Relevance of Plant Growth Promoting Microorganisms and Their Derived Compounds, in the Face of Climate Change
Abstract
:1. Introduction
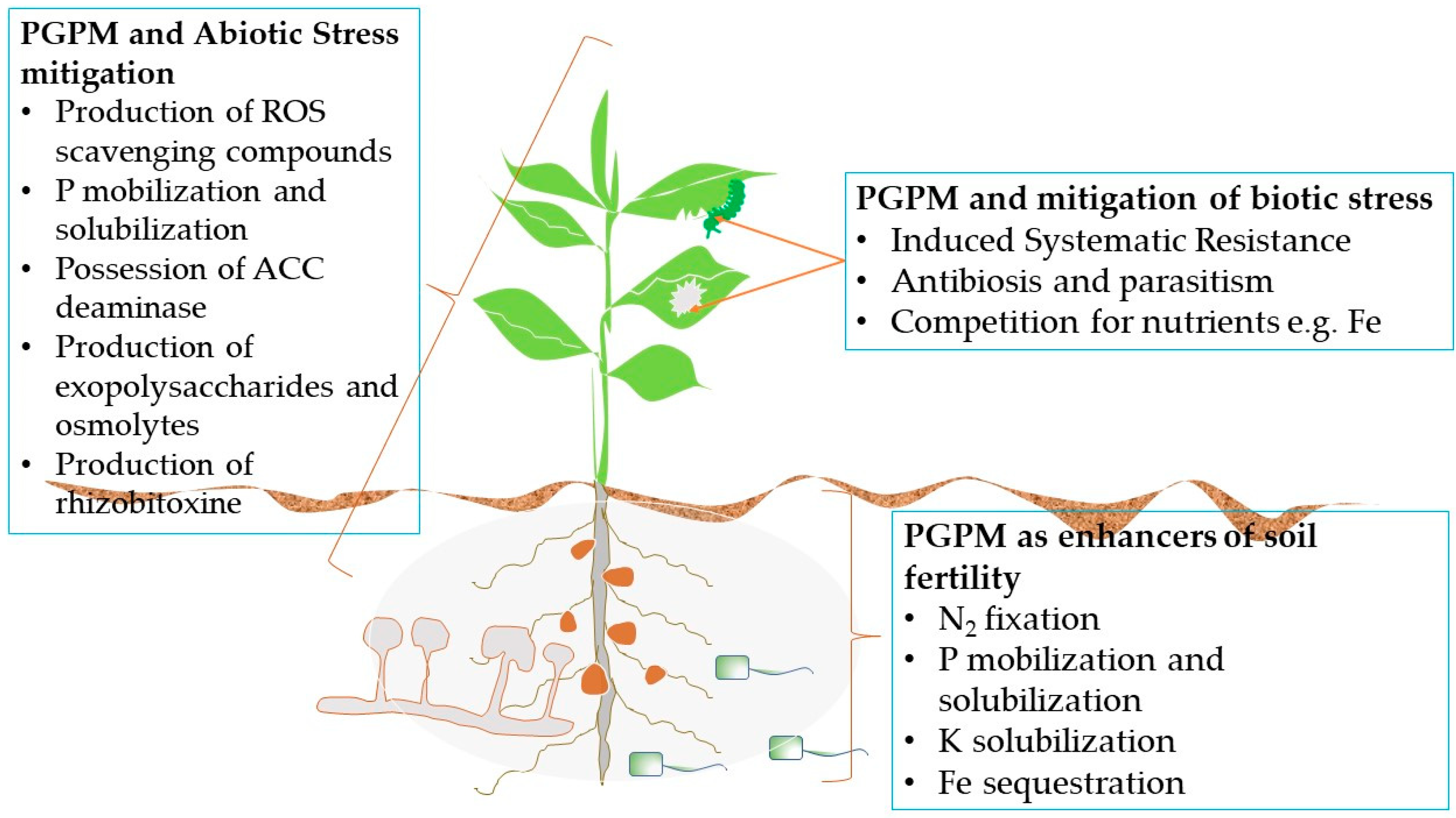
2. PGPM as Enhancers of Soil Fertility
2.1. Nitrogen Fixation
2.2. Phosphate Mobilisation and Solubilisation
2.3. Sequestering of Iron
2.4. Potassium Solubilisation
3. PGPM and Control of Plant Pests and Diseases
4. PGPM and Abiotic Stress
5. Commercialisation of Microbial Inoculants
Formulation of Microbial Inoculants for Commercial Purposes and Their Mode of Application
6. Limitations to Global Use of Microbial Inoculants
7. Microbial Consortia
8. Microbial Compounds as “Inoculants”
9. Microbial Cells or Microbial Compounds?
10. Way Forward and Recommendations
11. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lott, F.C.; Christidis, N.; Stott, P.A. Can the 2011 East African drought be attributed to human-induced climate change? Geophys. Res. Lett. 2013, 40, 1177–1181. [Google Scholar] [CrossRef]
- Rossi, F.; Olguın, E.J.; Diels, L.; de Philippis, R. Microbial fixation of CO2 in waterbodies and in drylands to combat climate change, soil loss and desertification. New Biotechnol. 2015, 32, 109–120. [Google Scholar] [CrossRef]
- Bradley, B.A.; Curtis, C.A.; Chambers, J.C. Bromus Response to Climate and Projected Changes with Climate Change. In Exotic Brome-Grasses in Arid and Semiarid Ecosystems of the Western US; Germino, M., Chambers, J., Brown, C., Eds.; Springer Series on Environmental Management; Springer: Cham, Switzerland, 2016; pp. 257–274. [Google Scholar] [CrossRef]
- Richards, M.B.; Wollenberg, E.; van Vuuren, D. National contributions to climate change mitigation from agriculture: Allocating a global target. Clim. Policy 2018, 18, 1271–1285. [Google Scholar] [CrossRef] [Green Version]
- Loboguerrero, A.M.; Campbell, B.M.; Cooper, P.J.M.; Hansen, J.W.; Rosenstock, T.; Wollenberg, E. Food and Earth Systems: Priorities for Climate Change Adaptation and Mitigation for Agriculture and Food Systems. Sustainability 2019, 11, 1372. [Google Scholar] [CrossRef] [Green Version]
- IPCC. 2014: Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Pachauri, R.K., Meyer, L.A., Eds.; IPCC: Geneva, Switzerland, 2014; p. 151. [Google Scholar]
- Porter, J.R.; Xie, L.; Challinor, A.J.; Cochrane, K.; Howden, S.M.; Iqbal, M.M.; Lobell, D.B.; Travasso, M.I. Food security and food production systems. In Climate Change 2014: Impacts, Adaptation, and Vulnerability Part A: Global and Sectoral Aspects. Contribution of Working GroupII to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Field, C.B., Barros, V.R., Dokken, D.J., Mach, K.J., Mastrandrea, M.D., Bilir, T.E., Chatterjee, M., Ebi, K.L., Estrada, Y.O., Genova, R.C., et al., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014; pp. 485–533. [Google Scholar]
- Dawson, T.P.; Perryman, A.H.; Osborne, T.M. Modelling impacts of climate change on global food security. Clim. Chang. 2016, 134, 429–440. [Google Scholar] [CrossRef]
- Bouwer, L.M.; Bubeck, P.; Aerts, J.C. Changes in future flood risk due to climate and development in a Dutch polder area. Glob. Environ. Chang. 2010, 20, 463–471. [Google Scholar] [CrossRef]
- Mirza, M.M.Q. Climate change, flooding in South Asia and implications. Reg. Environ. Chang. 2011, 11, 95–107. [Google Scholar] [CrossRef]
- Nam, W.; Hayes, M.J.; Svoboda, M.D.; Tadesse, T.; Wilhite, D.A. Drought hazard assessment in the context of climate change for South Korea. Agric. Water Manag. 2015, 160, 106–117. [Google Scholar] [CrossRef]
- Barea, J.M. Future challenges and perspectives for applying microbial biotechnology in sustainable agriculture based on a better understanding of plant-microbiome interactions. J. Soil Sci. Plant Nutr. 2015, 15, 261–282. [Google Scholar] [CrossRef]
- Gupta, G.; Parihar, S.S.; Ahirwar, N.K.; Snehi, S.K.; Singh, V. Plant Growth Promoting Rhizobacteria (PGPR): Current and Future Prospects for Development of Sustainable Agriculture. Microb. Biochem. Technol. 2015, 7, 96–102. [Google Scholar] [CrossRef]
- Bender, S.F.; Wagg, C.; van der Heijden, M.G.A. An Underground Revolution: Biodiversity and Soil Ecological Engineering for Agricultural Sustainability. Trends Ecol. Evol. 2016, 31, 440–452. [Google Scholar] [CrossRef] [PubMed]
- Babalola, O.O.; Glick, B.R. The use of microbial inoculants in African agriculture: Current practice and future prospects. J. Food Agric. Environ. 2012, 10, 540–549. [Google Scholar]
- Smith, D.L.; Subramanian, S.; Lamont, J.R.; Bywater-Ekegärd, M. Signaling in the phytomicrobiome: Breadth and potential. Front. Plant Sci. 2015, 6, 709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, D.L.; Gravel, V.; Yergeau, E. Signaling in the Phytomicrobiome. Front. Plant Sci. 2017, 8, 611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Backer, R.; Rokem, J.S.; Ilangumaran, G.; Lamont, J.; Praslickova, D.; Ricci, E.; Subramanian, S.; Smith, D.L. Plant Growth-Promoting Rhizobacteria: Context, Mechanisms of Action, and Roadmap to Commercialization of Bio stimulants for Sustainable Agriculture. Front. Plant Sci. 2018, 9, 1473. [Google Scholar] [CrossRef] [Green Version]
- Hartmann, A.; Rothballer, M.; Hense, B.A.; Schröder, P. Bacterial quorum sensing compounds are important modulators of microbe-plant interactions. Front. Plant Sci. 2014, 5, 131. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Cañizares, C.; Jorrín, B.; Poole, P.S.; Tkacz, A. Understanding the holobiont: The interdependence of plants and their microbiome. Curr. Opin. Microbiol. 2017, 38, 188–196. [Google Scholar] [CrossRef]
- Compant, S.; Samad, A.; Faist, H.; Sessitsch, A. A review on the plant microbiome: Ecology, functions and emerging trends in microbial applications. J. Adv. Res. 2019, 19, 29–37. [Google Scholar] [CrossRef]
- Berg, M.; Koskella, B. Nutrient and dose dependent protection against a plant pathogen. Curr. Biol. 2018, 28, 2487–2492. [Google Scholar] [CrossRef] [Green Version]
- Bashan, Y.; de-Bashan, L.E.; Prabhu, S.R.; Hernandez, J. Advances in plant growth-promoting bacterial inoculant technology: Formulations and practical perspectives (1998–2013). Plant Soil 2014, 378, 1–33. [Google Scholar] [CrossRef] [Green Version]
- Alori, E.T.; Dare, M.O.; Babalola, O.O. Microbial Inoculants for Soil Quality and Plant Health. In Sustainable Agriculture Reviews; Lichtfouse, E., Ed.; Springer: Cham, Switzerland, 2017; Volume 22, pp. 281–307. [Google Scholar] [CrossRef]
- Malusa, E.; Sas-Paszt, L.; Ciesielska, J. Technologies for Beneficial Microorganisms Inocula Used as Biofertilizers. Sci. World J. 2012, 2012, 491206. [Google Scholar] [CrossRef]
- Silva, E.C.; Nogueira, R.J.M.C.; Silva, M.A.; Albuquerque, M.B. Drought Stress and Plant Nutrition. Plant Stress 2011, 5, 32–41. [Google Scholar]
