Abstract
Climate change is altering the environment in which plants grow and survive. An increase in worldwide Earth surface temperatures has been already observed, together with an increase in the intensity of other abiotic stress conditions such as water deficit, high salinity, heavy metal intoxication, etc., generating harmful conditions that destabilize agricultural systems. Stress conditions deeply affect physiological, metabolic and morphological traits of plant roots, essential organs for plant survival as they provide physical anchorage to the soil, water and nutrient uptake, mechanisms for stress avoidance, specific signals to the aerial part and to the biome in the soil, etc. However, most of the work performed until now has been mainly focused on aerial organs and tissues. In this review, we summarize the current knowledge about the effects of different abiotic stress conditions on root molecular and physiological responses. First, we revise the methods used to study these responses (omics and phenotyping techniques). Then, we will outline how environmental stress conditions trigger various signals in roots for allowing plant cells to sense and activate the adaptative responses. Later, we discuss on some of the main regulatory mechanisms controlling root adaptation to stress conditions, the interplay between hormonal regulatory pathways and the global changes on gene expression and protein homeostasis. We will present recent advances on how the root system integrates all these signals to generate different physiological responses, including changes in morphology, long distance signaling and root exudation. Finally, we will discuss the new prospects and challenges in this field.
1. Introduction
Abiotic stresses, including drought, low or high temperature, salinity, UV-B, light intensities, flooding, heavy metal toxicity, nutrient deficiency, etc., seriously affect plant growth and yield. According to the Intergovernmental Panel on Climate Change IPCC-2014 (http://www.ipcc.ch/), climate change is modifying intensity, frequency, and spatiotemporal extents of the extreme weather events. This, together with the rapid worldwide increase in human population, being 7.3 billion people nowadays, and expected to reach 9.7 billion by 2050, according to the estimation of the United Nations (reviewed in [1]), makes food supply as one of the major challenges to cope with in the near future.
Although most studies on abiotic stress resistance mechanisms focus on aerial organs, mainly because of the difficulty to study roots in their natural environment, it has been pointed out that aboveground and belowground organs have distinct responses (reviewed in [2,3]), and physiological and molecular mechanisms leading to stress tolerance can be complementary but not identical among tissues and organs [4]. Taking this into consideration, novel strategies for the development of crops with improved tolerance to abiotic stress conditions, based on targeting specific tissues or organs (instead of the entire plant) are emerging (reviewed in [5]). Root is a key organ since it is involved in the uptake of water and nutrients, anchors the plant to the substrate and it is crucial for plant performance and crop productivity [6].
Perception of harmful conditions by different plant organs is the first step in the stress response. Abiotic stresses induce transcriptional changes in roots of many plant species [7]. It has been reported that antioxidant enzymes are upregulated in maize roots under water stress [8]. Under abiotic stress conditions, the synthesis of different metabolites (osmoprotectants, antioxidants, etc.) is induced to cope with the adverse conditions [9]. In addition, integration of environmental stimuli and physiological responses is mediated by an intricate network of plant hormones such as abscisic acid (ABA), jasmonates (JAs), salicylic acid (SA), or ethylene (ET) that modulate stress responses [10]. Auxins have a role in controlling root hair elongation and root branching under abiotic stress and have also been shown to participate in the positive regulation of drought stress tolerance through the arrangement of root architecture [11]. Stress perception, signaling and tolerance have been explored at the whole plant level (reviewed in [12]). However, the information related to the role of roots in these processes is much more limited. This study reviews recent findings in the biochemical, physiological and gene expression changes that take place in roots under different abiotic stress conditions, and the current progress in the recently developed platforms to achieve a global vision of plant stress response, which will be crucial in the future to develop breeding programs in a more targeted way.
2. Tools for the Study of Root Responses to Abiotic Stresses
Abiotic stresses affect roots at different levels and consequently, different tools to study root morphology and the regulatory networks that control root responses, including genomics, transcriptomics, proteomics, and metabolomics have been developed.
2.1. Phenotyping
Characterization of growth patterns is crucial since alteration in the root system architecture (RSA) is a critical adaptive strategy for crops to cope with abiotic stresses. As root phenotyping in the field is an important challenge, many works focus on root traits of plants grown in laboratories or under artificially controlled conditions, in gel medium or hydroponic solution [13,14].
Traditionally, root phenotyping has been achieved with 2D images, obtained from photographs or scans, and processed with informatic programs as WinRHIZO or free alternatives as SmartRoot or IJ-Rhizo macro for ImageJ, that allow the measurement of total and individual root length, root diameter, number of roots and the angles between primary and secondary roots [15]. An interesting tool for 2D phenotyping is the use of rhizotrons, growth chambers with transparent windows that allow continuous image acquisition while plant is growing, mimicking field conditions [16].
Recently, 3D imaging techniques have been developed and they allow phenotyping plant roots at field conditions. Some of these techniques are reviewed in [17] and also illustrated in Figure 1. The information obtained from phenotyping platforms has been used to obtain tolerant lines to abiotic stress, as maize plants resistant to drought [18] or Arabidopsis halotolerant lines [19].
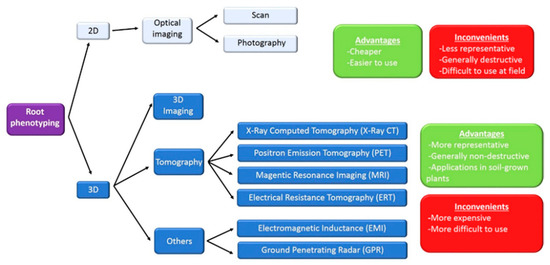
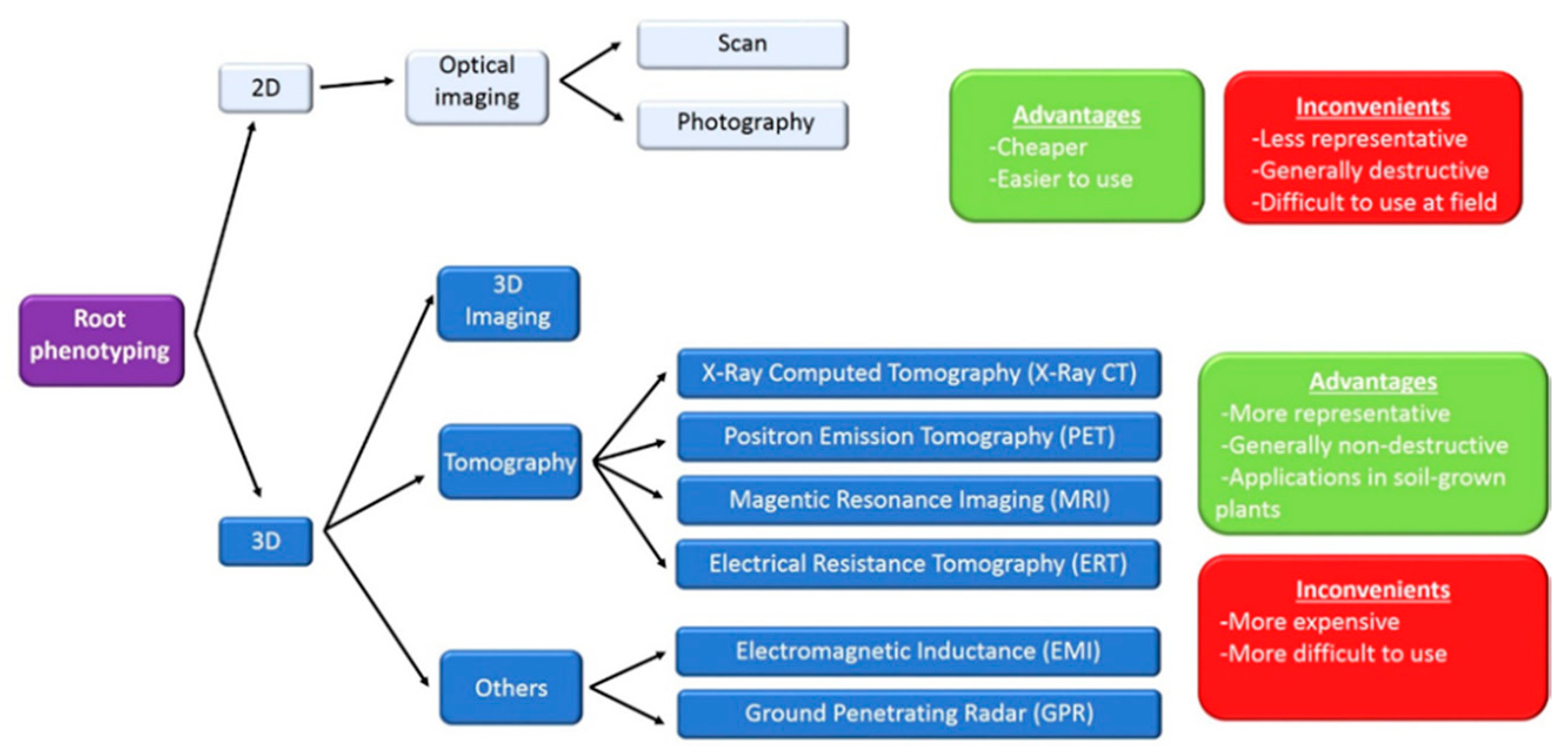
Figure 1.
Technologies for root phenotyping. At the top of the figure, classical non-destructive 2D techniques, based on optical imaging, are indicated. Plant root systems are three-dimensional (3D) structures with many features, difficult to quantify in 2D. Advances for phenotyping root architecture in 3D imaging are nowadays available. Non-destructive 3D root phenotyping includes tomographic techniques [X-ray Computed Tomography (X-ray CT); Positron Emission Tomography (PET); Magnetic Resonance Imaging (MRI) and Electrical Resistance Tomography (ERT)]. More recently, two geophysical approaches, Electromagnetic Inductance (EMI) and Ground Penetrating Radar (GPR), have been developed. Advantages and inconvenients of the different technologies are indicated.
2.2. Genomics and Transcriptomics
The massive genome sequencing of many species has allowed to identify gene families related to abiotic stress tolerance. Although most of the works focus on plant aerial organs, some of them remark the importance of their expression in roots. Most of these gene families are related to phytohormone signaling pathways whereas other are transcription factors or final targets of the signals. Some examples are WRKY (which encode proteins with the conserved domain WRKYGQK) [20], Calcium-Dependent Kinases (CDPKs) [21], BURPs (which encode proteins with the BURP domain) or HSPs [22]. In addition, Quantitative Trait Loci (QTL) related with the resistance to drought [23,24] or soil salinity [25] have been found [26]. Recently, several studies have reported the use of next generation sequencing platforms (NGS) to characterize plant response to abiotic stress conditions in agronomically important crops, considering root organs [27].
MicroRNAs (miRNAs) are single-chain and non-coding fragments of RNA with a length of 21–24 nucleotides, which are involved in the post-transcriptional regulation of gene expression [28]. Different kinds of analyses are used for miRNA determination, including microarray, RNA sequencing, northern blot and RT-PCR [29]. miRNAs have been proposed as key regulators of several abiotic stress-related processes.
The information obtained from these analyses, together with metabolomic studies, facilitates the development of neural networks that reveal specific pathways which are induced or repressed in roots under abiotic stress conditions such as drought [30], salinity [31], or heavy metal toxicity [32].
2.3. Proteomics
Proteomics utilizes two-dimensional (2-D) gel electrophoresis, mass spectrometry (MS), matrix-assisted laser desorption ionization–time of flight (MALDI TOF), western blot, and ELISA techniques in combination with bioinformatics tools to identify proteins and map their interactions in a cellular context. MS-based methods in combination with computational tools are capable of processing hundreds of peptide transitions simultaneously (reviewed in [33]). The effect of abiotic stress conditions in root proteomic profile has been largely studied in several plant species under different adverse situations, including drought [34,35,36]; salt stress [37], high temperatures [38], waterlogging [39,40,41]; toxic metal ions [42,43], and nutrient starvation [44,45]. Under these conditions, profiles of protein families related to cell division and expansion, C and N metabolism, signal transduction and redox balance are commonly altered. In addition, specific situations as heat stress or heavy metal ions induce the accumulation of specific protein families as Heat Shock Proteins (HSPs), or metallothioneins and phytochelatins, respectively [38,42]. Posttranslational modifications could be important for regulating protein activity under stress conditions [46].
2.4. Metabolomics
Metabolomics allows the systematic identification and quantification of low-molecular-weight molecules present in a tissue/organ offering a direct approach to know the interaction of the plant genome with the environment. Although many methodologies are used (spectrophotometry, Fourier transform, infrared spectroscopy or immunoassays), chromatography coupled to mass spectrophotometry is the most extended technique to analyze the metabolome [47]. Matrix-assisted laser desorption/ionization (MALDI–MSI) technique has been used in the last years to study the spatial distribution of secondary metabolites and small molecules in roots under salt stress [46,48].
In maize plants subjected to drought, root metabolism is not as altered as in leaves, indicating also that allocation of metabolites to shoots is reduced in sensitive lines [49]. High variation between organs was detected in the accumulation of sugars, amino acids and polyols in lentil plants under salt stress conditions, indicating distinct adaptation mechanisms [50]. Primary metabolism, including sugars, amino acids and organic acids is also induced in roots of other species such as soybean grown under water stress conditions [51]. This accumulation could contribute to an osmotic adjustment to avoid plant dehydration whereas secondary metabolites concentration, including phenolic compounds, is reduced in roots but increased in shoots, which is related to the high levels of antioxidants needed in leaves [52]. However, there is some controversy since it has also been reported that roots of soybean and tobacco plants highly induce their secondary metabolism under drought [53]. Roots of soybean plants subjected to salt stress accumulate high quantities of sugars, amino acids, fatty acids and organic acids, and the secondary metabolism of antioxidants is increased, revealing the importance of C and N metabolisms and the Krebs cycle in tolerant plants [54]. Sorghum plants grown under low N concentration reduced root content of phenylalanine, a precursor of SA, providing evidence of a reduced plant defense response under low N conditions [55].
