Cost-Effective and Time-Efficient Molecular Assisted Selection for PPV Resistance in Apricot Based on ParPMC2 Allele-Specific PCR
Abstract
:1. Introduction
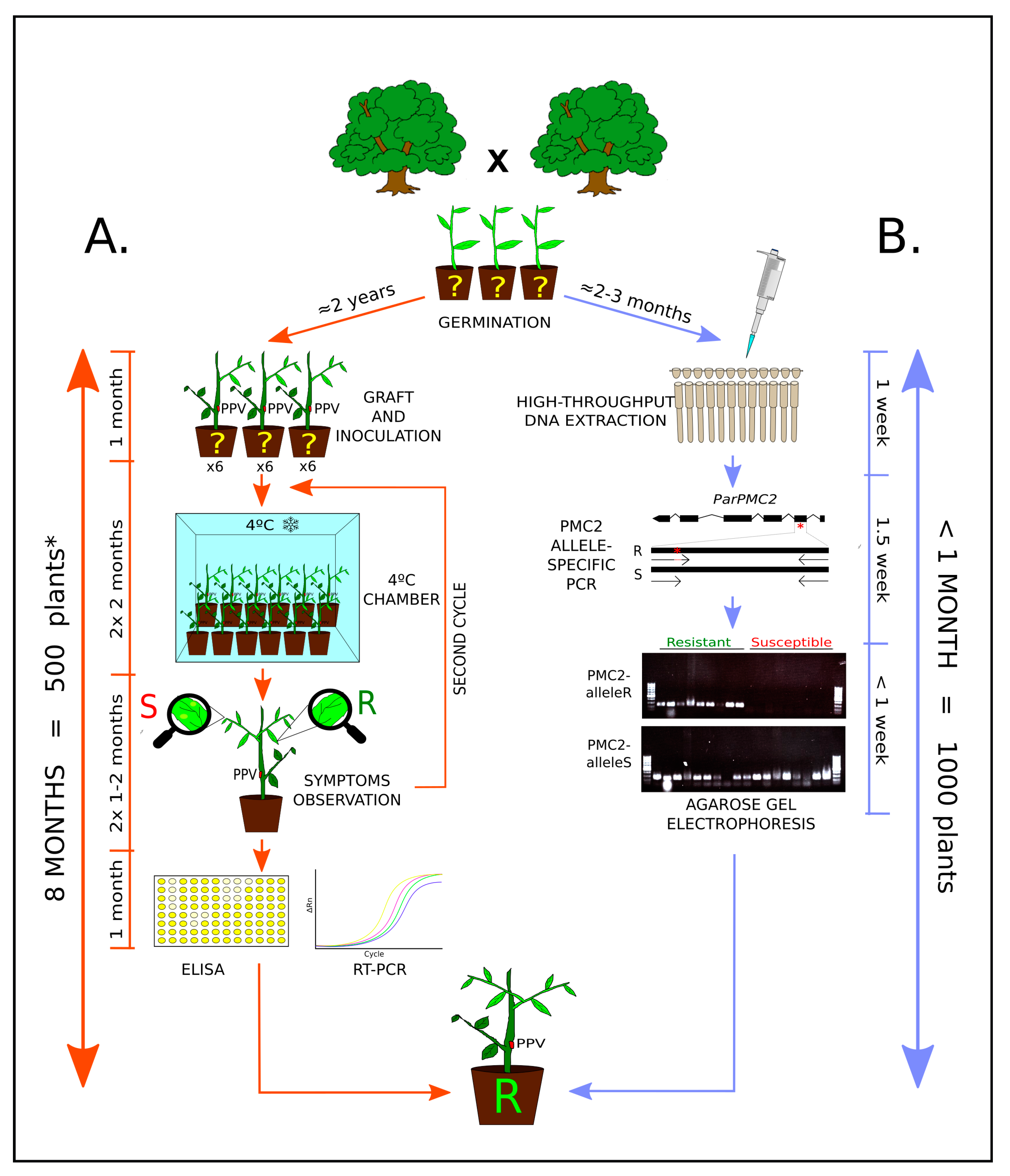
2. Materials and Methods
2.1. High-Throughput DNA Isolation in 96-Well Plate
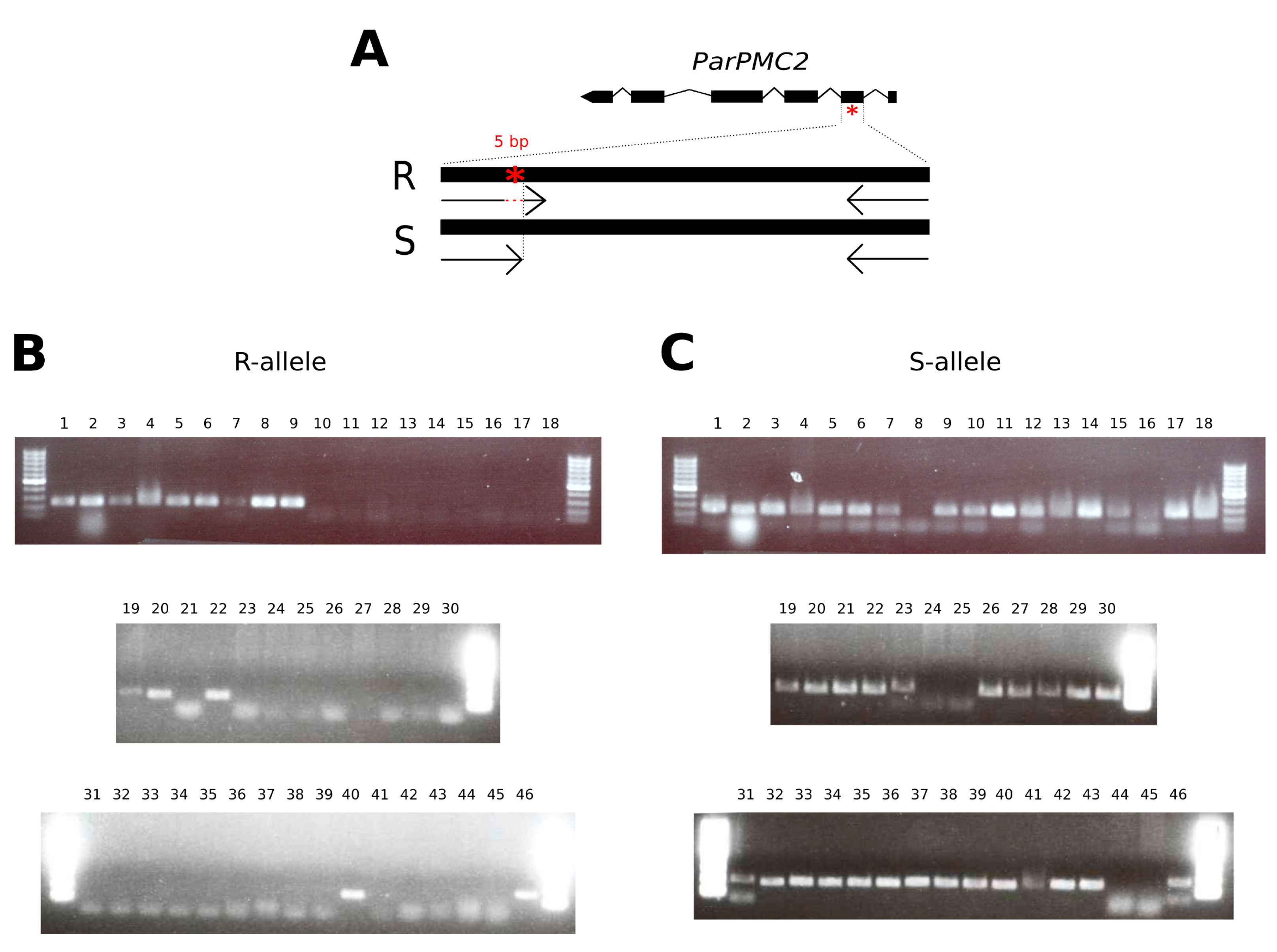
2.2. PMC2 Genotype by Allele-Specific PCR Assay
2.3. WGS Mapping and PMC2 Screening
3. Results and Discussion
3.1. High-Throughput DNA Extraction and ParPMC2-del Genotyping for MAS
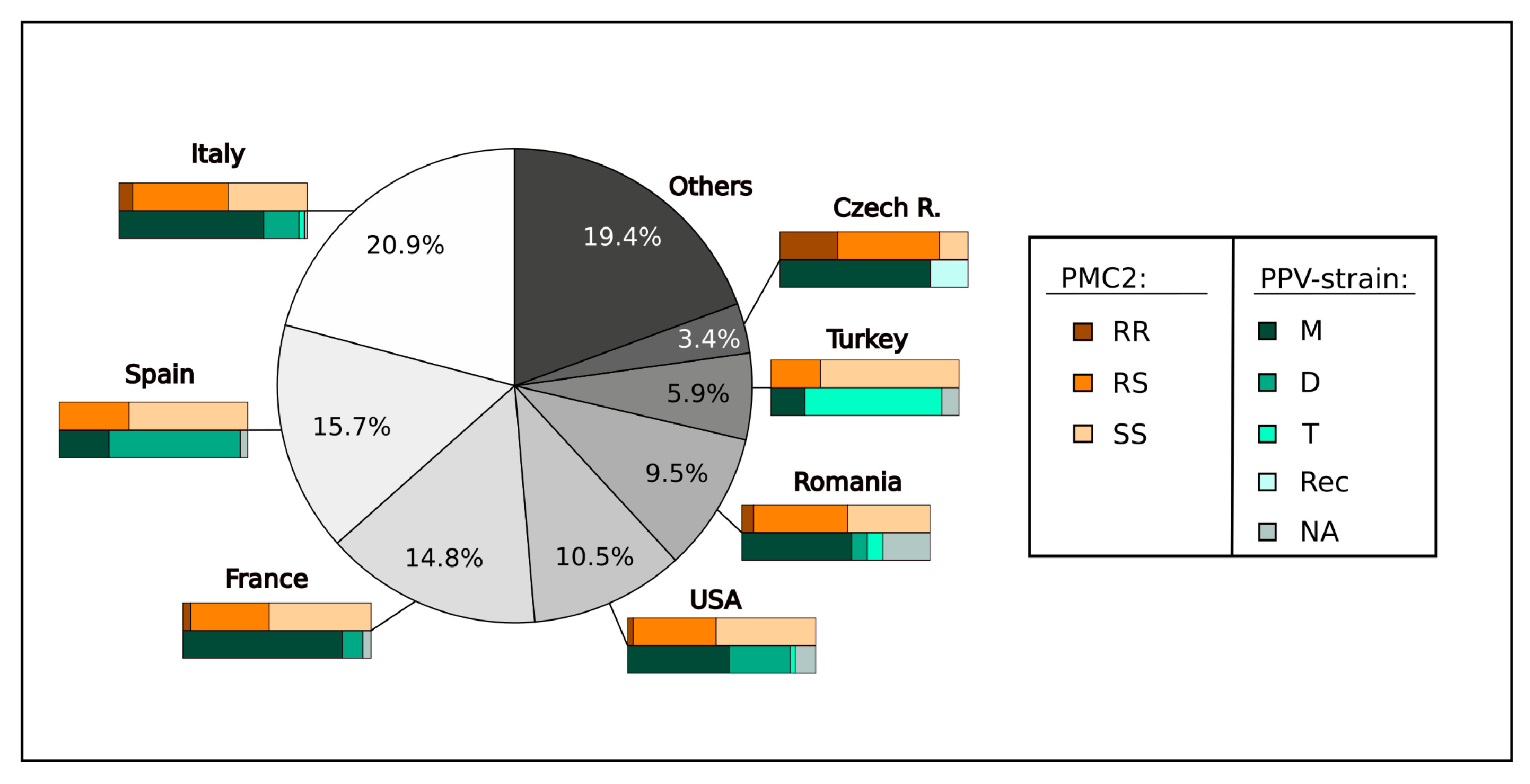
3.2. ParPMC2-del Highly Correlates with PPV Resistance in Apricot Germplasm
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rehder, A. Manual of Cultivated Trees and Shrubs Hardy in North America, 2nd ed.; The Macmillan Company: New York, NY, USA, 1940. [Google Scholar]
- Zhebentyayeva, T.N.; Ledbetter, C.; Burgos, L.; Llácer, G. Apricots. In Fruit Breeding, 1st ed.; Badenes, M.L., Byrne, D.H., Eds.; Springer: New York, NY, USA, 2012; Volume 3, pp. 415–458. [Google Scholar] [CrossRef]
- Moreno, M.A. Breeding and selection of Prunus rootstocks at the Aula Dei experimental station, Zaragoza, Spain. Acta Hortic. 2004, 658, 519–528. [Google Scholar] [CrossRef]
- García, J.A.; Cambra, M. Plum pox virus and sharka disease. Plant. Viruses 2007, 1, 69–79. [Google Scholar]
- García, J.A.; Glasa, M.; Cambra, M.; Candresse, T. Plum pox virus and sharka: A model potyvirus and a major disease. Mol. Plant. Pathol. 2014, 15, 226–241. [Google Scholar] [CrossRef]
- Chirkov, S.; Ivanov, P.; Sheveleva, A.; Zakubanskiy, A.; Osipov, G. New highly divergent Plum pox virus isolates infecting sour cherry in Russia. Virology 2017, 502, 56–62. [Google Scholar] [CrossRef]
- James, D.; Varga, A.; Sanderson, D. Genetic diversity of Plum pox virus: Strains, disease and related challenges for control. Can. J. Plant. Pathol. 2013, 35, 431–441. [Google Scholar] [CrossRef]
- Sihelská, N.; Glasa, M.; Šubr, Z.W. Host preference of the major strains of Plum pox virus—Opinions based on regional and world-wide sequence data. J. Integr. Agric. 2017, 16, 510–515. [Google Scholar] [CrossRef]
- Martínez-Gómez, P.; Dicenta, F.; Audergon, J.M. Behaviour of apricot (Prunus armeniaca L.) cultivars in the presence of Sharka (Plum pox potyvirus): A review. Agronomie 2000, 20, 407–422. [Google Scholar] [CrossRef] [Green Version]
- Dondini, L.; Lain, O.; Vendramin, V.; Rizzo, M.; Vivoli, D.; Adami, M.; Guidarelli, M.; Gaiotti, F.; Palmisano, F.; Bazzoni, A.; et al. Identification of QTL for resistance to Plum pox virus strains M and D in Lito and Harcot apricot cultivars. Mol. Breed. 2011, 27, 289–299. [Google Scholar] [CrossRef]
- Hurtado, M.A.; Romero, C.; Vilanova, S.; Abbott, A.G.; Llácer, G.; Badenes, M.L. Genetic linkage maps of two apricot cultivars (Prunus armeniaca L.) and mapping of PPV (sharka) resistance. Theor. Appl. Genet. 2002, 105, 182–191. [Google Scholar] [CrossRef]
- Lalli, D.A.; Abbott, A.G.; Zhebentyayeva, T.N.; Badenes, M.L.; Damsteegt, V.; Polák, J.; Krška, B.; Salava, J. A genetic linkage map for an apricot (Prunus armeniaca L.) BC1 population mapping Plum pox virus resistance. Tree Genet. Genomes 2008, 4, 481–493. [Google Scholar] [CrossRef]
- Lambert, P.; Dicenta, F.; Rubio, M.; Audergon, J.M. QTL analysis of resistance to sharka disease in the apricot (Prunus armeniaca L.) ‘Polonais’ x ‘Stark Early Orange’ F1 progeny. Tree Genet. Genomes 2007, 3, 299–309. [Google Scholar] [CrossRef]
- Marandel, G.; Pascal, T.; Candresse, T.; Decroocq, V. Quantitative resistance to Plum pox virus in Prunus davidiana P1908 linked to components of the eukaryotic translation initiation complex. Plant. Pathol. 2009, 58, 425–435. [Google Scholar] [CrossRef]
- Mariette, S.; Wong Jun Tai, F.; Roch, G.; Barre, A.; Chague, A.; Decroocq, S.; Groppi, A.; Laizet, Y.; Lambert, P.; Tricon, D.; et al. Genome-wide association links candidate genes to resistance to Plum pox virus in apricot (Prunus armeniaca). New Phytol. 2016, 209, 773–784. [Google Scholar] [CrossRef] [Green Version]
- Pilarova, P.; Marandel, G.; Decroocq, V.; Salava, J.; Krška, B.; Abbott, A.G. Quantitative trait analysis of resistance to Plum pox virus in the apricot F1 progeny ‘Harlayne’ x ‘Vestar’. Tree Genet. Genomes 2010, 6, 467–475. [Google Scholar] [CrossRef]