- DaMatta, F.; Loos, R.A.; Silva, E.A.; Loureiro, M.E.; Ducatti, C. Effects of soil water déficit and nitrogen nutrition on water relations and photosynthesis of pot-grown Coffea canephora Pierra. Trees 2002, 16, 555–558. [Google Scholar] [CrossRef]
- Tietema, A.; De Boer, W.; Riemer, L.; Verstraten, J.M. Nitrate production in nitrogen saturated acid forest soils: Vertical distributions and characteristics. Soil Biol. Biochem. 1992, 24, 235–240. [Google Scholar] [CrossRef]
- Tripathi, D.K.; Singh, S.; Gaur, S.; Singh, S.; Yadav, V.; Liu, S.; Singh, V.P.; Sharma, S.; Srivastava, P.; Prasad, S.M.; et al. Acquisition and Homeostasis of Iron in Higher Plants and Their Probable Role in Abiotic Stress Tolerance. Front. Environ. Sci. 2018, 5, 86. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.; Zheng, Q.; Shen, Q.; Guo, S. The Critical Role of Potassium in Plant Stress Response. Int. J. Mol. Sci. 2013, 14, 7370–7390. [Google Scholar] [CrossRef] [Green Version]
- Waraich, E.A.; Ahmad, R.; Halim, A.; Aziz, T. Alleviation of temperature stress by nutrient management in crop plants: A review. J. Soil Sci. Plant Nutr. 2012, 12, 221–244. [Google Scholar] [CrossRef] [Green Version]
- Waraich, E.A.; Ahmad, R.; Ashraf, M.Y.; Saifullah; Ahmad, M. Improving agricultural water use efficiency by nutrient management in crop plants. Acta Agriculturae Scandinavica. Sect. B Plant Soil Sci. 2011, 61, 291–304. [Google Scholar] [CrossRef]
- Karmakar, R.; Das, I.; Dutta, D.; Rakshit, A. Potential Effects of Climate Change on Soil Properties: A Review. Sci. Int. 2016, 4, 51–73. [Google Scholar] [CrossRef] [Green Version]
- Wu, F.U.; Bao, W.; Li, F.L.; Wu, N. Effects of water stress and nitrogen supply on leaf gas exchange and fluorescence parameters of Sophora davidii seedlings. Photosynthetica 2008, 46, 40–48. [Google Scholar] [CrossRef]
- Faye, I.; Diouf, O.; Guisse’, A.; Se`ne, M.; Diallo, N. Characterizing Root Responses to Low Phosphorus in Pearl Millet [Pennisetum glaucum (L.) R. Br.]. Agron. J. 2006, 98, 1187–1194. [Google Scholar] [CrossRef]
- Alori, E.T.; Fawole, O.B. Impact of chemical inputs on arbuscular mycorrhiza spores in soil: Response of AM Spores to fertilizer and herbicides. Alban J. Agric. Sci. 2017, 16, 10–13. [Google Scholar]
- Lal, R. Restoring Soil Quality to Mitigate Soil Degradation. Sustainability 2015, 7, 5875–5895. [Google Scholar] [CrossRef] [Green Version]
- Alori, E.T.; Babalola, O.O. Microbial Inoculants for Improving Crop Quality and Human Health in Africa. Front. Microbiol. 2018, 9, 2213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naamala, J.; Jaiswal, S.K.; Dakora, F.D. Microsymbiont diversity and phylogeny of native Bradyrhizobia associated with soybean (Glycine max L. Merr.) nodulation in South African soils. Syst. Appl. Microbiol. 2016, 39, 336–344. [Google Scholar] [CrossRef] [Green Version]
- Graham, J.H.; Leonard, R.T.; Menge, J.A. Membrane-Mediated Decrease in Root Exudation Responsible for Phorphorus Inhibition of Vesicular-Arbuscular Mycorrhiza Formation. Plant. Physiol. 1981, 68, 548–552. [Google Scholar] [CrossRef]
- Sprent, J.I.; Stephens, J.H.; Rupela, O.P. Environmental effects on nitrogen fixation. In World Crops: Cool Season Food Legumes; Summerfield, R.J., Ed.; Current Plant Science and Biotechnology in Agriculture; Springer: Dordrech, The Netherlands, 1988; Volume 5, pp. 801–810. [Google Scholar] [CrossRef]
- Marschner, H.; Dell, B. Nutrient uptake in mycorrhizal symbiosis. Plant Soil 1994, 159, 89–102. [Google Scholar] [CrossRef]
- Glick, B.R. Plant growth promoting bacteria: Mechanisms and applications. Scientifica 2012, 2012, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Goldstein, A.H.; Krishnaraj, P.U. Phosphate solubilizing microorganisms vs. phosphate mobilizing microorganisms: What separates a phenotype from a trait? In First International Meeting on Microbial Phosphate Solubilization. Developments in Plant and Soil Sciences; Velázquez, E., Rodríguez-Barrueco, C., Eds.; Springer: Dordrecht, The Netherlands, 2007; Volume 102, pp. 203–213. [Google Scholar] [CrossRef]
- Meding, S.M.; Zasoski, R.J. Hyphal-mediated transfer of nitrate, arsenic, cesium, rubidium, and strontium between arbuscular mycorrhizal forbs and grasses from a California oak woodland. Soil Biol. Biochem. 2008, 40, 126–134. [Google Scholar] [CrossRef]
- Hayat, R.; Ali, S.; Amara, U.; Khalid, R.; Ahmed, I. Soil beneficial bacteria and their role in plant growth promotion: A review. Ann. Microbiol. 2010, 60, 579–598. [Google Scholar] [CrossRef]
- Straker, C.J.; Mitchell, D.T. The activity and characterization of acid phosphatases in endomycorrhizal fungi of the Ericaceae. New Phytol. 1986, 104, 243–256. [Google Scholar] [CrossRef]
- Khan, M.S.; Zaidi, A.; Ahemad, M.; Oves, M.; Wani, P.A. Plant growth promotion by phosphate solubilizing fungi—Current perspective. Arch. Agron. Soil Sci. 2010, 56, 73–98. [Google Scholar] [CrossRef]
- Elias, F.; Woyessa, D.; Muleta, D. Phosphate Solubilisation Potential of Rhizosphere Fungi Isolated from Plants in Jimma Zone, Southwest Ethiopia. Int. J. Microbiol. 2016, 2016, 5472601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daly, D.H.; Velivelli, S.L.S.; Prestwich, B.D. The Role of Soil Microbes in Crop Biofortification. In Agriculturally Important Microbes for Sustainable Agriculture; Meena, V., Mishra, P., Bisht, J., Pattanayak, A., Eds.; Springer: Singapore, 2017. [Google Scholar] [CrossRef]
- Khan, A.; Singh, J.; Upadhayay, V.K.; Singh, A.V.; Shah, S. Microbial Biofortification: A Green Technology Through Plant Growth Promoting Microorganisms. In Sustainable Green Technologies for Environmental Management; Shah, S., Venkatramanan, V., Prasad, R., Eds.; Springer: Singapore, 2019. [Google Scholar] [CrossRef]
- Bhatti, T.M.; Yawar, W. Bacterial solubilization of phosphorus from phosphate rock containing sulfur-mud. Hydrometallurgy 2010, 103, 54–59. [Google Scholar] [CrossRef]
- Radzki, W.; Gutierrez Manero, F.J.; Algar, E.; Lucas Garcıa, J.A.; Garcıa-Villaraco, A.; Solano, B.R. Bacterial siderophores efficiently provide iron to iron-starved tomato plants in hydroponics culture. Antonie Van Leeuwenhoek 2013, 104, 321–330. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Johri, B.N. Growth promoting influence of siderophore-producing Pseudomonas strains GRP3A and PRS9 in maize (Zea mays L.) under iron limiting conditions. Microbiol. Res. 2003, 158, 243–248. [Google Scholar] [CrossRef]
- Yehuda, Z.; Shenker, M.; Romheld, V.; Marschner, H.; Hadar, Y.; Chen, Y. The Role of Ligand Exchange in the uptake of Iron from Microbial Siderophores by Cramineous Plants. Plant Physiol. 1996, 112, 1273–1280. [Google Scholar] [CrossRef] [Green Version]
- Nadeem, S.M.; Zahir, Z.A.; Naveed, M.; Ashghar, H.N.; Arshad, M. Rhizobacteria capable of producing ACC deaminase may mitigate salt stress in wheat. Soil Biol. Biochem. 2010, 74, 533–542. [Google Scholar] [CrossRef]
