Changes in Capsiate Content in Four Chili Pepper Genotypes (Capsicum spp.) at Different Ripening Stages
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Chemicals and Reagents
2.3. Fresh Pepper Extraction Procedure
2.4. UHPLC-Q-ToF-MS Identification of Capsinoids
2.5. UHPLC-PDA Analysis of Capsinoids
2.6. Statistical Analysis
3. Results and Discussion
3.1. Evolution of the Total Capsinoids Content
3.2. Comparison of Capsiate and Capsaicinoids Contents
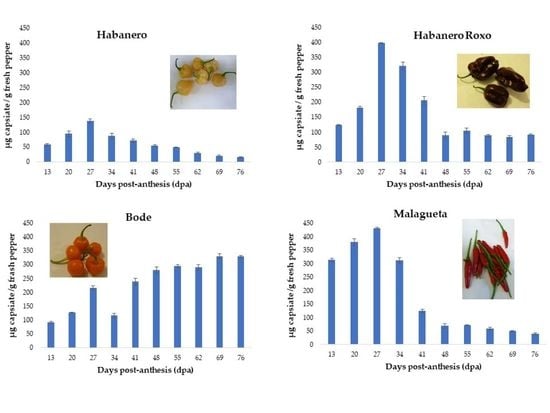
3.2.1. ‘Habanero’ pepper
3.2.2. ‘Habanero Roxo’ pepper
3.2.3. ‘Bode’ pepper
3.2.4. ‘Malagueta’ pepper
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mo, H.-S.; Jang, K.-S.; Hwang, J.-E.; Jeon, S.-G.; Kim, B. Horticultural and chemical quality characterization of accessions selected from four species of Capsicum. Hortic. Environ. Biotechnol. 2015, 56, 54–66. [Google Scholar] [CrossRef]
- Baenas, N.; Belović, M.; Ilic, N.; Moreno, D.A.; García-Viguera, C. Industrial use of pepper (Capsicum annum L.) derived products: Technological benefits and biological advantages. Food Chem. 2018, 274, 872–885. [Google Scholar] [CrossRef] [PubMed]
- Pino, J.; Gonzalez, M.; Ceballos, L.; Centurionyah, A.; Trujilloaguirre, J.; Latourneriemoreno, L.; Sauriduch, E. Characterization of total capsaicinoids, colour and volatile compounds of Habanero chilli pepper (Capsicum chinense Jack.) cultivars grown in Yucatan. Food Chem. 2007, 104, 1682–1686. [Google Scholar] [CrossRef]
- Davis, C.B.; Markey, C.E.; Busch, M.A.; Busch, K.W. Determination of Capsaicinoids in Habanero Peppers by Chemometric Analysis of UV Spectral Data. J. Agric. Food Chem. 2007, 55, 5925–5933. [Google Scholar] [CrossRef]
- Cisneros-Pineda, O.; Torres-Tapia, L.W.; Gutiérrez-Pacheco, L.C.; Contreras-Martín, F.; González-Estrada, T.; Peraza-Sánchez, S.R. Capsaicinoids quantification in chili peppers cultivated in the state of Yucatan, Mexico. Food Chem. 2007, 104, 1755–1760. [Google Scholar] [CrossRef]
- Cordell, G.A.; Araujo, O.E. Capsaicin: Identification, Nomenclature, and Pharmacotherapy. Ann. Pharmacother. 1993, 27, 330–336. [Google Scholar] [CrossRef] [PubMed]
- Chinn, M.S.; Sharma-Shivappa, R.R.; Cotter, J.L. Solvent extraction and quantification of capsaicinoids from Capsicum chinense. Food Bioprod. Process. 2011, 89, 340–345. [Google Scholar] [CrossRef]
- Sasahara, I.; Furuhata, Y.; Iwasaki, Y.; Inoue, N.; Sato, H.; Watanabe, T.; Takahashi, M. Assessment of the Biological Similarity of Three Capsaicin Analogs (Capsinoids) Found in Non-Pungent Chili Pepper (CH-19 Sweet) Fruits. Biosci. Biotechnol. Biochem. 2010, 74, 274–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macho, A.; Sancho, R.; Daddario, N.; Minassi, A.; Appendino, G. Non-pungent capsaicinoids from sweet pepper. Eur. J. Nutr. 2003, 42, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Snitker, S.; Fujishima, Y.; Shen, H.; Ott, S.; Pi-Sunyer, X.; Furuhata, Y.; Sato, H.; Takahashi, M. Effects of novel capsinoid treatment on fatness and energy metabolism in humans: Possible pharmacogenetic implications. Am. J. Clin. Nutr. 2008, 89, 45–50. [Google Scholar] [CrossRef] [Green Version]