2.5. Lipidomics
Lipidomics analyzes membrane lipid composition and has been used to decipher the role of lipids on tolerance to abiotic stress conditions [56]. Electrospray ionization tandem mass spectrometry (ESI-MS/MS) has been traditionally used for lipidomics although other mass spectrometry instruments such as HILIC-ESI-IT-TOF-MS/MS or UPLC-TripleTOF make lipid analyses more efficient and accurate [57]. Although this is a promising approach to study plant stress, its use in roots is limited. Yu et al. [58] reported changes in the levels of oxidized membrane lipids under salt stress conditions in barley. Recent studies have also analyzed the importance of glycosyl inositol phosphorylceramide (GIPC) sphingolipids of the plasma membrane in seedlings of salt-stressed plants by MALDI-MS [59].
Moreover, studies regarding root cell membrane have reported differences in plasma membrane viscosity and fluidity, highly influenced by plasma membrane/tonoplast intrinsic proteins [60]. Staining and microscope evaluation is usually used for these studies [61], as well as the analyses of K+ and H+ fluxes with selective electrodes [62]. Under osmotic and salt stress conditions, root cells exhibit a decrease of plasma membrane fluidity and an increase of its microviscosity [61,63] although at long term, there is an increase of membrane permeability induced by its degradation [62]. The most relevant analytical techniques used in each -omic approach are indicated in Figure 2.
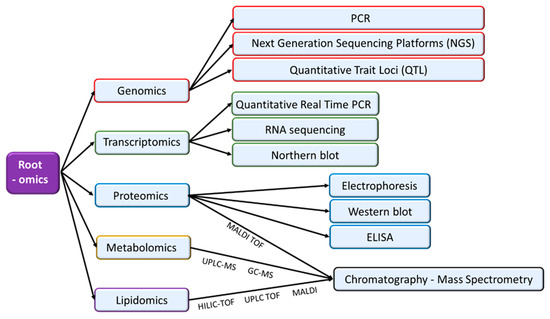
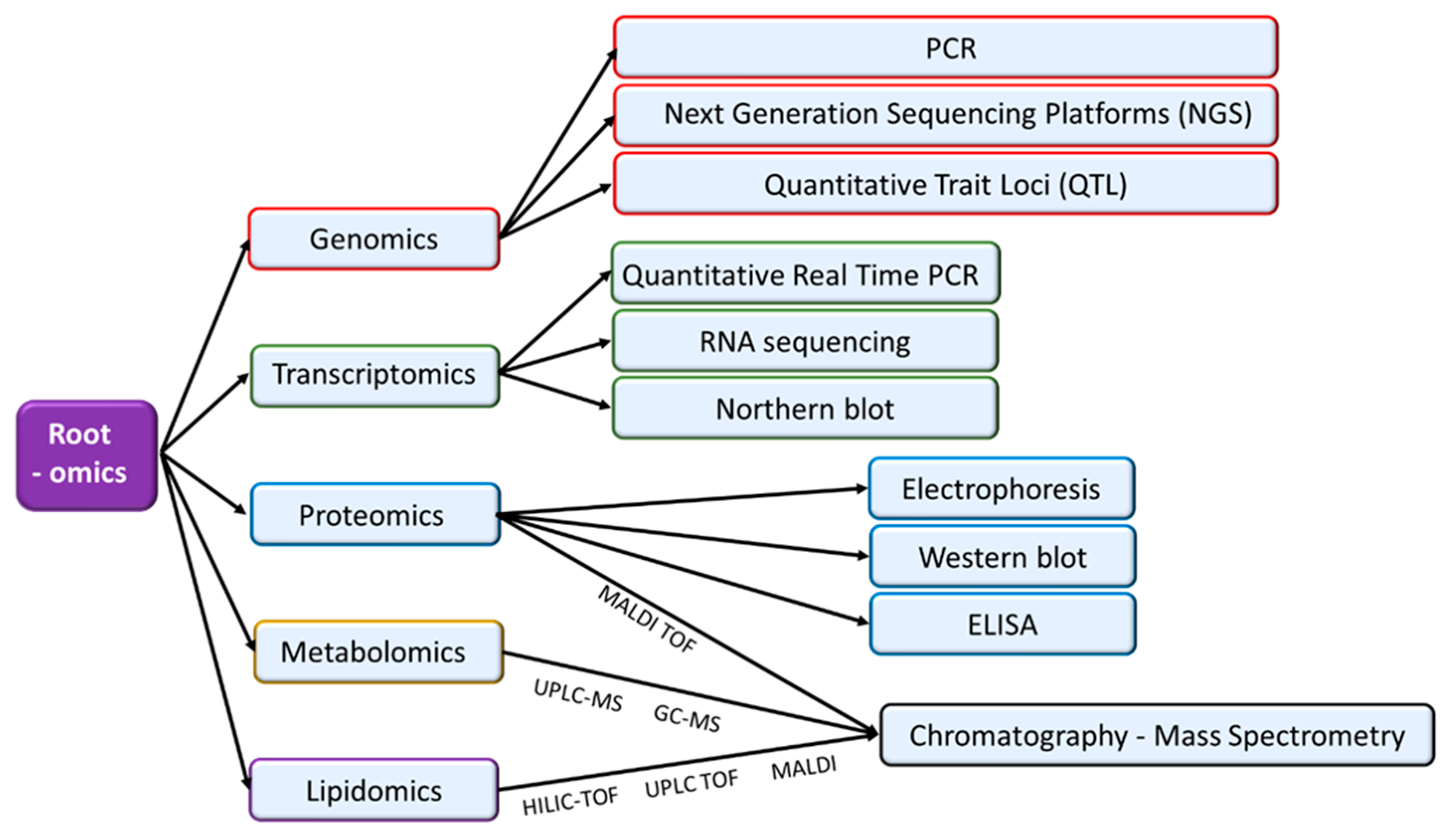
Figure 2.
Omic- approaches, including genomics, transcriptomics, proteomics, metabolomics and lipidomics, make possible active analyses of regulatory networks that control plant organ responses to adverse environmental conditions. In the figure, the most relevant analytical techniques used in each -omic approach are indicated.
3. Abiotic Stress Signaling
Signaling abiotic stress include mechanisms that should be able to link the sensing mechanism and the genetic response. Signal transduction pathways can be divided into four steps: signal perception, generation of second messengers (Ca2+, inositol phosphate and reactive oxygen species -ROS-), activation of secondary sensor proteins (phosphorylation cascade), and activation of transcription factors (TFs) or stress responsive genes (Figure 3).
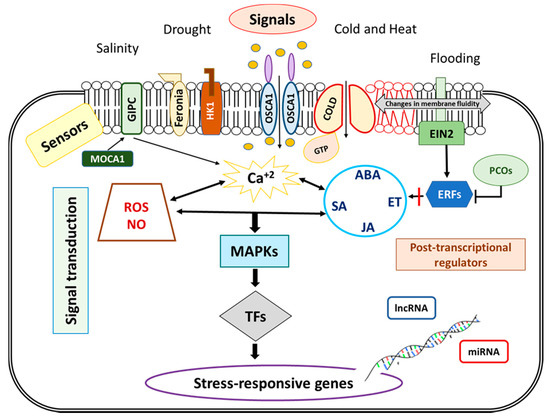
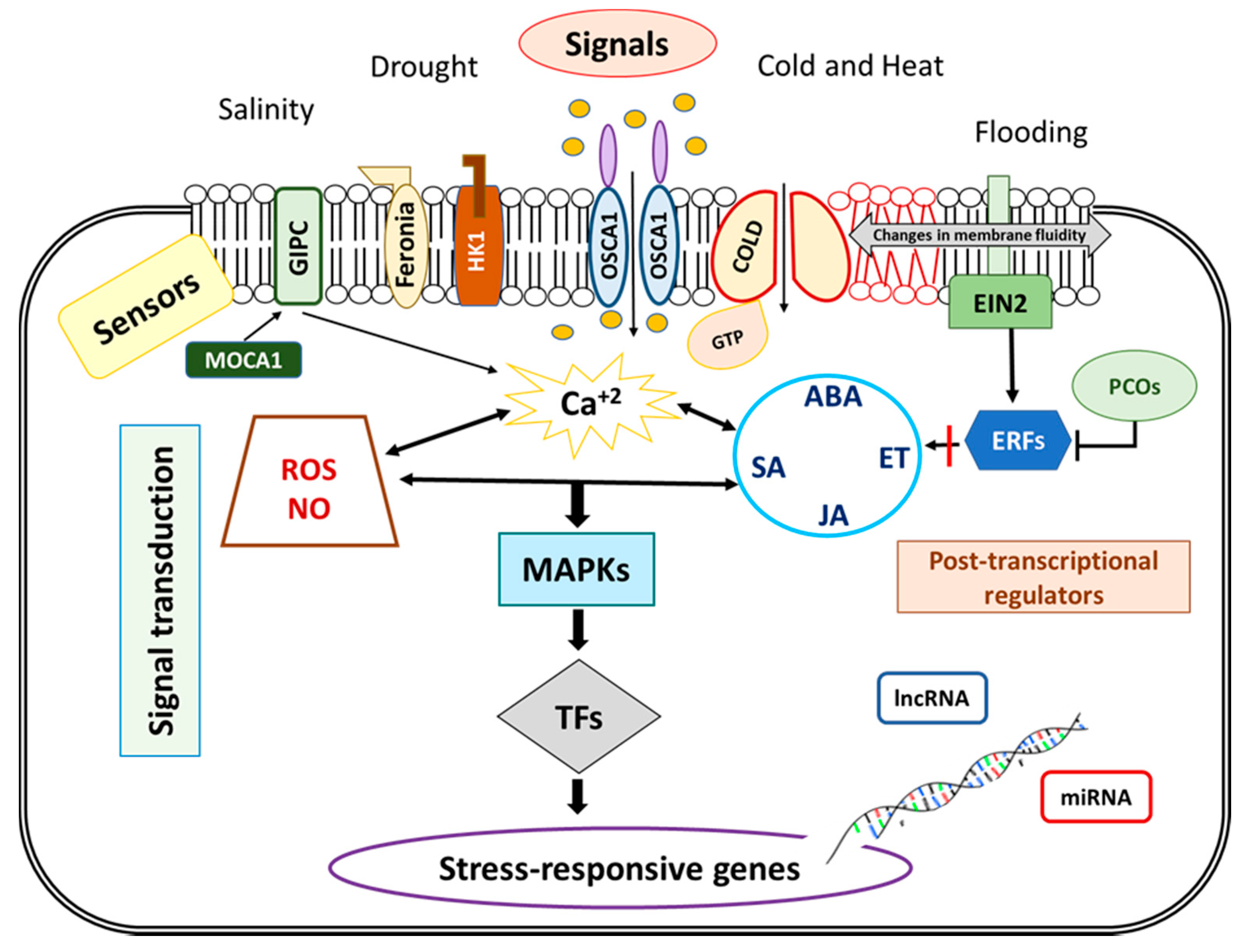
Figure 3.
Abiotic stress signaling in roots. To activate adaptative responses, in a first step environmental signals should be detected by sensors located at plasma membrane (as ion channels and other transporters, membrane-anchored receptor like kinases). Then, second messengers (Ca2+, inositol phosphate and Reactive Oxygen Species, ROS) amplify signals, and, in coordination with phytohormones (Abscisic Acid, ABA; Salicylic Acid, SA; Jasmonic Acid, JA, and Ethylene, ET) activate Mitogen-Activated Protein Kinases (MAPKs) signaling pathways and transcription factors (TFs) that modulate stress responsive genes expression. The expression of these genes is also posttranscriptionally regulated by non-coding RNAs (lncRNA and miRNA).
In roots, environmental stress conditions trigger various signals for allowing cells to sense and activate the adaptative responses. Signaling processes during stress happen at the cellular level and some organs built common signals. However, root is constituted by many types of cells that follow radial (rhizodermis, cortex, endoderm) and longitudinal (cap, meristematic, elongated and differentiated cells) patterns and these cell types could have different sensing for stress.
3.1. Stress Perception
Stress perception is the first step for which external stimuli activate diverse receptors/sensors to trigger signal transduction cascades. Although the absence of clear ligands makes difficult to identify these sensors, they seem crucial for developing stress tolerance [64].
Abiotic stress causes disturbances mainly to plasma membrane fluidity and it can modify cell membrane components to maintain their integrity and optimal function [65]. Thus, integral membrane proteins (channels and other transporters) and membrane-anchored receptor like kinases can be potential sensors [66,67]. Root capacity to sense variation in osmotic potential is basic to achieve an appropriate response to drought and high salinity. By using several mutants, Yuan et al. [68] identified hyperosmolarity-gated calcium-permeable channels (OSCA1) as a potential osmosensor. A recent study describes AtOSCA1 and AtOSCA3 as mechanosensitive ion channels that are activated by a conformational change promoted by membrane tension during osmotic stress [69]. Histidine Kinase 1 (HK1) was identified as a unique osmosensor also in Arabidopsis [70] but recent studies question its role in this process [71]. Under high salinity, the Feronia receptor kinase seems to function as external sensor in cell walls of Arabidopsis roots [72]. In addition, a recent study highlights the importance of GIPC sphingolipids in the plasma membrane by using monocation-induced Ca2+ increases 1 (moca 1) mutants. In Arabidopsis, MOCA1 was identified as a glucuronosyltransferase required for Ca2+ increase produced during salt stress signal transduction, related to GIPCs as monovalent-cation sensors [59].
