Comparison among Different Rewetting Strategies of Degraded Agricultural Peaty Soils: Short-Term Effects on Chemical Properties and Ecoenzymatic Activities
Abstract
1. Introduction
2. Materials and Methods
2.1. Case Study
2.2. Field Site Setup
2.3. Peat Sampling and Lab Analyses
2.4. Data Analysis
3. Results
3.1. Effects of Different Strategies of Rewetting on Chemical Properties of Peat
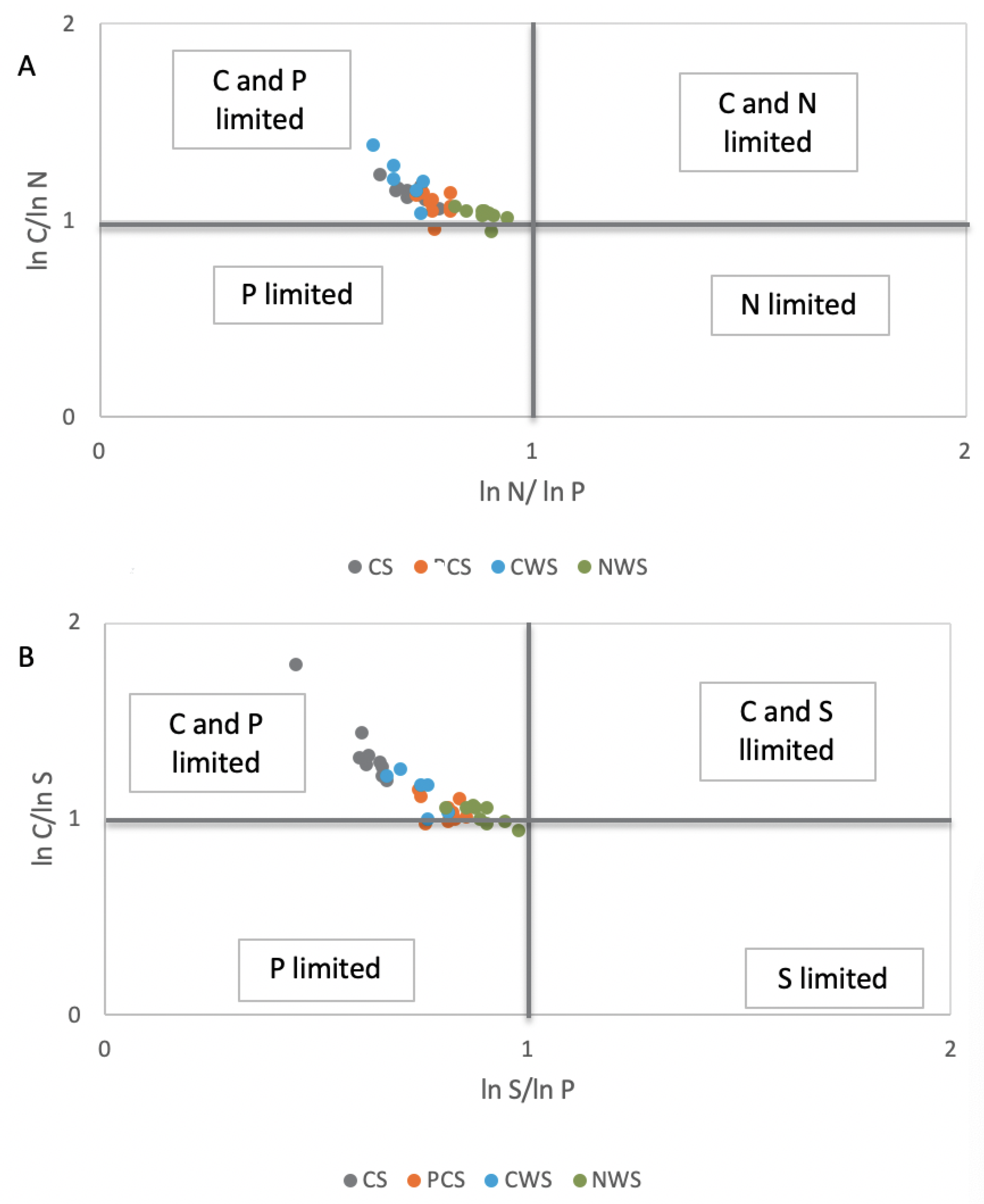
3.2. Effects of Different Strategies of Rewetting on Soil Enzymes Activities
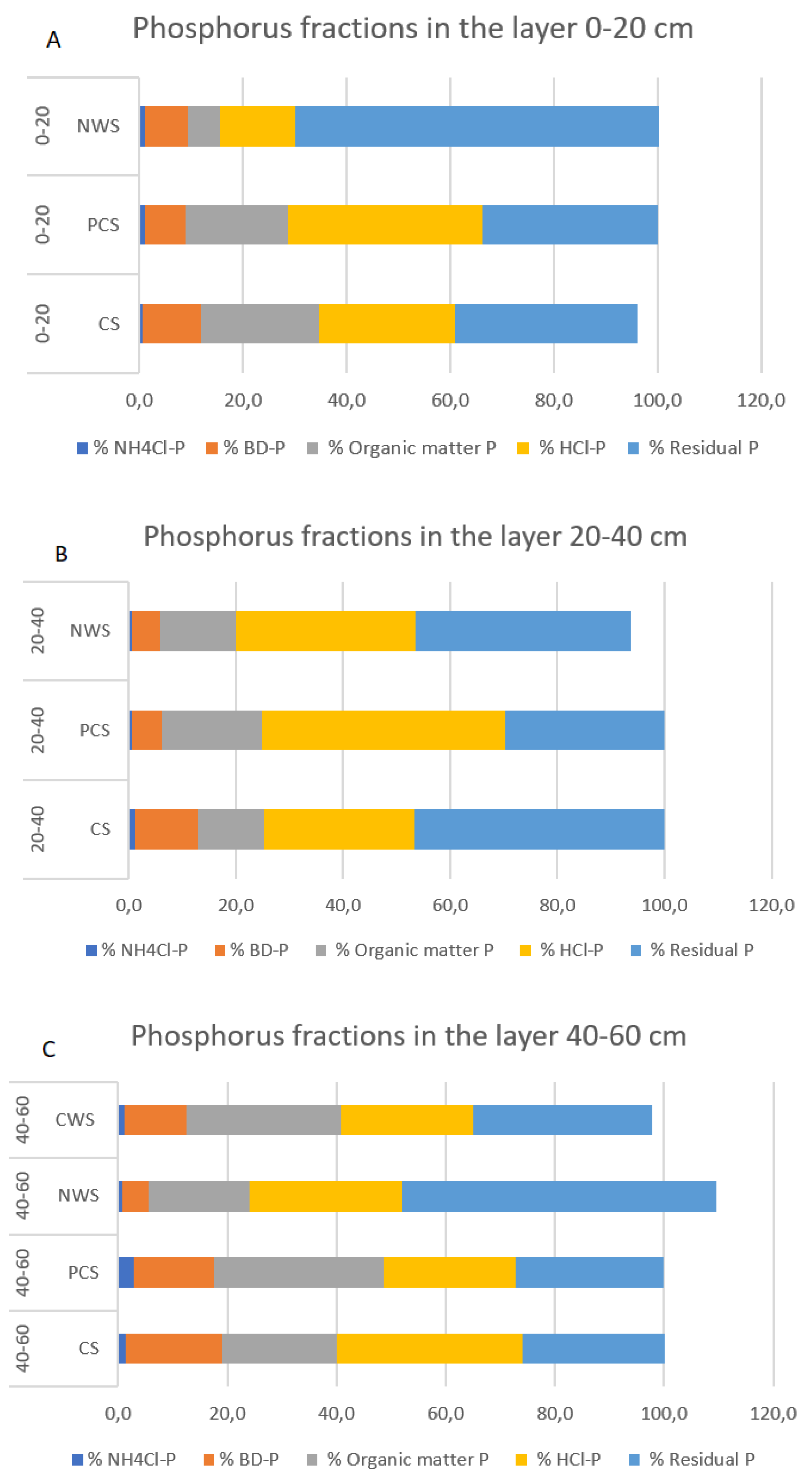
3.3. Effects of Different Strategies of Rewetting on Different Phosphorus Fractions
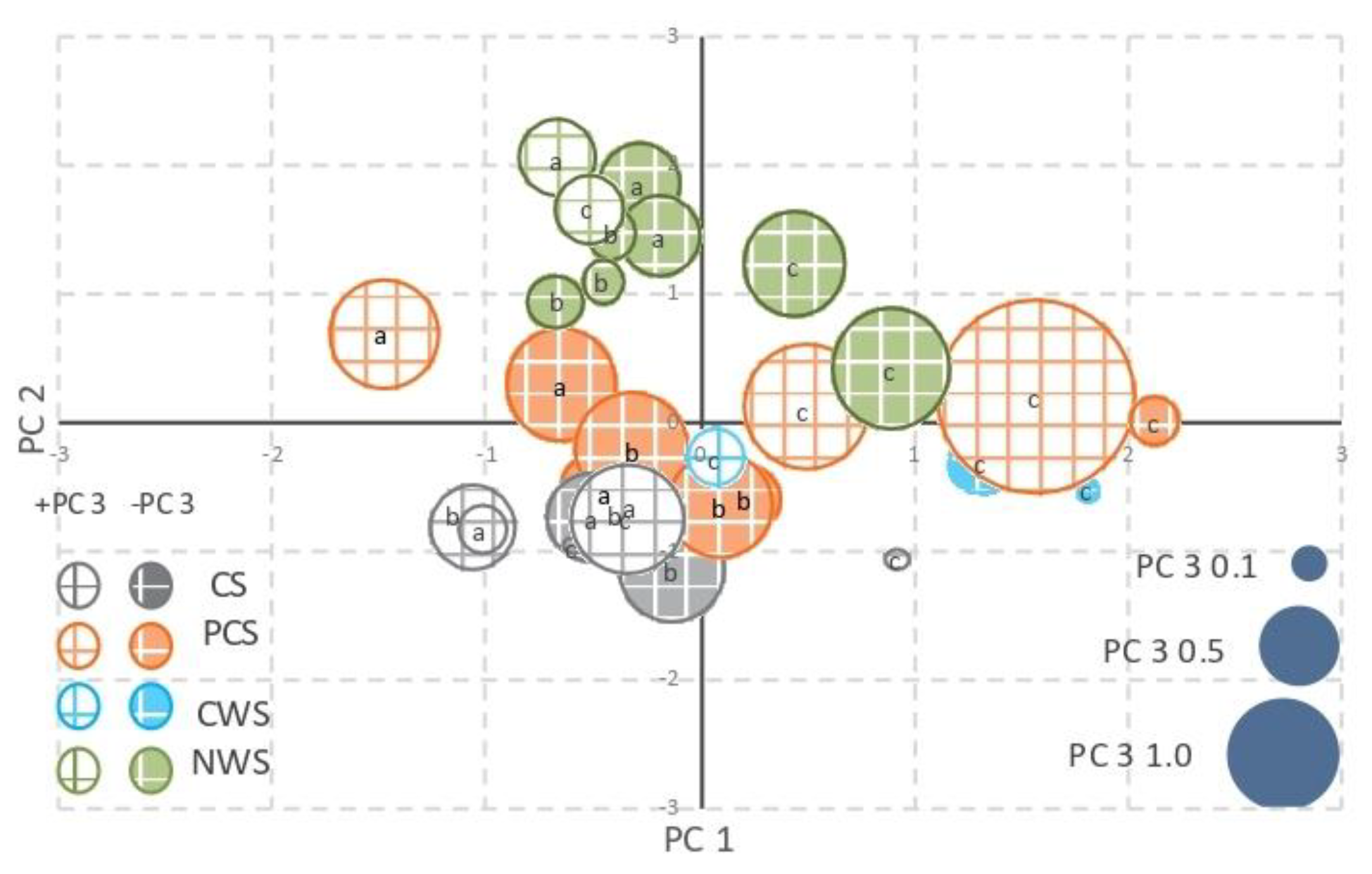
3.4. Principal Component Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Joosten, H. Peatlands, Climate Change Mitigation and Biodiversity Conservation: An Issue Brief on the Importance of Peatlands for Carbon and Biodiversity Conservation and the Role of Drained Peatlands as Greenhouse Gas Emission Hotspots; Nordic Council of Ministers: Copenhagen, Denmark, 2015; Volume 2015727. [Google Scholar]
- Joosten, H.; Clarke, D. Wise Use of Mires and Peatlands Background and Principles Including A Framework for Decision-Making; International Mire Conservation Group and International Peat Society: Saarijarvi, Finland, 2002. [Google Scholar]
- Lamb, D. Patterns of nitrogen mineralization in the forest floor of stands of pinus radiata on different soils. J. Ecol. 1975, 63, 615. [Google Scholar] [CrossRef]
- Damman, A.W.H. Regulation of nitrogen removal and retention in Sphagnum bogs and other peatlands. Oikos 1988, 51, 291. [Google Scholar] [CrossRef]
- Liu, H.; Zak, D.; Rezanezhad, F.; Lennartz, B. Soil degradation determines release of nitrous oxide and dissolved organic carbon from peatlands. Environ. Res. Lett. 2019, 14. [Google Scholar] [CrossRef]
- Nichols, D.S.; Keeney, D.R. Nitrogen and phosphorus release from decaying water milfoil. Hydrobiologia 1973, 42, 509–525. [Google Scholar] [CrossRef]
- Richardson, C.J.; Marshall, P.E. Processes controlling movement, storage, and export of phosphorus in a fen peatland. Ecol. Monogr. 1986, 56, 279–302. [Google Scholar] [CrossRef]
- Zak, D.; Gelbrecht, J. The mobilisation of phosphorus, organic carbon and ammonium in the initial stage of fen rewetting (a case study from NE Germany). Biogeochemistry 2007, 85, 141–151. [Google Scholar] [CrossRef]
- Wichtmann, W.; Wichmann, S. Environmental, social and economic aspects of a sustainable biomass production. J. Sustain. Energy Environ. Spec. Issue. 2011, 77–81. [Google Scholar]
- Tiemeyer, B.; Frings, J.; Kahle, P.; Köhne, S.; Lennartz, B. A comprehensive study of nutrient losses, soil properties and groundwater concentrations in a degraded peatland used as an intensive meadow—Implications for re-wetting. J. Hydrol. 2007, 345, 80–101. [Google Scholar] [CrossRef]
- Zak, D.; Gelbrecht, J.; Steinberg, C.E.W. Phosphorus Retention at the Redox Interface of Peatlands Adjacent to Surface Waters in Northeast Germany. Biogeochemistry 2004, 70, 357–368. [Google Scholar] [CrossRef]