- Aza-González, C.; Nuñez-Palenius, H.G.; Ochoa-Alejo, N. Molecular biology of capsaicinoid biosynthesis in chili pepper (Capsicum spp.). Plant Cell Rep. 2010, 30, 695–706. [Google Scholar] [CrossRef]
- Avellán, O.F.; Giménez, C.M.; Garcés-Claver, A. Evolución del conocimiento sobre la pungencia de la cebolla (Allium cepa L.) y del pimiento (Capsicum spp.): Desde sus orígenes hasta el potencial nutracéutico actual. Revisión bibliográfica. Inf. Tec. Econ. Agrar. 2018, 114, 99–118. [Google Scholar]
- Kobata, K.; Sugawara, M.; Mimura, M.; Yazawa, S.; Watanabe, T. Potent Production of Capsaicinoids and Capsinoids by Capsicum Peppers. J. Agric. Food Chem. 2013, 61, 11127–11132. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Sonoyama, T.; Muraga, Y.; Koeda, S.; Goto, T.; Yoshida, Y.; Yasuba, K. Multiple loss-of-function putative aminotransferase alleles contribute to low pungency and capsinoid biosynthesis in Capsicum chinense. Mol. Breed. 2015, 35, 142. [Google Scholar] [CrossRef]
- Kobata, K.; Todo, T.; Yazawa, S.; Iwai, K.; Watanabe, T. Novel Capsaicinoid-like Substances, Capsiate and Dihydrocapsiate, from the Fruits of a Nonpungent Cultivar, CH-19 Sweet, of Pepper (Capsicum annuum L.). J. Agric. Food Chem. 1998, 46, 1695–1697. [Google Scholar] [CrossRef]
- Singh, S.; Jarret, R.; Russo, V.; Majetich, G.; Shimkus, J.; Bushway, R.; Perkins, B. Determination of Capsinoids by HPLC-DAD in Capsicum Species. J. Agric. Food Chem. 2009, 57, 3452–3457. [Google Scholar] [CrossRef]
- Lang, Y.; Kisaka, H.; Sugiyama, R.; Nomura, K.; Morita, A.; Watanabe, T.; Tanaka, Y.; Yazawa, S.; Miwa, T. Functional loss of pAMT results in biosynthesis of capsinoids, capsaicinoid analogs, in Capsicum annuumcv. CH-19 Sweet. Plant J. 2009, 59, 953–961. [Google Scholar] [CrossRef]
- Tanaka, Y.; Hosokawa, M.; Miwa, T.; Watanabe, T.; Yazawa, S. Newly Mutated putative-aminotransferase in Nonpungent Pepper (Capsicum annuum) Results in Biosynthesis of Capsinoids, Capsaicinoid Analogues. J. Agric. Food Chem. 2010, 58, 1761–1767. [Google Scholar] [CrossRef]
- Han, K.; Jeong, H.-J.; Sung, J.; Keum, Y.S.; Cho, M.-C.; Kim, J.-H.; Kwon, J.-K.; Kim, B.-D.; Kang, B.-C. Biosynthesis of capsinoid is controlled by the Pun1 locus in pepper. Mol. Breed. 2012, 31, 537–548. [Google Scholar] [CrossRef]
- Park, Y.-J.; Nishikawa, T.; Minami, M.; Nemoto, K.; Iwasaki, T.; Matsushima, K. A low-pungency S3212 genotype of Capsicum frutescens caused by a mutation in the putative aminotransferase (p-AMT) gene. Mol. Genet. Genom. 2015, 290, 2217–2224. [Google Scholar] [CrossRef]
- López-López, R.; Inzunza-Ibarra, M.A.; Cohen, I.S.; Fierro-Alvarez, A.; Sifuentes-Ibarra, E. Water use efficiency and productivity of habanero pepper (Capsicum chinense Jacq.) based on two transplanting dates. Water Sci. Technol. 2015, 71, 885–891. [Google Scholar] [CrossRef] [PubMed]
- Jeeatid, N.; Techawongstien, S.; Suriharn, B.; Bosland, P.W.; Techawongstien, S. Light intensity affects capsaicinoid accumulation in hot pepper (Capsicum chinense Jacq.) cultivars. Hortic. Environ. Biotechnol. 2017, 58, 103–110. [Google Scholar] [CrossRef]