Changes in the environment temperatures affect the fluidity of phospholipid membranes [73]. In rice, Chilling tolerance divergence (Cold 1) is associated with G-proteins and mediates Ca2+ fluctuation induced by cold, indicating their role in sensing adverse conditions [74]. Phytochrome B has been described as a thermosensor in leaves but it is reasonable to propose a different sensor mechanism in roots, given that phytochrome B requires light to be activated [75,76]. These root sensors could be found among the new elements (DNA/chromatin structures, mRNAs, protein conformation changes) that have been recently proposed as thermosensors in plants [77].
3.2. Phytohormone Signaling
Abiotic stress induces changes on phytohormone levels in roots and these changes can be different from that observed in leaves. Moreover, as observed in Table 1, various phytohormones could participate in the responses to different stress conditions. This accounts for the complexity of the signaling process.

Table 1.
Changes in hormonal content in roots of plants under different abiotic stress conditions.
The importance of ABA in plant tolerance to drought is widely accepted but recent studies also show that a complex network of plant phytohormones performs with it [92,93]. In the past decade, core ABA signaling components were broadly characterized but only a few works addressed organ-specific signaling mechanisms. For instance, a high number of ABA receptors operate in tomato roots [94] whereas ABA treatment increased only the transcripts of ZmPYL1, ZmPYL2 and ZmPYL3 in maize roots [95]. In rice, the expression of OsPYL10 is upregulated by ABA treatment but downregulated under cold stress [90]. Therefore, additional species-specific characterization is required to understand the complexity of core ABA signaling [96].
In citrus and Arabidopsis roots, a transient increase of JA was recorded before ABA accumulation in response to water deficit [78,79]. In tomato roots, an interaction between ABA and SA was observed under water stress, where ABA seems to negatively regulate SA levels [82]. By using a split-root system in Gossypium hirsutum, it was showed that ABA signal could jump from salt-stressed to non-stressed roots. Saline roots could send through shoots some signals that increase 9-cis-epoxycarotenoid dioxygenase (NCED) expression and decrease that of cytochrome P450 family (CYP707A), enhancing ABA levels in non-stressed roots. This phytohormone could induce NADPH-Oxidase C (RBOHC) expression to produce H2O2, which downregulates NCED genes to prevent higher ABA accumulation in the non-salt-stressed roots [83]. In addition, JA biosynthesis rice mutants showed that uptake of Na+ in rice depends on jasmonates [97]. In tomato, JA-deficient mutant defenceless-1 (def1) increased levels of Na+ and reduced N levels in salt-stressed roots indicating a possible role of JA in N homeostasis. An increase in oxidative stress was also observed in this mutant, demonstrating that JA is involved in the protection response of plant during salt stress [86].
Ethylene is a key hormone mediating numerous important biological processes, including responses to abiotic stresses [98]. The membrane protein Ethylene Insensitive 2 (EIN2), a central regulator of ET signaling, controls the transduction of the ET signal from the endoplasmic reticulum membrane to the nucleus in Arabidopsis, and its phosphorylation inhibits ET signaling. After the signal cascade mediated by different EIN proteins, ET signals are delivered to ET Responsive Factors (ERFs), the last downstream components of the ethylene signaling pathway, which lead to the regulation of ET-controlled gene expression [99]. Hypoxia modifies transcript profiles of Group-VII ERFs (reviewed in [100]), which are part of a large family of proteins. Most of the VII-ERFs in plant species are degraded by N-end rules pathway in a process regulated by Plant Cysteine Oxidases (PCOs). These PCOs oxidize the amino terminal cysteine of ERF, in a way dependent on the intracellular oxygen levels, indicating the fate of the protein for degradation by proteasome [101]. Therefore, PCOs could be oxygen sensors under waterlogging stress [102].
Auxin mediates ET signaling to control root growth [103] and a recent study reveals that ET regulates organic acid secretion through auxin signaling in vine roots under alkali stress [62], indicating that ET is involved in responses to different stress conditions.
3.3. Signal Transduction and Stress-Induced Gene Expression
Second messengers as calcium and ROS are key elements to amplify signaling and the ROS/ Ca2+ waves together with phytohormones coordinate pulses of gene expression regulating plant responses to stress (reviewed in [104]).
Calcium plays a critical role in signaling nutrient availability [105] and cold stress response in plants [106]. Several studies have shown how Ca2+ signaling and its crosstalk with nitric oxide (NO), ROS and Mitogen-Activated Protein Kinases (MAPKs) signaling pathways are responsible for establishing cold tolerance in plants (reviewed in [107]). Interestingly, phytohormones act and regulate some of the genes and transcription factors needed for the stress response [27,108]. For example, the regulation of lateral root growth under drought stress seems to directly depend on the interaction of ABA and RBOHI in Arabidopsis roots [109].
Under high salinity conditions, the Salt Overly Sensitive (SOS) signaling pathway is the most important point to control ion homeostasis in roots and has been extensively studied. Briefly, influx of Na+ induces an increase of cytoplasmatic Ca2+ activating different transporters, increasing ROS levels and triggering ABA biosynthesis (reviewed in [110]). In Arabidopsis, AtANNEXIN 4 functions as a Ca2+ permeable transporter generating a calcium signal by interaction with SOS2-SOS3-like calcium-binding protein complex triggering the SOS pathway in response to salt stress [111]. A recent work, establishes that the calcineurin-B like protein 10 (CBL10)-interacting protein kinase 8 (CIPK8) complex regulates the plasma membrane Na+/H+ antiporter SOS1 in Arabidopsis roots, suggesting an additional branch of the SOS signaling pathway [112].
NO acts as a secondary messenger in plants, regulating protein function through a variety of different mechanisms [113]. In NH4+ treated rice plants an early NO burst in response to water stress, that promote the activities of antioxidant enzymes, has been described [114]. In addition, NO seems to be important in plant responses to heavy metal intoxication. For instance, Cd treatment in Arabidopsis induced an increase in NO levels, inhibiting auxin transport and root growth [115]. In addition, a recent work in cucumber roots determined that Cd treatment increases hydrogen sulphide (H2S) and H2O2 levels although their function under vascular H+-ATPase activity is opposite, indicating a different role during Cd stress for both signaling molecules [116].
Cold stress signaling is mostly regulated by the expression of Cold Responsive (COR) genes although the transcriptional cascade by temperature stress included other two components: C-repeat Binding Factors (CBF) and Inducer of CBF Expression (ICE) (reviewed in [117]). In wheat, ICE/CBF and COR genes were characterized and analyzed, showing that CBF genes are upregulated during different developmental stages in roots [118]. In Hevea brasiliensis, levels of HbCBF1 and HbCBF2 transcripts increased quickly after cold stress and JA treatment enhanced cold tolerance, indicating a possible role of this hormone in temperature signaling [119]. On the other hand, heat stress signaling is principally modulated by ROS and NO regulatory systems together with Heat Shock transcription Factors (HSFs), ABA and SA (reviewed in [120]) but more studies are necessary to explain hormone interaction in root responses to high temperature stress.
Flooding signal transduction is mainly modulated by VII-ERFs. In Arabidopsis, five groups have been described: Related to APETALA 2 12 (RAP2.12), RAP2.2, RAP2.3, hypoxia responsive 1 (HRE1) and HRE2 (reviewed in [102]). The VII ERFs are regulated by continuous proteasomal degradation in normoxia [121]. Hypoxia-Response Attenuator1 (HRA1) function under low O2 levels has also been analyzed [122]. The observed repression of RAP2.12 by a fast induction of HRA1 in young tissue could be related to the prevention of excessive expression of anaerobic genes. In addition, the study of universal stress protein 1 (hru 1.1) mutant in Arabidopsis determine that HRU1 interactions with other proteins are important for the regulation of ROS production under anoxia, probably through an interaction with RBOHD [123]. As a result, the coordination network of HRU1 and HRA1 proteins is fundamental for the flooding response. As in other stresses, Ca2+ is a key signaling molecule in Arabidopsis, maize, rice, and wheat under hypoxic conditions (reviewed in [102]). A recent study has revealed that ET could prevent VII-ERF proteolysis by increased production of the NO-scavenger Phytoglobin 1, preparing the plant for the response to hypoxia conditions, although the induction of the core hypoxia genes and second messengers as ROS or Ca2+ is necessary to induce a response [124].
MAPKs have an important role in the transduction of hormone signals and abiotic stresses but the relation between ROS and MAPKs activation is confusing. In tobacco, overexpression of the Populus trichocarpa MAPKK4 enhanced the activity of antioxidant enzymes, improving tolerance to salt stress [125]. A recent work in maize roots described that Cd treatment induced a rapid and transient ROS production following ZmMAPK6-1 and ZmMAPK3-1 activation, indicating that ROS accumulation may activate MAPKs cascade [126]. A comparative study between Arabidopsis and Brassica juncea under Ni treatment showed a different redox response, suggesting that increased Ni tolerance of Brassica juncea may be linked to reduced redox signaling [127].
Transcription factors are key components of signal transduction [128]. Under abiotic stress, different TFs, such as AP2/ERF and WRKY groups, mediate processes involved in tolerance to high salinity, cold and drought (reviewed in [129]). In citrus, Vives-Peris et al. [20] identified 50 putative WRKY TFs with particular expression patterns under different abiotic stress situations. In grapes, VvWRKY30 was shown to have a positive role in stress signaling [130]. Knockout plants of the NAC transcription factor (SlTAF) showed an increased sensitivity to salt stress, indicating a role of this NAC in signal transduction of salinity stress [131]. Overexpressing ABA-responsive element binding factors of sweet potato (IbABF4) in transgenic Arabidopsis seedlings enhanced salt and drought tolerance [132]. A novel stress responsive bZIP transcription factor (OsbZIP62) that seems to be regulated by ABA has been described [133]. In common bean, several PvDREB genes that increase their transcript levels after different abiotic stresses have been identified. PvDREB1F and PvDREB5A responded to salinity, cold and dehydration whereas PvDREB2A and PvDREB6B were only induced by cold and dehydration [134]. In rice, the overexpression of histone gene binding protein-1b (OsHBP1b) reduced ROS levels and modulated stress-related transcripts, increasing plant tolerance to multiple abiotic stresses [135]. Additional work is needed to elucidate the specific role of many TFs in roots.
Other genes codify proteins to remove harmful compounds (as antioxidant enzymes) or to protect the cell (as chaperones), whereas other transcripts are involved in coordinating a specific response. Under water stress, a comparative study between roots of two chickpea genotypes with different tolerance showed a remarkable induction of ABA-dependent (ABI5) and ABA-independent (DREB1A and DREB1C) genes in the tolerant genotype, as well as increased expression of many antioxidant enzymes and genes related with JA and ABA biosynthesis, indicating an adaptative response [136]. In wheat, water supply limitation induces the expression of lateral root density gene that decreases gibberellin levels by activating their catabolism, reducing lateral root growth, and improving plant response to drought [137]. In roots of soybean, some Calmodulin Binding Transcription Activator (GmCAMTAs) are important during early response to water stress and the overexpression of GmCAMTA12 in roots of Arabidopsis enhance drought survival [138]. Concerning to phytohormones, LeNCED1 overexpression, a key gene in ABA biosynthesis, improved salinity response [139] and the knockout of ABA 8’hydroxylase (OsABA8ox2), involved in ABA catabolism, enhanced drought tolerance [140]. In soybean, Salt induced1 (GmSIN1) overexpression induced GmNCED3 and GmRbhoB, improving salt tolerance, suggesting GmSIN1 gene could work as a modulator between ABA and ROS signaling [141]. A comparative analysis of roots and leaves of Thellungiella under cold stress has allowed to identify cold responsive genes that seem to be closely related to environmental adaptations [142]. In rice roots, common genes in response to Cd and As stress related with redox control, glutathione metabolism and transport activity have been identified [143].
Long non-coding RNAs (lncRNA) affect the expression of other genes [144]. In Arabidopsis, a nucleus-localized lncRNA DRIR is a positive regulator, enhancing plant responses to drought and salt stress [145], and the root-specific AtR8 increases accumulation under hypoxic conditions [146]. In rice, one hundred forty four lncRNA affect root development at early stage in response to Cd stress [147], and recently it has been reported that lncRNAs are involved in the regulation of key metabolic pathways in response to water stress in maize root tips [148].
miRNA are post-transcriptional regulators, essentially inhibiting gene expression [149]. miRNA analyses have been performed in roots of plants under drought or high salinity [150,151]. In salt-tolerant rice cultivars, miRNAs associated with maintenance of cellular homeostasis and development of root during salt stress have been identified [152]. The overexpression of eight miRNAs in Arabidopsis roots subjected to low oxygen conditions have been also reported [153]. Under hypoxic stress, miRNA72a was downregulated in maize roots [154] whereas in lotus it was upregulated [155]. Therefore, its expression was correlated to the species tolerance to waterloging (much higher in Lotus). Specific miRNAs were identified in maize under heat stress [149]. Gao et al. [156] provided new insights for the functional characterization of miRNAs, showing that Zma-miR393b and Zma-167f are key in auxin signaling under Cd+2 treatment in maize roots. A study in an apple rootstock evidenced that adventitious root formation is regulated by miRNAs [157].
4. Physiological Changes
Changes at molecular level induced by stressful situations lead to physiological responses such as the modification of root architecture (Figure 4) and root exudation pattern.