- Soriano, J.M.; Vera-Ruiz, E.; Vilanova, S.; Martínez-Calvo, J.; Llácer, G.; Badenes, M.L.; Romero, C. Identification and mapping of a locus conferring Plum pox virus resistance in two apricot-improved linkage maps. Tree Genet. Genomes 2008, 4, 391–402. [Google Scholar] [CrossRef] [Green Version]
- Soriano, J.M.; Domingo, M.L.; Zuriaga, E.; Romero, C.; Zhebentyayeva, T.; Abbott, A.G.; Badenes, M.L. Identification of simple sequence repeat markers tightly linked to Plum pox virus resistance in apricot. Mol. Breed. 2012, 30, 1017–1026. [Google Scholar] [CrossRef]
- Zhebentyayeva, T.N.; Reighard, G.L.; Lalli, D.; Gorina, V.M.; Krška, B.; Abbott, A.G. Origin of resistance to Plum pox virus in apricot: What new AFLP and targeted SSR data analyses tell. Tree Genet. Genomes 2008, 4, 403–417. [Google Scholar] [CrossRef]
- Zuriaga, E.; Soriano, J.M.; Zhebentyayeva, T.; Romero, C.; Dardick, C.; Cañizares, J.; Badenes, M.L. Genomic analysis reveals MATH gene(s) as candidate(s) for Plum pox virus (PPV) resistance in apricot (Prunus armeniaca L.). Mol. Plant Pathol. 2013, 14, 663–677. [Google Scholar] [CrossRef]
- Zuriaga, E.; Romero, C.; Blanca, J.M.; Badenes, M.L. Resistance to Plum pox virus (PPV) in apricot (Prunus armeniaca L.) is associated with down-regulation of two MATHd genes. BMC Plant Biol. 2018, 18, 25. [Google Scholar] [CrossRef] [Green Version]
- Rodamilans, B.; Valli, A.; García, J.A. Molecular plant-Plum pox virus interactions. Mol. Plant. Microbe Interact. 2020, 33, 6–17. [Google Scholar] [CrossRef]
- Rubio, M.; Ruiz, D.; Egea, J.; Martínez-Gómez, P.; Dicenta, F. Opportunities of marker assisted selection for Plum pox virus resistance in apricot breeding programs. Tree Genet. Genomes 2014, 10, 513–525. [Google Scholar] [CrossRef]
- Decroocq, S.; Chague, A.; Lambert, P.; Roch, G.; Audergon, J.M.; Geuna, F.; Chiozzotto, R.; Bassi, D.; Dondini, L.; Tartarini, S.; et al. Selecting with markers linked to the PPVres major QTL is not sufficient to predict resistance to Plum pox virus (PPV) in apricot. Tree Genet. Genomes 2014, 10, 1161–1170. [Google Scholar] [CrossRef]
- Moustafa, T.A.; Badenes, M.L.; Martínez-Calvo, J.; Llácer, G. Determination of resistance to sharka (plum pox) virus in apricot. Sci. Hort. 2001, 91, 59–70. [Google Scholar] [CrossRef]
- Lommel, S.A.; McCain, A.H.; Morris, T.J. Evaluation of indirect-linked immunosorbent assay for the detection of plant viruses. Phytopathology 1982, 72, 1018–1022. [Google Scholar] [CrossRef]
- Wetzel, T.; Candresse, T.; Ravelonandro, M.; Dunez, J. A polymerase chain reaction assay adapted to plum pox potyvirus detection. J. Virol. Methods 1991, 33, 355–365. [Google Scholar] [CrossRef]
- Passaro, M.; Geuna, F.; Bassi, D.; Cirilli, M. Development of a high-resolution melting approach for reliable and cost-effective genotyping of PPVres locus in apricot (P. armeniaca). Mol. Breed. 2017, 37, 74. [Google Scholar] [CrossRef]
- Ru, S.; Main, D.; Evans, K.; Peace, C. Current applications, challenges, and perspectives of marker-assisted seedling selection in Rosaceae tree fruit breeding. Tree Genet. Genomes 2015, 11, 8. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. A rapid isolation procedure for small quantities of fresh leaf tissue. Phytochem. Bull. 1987, 19, 11–15. [Google Scholar]
- Muñoz-Sanz, J.V.; Zuriaga, E.; Badenes, M.L.; Romero, C. A disulfide bond A-like oxidoreductase is a strong candidate gene for self-incompatibility in apricot (Prunus armeniaca) pollen. J. Exp. Bot. 2017, 68, 5069–5078. [Google Scholar] [CrossRef] [Green Version]
- Haas, B.J.; Papanicolaou, A.; Yassour, M.; Grabherr, M.; Blood, P.D.; Bowden, J.; Couger, M.B.; Eccles, D.; Li, B.; Lieber, M.; et al. De novo transcript sequence reconstruction from RNA-seq using the trinity platform for reference generation and analysis. Nat. Protoc. 2013, 8, 1494–1512. [Google Scholar] [CrossRef]
