Impact of Effective Microorganisms (EM) Application on the Physical Condition of Haplic Luvisol
Abstract
:1. Introduction
2. Materials and Methods
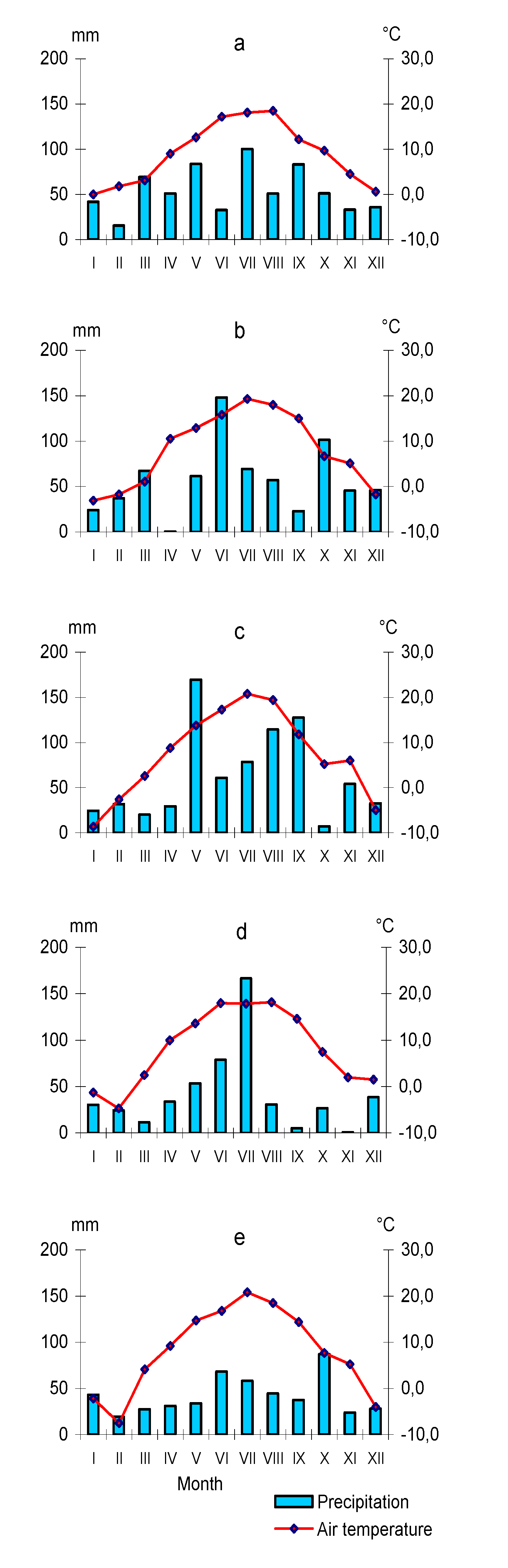
2.1. Study Area and Sampling
2.2. Analysis
- particle density (PD), with the pycnometric method [50] (Mg × m−3);
- bulk density (BD), with the gravimetric method, from the ratio of the mass of soil dried at 105 °C to the initial soil volume of 100 cm3 [51] (Mg × m−3);
- total porosity (TP) was calculated from the results of particle density (PD) and bulk density (BD), TP = 1 − BD/PD [52] (m3 × m−3);
- air capacity at the potential of −15.5 kPa (FAC) was derived from the results of total porosity (TP) and field water capacity (FC) (−15.5 kPa), FAC = TP − FC [52] (m3 × m−3);
- air permeability at the potential of -15.5 kPa (FAP) was measured using an apparatus for the measurement of the permeability of molding sand, LPiR-2e. The measurements were conducted on vertical (upward) airflow through the soil sample. The pressure head in the measurement chamber was 0.981 kPa (100 mm H2O), and the ambient temperature was stabilized (20 ± 1.0 °C). The relative air humidity was 40 ± 5%. The dynamic air viscosity (10−8 × m2 × Pa−1 × s−1) was not taken into account in the measurement results. The apparatus was produced by MULTISERW-Morek (Poland);
- soil moisture at sampling (SM) was calculated from the ratio of the mass of water contained in the soil during the sampling to the dry matter of soil dried at 105 °C [53] (kg × kg−1);
2.3. Statistical Analysis
3. Results and Discussion
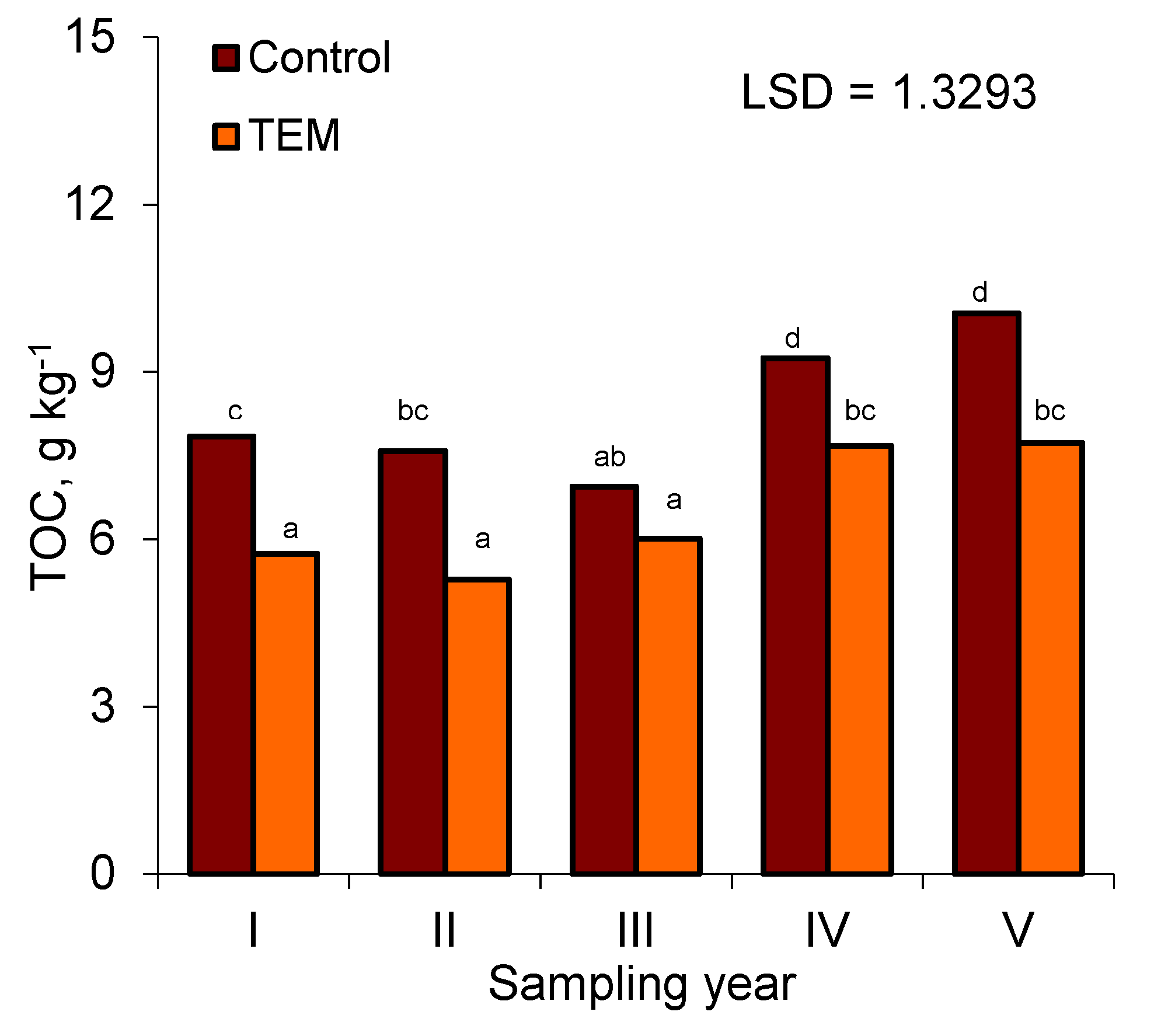
3.1. Total Organic Carbon (TOC)
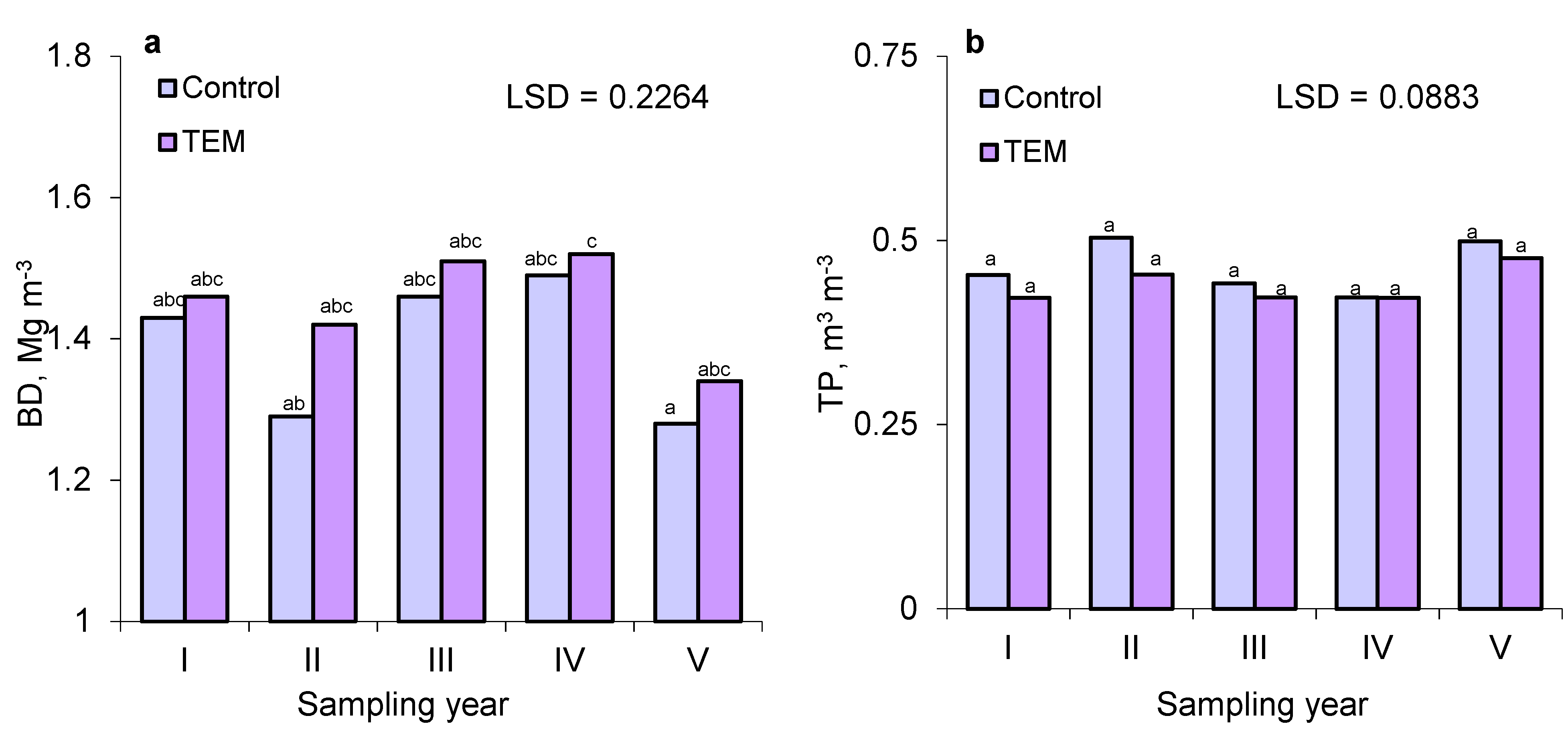
3.2. Bulk Density (BD), Total Porosity (TP), and Soil Air Properties (FAC and FAP)
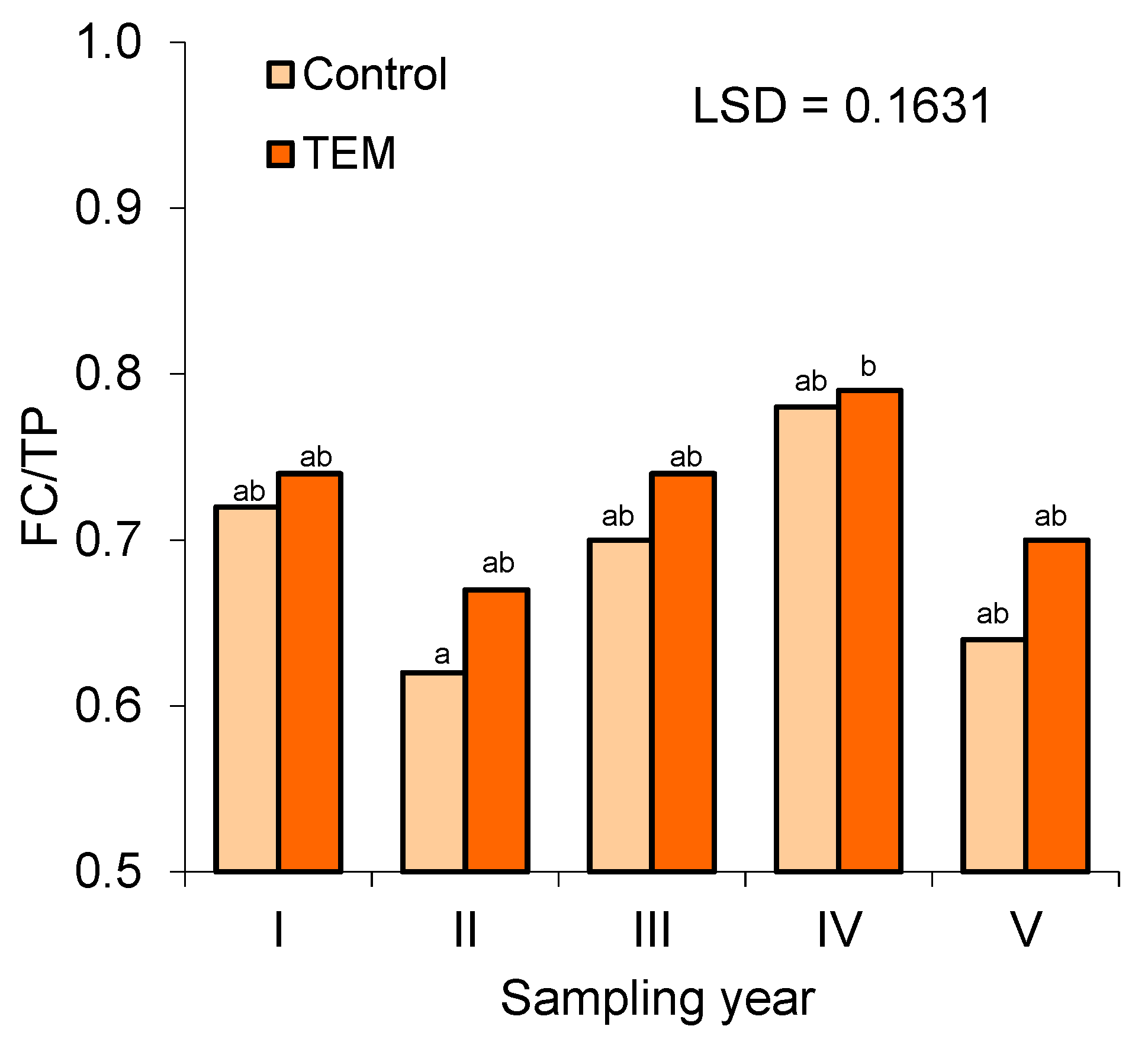
3.3. Soil Water Properties (SM, FC, AWC, and UWC) and the FC/TP Ratio
3.4. Aggregate Stability (A5–10, A1–5, and A<1) and Mean Weight Diameter (MWD) of Aggregates
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lal, R. Restoring Soil Quality to Mitigate Soil Degradation. Sustainability 2015, 7, 5875–5895. [Google Scholar] [CrossRef] [Green Version]
- Blum, W.E.H. Basic concepts: Degradation, Resilience, and Rehabilitation. In Methods for Assessment of Soil Degradation; CRC Press: Boca Raton, FL, USA; New York, NY, USA, 1998. [Google Scholar]
- Lal, R. Soil and sustainability agriculture. A review. Agron. Sustain. Dev. 2008, 28, 57–65. [Google Scholar] [CrossRef]
- Lal, R. Soils and world food security. Soil Tillage Res. 2009, 102, 1–4. [Google Scholar] [CrossRef]
- Lorenz, K.; Lal, R.; Ehlers, K. Soil organic carbon stock as an indicator for monitoring land and soil degradation in relation to United Nations’ Sustainable Development Goals. Land Degrad. Dev. 2019, 30, 824–838. [Google Scholar] [CrossRef]
- Pranagal, J. Intensity of agricultural land use and soil degradation processes. Adv. Agric. Sci. Prob. Issues 2009, 535, 321–329. [Google Scholar]
- Dahm, H.; Wrótniak-Drzewiecka, W.; Pauter, A. Microbial biofertilizers. In Physical, Chemical and Biological Processes in Soils; Szajdak, L.W., Karabanow, A.K., Eds.; Prodruk: Poznań, Poland, 2010; pp. 537–547. [Google Scholar]
- Nannipieri, P.; Falchini, L.; Landi, L.; Pietramellara, G. Management of soil microbiota. In Biological Resource Management; Balazs, E., Galante, E., Lynch, J.M., Shepers, J.S., Werner, D., Toutant, J.P., Werry, P.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2000; pp. 237–255. [Google Scholar]
- Rodrigez, H.; Fraga, R. Phosphate solubilizing bacteria and their role in plant growth promotion. Botechnol. Adv. 1999, 17, 319–339. [Google Scholar] [CrossRef]
- Vessey, J.K. Plant growth promoting rhizobacteria as biofertilizers. Plant Soil 2003, 255, 571–586. [Google Scholar] [CrossRef]