- Budiman, I.; Sari, E.N.; Hadi, E.E.; Siahaan, H.; Januar, R.; Hapsari, R.D. Progress of paludiculture projects in supporting peatland ecosystem restoration in Indonesia. Glob. Ecol. Conserv. 2020, 23, e01084. [Google Scholar] [CrossRef]
- Cougnon, M.; De Frenne, P.; Bommelé, L.; De Cauwer, B.; Verheyen, K.; Reheul, D. The short term agronomic impact of raising canal water levels in grassland areas: A case study in the Belgian polders. Agric. Ecosyst. Environ. 2011, 144, 159–166. [Google Scholar] [CrossRef]
- Nurulita, Y.; Adetutu, E.M.; Gunawan, H.; Zul, D.; Ball, A.S. Restoration of tropical peat soils: The application of soil microbiology for monitoring the success of the restoration process. Agric. Ecosyst. Environ. 2016, 216, 293–303. [Google Scholar] [CrossRef]
- Peacock, M.; Gauci, V.; Baird, A.; Burden, A.; Chapman, P.; Cumming, A.; Evans, J.G.; Grayson, R.; Holden, J.; Kaduk, J.; et al. The full carbon balance of a rewetted cropland fen and a conservation-managed fen. Agric. Ecosyst. Environ. 2019, 269, 1–12. [Google Scholar] [CrossRef]
- Urbanová, Z.; Straková, P.; Kastovska, E. Response of peat biogeochemistry and soil organic matter quality to rewetting in bogs and spruce swamp forests. Eur. J. Soil Boil. 2018, 85, 12–22. [Google Scholar] [CrossRef]
- Allison, S.D. Cheaters, diffusion and nutrients constrain decomposition by microbial enzymes in spatially structured environments. Ecol. Lett. 2005, 8, 626–635. [Google Scholar] [CrossRef]
- Hill, B.H.; Elonen, C.M.; Seifert, L.R.; May, A.A.; Tarquinio, E. Microbial enzyme stoichiometry and nutrient limitation in US streams and rivers. Ecol. Indic. 2012, 18, 540–551. [Google Scholar] [CrossRef]
- Chen, H.; Li, D.; Xiao, K.; Wang, K. Soil microbial processes and resource limitation in karst and non-karst forests. Funct. Ecol. 2018, 32, 1400–1409. [Google Scholar] [CrossRef]
- Hill, B.; Elonen, C.; Jicha, T.; Kolka, R.; Lehto, L.; Sebestyen; Seifert-Monson, L. Ecoenzymatic stoichiometry and microbial processing of organic matter in northern bogs and fens reveals a common P-limitation between peatland types. Biogeochemistry 2014, 120, 203–224. [Google Scholar]
- Li, T.; Bu, Z.; Liu, W.; Zhang, M.; Peng, C.; Zhu, Q.; Shi, S.; Wang, M. Weakening of the ‘enzymatic latch’ mechanism following long-term fertilization in a minerotrophic peatland. Soil Biol. Biochem. 2020, 136, 107528. [Google Scholar] [CrossRef]
- Yan, Z.; Li, Y.; Wu, H.; Zhang, K.; Hao, Y.; Wang, J.; Zhang, X.; Yan, L.; Kang, X. Different responses of soil hydrolases and oxidases to extreme drought in an alpine peatland on the Qinghai-Tibet Plateau, China. Eur. J. Soil Boil. 2020, 99, 103195. [Google Scholar] [CrossRef]
- Giannini, V.; Silvestri, N.; Dragoni, F.; Pistocchi, C.; Sabbatini, T.; Bonari, E. Growth and nutrient uptake of perennial crops in a paludicultural approach in a drained Mediterranean peatland. Ecol. Eng. 2017, 103, 478–487. [Google Scholar] [CrossRef]