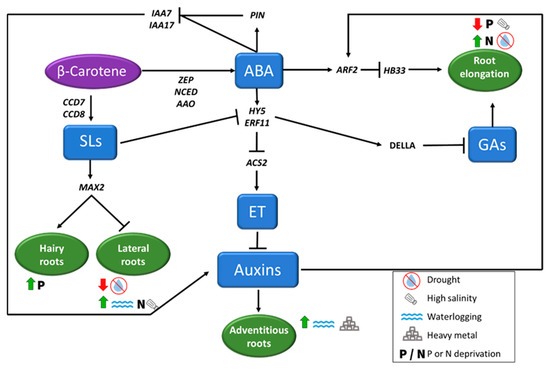
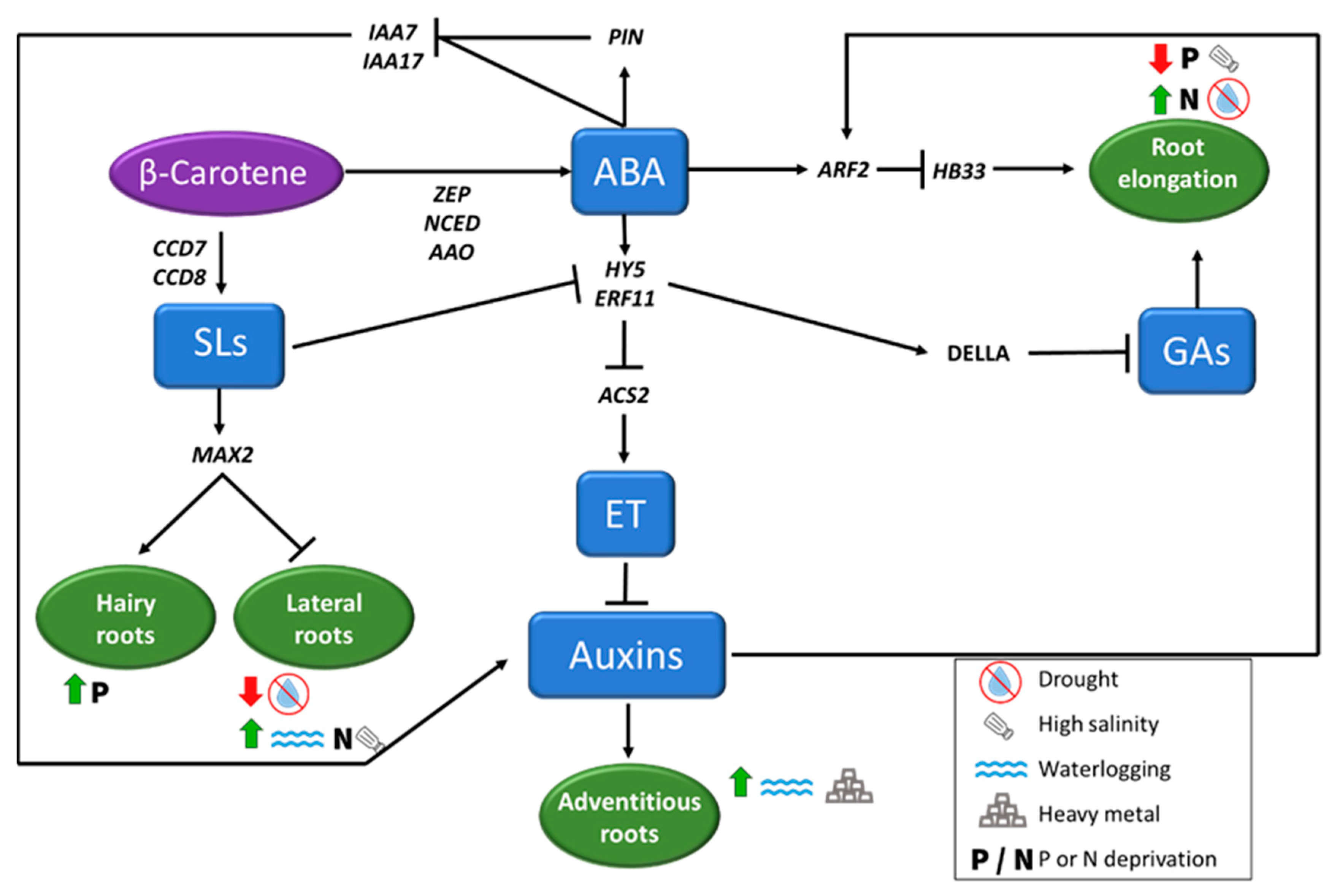
Figure 4.
Root architecture (considering root elongation, and hairy, lateral, and adventitious roots formation) under abiotic stress conditions is regulated by phytohormones, that induce or repress the process depending on the adverse condition. Abscisic Acid (ABA) plays a key role in regulating root cell elongation in different ways, repressing Gibberellins (GAs) through an increase of DELLA proteins concentration or inhibiting auxins biosynthesis. Auxins and Strigolactones (SLs), also play important role in root architecture, inducing or repressing the formation of new roots depending on the adverse environmental situation, as indicated in the figure.
4.1. Root Architecture
Plants are able to sense soil physicochemical parameters and consequently adjust their development in order to adapt to environmental adverse situations [158], adjusting their genetic program to post-embryonic root development, following different strategies depending on the applied stress [159]. Under water stress, the development of lateral roots is inhibited, but not its initiation, and the development of primary roots increased depending on hydrotropisms, being this adaptation highly modulated by ABA [159]. Under high salinity conditions, it is essential to keep low contents of cytosolic Na+ and Cl− ions to ensure plant adaptation and survival, having an important role the compartmentalization of these ions and their transport through membranes [160]. Under waterlogging conditions, the formation of lateral roots and aerenchyma in the root cortex to increase the internal concentration of O2 has been widely reported in species such as rice or maize, which is induced by ET and also regulated by ROS [85].
When plants face nutrient starvation, root morphology is also affected, and its area usually increases. However, specific effects depend on the element supplied in lower quantities, as the root response is focused on the assimilation of the specific nutrient [161]. One of the most studied deficiencies is P, which decreases the growth of the primary roots, while induces the expansion of secondary roots, hairy root growth and the formation of cluster roots, due to variations in endogenous levels of sugars and phytohormones [162]. New studies with 3D scanning under low N concentrations reveal the enhancement of the number of cells in root meristematic zone, an increase in cell elongation [163], and an increased growth of lateral roots, being this highly influenced by strigolactones (SLs) [161].
Most of the changes in root architecture are regulated by auxins and SLs, inducing or repressing the formation of new roots depending on the adverse environmental situation [164]. Both groups of plant hormones are closely related, being PIN, MAX2, and SHY2 some of the genes involved in this crosstalk. The role of ABA in root architecture is clearly important under abiotic stress conditions but with a changing role depending on the endogenous content, regulating root length, and lateral root development [165]. Among the mechanisms of root cell elongation inhibition, ABA is involved in different interactions, including the repression of ACS2 expression through HY5 and ERF11 [8]; the increase in the concentration of DELLA proteins (gibberellin repressors, [166]); or the inhibition of auxin biosynthesis through inhibiting IAA7 and IAA17 and inducing the expression of PIN gene family (Figure 4, [167]). In contrast, ABA is known to be an inducer of ARF2 expression that inhibits root cell elongation through HB33 inhibition [168]. Under osmotic stress, ABA is also involved in root xylem differentiation to protoxylem and metaxylem and inhibition of lateral root formation [169].
4.2. Root Exudation Pattern
Abiotic stress conditions can modify root exudation patterns, through mechanisms like direct diffusion through root membranes, the transport through ionic channels, vesicles transport, or membrane transporters as ABC or MATE, depending on the chemical properties of the exuded metabolites [170]. Among genes involved in this process, PDR2, PDR6, MRP2, PGP4-1 and ABCG30 are the most studied in the ABC family whereas in MATE family, FRD3, among others, is responsible for citrate root exudation in response to Al stress as a mechanism to chelate the toxic ion [171].
The exudation rate generally increases under abiotic stress conditions as occurs with primary metabolites as amino acids and sugars, which are released to the rhizosphere when plants are subjected to drought [172], high salinity [173] or Pb toxicity [174]. Compounds from the secondary metabolism are also exuded in response to drought [172], salt stress [175], heat stress [176], Al toxicity [177] and flooding [178]. Nutrient deficiencies also affect exudation rates of primary metabolites. Carvalhais et al. [179] concluded that while N starvation does not affect exudation at large grade, K deficiency reduced carbohydrate exudation and P starvation had the opposite effect.
However, it has been reported that root exudate composition may vary depending on the stress duration, severity, and plant tolerance. Therefore, under short-term drought, relative C exudation increased but at the long-term, exudation was highly variable and even decreased [180]. In addition, some metabolites as proline are usually exuded under drought conditions [181] and can be used for the detection of plant stress [89,181].
All these metabolites can also lead to microbiota recruitment, stablishing mutualistic relationships between plants and mycorrhizal fungi or plant growth promoting rhizobacteria [170]. This microbiota can induce a large variety of benefits for the plants through different mechanisms, including biofilm formation, that favors humidity retention around roots and protects the plant; production of phytohormones; fixation of atmospheric N; soil nutrient solubilization due to the release of organic acids; siderophore production; etc. [182].
An important aspect which is beyond the scope of this review is communication between roots and aerial organs [183]. Role of hormones and RNA molecules as signals among aerial and underground organs under abiotic stress conditions has been extensively studied [183,184,185,186,187,188].
5. Conclusions and Perspectives
Plants respond to environmental adverse conditions through specific pathways including stress sensing, signal transduction, the activation of several stress-responsive genes and regulating specific metabolite synthesis. Roots play a key role as the site of unique metabolic activities and, in many cases, as major contributors to secondary metabolites production in the whole plant. However, the underground location of this organ has hindered its study for decades. Consequently, the root phenotypes obtained from hydroponic and gel/agar systems do not really reflect the exact growth and development in soil. Recently, new non-destructive phenotyping approaches to study the root system architecture in natural soils and in complex environments make possible more reliable measurements of root traits.
In the past years, -omic technologies have provided relevant information on the genes and biochemical pathways that control plant responses to abiotic stresses, first in model plants, and more recently in agronomically important crops. The integration of data obtained from transcriptomic, proteomic, metabolic, phenotypic and physiological studies will provide relevant information for a better understanding of complex molecular networks underlying the mechanism of abiotic tolerance in plants Although the combination of all these approaches will help in the deciphering of stress resistance mechanisms, this will generate a large amount of data whose handling, interpretation and analysis will be a major challenge in the near future.
Under adverse conditions, the induction of myriad proteins occurs. Identifying these elements, that could be target for future breeding programs, including transformation methods and CRISPR/Cas9 gene editing techniques would lead to plants more resistant to the increasingly severe abiotic stress conditions to which they are subjected.