- Meena, V.S.; Maurya, B.R.; Prakash, J. Does a rhizospheric microorganism enhance K+ availability in agricultural soils? Microbiol. Res. 2014, 169, 337–347. [Google Scholar] [CrossRef]
- Cardoso, I.M.; Kuyper, T.W. Mycorrhizas and tropical soil fertility. Agric. Ecosyst. Environ. 2006, 116, 72–84. [Google Scholar] [CrossRef]
- Jalili, F.; Khavazi, K.; Pazira, E.; Nejati, A.; Rahmani, H.A.; Sadaghiani, H.R.; Miransari, M. Isolation and characterization of ACC deaminase-producing fluorescent pseudomonads, to alleviate salinity stress on canola (Brassica napus L.) growth. J. Plant Physiol. 2008, 166, 667–674. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Charles, T.C.; Glick, B.R. Amelioration of high salinity stress damage by plant growth promoting bacterial endophytes that contain ACC deaminase. Plant Physiol. Biochem. 2014, 80, 160–167. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Montano, F.; Alías-Villegas, C.; Bellogín, R.A.; del Cerro, P.; Espuny, M.R.; Jiménez-Guerrero, I.; López-Baena, F.J.; Ollero, F.J.; Cubo, T. Plant growth promotion in cereal and leguminous agricultural important plants: From microorganism capacities to crop production. Microbiol. Res. 2014, 169, 325–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jha, C.K.; Saraf, M. Plant growth promoting Rhizobacteria (PGPR): A review. E3 J. Agric. Res. Dev. 2015, 5, 0108–0119. [Google Scholar]
- Dodd, I.C.; Ruiz-Lozano, J.M. Microbial Enhancement of crop resource use efficiency. Curr. Opin. Biotechnol. 2012, 23, 236–242. [Google Scholar] [CrossRef]
- Hassen, A.I.; Bopape, F.L.; Sanger, L.K. Microbial Inoculants as Agents of Growth Promotion and Abiotic Stress Tolerance in Plants. In Microbial Inoculants in Sustainable Agricultural Productivity; Singh, D., Singh, H., Prabha, R., Eds.; Springer: New Delhi, India, 2016; pp. 23–36. [Google Scholar] [CrossRef]
- Recep, K.; Fikrettin, S.; Erkol, D.; Cafer, E. Biological control of the potato dry rot caused by Fusarium species using PGPR strains. Biol. Control 2009, 50, 194–198. [Google Scholar] [CrossRef]
- Moussa, T.A.A.; Almaghrabi, O.A.; Abdel-Moneim, T.S. Biological control of the wheat root rot caused by Fusarium graminearum using some PGPR strains in Saudi Arabia. Ann. Appl. Biol. 2013, 163, 72–81. [Google Scholar] [CrossRef]
- Díaz, A.; Smith, A.; Mesa, P.; Zapata, J.; Caviedes, D.; Cotes, A.M. Control of Fusarium Wilt in Cape Gooseberry by Trichoderma koningiopsis and PGPR; Pertot, I., Elad, Y., Barka, E.A., Clément, C., Eds.; Working Group Biological Control of Fungal and Bacterial Plant Pathogens; IOBC Bulletin: Dijon, France, 2013; Volume 86, pp. 89–94. [Google Scholar]
- Vanitha, S.; Ramjegathesh, R. Bio Control Potential of Pseudomonas fluorescens against Coleus Root Rot Disease. J. Plant Pathol. Microb. 2014, 5, 216. [Google Scholar] [CrossRef]
- Dixit, R.; Agrawal, L.; Gupta, S.; Kumar, M.; Yadav, S.; Chauhan, P.S.; Nautiyal, C.S. Southern blight disease of tomato control by 1-aminocyclopropane1-carboxylate (ACC) deaminase producing Paenibacillus lentimorbus B30488. Plant Signal. Behav. 2016, 11, e1113363. [Google Scholar] [CrossRef] [Green Version]
- Li, X.L.; George, E.; Marschner, H. Extension of the phosphorus depletion zone in VA-mycorrhizal white clover in a calcareous soil. Plant Soil 1991, 136, 41–48. [Google Scholar] [CrossRef]
- Wu, Y.; Lin, H.; Lin, Y.; Shi, J.; Xue, S.; Hung, Y.; Chen, Y.; Wang, H. Effects of biocontrol bacteria Bacillus amyloliquefaciens LY-1 culture broth on quality attributes and storability of harvested litchi fruit. Postharvest Biol. Technol. 2017, 132, 81–87. [Google Scholar] [CrossRef]
- Zhao, D.; Zhao, H.; Zhao, D.; Zhua, X.; Wang, Y.; Duan, Y.; Xuan, Y.; Chen, L. Isolation and identification of bacteria from rhizosphere soil and their effect on plant growth promotion and root-knot nematode disease. Biol. Control 2018, 119, 12–19. [Google Scholar] [CrossRef]
- de Vrieze, M.; Germanier, F.; Vuille, N.; Weisskopf, L. Combining Different Potato-Associated Pseudomonas Strains for Improved Biocontrol of Phytophthora infestans. Front. Microbiol. 2018, 9, 2573. [Google Scholar] [CrossRef] [PubMed]
- Berendsen, R.L.; Vismans, G.; Yu, K.; Song, Y.; de Jonge, R.; Burgman, W.P.; Burmølle, M.; Herschend, J.; Bakker, P.A.; Pieterse, C.M. Disease-induced assemblage of a plant-beneficial bacterial consortium. ISME J. 2018, 12, 1496–1507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gadhave, K.R.; Hourston, J.E.; Gange, A.C. Developing Soil Microbial Inoculants for Pest Management: Can One Have Too Much of a Good Thing? J. Chem. Ecol. 2016, 42, 348–356. [Google Scholar] [CrossRef]
- Schausberger, P.; Peneder, S.; Juerschik, S.; Hoffmann, D. Mycorrhiza changes plant volatiles to attract spidermite enemies. Funct. Ecol. 2012, 26, 441–449. [Google Scholar] [CrossRef]
- Pangesti, N.; Weldegergis, B.T.; Langendorf, B.; van Loon, J.J.; Dicke, M.; Pineda, A. Rhizobacterial colonization of roots modulates plant volatile emission and enhances the attraction of a parasitoid wasp. to host-infested plants. Oecologia 2015, 178, 1169–1180. [Google Scholar] [CrossRef] [Green Version]
- Herman, M.A.B.; Nault, B.A.; Smart, C.D. Effects of plant growth−promoting rhizobacteria on bell pepper production and green peach aphid infestations in New York. Crop Prot. 2008, 27, 996–1002. [Google Scholar] [CrossRef]
- Velivelli, S.L.S.; Sessitsch, A.; Prestwich, B.D. The Role of Microbial Inoculants in Integrated Crop Management Systems. Potato Res. 2014, 57, 291–309. [Google Scholar] [CrossRef]
- Karthiba, L.; Saveetha, K.; Suresh, S.; Raguchander, T.; Saravanakumar, D.; Samiyappan, R. PGPR and entomopathogenic fungus bioformulation for the synchronous management of leaffolder pest and sheath blight disease of rice. Pest. Manag. Sci. 2010, 66, 555–564. [Google Scholar] [CrossRef]
- Bano, A.; Muqarab, R. Plant defence induced by PGPR against Spodoptera litura in tomato (Solanum lycopersicum L.). Plant Biol. 2017, 19, 406–412. [Google Scholar] [CrossRef] [PubMed]
- Xiang, N.; Lawrence, K.S.; Kloepper, J.W.; Donald, P.A.; McInroy, J.A. Biological control of Heterodera glycines by spore-forming plant growth-promoting rhizobacteria (PGPR) on soybean. PLoS ONE 2017, 12, e0181201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viljoen, J.F.; Labuschagne, N.; Fourie, H.; Sikora, R.A. Biological control of the root-knot nematode Meloidogyne incognita on tomatoes and carrots by plant growth-promoting rhizobacteria. Trop Plant Pathol. 2019, 44, 284–291. [Google Scholar] [CrossRef]
- Frankowski, J.; Lorito, M.; Scala, F.; Schmid, R.; Berg, G.; Bahl, H. Purification and properties of two chitinolytic enzymes of Serratia plymuthica HRO-C48. Arch. Microbiol. 2001, 176, 421–426. [Google Scholar] [CrossRef]
