Biochar-Ca and Biochar-Al/-Fe-Mediated Phosphate Exchange Capacity are Main Drivers of the Different Biochar Effects on Plants in Acidic and Alkaline Soils
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nutrients Content in Biochar
2.2. Attenuated Total Reflection-Fourier Transform Infrared Spectroscopy
2.3. Nuclear Magnetic Resonance Spectroscopies
2.4. Scanning Electron Microscopy (SEM)
2.5. Biochar Integration on Granulated Fertilizers and Growth Chamber Experiment
2.6. Biochar Adsorption Isotherm Kinetics
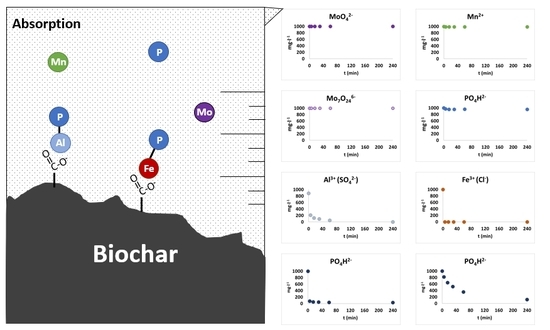
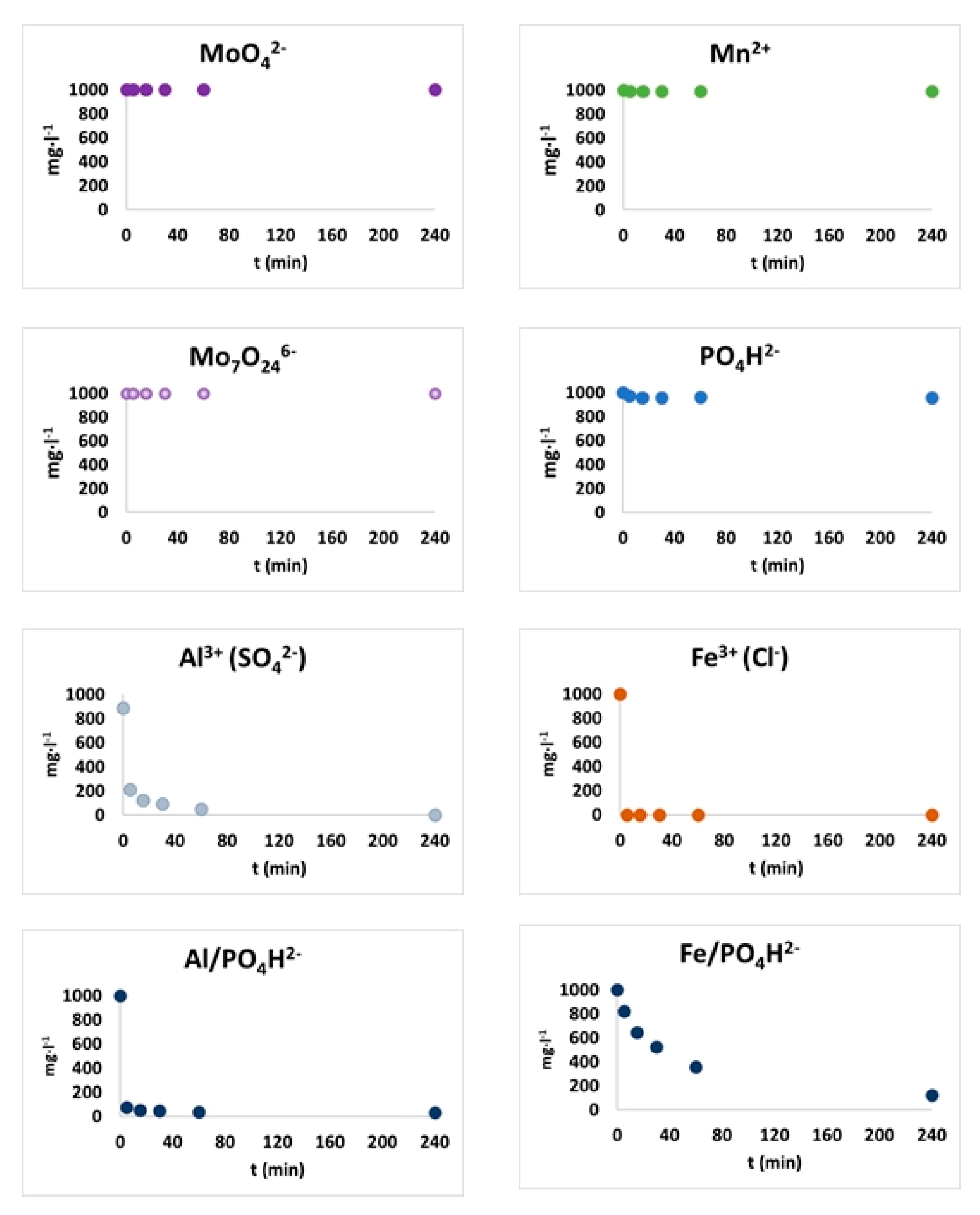
2.7. Biochar-Soil Solution Interactions
2.8. Greenhouse Experiment
2.9. Statistical Analysis
3. Results
3.1. Biochar Characterization
3.2. Growth Chamber Experiment
3.3. Biochar-Nutrients Adsorption Isotherm Kinetics
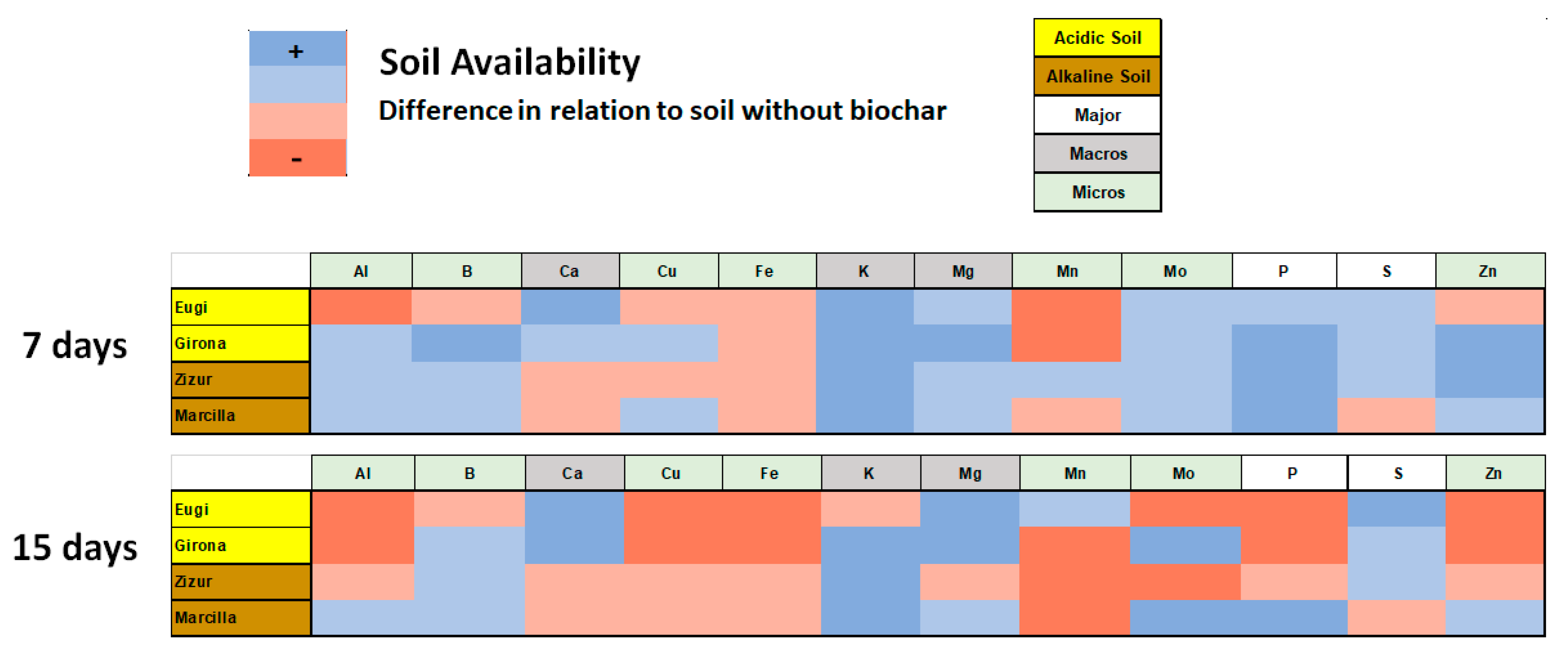
3.4. Biochar-Soil Solution Interactions
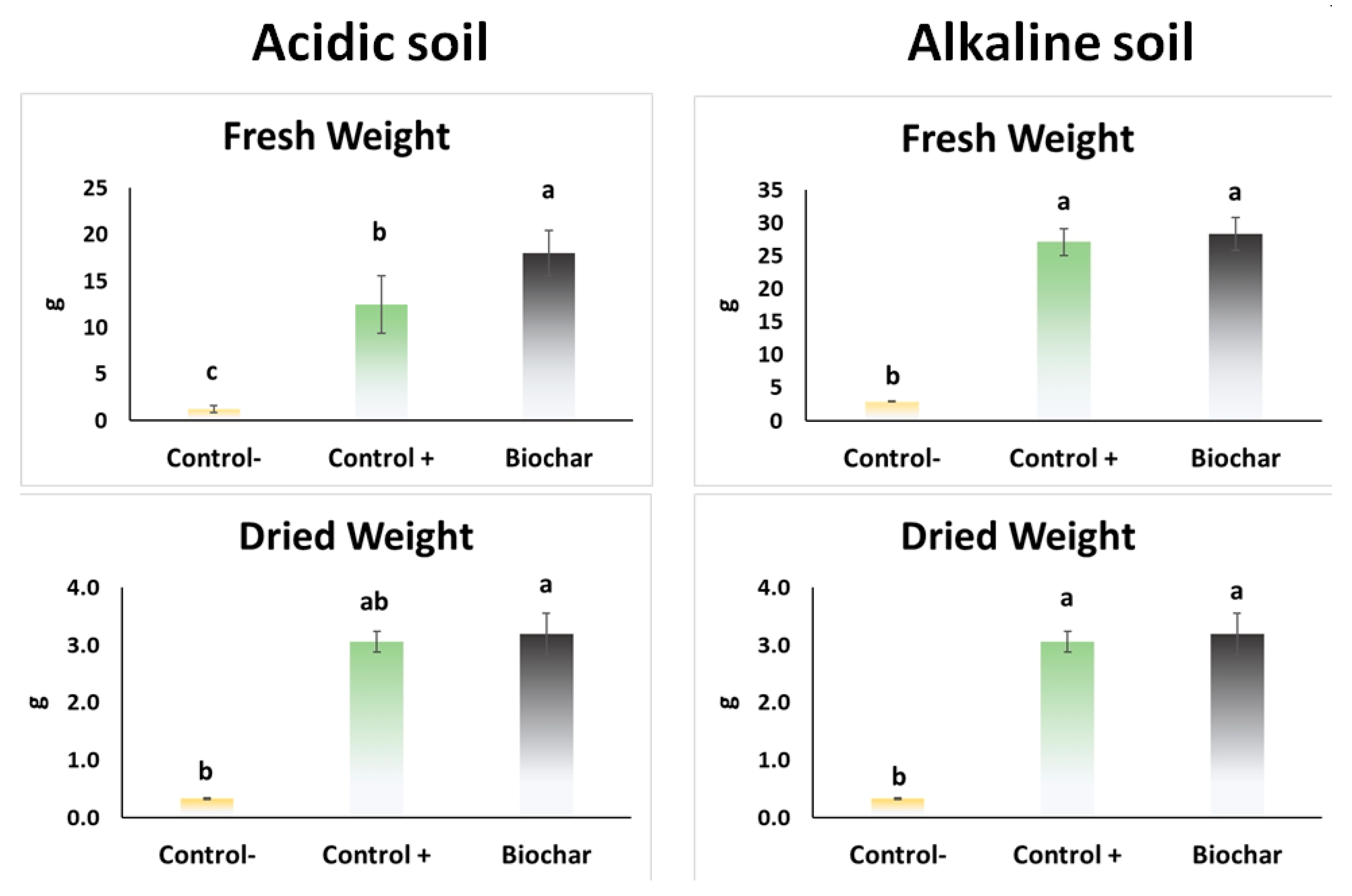
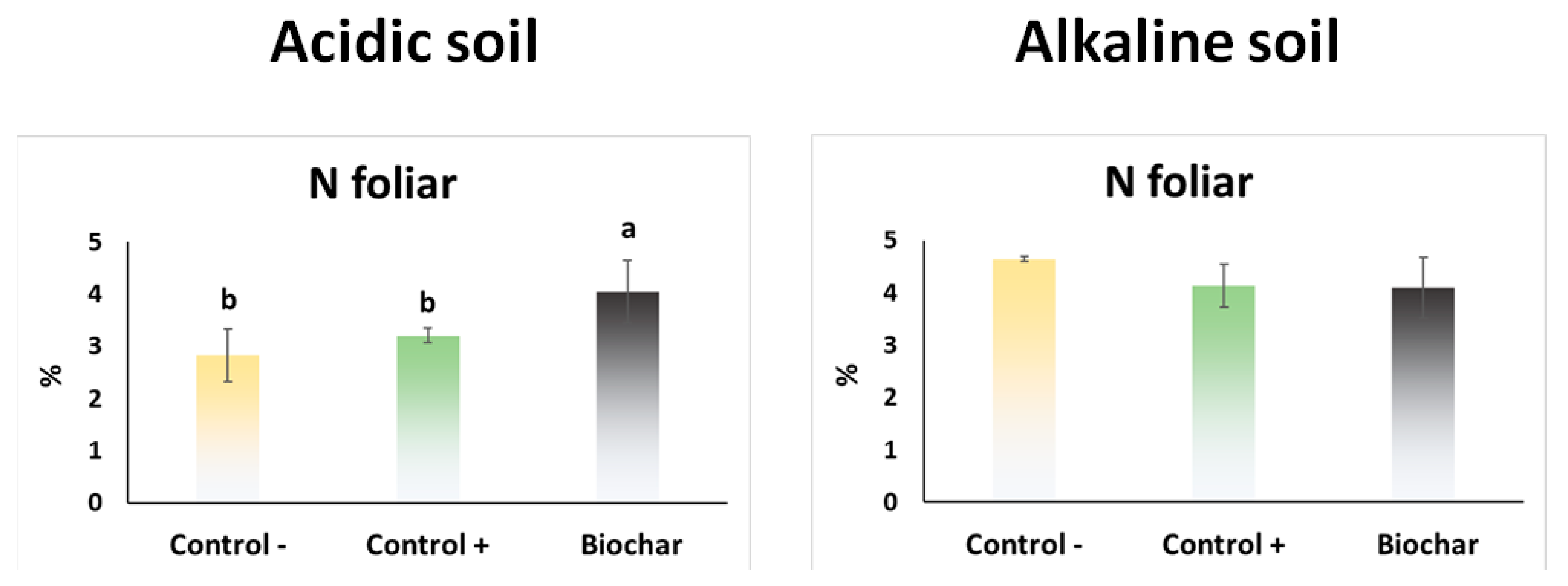
3.5. Greenhouse Experiment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lone, A.H.; Najar, G.R.; Ganie, M.A.; Sofi, J.A.; Ali, T. Biochar for Sustainable Soil Health: A Review of Prospects and Concerns. Pedosphere 2015, 25, 639–653. [Google Scholar] [CrossRef]
- Subedi, R.; Bertora, C.; Zavattaro, L.; Grignani, C. Crop response to soils amended with biochar: Expected benefits and unintended risks. Ital. J. Agron. 2017, 12, 161–173. [Google Scholar] [CrossRef] [Green Version]
- Lehmann, J.; Joseph, S. Biochar for Environmental Management; Taylor & Francis Group, Routledge: London, UK, 2015; ISBN 9781844076581. [Google Scholar]
