Abstract
To explore the molecular mechanism through which the novel fungicide N-(naphthalen-1-yl) phenazine-1-carboxamide (NNPCN) inhibits Rhizoctonia solani, we clarified the target and mode of action, explored lead compounds, and developed novel fungicides. Methods: Growth observation, scanning electron microscopy, transmission electron microscopy, transcriptome sequencing technology, quantitative real-time PCR (qRT-PCR), physiological and biochemical determination, and reverse molecular docking technology were used to study the effects of this compound on the microscopic morphology of R. solani. The differentially expressed genes (DEGs), functions, and metabolic pathways were analyzed. The genes displaying significant differences were randomly selected for qRT-PCR verification and confirmed by physiological and biochemical determination to construct their binding mode with key targets. The results showed that the mycelium treated with NNPCN produced a red secretion and exhibited progressive creeping growth. Under a scanning electron microscope, hyphal swelling, uneven thickness, fractures, deformities, and hyphal surface warts increased. Under a transmission electron microscope, the cell wall was separated, the subcellular organelles were disintegrated, and the septum disappeared. Furthermore, there were 6838 DEGs under NNPCN treatment, including 291 significant DEGs, of which 143 were upregulated and 148 downregulated. Ten DEGs were randomly selected for qRT-PCR verification, and the gene expression trend was consistent with the transcriptome sequencing results. Gene Ontology enrichment analysis showed that the DEGs were significantly enriched in cell wall glucan decomposition and metabolism, cell membrane synthesis, metabolism, composition, organic hydroxyl compounds, oxidoreductase activity, and transition metal ion binding. Metabolic pathway enrichment analysis showed that there were 16 significant metabolic pathways, such as steroid biosynthesis and ABC transporters. Further study found that genes, such as the glycosyl hydrolase family 10 domain-containing protein, which is related to glucan catabolic process function as tied to the cell wall, were downregulated. Lipid oxidation, modification, and other genes related to the cell membrane were also downregulated. Secondly, genes related to lipid modification, lipid metabolism processes, integral components of the membrane, and other ABC transporters were downregulated. Fatty-acid oxidation and carbohydrate metabolic processes, which are related to antioxidant and metabolic functions, displayed significant differences in their target genes. Nitrite reductase [NADH] activity and mitochondrial organization gene expression were downregulated. These results revealed that target genes may involved in the cell wall, cell membrane, antioxidant and metabolism, nitrogen metabolism, and mitochondria. The results of the physiological and biochemical tests showed that NNPCN decreased the β-1,3-glucanase, malondialdehyde, and ATPase activities and nucleic acid leakage but increased the activity of nitrate reductase. The results of the reverse molecular docking showed that NNPCN could freely bind to target proteins such as β-1,3-glucanase, ABC transporter, and NADPH nitrate reductase, whereby NNPCN could bind to glucanase via van der Waals and electrostatic forces and to ABC transporter and NADPH nitrate reductase via hydrogen bonding. Conclusion: The mechanism via which NNPCN inhibits R. solani may be related to the cell wall structure, cell membrane damage, antioxidant activity, and metabolism.
1. Introduction
Rice sheath blight is a fungal disease caused by Rhizoctonia solani. This disease is most harmful at the late tillering, heading, and full heading stages of rice, and it primarily harms the leaf sheath at the base of the rice and forms a “cloud-like” disease spot, resulting in white panicles, millet grains, and a decrease in the 1000-grain weight. In general, rice sheath blight can cause a 10–20% yield reduction and, in serious cases, cause nearly 45% yield reduction every growing season, which threatens the security of rice production [1]. The pathogen has a wide host range and strong viability. It can also cause potato nevus and root rot diseases of soybean, maize, onion, etc., as well as lower plants growth, yields, and quality; it is considered one of the most destructive plant pathogens [2,3,4,5]. The sclerotium that falls into the soil becomes the initial source of infection for the next season. As the pathogen is primarily stored in the soil, it is not easy to detect during the early stage of the disease, and it is often a serious infection source for the following season. Therefore, it is difficult to eradicate and prevent this disease [6,7]. At present, the primary measures for controlling rice sheath blight during production include selecting resistant varieties, strengthening cultivation management, using new detection technology, rational close planting, screening for sclerotia, improving soil nutrition, and regulating water and fertilizer management [8,9,10,11,12,13,14,15,16]. Among the many control strategies, the application of fungicides is effective for preventing and controlling rice sheath blight. However, with the frequent occurrence of rice sheath blight, the long-term repeated use of fungicides will increase the risk of fungicide resistance, and the dosage and costs of fungicides are also increasing each year [17,18]. Furthermore, the available options for rice sheath blight fungicides are limited, and the fungicides are highly regulated. The traditional rice sheath blight control agent jinggangmycin has been used for nearly 40 years, but its efficacy has displayed a downward trend. The EU classifies it as an antibiotic, and its future use will be increasingly restricted [19]. Therefore, developing novel, efficient, and low-risk fungicides for controlling rice sheath blight is paramount.
Natural products with broad-spectrum activity from microorganisms can be used to develop novel fungicides. These microorganisms are natural compound repositories, containing many novel structures and lead compounds with potential application. Many pesticides are derived from natural products, such as ningnanmycin, chloramphenicol, and pyrimidine nucleoside antibiotics. These natural drugs often have new carbon skeletons, good pharmacophores, and efficient biological activities, which have attracted the interest of drug developers [20,21,22].
Our group has been engaged in developing novel fungicides for plant diseases for a long time and has developed a variety of candidate lead compounds with biological activity. Novel fungicide N-(naphthalen-1-yl) phenazine-1-carboxamide (molecular formula: C23H15N3O; molecular weight: 349.38; abbreviation: NNPCN) is one of the most promising fungicides developed by our research group [23]. NNPCN was synthesized by biomimetic synthesis of the natural precursor compound phenazine-1-carboxylic acid. Previous studies have found that it has a significant inhibitory effect on R. solani, showing an inhibition rate of 87.64% and a 50% effective concentration (EC50) of 4.25 μg/mL, which are indicative of its potential as a novel fungicide. The inhibition effect of this compound is better than that reported for 1-methyl-phenazine (EC50 4.35 μg/mL) by Xiong [24], and it has the potential for further development (Supplementary Figure S1). However, the underlying mechanism is not clear. How does it act on cells? Are there new targets? The relevant data are not available, limiting the application of these compounds [23]. In view of this scientific gap, this study used ultrastructure observation, transcriptome sequencing technology, fluorescence-based quantitative PCR, physiological and biochemical tests, and reverse molecular docking technology to evaluate the microscopic morphology, target, and pathway of NNPCN in its inhibition of R. solani, as well as its drug–target binding mode. This was done with the aim of systematically clarifying the target of NNPCN and clarifying its mechanism of action and the drug–target binding mode, providing a basis for the development of green, efficient, and low-toxicity novel fungicides.