- Martyniuk, S.; Księżak, J. Evaluation of pseudo-microbial biopreparations used in crop production. Pol. J. Agron. 2011, 6, 27–33. [Google Scholar]
- Panhurst, C.E.; Rogers, S.L.; Gupta, V.S.R. Microbial parameters for monitoring soil pollution. In Environmental Biomonitoring; Lynch, J.M., Wiseman, A., Eds.; Cambridge University Press: Cambridge, UK, 1998; pp. 46–69. [Google Scholar]
- Amézketa, E. Soil aggregate stability: A review. J. Sustain. Agric. 1999, 14, 82–151. [Google Scholar] [CrossRef]
- Bronick, C.J.; Lal, R. Soil structure and management: A review. Geoderma 2005, 124, 3–22. [Google Scholar] [CrossRef]
- Pagliai, M.; Vignozzi, N.; Pellegrini, S. Soil structure and the effect of management practices. Soil Tillage Res. 2004, 79, 131–143. [Google Scholar] [CrossRef]
- Pranagal, J.; Podstawka-Chmielewska, E.; Słowińska-Jurkiewicz, A. Influence of EM on selected physical properties of a Haplic Podzol during a ten-year fallow period. Pol. J. Environ. Stud. 2007, 16, 875–880. [Google Scholar]
- Pranagal, J.; Podstawka-Chmielewska, E. Physical properties of a Rendzic Phaeozem during a ten-year period of fallowing under the conditions of south-eastern Poland. Geoderma 2012, 189–190, 262–267. [Google Scholar] [CrossRef]
- Shukla, M.K.; Lal, R. Air permeability of soil. In Encyclopedia of Soil Science; Lal, R., Ed.; Marcel Dekker: New York, NY, USA, 2006; pp. 60–63. [Google Scholar]
- Higa, T. Revolution in the Protection of Our Planet; Foundation–SGGW Development: Warsaw, Poland, 2003; 152p. (In Polish) [Google Scholar]
- Kaczmarek, Z.; Wolna-Maruwka, A.; Jakubus, M. Changes of the number of selected microorganism groups and enzymatic activity in the soil inoculated with Effective Microorganisms (EM). J. Res. Appl. Agric. Eng. 2008, 53, 122–127. [Google Scholar]
- Kucharski, J.; Jastrzębska, E. The role of Effective Microorganisms (EM) and soil microorganisms in shaping microbial properties of soil. Adv. Agric. Sci. Prob. Issues 2005, 507, 315–322. [Google Scholar]
- Schenck zu Schweinsberg-Mickan, M.; Müller, T. Impact of effective microorganisms and other biofertilizers on soil microbial characteristics, organic-matter decomposition, and plant growth. J. Plant Nut. Soil Sci. 2009, 172, 704–712. [Google Scholar] [CrossRef]
- Kocoń, A.; Gałązka, A. Effect of preparations with effective microorganisms on physicochemical properties of soil and on plant yields. Stud. Rep. IUNG-PIB 2015, 45, 113–125. (In Polish) [Google Scholar]
- Gajewski, P.; Kaczmarek, Z.; Owczarzak, W.; Jakubus, M. Impact of sludge and municipal compost additions as well as effective microorganisms on selected properties of the oro-humus mineral soil level. Part II. Structure condition. J. Res. Appl. Agric. Eng. 2012, 57, 87–91. [Google Scholar]
- Gajewski, P.; Kaczmarek, Z.; Owczarzak, W.; Jakubus, M.; Mocek, A. Impact of addition of organic additives and EM-A preparation on physical, chemical and structural state of the arable-humus soil horizon. Part III. Structure condition. J. Res. Appl. Agric. Eng. 2013, 58, 119–123. [Google Scholar]
- Gajewski, P. Influence of the EM-A preparation on the structure properties in various mineral soils. Soil Sci. Ann. 2016, 67, 179–184. [Google Scholar] [CrossRef] [Green Version]
- Gajewski, P.; Kaczmarek, Z.; Owczarzak, W.; Glina, B.; Mocek-Płóciniak, A.; Gaweł, E.; Grzelak, M.; Świerk, D. The influence of the EM-A preparation on the properties of structure in arable mineral soils. Fresenius Environ. Bull. 2016, 25, 4184–4191. [Google Scholar]