- Solaiman, Z.M.; Anawar, H.M. Application of Biochars for Soil Constraints: Challenges and Solutions. Pedosphere 2015, 25, 631–638. [Google Scholar] [CrossRef]
- Anawar, H.M.; Akter, F.; Solaiman, Z.M.; Strezov, V. Biochar: An Emerging Panacea for Remediation of Soil Contaminants from Mining, Industry and Sewage Wastes. Pedosphere 2015, 25, 654–665. [Google Scholar] [CrossRef]
- Trevisan, S.; Francioso, O.; Quaggiotti, S.; Nardi, S. Humic substances biological activity at the plant-soil interface: From environmental aspects to molecular factors. Plant Signal. Behav. 2010, 5, 635–643. [Google Scholar] [CrossRef] [Green Version]
- Barthod, J.; Rumpel, C.; Dignac, M.F. Composting with additives to improve organic amendments. A review. Agron. Sustain. Dev. 2018, 38. [Google Scholar] [CrossRef] [Green Version]
- Salem, T.M.; Refaie, K.M.; Abd, A.E.H.E.G.; Sherif, E.-L.; Eid, M.A.M. Biochar application in alkaline soil and its effect on soil and plant. Acta Agric. Slov. 2019, 114, 85–96. [Google Scholar] [CrossRef] [Green Version]
- Smith, N.J.H. Anthrosols and Human Carrying Capacity in Amazonia. Ann. Assoc. Am. Geogr. 2016, 70, 553–566. [Google Scholar] [CrossRef]
- Masiello, C.A.; Chen, Y.; Gao, X.; Liu, S.; Cheng, H.Y.; Bennett, M.R.; Rudgers, J.A.; Wagner, D.S.; Zygourakis, K.; Silberg, J.J. Biochar and microbial signaling: Production conditions determine effects on microbial communication. Environ. Sci. Technol. 2013, 47, 11496–11503. [Google Scholar] [CrossRef] [Green Version]