2. Materials and Methods
2.1. Test Strains
R. solani was provided by the Department of Biological and Control Resources Mining and Utilization of Hunan Agricultural University. This strain was isolated from infected rice, belonged to R. solani AG1 IA, and could induce rice sheath blight [25].
2.2. Test Reagents and Culture Medium
The novel fungicide NNPCN (95%) was produced by the Center for Biological Pesticide Research at Hunan Agricultural University [23]. Potato glucose medium was purchased from Haibo Biotechnology Co., Ltd, Qingdao, China. Phosphate buffer was purchased from Sinopharm Chemical Reagent Co., Ltd.
2.3. Microscopy Morphology
NNPCN was dissolved in trace acetone (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) and then mixed with sterile water to prepare the stock solution at a concentration of 400 μg/mL. The stock solution (1 mL) was mixed with 9 mL of potato dextrose agar medium for a final concentration of 40 μg/mL. The mixture was shaken evenly to prepare the treatment plates, and drug-free medium was used as the control. A fungal plug of R. solani with a diameter of 5 mm was punched and inoculated into the center of the infected plate. Each treatment was repeated three times and then cultured in the dark at 24–26 °C for 48 h. The colony growth on the control and treatment plates was observed, and the growth characteristics were described. In addition, samples were taken from the treatment and control groups using the Yan and Xie method and then fixed with an G1102 electron microscope fixation solution (Servicebio Technology Co., Ltd., Wuhan, China). The mycelium morphology was observed under a scanning electron microscope and a transmission electron microscope, and the ultrastructural characteristics of the mycelium were described [26,27].
2.4. Pretreatment of NNPCN on R. solani Transcription
NNPCN was added to potato dextrose broth (PDB) at a final concentration of 40 µg/mL using the method described in Section 2.3. A fungal plug of R. solani was inoculated into drug-containing PDB medium and drug-free PDB medium. Each group was repeated three times and placed in a 24–26 °C incubator for dark culture for 48 h. The mycelium was collected, sterilized water was used to rinse it clean, and then it was dried and placed in a sample tube, frozen in liquid nitrogen, and preserved at −80 °C for testing.
2.5. Transcriptome Sequencing of R. solani
One strain was used for analyses. Three samples were controls and three samples were treated with NNPCN (40 μg/mL). The total RNA of R. solani was extracted using the TRIzol (Solabao Technology Co., Ltd., Beijing, China) method, and the RNA quantity and integrity were assessed using a Qubit 2.0 (Thermo Fisher Scientific, Waltham, MA, USA) and Agilent 2100 system (Agilent Technologies, Waldbron, Germany) [28]. Libraries were generated using an NEB Next® Ultra™ Nondirectional RNA Library Prep Kit for Illumina® (New England Biolabs, Ipswich, MA, USA) following the manufacturer’s recommendations. Briefly, eukaryotic mRNA was enriched by magnetic beads with oligos (dT). Subsequently, fragmentation buffer was added to break the mRNA into short segments, and the mRNA was used as a template to synthesize single-strand cDNA with random hexamers of six bases. Then, buffer, dNTPs, DNA polymerase I, and RNaseH were added to synthesize double-stranded cDNA, and AMPure XP beads (Beckman Coulter, Inc.; Beckman A63881) were used to purify double-stranded cDNA. Purified double-stranded cDNA ends were repaired, a tail was added and connected to the sequencing adapter, and then AMPure XP beads were used for fragment size selection. Finally, PCR amplification was performed, and the PCR products were purified with AMPure XP (A63881, Beckman Coulter, Inc., Brea, CA, USA) beads to obtain the final library. After the library construction was completed, a Qubit 2.0 was used for a preliminary quantification, and the library was diluted to 1 ng/µL. Subsequently, an Agilent 2100 was used to detect the size of the inserted fragment of the library. Once the inserted fragment met the expectation, quantitative real-time PCR (qRT-PCR) was used to accurately quantify the effective concentration of the library (effective concentration of the library >2 nM) to ensure the quality of the library. After the library was qualified, different libraries were pooled into a flow cell according to the effective concentration and the requirement of the target offline data volume. After cBOT clustering, the Illumina high-throughput sequencing platform (HiSeq 2000) was used for paired-end sequencing (PE150).
2.6. Sequencing Data Filtering
To ensure the quality of the bioinformatics analysis, raw reads were filtered using Fastp software, and the low-quality reads with connectors were removed to obtain clean reads [29]. Hisat2 was used to compare the filtered reads to the reference genome to obtain the SAM/BAM file for each sample comparison [30,31]. StringTie software was used to splice clean reads. The spliced transcript sequences were used as reference sequences for subsequent analysis [32,33].
2.7. Differential Gene Expression Characteristics
RESM software (v1.3.1) was used to count the number of reads matched to each gene. The FPKM (expected number of fragments per kilobase of transcript sequence per million base pairs of sequencing) of each gene was calculated using the length of the gene and the number of reads matched to the gene. EdgeR (v3.20.2) software was used to analyze the difference in gene expression between the NNPCN-treated and control samples. Genes with false discovery rate (FDR) values less than or equal to 0.05 and a |log2FC|greater than or equal to 1 were selected as candidate differentially expressed genes (DEGs) [34,35].
2.8. Enrichment Analysis
ClusterProfiler (v1.10.0) software was used for Gene Ontology (GO) enrichment analysis of the DEGs, and the gene length preference was corrected. The GO analysis classified the genes with corrected p-values of less than 0.05 into DEGs. In addition, the software was used to analyze the significant enrichment of biochemical metabolic pathways and signal transduction pathways of the DEGs. Taking the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway as the unit, the hypergeometric test was used to determine the pathway of significant enrichment for DEGs relative to all the annotated genes [36].