- Verde, I.; Jenkins, J.; Dondini, L.; Micali, S.; Pagliarani, G.; Vendramin, E.; Paris, R.; Aramini, V.; Gazza, L.; Rossini, L.; et al. The Peach v2.0 release: High-resolution linkage mapping and deep resequencing improve chromosome-scale assembly and contiguity. BMC Genom. 2017, 18, 225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with bowtie 2. Nat. Meth. 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thorvaldsdóttir, H.; Robinson, J.T.; Mesirov, J.P. Integrative Genomics Viewer (IGV): High-performance genomics data visualization and exploration. Brief. Bioinform. 2013, 14, 178–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peace, C. DNA-informed breeding of rosaceous crops: Promises, progress and prospects. Hortic. Res. 2017, 4, 17006. [Google Scholar] [CrossRef] [Green Version]
- Edge-Garza, D.A.; Luby, J.J.; Peace, C. Decision support for cost-efficient and logistically feasible marker-assisted seedling selection in fruit breeding. Mol. Breed. 2015, 35, 223. [Google Scholar] [CrossRef]
- Audergon, J.M.; Blanc, A.; Gilles, F.; Broquaire, J.M.; Clauzel, G.; Gouble, B.; Grotte, M.; Reich, M.; Bureau, S.; Pitiot, C. New recent selections issued from INRA’s apricot breeding programme. Acta Hortic. 2010, 862, 179–182. [Google Scholar] [CrossRef]
- Bassi, D.; Audergon, J.M. Apricot breeding: Update and perspectives. Acta Hortic. 2006, 701, 279–294. [Google Scholar] [CrossRef]
- Bassi, D.; Bellini, E.; Guerriero, R.; Monastra, F.; Pennone, F. Apricot breeding in Italy. Acta Hortic. 1995, 384, 47–54. [Google Scholar] [CrossRef]
- Egea, J.; Dicenta, F.; Burgos, L.; Martínez-Gómez, P.; Rubio, M.; Campoy, J.A.; Ortega, E.; Patiño, J.L.; Nortes, L.; Molina, A.; et al. New apricot cultivars from CEBAS-CSIC (Murcia, Spain) breeding programme. Acta Hortic. 2010, 862, 113–118. [Google Scholar] [CrossRef]
- Martínez-Calvo, J.; Font, A.; Llácer, G.; Badenes, M.L. Apricot and Peach breeding programs from the IVIA. Acta Hortic. 2009, 814, 185–188. [Google Scholar] [CrossRef]
- Gürcan, K.; Ceylan, A. Strain identification and sequence variability of Plum pox virus in Turkey. Turkish J. Agric. 2016, 40, 746–760. [Google Scholar] [CrossRef]
- Decroocq, S.; Cornille, A.; Tricon, D.; Babayeva, S.; Chague, A.; Eyquard, J.P.; Karychev, R.; Dolgikh, S.; Kostritsyna, T.; Li, S.; et al. New insights into the history of domesticated and wild apricots and its contribution to Plum pox virus resistance. Mol. Ecol. 2016, 25, 4712–4729. [Google Scholar] [CrossRef] [PubMed]
- Dosba, F.; Orliac, S.; Dutranoy, F.; Maison, P.; Massonie, G.; Audergon, J.M. Evaluation of resistance to Plum pox virus in apricot trees. Acta Hortic. 1992, 309, 211–219. [Google Scholar] [CrossRef]
- Karayiannis, I.; Mainou, A. Resistance to Plum pox virus in apricots. EPPO Bull. 1994, 24, 761–766. [Google Scholar] [CrossRef]
- Martínez-Gómez, P.; Rubio, M.; Dicenta, F. Evaluation of resistance to Plum pox virus of North American and European apricot cultivars. HortScience 2003, 38, 568–569. [Google Scholar] [CrossRef] [Green Version]
- Egea, J.; Rubio, M.; Campoy, J.A.; Dicenta, F.; Ortega, E.; Nortes, M.D.; Martínez-Gómez, P.; Molina, A.; Molina, A., Jr.; Ruiz, D. ‘Mirlo Blanco’, ‘Mirlo Anaranjado’, and ‘Mirlo Rojo’: Three new very early-season apricots for the fresh market. HortScience 2010, 45, 1893–1894. [Google Scholar] [CrossRef] [Green Version]
- Passaro, M. Cost-Effective Use of Molecular Markers in the Practical Resolution of Common Horticultural Challenges. Ph.D. Thesis, Agriculture, Environment and Bioenergy in Universitá Degli Studi Di Milano, Milan, Italy, 2016. [Google Scholar]
- Krška, B.; Salava, J.; Polák, J. Breeding for resistance: Breeding for Plum pox virus resistant apricots (Prunus armeniaca L.) in the Czech Republic. EPPO Bull. 2006, 36, 330–331. [Google Scholar] [CrossRef]
- Krška, B.; Salava, J.; Polák, J.; Komínek, P. Genetics of resistance to Plum pox virus in apricot. Plant Protect. Sci. 2002, 38, 180–182. [Google Scholar] [CrossRef] [Green Version]
- Krška, B.; Vachun, Z.; Nečas, T.; Ondrásek, I. New Sharka resistant apricots at the Horticultural Faculty in Lednice. Acta Hortic. 2015, 1063, 105–110. [Google Scholar] [CrossRef]
- Rankovic, M.; Duli-Markovic, I.; Paunovic, S. Sharka virus in apricot and its diagnosis. Acta Hortic. 1999, 488, 783–786. [Google Scholar] [CrossRef]
- CEP INNOVATION Website. Available online: https://cepinnovation-novadi.com/variete/anegat/ (accessed on 29 July 2020).