- Lashari, M.S.; Ye, Y.; Ji, H.; Kibue, G.W.; Lu, H.; Zheng, J.; Pan, G. Biochar—Manure compost in conjunction with pyroligneous solution alleviated salt stress and improved leaf bioactivity of maize in a saline soil from central China: A 2-year field experiment. Sci. Food Agric. 2018. [Google Scholar] [CrossRef]
- Ni, B.J.; Huang, Q.S.; Wang, C.; Ni, T.Y.; Sun, J.; Wei, W. Competitive adsorption of heavy metals in aqueous solution onto biochar derived from anaerobically digested sludge. Chemosphere 2019, 219, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Zhou, N.; Zu, J.; Feng, Q.; Chen, H.; Li, J.; Zhong, M.E.; Zhou, Z.; Zhuang, S. Effect of pyrolysis condition on the adsorption mechanism of heavy metals on tobacco stem biochar in competitive mode. Environ. Sci. Pollut. Res. 2019, 26, 26947–26962. [Google Scholar] [CrossRef] [PubMed]
- Hiller, E.; Fargašová, A.; Zemanová, L.; Bartal, M. Influence of Wheat Ash on the MCPA Immobilization in Agricultural Soils. Bull. Environ. Contam. Toxicol. 2007, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, Y.; Xu, Y.; Lu, X. Biochar phosphorus fertilizer effects on soil phosphorus availability. Chemosphere 2020, 244, 125471. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; DeLuca, T.H. Influence of Biochar on Soil Nutrient Transformations, Nutrient Leaching, and Crop Yield. Adv. Plants Agric. Res. 2016, 4, 348–362. [Google Scholar] [CrossRef] [Green Version]
- Jay, C.N.; Fitzgerald, J.D.; Hipps, N.A.; Atkinson, C.J. Why short-term biochar application has no yield benefits: Evidence from three field-grown crops. Soil Use Manag. 2015, 31, 241–250. [Google Scholar] [CrossRef]
- Dos Santos, S.R.; Filho, J.F.L.; Vergütz, L.; Melo, L.C.A. Biochar association with phosphate fertilizer and its influence on phosphorus use efficiency by maize. Cienc. Agrotec. 2019, 43. [Google Scholar] [CrossRef] [Green Version]
