvense). Weed Technol. 2004, 18, 784–794. [Google Scholar] [CrossRef]
- Province of Alberta. Alberta Weed Control Act. 2008. Available online: http://www.qp.alberta.ca/1266.cfm?page=W05P1.cfm&leg_type=Acts&isbncln=9780779760602 (accessed on 29 April 2014).
- Bork, E.W.; Grekul, C.W.; DeBrujin, S.L. Extended pasture forage sward responses to Canada thistle (Cirsium arvense) control using herbicides and fertilization. Crop Prot. 2007, 26, 1546–1555. [Google Scholar] [CrossRef]
- Miller, A.J.; Bork, E.W.; Hall, L.M.; Summers, B. Long-term forage dynamics in pastures sprayed with residual broadleaf herbicide: A test of legume recovery. Can. J. Plant Sci. 2015, 95, 43–53. [Google Scholar] [CrossRef]
- Lindenmayer, R.B.; Nissen, S.J.; Westra, P.P.; Shaner, D.L.; Brunk, G. Aminocyclopyrachlor absorption, translocation and metabolism in field bindweed (Convolvulus arvensis). Weed Sci. 2013, 61, 63–67. [Google Scholar] [CrossRef]
- Strachan, S.D.; Nanita, S.C.; Ruggiero, M.; Casini, M.S.; Heldreth, K.M.; Hageman, L.H.; Flanigan, H.A.; Ferry, N.M.; Pentz, A.M. Correlation of chemical analysis of residual levels of aminocyclopyrachlor in soil to biological responses of alfalfa, cotton, soybean, and sunflower. Weed Technol. 2011, 25, 239–244. [Google Scholar] [CrossRef]
- Conklin, K.L.; Lym, R.G. Effect of temperature and moisture on aminocyclopyrachlor soil half-life. Weed Technol. 2013, 27, 552–556. [Google Scholar] [CrossRef]
- Lewis, D.F.; Jeffries, M.D.; Strek, H.J.; Richardson, R.J.; Yelverton, F.H. Effect of ambient moisture on aminocyclopyrachlor efficacy. Weed Technol. 2013, 27, 317–322. [Google Scholar] [CrossRef]
- Oliveira, R.S., Jr.; Alonso, D.G.; Koskinen, W.C. Sorption—Desorption of aminocyclopyrachlor in selected Brazilian soils. J. Agric. Food Chem. 2011, 59, 4045–4050. [Google Scholar] [CrossRef] [PubMed]
- Senseman, S.A. Herbicide Handbook, 9th ed.; Weed Science Society of America: Champagne, IL, USA, 2007. [Google Scholar]
- Finkelstein, B.L.; Armel, G.R.; Bolgunas, S.A.; Clark, D.A.; Claus, J.S.; Crosswicks, R.J.; Hirata, C.M.; Hollingshaus, G.J.; Koeppe, M.K.; Rardon, P.L.; et al. Discovery of aminocyclopyrachlor (proposed common name) (DPX-MAT28): A new broadspectrum auxinic herbicide. In Proceedings of the 236th ACS National Meeting, Philadelphia, PA, USA, 17–21 August 2008; American Chemical Society: Washington, DC, USA. [Google Scholar]
- Lindenmayer, R.B. Understanding Aminocyclopyrachlor Behavior in Soil and Plants. Ph.D. Thesis, Colorado State University, Fort Collins, CO, USA, 2012; p. 82. [Google Scholar]
- USEPA. Environmental Fate and Ecological Risk Assessment for the Registration of Aminopyralid; Office of Prevention, Pesticides, and Toxic Substances: Washington, DC, USA, 2005; p. 151. [Google Scholar]
- USEPA. Registration of the New Active Ingredient Aminocyclopyrachlor for Use on Non-Crop Areas, Sod Farms, Turf, and Residential Lawns; U.S. Environmental Protection Agency, Office of Pesticide Programs, Registration Division: Washington, DC, USA, 2010; p. 6.