- Menichini, F.; Tundis, R.; Bonesi, M.; Loizzo, M.; Conforti, F.; Statti, G.; De Cindio, B.; Houghton, P. The influence of fruit ripening on the phytochemical content and biological activity of Capsicum chinense Jacq. cv Habanero. Food Chem. 2009, 114, 553–560. [Google Scholar] [CrossRef]
- Barbero, G.F.; Ruiz, A.G.; Liazid, A.; Palma, M.; Vera, J.C.; Barroso, C.G.; Lovillo, M.P. Evolution of total and individual capsaicinoids in peppers during ripening of the Cayenne pepper plant (Capsicum annuum L.). Food Chem. 2014, 153, 200–206. [Google Scholar] [CrossRef]
- De Aguiar, A.C.; Coutinho, J.P.; Barbero, G.F.; Godoy, H.T.; Martínez, J. Comparative Study of Capsaicinoid Composition in Capsicum Peppers Grown in Brazil. Int. J. Food Prop. 2015, 19, 1292–1302. [Google Scholar] [CrossRef] [Green Version]
- The Food and Agriculture Organization (FAO). Global Forest Resources Assessment 2015; FAO: Rome, Italy, 2016. [Google Scholar]
- Soares, R.S.; Ribeiro, C.S.D.C.; Ragassi, C.F.; De Carvalho, S.I.C.; Maldonade, I.R.; Filho, J.G.D.S.; Braz, L.T.; Reifschneider, F.J.B. New Brazilian lines of Habanero pepper (Capsicum chinense): Morpho-agronomic and biochemical characterization in different environments. Sci. Hortic. 2020, 261, 108941. [Google Scholar] [CrossRef]
- Pino, J.; Sauri-Duch, E.; Marbot, R. Changes in volatile compounds of Habanero chile pepper (Capsicum chinense Jack. cv. Habanero) at two ripening stages. Food Chem. 2006, 94, 394–398. [Google Scholar] [CrossRef]
- Alvares-Bianchi, P.; Almeida da Silva, L.R.; da Silva Alencar, A.A.; Araújo Diniz Santos, P.H.; Pimenta, S.; Pombo Sudré, C.; Erpen-Dalla Corte, L.; Azeredo Gonçalves, L.S.; Rodrigues, R. Biomorphological Characterization of Brazilian Capsicum Chinense Jacq. Germplasm. Agronomy 2020, 10, 447. [Google Scholar] [CrossRef] [Green Version]
- Sosa-Moguel, O.; Pino, J.A.; Ayora-Talavera, G.; Sauri-Duch, E.; Cuevas-Glory, L. Biological activities of volatile extracts from two varieties of Habanero pepper (Capsicum chinense Jacq.). Int. J. Food Prop. 2017, 20, S3042–S3051. [Google Scholar] [CrossRef] [Green Version]
- Gonçalves, V.D.; Müller, D.H.; Fava, C.L.F.; Camili, E.C. Physiological ripeness of pepper ‘Bode Vermelha’ seeds. Rev. Caatinga 2015, 28, 137–146. [Google Scholar] [CrossRef] [Green Version]
- Rossato, M.; Santiago, T.R.; Lopes, C.A. Reaction of Capsicum peppers commercialized in the Federal District to bacterial wilt. Hortic. Bras. 2018, 36, 173–177. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos, P.; Aguiar, A.C.; Barbero, G.F.; A Rezende, C.; Martínez, J. Supercritical carbon dioxide extraction of capsaicinoids from malagueta pepper (Capsicum frutescens L.) assisted by ultrasound. Ultrason. Sonochem. 2015, 22, 78–88. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, S.; Bianchetti, L.; Ragassi, C.F.; Ribeiro, C.; Reifschneider, F.; Buso, G.; Faleiro, F. Genetic variability of a Brazilian Capsicum frutescens germplasm collection using morphological characteristics and SSR markers. Genet. Mol. Res. 2017, 16, 16039689. [Google Scholar] [CrossRef] [PubMed]