- Solaiman, Z.M.; Abbott, L.K.; Murphy, D.V. Biochar phosphorus concentration dictates mycorrhizal colonisation, plant growth and soil phosphorus cycling. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Novotny, E.H.; Auccaise, R.; Velloso, M.H.R.; Corrêa, J.C.; Higarashi, M.M.; Abreu, V.M.N.; Rocha, J.D.; Kwapinski, W. Characterization of phosphate structures in biochar from swine bones. Pesqui. Agropecu. Bras. 2012, 47, 672–676. [Google Scholar] [CrossRef] [Green Version]
- Puga, A.P.; Grutzmacher, P.; Cerri, C.E.P.; Ribeirinho, V.S.; Andrade, C.A. de Biochar-based nitrogen fertilizers: Greenhouse gas emissions, use efficiency, and maize yield in tropical soils. Sci. Total Environ. 2020, 704, 135375. [Google Scholar] [CrossRef]
- Shen, Y.S.; Wang, S.L.; Tzou, Y.M.; Yan, Y.Y.; Kuan, W.H. Removal of hexavalent Cr by coconut coir and derived chars—The effect of surface functionality. Bioresour. Technol. 2012, 104, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Ma, L.Q.; Zhu, Y.; Li, Y.; Gu, B. Mechanistic investigation of mercury sorption by Brazilian pepper biochars of different pyrolytic temperatures based on x-ray photoelectron spectroscopy and flow calorimetry. Environ. Sci. Technol. 2013, 47, 12156–12164. [Google Scholar] [CrossRef]
- Glusker, P. Citrate Conformation and Chelation: Enzymatic Implications. Acc. Chem. Res. 1980, 13, 345–352. [Google Scholar] [CrossRef]