- Pistocchi, C.; Silvestri, N.; Rossetto, R.; Sabbatini, T.; Guidi, M.; Baneschi, I.; Bonari, E.; Trevisan, D. A Simple Model to Assess Nitrogen and Phosphorus Contamination in Ungauged Surface Drainage Networks: Application to the Massaciuccoli Lake Catchment, Italy. J. Environ. Qual. 2012, 41, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World Map of the Köppen-Geiger climate classification updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef]
- USDA-SCS Agric. Soil Survey Staff. In Soil Taxonomy: A Basic System of Soil Classification for Making and Interpreting Soil Surveys; U.S. Govt. Print. Office: Washington, DC, USA, 1975; Agric. Handb., Number 436. [Google Scholar]
- IUSS Working Group WRB. World Reference Base for Soil Resources; World. Soil Resources Reports, Number 103; FAO: Rome, Italy, 2006. [Google Scholar]
- Pellegrino, E.; Bosco, S.; Ciccolini, V.; Pistocchi, C.; Sabbatini, T.; Silvestri, N.; Bonari, E. Agricultural abandonment in Mediterranean reclaimed peaty soils: Long-term effects on soil chemical properties, arbuscular mycorrhizas and CO2 flux. Agric. Ecosyst. Environ. 2015, 199, 164–175. [Google Scholar] [CrossRef]
- Giannini, V.; Bertacchi, A.; Bonari, E.; Silvestri, N. Recolonisation by Spontaneous Vegetation of a Rewetted Peatland after Topsoil Removal: A Focus on Biomass Production and Nutrient Uptake. Wetlands 2019, 39, 1079–1087. [Google Scholar] [CrossRef]
- Giannini, V.; Bertacchi, A.; Bonari, E.; Silvestri, N. Rewetting in Mediterranean reclaimed peaty soils and its potential for phyto-treatment use. J. Environ. Manag. 2018, 208, 92–101. [Google Scholar] [CrossRef]
- Silvestri, N.; Giannini, V.; Dragoni, F.; Bonari, E. A multi-adaptive framework for the crop choice in paludicultural cropping systems. Ital. J. Agron. 2016, 11, 69–76. [Google Scholar] [CrossRef]
- Moorhead, D.L.; Sinsabaugh, R.L.; Hill, B.H.; Weintraub, M.N. Vector analysis of ecoenzyme activities reveal constraints on coupled C, N and P dynamics. Soil Boil. Biochem. 2016, 93, 1–7. [Google Scholar] [CrossRef]
- Könönen, M.; Jauhiainen, J.; Laiho, R.; Kusin, K.; Vasander, H. Physical and chemical properties of tropical peat under stabilised land uses. Mires Peat 2015, 16, 1–13. [Google Scholar]
- Murphy, J.; Riley, J. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Zak, D.; Gelbrecht, J.; Wagner, C.; Steinberg, C.E.W. Evaluation of phosphorus mobilization potential in rewetted fens by an improved sequential chemical extraction procedure. Eur. J. Soil Sci. 2008, 59, 1191–1201. [Google Scholar] [CrossRef]
- Ciavatta, C.; Govi, M.; Antisari, L.; Sequi, P. Characterization of humified compounds by extraction and fractionation on solid polyvinylpyrrolidone. J. Chromatogr. A 1990, 509, 141–146. [Google Scholar] [CrossRef]
- Vepsäläinen, M.; Kukkonen, S.; Vestberg, M.; Sirviö, H.; Niemi, R.M. Application of soil enzyme activity test kit in a field experiment. Soil Boil. Biochem. 2001, 33, 1665–1672. [Google Scholar] [CrossRef]