Author Contributions
V.V.-P., M.F.L.-C. and R.M.P.-C. draw figures, all authors have contributed to the conception of the review, to search for bibliography and to write the manuscript. A.G.-C. coordinated the work and revised the last version. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Universitat Jaume I, grant number (UJI-B2019-11) and Generalitat Valenciana, grant number (AICO/2019/150).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Yang, W.; Feng, H.; Zhang, X.; Zhang, J.; Doonan, J.H.; Batchelor, W.D.; Xiong, L.; Yan, J. Crop phenomics and high-throughput phenotyping: Past decades, current challenges, and future perspectives. Mol. Plant 2020, 13, 187–214. [Google Scholar] [CrossRef] [PubMed]
- Arbona, V.; Manzi, M.; Zandalinas, S.I.; Vives-Peris, V.; Pérez-Clemente, R.M.; Gómez-Cadenas, A. Physiological, metabolic, and molecular responses of plants to abiotic stress. In Stress Signaling in Plants: Genomics and Proteomics Perspective; Sarwat, M., Ahmad, A., Abdin, M., Ibrahim, M., Eds.; Springer: Cham, Switzerland, 2017; Volume 2, pp. 1–35. [Google Scholar]
- Ambroise, V.; Legay, S.; Guerriero, G.; Hausman, J.-F.; Cuypers, A.; Sergeant, K. The roots of plant frost hardiness and tolerance. Plant Cell Physiol. 2020, 61, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Minocha, R.; Majumdar, R.; Minocha, S.C. Polyamines and abiotic stress in plants: A complex relationship. Front. Plant Sci. 2014, 5, 175. [Google Scholar] [CrossRef] [PubMed]
- Seo, D.H.; Seomun, S.; Choi, Y.D.; Jang, G. Root development and stress tolerance in rice: The key to improving stress tolerance without yield penalties. Int. J. Mol. Sci. 2020, 21, 1807. [Google Scholar] [CrossRef] [PubMed]
- Grossman, J.D.; Rice, K.J. Evolution of root plasticity responses to variation in soil nutrient distribution and concentration. Evol. Appl. 2012, 5, 850–857. [Google Scholar] [CrossRef]
- Yoo, Y.-H.; Nalini Chandran, A.K.; Park, J.-C.; Gho, Y.-S.; Lee, S.-W.; An, G.; Jung, K.-H. OsPhyB-mediating novel regulatory pathway for drought tolerance in rice root identified by a global RNA-Seq transcriptome analysis of rice genes in response to water deficiencies. Front. Plant Sci. 2017, 8, 580. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, L.; Yu, Y.; Quan, R.; Zhang, Z.; Zhang, H.; Huang, R. The ethylene response factor AtERF11 that is transcriptionally modulated by the bZIP transcription factor HY5 is a crucial repressor for ethylene biosynthesis in Arabidopsis. Plant J. 2011, 68, 88–99. [Google Scholar] [CrossRef]
- Singh, M.; Kumar, J.; Singh, S.; Singh, V.P.; Prasad, S.M. Roles of osmoprotectants in improving salinity and drought tolerance in plants: A review. Rev. Envrion. Sci. Biotechnol. 2015, 14, 407–426. [Google Scholar] [CrossRef]
- Arif, Y.; Sami, F.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salicylic acid in relation to other phytohormones in plant: A study towards physiology and signal transduction under challenging environment. Environ. Exp. Bot. 2020, 175, 104040. [Google Scholar] [CrossRef]
- Li, X.; Chen, R.; Chu, Y.; Huang, J.; Jin, L.; Wang, G.; Huang, J. Overexpression of RCc3 improves root system architecture and enhances salt tolerance in rice. Plant Physiol. Biochem. 2018, 130, 566–576. [Google Scholar] [CrossRef]
- Meraj, T.A.; Fu, J.; Raza, M.A.; Zhu, C.; Shen, Q.; Xu, D.; Wang, Q. Transcriptional factors regulate plant stress responses through mediating secondary metabolism. Genes 2020, 11, 346. [Google Scholar] [CrossRef] [PubMed]
- Qiao, S.; Fang, Y.; Wu, A.; Xu, B.; Zhang, S.; Deng, X.; Djalovic, I.; Siddique, K.H.M.; Chen, Y. Dissecting root trait variability in maize genotypes using the semi-hydroponic phenotyping platform. Plant Soil 2019, 439, 75–90. [Google Scholar] [CrossRef]
- Yoshino, K.; Numajiri, Y.; Teramoto, S.; Kawachi, N.; Tanabata, T.; Tanaka, T.; Hayashi, T.; Kawakatsu, T.; Uga, Y. Towards a deeper integrated multi-omics approach in the root system to develop climate-resilient rice. Mol. Breed. 2019, 39, 165. [Google Scholar] [CrossRef]
- Pierret, A.; Gonkhamdee, S.; Jourdan, C.; Maeght, J.L. IJ_Rhizo: An open-source software to measure scanned images of root samples. Plant Soil 2013, 373, 531–539. [Google Scholar] [CrossRef]
- Schnepf, A.; Leitner, D.; Landl, M.; Lobet, G.; Mai, T.H.; Morandage, S.; Sheng, C.; Zörner, M.; Vanderborght, J.; Vereecken, H. CRootBox: A structural-functional modelling framework for root systems. Ann. Bot. 2018, 121, 1033–1053. [Google Scholar] [CrossRef]
- Atkinson, J.A.; Pound, M.P.; Bennett, M.J.; Wells, D.M. Uncovering the hidden half of plants using new advances in root phenotyping. Curr. Opin. Biotechnol. 2019, 55, 1–8. [Google Scholar] [CrossRef]
- Guo, J.; Li, C.; Zhang, X.; Li, Y.; Zhang, D.; Shi, Y.; Song, Y.; Li, Y.; Yang, D.; Wang, T. Transcriptome and GWAS analyses reveal candidate gene for seminal root length of maize seedlings under drought stress. Plant Sci. 2020, 292, 110380. [Google Scholar] [CrossRef]
- Deolu-Ajayi, A.O.; Meyer, A.J.; Haring, M.A.; Julkowska, M.M.; Testerink, C. Genetic loci associated with early salt stress responses of roots. Iscience 2019, 21, 458–473. [Google Scholar] [CrossRef]
- Vives-Peris, V.; Marmaneu, D.; Gómez-Cadenas, A.; Pérez-Clemente, R.M. Characterization of Citrus WRKY transcription factors and their responses to phytohormones and abiotic stresses. Biol. Plant. 2018, 62, 33–44. [Google Scholar] [CrossRef]
- Baba, A.I.; Rigó, G.; Andrási, N.; Tietz, O.; Palme, K.; Szabados, L.; Csépl, Á. Striving towards abiotic stresses: Role of the plant CDPK superfamily members. In International Climate Protection; Palocz-Andresen, M., Szalay, D., Gostzom, A., Sípos, L., Taligás, T., Eds.; Springer: Cham, Switzerland, 2019; pp. 99–105. [Google Scholar]
- Nasir, F.; Tian, L.; Shi, S.; Bahadur, A.; Batool, A.; Ma, L.; Gao, Y.; Tian, C. Asian cultivated rice domestication supresses the expression of abiotic stress- and reactive oxygen species scavenging-related genes in roots. Pak. J. Bot. 2019, 51, 49–54. [Google Scholar] [CrossRef]
- Bharti, S.; Balyan, H.S.; Gupta, P.K. Quantitative trait loci analysis for some root traits in Bread Wheat (Triticum aestivum L.). Int. J. Agric. Sci. 2014, 4, 214–221. [Google Scholar]
- Christopher, J.; Christopher, M.; Jennings, R.; Jones, S.; Fletcher, S.; Borrell, A.; Manschadi, A.M.; Jordan, D.; Mace, E.; Hammer, G. QTL for root angle and number in a population developed from bread wheats (Triticum aestivum) with contrasting adaptation to water-limited environments. Theor. Appl. Genet. 2013, 126, 1563–1574. [Google Scholar] [CrossRef] [PubMed]
- Ahmadi, J.; Fotokian, M.H. Identification and mapping of quantitative trait loci associated with salinity tolerance in rice (Oryza sativa) using SSR markers. Iran. J. Biotechnol. 2011, 9, 21–30. [Google Scholar]
- Ahmad, H.M.; Mahmood, U.-R.; Azeem, F.; Tahir, N.; Iqbal, M.S. QTL mapping for crop improvement against abiotic stresses in cereals. J. Anim. Plant Sci. 2018, 28, 1558–1573. [Google Scholar]
- Zou, C.; Chen, A.; Xiao, L.; Muller, H.M.; Ache, P.; Haberer, G.; Zhang, M.; Jia, W.; Deng, P.; Huang, R.; et al. A high-quality genome assembly of quinoa provides insights into the molecular basis of salt bladder-based salinity tolerance and the exceptional nutritional value. Cell Res. 2017, 27, 1327–1340. [Google Scholar] [CrossRef]
- Hajdarpašić, A.; Ruggenthaler, P. Analysis of miRNA expression under stress in Arabidopsis thaliana. Bosn. J. Basic Med. Sci. 2012, 12, 169–176. [Google Scholar] [CrossRef][Green Version]
- Wani, S.H.; Kumar, V.; Khare, T.; Tripathi, P.; Shah, T.; Ramakrishna, C.; Aglawe, S.; Mangrauthia, S.K. miRNA applications for engineering abiotic stress tolerance in plants. Biologia 2020, in press. [Google Scholar] [CrossRef]
- Dash, M.; Yordanov, Y.S.; Georgieva, T.; Wei, H.; Busov, V. Gene network analysis of poplar root transcriptome in response to drought stress identifies a PtaJAZ3PtaRAP2.6-centered hierarchical network. PLoS ONE 2018, 13, e0208560. [Google Scholar] [CrossRef]
- Chandran, A.K.N.; Kim, J.W.; Yoo, Y.H.; Park, H.L.; Kim, Y.J.; Cho, M.H.; Jung, K.H. Transcriptome analysis of rice-seedling roots under soil–salt stress using RNA-Seq method. Plant Biotechnol. Rep. 2019, 13, 567–578. [Google Scholar] [CrossRef]
- De Smet, S.; Cuypers, A.; Vangronsveld, J.; Remans, T. Gene networks involved in hormonal control of root development in Arabidopsis thaliana: A framework for studying its disturbance by metal stress. Int. J. Mol. Sci. 2015, 16, 19195–19224. [Google Scholar] [CrossRef]
- Chaudhary, J.; Khatri, P.; Singla, P.; Kumawat, S.; Kumari, A.; Vinaykumar, R.; Vikram, A.; Jindal, S.K.; Kardile, H.; Kumar, R.; et al. Advances in omics approaches for abiotic stress tolerance in tomato. Biology 2019, 8, 90. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Mishra, S.K.; Misra, S.; Pandey, V.; Agrawal, L.; Nautiyal, C.S.; Chauhan, P.S. Revealing the complexity of protein abundance in chickpea root under drought-stress using a comparative proteomics approach. Plant Physiol. Biochem. 2020, 151, 88–102. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Sangireddy, S.R.; Yu, C.-L.; Hui, D.; Howe, K.; Fish, T.; Thannhauser, T.W.; Zhou, S. Comparative proteomics of root apex and root elongation zones provides insights into molecular mechanisms for drought stress and recovery adjustment in switchgrass. Proteomes 2020, 8, 3. [Google Scholar] [CrossRef]
- Prinsi, B.; Negri, A.S.; Failla, O.; Scienza, A.; Espen, L. Root proteomic and metabolic analyses reveal specific responses to drought stress in differently tolerant grapevine rootstocks. BMC Plant Biol. 2018, 18, 12. [Google Scholar] [CrossRef]
- Sun, X.; Wang, Y.; Xu, L.; Li, C.; Zhang, W.; Luo, X.; Jiang, H.; Liu, L. Unraveling the root proteome changes and its relationship to molecular mechanism underlying salt stress response in radish (Raphanus sativus L.). Front. Plant Sci. 2017, 8, 1192. [Google Scholar] [CrossRef]
- Giri, A.; Heckathorn, S.; Mishra, S.; Krause, C. Heat stress decreases levels of nutrient-uptake and -assimilation proteins in tomato roots. Plants 2017, 6, 6. [Google Scholar] [CrossRef] [PubMed]
- Luan, H.; Shen, H.; Pan, Y.; Guo, B.; Lv, C.; Xu, R. Elucidating the hypoxic stress response in barley (Hordeum vulgare L.) during waterlogging: A proteomics approach. Sci. Rep. 2018, 8, 9655. [Google Scholar] [CrossRef] [PubMed]
- Pan, R.; He, D.; Xu, L.; Zhou, M.; Li, C.; Wu, C.; Xu, Y.; Zhang, W. Proteomic analysis reveals response of differential wheat (Triticum aestivum L.) genotypes to oxygen deficiency stress. BMC Genom. 2019, 20, 60. [Google Scholar] [CrossRef]
- Xu, J.; Qiao, X.; Tian, Z.; Zhang, X.; Zou, X.; Cheng, Y.; Lu, G.; Zeng, L.; Fu, G.; Ding, X.; et al. Proteomic analysis of rapeseed root response to waterlogging stress. Plants 2018, 7, 71. [Google Scholar] [CrossRef]
- Hasan, M.K.; Cheng, Y.; Kanwar, M.K.; Chu, X.Y.; Ahammed, G.J.; Qi, Z.Y. Responses of plant proteins to heavy metal stress—A review. Front. Plant Sci. 2017, 8, 1492. [Google Scholar] [CrossRef]
- Chen, Z.; Zhu, D.; Wu, J.; Cheng, Z.; Yan, X.; Deng, X.; Yan, Y. Identification of differentially accumulated proteins involved in regulating independent and combined osmosis and cadmium stress response in Brachypodium seedling roots. Sci. Rep. 2018, 8, 7790. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Ren, Y.; Li, J.; Li, L.; Chen, S.; Wang, Z.; Xin, Z.; Chen, F.; Lin, T.; Cui, D.; et al. Comparative proteomic analysis provides new insights into low nitrogen-promoted primary root growth in hexaploid wheat. Front. Plant Sci. 2019, 10, 151. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.G.; Wang, Y.; Lee, C.H.; Mun, B.G.; Kim, P.J.; Lee, S.Y.; Kim, Y.C.; Kang, K.Y.; Rakwal, R.; Agrawal, G.K.; et al. A comparative proteomics survey of proteins responsive to phosphorous starvation in roots of hydroponically-grown rice seedlings. J. Korean Soc. Appl. Biol. Chem. 2011, 54, 667–677. [Google Scholar] [CrossRef]