- Milatović, D.; Nikolić, D.; Krška, B. Testing of self-(in)compatibility in apricot cultivars from European breeding programmes. HortScience 2013, 40, 65–71. [Google Scholar] [CrossRef] [Green Version]
- Brans, Y. Evaluation de la sensibilité de cultivars d’abricotier à la Sharka en zone confinée. Présentation de l’essai Ctifl 2012–2015. In Proceedings of the Rencontres Phytosanitaires Ctifl/DGAL—SDQPV Fruits à Noyau Ctifl Balandran, Bellegarde, France, 16 October 2016. [Google Scholar]
- Babini, A.R.; Vicchi, V.; Missere, D. L’esame delle cultivar tolleranti alla sharka. Ermes Agricoltura 2010. Available online: http://www.crpv.it/doc/549738/DLFE-9612.pdf (accessed on 29 August 2020).
- Finn, C.E.; Clark, J.R. Register of New Fruit and Nut Cultivars List 44. HortScience 2008, 43, 1321–1343. [Google Scholar] [CrossRef] [Green Version]
- Krška, B.; Vachůn, Z. Apricot Breeding at the Faculty of Horticulture in Lednice. Agronomy 2016, 6, 27. [Google Scholar] [CrossRef] [Green Version]
- Krška, B. Genetic resources of apricot for adaptability improvement and breeding. Acta Hortic. 2010, 862, 203–208. [Google Scholar] [CrossRef]
- Gürcan, K.; Çetinsağ, N.; Pınar, H.; Macit, T. Molecular and biological assessment reveals sources of resistance to Plum pox virus—Turkey strain in Turkish apricot (Prunus armeniaca) germplasm. Sci. Hortic. 2019, 252, 348–353. [Google Scholar] [CrossRef]
- Drogoudi, P. ΕΥΠAΘΕΙA ΠOΙΚΙΛΙΩΝ ΒΕΡΙΚOΚΙAΣ ΣΤOΝ ΙO ΤHΣ ΕΥΛOΓΙAΣ ΤHΣ ΔAΜAΣΚHΝΙAΣ (PPV) (Sensitivity of apricot varieties against PPV). Π. Δρογούδη 28o Συνέδριο Ελληνικής Εταιρείας πιστήμης Oπωροκηπευτικών ’50 χρόνια από την ίδρυση της ΕΕΕO’ . In Proceedings of the 28th Conference of the Hellenic Fruit and Vegetable Credit Society ‘50 Years Since the Founding of EEEO’, Thessaloniki, Greece, 16–20 October 2017. [Google Scholar]
- Goffreda, J.C.; Voordeckers, A.; Butenis-Vorsa, L.; Cowgill, W.P., Jr.; Maletta, M.H.; Frecon, J.L. NJA53 apricot. HortScience 1995, 30, 389–390. [Google Scholar] [CrossRef]
- Faggioli, F.; Barba, M. Screening of stone fruit germplasm for resistance to Plum Pox Potyvirus. Adv. Hortic. Sci. 1996, 10, 91–94. [Google Scholar]
- Poggi Pollini, C.; Bianchi, L.; Babini, A.; Vicchi, V.; Liverani, A.; Brandi, F.; Giunchedi, L.; Autonell, C.; Ratti, C. Evaluation of Plum pox virus infection on different stone fruit tree varieties. J. Plant. Pathol. 2008, 90, S27–S31. [Google Scholar]
- Brooks, R.M.; Olmo, H.P. The Brooks and Olmo Register of Fruit and Nut Varieties, 3rd ed.; ASHS Press: Alexandria, VA, USA, 1997. [Google Scholar]
- Leccese, A.; Bartolini, S.; Viti, R. Genotype harvest season, and cold storage influence on fruit quality and antioxidant properties of apricot. Int. J. Food Prop. 2012, 15, 864–879. [Google Scholar] [CrossRef]
- Fuchs, E.; Grünzig, M.; Kegler, H. Investigation on the Plum pox virus resistance in different apricot genotypes. Acta Virol. 1998, 42, 222–225. [Google Scholar]
- Polák, J.; Oukropec, I.; Krška, B.; Pívalová, J.; Miller, W. Difference in reactions of apricot and peach cultivars to Plum pox virus: Serological and symptomatological evaluation. HortScience 2003, 30, 129–134. [Google Scholar] [CrossRef] [Green Version]
- Audergon, J.M.; Dosba, F.; Karayiannis, I.; Dicenta, F. Amélioration de l’abricotier pour la résistance à la sharka. EPPO Bull. 1994, 24, 741–748. [Google Scholar] [CrossRef]
- Mesarović, J.; Trifković, J.; Tosti, T.; Akšić, M.F.; Milatović, D.; Ličina, V.; Milojković-Opsenica, D. Relationship between ripening time and sugar content of apricot (Prunus armeniaca L.) kernels. Acta Physiol. Plant. 2018, 40, 157. [Google Scholar] [CrossRef]
- Krška, B.; Oukropec, I.; Polak, J.; Kominek, P. The evaluation of apricot (Prunus armeniaca L.) cultivars and hybrids resistant to Sharka. Acta Hortic. 2000, 538, 143–146. [Google Scholar] [CrossRef]
- Salazar, J.A.; Rubio, M.; Ruiz, D.; Tartarini, S.; Martínez-Gómez, P.; Dondini, L. SNP development for genetic diversity analysis in apricot. Tree Genet. Genomes 2015, 11, 15. [Google Scholar] [CrossRef]
- Syrgiannidis, G.; Mainou, A. Two new apricot varieties resistant to Sharka (Plum pox virus) disease created by crossing. In Agriculure, Proceedings of the Programme de recherche Agrimed. Deuxiemes Rencontres sur L’abricotier, Avignon, France, 27–31 May 1991; CEC Commission of the European Communities: Luxembourg, 1993. [Google Scholar]
- Egea, J.; Ruiz, D.; Dicenta, F.; Burgos, L. Murciana apricot. HortScience 2005, 40, 254–255. [Google Scholar] [CrossRef]
- Ledbetter, C.A.; Peterson, S.J. ‘Apache’ and ‘Kettleman’: Two early season apricots for the fresh market. HortScience 2005, 40, 2202–2203. [Google Scholar] [CrossRef]
- Trandafirescu, M.; Dumitru, L.M.; Trandafirescu, I. Evaluating the Resistance to the Plum pox virus of Some Apricot Tree Cultivars and Hybrids in South-Eastern Romania. Proc. Latvian Acad. Sci. 2013, 67, 203–206. [Google Scholar] [CrossRef] [Green Version]
- Egea, J.; Ruiz, D.; Martínez-Gómez, P. Influence of rootstock on the productive behaviour of ‘Orange Red’ apricot under Mediterranean conditions. Fruits 2004, 59, 367–373. [Google Scholar] [CrossRef] [Green Version]
- Karayannis, I.; Di Terlizzi, B.; Audergon, J.M. Susceptibility of apricot cultivars to Plum pox virus. Acta Hortic. 1999, 488, 753–760. [Google Scholar] [CrossRef]
- Halász, J. Molecular Background of the S-locus Controlled Self-Incompatibility in Apricot. Ph.D. Thesis, Department of Genetics and Plant Breeding, Corvinus University, Budapest, Hungary, 2007. [Google Scholar]
- Ledbetter, C.A.; Ramming, D.W. Apricot cv. Robada. United. States Patent USPP9890P, 13 May 1997. [Google Scholar]
- Karayiannis, I.; Ledbetter, C.A. Susceptibility of certain apricot and plumcot cultivars to Plum pox virus infection. Acta Hortic. 2009, 825, 153–156. [Google Scholar] [CrossRef] [Green Version]
- Egea, J.; Dicenta, F.; Burgos, L. ‘Rojo Pasión’ Apricot. HortScience 2004, 39, 1490–1491. [Google Scholar] [CrossRef]
- Egea, J.; Dicenta, F.; Martínez-Gómez, P.; Burgos, L. Selene apricot. HortScience 2004, 39, 192–1493. [Google Scholar] [CrossRef]
- Syrgiannidis, G. Selection of two apricot varieties resistant to Sharka virus. Acta Phytopathol. Acad. Sci. Hung. 1980, 15, 85–87. [Google Scholar] [CrossRef]
- Adascalului, M.; Hoza, D.; Ion, L. Behaviour study for pollination a Romanian apricot varieties using different source of resistance to Sharka. J. Hortic. For. Biotechnol. 2014, 18, 13–17. [Google Scholar]
- Ion, L.; Asănică, A.; Moale, C. Studies of resistance to Sharka in several Romanian apricot progenies. In Proceedings of the International Conference on Chemical, Agricultural and Biological Sciences (CABS-2015), Istanbul, Turkey, 4–5 September 2015. [Google Scholar]