2.9. Verification of Differentially Expressed Genes by qRT-PCR
Following the fungal culture conditions in Section 2.4, total RNA was extracted as described in Section 2.5. The primers are shown in Table 1. The genomic DNA was first removed by recombinant DNase I (2270B, Takara Biomedical Technology Co., Ltd., Dalian, China), the mixture was prepared in an RNase-free centrifuge tube: 1 μL of 10 × DNase Buffer, 1 μg of template RNA (<8 μL), and 1 μL of DNase I, while RNase-free ddH2O was added to 10 μL. The mixture was gently blown and mixed with a liquid shifter at 37 °C for 30 min. Then, the first-strand cDNA was synthesized by Prime Script II First-Strand cDNA Synthesis Kit (6210B, Takara Biomedical Technology Co., Ltd., Dalian, China). The reaction mixture was prepared in a PCR tube: 1 μL of random 6-mers (50 μM), 1 μL of dNTP mixture (10 mM each), and <5 μg of template RNA, while RNase-free ddH2O was added to 10 μL, which was reacted at 65 °C for 5 min and cooled in an ice bath. The following reverse transcription reaction solutions were prepared in the first step reaction tube: 10 μL of the reaction solution during the first step, 4 μL of 5× PrimeScript II Buffer, 0.5 μL of RNase Inhibitor (40 U/μL) (20 U), and 1 μL of PrimeScript II RTase (200 U/μL), supplemented with RNase-free ddH2O to 20 μL. The reverse transcription reaction conditions included 30 °C for 10 min, 42 °C for 30 min, and 95 °C for 5 min, followed by ice bath cooling. Finally, fluorescent quantitative PCR was performed by SYBR Premix Ex Taq (RR420A, Takara Biomedical Technology Co., Ltd., Dalian, China). The reaction system was prepared as follows (in a total volume of 20 μL): SYBR Premix Ex Taq (2×) 10 μL, forward primer (10 μM) 0.4 μL, and reverse primer (10 μM) 0.4 μL, template (cDNA) 1 μL, supplemented with ddH2O to 20 μL. The two-step fluorescent quantitative PCR amplification conditions included an amplification curve of one cycle at 95 °C for 30 s, followed by 40 cycles of 95 °C for 5 s and 60 °C 20 s, followed by a 72 °C single-point detection signal. The conditions for the dissolution curve were 95 °C for 0 s, 65 °C for 15 s, and 95 °C for 0 s as a continuous detection signal. The 2−ΔΔCt method was used for the relative quantitative analysis of the data, where ΔCt = Ct (target gene) − Ct (reference gene), ΔΔCt = ΔCt (detection group) −ΔCt (control group), and 2−ΔΔCt = the relative expression of the target gene in the detection group. The gene-specific primers were designed using Primer Premier v6.0 software (Premier Biosoft Company, San Francisco, CA, USA). Using 18S rRNA as the internal reference, 10 genes with significant differences were randomly selected. The primers were designed and verified by qRT-PCR. Other operations were performed according to the conventional method.

Table 1.
Oligonucleotide primers used in this study.
2.10. β-1,3-glucanase Activity
A total of 1 g of mycelium in the logarithmic growth phase was weighed and mixed with each of 10 μg/mL and 40 μg/mL NNPCN for a final volume of 10 mL, and the same amount of buffer was used as the control. After 24 h of treatment, the samples were collected and centrifuged to remove the R. solani. The effect of NNPCN on the β-1,3-glucanase activity of R. solani was studied in accordance with the method of Jiang [37].
2.11. Nucleic Acid Leakage
A total of 1 g of mycelium in the logarithmic growth phase was weighed and mixed with 10 μg/mL and 40 μg/mL NNPCN for a final volume of 10 mL, and the same amount of buffer was used as the control. Samples were taken at 0, 2, 4, 6, 8, 12, and 24 h. The cells were removed by centrifugation, and the absorbance of the extracellular fluid at 260 nm was detected. Each treatment was repeated three times [38].
2.12. Lipid Peroxidation
Three pieces of fungal plugs with diameters of 5 mm were inoculated in 100 mL of PDB and cultured at 25 °C and 180 r/min for 16 h. NNPCN was added to reach final concentrations of 0, 10, and 40 μg/mL and then cultured for 48 h. The culture was centrifuged at 4000 r/min to obtain the supernatant. Two milliliters of fermentation supernatant was mixed with 2 mL of 0.6% thiobarbituric acid solution, which was then plugged, placed in boiling water for 15 min, cooled rapidly, and then centrifuged. The absorbance values at OD532 nm and OD450 nm were measured, and the malondialdehyde (MDA) content was calculated as MDA (μmol/L) = 6.45OD532 − 0.56OD450 [39].
2.13. ATPase Activity
The mycelium from the 1 g culture for 24 h was mixed with each of 10 μg/mL and 40 μg/mL NNPCN to a final volume of 10 mL. The same amount of PDB was used as the control. Each treatment was repeated three times. The mycelia were sampled at 48 h and then obtained by centrifugation. The mycelia were treated with an ATP enzyme assay kit (A016–1-1, Jiancheng Bioengineering Institute, Nanjing, China) according to the manufacturer’s instructions. In addition, the ATPase activity was measured.
2.14. Nitrate Reductase Activity
The mycelium from the 1 g cultured for 24 h was separately mixed with 10 μg/mL and 40 μg/mL NNPCN to a final volume of 10 mL. The same amount of PDB was used as the control. Each treatment was repeated three times. Samples were collected at 48 h, and the mycelium was removed by centrifugation. The mycelium samples were treated with a nitrate reductase test kit (A096-1-1, Nanjing Jiancheng Bioengineering Institute), and the nitrate reductase activity was measured.
2.15. Receptor Homology Modeling and Molecular Docking Effect of NNPCN
According to the results of the ultrastructure observation and transcriptome testing, four target genes with significant differences, downregulated expression, and suspected high levels were selected from the cell wall, cell membrane, mitochondrial functional genes, and metabolic pathway quotient. The target protein model was constructed by the trRosetta method, and the binding mode of NNPCN with the target protein was studied with reference to the methods of Liu and Liu [40,41].
2.16. Data Treatment
All of the data were preliminarily sorted using Excel (Microsoft Corp., Redmond, MA, USA), and DPS v6.55 version (Data Processing System, sponsored by Tang Qiyi from Zhejiang University, Hangzhou, China) was used to compare the data using Duncan’s new complex range method.
3. Results
3.1. Effect of NNPCN on the Micromorphology of R. solani
According to microscopy, the fungal colonies not treated with NNPCN grew normally, the hyphae flourished, and there was no observed deformity or distortion (Figure 1 CK). The mycelium treated with NNPCN secreted red substances (Figure 1, NNPCN-treated R. solani 1), and the colonies showed progressive, creeping growth and a slow growth speed (Figure 1, NNPCN-treated R. solani 2–3). SEM observations showed a smooth mycelium without NNPCN treatment, with the mycelium growing loose and standing upright, a uniform base and top, unbroken hyphae that exhibited no swelling, a mycelium with a diaphragm with a long spacing, and a mycelium with a smooth surface (Figure 2, CK1–CK3). There was deformity, distortion, and swelling upon treatment with NNPCN; the mycelium diaphragm spacing was smaller and prone to fracture, the surface of the mycelium was rough, and there were many protrusions (Figure 2, NNPCN treated R. solani 1–3). In cells not treated with NNPCN, transmission radio lens observations showed complete structures, massless wall separation, and mostly complete cell diaphragms (Figure 3, CK1–CK3). Cells treated with NNPCN showed mass wall separation, fractured and uneven mycelia, and cell diaphragm disappearance (Figure 3, NNPCN-treated R. solani 1-3). The results indicated that NNPCN affected the microscopic mycelium morphology of R. solani.