- Kim, Y.C.; Jung, H.; Kim, K.Y.; Park, S.K. An effective biocontrol bioformulation against Phytophthora blight of pepper using growth mixtures of combined chitinolytic bacteria under different field conditions. Eur. J. Plant Pathol. 2008, 120, 373–382. [Google Scholar] [CrossRef]
- Singh, P.P.; Shin, Y.C.; Park, C.S.; Chung, Y.R. Biological control of Fusarium wilt of cucumber by chitinolytic bacteria. Phytopathology 1999, 89, 92–99. [Google Scholar] [CrossRef] [Green Version]
- Leeman, M.; Ouder, F.M.D.; Pelt, J.A.V.; Dirk, F.P.M.; Steij, H.; Bakker, P.A.; Schippers, B. Iron availability affects induction of systemic resistance to Fusarium wilt of radishes by Pseudomonas fluorescens. Phytopathology 1996, 86, 149–155. [Google Scholar] [CrossRef]
- Buysens, S.; Heungens, K.; Poppe, J.; Höftee, M. Involvement of pyochelin and pyoverdin in suppression of Pythium-induced damping-off of tomato by Pseudomonas aeruginosa 7NSK2. Appl. Environ. Microbiol. 1996, 62, 865–871. [Google Scholar] [CrossRef] [Green Version]
- Khandelwal, S.R.; Manwar, A.V.; Chaudhari, B.L.; Chincholkar, S.B. Siderophorogenic bradyrhizobia boost yield of soybean. Appl. Biochem. Biotechnol. 2002, 102, 155–168. [Google Scholar] [CrossRef]
- Toklikishvili, N.; Dandurishvili, M.; Tediashvili, N.; Lurie, G.S.; Szegedi, E.; Glick, B.R.; Chermin, L.N. Inhibitory effect of ACC deaminase-producing bacteria on crown gall formation in tomato plants infected by Agrobacterium tumefaciens or A. vitis. Plant Pathol. 2010, 59, 1023–1030. [Google Scholar] [CrossRef]
- Niu, D.D.; Zheng, Y.; Zheng, L.; Jiang, C.H.; Zhou, D.M.; Guo, J.H. Application of PSX biocontrol preparation confers root-knot nematode management and increased fruit quality in tomato under field conditions. Biocontrol. Sci. Technol. 2016, 26, 174–180. [Google Scholar] [CrossRef]
- Santhanam, S.; Luu, V.T.; Weinhold, A.; Goldberg, J.; Oh, Y.; Baldwin, I.T. Native root-associated bacteria rescue a plant from a sudden-wilt disease that emerged during continuous cropping. Proc. Natl. Acad. Sci. USA 2015, 112, E5013–E5020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunziker, L.; Bönisch, D.; Groenhagen, U.; Bailly, A.; Schulz, S.; Weisskopf, L. Pseudomonas strains naturally associated with potato plants produce volatiles with high potential for inhibition of Phytophthora infestans. Appl. Environ. Microbiol. 2015, 81, 821–830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guyer, A.; de Vrieze, M.; Bönisch, D.; Gloor, R.; Musa, T.; Bodenhausen, N.; Bailly, A.; Weisskopf, L. The Anti-Phytophthora Effect of Selected Potato-Associated Pseudomonas Strains: From the Laboratory to the Field. Front. Microbiol. 2015, 6, 1309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- New, P.B.; Kerr, A. Biological Control of Crown Gall: Field Measurements and Glasshouse Experiments. J. Appl. Buct. 1972, 35, 279–287. [Google Scholar] [CrossRef]
- Allizadeh, H.; Behboudi, K.; Masoud, A.; Javan-Nikkhah, M.; Zamioudis, C.; Corné, M.J.P.; Bakker, A.H.M. Induced systemic resistance in cucumber and Arabidopsis thaliana by the combination of Trichoderma harzianum Tr6 and Pseudomonas sp. Ps14. Biol. Control 2013, 65, 14–23. [Google Scholar] [CrossRef]
- Durgadevi, D.; Srivignesh, S.; Sankaralingam, A. Effect of Consortia Bioformulation of Rhizobacteria on Induction of Systemic Resistance in Tuberose against Peduncle Blight Disease. Int. J. Bio Resour. Stress Manag. 2018, 9, 510–517. [Google Scholar] [CrossRef]
- Takishita, Y.; Charron, J.B.; Smith, D.L. Biocontrol Rhizobacterium Pseudomonas sp. 23S Induces Systemic Resistance in Tomato (Solanum lycopersicum L.) Against Bacterial Canker Clavibacter michiganensis subsp. michiganensis. Front. Microbiol. 2018, 9, 2119. [Google Scholar] [CrossRef]
- Kumar, S.; Chauhan, P.S.; Agrawal, L.; Raj, R.; Srivastava, A.; Gupta, S.; Mishra, S.K.; Yadav, S.; Singh, P.C.; Raj, S.K.; et al. Paenibacillus lentimorbus Inoculation Enhances Tobacco Growth and Extenuates the Virulence of Cucumber mosaic virus. PLoS ONE 2016, 11, e0149980. [Google Scholar] [CrossRef]
- Schuhegger, R.; Ihring, A.; Gantner, S.; Bahnweg, G.; Knappe, C.; Vogg, G.; Hutzler, P.; Schmid, M.; Breusegem, F.V.; Eberl, L.; et al. Induction of systemic resistance in tomato by N-acyl-L-homoserine lactone-producing rhizosphere bacteria. Plant Cell Environ. 2006, 29, 909–918. [Google Scholar] [CrossRef]
- Baffoni, L.; Gaggia, F.; Dalanaj, N.; Prodi, A.; Nipoti, P.; Pisi, A.; Biavati, B.; Gioia, D.D. Microbial inoculants for the biocontrol of Fusarium spp. in durum wheat. BMC Microbiol. 2015, 15, 242. [Google Scholar] [CrossRef] [PubMed]
- Martinuz, A.; Schouten, A.; Menjivar, R.; Sikora, R. Effectiveness of systemic resistance toward Aphis gossypii (Hom., Aphididae) as induced by combined applications of the endophytes Fusarium oxysporum Fo162 and Rhizobium etli G12. Biol. Control 2012, 62, 206–212. [Google Scholar] [CrossRef]
- Valenzuela-Soto, J.H.; Estrada-Hernandez, M.G.; Ibarra-Laclette, E.; Delano-Frier, J.P. Inoculation of tomato plants (Solanum lycopersicum) with growth-promoting Bacillus subtilis retards whitefly Bemisia tabaci development. Planta 2010, 231, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Bist, V.; Srivastava, S.; Singh, P.C.; Trivedi, P.K.; Asif, M.H.; Chauhan, P.S.; Nautiyal, C.S. Unraveling aspects of Bacillus amyloliquefaciens mediated enhanced production of rice under biotic stress of Rhizoctonia solani. Front. Plant Sci. 2016, 7, 587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collins, M.; Knutti, R.; Arblaster, J.; Dufresne, L.; Fichefet, T.; Friedlingstein, P.; Gao, X.; Gutowski, W.J.; Johns, T.; Krinner, G.; et al. Long-term Climate Change: Projections, Commitments and Irreversibility. In Climate Change 2013: The Physical Science Basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Cambridge University Press: New York, NY, USA, 2013; pp. 1029–1136. [Google Scholar]
- Xu, D.Y.; Kang, X.W.; Zhuang, D.F.; Pan, J.J. Multi-scale quantitative assessment of the relative roles of climate change and human activities in desertification—A case study of the Ordos Plateau, China. J. Arid Environ. 2010, 74, 498–507. [Google Scholar] [CrossRef]
- Tank, N.; Saraf, M. Salinity-resistant plant growth promoting rhizobacteria ameliorates sodium chloride stress on tomato plants. J. Plant Interact. 2010, 5, 51–58. [Google Scholar] [CrossRef]
- Rousk, J.; Elyaagubi, F.K.; Jones, D.L.; Godbold, D.L. Bacterial salt tolerance is unrelated to soil salinity across an arid agroecosystem salinity gradient. Soil Biol. Biochem. 2011, 43, 1881–1887. [Google Scholar] [CrossRef]
- Egamberdieva, D.; Lugtenberg, B. Use of Plant Growth-Promoting Rhizobacteria to Alleviate Salinity Stress in Plants. In Use of Microbes for the Alleviation of Soil Stresses; Miransari, M., Ed.; Springer Science + Business Media: New York, NY, USA, 2014; pp. 73–96. [Google Scholar] [CrossRef]