- Elibüyük, S.; Erdiller, G. The susceptibility of some apricot and plum varieties to Plum pox (sharka) virus. Acta Hortic. 1995, 384, 549–552. [Google Scholar] [CrossRef]
- Rodríguez, J.; Andrés, V.; Gil, L.; Martínez, J.; Hita, I. Sensibilidad a Sharka en Variedades de Albaricoquero de Murcia; Frutales Hueso Fund. La Caixa: Barcelona, Spain, 1995; pp. 56–64. [Google Scholar]
- Dosba, F.; Lansac, M.; Maison, P.; Massonie, G.; Audergon, J.M. Tolerance to Plum pox virus in apricot. Acta Hort. 1988, 235, 275–281. [Google Scholar] [CrossRef]
- Dondini, L.; Lain, O.; Geuna, F.; Banfi, R.; Gaiotti, F.; Tartarini, S.; Bassi, D.; Testolin, R. Development of a new SSR-based linkage map in apricot and analysis of synteny with existing Prunus maps. Tree Genet. Genomes 2007, 3, 239–249. [Google Scholar] [CrossRef]
- Martínez-Gómez, P.; Dicenta, F. Evaluation of resistance of apricot cultivars to a Spanish isolate of plum pox potyvirus (PPV). Plant. Breed. 2000, 119, 179–181. [Google Scholar] [CrossRef]
- Karayiannis, I. Susceptibility of apricots cultivars to Plum pox virus in Greece. Acta Hortic. 1989, 235, 271–274. [Google Scholar] [CrossRef]
- Balan, V.; Stoian, E. Susceptibility of certain apricot-tree to the Plum pox virus pathogenic action. Acta Hortic. 1995, 384, 565–569. [Google Scholar] [CrossRef]
- Avinent, L.; Hermoso de Mendoza, A.; Llácer, G.; García, S. Transmisión del virus de la sharka y sensibilidad varietal en albaricoquero. In Proceedings of the II Congreso Ibérico Ciencias Hortícolas, Zaragoza, Spain, 27–30 April 1993. [Google Scholar]
- Maghuly, F.; Borroto-Fernandez, E.; Ruthner, S.; Pedryc, A.; Laimer, M. Microsatellite variability in apricots (Prunus armeniaca L.) reflects their geographic origin and breeding history. Tree Genet. Genomes 2005, 1, 151–165. [Google Scholar] [CrossRef]
- Audergon, J.M.; Morvan, G.; Dicenta, F.; Chastelliere, G.; Karayiannis, I. A method to determine the susceptibility of apricot to Plum pox virus. Acta Hortic. 1995, 384, 575–579. [Google Scholar] [CrossRef]
- Egea, J.; Burgos, L. Detecting cross-incompatibility of three North American apricot cultivars and establishing the first incompatibility group in apricot. J. Am. Soc. Hort. Sci. 1996, 121, 1002–1005. [Google Scholar] [CrossRef] [Green Version]
- McLaren, J. Apricot tree, ‘F168 cv’. United. States Patent USPP16071P2, 25 October 2005. [Google Scholar]
- Dicenta, F.; Audergon, J.M. Localization of Plum pox virus (PPV) in tissues of susceptible and resistant apricot cultivars. Phytopathol. Med. 1995, 34, 83–87. [Google Scholar]
- Lachkar, A.; Mlika, M. New apricot varieties selected from the Tunisian breeding programme. Acta Hort. 2006, 717, 189–192. [Google Scholar] [CrossRef]
- Dosba, F.; Denise, F.; Audergon, J.M.; Maison, P.; Massonie, G. Plum pox virus resistance of apricot. Acta Hortic. 1991, 293, 569–580. [Google Scholar] [CrossRef]
- Egea, J.; Campoy, J.A.; Dicenta, F.; Burgos, L.; Patiño, J.L.; Ruiz, D. ‘Estrella’ and ‘Sublime’ apricot cultivars. HortScience 2009, 44, 469–470. [Google Scholar] [CrossRef] [Green Version]
- Eynard, A.; Roggero, P.; Lenzi, R.; Conti, M.; Milne, R.G. Test for pollen and seed transmission on Plum pox virus (Sharka) in two apricot cultivars. Adv. Hortic. Sci. 1991, 5, 104–106. [Google Scholar]
- Gallois, J.L.; Moury, B.; German-Retana, S. Role of the Genetic Background in Resistance to Plant Viruses. Int. J. Mol. Sci. 2018, 19, 2856. [Google Scholar] [CrossRef] [Green Version]
- Polák, J.; Kominek, P.; Jokes, M.; Oukropec, I.; Krška, B. The evaluation of resistance of apricots to Plum pox virus by ELISA and ISEM. Acta Hortic. 1995, 386, 285–289. [Google Scholar] [CrossRef]
- Layne, R.E.C.; Hunter, D.M. ‘AC Harostar’ apricot. HortScience 2003, 38, 140–141. [Google Scholar] [CrossRef] [Green Version]
- Hegedűs, A.; Lénárt, J.; Halász, J. Sexual incompatibility in Rosaceae fruit tree species: Molecular interactions and evolutionary dynamics. Biol. Plant 2012, 56, 201–209. [Google Scholar] [CrossRef]
- Austin, P.T. Pollination of Sundrop Apricot. Ph.D. Thesis, Massey University, Auckland, New Zealand, 1995. [Google Scholar]
- Egea, J.; Ruiz, D.; Burgos, L. “Dorada” apricot. HortScience 2005, 40, 1919–1920. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-Sanz, J.V.; Zuriaga, E.; López, I.; Badenes, M.L.; Romero, C. Self-(in)compatibility in apricot germplasm is controlled by two major loci, S and M. BMC Plant. Biol. 2017, 17, 82. [Google Scholar] [CrossRef] [Green Version]
- Corrin, A.A. “Ruby” Apricot Tree. United. States Patent USPP8177, 16 March 1993. [Google Scholar]
- Zaiger, C.F. Apricot Tree (Spring Giant). United. States Patent USPP5138, 15 November 1983. [Google Scholar]
- Dicenta, F.; Audergon, J.M. Inheritance of resistance to plum pox potyvirus (PPV) in ‘Stella’ apricot seedlings. Plant. Breed. 1998, 117, 579–581. [Google Scholar] [CrossRef]
- Tartarini, S.; Sansavini, S.; Vinatzer, B.; Gennari, F.; Domizi, C. Efficiency of marker assisted selection (MAS) for the Vf scab resistance gene. Acta Hortic. 2000, 538, 549–552. [Google Scholar] [CrossRef]
| Name | Country a | Origin | Pedigree | PPV Resistance Phenotype b | PPV Strain Used | First Phenotype Ref | PMC2 Genotype c | PMC2 Genotype Ref |
|---|---|---|---|---|---|---|---|---|
| A4316 | IT | R | M | [15] | RS | WGS | ||
| A4804 | IT | R | M | [15] | RS | WGS | ||
| Adriana (= Le-3241) | CR | Horticulture Faculty, Lednice | Vestar × SEO [50] | R | M | [51] | RR | [24] |
| Rec | [52] | |||||||
| Alfred (= NY345) | USA | Geneva, NY State Expt Sta, by Robert C. Lamb | OP seedling of selection from (Doty × Geneva) | R | M | [53] | RS | WGS |
| Andswee | IR | R | M | [15] | RS | WGS | ||
| Anegat | FR | INRA, CEP Innovation | R | M/D | [54] | RS | [49] | |
| Bergarouge (= Avirine A2914) | FR | INRA | Bergeron × Orange Red [55] | R | D | [23] | RS | [49] |
| M | [56] | |||||||
| Bergeval (= Aviclo, A3950) | FR | INRA | R | M | [56] | RS | [49] | |
| BO03615011 | IT | Goldrich × Harlayne [28] | R | M* | [49] | RS | [49] | |
| BO03615025 | IT | Goldrich × Harlayne [28] | R | M* | [49] | RR | [49] | |
| BO03615034 | IT | Goldrich × Harlayne [28] | R | M* | [28] | RR | [28] | |
| BO03615049 | IT | Goldrich × Harlayne [28] | R | M* | [28] | RR | [28] | |
| BO03615053 | IT | Goldrich × Harlayne [28] | R | M* | [28] | RS | [28] | |
| BO03615070 | IT | Goldrich × Harlayne [28] | R | M* | [49] | RR | [49] | |
| BO04624031 | IT | Portici × Goldrich [28] | R | M* | [28] | RS | [28] | |
| BO04624039 | IT | Portici × Goldrich [28] | R | M* | [49] | SS | [49] | |
| BO05636034 | IT | Kyoto × Priscilla [28] | R | M* | [28] | RS | [28] | |