- Wu, T.; Gruissem, W.; Bhullar, N.K. Plant Science Facilitated citrate-dependent iron translocation increases rice endosperm iron and zinc concentrations. Plant Sci. 2018, 270, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.F.; Ryan, P.R.; Delhaize, E.; Ryan, P.R.; Delhaize, E. Aluminium tolerance in plants and the complexing role of organic acids. Trends Plant Sci. 2001, 6, 273–278. [Google Scholar] [CrossRef]
- Hüppi, R.; Felber, R.; Neftel, A.; Six, J.; Leifeld, J. Effect of biochar and liming on soil nitrous oxide emissions from a temperate maize cropping system. Soil 2015, 1, 707–717. [Google Scholar] [CrossRef]
- Zhou, L.; Xu, D.; Li, Y.; Pan, Q.; Wang, J.; Xue, L.; Howard, A. Phosphorus and nitrogen adsorption capacities of biochars derived from feedstocks at different pyrolysis temperatures. Water 2019, 11, 1559. [Google Scholar] [CrossRef] [Green Version]
- Gerke, J. Humic (organic matter)-Al(Fe)-phosphate complexes: An underestimated phosphate form in soils and source of plant-available phosphate. Soil Sci. 2010, 175, 417–425. [Google Scholar] [CrossRef]
- Wang, H.; Zhu, J.; Fu, Q.L.; Xiong, J.W.; Hong, C.; Hu, H.Q.; Violante, A. Adsorption of phosphate onto ferrihydrite and ferrihydrite-humic acid complexes. Pedosphere 2015, 25, 405–414. [Google Scholar] [CrossRef]
- Guardado, I.; Urrutia, O.; García-Mina, J.M. Size distribution, complexing capacity, and stability of phosphate-metal-humic complexes. J. Agric. Food Chem. 2007, 55, 408–413. [Google Scholar] [CrossRef]
- Li, H.; Dong, X.; da Silva, E.B.; de Oliveira, L.M.; Chen, Y.; Ma, L.Q. Mechanisms of metal sorption by biochars: Biochar characteristics and modifications. Chemosphere 2017, 178, 466–478. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Liu, Y.; Chen, Y.; Wang, S.; Wang, M.; Xie, T.; Wang, G. Biochar amendment immobilizes lead in rice paddy soils and reduces its phytoavailability. Sci. Rep. 2016, 6, 1–8. [Google Scholar] [CrossRef]
