Benzoxazinoids Biosynthesis in Rye (Secale cereale L.) Is Affected by Low Temperature
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. RNA Isolation and cDNA Synthesis
2.3. Quantitative Real-Time PCR Analysis
2.4. Biochemical Analysis
2.5. Statistical Analysis
- 21-day-old seedlings vs. 70-day-old untreated plants;
- 21-day-old seedlings vs. 70-day-old cold-treated plants;
- 70-day-old untreated plants vs. 70-day-old cold-treated plants;
- 77-day-old untreated plants vs. 77-day-old cold-treated plants;
- 70-day-old untreated plants vs. 77-day-old untreated plants;
- 70-day-old cold-treated plants vs. 77-day-old cold-treated plants.
3. Results
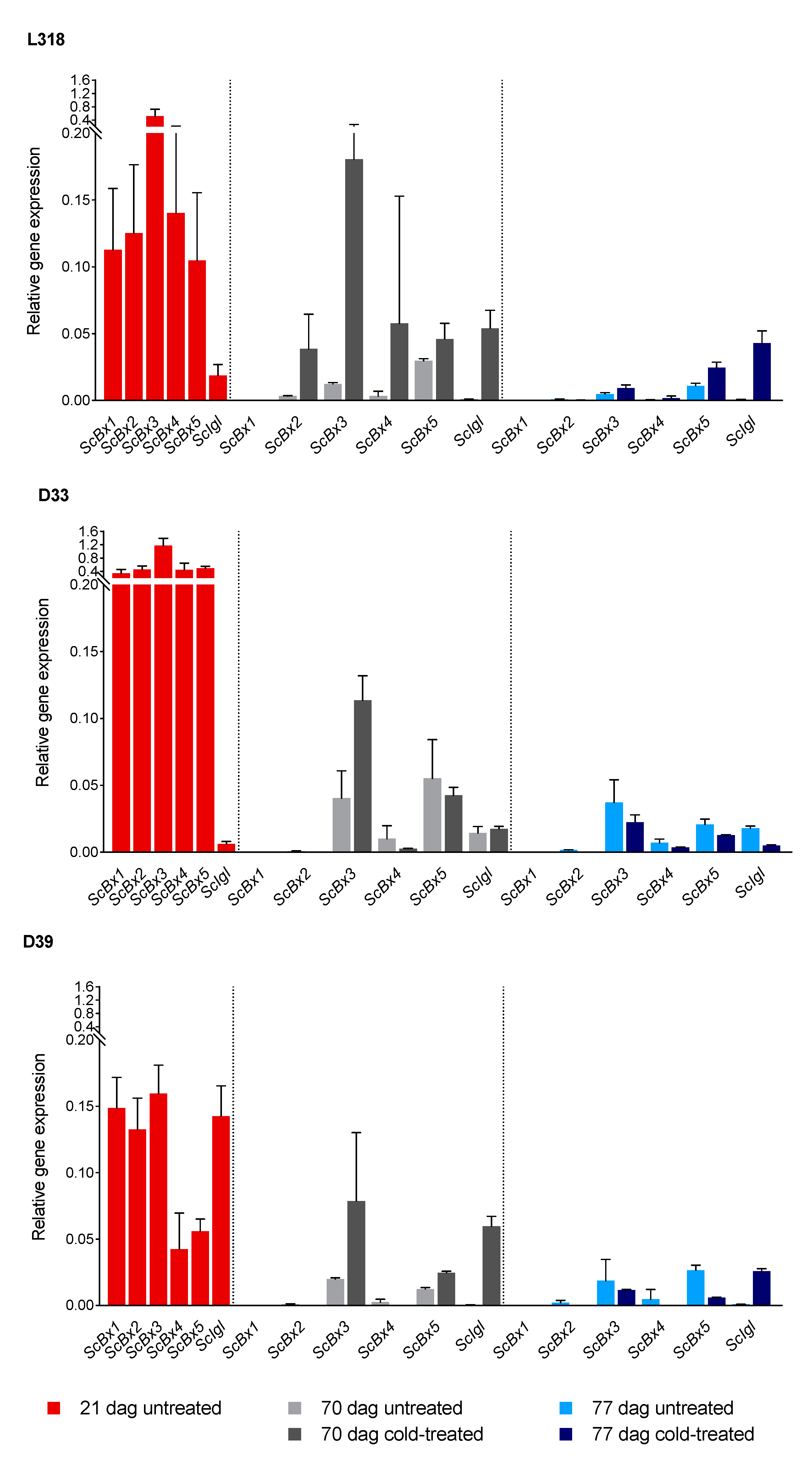
3.1. Effect of Low Temperature on Gene Expression
3.2. Effect of Low Temperature on BX Concentration
4. Discussion
Do the Changes in Gene Expression Correspond to Changes in BX Synthesis?