| BO06609012 | IT | Silvercot × Bora [28] | R | M* | [49] | RS | [49] | |
| BO06609013 | IT | Silvercot × Bora [28] | R | M* | [49] | RS | [49] | |
| BO06609024 | IT | Silvercot × Bora [28] | R | M* | [49] | RS | [49] | |
| BO06609033 | IT | Silvercot × Bora [28] | R | M* | [49] | RS | [49] | |
| BO06609036 | IT | Silvercot × Bora [28] | R | M* | [49] | RS | [49] | |
| BO06609037 | IT | Silvercot × Bora [28] | R | M* | [49] | RS | [49] | |
| BO06609039 | IT | Silvercot × Bora [28] | R | M* | [49] | RS | [49] | |
| BO06609045 | IT | Silvercot × Bora [28] | R | M* | [49] | RS | [49] | |
| BO06609048 | IT | Silvercot × Bora [28] | R | M* | [28] | RS | [28] | |
| BO06609055 | IT | Silvercot × Bora [28] | R | M* | [28] | RS | [28] | |
| BO06609060 | IT | Silvercot × Bora [28] | R | M* | [49] | RS | [49] | |
| BO06609068 | IT | Silvercot × Bora [28] | R | M* | [49] | RS | [49] | |
| BO06609074 | IT | Silvercot × Bora [28] | R | M* | [49] | RS | [49] | |
| BO06609079 | IT | Silvercot × Bora [28] | R | M* | [49] | RS | [49] | |
| BO06609083 | IT | Silvercot × Bora [28] | R | M* | [49] | RS | [49] | |
| BO06609087 | IT | Silvercot × Bora [28] | R | M* | [49] | RS | [49] | |
| BO06609099 | IT | Silvercot × Bora [28] | R | M* | [49] | RS | [49] | |
| BO06609104 | IT | Silvercot × Bora [28] | R | M* | [49] | RS | [49] | |
| BO06609113 | IT | Silvercot × Bora [28] | R | M* | [49] | RS | [49] | |
| BO06609129 | IT | Silvercot × Bora [28] | R | M* | [49] | RS | [49] | |
| BO06609133 | IT | Silvercot × Bora [28] | R | M* | [49] | RS | [49] | |
| BO06609136 | IT | Silvercot × Bora [28] | R | M* | [49] | RS | [49] | |
| BO96621002 | IT | Goldrich × Lito [28] | R | M | [57] | RR | [28] | |
| BO96621030 | IT | Goldrich × Lito [28] | R | M | [57] | RS | [28] | |
| Bora (BO90610010) | IT | University of Bologna and Milan, by D. Bassi | Early Blush × PA 7005-2 [58] | R | M/D | [58] | RS | [21,28] |
| Candela (= LE-2927) | CR | Horticulture Faculty, Lednice | Hungarian Best × SEO [59] | R | M | [60] | RR | [49] |
| Cebir | TU | R | T | [61] | RS | [61] | ||
| Congat | FR | INRA, CEP Innovation | R | - | [62] | RS | [49] | |
| Early Blush (= RUTBHART, NJA53, Aurora46) | US | Rutgers Horticultural Research Farm, New Brunswick, N.J. | RR17–62 × NJA-13 [63] | R | D | [64] | RS | PCR; [21,28,61] |
| M | [65] | |||||||
| T | [61] | |||||||
| Farlis | FR | Marie-France BOIS, France (IPS) | R | M* | [28,49] | RS | [28] | |
| Farmingdale (=NY346) | USA | Geneva, NY State Expt Sta, by Robert C. Lamb | OP seedling of selection from (Doty × Geneva) [66] | R | M | [53] | RS | [28] |
| Flavor cot (=Bayoto) | USA | Washington State University Research, by Tom Toyama | R | M | [57] | RS | [28] | |
| Flopria | FR | PSB Producción Vegetal S.L. | R | M* | [28] | RS | PCR; [28] | |
| GG9310 | SP | IVIA, Moncada, Valencia | Goldrich × Ginesta [18] | R | D | IVIA | RS | PCR |
| GG9318 | SP | IVIA, Moncada, Valencia | Goldrich × Ginesta [18] | R | D | IVIA | RS | PCR |
| GG937 | SP | IVIA, Moncada, Valencia | Goldrich × Ginesta [18] | R | D | IVIA | RS | PCR |
| GG941 | SP | IVIA, Moncada, Valencia | Goldrich × Ginesta [18] | R | D | IVIA | RS | PCR |
| GG979 | SP | IVIA, Moncada, Valencia | Goldrich × Ginesta [18] | R | D | IVIA | RS | PCR |
| GG9869 | SP | IVIA, Moncada, Valencia | Goldrich × Ginesta [18] | R | D | IVIA | RS | PCR |
| Gilgat | FR | INRA / CEP INNOVATION | R | M* | [28]; [49] | RS | [28] | |
| GP9817 | SP | IVIA, Moncada, Valencia | Goldrich × Palau [18] | R | D | IVIA | RS | PCR |
| Dama Rosa (GG9871) | SP | IVIA, Moncada, Valencia | Goldrich × Ginesta [18] | R | D | IVIA | RS | PCR; [49] |
| Dama Taronja (GK988) | SP | IVIA, Moncada, Valencia | Goldrich × Katy [18] | R | D | IVIA | RS | PCR; [49] |
| Dulcinea | IT | Pisa University | Moniqui OP [67] | R | D | [64] | SS | PCR; [49] |
| Fracasso | IT | Tolerant | T | [61] | SS | [61] | ||
| Harlayne | C | Agr. Canada, Res. Station, Harrow, Ontario, by REC Layne | V51092 ((Reliable × OP) × OP) × Sun Glo [66] | R | D | [68] | RS | PCR; [20,21,24,28,61] |
| M | [45] | |||||||
| Harval (=HW437) | C | Agr. Canada, Res. Station, Harrow, Ontario, by REC Layne | Veecot × HW435 (Rouge du Roussillon × NJA2 (Morden604 OP)) [66] | R | M | [69] | RS | [28] |
| Henderson | USA | Geneva, NY, by GW Henderson | Unknown [66] | R | M | [46] | RS | PCR; [21] |
| D | [70] | |||||||
| Kaniş (=M2252) | TU | R | T | [61] | SS | [61] | ||
| Karum | TU | R | T | [61] | RS | [61] | ||
| Lady cot (=HYB 3-3) | FR | COT International | R | M* | [28] | RS | [28] | |
| Laycot | C | V51092 ((Reliable o.p.) o.p.) × NJA1 [71] | R | M | [15] | RR | WGS | |
| LE-2904 | CR | Horticulture Faculty, Lednice | Velkopavlovická × SEO [19] | R | M | [72] | RS | [49] |
| LE-3205 | CR | Horticulture Faculty, Lednice | R | M* | [49] | RR | [49] | |
| Le-3246 | CR | Horticulture Faculty, Lednice | Vestar × SEO [51] | R | M | [51] | RS | [24] |
| LE-3662 | CR | Horticulture Faculty, Lednice | R | M | [72] | RR | [49] | |
| Lifos | TU | R | T | [61] | RS | [61] | ||
| Lillycot | FR | SDR Fruit Llc (US) | Unknown [73] | R | M* | [28] | RS | [28] |
| Lito | GR | SEO × Tirynthos [18] | R | M | [74] | RS | PCR; [24,28] | |
| D | IVIA | |||||||
| Mediabel (=Mediabell) | FR | Newcot and IPS | R | M* | [28] | RS | [28] | |
| Mirlo Naranja (= Mirlo anaranjado) | SP | CEBAS-CSIC, Murcia | Rojo Pasión × Búlida Precoz [48] | R | D | [48] | RS | PCR |
| SS | [49] | |||||||
| Mirlo Blanco | SP | CEBAS-CSIC, Murcia | Rojo Pasión × Búlida Precoz [48] | R | D | [48] | RS | [28] |
| Mirlo Rojo | SP | CEBAS-CSIC, Murcia | Rojo Pasión × Búlida Precoz [48] | R | D | [48] | RS | PCR; [49] |
| Mogador | SP | PSB Producción Vegetal S.L. | R | M* | [28,49] | RS | PCR; [28] | |
| Moixent (=GM961) | SP | IVIA, Valencia | Goldrich × Mitger [18] | R | D | IVIA | RS | PCR; [49] |
| Murciana | SP | CEBAS-CSIC, Murcia | Orange Red × Currot [73] | R | D | [75] | RS | WGS; PCR; [49] |
| M | [15] | |||||||
| Nikitskii | UKR | R | M | [15] | RS | WGS | ||
| NJA42 | USA | New Jersey | NJA12 × NJA13 [76] | R | ? | [77] | RS | PCR |
| Orange Red (=Barth; NJA-32) | USA | New Jersey | Lasgerdi Mashhad × NJA2 (= Morden 604 OP) [78] | R | D | [68] | RS | PCR; [21] |
| M | [79] | |||||||
| Pandora | GR | SEO × Tirynthos [18] | R | M | [74] | RS | PCR | |
| D | [47] | |||||||
| Pelese di Giovanniello | IT | Tolerant | D | [64] | SS | [49] | ||
| Perla | SP | Murcia | R | D | [64] | SS | PCR | |
| Petra (BO88617102) | IT | University of Bologna and Milan, Italy, by D Bassi | Goldrich × Pelese di Giovanniello [73] | R | M* | [28] | RS | [28] |
| Precoce d’Imola | IT | tolerant | D | [64] | SS | WGS | ||