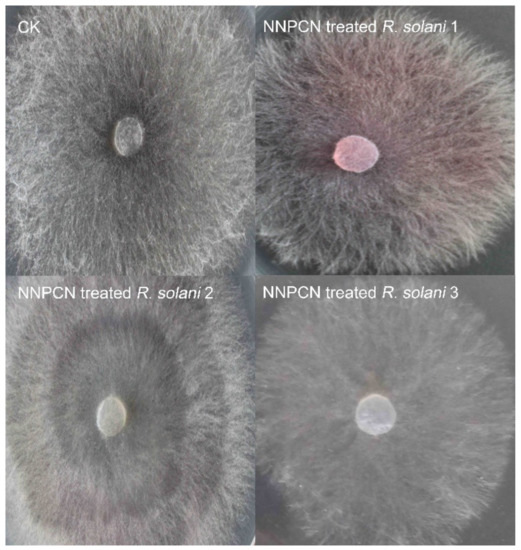
Figure 1.
Effect of the novel fungicide NNPCN on the colony morphology of R. solani (48 h). Note: CK: the control mycelium grew normally; NNPCN-treated R. solani 1: light-red secretions on the surface of mycelium treated with the compound; NNPCN-treated R. solani 2: progressive growth ring of mycelium treated with the compound; NNPCN-treated R. solani 3: propagation of mycelium treated with the compound.

Figure 2.
Scanning electron microscopy of the novel fungicide NNPCN inhibiting mycelial morphology of R. solani (48 h). Note: CK1: control mycelium was uniform, no fracture; NNPCN-treated R. solani 1: compound treatment mycelia were fractured and uneven in thickness; CK2: the control mycelium was smooth with no swelling; NNPCN-treated R. solani 2: compound treatment resulted in mycelium swelling and distortion; CK3: the surface of the control mycelium was smooth; NNPCN-treated R. solani 3: verrucous protrusions on the surface of the mycelium were observed under treatment with the compound.

Figure 3.
Transmission electron microscopy of the novel fungicide NNPCN inhibiting the mycelial morphology of R. solani (48 h). Note: CK1: control cell wall without damage; NNPCN-treated R. solani 1: cytoplasmic wall separation when treated with the compound; CK2: the control subcellular organelles were basically complete; NNPCN-treated R. solani 2: the cell subcellular organelles were disintegrated; CK3: the control cell membrane was clear; NNPCN-treated R. solani 3: the drug-treated cell membrane disappeared.
3.2. Data Quality Control and Evaluation
The raw data from processing groups 1, 2, and 3, and controls 1, 2, and 3 were sequenced. After removing the low-quality data, there were 22,582,425, 26,800,753, 20,692,153, 25,126,831, 19,678,982, and 20,868,689 clean reads, respectively. The percentages of the ratio of the Q20 (percentage of base mass greater than 20 to total base ratio) were 98.70%, 98.65%, 98.69%, 98.50%, 98.26%, and 98.91%. The percentages of the ratio of the Q30 (percentage of base mass greater than 30 to total base ratio) were 95.04%, 94.89%, 95.00%, 94.52%, 93.91%, and 95.65% (Supplementary Table S1). These results indicate that the data obtained by sequencing were of good quality and met the follow-up test requirements.
3.3. Analysis of Gene Expression Characteristics and Difference Levels
As indicated in Figure 4, there were 6838 DEGs between the processing and control groups, including 3384 upregulated genes and 3454 downregulated genes, and there was differential expression with small absolute values and significant differences. There were 291 genes for which the absolute value of log2 fold change was greater than the 1143 upregulated genes and 148 downregulated genes.

Figure 4.
Overall distribution of the DEGs. Note: black dots: genes with no significant difference; red dots: significantly different genes.
3.4. GO Functional Clustering Analysis
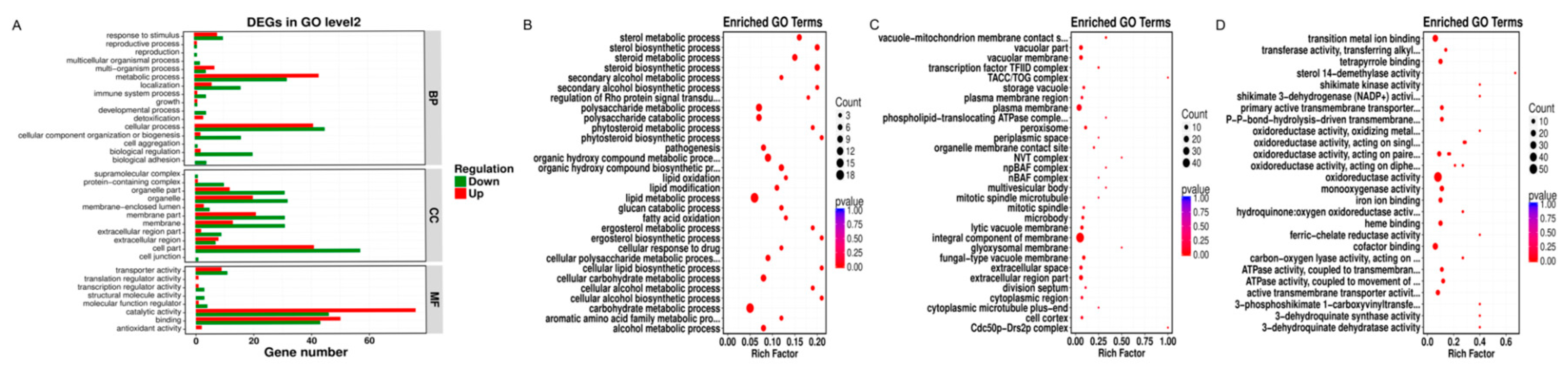
Our GO functional enrichment analysis showed that the DEGs were primarily divided into three categories, namely, biological processes, cellular components, and molecular functions, and they were further divided into 16, 11, and eight subcategories (Figure 5A). In the biological processes category, most DEGs were associated with cell physiological process, followed by metabolic process and biological regulation, with 86, 75, and 22 DEGs, respectively, accounting for 29.55%, 25.77%, and 7.56% of the total DEGs. In the cell component category, the largest number of DEGs was associated with cell components, followed by membrane components and organelles. The corresponding DEGs were 98, 52, and 52, accounting for 33.68%, 17.87%, and 17.87% of the total DEGs, respectively. In the cell physiological process category, biological regulation, cell components, membrane elements, organelles, organelle part, membrane, extracellular region, transporter activity, molecular function regulator, and other subclasses had a significantly higher number of downregulated genes than upregulated genes, indicating that the target genes and functions of R. solani were inhibited by NNPCN, thus preventing the growth and amplification of R. solani. In the metabolic process category, multi-organism process, catalytic activity, binding function, and other subclasses had a significantly higher number of upregulated genes than downregulated genes, indicating that NNPCN treatment could reduce the damage caused by NNPCN fungicide and actively improve the metabolic ability of R. solani.