- Olguín-Rojas, J.A.; Fayos, O.; Vázquez-León, L.A.; Palma, M.; Rodríguez-Jiménes, G.D.C.; Palma, M.; Garcés-Claver, A.; Barbero, G.F. Progression of the Total and Individual Capsaicinoids Content in the Fruits of Three Different Cultivars of Capsicum chinense Jacq. Agronomy 2019, 9, 141. [Google Scholar] [CrossRef] [Green Version]
- Fayos, O.; De Aguiar, A.C.; Jiménez-Cantizano, A.; Palma, M.; Garcés-Claver, A.; Martínez, J.; Mallor, C.; Ruiz-Rodríguez, A.; Palma, M.; Barroso, C.G.; et al. Ontogenetic Variation of Individual and Total Capsaicinoids in Malagueta Peppers (Capsicum frutescens) during Fruit Maturation. Molecules 2017, 22, 736. [Google Scholar] [CrossRef]
- Toma, M.; Vinatoru, M.; Paniwnyk, L.; Mason, T. Investigation of the effects of ultrasound on vegetal tissues during solvent extraction. Ultrason. Sonochem. 2001, 8, 137–142. [Google Scholar] [CrossRef]
- Barbero, G.F.; Molinillo, J.M.G.; Varela, R.M.; Palma, M.; Macías, F.A.; Barroso, C.G. Application of Hansch’s Model to Capsaicinoids and Capsinoids: A Study Using the Quantitative Structure−Activity Relationship. A Novel Method for the Synthesis of Capsinoids. J. Agric. Food Chem. 2010, 58, 3342–3349. [Google Scholar] [CrossRef]
- Vázquez-Espinosa, M.; González-De-Peredo, A.V.; Palma, M.; Barroso, C.G.; Palma, M.; Barbero, G.F.; Espada-Bellido, E.; González-De-Peredo, A.V. Optimizing and Comparing Ultrasound- and Microwave-Assisted Extraction Methods Applied to the Extraction of Antioxidant Capsinoids in Peppers. Agronomy 2019, 9, 633. [Google Scholar] [CrossRef] [Green Version]
- Vázquez-Espinosa, M.; Olguín-Rojas, J.A.; Fayos, O.; González-De-Peredo, A.V.; Espada-Bellido, E.; Palma, M.; Barroso, C.G.; Barbero, G.F.; Garcés-Claver, A.; Palma, M. Influence of Fruit Ripening on the Total and Individual Capsaicinoids and Capsiate Content in Naga Jolokia Peppers (Capsicum chinense Jacq.). Agronomy 2020, 10, 252. [Google Scholar] [CrossRef] [Green Version]
- Fayos, O.; Ochoa-Alejo, N.; De La Vega, O.M.; Savirón, M.; Orduna, J.; Mallor, C.; Barbero, G.F.; Garcés-Claver, A. Assessment of Capsaicinoid and Capsinoid Accumulation Patterns during Fruit Development in Three Chili Pepper Genotypes (Capsicum spp.) Carrying Pun1 and pAMT Alleles Related to Pungency. J. Agric. Food Chem. 2019, 67, 12219–12227. [Google Scholar] [CrossRef]
- Jang, S.; Han, K.; Jo, Y.D.; Jeong, H.-J.; Siddique, M.I.; Kang, B.-C. Substitution of a Dysfunctional pAMT Allele Results in Low-Pungency but High Levels of Capsinoid in Capsicum chinense ‘Habanero’. Plant Breed. Biotechnol. 2015, 3, 119–128. [Google Scholar] [CrossRef] [Green Version]
- Jarret, R.L.; Bolton, J.; Perkins, L.B. 509-45-1, a Capsicum annuum Pepper Germplasm Containing High Concentrations of Capsinoids. HortScience 2014, 49, 107–108. [Google Scholar] [CrossRef] [Green Version]
- Chan, C.-H.; Yusoff, R.; Ngoh, G.-C.; Kung, F.W.-L. Microwave-assisted extractions of active ingredients from plants. J. Chromatogr. A 2011, 1218, 6213–6225. [Google Scholar] [CrossRef] [PubMed]
- A Arce-Rodriguez, M.L.; Ochoa-Alejo, N. Silencing AT3 gene reduces the expression of pAmt, BCAT, Kas, and Acl genes involved in capsaicinoid biosynthesis in chili pepper fruits. Boil. Plant. 2015, 59, 477–484. [Google Scholar] [CrossRef]