5. Conclusions
- The cultivation for seven weeks at 4 °C causes a decrease of BX concentration and expression level of related genes compared with those on the 21st day after seed germination.
- The decrease over time in BX concentration and in expression level of related genes is greater in cold-treated than in untreated plants.
- Manipulating the duration of the vernalization period can significantly affect the production of BXs in rye.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Frey, M.; Schullehner, K.; Dick, R.; Fiesselmann, A.; Gierl, A. Benzoxazinoid biosynthesis, a model for evolution of specialized metabolic pathways in plants. Phytochemistry 2009, 70, 1645–1651. [Google Scholar] [CrossRef] [PubMed]
- Niemeyer, H.M. Hydroxamic acids derived from 2-hydroxy-2H-1,4-benzoxazin-3(4H)-one: Key defense chemicals of cereals. J. Agric. Food Chem. 2009, 57, 1677–1696. [Google Scholar] [CrossRef] [PubMed]
- Makowska, B.; Bakera, B.; Rakoczy-Trojanowska, M. The genetic background of benzoxazinoid biosynthesis in cereals. Acta Physiol. Plant 2015, 37, 176. [Google Scholar] [CrossRef] [Green Version]
- Wouters, F.C.; Blanchett, B.; Gershenzo, J.; Vassão, D.G. Plant defense and herbivore counter-defense: Benzoxazinoids and insect herbivores. Phytochem. Rev. 2016, 15, 1127–1151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, S.; Richter, A.; Jander, G. Beyond Defense: Multiple Functions of Benzoxazinoids in Maize Metabolism. Plant Cell Physiol. 2018, 59, 1528–1537. [Google Scholar] [CrossRef]
- Chu, H.Y.; Wegel, E.; Osbourn, A. The plant genome: An evolutionary view on structure and function, from hormones to specialized metabolism: The emergence of metabolic gene clusters in plants. Plant J. 2011, 66, 66–79. [Google Scholar] [CrossRef]
- Meihls, L.N.; Handrick, V.; Glauser, G.; Barbier, H.; Kaur, H.; Haribal, M.M.; Lipka, A.E.; Gershenzon, J.; Buckler, E.S.; Erb, M.; et al. Natural variation in maize aphid resistance is associated with 2,4-dihydroxy-7-methoxy-1,4-benzoxazin-3-one glucoside methyltransferase activity. Plant Cell 2013, 25, 2341–2355. [Google Scholar] [CrossRef] [Green Version]
- Bakera, B.; Rakoczy-Trojanowska, M. Isolation and structural analysis of the Bx6 and Bx7 genes controlling the biosynthesis of benzoxazinoids in rye (Secale cereale L.). Acta Physiol. Plant 2020, 42, 56. [Google Scholar] [CrossRef] [Green Version]
- Frey, M.; Huber, K.; Park, W.J.; Sicker, D.; Lindberg, P.; Meeley, R.B.; Simmons, C.R.; Yalpani, N.; Gierl, A. A 2-oxoglutarate-dependent Dioxygenase Is Integrated in DIMBOA-biosynthesis. Phytochemistry 2003, 62, 371–376. [Google Scholar] [CrossRef]
- Jonczyk, R.; Schmidt, H.; Osterrieder, A.; Fiesselmann, A.; Schullehner, K.; Haslbeck, M.; Sicker, D.; Hofmann, D.; Yalpani, N.; Simmons, C.; et al. Elucidation of the final reactions of DIMBOA-glucoside biosynthesis in maize: Characterization of Bx6 and Bx7. Plant Physiol. 2008, 146, 1053–1063. [Google Scholar] [CrossRef] [Green Version]
- Rad Von, U.; Huttl, R.; Lottspeich, F.; Gierl, A.; Frey, M. Two glucosyltransferases are involved in detoxification of benzoxazinoids in maize. Plant J. 2001, 28, 633–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dick, R.; Rattei, T.; Haslbeck, M.; Schwab, W.; Gierl, A.; Frey, M. Comparative analysis of benzoxazinoid biosynthesis in monocots and dicots: Independent recruitment of stabilization and activation functions. Plant Cell 2012, 24, 915–928. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babcock, G.D.; Esen, A. Substrate specificity of maize b-glucosidase. Plant Sci. 1994, 101, 31–39. [Google Scholar] [CrossRef]