- Pourret, O.; Houben, D. Characterization of metal binding sites onto biochar using rare earth elements as a fingerprint. Heliyon 2018, 4, e00543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahmoud, E.; El-Beshbeshy, T.; El-Kader, N.A.; El Shal, R.; Khalafallah, N. Impacts of biochar application on soil fertility, plant nutrients uptake and maize (Zea mays L.) yield in saline sodic soil. Arab. J. Geosci. 2019, 12. [Google Scholar] [CrossRef]
- Rodrigues, M.A.; Garmus, T.; Arrobas, M.; Gonçalves, A.; Silva, E.; Rocha, L.; Pinto, L.; Brito, C.; Martins, S.; Vargas, T.; et al. Combined biochar and organic waste have little effect on chemical soil properties and plant growth. Span. J. Soil Sci. 2019, 9, 199–211. [Google Scholar] [CrossRef]
- Kraska, P.; Oleszczuk, P.; Andruszczak, S.; Kwiecińska-Poppe, E.; Różyło, K.; Pałys, E.; Gierasimiuk, P.; Michałojć, Z. Effect of various biochar rates on winter rye yield and the concentration of available nutrients in the soil. Plant Soil Environ. 2016, 62, 483–489. [Google Scholar] [CrossRef] [Green Version]
- Hagemann, N.; Joseph, S.; Schmidt, H.P.; Kammann, C.I.; Harter, J.; Borch, T.; Young, R.B.; Varga, K.; Taherymoosavi, S.; Elliott, K.W.; et al. Organic coating on biochar explains its nutrient retention and stimulation of soil fertility. Nat. Commun. 2017, 8, 1–11. [Google Scholar] [CrossRef]


| 13C-NMR Spectroscopy (%) | 1H-NMR Spectroscopy (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Alkyl C | O-alkyl C | Arom. C | Phen. C | Carbox. C | Alkyl H | O-alkyl H | Arom. H 1 | Carbox. H |
