Dependence of Weed Composition on Cultivated Plant Species and Varieties in Energy-Tree and -Grass Plantations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Experiment Design
2.2. Description of Species and Varieties
2.3. Sampling and Data Analysis
3. Results
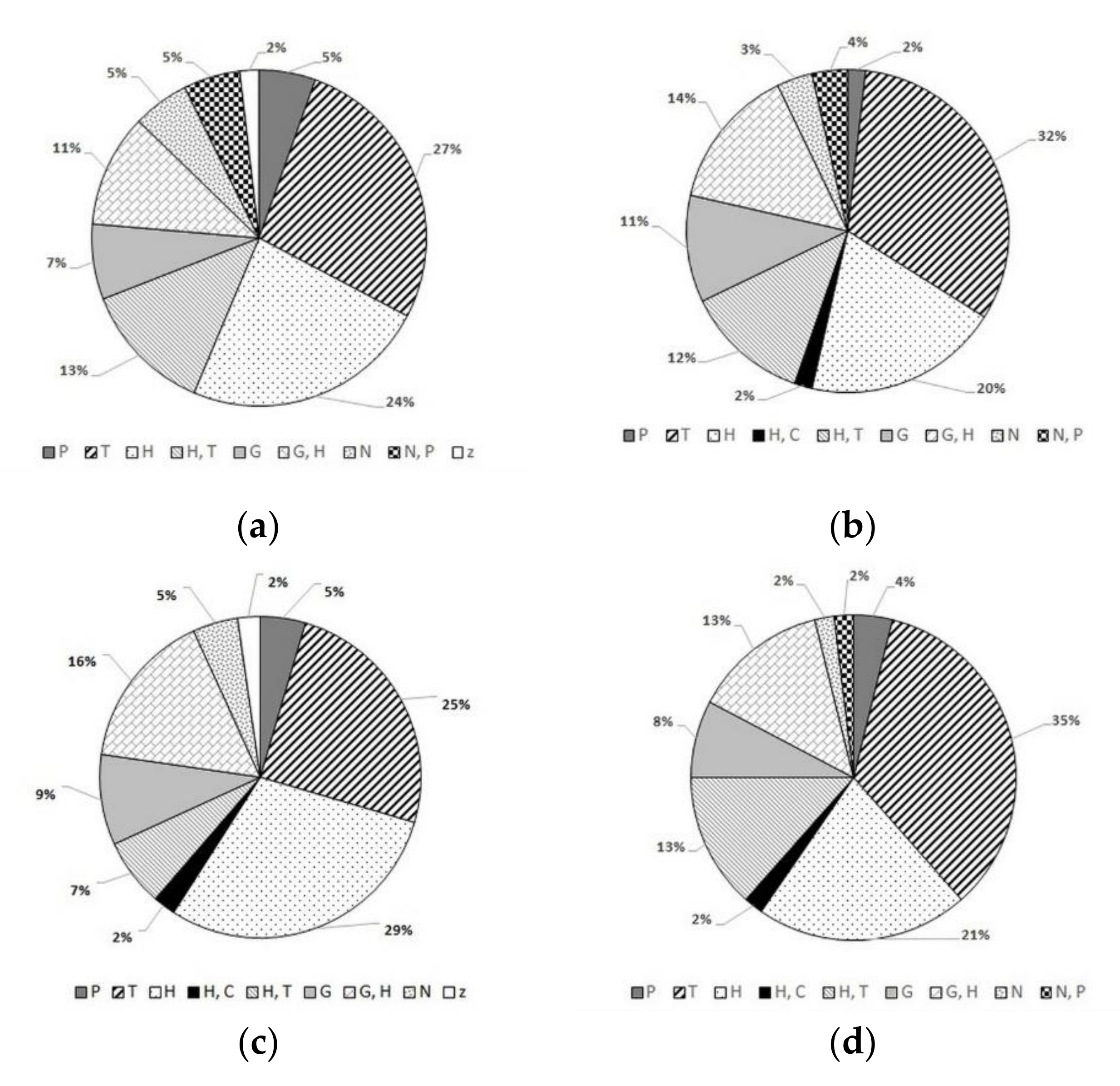
3.1. Species Composition and Habitat Preferences
3.2. Life Cycle
3.3. Life Forms
3.4. Ground-Flora Evaluation According to Ecological Demands of Species
- Light requirements. Species ranged from shade-tolerating and semi-shade-tolerating to light-demanding. The highest light-requirement category included five species: Arctium lappa, Dipsacus fullonum, Lactuca serriola, Lycium barbarum, and Symphyotrichum novi-belgii. Shade-tolerating to semi-shade-tolerating species were tree species Acer pseudoplatanus, Fraxinus excelsior and Prunus avium, and herb species Geum urbanum. The average values of Ellenberg’s indicator numbers for weeds in different energy crops ranged from 6.81 to 6.98, suggesting that most species had higher light requirements, often growing in full light, but they tolerated the shade in energy plantations well.