- Mikkelson, J.R.; Lym, R.G. Aminopyralid soil residues affect crop rotation in North Dakota soils. Weed Technol. 2011, 25, 422–429. [Google Scholar] [CrossRef]
- Vough, L.R.; Marten, G.C. Influence of soil moisture and ambient temperature on yield and quality of alfalfa forage. Agron. J. 1971, 63, 40–42. [Google Scholar] [CrossRef]
- Evans, D.R.; Williams, T.A.; Evans, S.A. Evaluation of white clover varieties under grazing and their role in farm systems. Grass Forage Sci. 1992, 47, 342–352. [Google Scholar] [CrossRef]
- Brummer, E.C.; Moore, K.J. Persistence of perennial cool-season grass and legume cultivars under continuous grazing by beef cattle. Agron. J. 2000, 92, 466–471. [Google Scholar] [CrossRef]
- Seguin, P.; Peterson, P.R.; Sheaffer, C.C.; Smith, D.L. Physical sod suppression as an alternative to herbicide use in pasture renovation with clovers. Can. J. Plant Sci. 2001, 81, 255–263. [Google Scholar] [CrossRef]
- Katepa-Mupondwa, F.; Singh, A.; Smith, S.R., Jr.; McCaughey, W.P. Grazing tolerance of alfalfa (Medicago spp.) under continuous and rotational stocking systems in pure stands and in mixture with meadow bromegrass (Bromus riparious Rehm. syn. B. biebersteinii Roem & Schult). Can. J. Plant Sci. 2002, 82, 337–347. [Google Scholar]
- Schwinning, S.; Parsons, A.J. Analysis of the coexistence mechanisms for grasses and legumes in grazing systems. J. Ecol. 1996, 84, 799–813. [Google Scholar] [CrossRef]
- SAS Institute Inc. SAS/STAT® 9.2 User’s Guide; SAS Institute Inc.: Cary, NC, USA, 2008. [Google Scholar]
- Renz, M.J. Establishment of forage grasses and legumes after fall herbicide applications. Forage Grazinglands 2010, 8. [Google Scholar] [CrossRef]
- Aasen, A.; Bjorge, M. Alberta Forage Manual; Agdex 120/20-1; Alberta Agriculture and Rural Development: Edmonton, AB, Canada, 2009; p. 354. [Google Scholar]
- Gabruck, D.T.; Bork, E.W.; Hall, L.M.; King, J.R.; Hare, D.D. Interspecific relationships between white clover, Kentucky bluegrass and Canada thistle during establishment. Agron. J. 2013, 105, 1467–1474. [Google Scholar] [CrossRef]
- Lane, L.A.; Ayres, J.F.; Lovett, J.V. The pastoral significance, adaptive characteristics, and grazing value of white clover (Trifolium repens L.) in dryland environments in Australia: A review. Aust. J. Exp. Agric. 2000, 40, 1033–1046. [Google Scholar] [CrossRef]
- Frame, J. Forage Legumes for Temperate Grasslands; Science Publishers Inc.: Enfield, NH, USA, 2005; p. 309. [Google Scholar]
- Picton, P.; Farenhorst, A. Factors influencing 2,4-D sorption and mineralization in soil. J. Environ. Sci. Health 2004, 39, 367–379. [Google Scholar] [CrossRef]
- Veeh, R.H.; Inskeep, W.P.; Camper, A.K. Soil depth and temperature effects on microbial degradation of 2,4-D. J. Environ. Qual. 1996, 25, 5–12. [Google Scholar] [CrossRef]
- Parker, L.W.; Doxtader, K.G. Kinetics of the microbial degradation of 2,4-D in soil: Effects of temperature and moisture. J. Environ. Qual. 1983, 12, 533–558. [Google Scholar] [CrossRef]
- Pyle, L.A.; Hall, L.M.; Bork, E.W. Soil properties in northern temperate pasture do not vary with management practices and are independent of rangeland health. Can. J. Soil Sci. 2019, 99, 495–507. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).