Figure 5.
Gene Ontology enrichment analysis. (A) GO term level 2 differential gene number; (B) GO enrichment of differentially expressed genes in the BP process; (C) GO enrichment of differential genes in the CC process; (D) GO enrichment of differential genes in the MF process.
Under the biological process classification, the DEGs were significantly enriched in the carbohydrate metabolic process, lipid metabolic process, organic hydroxy compound biosynthetic and metabolic process, and glucan catabolic process polysaccharide metabolic and catabolic process. Most of these processes had more downregulated genes than upregulated genes, showing that NNPCN inhibited the gene expression of carbohydrate metabolism, lipids, and organic hydroxyl compounds. Various DEGs were significantly enriched in the steroid biosynthetic and metabolic process and sterol biosynthetic and metabolic process categories, and the associated DEGs were upregulated, while a relatively small amount of downregulation expression was not enriched by the GO database, as shown in Figure 5B. The synthesis and metabolic processes of the rice grain blight membrane resisted NNPCN damage, which may be related to its resistance.
Under the cell-group classification, the DEGs were significantly enriched in the integral component of membrane, plasma membrane, vacuolar membrane, and extracellular region part. Of these, DEGs were downregulated in 29 genes associated with the integral component of the membrane and showed upregulated expression of 19 genes. The primary categories with strong differential gene enrichment were the glyoxysomal membrane, plasma membrane, and peroxisome. A total of 18 genes were downregulated, and four genes were upregulated (Figure 5C). NNPCN inhibited partial component gene expression of the membrane, damaging the composition of the membrane.
Under the molecular function classification, the DEGs were significantly enriched in oxidoreductase activity, transition metal ion binding, cofactor binding, and monooxygenase activity (Figure 5D). The primary genes with strong differential gene enrichment included ATPase activity coupled to the movement of substances, heme binding, tetrapyrrole binding, iron ion binding, ATPase activity, coupling to transmembrane movement of substances, shikimate dehydrogenase (NADP+), shikimate activity, and active transmembrane transporter activity. Both genes were upregulated in these GO terms; there was also gene downregulation, and the existing inhibitory genes in the key genes demonstrated the effects of NNPCN. Resistant genes were also detected.
3.5. KEGG Enrichment Analysis
Through KEGG database analysis, significant metabolic pathways in the DEGs were identified, and the physiological and biochemical processes in which the DEGs participated were explored. The results showed that NNPCN primarily affected environmental information processing, cellular processes, organismal systems, and the genetic information processing of R. solani. The number of downregulated genes in these pathways was higher than the number of upregulated genes, indicating that these pathways may be acting as targets. The number of upregulated genes in metabolism and other channels was higher than the number of downregulated genes. To show that a given pathway is designed to resist destruction by NNPCN, enhanced repair capabilities may be related to the resistance of some rice strains (Figure 6A). There were 16 metabolic pathways in the samples treated with NNPCN that exhibited significant metabolic pathway changes. The most prominent metabolic pathways were steroid biosynthesis, ABC transporters, phenylpropanoid biosynthesis, and cyanoamino acid metabolism. There was strong enrichment in valine, leucine, and isoleucine degradation, metabolism of xenobiotics by cytochrome P450, plant hormone signal transduction, fatty-acid degradation, and aminobenzoate degradation (Figure 6B). These metabolic pathways may be potential targets of NNPCN.

Figure 6.
KEGG enrichment analysis. (A) KEGG pathway and a class of differential genes; (B) enrichment of differential genes by KEGG pathway analysis.
3.6. Key Genes Involved in the Cell Wall
NNPCN led to changes in the composition and structure of the R. solani cell wall. There was downregulated gene expression in the glycosyl hydrolase family 10 domain-containing protein (AG1IA_04663, ghf) and cyclic nucleotide-binding domain-containing protein (AG1IA_09068, cnb), while the chitin biosynthetic process function displayed reduced gene expression in the glycosyltransferase family 2 protein (AG1IA_09260, gfp), laccase precursor (AG1IA_03152, lp), and homeobox domain-containing protein (AG1IA_05765, hdc). The above genes may be the targets for NNPCN. However, beta-glucosidase (AG1IA_03090, bg) is in the phenylpropanoid biosynthesis pathway. This gene may be related to anti-NNPCN resistance.
3.7. Key Genes Involved in the Cell Membrane
The gene expression of lipid oxidation, lipid modification, and peroxysomal citrate synthase (AG1IA_05287, pcs) was downregulated in R. solani treated with NNPCN, followed by lipid modification, sacI-like domain-containing protein (AG1IA_05039, sdc), and alpha/beta hydrolase family domain-containing protein (AG1IA_02292, ahf). Secondly, the integral component of the membrane, ABC transporter domain-containing protein (AG1IA_04148, atd), Ecl1 domain-containing protein (AG1IA_01132, edc), ABC transporter CDR4 (AG1IA_01784, apc), phospholipid-transporting ATPase, putative (AG1IA_06826, ap), alpha/beta hydrolase fold domain-containing protein (AG1IA_06030, ahf), and other genes in the plasma membrane were downregulated, and the peroxisomal membrane protein (AG1IA_04529, pmp) in the glyoxysomal membrane was also downregulated. The downregulation of cell membrane lipid oxidation, modification, metabolism, membrane components, and ABC transporter-related genes suggested that these genes may be potential targets of NNPCN. In contrast, the cytochrome P450 (AG1IA_08569, p450), delta24 (24−1) sterol reductase (AG1IA_02524, dsr), GCC2 and GCC3 domain-containing protein (AG1IA_09056, AG1IA_01832), C-8 sterol isomerase (AG1IA_05701, csi), delta-sterol C-methyltransferase (AG1IA_06235, dsc), and other steroid-associated genes were upregulated in the cell membrane. In addition, the lanosterol synthase (AG1IA_02324, lsn) genes in steroids and C-4 methyl esterol oxidase (AG1IA_07096, sce), delta 24 (24–1) sterol reductase (AG1IA_02524, dsr), and delta-sterol C-methyltransferase (AG1IA_06235, dsc) genes in ergosterol were upregulated. Cytochrome P450 (AG1IA_08569, p450), protein tyrosine kinase domain-containing protein (AG1IA_07367, kdcp), and GPR1/FUN34/yaaH family domain-containing protein (AG1IA_06340, gfd) were also upregulated. This result indicated that the upregulation of sterol, steroids, ergosterol, cytochrome P450, and other genes in the cell membrane might be related to the repair of the cell membrane by pathogens.