- Temperature requirements. Only one species, Juglans regia, belongs to a species with high-temperature requirements, and one species, Senecio nemorensis, is a species with low-temperature requirements. The average value of the indicator numbers for each variant ranged from 5.76 to 5.93, and most species extended from the hills to the lower mountain locations (mild-to-warm climate).
- Continentality. The average continentality-indicator values for each variant ranged between 4.08 and 4.21, indicating that most species are typical for the suboceanic climate of Central Europe (with continental to subcontinental species Calamagrostis epigejos, Chenopodium ficifolium, Elymus repens, Lactuca serriola, Lepidium draba, Plantago media, and Senecio nemorensis; and maritimity species Heracleum sphondylium, Juglans regia, and Symphyotrichum novi-belgii,).
- Moisture requirements. The average indicator values for different energy-plant stands varied from 5.03 to 5.07, which indicated that most occurring species require moist soils (lacking in wet and in frequently dry soils). One species required wet soils (Silene baccifera), and three species were drought-tolerant (Euphorbia cyparissias, Lepidium draba, and Veronica spicata).
- Soil-reaction requirements. The average indicator values for each variant ranged from 6.79 to 7.1, which indicated species that mostly prefer alkalic soils (pH 6.5–8). Mentha longifolia was one species demanding soils very rich in calcium, and Viola canina is a species that requires acidic soils.
- Nitrogen requirements. We identified three species that occur in soils with high nitrogen concentrations (Calystegia sepium, Sambucus nigra, and Symphyotrichum novi-belgii), and three species of soils that occur in areas poor in nitrogen (Hypericum maculatum, Veronica spicata, and Viola canina). The average indicator values for each energy-plant stand was in the range of 6.39–6.7, so most species were typical species occurring in medium-to-rich nitrogen soils.
3.5. Weed-Species Harmfulness
4. Discussion
5. Conclusions
- the total number of vascular plant species in SRC and miscanthus-stand understories (all plots) was 98. The highest number of species was recorded for the research plots with Tordis and Inger willow, and the lowest for the Pegaso poplar. Perennial species dominated, 10 of which were found in all plots. Ruderal therophytes and hemicryptophytes prevailed;
- weeds represented 49 species among all identified vascular plant species in the SRC and miscanthus understory, 46.94% of which were very harmful. Poplar plantation hosted the highest number of “very dangerous weeds”, the least of them was observed in miscanthus stands (willow stands were intermediate). Non-native invasive species were also recorded in the ground flora;
- according to ecological requirements of weeds, most species have higher light requirements. For temperature requirements, the presented species are predominantly mild-to-warm suboceanic species. Species that prefer freshly moist alkalic soils that are medium-to-rich in mineral nitrogen predominated;
- weeds of the Tordis willow variety were the least influenced by the environmental conditions of the taxon of the energy plant. Weeds of the Inger willow variety were mainly defined by the soil reaction. Weeds in the undergrowth of miscanthus (Miscanthus × giganteus) and poplar trees (Pegaso) had the greatest affinity to soil nitrogen content and temperature requirements.