- Kholghi, M.; Toorchi, M.; Bandehagh, A.; Ostendorp, A.; Ostendorp, S.; Hanhart, P.; Kehr, J. Comparative proteomic analysis of salt-responsive proteins in canola roots by 2-DE and MALDI-TOF MS. Biochim. Biophys. Acta Proteins Proteomics 2019, 1867, 227–236. [Google Scholar] [CrossRef]
- Arbona, V.; Manzi, M.; de Ollas, C.; Gómez-Cadenas, A. Metabolomics as a tool to investigate abiotic stress tolerance in plants. Int. J. Mol. Sci. 2013, 14, 4885–4911. [Google Scholar] [CrossRef]
- Sarabia, L.D.; Boughton, B.A.; Rupasinghe, T.; van de Meene, A.M.L.; Callahan, D.L.; Hill, C.B.; Roessner, U. High-mass-resolution MALDI mass spectrometry imaging reveals detailed spatial distribution of metabolites and lipids in roots of barley seedlings in response to salinity stress. Metabolomics 2018, 14, 63. [Google Scholar] [CrossRef]
- Kang, Z.; Babar, M.A.; Khan, N.; Guo, J.; Khan, J.; Islam, S.; Shrestha, S.; Shahi, D. Comparative metabolomic profiling in the roots and leaves in contrasting genotypes reveals complex mechanisms involved in post-anthesis drought tolerance in wheat. PLoS ONE 2019, 14, e0213502. [Google Scholar] [CrossRef]
- Skliros, D.; Kalloniati, C.; Karalias, G.; Skaracis, G.N.; Rennenberg, H.; Flemetakis, E. Global metabolomics analysis reveals distinctive tolerance mechanisms in different plant organs of lentil (Lens culinaris) upon salinity stress. Plant Soil 2018, 429, 451–468. [Google Scholar] [CrossRef]
- Ullah, N.; Yüce, M.; Neslihan Öztürk, G.Z.; Budak, H. Comparative metabolite profiling of drought stress in roots and leaves of seven Triticeae species. BMC Genom. 2017, 18, 969. [Google Scholar] [CrossRef]
- Mundim, F.M.; Pringle, E.G. Whole-plant metabolic allocation under water stress. Front. Plant Sci. 2018, 9, 852. [Google Scholar] [CrossRef]
- Rabara, R.C.; Tripathi, P.; Rushton, P.J. Comparative metabolome profile between tobacco and soybean grown under water-stressed conditions. Biomed. Res. Int. 2017, 2017, 3065251. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Guo, R.; Jiao, Y.; Jin, X.; Zhang, H.; Shi, L. Comparison of salt tolerance in Soja based on metabolomics of seedling roots. Front. Plant Sci. 2017, 8, 1101. [Google Scholar] [CrossRef] [PubMed]
- Sheflin, A.M.; Chiniquy, D.; Yuan, C.; Goren, E.; Kumar, I.; Braud, M.; Brutnell, T.; Eveland, A.L.; Tringe, S.; Liu, P.; et al. Metabolomics of sorghum roots during nitrogen stress reveals compromised metabolic capacity for salicylic acid biosynthesis. Plant Direct 2019, 3, e00122. [Google Scholar] [CrossRef] [PubMed]
- Natera, S.H.A.; Hill, C.B.; Rupasinghe, T.W.T.; Roessner, U. Salt-stress induced alterations in the root lipidome of two barley genotypes with contrasting responses to salinity. Funct. Plant Biol. 2016, 43, 207. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Liu, H.; Jin, C.; Li, Q.; Guo, L. An efficient and comprehensive plant glycerolipids analysis approach based on high-performance liquid chromatography-quadrupole time-of-flight mass spectrometer. Plant Direct 2019, 3, 1–13. [Google Scholar] [CrossRef]
- Yu, D.; Boughton, B.A.; Hill, C.B.; Feussner, I.; Roessner, U.; Rupasinghe, T.W.T. Insights into oxidized lipid modification in barley roots as an adaptation mechanism to salinity stress. Front. Plant Sci. 2020, 11, 1. [Google Scholar] [CrossRef]
- Jiang, Z.; Zhou, X.; Tao, M.; Yuan, F.; Liu, L.; Wu, F.; Wu, X.; Xiang, Y.; Niu, Y.; Liu, F.; et al. Plant cell-surface GIPC sphingolipids sense salt to trigger Ca2+ influx. Nature 2019, 572, 341–346. [Google Scholar] [CrossRef]
- Quiroga, G.; Erice, G.; Ding, L.; Chaumont, F.; Aroca, R.; Ruiz-Lozano, J.M. The arbuscular mycorrhizal symbiosis regulates aquaporins activity and improves root cell water permeability in maize plants subjected to water stress. Plant Cell Environ. 2019, 42, 2274–2290. [Google Scholar] [CrossRef]
- Guo, Q.; Liu, L.; Barkla, B.J. Membrane lipid remodeling in response to salinity. Int. J. Mol. Sci. 2019, 20, 4264. [Google Scholar] [CrossRef]
- Palm, E.; Nissim, W.G.; Giordano, C.; Mancuso, S.; Azzarello, E. Root potassium and hydrogen flux rates as potential indicators of plant response to zinc, copper and nickel stress. Environ. Exp. Bot. 2017, 143, 38–50. [Google Scholar] [CrossRef]
- Couchoud, M.; Der, C.; Girodet, S.; Vernoud, V.; Prudent, M.; Leborgne-Castel, N. Drought stress stimulates endocytosis and modifies membrane lipid order of rhizodermal cells of Medicago truncatula in a genotype-dependent manner. BMC Plant Biol. 2019, 19, 221. [Google Scholar] [CrossRef] [PubMed]
- Lamers, J.; van der Meer, T.; Testerink, C. How plants sense and respond to stressful environments. Plant Physiol. 2020, 182, 1624–1635. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Tian, B.; Zhang, F.; Tao, F.; Li, W. Plant adaptation to frequent alterations between high and low temperatures: Remodeling of membrane lipids and maintenance of unsaturation levels. Plant Cell Environ. 2011, 34, 1431–1442. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Niu, H.; Liang, M.; Zhai, Y.; Huang, W.; Ding, Q.; Du, Y.; Lu, M. A wall-associated kinase gene CaWAKL20 from pepper negatively modulates plant thermotolerance by reducing the expression of ABA-responsive genes. Front. Plant Sci. 2019, 10, 591. [Google Scholar] [CrossRef]
- Xiang, G.; Ma, W.; Gao, S.; Jin, Z.; Yue, Q.; Yao, Y. Transcriptomic and phosphoproteomic profiling and metabolite analyses reveal the mechanism of NaHCO3-induced organic acid secretion in grapevine roots. BMC Plant Biol. 2019, 19, 383. [Google Scholar] [CrossRef]
- Yuan, F.; Yang, H.; Xue, Y.; Kong, D.; Ye, R.; Li, C.; Zhang, J.; Theprungsirikul, L.; Shrift, T.; Krichilsky, B.; et al. OSCA1 mediates osmotic-stress-evoked Ca2+ increases vital for osmosensing in Arabidopsis. Nature 2014, 514, 367–371. [Google Scholar] [CrossRef]
- Zhang, M.; Wang, D.; Kang, Y.; Wu, J.-X.; Yao, F.; Pan, C.; Yan, Z.; Song, C.; Chen, L. Structure of the mechanosensitive OSCA channels. Nat. Struct. Mol. Biol. 2018, 25, 850–858. [Google Scholar] [CrossRef]
- Nagaraj, K.M.; Jane, W.N.; Verslues, P.E. Role of the putative osmosensor arabidopsis Histidine Kinase1 in dehydration avoidance and low-water- potential response. Plant Physiol. 2013, 161, 942–953. [Google Scholar]
- Sussmilch, F.C.; Brodribb, T.J.; McAdam, S.A.M. Up-regulation of NCED3 and ABA biosynthesis occur within minutes of a decrease in leaf turgor but AHK1 is not required. J. Exp. Bot. 2017, 68, 2913–2918. [Google Scholar] [CrossRef]
- Feng, W.; Kita, D.; Peaucelle, A.; Cartwright, H.N.; Doan, V.; Duan, Q.; Liu, M.C.; Maman, J.; Steinhorst, L.; Schmitz-Thom, I.; et al. The FERONIA receptor kinase maintains cell-wall integrity during salt stress through Ca2+ signaling. Curr. Biol. 2018, 28, 666–675. [Google Scholar] [CrossRef]
- Hou, Q.; Ufer, G.; Bartels, D. Lipid signalling in plant responses to abiotic stress. Plant Cell Environ. 2016, 39, 1029–1048. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Dai, X.; Xu, Y.; Luo, W.; Zheng, X.; Zeng, D.; Pan, Y.; Lin, X.; Liu, H.; Zhang, D.; et al. COLD1 confers chilling tolerance in rice. Cell 2015, 160, 1209–1221. [Google Scholar] [CrossRef] [PubMed]
- Bellstaedt, J.; Trenner, J.; Lippmann, R.; Poeschl, Y.; Zhang, X.; Friml, J.; Quint, M.; Delker, C. A mobile auxin signal connects temperature sensing in cotyledons with growth responses in hypocotyls. Plant Physiol. 2019, 180, 757–766. [Google Scholar] [CrossRef] [PubMed]
- Legris, M.; Klose, C.; Burgie, E.S.; Rojas, C.C.; Neme, M.; Hiltbrunner, A.; Wigge, P.A.; Schäfer, E.; Vierstra, R.D.; Casal, J.J. Phytochrome B integrates light and temperature signals in Arabidopsis. Science 2016, 354, 897–900. [Google Scholar] [CrossRef] [PubMed]
- Vu, L.D.; Gevaert, K.; De Smet, I. Feeling the heat: Searching for plant thermosensors. Trends Plant Sci. 2019, 24, 210–219. [Google Scholar] [CrossRef]
- de Ollas, C.; Hernando, B.; Arbona, V.; Gómez-Cadenas, A. Jasmonic acid transient accumulation is needed for abscisic acid increase in citrus roots under drought stress conditions. Physiol. Plant. 2013, 147, 296–306. [Google Scholar] [CrossRef]
- de Ollas, C.; Arbona, V.; Gómez-Cadenas, A. Jasmonoyl isoleucine accumulation is needed for abscisic acid build-up in roots of Arabidopsis under water stress conditions. Plant Cell Environ. 2015, 38, 2157–2170. [Google Scholar] [CrossRef]
- Van Ha, C.; Leyva-Gonzalez, M.A.; Osakabe, Y.; Tran, U.T.; Nishiyama, R.; Watanabe, Y.; Tanaka, M.; Seki, M.; Yamaguchi, S.; Dong, N.V.; et al. Positive regulatory role of strigolactone in plant responses to drought and salt stress. Proc. Natl. Acad. Sci. USA 2014, 111, 851–856. [Google Scholar]
- Mahouachi, J.; Arbona, V.; Gómez-Cadenas, A. Hormonal changes in papaya seedlings subjected to progressive water stress and re-watering. Plant Growth Regul. 2007, 53, 43–51. [Google Scholar] [CrossRef]
- Muñoz-Espinoza, V.A.; López-Climent, M.F.; Casaretto, J.A.; Gómez-Cadenas, A. Water stress responses of tomato mutants impaired in hormone biosynthesis reveal abscisic acid, jasmonic acid and salicylic acid interactions. Front. Plant Sci. 2015, 6, 997. [Google Scholar] [CrossRef]
- Kong, X.; Luo, Z.; Dong, H.; Eneji, A.E.; Li, W. H2O2 and ABA signaling are responsible for the increased Na+ efflux and water uptake in Gossypium hirsutum L. roots in the non-saline side under non-uniform root zone salinity. J. Exp. Bot. 2016, 67, 2247–2261. [Google Scholar] [CrossRef] [PubMed]
- Arbona, V.; Gómez-Cadenas, A. Hormonal modulation of citrus responses to flooding. J. Plant Growth Regul. 2008, 27, 241–250. [Google Scholar] [CrossRef]
- Pedersen, O.; Sauter, M.; Colmer, T.D.; Nakazono, M. Regulation of root adaptive anatomical and morphological traits during low soil oxygen. New Phytol. 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Abouelsaad, I.; Renault, S. Enhanced oxidative stress in the jasmonic acid-deficient tomato mutant def-1 exposed to NaCl stress. J. Plant Physiol. 2018, 226, 136–144. [Google Scholar] [CrossRef] [PubMed]
- López-Climent, M.F.; Arbona, V.; Pérez-Clemente, R.M.; Gómez-Cadenas, A. Effects of cadmium on gas exchange and phytohormone contents in citrus. Biol. Plant. 2011, 55, 187–190. [Google Scholar] [CrossRef]
- Bankaji, I.; Sleimi, N.; López-Climent, M.F.; Perez-Clemente, R.M.; Gomez-Cadenas, A. Effects of combined abiotic stresses on growth, trace element accumulation, and phytohormone regulation in two halophytic species. J. Plant. Growth Regul. 2014, 33, 632–643. [Google Scholar] [CrossRef]
- Vives-Peris, V.; Gómez-Cadenas, A.; Pérez-Clemente, R.M. Citrus plants exude proline and phytohormones under abiotic stress conditions. Plant Cell Rep. 2017, 36, 1971–1984. [Google Scholar] [CrossRef]
- Verma, R.K.; Santosh Kumar, V.V.; Yadav, S.K.; Pushkar, S.; Rao, M.V.; Chinnusamy, V. Overexpression of ABA receptor PYL10 gene confers drought and cold tolerance to indica rice. Front. Plant Sci. 2019, 10, 1488. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Rivero, R.M.; Martínez, V.; Gómez-Cadenas, A.; Arbona, V. Tolerance of citrus plants to the combination of high temperatures and drought is associated to the increase in transpiration modulated by a reduction in abscisic acid levels. BMC Plant Biol. 2016, 16, 105. [Google Scholar] [CrossRef]
- Balfagón, D.; Sengupta, S.; Gómez-Cadenas, A.; Fritschi, F.B.; Azad, R.K.; Mittler, R.; Zandalinas, S.I. Jasmonic acid is required for plant acclimation to a combination of high light and heat stress. Plant Physiol. 2019, 181, 1668–1682. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Mittler, R.; Balfagón, D.; Arbona, V.; Gómez-Cadenas, A. Plant adaptations to the combination of drought and high temperatures. Physiol. Plant. 2018, 162, 2–12. [Google Scholar] [CrossRef] [PubMed]