3.8. Other Antioxidant and Metabolism-Related Genes
The expression of peroxysomal citrate synthase (AG1IA_05287, pcs), putative acyl-coenzyme, oxidase 3.2, peroxisome (AG1IA_09252, pcop), and other genes involved in the biological function of R. solani treated with NNPCN was downregulated. The gene expression of the arom polypeptide (AG1IA_04892, app) involved in the biological function of the aromatic amino acid family metabolic process was downregulated. The expression of NADPH reductase (AG1IA_09410, nadh) in the biological function of NADPH reductase [NAD (P) H] activity was downregulated. The gene expression of PIF1 domain-containing protein (AG1IA_06722, pdc) was downregulated in the biological function of mtDNA organization. d-Xylulose kinase (AG1IA_02281, dki), cyclic nucleotide-binding domain-containing protein (AG1IA_09068, cnbd), glycosyl hydrolase family 10 domain-containing protein (AG1IA_04663, ghfd), pectate lyase domain-containing protein (AG1IA_03046, pldc), and pectate lyase domain-containing protein (AG1IA_06604, pldcp) were downregulated during carbohydrate metabolism. The above genes may be potential targets of NNPCN. However, beta-glucosidase (AG1IA_03090, bgl), methyltransferase type 11 (AG1IA_02081, mtt), glycoside hydrolase family 13 protein (AG1IA_06735, ghf), glycoside hydrolase family 15 protein (AG1IA_02474, glhf), glycosyltransferase family 8 protein (AG1IA_07325, gsf), trehalose phosphorylase (AG1IA_06016, tpf), and major alcohol dehydrogenase (AG1IA_00808, mad) were upregulated in this pathway. The glutaredoxin domain-containing protein (AG1IA_02038, gdc) gene, which is involved in the biological function of glutathione dehydrogenase (ascorbate) activity, was upregulated. Glutaredoxin domain-containing protein (AG1IA_02038, gldc) and glutathione S-transferase (AG1IA_00715, gls), which are involved in the biological function of glutathione transferase activity, were upregulated. The gene expression of glutathione S-transferase (AG1IA_00715, gls) in the biological function of peroxidase activity was upregulated. The expression of mannitol-1-phosphate dehydrogenase MPDH1 (AG1IA_05689, mpd) was upregulated in NADH oxidation biological function. The expression of the adenosine deaminase (AG1IA_03187, ade) gene was upregulated within the biological function of adenosine receptor binding. The gene expression of mannitol-1-phosphate dehydrogenase MPDH1 (AG1IA_05689, mpd) was upregulated within the biological function of alcohol dehydrogenase (NAD) activity. The expression of the beta-glucosidase (AG1IA_03090, bgl) gene was upregulated in the biological function of scopolin beta-glucosidase activity. The upregulation of these genes suggests that the pathogen resists NNPCN damage through antioxidant or energy metabolism processes.
3.9. qRT-PCR Validation
Ten genes were randomly selected from the above metabolic pathway for qRT-PCR verification, which showed that the qRT-PCR validation results with 18S rRNA as the internal parameter were consistent with the transcriptome determination expression trends (Figure 7), indicating high sequencing quality.

Figure 7.
Quantitative RT-PCR relative expression of R. solani differentially expressed genes after NNPCN treatment (CK: control; CL: 40 μg/mL; * p-value < 0.05).
3.10. Effect of NNPCN on the β-1,3-glucanase Activity of R. solani
Figure 8A shows that NNPCN inhibited the activity of β-1,3-glucanase in R. solani. The higher the concentration of NNPCN, the better the activity of β-1,3-glucanase, and it varied significantly between treatments (p < 0.05). When NNPCN reached a concentration of 40 μg/mL, its enzyme activity was 115.23 U/mL, 0.79 times that of the control enzyme activity. NNPCN may, therefore, reduce the activity of β-1,3-glucanase, resulting in blocked decomposition. This study confirms the reduction in key gene expression in the β-1,3-glucanase found in transcriptional sequencing.

Figure 8.
Effect of NNPCN on the enzymatic activity of R. solani. (A) β-1,3-glucanase; (B) ATPase; (C) NADH nitrate reductase. Note: Columns followed by different letters indicate significantly different scores in the same phase according to Duncan’s multiple range tests at the p < 0.05 significance level.
3.11. Effect of NNPCN on Nucleic Acid Leakage in R. solani
Figure 9 shows that NNPCN affected the permeability of the stria cell membranes. The extracellular nucleic acid content of the R. solani treated with NNPCN was higher than that in the control group and varied significantly between the treatments (p < 0.05). Secondly, a higher concentration of NNPCN led to higher nucleic acid content in the extracellular fluid of R. solani. When the concentration of NNPCN was 40 μg/mL, the extracellular nucleic acid content was highest and was significantly different (p < 0.05). As the experiment progressed, the unified time period was reached. This finding suggests that NNPCN can enhance cell membrane permeability, thus causing nucleic acid exudation, suggesting that the cell membrane may be the target of its action.

Figure 9.
Effect of NNPCN on nucleic acid leakage by R. solani. Note: Different lowercase letters indicate significantly different scores in the same phase according to Duncan’s multiple range tests at the p < 0.05 level.
3.12. Effect of NNPCN on the Lipid Peroxidation of R. solani
The malonaldehyde (MDA) content can reflect the degree of intra-lipid peroxidation and indirectly reflect the extent of cell damage. Since MDA is a small water-soluble molecule, the membrane is released into extracellular culture when destroyed. Mycelium treated with NNPCN showed increased propylene dialdehyde in culture compared with the control group and varied significantly (p < 0.05). The concentration of NNPCN was positively associated with the MDA content of R. solani, in which the 40 μg/mL treatment yielded 1.34 times the concentration of the 10 μg/mL treatment (Table 2). This rule is largely consistent with intracellular nucleic acid leakage, suggesting that NNPCN treatment caused damage to the cell membrane.

Table 2.
Effect of NNPCN on malonaldehyde in R. solani.
3.13. Effect of NNPCN on the ATPase Activity of R. solani
Figure 8B shows that the activity of the ATP enzyme treated with NNPCN was inhibited and varied significantly between the different treatments (p < 0.05). A higher concentration of NNPCN led to better ATP activity. When the NNPCN concentration was 40 μg/mL, the enzyme activity was 0.31 U/mg prot, which was 0.63 times that of the control enzyme. NNPCN could, thus, inhibit ATP enzyme activity, resulting in insufficient ATP supply and slow metabolism. This study confirms that the transcriptional sequencing findings are connected to ATP in terms of reduced gene expression.
3.14. Effect of NNPCN on the Nitrate Reductase Activity of R. solani
Figure 8C shows that NNPCN may significantly enhance nitrate reductase activity in the striatum compared to the control (p < 0.05). The concentration of NNPCN was negatively correlated with the nitrate reductase activity and varied significantly between treatments (p < 0.05). When the NNPCN concentration was 40 μg/mL, the maximum enzyme activity was 0.13 μmol/h/g, which was 2.17 times that of the control enzyme. Thus, NNPCN could improve nitrate reductase activity. This is inconsistent with the downregulation of key gene expression in intracellular nitrate reductase found by the transcriptome sequencing analysis.