| Priboto (=Zebra) | FR | bud mutation of Goldrich [80] | R | M | [15] | RS | WGS; [49] | |
| Pricia | FR | Marie-France BOIS, France (IPS) | R | M* | [28,49] | RS | [28] | |
| Pseudo Royal | USA | R | M | [15] | RS | WGS | ||
| Robada (= K106-2) | USA | Parlier, California | Orange Red × K113-40 (ancestry includes Blenheim, Blush and Perfection) [81] | R | M | [82] | RS | WGS |
| Rojo Pasión | SP | CEBAS-CSIC | Orange Red × Currot [83] | R | D | [83] | RS | PCR; [49] |
| Rosa | SP | CEBAS-CSIC, Murcia | Orange Red × Palsteyn [73] | R | D | [41] | RS | [49] |
| Tolerant | [23] | |||||||
| Rubista | FR | Marie-France BOIS, France (IPS) | R | M* | [28,49] | RS | [28] | |
| Sabbatani (= Selezione Sabbatani?) | IT | R | D | [64] | SS | [49] | ||
| Selene | SP | CEBAS-CSIC | Goldrich × A2564 (=Screara × SEO) [18] | R | D | [84] | RS | PCR; [49] |
| SEOP934 | SP | IVIA | SEO × Palau [18] | R | D | IVIA | RS | PCR |
| Spring Blush (= EA3126TH) | FR | Escande EARL | R | M* | [57] | RR | [49] | |
| Stark Early Orange (= SEO, Earle Orange) | USA | Grandview, Washington, by WL Roberts | Unknown [66] | R | M | [85] | RS | PCR; [20,21,24,28,61] |
| D | [70] | |||||||
| Stella | USA | Unknown [18] | R | M | [85] | RR | PCR; [21] | |
| D | [70] | |||||||
| Sunglo (= Sun Glo) | USA | Columbia & Okanogan Nursery Co. | Unknown [66] | R | M | [45] | RS | PCR |
| D | [47] | SS | WGS | |||||
| Sunnycot (= 97-3-203) | USA | SDR FRUIT LLC – USA | R | [62] | RS | [49] | ||
| Traian | RO | R | D | [86] | RS | PCR; [87] | ||
| Tsunami (= EA 5016) | FR | Escande EARL | R | M* | [28] | RS | [28] | |
| Wonder Cot (= RM 7) | USA | SDR FRUIT LLC – USA | R | M* | [28] | RS | [28] | |
| Zard | CA | R | T | [61] | RS | [61] |
| Cultivar | Country a | Origin | Pedigree | PPV Resistance Phenotype b | PPV Strain Used | First Phenotype Ref | PMC2 Genotype c | PMC2 Genotype Ref |
|---|---|---|---|---|---|---|---|---|
| A3521 | IR | S | M | [15] | SS | WGS | ||
| A3522 | IR | S | M | [15] | SS | WGS | ||
| Amabile Vecchoni | IT | Seedling by Prof. F. Scaramuzzi | Unknown [67] | S | M | [45] | SS | [49] |
| Aprikoz | TR | S | M | [88] | SS | PCR | ||
| Arrogante | SP | Murcia | S | D | [89] | SS | [21] | |
| Avikaline | FR | S | M | [15] | SS | WGS | ||
| Bebecou (Bebeco) | GR | Unknown [18] | S | M/D | [90] | SS | PCR; [21,28] | |
| Bella Di Imola | IT | Spontaneous seedling [23] | S | D | [64] | SS | [28] | |
| Bergeron | FR | Saint-Cyr-au-Mont-d’Or, Lyon | Spontaneous seedling [23] | S | M | [90] | SS | PCR; [21] |
| Big Red (EA4006) | FR | Escande EARL, France | S | M | [57] | RS | [28] | |
| BO04624042 | IT | Portici × Goldrich [28] | S | M* | [28] | SS | [28] | |
| BO04624043 | IT | Portici × Goldrich [28] | S | M* | [28] | SS | [28] | |
| BO06609003 | IT | Silvercot × Bora [28] | S | M* | [49] | RS | [49] | |
| BO81604311 | IT | San Castrese × Reale di Imola [73] | S | D | [91] | SS | [24] | |
| BO96621021 | IT | Goldrich × Lito [28] | S | M* | [28] | RS | [28] | |
| Boucheran Boutard | FR | S | M | [15] | SS | WGS | ||
| Búlida | SP | Murcia | Unknown [73] | S | D | [92] | SS | PCR; [21] |
| M | [93] | |||||||
| Cafona | IT | Vesuvian area | S | M | [94] | SS | WGS | |
| D | [64] | |||||||
| CAID AGDZ n2 | MO | S | M | [15] | SS | WGS | ||
| Canino | SP | Valencia | Unknown [18] | S | D | [95] | SS | PCR; [20,21] |
| M | [90] | |||||||
| Castlebrite (=K111-6) | USA | USDA, Fresno, California | OP seedling of B60-12 (= Perfection × Castleton) [66] | S | M | [45] | SS | PCR |
| Ceglédi Bíbor | HU | Cegléd Horticultural Research Institute | Chance seedling [96] | S | M | [46] | SS | [28] |
| Colorado (Colorao 43-15) | SP | PSB Producción Vegetal SL | Unknown | S | M* | [49] | SS | PCR; [28] |
| D | [89] | |||||||
| Corbató | SP | Valencia | S | D | [95] | SS | PCR | |
| M | [46] | |||||||
| Currot | SP | Valencia | Unknown [18] | S | D | [95] | SS | PCR |
| M | [46] | |||||||
| Estrella | SP | CEBAS-CSIC | Orange Red × Z211-18 (= Goldrich × Pepito del Rubio) [23] | S | D | [23] | SS | PCR; [49] |
| Faralia | FR | Marie-France BOIS, IPS | S | M* | [28] | SS | [28] | |
| Farclo | FR | Marie-France BOIS, IPS | S | M | [57] | SS | [28] | |
| Favorit | RO | S | M | [94,97] | SS | [49] | ||
| Geç Abligoz | TR | S | T | [61] | SS | [61] | ||
| Ginesta | SP | Valencia | Unknown [18] | S | D | [95] | SS | PCR |
| M | [46] | |||||||
| Dama Vermella (HG9869) | SP | IVIA | Harcot × Ginesta [18] | S | D | IVIA | SS | PCR; [49] |
| Hacıhaliloğlu | TR | S | T | [61] | SS | [61] | ||
| Hargrand (= HW410) | C | Richard EC Layne, Agr. Canada, Res. Station | V51092 ((Reliable × OP) × OP) × NJA1 (Phelps × Perfection) [66] | S | M | [45] | SS | [21] |
| Hasanbey | TR | S | M | [45] ¹ | SS | PCR | ||
| Hungarian Best = (Best of Hungary?) | HU/RO | S | T | [61] | SS | [61] | ||
| M | [94] | |||||||
| Katy | USA | Zaiger’s Genetics⁴ | S | D | [18] | SS | PCR; [21] | |
| Krasnoshchekii | UKR | Advanced/improved cultivar | S | D | [20] | SS | [20,21] | |
| Kyoto (= Kioto) | FR | Escande | Unknown [73] | S | M* | [28] | SS | [28] |
| Lambertin-1 | USA | USDA, Fresno, California | A95-45 × B69-85 (=Perfection × Royal) [98] | S | M | [45] | SS | [21] |
| Larclyd (= F168 cv; Jenny Cot) | NZ | Central Otago | Sundrop × Moorpark [99] | S | M | [15] | SS | WGS |
| Le-3218 | CR | Faculty of Horticulture in Lednice | Vestar × SEO [51] | S | M | [51] | SS | [24] |
| Luizet (= Suchet; Hatif du clos; Abricot du Clos) | FR | Spontaneous seedling [71] | S | M | [93] | SS | WGS | |
| Luna | IT | S | M* | [28] | RS | [28] | ||
| Madarska Narijlepsia | SL | S | M | [15] | SS | WGS | ||
| Magic cot (= RM 22) | USA | SDR FRUIT LLC - USA | Unknown [23] | S | D | [23] | SS | [49] |
| Manicot | FR | S | D | [97,100] | SS | WGS | ||
| M | [15] | |||||||
| Maravilla | SP | CEBAS-CSIC, Murcia | Orange Red × Z211-18 (= Goldrich × Pepito) [23] | S | D | [23] | SS | PCR; [49] |
| Mari de Cenad | RO | Unknown | S | [86] | RS | PCR | ||
| Markuleşti | TR | S | T | [61] | SS | [61] | ||
| Marlén | CR | Horticulture Faculty, Lednice | clone of Hungarian Best [59] | S | Rec | [14] | SS | PCR; [24] |
| Marouch 14 | MO | Local landrace | S | M | [15] | SS | WGS | |
| Marouch 4 | MO | S | M | [15] | SS | WGS | ||
| Mei Hwang | CH | Traditional cultivar/landrace | S | M | [15] | SS | WGS | |
| Mektep | TR | S | T | [61] | SS | [61] | ||
| Mektep 8 | TR | S | T | [61] | SS | [61] | ||
| Mitger | SP | Castellón [30] | Unknown [18] | S | D | [95] | SS | PCR |
| M | [46] | |||||||
| Monaco Bello | IT | S | M | [97] | SS | WGS | ||
| Moniqui | SP | Murcia | Unknown | S | M | [90] | SS | PCR; [21,24] |