- Marx, M.-C.; Wood, M.; Jarvis, S. A microplate fluorimetric assay for the study of enzyme diversity in soils. Soil Boil. Biochem. 2001, 33, 1633–1640. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Hill, B.H.; Shah, J.J.F. Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature 2009, 462, 795–798. [Google Scholar] [CrossRef]
- Fujita, K.; Miyabara, Y.; Kunito, T. Microbial biomass and ecoenzymatic stoichiometries vary in response to nutrient availability in an arable soil. Eur. J. Soil Boil. 2019, 91, 1–8. [Google Scholar] [CrossRef]
- Inglett, P.; Reddy, K.; Corstanje, R. Anaerobic Soils: Encyclopedia of Soils in the Environment; Elsevier: Amsterdam, The Netherlands, 2005; pp. 72–78. [Google Scholar]
- Harpenslager, S.F.; Elzen, E.V.D.; Kox, M.A.R.; Smolders, A.J.; Ettwig, K.F.; Lamers, L.P. Rewetting former agricultural peatlands: Topsoil removal as a prerequisite to avoid strong nutrient and greenhouse gas emissions. Ecol. Eng. 2015, 84, 159–168. [Google Scholar] [CrossRef]
- Barbagli, A. Analisi delle Interazioni Acqua-Suolo nella Fitodepurazione delle Acque di Drenaggio e nella Ricarica delle Falde Analysis of Water-Soil Interaction in Drainage Water Phyto-Treatment and in Aquifer Recharge Schemes. Ph.D. Thesis, Scuola Superiore Sant’Anna, Pisa, Italy, 2017. [Google Scholar]
- Höll, B.S.; Fiedler, S.; Jungkunst, H.F.; Kalbitz, K.; Freibauer, A.; Drösler, M.; Stahr, K. Characteristics of dissolved organic matter following 20 years of peatland restoration. Sci. Total Environ. 2009, 408, 78–83. [Google Scholar] [CrossRef]
- Chen, H.; Li, D.; Mao, Q.; Xiao, K.; Wang, K. Resource limitation of soil microbes in karst ecosystems. Sci. Total Environ. 2019, 650, 241–248. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, C.; Chen, Z.; Zhang, G.; Chen, L.; Wu, Z. Stoichiometric analyses of soil nutrients and enzymes in a cambisol soil treated with inorganic fertilizers or manures for 26 years. Geoderma 2019, 353, 382–390. [Google Scholar] [CrossRef]
- Sinsabaugh, R.S. Enzymic analysis of microbial pattern and process. Boil. Fertil. Soils 1994, 17, 69–74. [Google Scholar] [CrossRef]
- Bünemann, E.K.; Condron, L.M. Phosphorus and Sulphur Cycling in Terrestrial Ecosystems. In Soil Biology; Springer: Berlin/Heidelberg, Germany, 2007; Volume 10, pp. 65–92. [Google Scholar]
- Pinsonneault, A.; Moore, T.R.; Roulet, N.T.; Lapierre, J. Biodegradability of Vegetation-Derived Dissolved Organic Carbon in a Cool Temperate Ombrotrophic Bog. Ecosystems 2016, 19, 1023–1036. [Google Scholar] [CrossRef]
- Pinsonneault, A.; Moore, T.R.; Roulet, N.T. Effects of long-term fertilization on peat stoichiometry and associated microbial enzyme activity in an ombrotrophic bog. Biogeochemistry 2016, 129, 149–164. [Google Scholar] [CrossRef]
- Brouns, K.; Keuskamp, J.A.; Potkamp, G.; Verhoeven, J.T.; Hefting, M. Peat origin and land use effects on microbial activity, respiration dynamics and exo-enzyme activities in drained peat soils in the Netherlands. Soil Boil. Biochem. 2016, 95, 144–155. [Google Scholar] [CrossRef]