- Bernal, M.A.; Calderón, A.A.; Ferrer, M.A.; De Cáceres, F.M.; Barceló, A.R. Oxidation of Capsaicin and Capsaicin Phenolic Precursors by the Basic Peroxidase Isoenzyme B6 from Hot Pepper. J. Agric. Food Chem. 1995, 43, 352–355. [Google Scholar] [CrossRef]
- Contreras-Padilla, M.; Yahia, E.M. Changes in Capsaicinoids during Development, Maturation, and Senescence of Chile Peppers and Relation with Peroxidase Activity. J. Agric. Food Chem. 1998, 46, 2075–2079. [Google Scholar] [CrossRef]
- Lema, A.; Martínez-Cortés, T.; Garcés, A.; Mallor Giménez, C.; Fayos, O.; Barbero, G.F.; Silvar, C.; Pomar, F. 5-5′ dicapsiate: Product of the oxidation of capsiate by cationic peroxidases from pepper (Capsicum annuum L.). In Proceedings of the XVIth EUCARPIA Capsicum and Eggplant Working Group Meeting, Kecskemét, Hungary, 12–14 September 2016; pp. 500–505, ISBN 978-615-5270-27-7. [Google Scholar] [CrossRef]
- Sutoh, K.; Kobata, K.; Watanabe, T. Stability of Capsinoid in Various Solvents. J. Agric. Food Chem. 2001, 49, 4026–4030. [Google Scholar] [CrossRef]
- Tanaka, Y.; Yoneda, H.; Hosokawa, M.; Miwa, T.; Yazawa, S. Application of marker-assisted selection in breeding of a new fresh pepper cultivar (Capsicum annuum) containing capsinoids, low-pungent capsaicinoid analogs. Sci. Hortic. 2014, 165, 242–245. [Google Scholar] [CrossRef]
- Harvell, K.P.; Bosland, P.W. The Environment Produces a Significant Effect on Pungency of Chiles. HortScience 1997, 32, 32. [Google Scholar] [CrossRef] [Green Version]
- Lima, M.; Carvalho, S.; Ragassi, C.F.; Bianchetti, L.; Faleiro, F.; Reifschneider, F. Characterization of a pepper collection (Capsicum frutescens L.) from Brazil. Genet. Mol. Res. 2017, 16, 16039704. [Google Scholar] [CrossRef]
- Ohyama, K.; Nogusa, Y.; Shinoda, K.; Suzuki, K.; Bannai, M.; Kajimura, S. A Synergistic Antiobesity Effect by a Combination of Capsinoids and Cold Temperature Through Promoting Beige Adipocyte Biogenesis. Diabetes 2016, 65, 1410–1423. [Google Scholar] [CrossRef] [PubMed] [Green Version]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vázquez-Espinosa, M.; Fayos, O.; V. González-de-Peredo, A.; Espada-Bellido, E.; Ferreiro-González, M.; Palma, M.; Garcés-Claver, A.; F. Barbero, G. Changes in Capsiate Content in Four Chili Pepper Genotypes (Capsicum spp.) at Different Ripening Stages. Agronomy 2020, 10, 1337. https://doi.org/10.3390/agronomy10091337
Vázquez-Espinosa M, Fayos O, V. González-de-Peredo A, Espada-Bellido E, Ferreiro-González M, Palma M, Garcés-Claver A, F. Barbero G. Changes in Capsiate Content in Four Chili Pepper Genotypes (Capsicum spp.) at Different Ripening Stages. Agronomy. 2020; 10(9):1337. https://doi.org/10.3390/agronomy10091337
Chicago/Turabian StyleVázquez-Espinosa, Mercedes, Oreto Fayos, Ana V. González-de-Peredo, Estrella Espada-Bellido, Marta Ferreiro-González, Miguel Palma, Ana Garcés-Claver, and Gerardo F. Barbero. 2020. "Changes in Capsiate Content in Four Chili Pepper Genotypes (Capsicum spp.) at Different Ripening Stages" Agronomy 10, no. 9: 1337. https://doi.org/10.3390/agronomy10091337