- Shrivastava, P.; Kumar, R. Soil salinity: A serious environmental issue and plant growth promoting bacteria as one of the tools for its alleviation. Saudi J. Biol. Sci. 2015, 22, 123–131. [Google Scholar] [CrossRef] [Green Version]
- Yan, N.; Marschner, P.; Cao, W.; Zuo, C.; Qin, W. Influence of salinity and water content on soil microorganisms. Int. Soil Water Conserv. Res. 2015, 3, 316–323. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Liu, Y.; Wu, G.; Njeri, K.V.; Shen, O.; Zhang, N.; Zhang, R. Induced maize salt tolerance by rhizosphere inoculation of Bacillus amyloliquefaciens SQR9. Physiol. Plant 2016, 158, 34–44. [Google Scholar] [CrossRef]
- Subramanian, P.; Mageswari, A.; Kim, K.; Lee, Y.; Sa, T. Psychrotolerant endophytic Pseudomonas sp. strains OB155 and OS261 induced chilling resistance in tomato plants (Solanum lycopersicum mill.) by activation of their antioxidant capacity. Mol. Plant Microbe Interact. 2015, 28, 1073–1081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiwari, S.; Lata, C.; Chauhan, S.P.; Chandra Shekhar Nautiyal, C.P. Pseudomonas putida attunes morphophysiological, biochemical and molecular responses in Cicer arietinum L. during drought stress and recovery. Plant Physiol. Biochem. 2016, 99, 108–117. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Dodd, I.C.; Belimov, A.A.; Jiang, F. Rhizosphere bacteria containing 1-aminocyclopropane-1-carboxylate deaminase increase growth and photosynthesis of pea plants under salt stress by limiting Na+ accumulation. Funct. Plant Biol. 2016, 43, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Bhartirt, N.; Pandey, S.S.; Barnawal, D.; Patel, V.K.; Kalra, A. Plant growth promoting rhizobacteria Dietzia natronolimnaea modulates the expression of stress responsive genes providing protection of wheat from salinity stress. Sci. Rep. 2016, 6, 34768. [Google Scholar] [CrossRef] [Green Version]
- Subramanian, S.; Ricci, E.; Souleimanov, A.; Smith, D.L. A proteomic approach to lip-chitooligosaccharide and thuricin 17 effects on soybean germination under salt stress. PLoS ONE 2016, 11, e0160660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rolli, E.; Marasco, R.; Vigani, G.; Ettoumi, B.; Mapelli, F.; Deangelis, M.L.; Gandolfi, C.; Casati, F.; Previtali, F.; Gerbino, R.; et al. Improved plant resistance to drought is promoted by the root-associated microbiome as a water stress-dependent trait. Environ. Microbiol. 2015, 17, 316–331. [Google Scholar] [CrossRef]
- Molina-Romero, D.; Baez, A.; Quintero-Hernández, V.; Castañeda-Lucio, M.; Fuentes-Ramírez, L.E.; Bustillos-Cristales, M.D.R.; Rodríguez-Andrade, O.; Morales-García, Y.E.; Munive, A.; Muñoz-Rojas, J. Compatible bacterial mixture, tolerant to desiccation, improves maize plant growth. PLoS ONE 2017, 12, e0187913. [Google Scholar] [CrossRef]
- Akhtar, S.S.; Amby, D.B.; Hegelund, J.N.; Fimognari, L.; Großkinsky, D.K.; Westergaard, J.C.; Muller, R.; Melba, L.; Liu, F.; Roitsch, T. Bacillus licheniformis FMCH001 increases water use efficiency via growth stimulation in both normal and drought conditions. Front. Plant Sci. 2020, 11, 297. [Google Scholar] [CrossRef]
- Yang, A.; Akhtar, S.S.; Fu, Q.; Naveed, M.; Iqbal, S.; Roitsch, T.; Jacobsen, S.E. Burkholderia Phytofirmans PsJN Stimulate Growth and Yield of Quinoa under Salinity Stress. Plants 2020, 9, 672. [Google Scholar] [CrossRef]
- Zahran, H.H. Rhizobium-Legume Symbiosis and Nitrogen Fixation under Severe Conditions and in an Arid Climate. Microbiol. Mol. Biol. Rev. 1999, 63, 968–989. [Google Scholar] [CrossRef] [Green Version]
- Etesami, H.; Mirseyedhosseini, H.; Alikhani, H.A. Bacterial biosynthesis of 1-aminocyclopropane-1-caboxylate (ACC) deaminase, a useful trait to elongation and endophytic colonization of the roots of rice under constant flooded conditions. Physiol. Mol. Biol. Plants 2014, 20, 425–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, S.M.; Khan, A.L.; Waqs, M.; You, Y.H.; Hamayun, M.; Joo, G.; Shahzad, R.; Choi, K.; Lee, I.J. Gibberellin-producing Serratia nematodiphila PEJ1011 ameliorates low temperature stress in Capsicum annuum L. Eur. J. Soil Biol. 2015, 68, 85–93. [Google Scholar] [CrossRef]
- Fernandez, O.; Theocharis, A.; Bordiec, S.; Feil, R.; Jacquens, L.; Clément, C.; Fontaine, F.; Barka, E.A. Burkholderia phytofirmans PsJN Acclimates Grapevine to Cold by Modulating Carbohydrate Metabolism. Mol. Plant Microbe Interact. 2012, 25, 496–504. [Google Scholar] [CrossRef] [Green Version]
- Barnawal, D.; Bharti, N.; Maji, D.; Chanotiya, C.S.; Kalra, A. 1-Aminocyclopropane-1-carboxylic acid (ACC) deaminase containing rhizobacteria protect Ocimum sanctum plants during water logging stress via reduced ethylene generation. Plant Physiol. Biochem. 2012, 58, 227–235. [Google Scholar] [CrossRef] [PubMed]
- Bano, A.; Fatima, M. Salt tolerance in Zea mays (L.) following inoculation with Rhizobium and Pseudomonas. Biol. Fertil. Soils 2009, 45, 405–413. [Google Scholar] [CrossRef]
- Karthikeyan, B.; Joe, M.M.; Islam, M.D.R.; Sa, T. ACC deaminase containing diazotrophic endophytic bacteria ameliorate salt stress in Catharanthus roseus through reduced ethylene levels and induction of antioxidative defense systems. Symbiosis 2012, 56, 77–86. [Google Scholar] [CrossRef]
- Mallick, I.; Bhattacharyya, C.; Mukherji, S.; Sarkar, S.C.; Mukhopadhyay, U.K.; Ghosh, A. Effective rhizoinoculation and biofilm formation by arsenic immobilizing halophilic plant growth promoting bacteria (PGPB) isolated from mangrove rhizosphere: A step towards arsenic rhizoremediation. Sci. Total Environ. 2018, 610, 1239–1250. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, P.; Singh, P.C.; Mishra, A.; Srivastava, S.; Chauhan, R.; Awasthi, S.; Mishra, S.; Dwivedi, S.; Tripathi, P.; Kalra, A.; et al. Arsenic tolerant Trichoderma sp. reduces arsenic induced stress in chickpea (Cicer arietinum). Environ. Pollut. 2017, 223, 137–145. [Google Scholar] [CrossRef]
- Sánchez-Yáñez, J.M.; Alonso-Bravo, J.N.; Dasgupta-Schuber, N.; Márquez-Benavides, L. Bioremediation of soil contaminated by waste motor oil in 55,000 and 65,000 and phytoremediation by Sorghum bicolor inoculated with Burkholderia cepacia and Penicillium chrysogenum. J. Selva Andina Biosph. 2015, 3, 86–94. [Google Scholar]
- Sarkar, J.; Chakraborty, B.; Chakraborty, U. Plant Growth Promoting Rhizobacteria Protect Wheat Plants Against Temperature Stress Through Antioxidant Signalling and Reducing Chloroplast and Membrane Injury. J. Plant Growth Regul. 2018, 37, 1396–1412. [Google Scholar] [CrossRef]
- Islam, F.; Yasmeen, T.; Ali, O.; Ali, S.; Arif, S.M.; Sabir Hussain, S.; Rizv, H. Influence of Pseudomonas aeruginosa as PGPR on oxidative stress tolerance in wheat under Zn stress. Ecotoxicol. Environ. Saf. 2014, 104, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Berninger, T.; Lopez, O.G.; Bejarano, A.; Preininger, C.; Sessitsch, A. Maintenance and assessment of cell viability in formulation of non-sporulating bacterial inoculants. Microb. Biotechnol. 2018, 11, 277–301. [Google Scholar] [CrossRef] [Green Version]