- Romanowicz, K.J.; Kane, E.S.; Potvin, L.R.; Daniels, A.L.; Kolka, R.K.; Lilleskov, E.A. Understanding drivers of peatland extracellular enzyme activity in the PEATcosm experiment: Mixed evidence for enzymic latch hypothesis. Plant Soil 2015, 397, 371–386. [Google Scholar] [CrossRef]
- Saraswati, S.; Parsons, C.T.; Strack, M. Access roads impact enzyme activities in boreal forested peatlands. Sci. Total Environ. 2019, 651, 1405–1415. [Google Scholar] [CrossRef]
- Parsons, C.T.; Rezanezhad, F.; David, W.; David, W.; O’Connell; Van Cappellen, P.; O’Connell, D.W. Sediment phosphorus speciation and mobility under dynamic redox conditions. Biogeosciences 2017, 14, 3585–3602. [Google Scholar] [CrossRef]
- Wang, S.; Zhou, K.; Mori, T.; Mo, J.; Zhang, W. Effects of phosphorus and nitrogen fertilization on soil arylsulfatase activity and sulfur availability of two tropical plantations in southern China. For. Ecol. Manag. 2019, 453, 117613. [Google Scholar] [CrossRef]
- Kunito, T.; Shiroma, T.; Moro, H.; Sumi, H. Annual variation in soil enzyme activity in a paddy field: Soil temperature and nutrient availability are important for controlling enzyme activities. Appl. Environ. Soil Sci. 2018, 2018, 1–7. [Google Scholar] [CrossRef]
- Lu, S.; Han, S.; Du, Y.; Liu, H.; Nie, H.; Luo, X.; Huang, Q.; Chen, W. The shift of sulfate-reducing bacterial communities from the upland to the paddy stage in a rapeseed-rice rotation system, and the effect from the long-term straw returning. Appl. Soil Ecol. 2018, 124, 124–130. [Google Scholar] [CrossRef]
- Ye, R.; Jin, Q.; Bohannan, B.; Keller, J.K.; McAllister, S.A.; Bridgham, S.D. pH controls over anaerobic carbon mineralization, the efficiency of methane production, and methanogenic pathways in peatlands across an ombrotrophic–minerotrophic gradient. Soil Boil. Biochem. 2012, 54, 36–47. [Google Scholar] [CrossRef]
- Wittmann, C.; Kähkönen, M.A.; Ilvesniemi, H.; Kurola, J.; Salkinoja-Salonen, M.S. Areal activities and stratification of hydrolytic enzymes involved in the biochemical cycles of carbon, nitrogen, sulphur and phosphorus in podsolized boreal forest soils. Soil Boil. Biochem. 2004, 36, 425–433. [Google Scholar] [CrossRef]
- Grasset, C.; Rodriguez, C.; Delolme, C.; Marmonier, P.; Bornette, G. Can soil organic carbon fractions be used as functional indicators of wetlands? Wetlands 2017, 37, 1195–1205. [Google Scholar] [CrossRef]
- Freeman, C.; Fenner, N.; Shirsat, A.H. Peatland geoengineering: An alternative approach to terrestrial carbon sequestration. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 2012, 370, 4404–4421. [Google Scholar] [CrossRef] [PubMed]
- Freeman, C.; Ostle, N.; Kang, H.; Ostle, N. An enzymic ’latch’ on a global carbon store. Nature 2001, 409, 149. [Google Scholar] [CrossRef] [PubMed]
- Freeman, C.; Ostle, N.; Fenner, N.; Kang, H. A regulatory role for phenol oxidase during decomposition in peatlands. Soil Boil. Biochem. 2004, 36, 1663–1667. [Google Scholar] [CrossRef]