- Oikawa, A.; Ishihara, A.; Iwamura, H. Induction of HDMBOA-Glc accumulation and DIMBOA-Glc4-O-methyltransferase by jasmonic acid in poaceous plants. Phytochemistry 2002, 61, 331–337. [Google Scholar] [CrossRef]
- Sue, M.; Nakamura, C.; Nomura, T. Dispersed benzoxazinone gene cluster: Molecular characterization and chromosomal localization of glucosyltransferase and glucosidase genes in wheat and rye. Plant Physiol. 2011, 157, 985–997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niculaes, C.; Abramov, A.; Hannemann, L.; Frey, M. Plant protection by benzoxazinoids–recent insights into biosynthesis and function. Agronomy 2018, 8, 143. [Google Scholar] [CrossRef] [Green Version]
- Handrick, V.; Robert, C.A.M.; Ahern, K.R.; Zhou, S.; Machado, R.A.R.; Maag, D.; Glauser, G.; Fernandez-Penny, F.E.; Chandran, J.N.; Rodgers-Melnik, E.; et al. Biosynthesis of 8-O-methylated benzoxazinoid defence compounds in maize. Plant Cell Online 2016, 28, 1682–1700. [Google Scholar] [CrossRef] [Green Version]
- Nomura, T.; Ishihara, A.; Imaishi, H.; Endo, T.R.; Ohkawa, H.; Iwamura, H. Molecular characterization and chromosomal localization of cytochrome P450 genes involved in the biosynthesis of cyclic hydroxamic acids in hexaploid wheat. Mol. Genet. Genom. 2002, 267, 210–217. [Google Scholar] [CrossRef]
- Nomura, T.; Ishihara, A.; Imaishi, H.; Ohkawa, H.; Endo, T.R.; Iwamura, H. Rearrangement of the genes for the biosynthesis of benzoxazinones in the evolution of Triticeae species. Planta 2003, 217, 776–782. [Google Scholar] [CrossRef]
- Nomura, T.; Ishihara, A.; Yanagita, R.C.; Endo, T.R.; Iwamura, H. Three genomes differentially contribute to the biosynthesis of benzoxazinones in hexaploid wheat. Proc. Natl. Acad. Sci. USA 2005, 102, 16490–16495. [Google Scholar] [CrossRef] [Green Version]
- Nomura, T.; Nasuda, S.; Kawaura, K.; Ogihara, Y.; Kato, N.; Sato, F.; Kojima, T.; Toyoda, A.; Iwamura, H.; Endo, T.R. Structures of the three homoeologous loci of wheat benzoxazinone biosynthetic genes TaBx3 and TaBx4 and characterization of their promoter sequences. Theor. Appl. Genet. 2008, 116, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Frey, M.; Chomet, P.; Glawischnig, E.; Stettner, C.; Grün, S.; Winklmair, A.; Eisenreich, W.; Bacher, A.; Meeley, R.B.; Briggs, S.P.; et al. Analysis of a chemical plant defence mechanism in grasses. Science 1997, 277, 696–699. [Google Scholar] [CrossRef] [PubMed]
- Grün, S.; Frey, M.; Gierl, A. Evolution of the Indole Alkaloid Biosynthesis in the Genus Hordeum: Distribution of Gramine and DIBOA and Isolation of the Benzoxazinoid Biosynthesis Genes FromHordeum Lechleri. Phytochemistry 2005, 66, 1264–1272. [Google Scholar] [CrossRef] [PubMed]
- Nikus, J.; Esen, A.; Jonsson, L.M.V. Cloning of a plastidic rye (Secale cereale) b-glucosidase cDNA and its expression in Escherichia coli. Physiol. Plant 2003, 118, 337–345. [Google Scholar] [CrossRef]