- Mehnaz, S. An Overview of Globally Available Bioformulations. In Bioformulations: For Sustainable Agriculture; Arora, N., Mehnaz, S., Balestrini, R., Eds.; Springer: New Delhi, India, 2016; pp. 267–281. [Google Scholar] [CrossRef]
- Arthur, S.; Dara, S.K. Microbial biopesticides for invertebrate pests and their markets in the United States. J. Invertebr. Pathol. 2018, 165, 13–21. [Google Scholar] [CrossRef]
- Babalola, O.O.; Glick, B.R. Indigenous African agriculture and plant associated microbes: Current practice and future transgenic prospects. Sci. Res. Essays 2012, 7, 2431–2439. [Google Scholar]
- Aremu, B.R.; Alori, E.T.; Kutu, R.F.; Babalola, O.O. Potentials of Microbial Inoculants in Soil Productivity: An Outlook on African Legumes. In Microorganisms for Green Revolution. Microorganisms for Sustainability; Panpatte, D., Jhala, Y., Vyas, R., Shelat, H., Eds.; Springer: Singapore, 2017; Volume 6. [Google Scholar] [CrossRef]
- Souleimanov, A.; Prithiviraj, B.; Smith, D. The major Nod factor of Bradyrhizobium japonicum promotes early growth of soybean and corn. J. Exp. Bot. 2002, 53, 1929–1934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gray, E.J.; Lee, K.D.; Souleimanov, A.M.; Di Falco, M.R.; Zhou, X.; Ly, A.; Charles, T.C.; Driscoll, B.T.; Smith, D.L. A novel bacteriocin, thuricin 17, produced by plant growth promoting rhizobacteria strain Bacillus thuringiensis NEB17: Isolation and classification. J. Appl. Microbiol. 2006, 100, 545–554. [Google Scholar] [CrossRef]
- Subramanian, S.; Souleimanov, A.; Smith, D.L. Proteomic Studies on the Effects of Lipochitooligosaccharide and Thuricin 17 under Unstressed and Salt Stressed Conditions in Arabidopsis thaliana. Front. Plant Sci. 2016, 7, 1314. [Google Scholar] [CrossRef]
- Arunachalam, S.; Schwinghamer, T.; Dutilleul, P.; Smith, D.L. Multi-Year Effects of Biochar, Lipo-Chitooligosaccharide, Thuricin 17, and Experimental Bio-Fertilizer for Switchgrass. Agron. J. 2018, 110, 77–84. [Google Scholar] [CrossRef]
- Navarro, M.O.P.; Piva, A.C.M.; Simionato, A.S.; Spago, F.R.; Modolon, F.; Emiliano, J.; Azul, A.M.; Chryssafidis, A.L.; Andrade, G. Bioactive Compounds Produced by Biocontrol Agents Driving Plant Health. In Microbiome in Plant Health and Disease; Kumar, V., Prasad, R., Kumar, M., Choudhary, D., Eds.; Springer: Singapore, 2019; pp. 337–374. [Google Scholar] [CrossRef]
- Prudent, M.; Salon, C.; Souleimanov, S.A.; Emery, R.J.N.; Smith, D.L. Soybean is less impacted by water stress using Bradyrhizobium japonicum and thuricin-17 from Bacillus thuringiensis. Agron. Sustain. Dev. 2015, 35, 749–757. [Google Scholar] [CrossRef] [Green Version]
- Schwinghamer, T.; Souleimanov, A.; Dutilleul, P.; Smith, D.L. Supplementation with solutions of lipo-chitooligosaccharide Nod Bj V (C18:1, MeFuc) and thuricin 17 regulates leaf arrangement, biomass, and root development of canola (Brassica napus [L.]). Plant Growth Regul. 2015, 78, 31–41. [Google Scholar] [CrossRef]
- Yuan, L.; Li, Y.; Wang, Y.; Zhang, X.; Xu, Y. Optimization of critical medium components using response surface methodology for phenazine-1-carboxylic acid production by Pseudomonas sp. M-18Q. J. Biosci. Bioeng. 2008, 3, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Pan, X.; Luo, J.; Wu, J.; Zhou, Z.; Liang, X.; He, Y.; Zhou, M. Effects of phenazine-1-carboxylic acid on the biology of the plant-pathogenic bacterium Xanthomonas oryzae pv. Oryzae. Pestic. Biochem. Physiol. 2015, 117, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Mciver, J.; Yang, Y.; Bai, Y.; Schultz, B.; Mciver, A. Foliar application of lipochitooligosaccharides (Nod factors) to tomato (Lycopersicon esculentum) enhances flowering and fruit production. Can. J. Plant Sci. 2007, 87, 365–372. [Google Scholar] [CrossRef]
- Duke, S.O.; Lydon, J. Herbicides from Natural Compounds. Weed Technol. 1987, 1, 122–128. [Google Scholar] [CrossRef]
- Zhang, Z.K.; Huber, D.J.; Qu, H.X.; Yun, Z.; Wang, H.; Huang, Z.H.; Huang, H.; Jiang, Y.M. Enzymatic browning and antioxidant activities in harvested litchi fruit as influenced by apple polyphenols. Food Chem. 2015, 171, 191–199. [Google Scholar] [CrossRef]
- Shanmugaiah, V.; Mathivanan, N.; Varghese, B. Purification, crystal structure and antimicrobial activity of phenazine-1-carboxamide produced by a growth-promoting biocontrol bacterium, Pseudomonas aeruginosa MML2212. J. Appl. Microbiol. 2010, 108, 703–711. [Google Scholar] [CrossRef]
- Huang, H.; Sun, L.; Bi, K.; Zhong, G.; Hu, M. The effect of phenazine-1-carboxylic acid on the morphological, physiological, and molecular characteristics of Phellinus noxius. Molecules 2016, 21, 613. [Google Scholar] [CrossRef] [Green Version]
- Puopolo, G.; Masi, M.; Raio, A.; Andolfi, A.; Zoina, A.; Cimmino, A.; Evidente, A. Insights on the susceptibility of plant pathogenic fungi to phenazine-1-carboxylic acid and its chemical derivatives. Nat. Prod. Res. 2013, 27, 956–966. [Google Scholar] [CrossRef]
- Gheorghe, I.; Popa, M.; Marutescu, L.; Saviuc, C.; Lazar, V.; Chifiriuc, M.C. Lessons from interregn communication for development of novel, ecofriendly pesticides. In New Pesticides and Soil Sensors; Grumezescu, A.M., Ed.; Academic Press: London, UK, 2017; pp. 1–46. [Google Scholar] [CrossRef]
- Rane, M.R.; Sarode, P.D.; Chaudhari, B.L.; Chincholkar, S.B. Exploring antagonistic metabolites of established biocontrol agent of marine origin. Appl. Biochem. Biotechnol. 2008, 151, 665–675. [Google Scholar] [CrossRef]
- Kare, E.; Arora, N.K. Dual activity of pyocyanin from Pseudomonas aeruginosa—Antibiotic against phytopathogen and signal molecule for biofilm development by rhizobia. Can. J. Microbiol. 2011, 57, 708–713. [Google Scholar] [CrossRef]
- Jung, B.K.; Hong, S.J.; Park, G.S.; Kim, M.C.; Shin, J.H. Isolation of Burkholderia cepacia JBK9 with plant growth–promoting activity while producing pyrrolnitrin antagonistic to plant fungal diseases. Appl. Biol. Chem. 2018, 61, 173–180. [Google Scholar] [CrossRef] [Green Version]
- Okada, A.; Banno, S.; Ichiishi, A.; Kimura, M.; Yamaguchi, I.; Fujimura, M. Pyrrolnitrin interferes with osmotic signal transduction in Neurospora crassa. J. Pestic. Sci. 2005, 30, 378–383. [Google Scholar] [CrossRef] [Green Version]
- Han, J.W.; Kim, J.D.; Lee, J.M.; Ham, J.H.; Lee, D.; Kim, B.S. Structural elucidation and antimicrobial activity of new phencomycin derivatives isolated from Burkholderia glumae strain 411gr6. J. Antibiot. 2014, 67, 721. [Google Scholar] [CrossRef]
- Deng, P.; Foxfire, A.; Xu, J.; Baird, S.M.; Jia, J.; Delgado, K.H.; Shin, R.; Smith, L.; Lu, S.E. Siderophore product ornibactin is required for the bactericidal activity of Burkholderia contaminans MS14. Appl. Environ. Microbiol. 2017, 83, e00051-17. [Google Scholar] [CrossRef] [Green Version]