Author Contributions
Funding
Conflicts of Interest
References
- Fehér, A.; Končeková, L.; Glemnitz, M.; Berger, G.; Pfeffer, H.; Herzon, I. Maintaining and promoting biodiversity. In Sustainable Agriculture; Jakobson, C., Ed.; Baltic University Press: Uppsala, Sweden, 2012; pp. 371–387. ISBN 978-91-86189-10-5. [Google Scholar]
- Benton, T.G.; Vickery, J.A.; Wilson, J.D. Farmland biodiversity: Is habitat heterogeneity the key? Trends Ecol. Evol. 2003, 18, 182–188. [Google Scholar] [CrossRef]
- Baum, S.; Bolte, A.; Weih, M. High value of short rotation coppice plantations for phytodiversity in rural landscapes. Glob. Chang. Biol. Bioenergy 2012, 7, 728–738. [Google Scholar] [CrossRef]
- Feledyn-Szewczyk, B.; Matyka, M.; Staniak, M. Comparison of the effect of perennial energy crops and agricultural crops on weed flora diversity. Agronomy 2019, 9, 695. [Google Scholar] [CrossRef] [Green Version]
- Vanbeveren, S.P.P.; Ceulemans, R. Biodiversity in short rotation coppice. Renew. Sustain. Energy Rev. 2019, 111, 34–43. [Google Scholar] [CrossRef]
- Kuzovkina, Y.A.; Quigley, M.F. Willows beyond wetlands: Uses of Salix, L. species for environmental projects. Water Air Soil Pollut. 2005, 162, 183–204. [Google Scholar] [CrossRef]
- Gustaffson, L. Plant conservation aspects of energy forestry—A new type of land use in Sweden. For. Ecol. Manag. 1987, 21, 141–161. [Google Scholar] [CrossRef]
- Sage, R.B. Weed competition in willow coppice crops: The cause and extent of yield losses. Weed Res. 1999, 39, 399–411. [Google Scholar] [CrossRef]
- Semere, T.; Slater, F.M. Ground flora, small mammal and bird species diversity in miscanthus (Miscanthus × giganteus) and reed canary-grass (Phalaris arundinacea) fields. Biomass Bioenergy 2007, 31, 20–29. [Google Scholar] [CrossRef]
- Clapham, S.J.; Slater, F.M. The biodiversity of established biomass grass crops. Asp. Appl. Biol. 2008, 90, 325–329. [Google Scholar]
- Dauber, J.; Jones, M.B.; Stout, J.C. The impact of biomass crop cultivation on temperate biodiversity. Glob. Chang. Biol. Bioenergy 2010, 2, 289–309. [Google Scholar] [CrossRef]
- Baum, S. Phytodiversity in Short Rotation Coppice Plantations. Ph.D. Dissertation, Fakultät für Forstwissenschaften und Waldökologie, Georg-August-Universität, Göttingen, Germany, 2012. [Google Scholar]
- Langeveld, H.; Quist-Wessel, F.; Dimitriou, I.; Aronsson, P.; Baum, C.; Schulz, U.; Bolte, A.; Baum, S.; Kohn, J.; Weih, M.; et al. Assessing environmental impacts of short rotation coppice (SRC) expansion: Model definition and preliminary models. Bioenergy Res. 2012, 5, 621–635. [Google Scholar] [CrossRef]
- Pučka, I.; Lazdiņa, D.; Bebre, I. Ground flora in plantations of three years old short rotation willow coppice. Agron. Res. 2016, 14, 1450–1466. [Google Scholar]
- Volk, T.A.; Verwijst, T.; Tharakan, P.J.; Abrahamson, L.P.; White, E.H. Growing fuel: A sustainability assessment of willow biomass crops. Front. Ecol. Environ. 2004, 2, 411. [Google Scholar] [CrossRef]
- Dornburg, V.; Faaij, A.; Verweij, P.; Langeveld, H.; van de Ven, G.; Wester, F.; van Keulen, H.; van Diepen, K.; Meeusen, M.; Banse, M.; et al. Climate Change Scientific Assessment and Policy Analysis: Biomass Assessment. Assessment of Global Biomass Potentials and Their Links to Food, Water, Biodiversity, Energy Demand and Economy—Main Report; Lysen, E., van Egmond, S., Eds.; Netherlands Environmental Assessment Agency MNP: Bilthoven, The Netherlands, 2008; Available online: http://www.globalbioenergy.org/uploads/media/0801_WAB_-_Biomass_assessment.pdf (accessed on 12 September 2011).
- Baker, H.G. The Evolution of Weeds. Annu. Rev. Ecol. Syst. 1974, 5, 1–24. [Google Scholar] [CrossRef]
- Crawley, J.M. Biodiversity. In Plant Ecology; Crawley, J.M., Ed.; Blackwell Science: Oxford, UK, 1997; pp. 595–632. ISBN 0-632-03639-7. [Google Scholar]
- Genovesi, P.; Shine, C. European Strategy on Invasive Alien Species; Council of Europe: Strasbourg, France, 2004; Available online: https://www.cbd.int/doc/external/cop-09/bern-01-en.pdf (accessed on 15 July 2019).