- Bakera, B.; Makowska, B.; Groszyk, J.; Niziołek, M.; Orczyk, W.; Bolibok-Brągoszewska, H.; Hromada-Judycka, A.; Rakoczy-Trojanowska, M. Structural characteristics of ScBx genes controlling the biosynthesis of hydroxamic acids in rye (Secale cereale L.). J. Appl. Genet. 2015, 56, 287–298. [Google Scholar] [CrossRef] [Green Version]
- Tanwir, F.; Dionisio, G.; Adhikari, K.B.; Fomsgaard, I.S.; Gregersen, P.L. Biosynthesis and chemical transformation of benzoxazinoids in rye during seed germination and the identification of a rye Bx6-like gene. Phytochemistry 2017, 140, 95–107. [Google Scholar] [CrossRef]
- Wlazło, A.; Święcicka, M.; Koter, M.D.; Krępski, T.; Bolibok, L.; Stochmal, A.; Kowalczyk, M.; Rakoczy-Trojanowska, M. Genes ScBx1 and ScIgl–Competitors or Cooperators? Genes 2020, 20, 223. [Google Scholar] [CrossRef] [Green Version]
- Gianoli, E.; Papp, M.; Niemeyer, H.M. Costs and benefits of hydroxamic acids-related resistance in winter wheat against the bird cherry-oat aphid, Rhopalosiphum padi L. Ann. Appl. Biol. 1996, 129, 83–90. [Google Scholar] [CrossRef]
- Copaja, S.V.; Villarroel, E.; Bravo, H.R.; Pizarro, L.; Argandoña, V.H. Hydroxamic acids in Secale cereale L., and the relationship with their antifeedant and allelopathic properties. Z. Naturforsch. C. 2006, 61, 670–676. [Google Scholar] [CrossRef]
- Barrίa, B.N.; Copaja, S.V.; Niemeyer, H.M. Occurrence of DIBOA in wild Hordeum species and its relation to aphid resistance. Phytochemistry 1992, 31, 89–91. [Google Scholar] [CrossRef]
- Niemeyer, H.M.; Copaja, S.V.; Barrıa, B.N. The Triticeae as sources of hydroxamic acids, specialized metabolites in wheat conferring resistance against aphids. Hereditas 1992, 116, 295–299. [Google Scholar] [CrossRef]
- Frey, M.; Kliem, R.; Saedler, H.; Gierl, A. Expression of a cytochrome P450 gene family in maize. Mol. Gen. Genet. 1995, 246, 100–109. [Google Scholar] [CrossRef]
- Reberg-Horton, S.C.; Burton, J.D.; Danehower, D.A.; Ma, G.Y.; Monks, D.W.; Murphy, J.P.; Ranells, N.N.; Williamson, J.D.; Creamer, N.G. Changes over time in the allelochemical concentration of ten cultivars of rye (Secale cereale L.). J. Chem. Ecol. 2005, 31, 179–193. [Google Scholar] [CrossRef]
- La Hovary, C. Allelochemicals in Secale cereale: Biosynthesis and Molecular Biology of Benzoxazinones. 2011. Available online: https://repository.lib.ncsu.edu/handle/1840.16/6844 (accessed on 5 May 2020).
- Groszyk, J.; Kowalczyk, M.; Yanushevska, Y.; Stochmal, A.; Rakoczy-Trojanowska, M.; Orczyk, W. Identification and VIGS-based characterization of Bx1 ortholog in rye (Secale cereale L.). PLoS ONE 2017, 12, e0171506. [Google Scholar] [CrossRef]
- Zheng, Y.Q.; Zhao, Y.; Dong, F.S.; Yao, J.R.; Hurle, K. Relationship of DIMBOA concentration in wheat seedlings and its resistance to plant pathogens. Allelopath. J. 2005, 15, 137–143. [Google Scholar]
- Ahmad, S.; Veyrat, N.; Gordon-Weeks, R. Benzoxazinoid metabolites regulate innate immunity against aphids and fungi in maize. Plant Physiol. 2011, 157, 317–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, P.; Praz, C.; Li, B.; Singla, J.; Robert, C.A.; Kessel, B.; Scheuermann, D.; Lüthi, L.; Ouzunova, M.; Erb, M.; et al. Fungal resistance mediated by maize wall-associated kinase ZmWAK-RLK1 correlates with reduced benzoxazinoid concentration. New Phytol. 2019, 221, 976–987. [Google Scholar] [CrossRef] [Green Version]