| 19.8 | 30.9 | 28.2 | 10.9 | 10.2 | 40.3 | 24.9 | 30.05(6.65) | 3.75 |
| mg kg−1 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Al | B | Ca | Cu | Fe | K | Mg | Mn | Mo | Na | P | S | Zn |
| 1092 | 70.8 | 58,492 | 26.5 | 953 | 6601 | 6459 | 678 | <0.1 | 1005 | 1981 | 663 | 70.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baigorri, R.; San Francisco, S.; Urrutia, Ó.; García-Mina, J.M. Biochar-Ca and Biochar-Al/-Fe-Mediated Phosphate Exchange Capacity are Main Drivers of the Different Biochar Effects on Plants in Acidic and Alkaline Soils. Agronomy 2020, 10, 968. https://doi.org/10.3390/agronomy10070968
Baigorri R, San Francisco S, Urrutia Ó, García-Mina JM. Biochar-Ca and Biochar-Al/-Fe-Mediated Phosphate Exchange Capacity are Main Drivers of the Different Biochar Effects on Plants in Acidic and Alkaline Soils. Agronomy. 2020; 10(7):968. https://doi.org/10.3390/agronomy10070968
Chicago/Turabian StyleBaigorri, Roberto, Sara San Francisco, Óscar Urrutia, and José María García-Mina. 2020. "Biochar-Ca and Biochar-Al/-Fe-Mediated Phosphate Exchange Capacity are Main Drivers of the Different Biochar Effects on Plants in Acidic and Alkaline Soils" Agronomy 10, no. 7: 968. https://doi.org/10.3390/agronomy10070968
APA StyleBaigorri, R., San Francisco, S., Urrutia, Ó., & García-Mina, J. M. (2020). Biochar-Ca and Biochar-Al/-Fe-Mediated Phosphate Exchange Capacity are Main Drivers of the Different Biochar Effects on Plants in Acidic and Alkaline Soils. Agronomy, 10(7), 968. https://doi.org/10.3390/agronomy10070968