- Hill, S.B.; Ramsay, J. Weeds as Indicators of Soil Conditions. EAP Publications no 67. Ecological Agriculture Projects, McGill University. 1997. Available online: http://www.eap.mcgill.ca/publications/EAP67.htm (accessed on 7 July 2018).
- Greef, J.M.; Deuter, M. Syntaxonomy of Miscanthus × giganteus GREEF et DEU. Angew. Bot. 1993, 67, 87–90. [Google Scholar]
- Lewandowski, I.; Clifton-Brown, J.C.; Scurlock, J.M.O.; Huisman, W. Miscanthus: European experience with a novel energy crop. Biomass Bioenergy 2000, 19, 209–227. [Google Scholar] [CrossRef]
- Braun-Blanquet, J. Pflanzensoziologie. Grundzüge der Vegetationskunde, 3rd ed.; Springer: New York, NY, USA, 1964; ISBN 978-3-7091-8111-9. [Google Scholar]
- Mueller-Dombois, D.; Ellenberg, H. Aims and Methods of Vegetation Ecology; Wiley and Sons: New York, NY, USA, 1974; ISBN 0-471-62290-7. [Google Scholar]
- The Plant List Version 1.1. Available online: http://www.theplantlist.org (accessed on 16 August 2020).
- Líška, E.; Černuško, K.; Hunková, E.; Otepka, P. Biológia burín. (Biology of Weeds); VES SPU: Nitra, Slovakia, 2002; pp. 16–24. ISBN 80-8069-001-4. (In Slovak) [Google Scholar]
- Ellenberg, H.; Weber, H.E.; Düll, R.; Wirth, V.; Werner, W.; Paulißen, D. Zeigerwerte von Pflanzen in Mitteleuropa. Scripta Geobotanica 18, 3rd ed.; Erich Goltze KG: Göttingen, Germany, 2001; pp. 1–262. ISBN 10-3884525182. [Google Scholar]
- Dölle, M.; Schmidt, W. The relationship between soil seed bank, above-ground vegetation and disturbance intensity on old-field successional permanent plots. Appl. Veg. Sci. 2009, 12, 415–428. [Google Scholar] [CrossRef]
- Raunkiaer, C. The life-forms of plants and their bearing on geography. In The Life Forms of Plants and Statistical Plant Geography; Raunkiaer, C., Ed.; Clarendon Press: Oxford, UK, 1934; pp. 2–104. [Google Scholar]
- Ter Braak, C.J.F. Unimodal Models to Relate Species to Environment; DLO-Agricultural Mathematics Group: Wageningen, The Netherlands, 1996. [Google Scholar]
- Ter Braak, C.J.F.; Šmilauer, P. CANOCO Reference Manual and CanoDraw for Windows User’s Guide. Software for Canonical Community Ordination (Version 4.5); Microcomputer Power: Ithaca, NY, USA, 2002. [Google Scholar]
- Delarze, R.; Ciardo, F. Rote Liste-Arten in Pappelplantagen (Red List Species in Poplar Plantations); Informationsblatt Forschungsbereich Wald, WSL: Birmensdorf, Switzerland, 2002; Volume 9, pp. 3–4. (In German) [Google Scholar]
- Cunningham, M.D.; Bishop, J.D.; McKay, H.V.; Sage, R.B. ARBRE Monitoring-Ecology of Short Rotation Coppice; DTI: London, UK, 2004; p. 157. [Google Scholar]
- Baum, S.; Weih, M.; Bolte, A. Floristic diversity in short rotation coppice (SRC): Comparison between soil seed bank and recent vegetation. Appl. Agric. For. Res. 2013, 63, 221–228. [Google Scholar] [CrossRef]
- Britt, C.P.; Fowbert, J.; Mc Millan, S.D. The ground flora and invertebrate fauna of hybrid poplar plantations: Results of ecological monitoring in the PAMUCEAF project. Asp. Appl. Biol. 2007, 82, 83–90. [Google Scholar]
- Archaux, F.; Chevalier, R.; Berthelot, A. Towards practices favourable to plant diversity in hybrid poplar plantations. For. Ecol. Manag. 2010, 259, 2410–2417. [Google Scholar] [CrossRef]