- Ding, X.; Yang, M.; Huang, H.; Chuan, Y.; He, X.; Li, C.; Zhu, Y.; Zhu, S. Priming maize resistance by its neighbors: Activating 1,4-benzoxazine-3-ones synthesis and defense gene expression to alleviate leaf disease. Front. Plant Sci. 2015, 6, 830. [Google Scholar] [CrossRef] [Green Version]
- Morse, S.; Wratten, S.D.; Edwards, P.J.; Niemeyer, H.M. Changes in the hydroxamic acid concentration of maize leaves with time and after artificial damage; implications for insect attack. Ann. Appl. Biol. 1991, 119, 239–249. [Google Scholar] [CrossRef]
- Kruidhof, H.M.; Van Dam, N.M.; Ritz, C.; Lotz, L.A.P.; Kropff, M.J.; Bastiaans, L. Mechanical wounding under field conditions: A potential tool to increase the allelopathic inhibitory effect of cover crops on weeds? Europ. J. Agron. 2014, 52, 229–236. [Google Scholar] [CrossRef]
- Makleit, P. Changes in cyclic hydroxamic acid concentration of various rye varieties for the effect of abiotic stress. Acta Biol. Szeged. 2005, 49, 103–104. [Google Scholar]
- Rakoczy-Trojanowska, M.; Święcicka, M.; Bakera, B.; Kowalczyk, M.; Stochmal, A.; Bolibok, L. Co-cultivating rye with berseem clover affects benzoxazinoid production and expression of related genes. Crop Sci. 2020. [CrossRef]
- Mwaja, V.N.; Masiunas, J.B.; Weston, L.A. Effects of fertility on biomass, phytotoxicity, and allelochemical concentration of cereal rye. J. Chem. Ecol. 1995, 21, 81–96. [Google Scholar] [CrossRef] [PubMed]
- Epstein, W.W.; Rowsemitt, C.N.; Berger, P.J.; Negus, N.C. Dynamics of 6 methoxybenzoxazolinone in winter wheat, Effects of photoperiod and temperature. J. Chem. Ecol. 1986, 12, 2011–2021. [Google Scholar] [CrossRef] [PubMed]
- Gianoli, E.; Niemeyer, H.M. Environmental effects on the accumulation of hydroxamic acids in wheat seedlings: The importance of plant growth rate. J. Chem. Ecol. 1997, 23, 543–551. [Google Scholar] [CrossRef]
- Nie, C.R.; Luo, S.M.; Lin, C.X.; Zeng, R.S.; Huang, J.H.; Wang, J.W. Status of DIMBOA and phenolic acids in transgenic Bt corn. Aust. J. Agri. Res. 2005, 56, 833–837. [Google Scholar] [CrossRef]
- Rakoczy-Trojanowska, M.; Orczyk, W.; Krajewski, P.; Bocianowski, J.; Stochmal, A.; Kowalczyk, M. ScBx gene based association analysis of hydroxamate concentration in rye (Secale cereale L.). J. Appl. Genet. 2017, 58, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Świecicka, M.; Dmochowska-Boguta, M.; Orczyk, W.; Grądzielewska, A.; Stochmal, A.; Kowalczyk, M.; Bolibok, L.; Rakoczy-Trojanowska, M. Changes in benzoxazinoid contents and the expression of the associated genes in rye (Secale cereal L.) due to brown rust and the inoculation procedure. PLoS ONE 2020, 15, e0233807. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Stoskopf, N.C. Cereal Grain Crops; Reston Publishing: Reston VA, USA, 1985; pp. 403–414. [Google Scholar]
- Nuttonson, M.Y. Rye–Climate Relationships and the Use of Phenology in Ascertaining the Thermal and Photo-Thermal Requirements of Rye–Based on Data of North America and of Some Thermally Analogous Areas of North America in the Soviet Union, Finland, Poland, and Czechoslovakia; American Institute of Crop Ecology: Washington, DC, USA, 1957; p. 219. [Google Scholar]
- Hartman, T.A. Influence of daylength on vernalization of winter rye. NJAS Wagening. J. Life Sci. 1964, 12, 132–155. [Google Scholar] [CrossRef]