- Kaczmarek, Z.; Owczarzak, W.; Gajewski, P.; Mrugalska, L. Impact of effective microorganisms on selected physical and aqueous properties and the structure state of the arable-humus horizon of mineral soils at varying content of organic matter in them. Part I. Physical and water properties. J. Res. Appl. Agric. Eng. 2011, 56, 179–184. [Google Scholar]
- Dziamba, S.; Pranagal, J.; Wielgosz, E. Some properties of Haplic Luvisol after one-year application of Effective Microorganisms (EM). Adv. Agric. Sci. Prob. Issues 2009, 542, 139–145. [Google Scholar]
- Frąszczak, B.; Kleiber, T.; Klama, J. Impact of Effective Microorganisms on yields and nutrition of sweet basil (Ocimum basilicum L.) and microbiological properties of the substrate. Afr. J. Agric. Res. 2012, 7, 5756–5765. [Google Scholar] [CrossRef]
- Kleiber, T.; Starzyk, J.; Bosiacki, M. Effect of nutrient solution, Effective Microorganisms (EM-A), and assimilation illumination of plants on the induction of the growth of lettuce (Lactuca sativa L.) in hydroponic cultivation. Acta Agrobot. 2013, 66, 27–38. [Google Scholar] [CrossRef] [Green Version]
- Tyburski, J.; Łachacz, A. The effectiveness of agents improving heavy soils in organic farms. In Summary of Results of Studies on Organic Farming Realized in 2009; Ministry of Agriculture and Rural Development: Warsaw, Poland, 2010; Volume 318, pp. 267–276. (In Polish) [Google Scholar]
- Tołoczko, W.; Trawczyńska, A.; Niewiadomski, A. Content of organic compounds in soils fertilized with EM preparation. Soil Sci. Ann. 2009, 60, 97–101. [Google Scholar]
- Kowalska, J. Effect of fertilization and microbiological bio-stimulators on healthiness and yield of organic potato. Prog. Plant Prot. 2016, 56, 230–235. [Google Scholar] [CrossRef]
- Kaczmarek, Z.; Jakubus, M.; Grzelak, M.; Mrugalska, L. Impact of the addition of various doses of Effective Microorganisms to arable-humus horizons of mineral soils on their physical and water properties. J. Res. Appl. Agric. Eng. 2008, 53, 118–121. [Google Scholar]
- Van Vliet, P.C.J.; Bloem, J.; de Goede, R.G.M. Microbial diversity, nitrogen loss and grass production after addition of Effective Microorganisms® (EM) to slurry manure. Appl. Soil Ecol. 2006, 32, 188–198. [Google Scholar] [CrossRef]
- Mayer, J.; Scheid, S.; Widmer, F.; Fließach, A.; Oberholzer, H.-R. How effective are “Effective microorganisms® (EM)”? Results from a field study in temperate climate. Appl. Soil Ecol. 2010, 46, 230–239. [Google Scholar] [CrossRef]
- Khaliq, A.; Abbasi, M.K.; Hussain, T. Effects of integrated use of organic and inorganic nutrient sources with effective microorganisms (EM) on seed cotton yield in Pakistan. Bioresour Technol. 2006, 97, 967–972. [Google Scholar] [CrossRef] [PubMed]
- Ismail, S.M. Influence of effective microorganisms and green manure on soil properties and productivity of pearl millet alfalfa grown on sandy loam in Saudi Arabia. Afr. J. Microb. Res. 2013, 7, 375–382. [Google Scholar] [CrossRef] [Green Version]
- Dexter, A.R. Soil physical quality. Part I. theory, effects of soil texture, density, and organic matter, and effects on root growth. Geoderma 2004, 120, 201–214. [Google Scholar] [CrossRef]
- Dexter, A.R. Soil physical quality: Part II. Friability, tillage, tilth and hard-setting. Geoderma 2004, 120, 215–225. [Google Scholar] [CrossRef]
- Dexter, A.R. Soil physical quality: Part III: Unsaturated hydraulic conductivity and general conclusions about S-theory. Geoderma 2004, 120, 227–239. [Google Scholar] [CrossRef]
- Di Giuseppe, D.; Melchiorre, M.; Tessari, U.; Faccini, B. Relationship between particle density and soil bulk chemical composition. J. Soils Sediments 2016, 16, 909–915. [Google Scholar] [CrossRef]
- Reynolds, W.D.; Bowman, B.T.; Drury, C.F.; Tan, C.S.; Lu, X. Indicators of good soil physical quality: Density and storage parameters. Geoderma 2002, 110, 131–146. [Google Scholar] [CrossRef]