- Zak, D.; Roth, C.; Unger, V.; Goldhammer, T.; Fenner, N.; Freeman, C.; Jurasinski, G. Unraveling the importance of polyphenols for microbial carbon mineralization in rewetted riparian peatlands. Front. Environ. Sci. 2019, 7, 147. [Google Scholar] [CrossRef]
- Emsens, W.-J.; Aggenbach, C.J.; Smolders, A.J.; Van Diggelen, R. Topsoil removal in degraded rich fens: Can we force an ecosystem reset? Ecol. Eng. 2015, 77, 225–232. [Google Scholar] [CrossRef]
- Zak, D.; Meyer, N.; Cabezas, A.; Gelbrecht, J.; Mauersberger, R.; Tiemeyer, B.; Wagner, C.; McInnes, R. Topsoil removal to minimize internal eutrophication in rewetted peatlands and to protect downstream systems against phosphorus pollution: A case study from NE Germany. Ecol. Eng. 2017, 103, 488–496. [Google Scholar] [CrossRef]
- Allison, S.D.; Weintraub, M.N.; Gartner, T.B.; Waldrop, M.P. Evolutionary-Economic Principles as Regulators of Soil Enzyme Production and Ecosystem Function. In Heavy Metal Contamination of Soils; Springer: Berlin/Heidelberg, Germany, 2010; Volume 22, pp. 229–243. [Google Scholar]

| Parameters | Methods | Instruments |
|---|---|---|
| C, H, N | Gas chromatography after dry combustion at 1150 °C DIN EN 15104 (2010) | LECO Truspec CHN Analyzer (LECO Corporation, Saint Joseph, Michigan, USA) |
| Total P | [34] | Spectrophotometer—UNICAM UV 500 Thermo (Thermo Fisher Scientific, Waltham, Massachusetts, USA) |
| P fractionation | [35] | Spectrophotometer—UNICAM UV 500 Thermo (Thermo Fisher Scientific, Waltham, Massachusetts, USA) |
| Redox potential—ORP | Redox potential was measured in a slurry of soil:water (1:1, w/v) | InLab Redox Pro Argenthal TM (Mettler—Toledo, Columbus, Ohio USA) |
| pH | pH was measured in a soil: water extract (1:5, w/v) | Seven Multi—InLab pH Pro (Mettler—Toledo, Columbus, Ohio USA) |
| Electrical conductivity | Electrical conductivity was measured in a soil: water extract (1:2.5, w/v) | Seven Multi—In Lab EC Pro (Mettler—Toledo, Columbus, Ohio USA) |
| % Humic substances | [36] | LECO RC-412 multiphase carbon (LECO Corporation, St. Joseph, Michigan, USA) |
| Butyrate esterase, BE (EC 3.1.1.1) | [37,38] Substrate: 4-MUB–butyrate | Ultra-Turrax homogenizer, (IKA®-Werke GmbH & Co. KG, Staufen, Germany), Fluorimeter—Infinite F200 pro —plate reader (TECAN Männedorf, Zürich, Switzerland) |
| β-glucosidase, BG (EC 3.2.1.21) | [37,38] Substrate: 4-MUB-βd-glucoside | |
| Acid phosphatase, AP (EC 3.1.3.2) | [37,38] Substrate: 4-MUB-phosphate | |
| Proteases—Leucine aminopeptidase, LAP (EC 3.4.11.1); N-acetyl-b-D-glucosaminidase, NAG (EC 3.2.1.14) | [37,38] Substrate: Leucine-7-amino-4methylcoumarine; 4-MUB-N-acetyl-β-D-glucosaminide | |
| Arylsulphatase, AS (EC 3.1.6.1) | [37,38] Substrate: 4-MUB-sulphate |
| Management | |||||
|---|---|---|---|---|---|
| Layer | CS (a) | PCS (b) | CWS (c) | NWS (d) | |
| pH | 0–20 | 4.26 bD | 4.92 ad | 5.79 Ab | |
| 20–40 | 4.29 D | 5.09 | 5.28 A | ||