- Ye, Y.; Li, Q.; Fu, G.; Yuan, G.; Miao, J.; Lin, W. Identification of antifungal substance (Iturin A2) produced by Bacillus subtilis B47 and its effect on southern corn leaf blight. J. Integr. Agric. 2012, 11, 90–99. [Google Scholar] [CrossRef]
- Deravel, J.; Lemière, S.; Coutte, F.; Krier, F.; Hese, N.V.; Béchet, M.; Sourdeau, N.; Höfte, M.; Leprêtre, A.; Jacques, P. Mycosubtilin and surfactin are efficient, low ecotoxicity molecules for the biocontrol of lettuce downy mildew. Appl. Microbiol. Biotechnol. 2014, 98, 6255–6264. [Google Scholar] [CrossRef]
- Isaac, B.G.; Ayer, S.W.; Elliott, R.C.; Stonard, R.J. Herboxidiene: A potent phytotoxic polyketide from Streptomyces sp. A7847. J. Org. Chem. 1992, 57, 7220–7226. [Google Scholar] [CrossRef]
- Saxena, S.; Pandey, A.K. Microbial metabolites as eco-friendly agrochemicals for the next millennium. Appl. Microbiol. Biotechnol. 2001, 55, 395–403. [Google Scholar] [CrossRef]
- Tanaka, Y.; Omura, S. Agroactive compounds of microbial origin. Annu. Rev. Microbiol. 1993, 47, 57–87. [Google Scholar] [CrossRef]
| PGPM | Biotic Stress | Host Plant | Reference |
|---|---|---|---|
| Bacillus amyloliquefaciens LY-1 | Peronophythora litchii | Litchi (Litchi chinensis Sonn.) | [71] |
| Burkholderia cepacia | Fusarium oxysporum | Solanum tuberosum | [65] |
| Pseudomonas fluorescens | Fusarium graminearum | Triticum aestivum(wheat) cv. Tabuki | [66] |
| Pseudomonas fluorescens CHAO | Gaeumannomyces graminis var. tritici | Triticum sp. | [64] |
| Pseudomonas fluorescens CHAO | Thielaviopsis basicola | Nicotiana tabacum | [64] |
| Bacillus spp. | Heterodera glycines | Glycine max. | [82] |
| Serratia proteamaculans | Meloidogyne incognita | Solanum lycopersicum L. | [72] |
| Bacillus aryabhattai A08 | Meloidogyne incognita | Solanum lycopersicum L. | [83] |
| Serratia plymuthica HRO-C48 | Botrytis cinerea | _ | [84] |
| Serratia plymuthica strain C-1, Chromobacterium sp. strain C-61 and Lysobacter enzymogenes strain C-3 consortium | Phytophthora capsici | Cupsicum spp. | [85] |
| Paenibacillus sp. 300 + Streptomyces sp. 385, | Oxysporum f. sp. Cucumerinum | Cucumis sativus | [86] |
| Pseudomonas fluorescens WCS 358 | Fusarium oxysporum f sp. Raphani | Raphanus sativus | [87] |
| Pseudomonas fluorescens | Macrophomina phseolina | Coleus forskohlii Briq. | [68] |
| Pseudomonas aeruginosa 7NSK2 | Pythium splendens | Lycopersicon esculentum | [88] |
| Pseudomonas fluorescens | Pythium spp. | Triticum sp. | [64] |
| Pseudomonas fluorescens | Pythium ultimum | Gossypium sp. | [64] |
| Bradyrhizobium japonicum NCIM 2746 | Rhizopus sp. and, Fusarium sp. | Glycine max L. | [89] |
| Paenibacillus lentimorbus B30488 | Scelerotium rolfsii | Solunum lycopersicum L. | [69] |
| Pseudomonas putida UW4 | Agrobacterium tumefaciens | Solanum lycopersicum | [90] |
| Burkholderia phytofirmans PsJN | Agrobacterium tumefaciens | Solanum lycopersicum | [90] |
| Bacillus cereus PX35, Bacillus subtilis SM21 and Serrati asp. XY2 | Meloidogyne incognito | S. lycopersicum | [91] |
| Pseudomonas fluorescens strain S35 | Phytophthora infestans | Solanum tuberosum | [73] |
| Pseudomonas frederiksbergensis strain 49 and Pseudomonas fluorescens strain 19 consortium | Phytophthora infestans | Solanum tuberosum | [73] |
| Pseudomonas putida strain R32 | Phytophthora infestans | Solanum tuberosum | [73] |
| Pseudomonas chlororaphis spp. strain R47 | Phytophthora infestans | Solanum tuberosum | [73] |
| Pseudomonas spp. strain S49 | Phytophthora infestans | Solanum tuberosum | [73] |
| Bacillus and Pseudomonas spp. consortium | Fusarium oxysporum U3 and Alternaria sp. U10 | Nicotiana attenuata | [92] |
| Chaetomium sp. C72 and Oidodendron sp. Oi3 consortium | Fusarium oxysporum U3 and Alternaria sp. U10 | Nicotiana attenuata | [92] |
| Pseudomonas chlororaphis R47 | Phytophthora infestans | Solanum tuberosum | [69,93] |
| Pseudomonas fluorescens strain LBUM 636 | Phytophthora infestans | Solanum tuberosum | [94] |
| Agrobacterium radiobacter var radiobacter | Crown gall | Solunum lycopersicon | [95] |
| Tricoderma koningiopsis Th003 WP | Fusarium oxysporum | Physalis peruviana | [67] |
| Trichoderma harzianum Tr6 + Pseudomonas sp. Ps14 | Fusarium oxysporum f. sp. radicis cucumerinum | Cucumis sativus | [96] |
| Pseudomonas sp. Ps14 | Botrytis cinerea | Arabidopsis thaliana | [96] |
| Trichoderma harzianum Tr6 | Botrytis cinerea | Arabidopsis thaliana | [96] |
| Pseudomonas putida | Spodoptera litura | Solanum lycopersicum L. | [96] |
| Pseudomnas flourescences Pf1, Bacillus subtilis Bs and Trichoderma viridae Tv consortium | Lasiodiplodia theobromae | Polianthes tuberosa L. | [97] |
| Pseudomonas sp. 23S | Clavibacter michiganensis | Solanum lycopersicum L. | [98] |
| Peanibacillus lentimorbus B-30488 | cucumber mosaic virus | Nicotiana tabacum cv White burley | [99] |
| Serratia liquefaciens MG1 | Alternaria alternate | Solanum lycopersicum | [100] |
| Xanthomonas sp. WCS2014-23, Stenotrophomonas sp. WCS2014-113 and Microbacterium sp. WCS2014-259 | Hyaloperonospora arabidopsidis | Arabidopsis thaliana | [74] |
| Lactobacillus plantarum SLG17 and Bacillus amyloliquefaciens FLN13 | Fusarium spp. | Triticum durum | [101] |
| Fusarium oxysporum strain Fo162 | Aphis gossypii Glover | Cucurbita pepo | [102] |
| Rhizobium etli strain G12 | Aphis gossypii Glover | Cucurbita pepo | [102] |
| Bacillus subtilis strain BEB-DN | Bemisia tabaci | Solanum lycopersicum | [103] |
| Bacillus amyloliquefaciens (SN13) | Rhizoctonia solani | Rice (Oryza sativa) | [104] |
| Pseudomonas fluorescens Migula strains Pf1 and AH1 | Desmia funeralis | Oryza sativa | [80] |
| Pseudomonas putida and Rothia sp. | Spodoptera litura | Solanum lycopersicum | [81] |
| PGPM | Abiotic Stress | Host Plant | Reference |
|---|---|---|---|
| Pseudomonas putida MTCC5279 | Drought | chickpea (Cicer arietinum) | [114] |
| Pseudomonas fluorescens REN1 | Flooding | Rice (Oryza sativa) | [123] |
| Variovorax paradoxus 5C-2, | Salinity | Peas | [115] |
| Bacillus amyloliquefaciens SQR9 | salinity | Maize | [112] |
| Dietzia natronolimnaea | Salinity | Wheat (Triticum aestivum) | [116] |
| Serratia nematodiphila | Low temperature | pepper (Capsicum annum) | [124] |
| Burkholderia phytofirmans PsJN | Low temperature | grapevine (Vitis vinifera) | [125] |
| Pseudomonas vancouverensis | Low temperature | Tomato (Solanum lycopersicum) | [113] |
| Pseudomonas sp. S1 | drought | Capsicum annum | [118] |
| Pseudomonas sp. S1 | drought | Vitis vinifera | [118] |
| Achromobacter xylosoxidans | Flooding stress | Ocimumsanctum | [126] |
| Pseudomonas sp. 54RB + Rhizobium sp. Thal-8 | Salinity | Zea mays cv. Agaiti 2002 | [127] |
| Pseudomonas putida KT2440, Sphingomonas sp. OF178, Azospirillum brasilense Sp7 and Acinetobacter sp. EMM02) consortium | drought | Zea mays | [119] |