- Zieliński, K.; Krzewska, M.; Żur, I.; Juzoń, K.; Kopeć, P.; Nowicka, A.; Moravčiková, J.; Skrzypek, E.; Dubas, E. The effect of glutathione and mannitol on androgenesis in anther and isolated microspore cultures of rye (Secale cereale L.). Plant Cell Tiss. Organ Cult. 2020, 140, 577–592. [Google Scholar] [CrossRef] [Green Version]
- Sue, M.; Yamazaki, K.; Yajima, S.; Nomura, T.; Matsukawa, T.; Iwamura, H.; Miyamoto, T. Molecular and structural characterization of hexameric β-d-glucosidases in wheat and rye. Plant Physiol. 2006, 141, 1237–1247. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rice, C.P.; Park, Y.B.; Adam, F.; Abdul-Baki, A.A.; Teasdale, J.R. Hydroxamic acid concentration and toxicity of rye at selected growth stages. J. Chem. Ecol. 2005, 31, 1887–1905. [Google Scholar] [CrossRef]
- La Hovary, C.; Danehower, D.A.; Ma, G.; Reberg-Horton, C.; Williamson, J.D.; Baerson, S.R.; Burton, J.D. Phytotoxicity and benzoxazinone concentration in field grown cereal rye (Secale cereale L.). Int. J. Agron. 2016. [Google Scholar] [CrossRef]
- Gierl, A.; Frey, M. Evolution of benzoxazinone biosynthesis and indole production in maize. Planta 2001, 213, 493–498. [Google Scholar] [CrossRef] [PubMed]
- Zasada, I.A.; Meyer, S.L.F.; Halbrendt, J.M.; Rice, C. Activity of hydroxamic acids from Secale cereale against the plant-parasitic nematodes Meloidogyne incognita and Xiphinema americanum. Phytopathology 2005, 95, 1116–1121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giri, S.; Lathrop, R.G.; Obropta, C.C. Climate change vulnerability assessment and adaptation strategies through best management practices. J. Hydrol. 2020, 580, 124311. [Google Scholar] [CrossRef]
- Kitetu, G.M.; Ko, J.H. Climate Change on Agriculture in 2050: A CGE Approach. 2020. In Proceedings of the 23rd GTAP Annual Conference on Global Economic Analysis “Global Economic Analysis Beyond 2020”, Division of International & Area Studies, Busan, Korea, 17–19 June 2020. [Google Scholar]
- Wittchen, U.; Chmielewski, F.M. Phytoclimate of winter rye stands. Meteorol. Z. 2005, 14, 183–189. [Google Scholar] [CrossRef]
- Pomortsev, A.V.; Dorofeev, N.V.; Zorina, S.Y.; Katysheva, N.B.; Sokolova, L.G. The effect of planting date on Winter rye and triticale overwinter survival and yield in Eastern Siberia. In Proceedings of the IOP Conference Series: Earth and Environmental Science, Siberian Institute of Plant Physiology and Biochemistry, Krasnoyarsk, Russia, 22 June 2019. [Google Scholar]
- Xie, Y.; Wang, C.; Yang, W.; Yang, W.; Feng, M.; Qiao, X.; Song, J. Canopy hyperspectral characteristics and yield estimation of winter wheat (Triticum aestivum) under low temperature injury. Sci. Rep. 2020, 10, 244. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Algaba, J.; Sørensen, C.K.; Labouriau, R.; Justesen, A.F.; Hovmøller, M.S. Susceptibility of winter wheat and triticale to yellow rust influenced by complex interactions between vernalisation, temperature, plant growth stage and pathogen race. Agronomy 2020, 10, 13. [Google Scholar] [CrossRef] [Green Version]
- Kuwabara, C.; Imai, R. Molecular basis of disease resistance acquired through cold acclimation in overwintering plants. J. Plant Biol. 2009, 52, 19–26. [Google Scholar] [CrossRef]