- González-Guzmán, M.; Rodríguez, L.; Lorenzo-Orts, L.; Pons, C.; Sarrión-Perdigones, A.; Fernandez, M.A.; Peirats-Llobet, M.; Forment, J.; Moreno-Alvero, M.; Cutler, S.R.; et al. Tomato PYR/PYL/RCAR abscisic acid receptors show high expression in root, differential sensitivity to the abscisic acid agonist quinabactin, and the capability to enhance plant drought resistance. J. Exp. Bot. 2014, 65, 4451–4464. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Zhao, M.; Li, S.; Bai, X.; Li, J.; Meng, H.; Mu, Z. Contrasting transcriptional responses of PYR1/PYL/RCAR ABA receptors to ABA or dehydration stress between maize seedling leaves and roots. BMC Plant Biol. 2016, 16, 99. [Google Scholar] [CrossRef] [PubMed]
- Arbona, V.; Zandalinas, S.I.; Manzi, M.; González-Guzmán, M.; Rodriguez, P.L.; Gómez-Cadenas, A. Depletion of abscisic acid levels in roots of flooded Carrizo citrange (Poncirus trifoliata L. Raf. × Citrus sinensis L. Osb.) plants is a stress-specific response associated to the differential expression of PYR/PYL/RCAR receptors. Plant Mol. Biol. 2017, 93, 623–640. [Google Scholar] [CrossRef] [PubMed]
- Hazman, M.; Hause, B.; Eiche, E.; Nick, P.; Riemann, M. Increased tolerance to salt stress in OPDA-deficient rice ALLENE OXIDE CYCLASE mutants is linked to an increased ROS-scavenging activity. J. Exp. Bot. 2015, 66, 3339–3352. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Smith, J.A.C.; Harberd, N.P.; Jiang, C. The regulatory roles of ethylene and reactive oxygen species (ROS) in plant salt stress responses. Plant Mol. Biol. 2016, 91, 651–659. [Google Scholar] [CrossRef] [PubMed]
- Müller, M.; Munné-Bosch, S. Ethylene response factors: A key regulatory hub in hormone and stress signaling. Plant Physiol. 2015, 169, 32–41. [Google Scholar] [CrossRef]
- Hartman, S.; Sasidharan, R.; Voesenek, L.A.C.J. The role of ethylene in metabolic acclimations to low oxygen. New Phytol. 2019, in press. [Google Scholar] [CrossRef]
- Mendiondo, G.M.; Gibbs, D.J.; Szurman-Zubrzycka, M.; Korn, A.; Marquez, J.; Szarejko, I.; Maluszynski, M.; King, J.; Axcell, B.; Smart, K.; et al. Enhanced waterlogging tolerance in barley by manipulation of expression of the N-end rule pathway E3 ligase PROTEOLYSIS6. Plant Biotechnol. J. 2016, 14, 40–50. [Google Scholar] [CrossRef]
- Wang, F.; Chen, Z.H.; Shabala, S. Hypoxia sensing in plants: On a quest for ion channels as putative oxygen sensors. Plant Cell Physiol. 2017, 58, 1126–1142. [Google Scholar] [CrossRef]
- Li, J.; Xu, H.H.; Liu, W.C.; Zhang, X.W.; Lu, Y.T. Ethylene inhibits root elongation during alkaline stress through AUXIN1 and associated changes in auxin accumulation. Plant Physiol. 2015, 168, 1777–1791. [Google Scholar] [CrossRef] [PubMed]
- Kollist, H.; Zandalinas, S.I.; Sengupta, S.; Nuhkat, M.; Kangasjärvi, J.; Mittler, R. Rapid responses to abiotic stress: Priming the landscape for the signal transduction network. Trends Plant Sci. 2019, 24, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Kudla, J.; Becker, D.; Grill, E.; Hedrich, R.; Hippler, M.; Kummer, U.; Parniske, M.; Romeis, T.; Schumacher, K. Advances and current challenges in calcium signaling. New Phytol. 2018, 218, 414–431. [Google Scholar] [CrossRef]
- Yuan, P.; Yang, T.; Poovaiah, B.W. Calcium signaling-mediated plant response to cold stress. Int. J. Mol. Sci. 2018, 19, 3896. [Google Scholar] [CrossRef] [PubMed]
- Thor, K. Calcium—Nutrient and messenger. Front. Plant Sci. 2019, 10, 440. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Jin, C.; Wang, G.; Ji, J.; Guan, C.; Li, X. Enhancement of endogenous SA accumulation improves poor-nutrition stress tolerance in transgenic tobacco plants overexpressing a SA-binding protein gene. Plant Sci. 2020, 292, 110384. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Yan, J.; Yu, X.; Liang, Y.; Fang, L.; Scheller, H.V.; Zhang, A. The NADPH-oxidase AtRbohI plays a positive role in drought-stress response in Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 2017, 491, 834–839. [Google Scholar] [CrossRef]
- van Zelm, E.; Zhang, Y.; Testerink, C. Salt tolerance mechanisms of plants. Annu. Rev. Plant Biol. 2020, 71, 403–433. [Google Scholar] [CrossRef]
- Ma, L.; Ye, J.; Yang, Y.; Lin, H.; Yue, L.; Luo, J.; Long, Y.; Fu, H.; Liu, X.; Zhang, Y.; et al. The SOS2-SCaBP8 complex generates and fine-tunes an AtANN4-dependent calcium signature under salt stress. Dev. Cell 2019, 48, 697–709. [Google Scholar] [CrossRef]
- Yin, X.; Xia, Y.; Xie, Q.; Cao, Y.; Wang, Z.; Hao, G.; Song, J.; Zhou, Y.; Jiang, X. CBL10-CIPK8-SOS1, a novel SOS pathway, functions in Arabidopsis to regulate salt tolerance. J. Exp. Bot. 2020, 71, 1801–1814. [Google Scholar] [CrossRef]
- Sami, F.; Faizan, M.; Faraz, A.; Siddiqui, H.; Yusuf, M.; Hayat, S. Nitric oxide-mediated integrative alterations in plant metabolism to confer abiotic stress tolerance, NO crosstalk with phytohormones and NO-mediated post translational modifications in modulating diverse plant stress. Nitric Oxide 2018, 73, 22–38. [Google Scholar] [CrossRef]
- Cao, X.; Zhu, C.; Zhong, C.; Zhang, J.; Wu, L.; Jin, Q.; Ma, Q. Nitric oxide synthase-mediated early nitric oxide burst alleviates water stress-induced oxidative damage in ammonium-supplied rice roots. BMC Plant Biol. 2019, 19, 108. [Google Scholar] [CrossRef] [PubMed]
- Yuan, H.M.; Huang, X. Inhibition of root meristem growth by cadmium involves nitric oxide-mediated repression of auxin accumulation and signalling in Arabidopsis. Plant Cell Environ. 2016, 39, 120–135. [Google Scholar] [CrossRef] [PubMed]
- Kabała, K.; Zboińska, M.; Głowiak, D.; Reda, M.; Jakubowska, D.; Janicka, M. Interaction between the signaling molecules hydrogen sulfide and hydrogen peroxide and their role in vacuolar H+-ATPase regulation in cadmium-stressed cucumber roots. Physiol. Plant. 2019, 166, 688–704. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Shi, Y.; Yang, S. Advances and challenges in uncovering cold tolerance regulatory mechanisms in plants. New Phytol. 2019, 222, 1690–1704. [Google Scholar] [CrossRef]
- Guo, J.; Ren, Y.; Tang, Z.; Shi, W.; Zhou, M. Characterization and expression profiling of the ICE-CBF-COR genes in wheat. PeerJ 2019, 7, e8190. [Google Scholar] [CrossRef]
- Chen, W.J.; Wang, X.; Yan, S.; Huang, X.; Yuan, H.M. The ICE-like transcription factor HbICE2 is involved in jasmonate-regulated cold tolerance in the rubber tree (Hevea brasiliensis). Plant Cell Rep. 2019, 38, 699–714. [Google Scholar] [CrossRef]
- Katano, K.; Honda, K.; Suzuki, N. Integration between ROS regulatory systems and other signals in the regulation of various types of heat responses in plants. Int. J. Mol. Sci. 2018, 19, 3370. [Google Scholar] [CrossRef]
- van Dongen, J.T.; Licausi, F. Oxygen sensing and signaling. Annu. Rev. Plant Biol. 2015, 66, 345–367. [Google Scholar] [CrossRef]
- Giuntoli, B.; Lee, S.C.; Licausi, F.; Kosmacz, M.; Oosumi, T.; van Dongen, J.T.; Bailey-Serres, J.; Perata, P. A trihelix DNA binding protein counterbalances hypoxia-responsive transcriptional activation in Arabidopsis. PLoS Biol. 2014, 12, e1001950. [Google Scholar] [CrossRef]
- Gonzali, S.; Loreti, E.; Cardarelli, F.; Novi, G.; Parlanti, S.; Pucciariello, C.; Bassolino, L.; Banti, V.; Licausi, F.; Perata, P. Universal stress protein HRU1 mediates ROS homeostasis under anoxia. Nat. Plants 2015, 1, 15151. [Google Scholar] [CrossRef] [PubMed]
- Hartman, S.; Liu, Z.; van Veen, H.; Vicente, J.; Reinen, E.; Martopawiro, S.; Zhang, H.; van Dongen, N.; Bosman, F.; Bassel, G.W.; et al. Ethylene-mediated nitric oxide depletion pre-adapts plants to hypoxia stress. Nat. Commun. 2019, 10, 4020. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, R.; Gou, L.; Si, Y.; Guan, Q. Overexpression of Populus trichocarpa mitogen-activated protein kinase kinase4 enhances salt tolerance in tobacco. Int. J. Mol. Sci. 2017, 18, 2090. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, L.; Qi, J.; Dang, P.; Xia, T. Cadmium activates ZmMPK3-1 and ZmMPK6-1 via induction of reactive oxygen species in maize roots. Biochem. Biophys. Res. Commun. 2019, 516, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Kolbert, Z.; Oláh, D.; Molnár, Á.; Szőllősi, R.; Erdei, L.; Ördög, A. Distinct redox signalling and nickel tolerance in Brassica juncea and Arabidopsis thaliana. Ecotoxicol. Environ. Saf. 2020, 189, 109989. [Google Scholar] [CrossRef] [PubMed]
- Lai, X.; Stigliani, A.; Vachon, G.; Carles, C.; Smaczniak, C.; Zubieta, C.; Kaufmann, K.; Parcy, F. Building transcription factor binding site models to understand gene regulation in plants. Mol. Plant 2019, 12, 743–763. [Google Scholar] [CrossRef]
- Sun, H.; Sun, X.; Wang, H.; Ma, X. Advances in salt tolerance molecular mechanism in tobacco plants. Hereditas 2020, 157, 5. [Google Scholar] [CrossRef]
- Zhu, D.; Hou, L.; Xiao, P.; Guo, Y.; Deyholos, M.K.; Liu, X. VvWRKY30, a grape WRKY transcription factor, plays a positive regulatory role under salinity stress. Plant Sci. 2019, 280, 132–142. [Google Scholar] [CrossRef]
- Devkar, V.; Thirumalaikumar, V.P.; Xue, G.P.; Vallarino, J.G.; Turečková, V.; Strnad, M.; Fernie, A.R.; Hoefgen, R.; Mueller-Roeber, B.; Balazadeh, S. Multifaceted regulatory function of tomato SlTAF1 in the response to salinity stress. New Phytol. 2020, 225, 1681–1698. [Google Scholar] [CrossRef]
- Wang, W.; Qiu, X.; Yang, Y.; Kim, H.S.; Jia, X.; Yu, H.; Kwak, S.-S. Sweetpotato BZIP transcription factor IBABF4 confers tolerance to multiple abiotic stresses. Front. Plant Sci. 2019, 10, 630. [Google Scholar] [CrossRef]
- Yang, J.; Duan, G.; Li, C.; Liu, L.; Han, G.; Zhang, Y.; Wang, C. The crosstalks between jasmonic acid and other plant hormone signaling highlight the involvement of jasmonic acid as a core component in plant response to biotic and abiotic stresses. Front. Plant Sci. 2019, 10, 1349. [Google Scholar] [CrossRef] [PubMed]
- Konzen, E.R.; Recchia, G.H.; Cassieri, F.; Gomes Caldas, D.G.; Berny Mier, Y.; Teran, J.C.; Gepts, P.; Tsai, S.M. DREB genes from common bean (Phaseolus vulgaris L.) show broad to specific abiotic stress responses and distinct levels of nucleotide diversity. Int. J. Genom. 2019, 2019, 9520642. [Google Scholar] [CrossRef] [PubMed]
- Das, P.; Lakra, N.; Nutan, K.K.; Singla-Pareek, S.L.; Pareek, A. A unique bZIP transcription factor imparting multiple stress tolerance in rice. Rice 2019, 12, 58. [Google Scholar] [CrossRef] [PubMed]
- Bhaskarla, V.; Zinta, G.; Ford, R.; Jain, M.; Varshney, R.K.; Mantri, N. Comparative root transcriptomics provide insights into drought adaptation strategies in chickpea (Cicer arietinum L.). Int. J. Mol. Sci. 2020, 21, 1781. [Google Scholar] [CrossRef] [PubMed]
- Placido, D.F.; Sandhu, J.; Sato, S.J.; Nersesian, N.; Quach, T.; Clemente, T.E.; Staswick, P.E.; Walia, H. The LATERAL ROOT DENSITY gene regulates root growth during water stress in wheat. Plant Biotechnol. J. 2020, in press. [Google Scholar] [CrossRef] [PubMed]
- Noman, M.; Jameel, A.; Qiang, W.D.; Ahmad, N.; Liu, W.C.; Wang, F.W.; Li, H.Y. Overexpression of GmCAMTA12 enhanced drought tolerance in Arabidopsis and soybean. Int. J. Mol. Sci. 2019, 20, 4849. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Andújar, C.; Martínez-Pérez, A.; Ferrández-Ayela, A.; Albacete, A.; Martínez-Melgarejo, P.A.; Dodd, I.C.; Thompson, A.J.; Pérez-Pérez, J.M.; Pérez-Alfocea, F. Impact of overexpression of 9-cis-epoxycarotenoid dioxygenase on growth and gene expression under salinity stress. Plant. Sci. 2020, in press. [Google Scholar]
- Zhang, Y.; Wang, X.; Luo, Y.; Zhang, L.; Yao, Y.; Han, L.; Chen, Z.; Wang, L.; Li, Y. OsABA8ox2, an ABA catabolic gene, suppresses root elongation of rice seedlings and contributes to drought response. Crop J. 2020, in press. [Google Scholar] [CrossRef]