- Pranagal, J.; Oleszczuk, P.; Tomaszewska-Krojańska, D.; Kraska, P.; Różyło, K. Effect of biochar application on the physical properties of Haplic Podzol. Soil Tillage Res. 2017, 174, 92–103. [Google Scholar] [CrossRef]
- Cóndor-Golec, A.F.; Pérez, P.G.; Lokare, C. Effective Microorganisms: Myth or reality? Rev. Peru. Biol. 2007, 14, 315–319. [Google Scholar]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, Update 2015. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports No. 106; FAO: Rome, Italy, 2015. [Google Scholar]
- Domżał, H.; Pranagal, J. Pedological characteristics of research site for studying climate of the cultivated field. Adv. Agric. Sci. Prob. Issues 1995, 419, 9–14. [Google Scholar]
- Klute, A. Methods of Soil Analysis. 1. Physical and Mineralogical Methods; ASA-SSSA Inc.: Madison, WI, USA, 1986. [Google Scholar]
- Blake, G.R.; Hartge, K.H. Particle density. In Methods of Soil Analysis. 1. Physical and Mineralogical Methods; Klute, A., Ed.; ASA-SSSA Inc.: Madison, WI, USA, 1986; pp. 377–382. [Google Scholar]
- Blake, G.R.; Hartge, K.H. Bulk density. In Methods of Soil Analysis. 1. Physical and Mineralogical Methods; Klute, A., Ed.; ASA-SSSA Inc.: Madison, WI, USA, 1986; pp. 363–375. [Google Scholar]
- Danielson, R.E.; Sutherland, P.L. Porosity. In Methods of Soil Analysis. 1. Physical and Mineralogical Methods; Klute, A., Ed.; ASA-SSSA Inc.: Madison, WI, USA, 1986; pp. 443–460. [Google Scholar]
- Gardner, W.H. Water content. In Methods of Soil Analysis. 1. Physical and Mineralogical Methods; Klute, A., Ed.; ASA-SSSA Inc.: Madison, WI, USA, 1986; pp. 493–541. [Google Scholar]
- Cassel, D.K.; Nielsen, D.R. Field capacity and available water capacity. In Methods of Soil Analysis. 1. Physical and Mineralogical Methods; Klute, A., Ed.; ASA-SSSA Inc.: Madison, WI, USA, 1986; pp. 901–924. [Google Scholar]
- Canarache, A.; Vintila, I.; Munteanu, I. Elsevier’s Dictionary of Soil Science: Definitions in English with French, German, and Spanish Word Translations; Elsevier, BV: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Olness, A.; Clapp, C.E.; Liu, R.; Palazzo, A.J. Biosoilds and their effect on soil properties. In Handbook of Soil Conditioners; Wallace, A., Terry, R.E., Eds.; Marcel Dekker: New York, NY, USA, 1998; pp. 141–165. [Google Scholar]
- Reynolds, W.D.; Drury, C.F.; Yang, X.M.; Tan, C.S. Optimal soil physical quality inferred through structural regression and parameter interactions. Geoderma 2008, 146, 466–474. [Google Scholar] [CrossRef]
- Skopp, J.; Janson, M.D.; Doran, J.W. Steady—State aerobic microbial activity as a function of soil water content. Soil Sci. Soc. Am. J. 1990, 54, 1619–1625. [Google Scholar] [CrossRef] [Green Version]
- Kemper, W.D.; Rosenau, R.C. Aggregate stability and size distribution. In Methods of Soil Analysis. Part 1. Agronomy Monograph 9, 2nd ed.; Klute, A., Ed.; American Society of Agronomy: Madison, WI, USA, 1986; pp. 425–442. [Google Scholar]
- Drewry, J.J.; Cameron, K.C.; Buchan, G.D. Pasture yield and soil physical property responses to soil compaction from treading and grazing – a review. Aust. J. Soil Res. 2008, 46, 237–256. [Google Scholar] [CrossRef]
- Du, Z.; Liu, S.; Li, K.; Ren, T. Soil organic carbon and physical quality as influenced by long-term application of residue and mineral fertiliser in the North China Plain. Aust. J. Soil Res. 2009, 47, 585–591. [Google Scholar] [CrossRef]
- Paluszek, J. Criteria of evaluation of physical quality of polish arable soils. Acta Agrophys. 2011, 191, 1–139. [Google Scholar]
- Pranagal, J. The Physical State of Selected Silty Soils of on the Lublin Region. Ph.D. Thesis, University of Life Sciences in Lublin, Lublin, Poland, 2011; pp. 1–129. [Google Scholar]