- Gaudet, D.A.; Laroche, A.; Frick, M.; Davoren, J.; Puchalski, B.; Ergon, A. Expression of plant defence-related (PR-protein) transcripts during hardening and dehardening of winter wheat. Physiol. Mol. Plant Pathol. 2000, 57, 15–24. [Google Scholar] [CrossRef]



| Gene | Sequences (5′–3′) |
|---|---|
| ScBx1 | F: TCAAAACCTGAACACGTGAAGC |
| R: GCCTCTAGCCTTTTCAATCCTTC | |
| ScBx2 | F: CTCATGATTCCACACTTCTCCC |
| R: AGGCGTTTACAACGACACGA | |
| ScBx3 | F: CGGTCTCACTACGGATAACATCA |
| R: GAGCTCAGCCATGCCG | |
| ScBx4 | F: TTCTCTCAAGAAAGAGTACGGC |
| R: GGAGTATTCCAGCACCAGGA | |
| ScBx5 | F: GAAGCTCGTCAACACCCATCT |
| R: GCCAGGAACTCGCTCATGT | |
| ScIgl | F: AACACCAGCTACACCATCAGAG |
| R: GTGGGTTTACAGTCGCCCTA | |
| HvAct | F: CCCCTTTGAACCCAAAAGCC |
| R: GAAAGCACGGCCTGAATAGC |
| Inbred Line | Comparison | Gene | |||||
|---|---|---|---|---|---|---|---|
| ScBx1 | ScBx2 | ScBx3 | ScBx4 | ScBx5 | ScIgl | ||
| L318 | 1 | nd | ** | ** | ** | ** | - |
| 2 | nd | * | * | - | ** | ** | |
| 3 | nd | - | - | - | ** | ** | |
| 4 | nd | * | * | * | ** | ** | |
| 5 | nd | * | - | * | ** | - | |
| 6 | nd | * | - | - | ** | ** | |
| D33 | 1 | nd | ** | ** | ** | ** | ** |
| 2 | nd | nd | ** | ** | ** | ** | |
| 3 | nd | nd | ** | - | - | - | |
| 4 | nd | nd | * | * | ** | ** | |
| 5 | nd | ** | - | - | ** | - | |
| 6 | nd | nd | ** | ** | ** | ** | |
| D39 | 1 | nd | ** | * | * | * | ** |
| 2 | nd | nd | - | nd | - | ** | |
| 3 | nd | nd | * | nd | ** | ** | |
| 4 | nd | ** | * | nd | ** | ** | |
| 5 | nd | ** | - | - | ** | ** | |
| 6 | nd | nd | * | nd | - | - | |
| Inbred Line | Comparison | BX | |||||
|---|---|---|---|---|---|---|---|
| HBOA | GDIBOA | DIBOA | GDIMBOA | DIMBOA | MBOA | ||
| L318 | 1 | - | * | ** | ** | - | ** |
| 2 | ** | ** | ** | ** | ** | ** | |
| 3 | - | - | - | - | ** | ** | |
| 4 | ** | ** | ** | nd | nd | ** | |
| 5 | - | ** | - | ** | - | - | |
| 6 | - | ** | ** | nd | nd | - | |
| D33 | 1 | ** | ** | ** | ** | - | ** |
| 2 | * | ** | ** | ** | nd | ** | |
| 3 | ** | ** | ** | * | nd | ** | |
| 4 | ** | ** | ** | ** | - | ** | |
| 5 | - | ** | - | ** | - | * | |
| 6 | Ndr | ** | - | - | nd | - | |
| D39 | 1 | ** | ** | ** | ** | ** | nd |
| 2 | ** | ** | ** | ** | nd | ** | |
| 3 | ** | ** | ** | - | nd | nd | |
| 4 | ** | - | ** | nd | nd | ** | |
| 5 | * | - | - | * | - | nd | |
| 6 | - | ** | - | nd | nd | - | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakera, B.; Święcicka, M.; Stochmal, A.; Kowalczyk, M.; Bolibok, L.; Rakoczy-Trojanowska, M. Benzoxazinoids Biosynthesis in Rye (Secale cereale L.) Is Affected by Low Temperature. Agronomy 2020, 10, 1260. https://doi.org/10.3390/agronomy10091260
Bakera B, Święcicka M, Stochmal A, Kowalczyk M, Bolibok L, Rakoczy-Trojanowska M. Benzoxazinoids Biosynthesis in Rye (Secale cereale L.) Is Affected by Low Temperature. Agronomy. 2020; 10(9):1260. https://doi.org/10.3390/agronomy10091260
Chicago/Turabian StyleBakera, Beata, Magdalena Święcicka, Anna Stochmal, Mariusz Kowalczyk, Leszek Bolibok, and Monika Rakoczy-Trojanowska. 2020. "Benzoxazinoids Biosynthesis in Rye (Secale cereale L.) Is Affected by Low Temperature" Agronomy 10, no. 9: 1260. https://doi.org/10.3390/agronomy10091260
APA StyleBakera, B., Święcicka, M., Stochmal, A., Kowalczyk, M., Bolibok, L., & Rakoczy-Trojanowska, M. (2020). Benzoxazinoids Biosynthesis in Rye (Secale cereale L.) Is Affected by Low Temperature. Agronomy, 10(9), 1260. https://doi.org/10.3390/agronomy10091260