- Li, S.; Wang, N.; Ji, D.; Zhang, W.; Wang, Y.; Yu, Y.; Zhao, S.; Lyu, M.; You, J.; Zhang, Y.; et al. A GmSIn1/GmnCed3S/GmrboHBs feed-forward loop acts as a signal amplifier that regulates root growth in soybean exposed to salt stress. Plant Cell 2019, 31, 2107–2130. [Google Scholar] [CrossRef]
- Wang, J.; Zhang, Q.; Cui, F.; Hou, L.; Zhao, S.; Xia, H.; Qiu, J.; Li, T.; Zhang, Y.; Wang, X.; et al. Genome-wide analysis of gene expression provides new insights into cold responses in Thellungiella salsuginea. Front. Plant Sci. 2017, 8, 713. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, H.; Reinfelder, J.R.; Liang, X.; Sun, C.; Liu, C.; Li, F.; Yi, J. A transcriptomic (RNA-seq) analysis of genes responsive to both cadmium and arsenic stress in rice root. Sci. Total Environ. 2019, 666, 445–460. [Google Scholar] [CrossRef] [PubMed]
- Quinn, J.J.; Chang, H.Y. Unique features of long non-coding RNA biogenesis and function. Nat. Rev. Genet. 2016, 17, 47–62. [Google Scholar] [CrossRef]
- Qin, T.; Zhao, H.; Cui, P.; Albesher, N.; Xiong, L. A nucleus-localized long non-coding RNA enhances drought and salt stress tolerance. Plant Physiol. 2017, 175, 1321–1336. [Google Scholar] [CrossRef]
- Wu, J.; Okada, T.; Fukushima, T.; Tsudzuki, T.; Sugiura, M.; Yukawa, Y. A novel hypoxic stress-responsive long non-coding RNA transcribed by RNA polymerase III in Arabidopsis. RNA Biol. 2012, 9, 302–313. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Shi, S.; Jiang, N.; Khanzada, H.; Wassan, G.M.; Zhu, C.; Peng, X.; Xu, J.; Chen, Y.; Yu, Q.; et al. Genome-wide analysis of long non-coding RNAs affecting roots development at an early stage in the rice response to cadmium stress. BMC Genom. 2018, 19, 460. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Tan, Z.; Fang, T.; Tang, K.; Liang, K.; Qiu, F. A comprehensive transcriptomics analysis reveals long non-coding RNA to be involved in the key metabolic pathway in response to waterlogging stress in maize. Genes 2020, 11, 267. [Google Scholar] [CrossRef]
- He, J.; Jiang, Z.; Gao, L.; You, C.; Ma, X.; Wang, X.; Xu, X.; Mo, B.; Chen, X.; Liu, L. Genome-wide transcript and small RNA profiling reveals transcriptomic responses to heat stress. Plant Physiol. 2019, 181, 609–629. [Google Scholar] [CrossRef]
- Feng, J.; Wang, J.; Fan, P.; Jia, W.; Nie, L.; Jiang, P.; Chen, X.; Lv, S.; Wan, L.; Chang, S.; et al. High-throughput deep sequencing reveals that microRNAs play important roles in salt tolerance of euhalophyte Salicornia europaea. BMC Plant Biol. 2015, 15, 63. [Google Scholar] [CrossRef]
- Gentile, A.; Dias, L.I.; Mattos, R.S.; Ferreira, T.H.; Menossi, M. MicroRNAs and drought responses in sugarcane. Front. Plant Sci. 2015, 6, 58. [Google Scholar] [CrossRef]
- Goswami, K.; Mittal, D.; Gautam, B.; Sopory, S.K.; Sanan-Mishra, N. Mapping the salt stress-induced changes in the root miRNome in Pokkali rice. Biomolecules 2020, 10, 498. [Google Scholar] [CrossRef]
- Moldovan, D.; Spriggs, A.; Yang, J.; Pogson, B.J.; Dennis, E.S.; Wilson, I.W. Hypoxia-responsive microRNAs and trans-acting small interfering RNAs in Arabidopsis. J. Exp. Bot. 2010, 61, 165–177. [Google Scholar] [CrossRef]
- Azahar, I.; Ghosh, S.; Adhikari, A.; Adhikari, S.; Roy, D.; Shaw, A.K.; Singh, K.; Hossain, Z. Comparative analysis of maize root sRNA transcriptome unveils the regulatory roles of miRNAs in submergence stress response mechanism. Environ. Exp. Bot. 2020, 171, 103924. [Google Scholar] [CrossRef]
- Jin, Q.; Xu, Y.; Mattson, N.; Li, X.; Wang, B.; Zhang, X.; Jiang, H.; Liu, X.; Wang, Y.; Yao, D. Identification of submergence-responsive microRNAs and their targets reveals complex miRNA-mediated regulatory networks in lotus (Nelumbo nucifera Gaertn). Front. Plant Sci. 2017, 8, 6. [Google Scholar] [CrossRef]
- Gao, J.; Luo, M.; Peng, H.; Chen, F.; Li, W. Characterization of cadmium-responsive MicroRNAs and their target genes in maize (Zea mays) roots. BMC Mol. Biol. 2019, 20, 14. [Google Scholar] [CrossRef]
- Li, K.; Liu, Z.; Xing, L.; Wei, Y.; Mao, J.; Meng, Y.; Bao, L.; Han, M.; Zhao, C.; Zhang, D. miRNAs associated with auxin signaling, stress response, and cellular activities mediate adventitious root formation in apple rootstocks. Plant Physiol. Biochem. 2019, 139, 66–81. [Google Scholar] [CrossRef]
- Franco, J.A.; Bañón, S.; Vicente, M.J.; Miralles, J.; Martínez-Sánchez, J.J. Root development in horticultural plants grown under abiotic stress conditions—A review. J. Hortic. Sci. Biotechnol. 2011, 86, 543–556. [Google Scholar] [CrossRef]
- Sánchez-Calderón, L.; Ibarra-Cortés, M.E.; Zepeda-Jazo, I. Root Development and Abiotic Stress Adaptation. In Abiotic Stress—Plant Responses and Applications in Agriculture; Vahdati, K., Leslie, C., Eds.; IntechOpen: London, UK, 2013; pp. 135–167. [Google Scholar]
- Julkowska, M.M.; Koevoets, I.T.; Mol, S.; Hoefsloot, H.; Feron, R.; Tester, M.A.; Keurentjes, J.J.B.; Korte, A.; Haring, M.A.; de Boer, G.J.; et al. Genetic components of root architecture remodeling in response to salt stress. Plant Cell 2017, 29, 3198–3213. [Google Scholar] [CrossRef]
- Marzec, M.; Melzer, M. Regulation of root development and architecture by strigolactones under optimal and nutrient deficiency conditions. Int. J. Mol. Sci. 2018, 19, 1887. [Google Scholar] [CrossRef]
- Niu, Y.F.; Chai, R.S.; Jin, G.L.; Wang, H.; Tang, C.X.; Zhang, Y.S. Responses of root architecture development to low phosphorus availability: A review. Ann. Bot. 2013, 112, 391–408. [Google Scholar] [CrossRef]
- Qin, L.; Walk, T.C.; Han, P.; Chen, L.; Zhang, S.; Li, Y.; Hu, X.; Xie, L.; Yang, Y.; Liu, J.; et al. Adaption of roots to nitrogen deficiency revealed by 3D quantification and proteomic analysis. Plant Physiol. 2019, 179, 329–347. [Google Scholar] [CrossRef]
- Kapulnik, Y.; Koltai, H. Strigolactone involvement in root development, response to abiotic stress, and interactions with the biotic soil environment. Plant Physiol. 2014, 166, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.M. Abscisic acid: Hidden architect of root system structure. Plants 2015, 4, 548–572. [Google Scholar] [CrossRef] [PubMed]
- Rowe, J.H.; Topping, J.F.; Liu, J.; Lindsey, K. Abscisic acid regulates root growth under osmotic stress conditions via an interacting hormonal network with cytokinin, ethylene and auxin. New Phytol. 2016, 211, 225–239. [Google Scholar] [CrossRef]
- Yuan, T.T.; Xu, H.H.; Zhang, K.X.; Guo, T.T.; Lu, Y.T. Glucose inhibits root meristem growth via ABA INSENSITIVE 5, which represses PIN1 accumulation and auxin activity in Arabidopsis. Plant Cell Environ. 2014, 37, 1338–1350. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hua, D.; He, J.; Duan, Y.; Chen, Z.; Hong, X.; Gong, Z. Auxin response Factor2 (ARF2) and its regulated homeodomain gene HB33 mediate abscisic acid response in Arabidopsis. PLoS Genet. 2011, 7, e10022172. [Google Scholar] [CrossRef] [PubMed]
- Bloch, D.; Puli, M.R.; Mosquna, A.; Yalovsky, S. Abiotic stress modulates root patterning via ABA-regulated microRNA expression in the endodermis initials. Development 2019, 146, dev177097. [Google Scholar] [CrossRef]
- Vives-Peris, V.; de Ollas, C.; Gómez-Cadenas, A.; Pérez-Clemente, R.M. Root exudates: From plant to rhizosphere and beyond. Plant Cell Rep. 2020, 39, 3–17. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Z.; Gong, L.; Chen, A.; Liu, N.; Li, S.; Sun, H.; Yang, Z.; You, J. Functional characterization of three MATE genes in relation to aluminum-induced citrate efflux from soybean root. Plant Soil 2019, 443, 121–138. [Google Scholar] [CrossRef]
- Gargallo-Garriga, A.; Preece, C.; Sardans, J.; Oravec, M.; Urban, O.; Peñuelas, J. Root exudate metabolomes change under drought and show limited capacity for recovery. Sci. Rep. 2018, 8, 12696. [Google Scholar] [CrossRef]
- Tawaraya, K.; Horie, R.; Saito, S.; Wagatsuma, T.; Saito, K.; Oikawa, A. Metabolite profiling of root exudates of common bean under phosphorus deficiency. Metabolites 2014, 4, 599–611. [Google Scholar] [CrossRef]
- Luo, Q.; Wang, S.; Sun, L.N.; Wang, H. Metabolic profiling of root exudates from two ecotypes of Sedum alfredii treated with Pb based on GC-MS. Sci. Rep. 2017, 7, 39878. [Google Scholar] [CrossRef] [PubMed]
- Dardanelli, M.S.; de Córdoba, F.J.F.; Estévez, J.; Contreras, R.; Cubo, M.T.; Rodríguez-Carvajal, M.A.; Gil-Serrano, A.M.; López-Baena, F.J.; Bellogín, R.; Manyani, H.; et al. Changes in flavonoids secreted by Phaseolus vulgaris roots in the presence of salt and the plant growth-promoting rhizobacterium Chryseobacterium balustinum. Appl. Soil Ecol. 2012, 57, 31–38. [Google Scholar] [CrossRef]
- Pramanik, M.H.R.; Nagai, M.; Asao, T.; Matsui, Y. Effects of temperature and photoperiod on phytotoxic root exudates of cucumber (Cucumis sativus) in hydroponic culture. J. Chem. Ecol. 2000, 26, 1953–1967. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Lakshmanan, V.; Kobayashi, Y.; Asai, M.; Iuchi, S.; Kobayashi, M.; Bais, H.P.; Koyama, H. Overexpression of AtALMT1 in the Arabidopsis thaliana ecotype Columbia results in enhanced Al-activated malate excretion and beneficial bacterium recruitment. Plant Signal. Behav. 2013, 8, e25565. [Google Scholar] [CrossRef] [PubMed]
- Henry, A.; Doucette, W.; Norton, J.; Bugbee, B. Changes in crested wheatgrass root exudation caused by flood, drought, and nutrient stress. J. Environ. Qual. 2007, 36, 904–912. [Google Scholar] [CrossRef] [PubMed]
- Carvalhais, L.C.; Dennis, P.G.; Fedoseyenko, D.; Hajirezaei, M.R.; Borruss, R.; von Wirén, N. Root exudation of sugars, amino acids, and organic acids by maize as affected by nitrogen, phosphorus, potassium, and iron deficiency. J. Plant Nutr. Soil Sci. 2011, 174, 3–11. [Google Scholar] [CrossRef]
- Williams, A.; de Vries, F.T. Plant root exudation under drought: Implications for ecosystem functioning. New Phytol. 2020, 225, 1899–1905. [Google Scholar] [CrossRef]
- Vives-Peris, V.; Molina, L.; Segura, A.; Gómez-Cadenas, A.; Pérez-Clemente, R.M. Root exudates from citrus plants subjected to abiotic stress conditions have a positive effect on rhizobacteria. J. Plant Physiol. 2018, 228, 208–217. [Google Scholar] [CrossRef]
- Etesami, H.; Glick, B.R. Halotolerant plant growth-promoting bacteria: Prospects for alleviating salinity stress in plants. Environ. Exp. Bot 2020, in press. [Google Scholar] [CrossRef]
- Ko, D.; Helariutta, Y. Shoot–root communication in flowering plants. Curr. Biol. 2017, 27, R973–R978. [Google Scholar] [CrossRef]
- Manzi, M.; Lado, J.; Rodrigo, M.J.; Zacariás, L.; Arbona, V.; Gómez-Cadenas, A. Root ABA accumulation in long-term water-stressed plants is sustained by hormone transport from aerial organs. Plant Cell Physiol. 2015, 56, 2457–2466. [Google Scholar] [CrossRef] [PubMed]
- McAdam, S.A.M.; Manzi, M.; Ross, J.J.; Brodribb, T.J.; Gómez-Cadenas, A. Uprooting an abscisic acid paradigm: Shoots are the primary source. Plant Signal. Behav. 2016, 11, e1169359. [Google Scholar] [CrossRef] [PubMed]
- de Ollas, C.; Arbona, V.; Gómez-Cadenas, A.; Dodd, I.C. Attenuated accumulation of jasmonates modifies stomatal responses to water deficit. J. Exp. Bot. 2018, 69, 2103–2116. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Kong, X.; Zhang, Y.; Li, W.; Zhang, D.; Dai, J.; Fang, S.; Chu, J.; Dong, H. Leaf-derived jasmonate mediates water uptake from hydrated cotton roots under partial root-zone irrigation. Plant Physiol. 2019, 180, 1660–1676. [Google Scholar] [CrossRef]
- Lin, S.-I.; Chiang, S.-F.; Lin, W.-Y.; Chen, J.-W.; Tseng, C.-Y.; Wu, P.-C.; Chiou, T.-J. Regulatory network of microRNA399 and PHO2 by systemic signaling. Plant Physiol. 2008, 147, 732–746. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).