- Mentges, M.I.; Reichert, J.M.; Rodrigues, M.F.; Awe, G.O.; Mentges, L.R. Capacity and intensity soil aeration properties affected by granulometry, moisture, and structure in no-tillage soils. Geoderma 2016, 263, 47–59. [Google Scholar] [CrossRef]
- Olness, A.; Archer, D. Effect of organic carbon on available water in soil. Soil Sci. 2005, 170, 90–101. [Google Scholar] [CrossRef]
- Wang, W.; Li, J.; Su, L.; Wang, Q. Soil air permeability model based on soil physical basic parameters. Nongye Jixie Xuebao/Trans. Chin. Soc. Agric. Mach. 2015, 46, 125–130. [Google Scholar] [CrossRef]
- Asgarzadeh, H.; Mosaddeghi, M.R.; Dexter, A.R.; Mahboubi, A.A.; Neyshabouri, M.R. Determination of soil available water for plants: Consistency between laboratory and field measurements. Geoderma 2014, 226–227, 8–20. [Google Scholar] [CrossRef]
- Githinji, L. Effect of biochar application rate on soil physical and hydraulic properties of a sandy loam. Arch. Agron. Soil Sci. 2014, 60, 457–470. [Google Scholar] [CrossRef]
- Iversen, B.V.; Schjønning, P.; Poulsen, T.G.; Moldrup, P. In-situ, on-situ and laboratory measurements of soil air permeability: Boundary conditions and measurement scale. Soil Sci. 2001, 166, 97–106. [Google Scholar] [CrossRef]
- Kuncoro, P.H.; Koga, K.; Satta, N.; Muto, Y. A study on the effect of compaction on transport properties of soil gas and water I: Relative gas diffusivity, air permeability, and saturated hydraulic conductivity. Soil Tillage Res. 2014, 143, 172–179. [Google Scholar] [CrossRef]
- Darboux, F.; Le Bissonnais, Y. Changes in structural stability with soil surface crusting: Con-sequences for erodibility estimation. Eur. J. Soil Sci. 2007, 58, 1107–1114. [Google Scholar] [CrossRef]
- Le Bissonnais, Y. Aggregate stability and assessment of soil crustability and erodibility: I. Theory and methodology. Eur. J. Soil Sci. 1996, 47, 425–437. [Google Scholar] [CrossRef]
- Tisdall, J.M. Formation of soil aggregates and accumulation of soil organic matter. In Structure and Organic Matter Storage in Agricultural Soils; Carter, M.R., Stewart, D.A., Eds.; Lewis Publishers, CRC Press: Boca Raton, FL, USA, 1996; pp. 57–96. [Google Scholar]
- Tisdall, J.M.; Oades, J.M. Organic matter and water-stable aggregates in soils. J. Soil Sci. 1982, 33, 141–163. [Google Scholar] [CrossRef]
- Jones, C.A. Effect of soil texture on critical bulk densities for root growth. Soil Sci. Soc. Am. J. 1983, 47, 1208–1212. [Google Scholar] [CrossRef]
- Dexter, A.R.; Czyż, E.A. Applications of s-theory in the study of soil physical degradation and its consequences. Land Degrad. Dev. 2007, 18, 369–381. [Google Scholar] [CrossRef]
- Carter, M.R. Temporal variability of soil macroporosity in a fine sandy loam under mouldboard ploughing and direct drilling. Soil Tillage Res. 1988, 12, 37–51. [Google Scholar] [CrossRef]
- McQueen, D.J.; Shepherd, T.G. Physical changes and compaction sensitivity of a fine textured, poorly drained soil (Typic Endoaquept) under varying durations of cropping, Manawatu Region, New Zealand. Soil Tillage Res. 2002, 63, 93–107. [Google Scholar] [CrossRef]
- Kowda, W.A. Basics of Soil Science; PWRiL: Warsaw, Poland, 1984. [Google Scholar]
- Thompson, L.M.; Troeh, F.R. Soils and Soil Fertility; McGraw–Hill, Inc.: New York, NY, USA, 1978. [Google Scholar]
- Cockroft, B.; Olsson, K.A. Case study of soil quality in south-eastern Australia: Management of structure for roots in duplex soils. In Soil Quality for Crop Production and Ecosystem Health; Gregorich, E.G., Carter, M.R., Eds.; Developments in Soil Science; Elsevier: New York, NY, USA, 1997; Volume 25, pp. 339–350. [Google Scholar]
- Walczak, R.; Ostrowski, J.; Witkowska-Walczak, B.; Sławiński, C. Hydrophysical characteristics of Polish mineral arable soils. Acta Agrophys. 2002, 79, 1–64. [Google Scholar]