| Mono | USA | Le Grand, California, by FW Anderson | Perfection OP [66] | S | M | [93] | SS | [49] |
| Moongold (= Moongola?) | USA | University of Minessota | S | - | [77] | SS | PCR | |
| Moorpark (=Moor Park) | USA | S | M | [46] | SS | WGS | ||
| Morden 604 | C | Morden, Manitoba, by Canada Dept. Agr. Res. Sta. | Scout × McClure [66] | S | M | [15] | SS | WGS |
| Ninfa (BO81602075) | IT | University of Bologna and Milan, by D. Bassi | Ouardy × Tyrinthos [55] | S | M* | [28] | SS | PCR; [28,61] |
| T | [61] | |||||||
| Olimp | RO | S | M | [45] | SS | WGS; [49] | ||
| Orange Rubis (=Couloumine) | FR | Mallard | S | M | [57] | SS | [28] | |
| Ordubat B. | TR | S | T | [61] | SS | [61] | ||
| Ouardi | TU | INRAT, Ariana | Canino × Hamidi [101] | S | M | [46] | SS | [49] |
| Palsteyn (Palstein) | SA | Blenhein × Canino [73] | S | M | [102] | SS | WGS | |
| Palabras | SP | S | D | [95] | SS | PCR | ||
| M | [46] | |||||||
| Palau | SP | Unknown [18] | S | D | [95] | SS | PCR | |
| Paviot | FR | S | M | [93] | SS | WGS | ||
| Peche De Nancy | FR | S | M | [15] | SS | WGS | ||
| Perfection | USA | Waterville, Washington | Unknown [66] | S | M | [46] | SS | [21] |
| Piera | S | M | [65] | RS | PCR | |||
| Poizat | FR | S | M | [15] | SS | WGS | ||
| Polonais | FR | Spontaneous seedling [23] | S | M | [93] | SS | [24] | |
| Poppy | USA | Zaiger Genetics, Inc., Modesto, CA | 78EB575 × 123GD161 [58] | S | D | [23] | SS | [49] |
| Portici (= Pertini) | IT | Vesuvian area | Unknown; Local selection [23] | S | M | [46] | SS | PCR; [28] |
| D | [64] | |||||||
| Precoce Ampuis | FR | S | M | [15] | SS | WGS | ||
| Reale d’Imola | IT | Luizet OP [23] | S | M | [46] | SS | [21,24,49] | |
| D | [64] | |||||||
| Rojo de Carlet | SP | Valencia | S | D | [95] | SS | PCR | |
| M | [46] | |||||||
| Rouge Du Roussillon | FR | S | M | [45] | SS | WGS | ||
| Rouge De Fournes | FR | S | M | [15] | SS | WGS | ||
| Saturn | RO | S | M | [45] | SS | WGS | ||
| Screara | FR | S | D | [70] | SS | WGS | ||
| M | [45] | |||||||
| Şekerpare B. | TR | S | T | [61] | SS | [61] | ||
| Shalakh (=Yerevani, Erevani) | AR | Local selection [23] | S | M | [93] | SS | WGS; [20,21] | |
| Silistra × Ananas (Marculesti 43/1) | RO | S | M | [15] | SS | WGS | ||
| Sucre De Holub | HU | Bohême, by M. Holub | S | M | [15] | SS | WGS | |
| Sublime | SP | CEBAS-CSIC | Orange Red × Z211-18 (= Goldrich × Pepito del Rubio) [103] | S | D | [103] | SS | PCR; [49] |
| Super Rouge | FR | S | M | [15] | SS | WGS | ||
| Sweet Red | FR | S | M | [57] | SS | [49] | ||
| Szegedi mamut (=Szegadti Mamut?) | HU | Foki István and Kovács Imre | Hybrid of Cegledi orias, "Giant" group [96] | S | M | [94] | SS | [49] |
| Tabriz | TR | S | [86] | SS | PCR | |||
| Tadeo (= Taddeo) | SP | Valencia | S | D | [95] | SS | PCR | |
| M | [45] | |||||||
| Tardif De Bordaneil | FR | Unknown [23] | S | M | [46] | SS | WGS | |
| D | [64] | |||||||
| Tardif De Tain | FR | S | M | [15] | SS | WGS | ||
| Tonda di costigliole | IT | Piedmont | S | [104] | SS | [49] | ||
| Trevatt | AU | S | M | [45] | SS | PCR | ||
| Tyrinthos | GR | Unknown [18] | S | D | [70] | SS | PCR; WGS; [49] | |
| M | [97] | |||||||
| Uleanos | SP | Ulea, Murcia | S | D | [89] | SS | [49] | |
| Velázquez | SP | Murcia | S | D | [89] | SS | PCR; [21] | |
| Venus (= Venus 1414?) | RO | (Umberto × Ananas) × (Luizet × Umberto) [96] | S | M | [46] | SS | [49] | |
| Vestar | CR | Hungarian Best × mixture of pollen from Chinese cultivars [55] | S | M | [105] | RS | WGS; [24] | |
| Vivagold | C | Vineland Station, Ontario | Veecot × V49024 (= Geneva × Gibb) [66] | S | M | [15] | SS | WGS |
| Xirivello (=Chirivello) | SP | Valencia | Unknown | S | M | [46] | SS | PCR |
| Yilbat (=M2243) | TR | S | T | [61] | RS | [61] |
| Cultivar | Country a | Origin | Pedigree | PPV Resistance Phenotype b | PPV Strain Used | First Phenotype Ref | PMC2 Genotypec | PMC2 Genotype Ref |
|---|---|---|---|---|---|---|---|---|
| Badami | IR | S | M | [102] | SS | WGS | ||
| T | D | |||||||
| Farbaly | FR | Marie-France BOIS, IPS | S | M* | [28] | RS | [28] | |
| R | M | [56] | ||||||
| Goldrich | USA | USDA and Washington State University, Prosser, Washington | Sun Glo × Perfection [73] | R | D | [68] | RS | PCR; [20,21,24,28] |
| M | [45] | |||||||
| uncertain | M | [106] | ||||||
| D | [68] | |||||||
| S | D | [64] | ||||||
| Harcot | C | Agr. Canada, Res. Station, Harrow, Ontario, by REC Layne | (T2 (Geneva × Naramata) × Morden 604 (Scout × McClure)) × NJA1 (Phelps × Perfection) [66] | T? | - | [28] | RS | PCR; [21,24,28] |
| R | M | [90] | ||||||
| D | [70] | |||||||
| S | T | [61] | SS | [61] | ||||
| Incomparable de Malissard (= Valssard) | FR | Malissard, Valence | R | M | [15] | RS | WGS | |
| S | [57] | |||||||
| Pisana | IT | ICAPI 26/5 OP [55] | S | M* | [28] | SS | PCR; [28] | |
| R | M | [65] | ||||||
| R | D | [64] | ||||||
| S | D | [28] | ||||||
| Pieve (BO89608015) | IT | University of Bologna and Milan, by D. Bassi | Harcot × Reale di Imola [73] | S | M* | [28] | SS | [28] |
| R | M | [65] | ||||||
| San Castrese | IT | Naples | Unknown [73] | T | D | [64] | SS | WGS; [49] |
| S | M | [46] | ||||||
| Sulmona | RO | (Luizet × Re Umberto) × (Ananas × Ananas) [71] | S | M | [45] | SS | [49] | |
| R | - | [77] | ||||||
| Veecot | C | Ontario Dept Agr Res Inst, Vineland Station, Ontario, by OA Bradt | Reliable OP [18] | R | M | [45] | RS | PCR; [21] |
| S | [105] | |||||||
| T | D | [47] | ||||||
| Viceroy (=Viceroy_603_G?) | RO | R | - | [77] | SS | PCR | ||
| S | - | [86] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Polo-Oltra, Á.; Romero, C.; López, I.; Badenes, M.L.; Zuriaga, E. Cost-Effective and Time-Efficient Molecular Assisted Selection for PPV Resistance in Apricot Based on ParPMC2 Allele-Specific PCR. Agronomy 2020, 10, 1292. https://doi.org/10.3390/agronomy10091292
Polo-Oltra Á, Romero C, López I, Badenes ML, Zuriaga E. Cost-Effective and Time-Efficient Molecular Assisted Selection for PPV Resistance in Apricot Based on ParPMC2 Allele-Specific PCR. Agronomy. 2020; 10(9):1292. https://doi.org/10.3390/agronomy10091292
Chicago/Turabian StylePolo-Oltra, Ángela, Carlos Romero, Inmaculada López, María Luisa Badenes, and Elena Zuriaga. 2020. "Cost-Effective and Time-Efficient Molecular Assisted Selection for PPV Resistance in Apricot Based on ParPMC2 Allele-Specific PCR" Agronomy 10, no. 9: 1292. https://doi.org/10.3390/agronomy10091292
APA StylePolo-Oltra, Á., Romero, C., López, I., Badenes, M. L., & Zuriaga, E. (2020). Cost-Effective and Time-Efficient Molecular Assisted Selection for PPV Resistance in Apricot Based on ParPMC2 Allele-Specific PCR. Agronomy, 10(9), 1292. https://doi.org/10.3390/agronomy10091292