| 40–60 | 4.51b Cd | 5.30 a | 5.60 A | 5.31 a | |
| EC dS/m | 0–20 | 0.282 D | 0.609 d | 1.70 Ab | |
| 20–40 | 0.290 | 0.920 | 1.08 | ||
| 40–60 | 0.707 B | 3.17 ACD | 1.97 B | 1.08 B | |
| Redox Potential mV | 0–20 | 337 BD | 110 AD | −56.1 AB | |
| 20–40 | 327 BD | 113 A | -2.4 A | ||
| 40–60 | 313 BCD | 113 A | 30.6A | 9.7 A | |
| Total Carbon % C | 0–20 | 24.8 | 23.0 | 24.9 | |
| 20–40 | 24.5 | 24.8 | 25.4 | ||
| 40–60 | 28.7 BC | 39.4 AD | 37.4 AD | 27.5 BC | |
| Total Nitrogen % N | 0–20 | 1.42 | 1.44 | 1.47 | |
| 20–40 | 1.39 | 1.50 | 1.50 | ||
| 40–60 | 1.66 B | 2.04 AD | 1.90 D | 1.57 BC | |
| Total Phosphorus % P | 0–20 | 0.078 B | 0.122 AD | 0.095 B | |
| 20–40 | 0.079 | 0.090 | 0.094 | ||
| 40–60 | 0.055 | 0.044 D | 0.060 | 0.071 B | |
| Humic C %/TC | 0–20 | 35.2 D | 44.4 D | 59.3 AB | |
| 20–40 | 31.9 D | 37.6 D | 59.5 AB | ||
| 40–60 | 28.1 | 20.2 | 23.5 | 39.7 | |
| C/N mol | 0–20 | 20.4 | 18.6 | 19.8 | |
| 20–40 | 20.5 | 19.2 | 19.7 | ||
| 40–60 | 20.2 C | 22.5 | 23.0 Ad | 20.5 c | |
| C/P mol | 0–20 | 831 | 487 | 675 | |
| 20–40 | 801 | 758 | 695 | ||
| 40–60 | 1626 BD | 2444 ACD | 1665 BD | 1015 ABC | |
| N/P mol | 0–20 | 41.1 | 26.2 | 34.2 | |
| 20–40 | 39.5 | 40.4 | 35.5 | ||
| 40–60 | 81.3b D | 108a CD | 73.3 Bd | 50.7 ABc | |
| Management | |||||
|---|---|---|---|---|---|
| Layer | CS (a) | PCS (b) | CWS (c) | NWS (d) | |
| β-glucosidase mmol MUB kg−1 dw h−1 | 0–20 | 318 B | 627 Ad | 376 b | |
| 20–40 | 321 | 412 | 295 | ||
| 40–60 | 322 | 375 | 466 | 326 | |
| Phosphatase mmol MUB kg−1 dw h−1 | 0–20 | 1407 D | 1560 D | 624 AB | |
| 20–40 | 1469 | 1930 D | 918 B | ||
| 40–60 | 1729 D | 1488 D | 1746 D | 684 ABC | |
| Butirate esterase mmol MUB kg−1 dw h−1 | 0–20 | 1759 D | 1943 D | 865 AB | |
| 20–40 | 1929 D | 2173 D | 828 AB | ||
| 40–60 | 2145 D | 1621 d | 2078 D | 733 AbC | |
| Aryl sulphatase mmol MUB kg−1 dw h−1 | 0–20 | 49.5 BD | 293 A | 318 A | |
| 20-40 | 59.7 BD | 319 A | 250 A | ||
| 40–60 | 84.3 BCD | 317 Ac | 207 Ab | 397 Ac | |
| Protease mmol AMC kg−1 dw h−1 | 0–20 | 82.5 bD | 124 a | 159 A | |
| 20–40 | 58.0 BD | 110 A | 151 A | ||
| 40–60 | 72.1 bd | 114 aC | 68.2 BD | 119 aC | |
| Management | |||||
|---|---|---|---|---|---|
| Layer | CS (a) | PCS (b) | CWS (c) | NWS (d) | |
| % β-glucosidase | 0–20 | 17.1 D | 21.3 | 25.5 A | |
| 20–40 | 16.8 | 14.9 d | 20.2 b | ||
| 40–60 | 14.4 D | 13.6 cD | 19.2 b | 21.8 AB | |
| % Phosphatase | 0–20 | 76.1 BD | 60.6 AD | 43.1 AB | |
| 20–40 | 77.1 D | 69.6 D | 52.8 AB | ||
| 40–60 | 78.6 BCD | 67.0 AD | 68.4 AD | 44.6 ABC | |
| % Arylsulphatase | 0–20 | 2.49 BD | 12.9 A | 21.6 A | |
| 20–40 | 3.07 BD | 11.5 Ad | 16.9 Ab | ||
| 40–60 | 3.83 BcD | 14.0 AcD | 9.27 abD | 25.6 ABC | |
| % Protease | 0–20 | 4.38 D | 5.14 D | 10.8 AB | |
| 20–40 | 2.97 D | 2.97 D | 10.2 AB | ||
| 40–60 | 3.18 D | 3.18 cD | 3.16 bD | 8.06 ABc | |
| Management | |||||
|---|---|---|---|---|---|
| Layer | CS (a) | PCS (b) | CWS (c) | NWS (d) | |
| NH4Cl-P mg P/kg dw | 0–20 | 4.95 B | 13.7 A | 9.30 | |