- Drewry, J.J. Natural recovery of soil physical properties from treading damage of pastoral soils in New Zealand and Australia: A review. Agric. Ecosyst. Environ. 2006, 114, 159–169. [Google Scholar] [CrossRef]
- Grable, A.R.; Siemer, E.G. Effects of bulk density, aggregate size, and soil water suction on oxygen diffusion, redox potentials and elongation of corn roots. Soil Sci. Soc. Am. Proc. 1968, 32, 180–186. [Google Scholar] [CrossRef]
- Mayers, W.S.; Barrs, H.D. Roots in irrigated clay soil: Measurement techniques and responses to root zone conditions. Irrig. Sci. 1991, 12, 125–134. [Google Scholar]
- Mueller, L.; Kay, B.D.; Been, B.; Hu, C.; Zhang, Y.; Wolff, M.; Eulenstein, F.; Schindler, U. Visual assessment of soil structure: Part II. Implications of tillage, rotation and traffic on sites in Canada, China and Germany. Soil Tillage Res. 2008, 103, 188–196. [Google Scholar] [CrossRef]
- Poulsen, T.G.; Iversen, B.V.; Yamaguchi, T.; Moldrup, P.; Schjønning, P. Spatial and temporal dynamics of air permeability in a constructed fields. Soil Sci. 2001, 166, 153–162. [Google Scholar] [CrossRef]
- Kutílek, M. Soil hydraulic properties as related to soil structure. Soil Tillage Res. 2004, 79, 175–184. [Google Scholar] [CrossRef]
- Leśny, J. Meteorology and Climatology Research. Acta Agrophys. 2010, 184, 1–263. [Google Scholar]
- Petrosyants, M.A.; Kislov, A.V.; Semenov, E.K. Principal concepts in meteorology and climatology. Vestn. Mosk. Univ. S 5 Geogr. 2005, 1, 83–91. [Google Scholar]
- White, R.E. Principles and Practice of Soil Science, 4th ed.; Blackwell Publishing: Oxford, UK, 2006. [Google Scholar]
- Usowicz, B.; Usowicz, Ł. Point measurements of soil water content and its spatial distribution in cultivated fields. Acta Agrophys. 2004, 4, 573–588. [Google Scholar]
- Staricka, J.A.; Benoit, G.R. Freeze-drying effects on wet and dry soil aggregate stability. Soil Sci. Soc. Am. J. 1995, 59, 218–223. [Google Scholar] [CrossRef]
- Paluszek, J. Comparing the physical quality of Polish chernozems classified in various complexes of agricultural suitability. Pol. J. Environ. Stud. 2016, 25, 259–271. [Google Scholar] [CrossRef]
- Kaczmarek, Z.; Owczarzak, W.; Mrugalska, L.; Grzelak, M. The influence of effective microorganisms for some of physical and water properties on arable-humus horizons of mineral soils. J. Res. Appl. Agric. Eng. 2007, 52, 73–77. [Google Scholar]
- Craul, P.J. Urban Soils: Applications and Practices; Wiley: Toronto, ON, USA, 1999. [Google Scholar]
- Oades, J.M.; Waters, A.G. Aggregate hierarchy in soils. Aust. J. Soil Res. 1991, 29, 815–828. [Google Scholar] [CrossRef]
- Cosentino, D.; Chenu, C.; Le Bissonnais, Y. Aggregate stability and microbial community dynamics under drying-wetting cycles in a silt loam soil. Soil Biol. Biochem. 2006, 38, 2053–2062. [Google Scholar] [CrossRef]
- Malamoud, K.; McBratney, A.B.; Minasny, B.; Field, D.J. Modelling how carbon affect soil structure. Geoderma 2009, 149, 19–26. [Google Scholar] [CrossRef]
- Paluszek, J. Assessment of soil structure of Luvisols developed from loess classified in various complexes of agricultural suitability. Soil Sci. Ann. 2013, 64, 41–48. [Google Scholar] [CrossRef]
- Paluszek, J. Air-dry and water-stable soil aggregate distribution of Polish chernozems classified in various complexes of agricultural suitability. Pol. J. Environ. Stud. 2014, 23, 389–397. [Google Scholar]
- Barthès, B.; Roose, E. Aggregate stability as an indicator of soil susceptibility to runoff and erosion; validation at several levels. Catena 2002, 47, 133–149. [Google Scholar] [CrossRef] [Green Version]