- Birmele, J.; Kopp, G.; Brodbeck, F.; Konold, W.; Sauter, U.H. Successional changes of phytodiversity on a short rotation coppice plantation in Oberschwaben, Germany. Front. Plant Sci. 2015, 6, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Fehér, A.; Halmová, D.; Končeková, L. Gradient analysis of importance of spontaneously occurring vascular plant species in energy tree and grass stands. Acta Reg. Environ. 2013, 10, 33–35. [Google Scholar] [CrossRef] [Green Version]
- Verheyen, K.; Buggenhout, M.; Vangansbeke, P.; De Dobbelaere, A.; Verdonckt, P.; Bonte, D. Potential of short rotation coppice plantations to reinforce functional biodiversity in agricultural landscapes. Biomass Bioenergy 2014, 67, 435–442. [Google Scholar] [CrossRef]
- Welc, M.; Lundkvist, A.; Nordh, N.E.; Verwijst, T. Weed community trajectories in cereal and willow cultivations after termination of a willow short rotation coppice. Agron. Res. 2017, 15, 1795–1814. [Google Scholar] [CrossRef]
- Lososová, Z.; Chytrý, M.; Cimalová, Š.; Kropáč, Z.; Otýpková, Z.; Pyšek, P.; Tichý, L. Weed vegetation of arable land in central Europe: Gradients of diversity and species composition. J. Veg. Sci. 2004, 15, 415–422. [Google Scholar] [CrossRef]
- Fry, D.; Slater, F. The Biodiversity of Short Rotation Willow Coppice in the Welsh Landscape: A Report to the Institute of Biological, Environmental and Rural Sciences, Aberystwyth University for EU Project “Willows for Wales”; Aberystwyth University: Penglais, UK, 2009; Available online: https://www.aber.ac.uk/en/media/departmental/ibers/research/willowforwales/Biodiversity-of-src-coppice-in-the-Welsh-Landscape.pdf (accessed on 14 February 2012).

| Species Habitat Preferences | Willow Tordis | Willow Inger | Poplar Pegaso | Miscanthus × giganteus |
|---|---|---|---|---|
| Woodland | 18.97 | 10.34 | 13.33 | 7.55 |
| Arable land | 10.34 | 12.07 | 11.11 | 15.09 |
| Grassland | 25.86 | 31.03 | 35.56 | 28.30 |
| Ruderal sites | 12.07 | 12.07 | 13.33 | 13.21 |
| Arable land and ruderal sites | 17.24 | 17.24 | 11.11 | 20.75 |
| Grassland and ruderal sites | 5.17 | 6.90 | 4.44 | 7.55 |
| Not stated | 10.34 | 10.34 | 11.11 | 7.55 |
| Number of Weed Species | Willow Tordis | Willow Inger | Poplar Pegaso | Miscanthus × giganteus | |
|---|---|---|---|---|---|
| Willow variety Tordis | 58 | - | 0.526 | 0.288 | 0.542 |
| Willow variety Inger | 58 | 40 | - | 0.556 | 0.586 |
| Poplar variety Pegaso | 45 | 23 | 25 | - | 0.289 |
| Miscanthus × giganteus | 53 | 39 | 41 | 22 | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fehér, A.; Pintér, E.; Prus, P.; Končeková, L. Dependence of Weed Composition on Cultivated Plant Species and Varieties in Energy-Tree and -Grass Plantations. Agronomy 2020, 10, 1247. https://doi.org/10.3390/agronomy10091247
Fehér A, Pintér E, Prus P, Končeková L. Dependence of Weed Composition on Cultivated Plant Species and Varieties in Energy-Tree and -Grass Plantations. Agronomy. 2020; 10(9):1247. https://doi.org/10.3390/agronomy10091247
Chicago/Turabian StyleFehér, Alexander, Eduard Pintér, Piotr Prus, and Lýdia Končeková. 2020. "Dependence of Weed Composition on Cultivated Plant Species and Varieties in Energy-Tree and -Grass Plantations" Agronomy 10, no. 9: 1247. https://doi.org/10.3390/agronomy10091247