| 20–40 | 8.72 | 5.53 | 6.11 | ||
| 40–60 | 6.69 | 12.2 | 6.91 | 5.68 | |
| BD-P mg P/kg dw | 0–20 | 86.1 | 94.1 | 74.2 | |
| 20–40 | 89.6 | 52.8 | 47.9 | ||
| 40–60 | 97.7 | 60.6 | 73.5 | 29.3 | |
| NaOH-P (OM-P) mg P/kg dw | 0–20 | 172 d | 236 D | 61.5 aB | |
| 20–40 | 97.4 | 157 | 135 | ||
| 40–60 | 117 | 141 | 179 | 122 | |
| HCl-P mg P/kg dw | 0–20 | 204 | 459 d | 141 b | |
| 20–40 | 221 | 388 | 311 | ||
| 40–60 | 168 | 108 | 155 | 172 | |
| Residual P mg P/kg dw | 0–20 | 281 d | 416 D | 663 aB | |
| 20–40 | 378 | 294 | 381 | ||
| 40–60 | 164 d | 114 D | 181 D | 426 aBC | |
| PC1 | PC2 | PC3 | |
|---|---|---|---|
| pH | 0.580 | 0.570 | −0.039 |
| Electrical conductivity | 0.662 * | 0.238 | 0.502 |
| Overall microbial activity (butirate esterase) | 0.125 | −0.781 * | 0.051 |
| C enzyme (% b-glucosidase activity) | −0.265 | 0.712 * | −0.155 |
| P enzyme (% phosphatase activity) | 0.003 | −0.929 * | 0.202 |
| S enzyme (% arylsulphatase activity) | 0.176 | 0.836 * | −0.184 |
| N enzyme (% protease activity) | −0.108 | 0.832 * | −0.145 |
| Total Phosphorus | −0.513 | 0.223 | −0.480 |
| Redox Potential | −0.322 | −0.628 * | 0.158 |
| Total Nitrogen–Total Carbon | 0.747 * | −0.092 | 0.303 |
| C/N mol | 0.738 * | −0.104 | 0.109 |
| C/P mol–N/P mol | 0.748 * | −0.203 | 0.499 |
| Humic carbon | −0.306 | 0.704 * | −0.011 |
| % NH4Cl-P | 0.206 | 0.023 | 0.872 * |
| % BD-P | 0.044 | −0.240 | 0.800 * |
| % Organic matter P | 0.336 | −0.145 | 0.680 * |
| % Not available P | −0.224 | 0.334 | −0.716 * |
| Explained variance | 3.232 | 4.941 | 3.359 |
| Total proportionality | 0.190 | 0.291 | 0.198 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giannini, V.; Peruzzi, E.; Masciandaro, G.; Doni, S.; Macci, C.; Bonari, E.; Silvestri, N. Comparison among Different Rewetting Strategies of Degraded Agricultural Peaty Soils: Short-Term Effects on Chemical Properties and Ecoenzymatic Activities. Agronomy 2020, 10, 1084. https://doi.org/10.3390/agronomy10081084
Giannini V, Peruzzi E, Masciandaro G, Doni S, Macci C, Bonari E, Silvestri N. Comparison among Different Rewetting Strategies of Degraded Agricultural Peaty Soils: Short-Term Effects on Chemical Properties and Ecoenzymatic Activities. Agronomy. 2020; 10(8):1084. https://doi.org/10.3390/agronomy10081084
Chicago/Turabian StyleGiannini, Vittoria, Eleonora Peruzzi, Grazia Masciandaro, Serena Doni, Cristina Macci, Enrico Bonari, and Nicola Silvestri. 2020. "Comparison among Different Rewetting Strategies of Degraded Agricultural Peaty Soils: Short-Term Effects on Chemical Properties and Ecoenzymatic Activities" Agronomy 10, no. 8: 1084. https://doi.org/10.3390/agronomy10081084
APA StyleGiannini, V., Peruzzi, E., Masciandaro, G., Doni, S., Macci, C., Bonari, E., & Silvestri, N. (2020). Comparison among Different Rewetting Strategies of Degraded Agricultural Peaty Soils: Short-Term Effects on Chemical Properties and Ecoenzymatic Activities. Agronomy, 10(8), 1084. https://doi.org/10.3390/agronomy10081084