- Vermang, J.; Demeyer, V.; Cornelis, W.M. Aggregate stability and erosion response to antecedent water content of a loess soil. Soil. Sci. Soc. Am. J. 2009, 73, 718–726. [Google Scholar] [CrossRef]
- Domżał, H.; Pranagal, J. Aggregate stability of soil as an index of soil degradation caused by the agricultural use. Fragm. Agron. 1994, 3, 22–34. [Google Scholar]
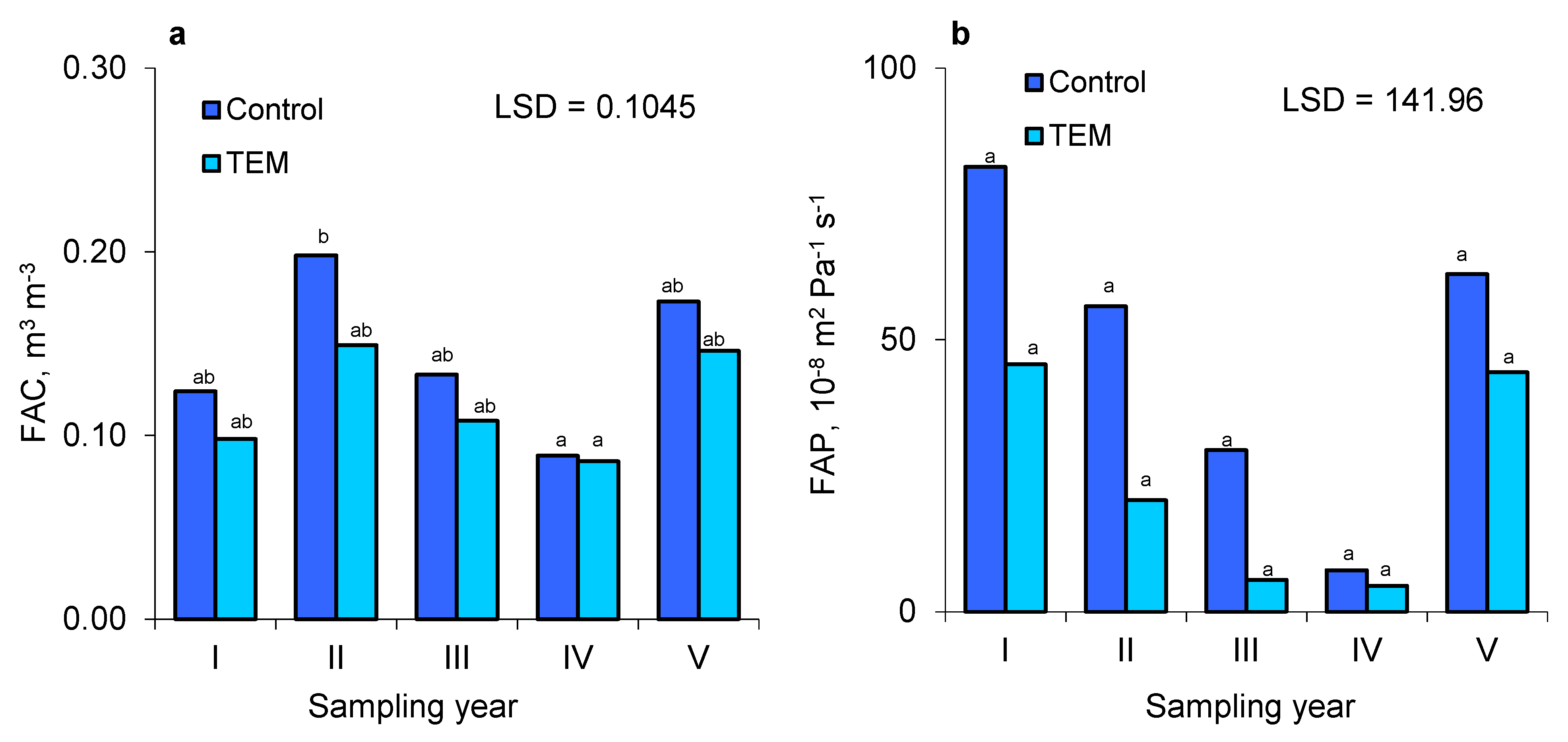


| Years | Date | TOC | BD | TP | FAC | FAP | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| g × kg−1 | Mg × m−3 | m3 × m−3 | m3 × m−3 | 10−8 × m2 × Pa−1 × s−1 | ||||||
| Control | TEM | Control | TEM | Control | TEM | Control | TEM | Control | ||
| I | i | 8.69 | 6.21 | 1.43 | 1.51 | 0.452 | 0.421 | 0.134 | 0.086 | 44.3 |
| ii | 7.95 | 5.66 | 1.47 | 1.47 | 0.437 | 0.437 | 0.098 | 0.071 | 3.1 | |
| iii | 6.87 | 5.36 | 1.38 | 1.39 | 0.471 | 0.467 | 0.141 | 0.136 | 195.9 | |
| II | i | 7.94 | 5.65 | 1.32 | 1.45 | 0.494 | 0.444 | 0.172 | 0.112 | 49.4 |
| ii | 7.39 | 5.32 | 1.15 | 1.39 | 0.559 | 0.467 | 0.278 | 0.178 | 46.9 | |
| iii | 7.42 | 4.86 | 1.41 | 1.43 | 0.46 | 0.452 | 0.143 | 0.157 | 72.2 | |
| III | i | 7.23 | 6.19 | 1.46 | 1.47 | 0.441 | 0.437 | 0.105 | 0.1 | 3.2 |
| ii | 5.99 | 5.88 | 1.45 | 1.55 | 0.444 | 0.406 | 0.145 | 0.096 | 17.2 | |
| iii | 6.26 | 5.94 | 1.46 | 1.5 | 0.441 | 0.425 | 0.149 | 0.128 | 68.6 | |
| IV | i | 9.86 | 7.95 | 1.48 | 1.53 | 0.433 | 0.414 | 0.087 | 0.075 | 2.9 |
| ii | 9.12 | 7.64 | 1.46 | 1.5 | 0.422 | 0.444 | 0.099 | 0.113 | 14.6 | |
| iii | 8.76 | 7.46 | 1.53 | 1.54 | 0.414 | 0.409 | 0.08 | 0.069 | 5.3 | |
| V | i | 10.11 | 7.65 | 1.29 | 1.31 | 0.494 | 0.489 | 0.16 | 0.167 | 35.6 |
| ii | 10.02 | 7.72 | 1.19 | 1.2 | 0.535 | 0.531 | 0.191 | 0.19 | 124.7 | |
| iii | 10.03 | 7.81 | 1.36 | 1.52 | 0.468 | 0.407 | 0.169 | 0.08 | 26 | |
| CV | 0.17 | 0.17 | 0.08 | 0.07 | 0.09 | 0.08 | 0.35 | 0.35 | 1.12 | |
| Properties | Control | TEM | LSD0.05 |
|---|---|---|---|
| TOC (g × kg−1) | 8.24a | 6.49b | 0.3498 |
| BD (Mg × m−3) | 1.39a | 1.45a | 0.0586 |
| TP (m3 × m−3) | 0.464a | 0.443a | 0.0223 |
| FAC (m3 × m−3) | 0.143a | 0.117a | 0.0275 |
| FAP (10−8 × m2 × Pa−1 × s−1) | 47.3a | 24.1a | 37.382 |
| SM (kg × kg−1) | 0.166a | 0.154a | 0.0291 |
| FC (m3 × m−3) | 0.321a | 0.321a | 0.0142 |
| AWC m3 × m−3) | 0.240a | 0.247a | 0.0134 |
| UWC (m3 × m−3) | 0.079a | 0.078a | 0.0053 |
| FC/TP | 0.69a | 0.73a | 0.0433 |
| A5–10, % | 3.31a | 0.89b | 0.9498 |
| A1–5, % | 15.03a | 6.38b | 2.4756 |
| A<1, % | 87.70a | 92.75b | 3.1660 |
| MWD, mm | 0.85a | 0.45b | 0.1104 |
| Properties | TOC | SM | BD | TP | FC | FC/TP | AWC | UWC | FAC | FAP | A5–10 | A1–5 | A<1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SM | −0.271 | ||||||||||||
| BD | −0.284 | 0.125 | |||||||||||
| TP | 0.288 | −0.106 | −0.988 * | ||||||||||
| FC | 0.355 | −0.335 | 0.083 | −0.084 | |||||||||
| FC/TP | 0.021 | −0.087 | 0.814 * | −0.828 * | 0.610 * | ||||||||
| AWC | 0.489 * | −0.392 * | −0.199 | 0.156 | 0.751 * | 0.305 | |||||||
| UWC | −0.316 | 0.083 | 0.653 * | −0.605 * | 0.144 | 0.536 * | −0.468 * | ||||||
| FAC | 0.072 | 0.061 | −0.892 * | 0.906 * | −0.444 | −0.952 * | −0.149 | −0.586 * | |||||
| FAP | 0.023 | 0.117 | −0.565 * | 0.573 * | 0.098 | −0.422 * | 0.115 | −0.179 | 0.486 * | ||||
| A5–10 | 0.511 * | 0.119 | −0.335 | 0.337 | −0.021 | −0.289 | −0.010 | −0.123 | 0.348 | 0.279 | |||
| A1–5 | 0.239 | 0.211 | −0.214 | 0.247 | −0.164 | −0.275 | −0.372 * | 0.181 | 0.324 | 0.075 | 0.788 * | ||
| A<1 | −0.302 | −0.199 | 0.245 | −0.273 | 0.137 | 0.284 | 0.308 | −0.127 | −0.338 | −0.121 | −0.860 * | −0.991 * | |
| MWD | 0.383 * | 0.163 | −0.210 | 0.228 | −0.086 | −0.233 | −0.241 | 0.110 | 0.282 | 0.077 | 0.886 * | 0.959 * | −0.979 * |
| Years | Date | SM | FC | AWC | UWC | FC/TP | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| kg × kg−1 | m3 × m−3 | m3 × m−3 | m3 × m−3 | ||||||||