| Achromobacter xylosoxidans | salinity | Catharanthus roseus | [128] |
| Burkholdera cepacia SE4 | salinity | Cucumis sativus L. | [124] |
| Pseudomonas putida (W2) | salinity | Triticum aestivum L. | [56] |
| Pseudomonas fluorescens (W17) | salinity | Triticum aestivum L. | [56] |
| Kocuria flava AB402 | Arsenic toxicity | Oryza sativum | [129] |
| Bacillus vietnamensis AB403 | Arsenic toxicity | Oryza sativum | [129] |
| Trichoderma spp. strain, M-35 | Arsenic toxicity | Cicer arietinum | [130] |
| Burkholderia cepacia and Penicillium chrysogenum consortium | waste motor oil toxicity | Sorghum bicolor | [131] |
| Bacillus safensis | High temperature | Triticum aestivum L. | [132] |
| Pseudomonas aeruginosa | Zn-induced oxidative stress | Triticum aestivum L. | [133] |
| Bacillus licheniformis (FMCH001) | oxidative stress Drought | Zea mays L. cv. Ronaldinho | [120] |
| Burkholderia phytofirmans PsJN | Salinity | Chenopodium quinoa Willd | [121] |
| Inoculant | Country | Producer | Use | Reference |
|---|---|---|---|---|
| Bacillus megaterium | Sri Lanka | BioPowerLanka | Phosphorus solubilisation | [135] |
| Pseudomonas striata, B. Polymyxa and B.megaterium consortium | India | AgriLife | Phosphorus solubilisation | [135] |
| Acidithiobacillus ferrooxidans | India | AgriLife | Iron mobilization | [135] |
| Trichoderma and Bradyrhizobium Spp (Excalibre-SA) consortium | USA | ABM® | N fixation Growth stimulation | [18] |
| BIODOZ® (B. japonicum) | Denmark | Novozymes | Nitrogen fixation | [134] |
| Cell-Tech® (B.japonicum) | Belgium | Monsanto (Bayer) | Nitrogen fixation | [134] |
| Nitragin® S. meliloti | Belgium | Monsanto BioAgTM (Bayer) | Nitrogen fixation | [134] |
| Cedomon® Pseudomonas chlororaphis | Sweden | BioAgriAB | Biopesticide | [134] |
| SheathguardTM Pseudomonas fluorescens | India | AgriLife | Biopesticide | [134] |
| Galltrol® -A Agrobacterium radiobacter | USA | AgBioChem | Biopesticide | [134] |
| HISTICK® Bradyrhizobium japonicum | Germany | BASF SE | Nitrogen fixation | [135] |
| Bacillus + Pseudomonas + Lactobacillus + Saccharomyces spp. | Canada | EVL Inc | Biostimulant | |
| Xen Tari (Bacillus thuringiensis) | USA | Valent USA | Biopesticide | [136] |
| VOTIVO FS seed treatment (Bacillus firmus) | USA | Bayer | Biopesticide | [136] |
| VectoLex FG (Bacillus sphaericus) | USA | Valent Biosciences | Biopesticide | [136] |
| Venerate XC (Burkholderia rinojensis) | USA | Marrone Bio Innovations | Biopesticide | [136] |
| Zequanox (Pseudomonas fluorescens) | USA | Marrone Bio Innovations | Biopesticide | [136] |
| BotaniGard ES/WP, Mycotrol (Beauveria bassiana) | USA | Lam International | Biopesticide | [136] |
| Naturalis L (Beauveria bassiana) | USA | Troy BioSciences | Biopesticide | [136] |
| BioCeres WP (Beauveria bassiana) | USA | BioSafe | Biopesticide | [136] |
| Met-52 EC and Met-52 G (Metarhizium brunneum (anisopliae s.L.) | USA | Novozymes | Biopesticide | [136] |
| MeloCon WG (Purpureocillium lilacinum) | USA | Bayer | Biopesticide | [136] |
| Cyd-X, Cyd-X HP (Cydia pomonella (CpGV) | USA | Certis USA | Biopesticide | [136] |
| FruitGuard (Plodia interpunctella GV | USA | Agrivir | Biopesticide | [136] |
| Serenade (Bacillus subtilis QST 713) | Agraquest | Biocontrol | [79] | |
| Bacillus firmus I-1582 WP5 (B. firmus I-1582) | Bayer Crop Science | Biocontrol | [79] | |
| Cedomon (Pseudomonas chlororaphis MA342) | Bioagri | [79] | ||
| Proradix (Pseudomonas sp. DSMZ 13134) | Sourcon–Padena Germany, Itary | Biocontrol | [79] | |
| Novodor (B. thuringiensis ssp. tenebrionis NB 176) | USA | Valent Bioscience | Biocontrol | [79] |
| Compound | Producing Microbe | Function | Comment | Reference |
|---|---|---|---|---|
| LCO | Bradyrhizobium japonicum | Biostimulant | Stimulates plant growth under stressed and non stressed conditions. | [117,146] |
| Thuricin17 | Bacillus thuringiensis | Biostimulant | Enhances growth of different crops eg Soybean in stressed and non stressed conditions | [141,142] |
| Anisomycin | Streptomyces sp. | herbicide | Effective against Digitaria spp. | [149] |
| Phenazine-1-carboxyamide (PCN) | Pseudomonas spp. | biocontrol | It is effective against; Fusarium oxysporum f. sp. Radicis-lycopersici, Xanthomonas oryzae pv. Oryzae, Rhizoctonia solani, Botrytis cinerea | [143,148,149,151] |
| Phenazine-1-carboxylic acid (PCA) | Pseudomonas spp. | biocontrol | It is effective against Fusarium oxysp.orum f. sp. Radicis-lycopersici, Colletotrichum orbiculare, Gaeumannomyces graminis var. tritici, Phytophthora capsici | [143,146,149,152,153] |
| Pyocyanin (PYO) | Pseudomonas spp. | biocontrol | Effective against: Sclerotium rolfsii, Macrophomina phaseolina | [154,155,156] |
| Pyrrolnitrin | Burkholderia pyrrocinia 2327 | biocontrol | It has antifungal properties against; Ralstonia solani, Phytophthora capsici, and Fusarium oxysporum | [157,158] |
| Phencomycin | Burkholderia glumae 411gr-6 | biocontrol | Effective against; Alternaria brassicicola, Aspergillus oryzae, Cladosporium cucumerinum, Colletotrichum gloeosporioides | [159] |
| Ornibactin | Burkholderia contaminans MS14 | biocontrol | Siderophore with biocontrol activity against Erwinia amylovora, Ralstonia solanacearum, Pseudomonas syringae B301, Clavibacter michiganensis subsp. michiganensis | [160] |
| Iturin A2 | Bacillus subtilis B47 | biocontrol | Effective against fungi; Bipolaris maydis | [161] |
| Mycosubtilin | Bacillus subtilis | biocontrol | Has anti fungal properties, effective against; Bremia lactucae | [162] |
| Herboxidiene | Streptomyces sp. A7847 | herbecide | Effective on a number of weed sp. | [163] |
| Phosphinothricin | Streptomyces hygroscopicus | herbecide | ||
| Cyanobacterin | Scytonema hofmanni | herbecide | Effective on cynobacteria, algae and higher plants | [164] |
| Avermectin | Streptomyces avermitilis | Insectide nematocide | Effective against Spider mites, Citrus red mite, horn worms, army worms, etc. | [165] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Naamala, J.; Smith, D.L. Relevance of Plant Growth Promoting Microorganisms and Their Derived Compounds, in the Face of Climate Change. Agronomy 2020, 10, 1179. https://doi.org/10.3390/agronomy10081179
Naamala J, Smith DL. Relevance of Plant Growth Promoting Microorganisms and Their Derived Compounds, in the Face of Climate Change. Agronomy. 2020; 10(8):1179. https://doi.org/10.3390/agronomy10081179
Chicago/Turabian StyleNaamala, Judith, and Donald L. Smith. 2020. "Relevance of Plant Growth Promoting Microorganisms and Their Derived Compounds, in the Face of Climate Change" Agronomy 10, no. 8: 1179. https://doi.org/10.3390/agronomy10081179
APA StyleNaamala, J., & Smith, D. L. (2020). Relevance of Plant Growth Promoting Microorganisms and Their Derived Compounds, in the Face of Climate Change. Agronomy, 10(8), 1179. https://doi.org/10.3390/agronomy10081179