3.15. Molecular Docking of NNPCN and Target Protein
The trRosetta method was used to construct the AG1IA-04663 protein model, and the overall quality factor score of ERRAT was 90.10, as evaluated by SAVES. The VERIFY3D evaluation results showed that 89.35% of the residues had 3D–1D scores ≥0.2, which met the modeling requirements. The trRosetta method was used to construct the AG1IA-06030 protein model, and the results showed that the confidence level of the protein model was 0.77. The overall quality factor score by ERRAT was 89.12, as evaluated by SAVES. The VERIFY3 D evaluation results showed that the average 3D–1D score of 98.80% of the residues was ≥0.2, which also met the modeling requirements. The trRosetta method was used to construct the AG1IA-09410 protein model, and the confidence level of the protein model was 0.32. The overall quality factor score of ERRAT was 90.68, as evaluated by SAVES. The VERIFY3D evaluation results showed that 91.14% of the residual groups had a 3D–1D score ≥0.2, which met the modeling requirements. Therefore, the tertiary structure of the target protein constructed by the trRosetta method was stable and reliable and could be used for subsequent analysis.
To verify the binding of NNPCN to the target protein, its binding mode to AG1IA_04663, AG1IA_06030, and AG1IA_09410 was studied by the molecular docking method. NNPCN could dock with AG1IA_04663, AG1IA_06030, and AG1IA_09410, where binding to AG1IA_06030 was best and had a free energy of −8.07 kJ·mol−1, AG1IA_04663 was second, with a free energy of −6.36 kJ·mol−1, and AG1IA_09410 was third, with a free energy of −5.93 kJ·mol−1. NNPCN may not form a hydrogen bond with AG1IA_04663 and may act through van der Waals forces and electrostatic forces (Figure 10A). NNPCN may form a hydrogen bond with Glu395 of AG1IA_06030 (Figure 10B) and a hydrogen bond with Leu550 of AG1IA_09410 (Figure 10C), indicating that the binding process was spontaneous and may function through a hydrogen bond.

Figure 10.
Theoretical binding mode between NNPCN and the target receptor, with the result shown by PyMoL 1.7.6. (A) Glucan catabolic enzyme; (B) ABC transporter protein; (C) NADH nitrite reductase.
In conclusion, the mechanism of NNPCN inhibiting R. solani may first destroy the cell wall structure, damage the cell membrane transport and the synthesis of key substances, cause the leakage of intracellular substances (such as DNA, malondialdehyde) and the inactivation of ATPase, and finally lead to cell apoptosis (Figure 11).
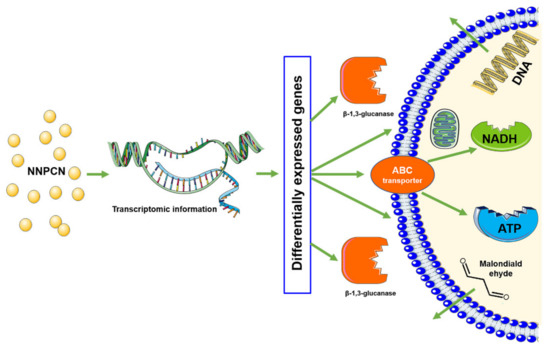
Figure 11.
An inhibition mechanism model of cell wall structure, cell membrane damage, antioxidant activity, and metabolism of R. solani treated with NNPCN.
4. Discussion
Exploring new fungicides has potential application in the discovery of a new action target, improving the efficiency of drugs, increasing crop yields, assisting in structurally modifying fungicides, adjusting the components of drug use, reducing the resistance of pathogens, and saving costs. It is also of great significance in promoting the creation of new types of pesticides with high efficiency, low toxicity, and environmentally friendly characteristics [42,43,44,45]. Our findings showed that NNPCN caused diversification of the colony and mycelial morphology of R. solani. When treated with NNPCN, red water-soluble material appeared in the colony, which may be effective for dilution, neutralization, and decomposition. Treating the hyphae resulted in progressive growth, which may be because NNPCN changed their growth pattern. To resist the toxicity of the drug, the hyphae adopted curve-shaped growth instead of linear growth. The hyphal group and the external hyphae first died to protect the internal hyphae so that hyphal growth could be preserved. When treated with NNPCN, the stolon was increased. This is possibly because the drug diffused around the surface of the medium, which inhibited the growth of the stolon. Previous studies suggested that fungicide could inhibit hyphal growth by distorting and deforming the hyphae, increasing hyphal density, increasing the number of hyphal branches, and breaking the hyphae. Reports on changes in the colonial morphology, abnormal extracellular metabolism in the hyphae, and increased aerial mycelium are rare [46]. Our findings confirmed that NNPCN has multitarget characteristics and can block gene expression, resulting in diverse colony morphologies and changes in mycelial metabolism. However, how these changes occur requires further examination in the future.
The scanning electron microscopy observations indicated that NNPCN could create hyphal fractures. The reason may be that NNPCN destroyed the structure of the glucan or the chitin in the cell wall, which resulted in a collapse. Secondly, the mycelial treated with NNPCN had an uneven thickness, possibly because the expression of some key substances in hyphal nutrition was affected by NNPCN; therefore, the normal and abnormal expressions of hyphal nutrition are interwoven. Notably, the increasing verrucous bulges on the cell wall surface again proved that the normal expression of cell wall of R. solani was influenced by NNPCN, which forced the key gene in cells to be expressed abnormally, leading to hyphal development anomalies. These results differ from those indicated by previous research. NNPCN did not shrivel the hyphae or thicken the cell wall. However, it could break the hyphae, unevenly thicken the mycelium, and increase the verrucous bulges on the cell walls. Various signs show that it has a stronger destructive power than other phenazine fungicides [47].
The transmission electron microscopy results indicated that NNPCN could cause R. solani wall separation in the cells. NNPCN may bind with substances on the cell wall or cell membrane, such that intracellular dehydration or the leakage of key substances is related. Secondly, this compound could disappear or break up the diaphragms and subcellular organelles. Perhaps the compound enters the cell through ion channels or carrier protein channels on the cell membrane and binds or collides with intracellular organelles, resulting in the disintegration of subcellular organelles. This research differs from earlier studies as NNPCN has multiple targets, causing subcellular structure damage, eventually leading to mycelial apoptosis [48].