| Control | TEM | Control | TEM | Control | TEM | Control | TEM | Control | TEM | ||
| I | i | 0.095 | 0.081 | 0.318 | 0.335 | 0.229 | 0.245 | 0.089 | 0.091 | 0.7 | 0.79 |
| ii | 0.151 | 0.144 | 0.338 | 0.314 | 0.247 | 0.223 | 0.091 | 0.091 | 0.77 | 0.72 | |
| iii | 0.213 | 0.202 | 0.332 | 0.329 | 0.242 | 0.243 | 0.086 | 0.088 | 0.7 | 0.71 | |
| II | i | 0.199 | 0.177 | 0.323 | 0.332 | 0.213 | 0.26 | 0.079 | 0.073 | 0.65 | 0.75 |
| ii | 0.189 | 0.185 | 0.284 | 0.289 | 0.21 | 0.227 | 0.07 | 0.063 | 0.51 | 0.62 | |
| iii | 0.193 | 0.161 | 0.318 | 0.294 | 0.231 | 0.215 | 0.086 | 0.08 | 0.69 | 0.65 | |
| III | i | 0.168 | 0.164 | 0.337 | 0.335 | 0.251 | 0.262 | 0.085 | 0.075 | 0.76 | 0.77 |
| ii | 0.175 | 0.166 | 0.303 | 0.308 | 0.211 | 0.212 | 0.088 | 0.098 | 0.68 | 0.76 | |
| iii | 0.184 | 0.174 | 0.295 | 0.296 | 0.218 | 0.222 | 0.074 | 0.075 | 0.67 | 0.7 | |
| IV | i | 0.129 | 0.122 | 0.346 | 0.335 | 0.263 | 0.246 | 0.083 | 0.09 | 0.79 | 0.81 |
| ii | 0.141 | 0.138 | 0.321 | 0.33 | 0.241 | 0.251 | 0.082 | 0.08 | 0.76 | 0.74 | |
| iii | 0.216 | 0.203 | 0.334 | 0.338 | 0.245 | 0.258 | 0.086 | 0.082 | 0.81 | 0.83 | |
| V | i | 0.154 | 0.138 | 0.333 | 0.322 | 0.279 | 0.258 | 0.055 | 0.064 | 0.67 | 0.66 |
| ii | 0.102 | 0.091 | 0.347 | 0.341 | 0.28 | 0.288 | 0.064 | 0.053 | 0.65 | 0.65 | |
| iii | 0.176 | 0.172 | 0.286 | 0.316 | 0.239 | 0.259 | 0.06 | 0.068 | 0.61 | 0.8 | |
| CVs | 0.22 | 0.23 | 0.06 | 0.05 | 0.09 | 0.09 | 0.14 | 0.16 | 0.11 | 0.09 | |
| Years | Date | Water-Stable Aggregate Distributions, % | MWD | ||||||
|---|---|---|---|---|---|---|---|---|---|
| A5–10 | A1–5 | A<1 | mm | ||||||
| mm | mm | mm | |||||||
| Control | TEM | Control | TEM | Control | TEM | Control | TEM | ||
| I | i | 2.12 | 0.24 | 9.92 | 8.72 | 87.96 | 91.04 | 0.62 | 0.44 |
| ii | 2.56 | 0.12 | 16.12 | 6.6 | 81.32 | 93.28 | 0.8 | 0.43 | |
| iii | 2.84 | 0.44 | 10.56 | 4.04 | 86.6 | 95.52 | 0.63 | 0.33 | |
| II | i | 2.47 | 0.22 | 18.08 | 6.23 | 79.45 | 93.55 | 0.82 | 0.43 |
| ii | 4.74 | 1.08 | 30.42 | 6.45 | 64.84 | 92.47 | 1.33 | 0.48 | |
| iii | 7.02 | 1.78 | 25.93 | 9.81 | 67.05 | 88.41 | 1.27 | 0.61 | |
| III | i | 0.77 | 0.6 | 9.21 | 6.12 | 90.02 | 93.28 | 0.55 | 0.41 |
| ii | 1.93 | 0.5 | 10.42 | 5.45 | 87.65 | 94.05 | 0.71 | 0.4 | |
| iii | 4.02 | 0.5 | 18.93 | 6.92 | 77.05 | 92.58 | 0.98 | 0.44 | |
| IV | i | 5.96 | 2.24 | 18.24 | 12.61 | 75.8 | 85.15 | 1.23 | 0.7 |
| ii | 1.84 | 0.89 | 15.26 | 8.48 | 82.9 | 90.63 | 0.88 | 0.54 | |
| iii | 3.56 | 1.02 | 15.59 | 5.89 | 80.85 | 93.13 | 0.92 | 0.48 | |
| V | i | 3.76 | 0.28 | 9.56 | 2.27 | 86.68 | 97.45 | 0.71 | 0.32 |
| ii | 2.78 | 2.77 | 9.76 | 3.98 | 87.46 | 93.45 | 0.69 | 0.43 | |
| iii | 3.28 | 0.64 | 7.41 | 2.19 | 89.91 | 97.18 | 0.54 | 0.33 | |
| CVs | 0.22 | 0.18 | 0.39 | 0.43 | 0.08 | 0.07 | 0.26 | 0.27 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pranagal, J.; Ligęza, S.; Smal, H. Impact of Effective Microorganisms (EM) Application on the Physical Condition of Haplic Luvisol. Agronomy 2020, 10, 1049. https://doi.org/10.3390/agronomy10071049
Pranagal J, Ligęza S, Smal H. Impact of Effective Microorganisms (EM) Application on the Physical Condition of Haplic Luvisol. Agronomy. 2020; 10(7):1049. https://doi.org/10.3390/agronomy10071049
Chicago/Turabian StylePranagal, Jacek, Sławomir Ligęza, and Halina Smal. 2020. "Impact of Effective Microorganisms (EM) Application on the Physical Condition of Haplic Luvisol" Agronomy 10, no. 7: 1049. https://doi.org/10.3390/agronomy10071049
APA StylePranagal, J., Ligęza, S., & Smal, H. (2020). Impact of Effective Microorganisms (EM) Application on the Physical Condition of Haplic Luvisol. Agronomy, 10(7), 1049. https://doi.org/10.3390/agronomy10071049