How does NNPCN effect mycelium apoptosis? Further study showed that the compound could downregulate the expression of β-glucanase, chitinase, and other related genes in the cell wall macromolecular metabolic pathway of R. solani. This finding indicates that the compound affected the structural synthesis and degradation of the cell wall, resulting in abnormal growth, revealing that the cell wall may be one of its targets. The possible reasons for the uneven, broken, and verrucous processes of the mycelia treated with NNPCN were explained at the molecular level. This study differs from an earlier study in that the mechanism of phenazine-1-formamide is different, as NNPCN may act on cell wall glucan and has multiple target sites [49]. It is well known that the components of the fungal cell wall are glucan and chitin, the components of the plant cell wall are cellulose and pectin, and the components of the animal cell are not the same. If the target of novel fungicidal compounds is the cell wall, it is safe for animal and plant cells, which has been the focus of novel pesticide development [50,51]. This result indicates that NNPCN has broad development prospects as a candidate pesticide for rice sheath blight. The primary components of the fungal cell membrane are lipids, proteins, enzymes, and sterols, which primarily have material, energy, and information exchange roles with the outside world [52]. In general, researchers can quickly understand the effect of NNPCN on the cell membrane using transcriptome technology. KEGG enrichment analysis helps to understand the related biological functions and pathways of differentially expressed genes, easily address the research objectives, and identify the objects that should be paid attention to in follow-up research [53,54]. The KEGG enrichment results indicated that NNPCN had a strong influence on the functions of sterol, steroids, ABC transporters, and lipids in the cell membrane pathway of R. solani, and the expression of genes encoding lipid oxidation, modification, metabolism, membrane composition, and ABC transporters was downregulated. Since the cell membrane is responsible for the exchange of intracellular and extracellular substances, the carrier protein channel plays a key role. This study confirmed that NNPCN may affect the expression of ABC transporters and the exchange of substances, indicating that the target of NNPCN is on the cell membrane. This assumption is consistent with the results of another study indicating that the action sites of phenazine-1-carboxylic acid, a similar compound, on the membrane may have some identical pharmacophores [55]. However, it was reported that the antifungal mechanism of a similar compound, phenazine-1-carboxylic acid, involved destruction of the vesicle trafficking and autophagy of host cells, which may be due to different pharmacodynamic groups. However, this difference requires further study with respect to the structure–activity relationship [56].
In general, the weakening of the intracellular antioxidant capacity and the energy metabolism system means that cells face apoptosis. This study confirmed that NNPCN had some effects on the antioxidant and energy metabolism of R. solani. This compound can downregulate the expression of genes related to cell fatty-acid oxidation, carbohydrate metabolism, aromatic amino-acid metabolism, and nitrite reductase, which more or less affect cell oxidative phosphorylation, glycolysis, and the tricarboxylic acid cycle. An earlier study also confirmed that glycolysis and oxidative phosphorylation may be targets of fungicides [57]. This observation indicates that oxidative phosphorylation, glycolysis, and the tricarboxylic acid cycle may be the potential targets of NNPCN.
The possible targets of NNPCN were verified by physiological and biochemical methods. The results showed that the glucanase activity decreased following treatment with NNPCN. This result might explain the downregulation of genes related to glucan decomposition. The leakage of nucleic acids from mycelia treated with NNPCN revealed that it might destroy the structure of the cell membrane. However, the way in which it causes this destruction, from a physiological and biochemical perspective, is not clearly understood and requires further study. The ATP enzyme activity and nitrate reductase activity of the mycelium treated with NNPCN were significantly decreased, indicating that intracellular energy synthesis and nitrite metabolism ability were weakened, resulting in the accumulation of toxic substances and apoptosis.
NNPCN was selected as the key target for glucanases, ABC transporters, and NADPH nitrate reductases related to the cell wall, cell membrane, and cell antioxidant and energy metabolism, and the binding mode of the ligand and receptor was studied by molecular docking. The results showed that NNPCN could bind well to glucanase, the ABC transporter, and NADPH nitrate reductase, and it could bind to glucanase via van der Waals and electrostatic forces. NNPCN could bind to ABC transporters and NADPH nitrate reductase via hydrogen bonding. This study confirmed the potential targets of NNPCN. However, the primary and secondary binding of the compound to the target, the length of the binding time, and the sequence of binding were not studied in depth due to time limitations; thus, they require additional analysis in the future.
In addition, although this study systematically explored the antifungal molecular mechanism of NNPCN using a variety of methods, such as ultrastructure observation, transcriptome sequencing technology, physiological and biochemical tests, and molecular docking methods, further verification is still required, such as the changes in the proteome, modification group, and metabolic activity. Secondly, this study explored the gene functions and metabolic pathways that were greatly affected by NNPCN, but a large number of genes with significant differences were not completely resolved, and a large number of suspected target genes remain to be explored. Thirdly, whether NNPCN truly acts on the key target genes of R. solani must still be verified by gene function. In the future, CRISPR/Cas9 or homologous recombination technology can be used to knock out the key genes, following which the drug can be used to act on the resulting mutant to observe changes in antifungal effects [58]. Lastly, the current test methods cannot achieve 100% seamless docking, and the relationship between the observed phenomena and the subsequent test results can only be reasonably inferred. Therefore, whether seamless docking can be achieved needs to be further explored.
NNPCN can act on the cell wall, cell membrane, and cell antioxidant and energy metabolism of R. solani. It has the characteristics of multiple targets, and it has a unique advantage in that the pathogen does not easily exhibit resistance.
5. Conclusions
NNPCN activity was associated with red secretion in treated R. solani mycelium, mycelium swelling, uneven thickness, fractures, increased verrucous protrusions on the surface of the mycelium, separation of the cytoplasm wall, disintegration of the subcellular organelles, and disappearance of the septum. There were 291 significantly different genes in the samples treated with NNPCN, including both downregulated and upregulated genes, and the expression trend of 10 DEGs genes was consistent with the results of the transcriptome sequencing. The GO enrichment analysis showed that the DEGs were significantly enriched in cell wall glucan decomposition and metabolism, cell membrane synthesis, metabolism, composition, organic hydroxyl compounds, oxidoreductase activity, and transition metal ion binding. The KEGG pathway enrichment analysis showed that the significant metabolic pathways of the DEGs were mainly primarily enriched in steroid biosynthesis, ABC transporters, and other pathways. Further studies revealed that the downregulated expression of genes related to glucan catabolic process function related to the cell wall may be a potential target. The downregulated expression of genes related to lipid oxidation, modification, and ABC transporters in the plasma membrane indicated that NNPCN acted as the target gene in the cells. Genes related to cellular antioxidant and metabolic functions, such as fatty-acid oxidation and carbohydrate metabolism, as well as genes related to antioxidants and metabolism, had significant differences in target genes. Nitrite reductase [NADH] activity and mitochondrial organization gene expression were downregulated. The results of the physiological and biochemical tests showed that NNPCN could decrease the activities of β-1,3-glucanase, nucleic acid leakage, MDA, and ATPase, but increase the activity of nitrate reductase. The reverse molecular docking results showed that NNPCN was free to bind to target proteins such as β-1,3-glucanase, ABC transporter, and NADH nitrate reductase, binding to glucanase via van der Waals forces and electrostatic forces and to ABC transporter and NADH nitrate reductase via hydrogen bonding.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/agronomy11122532/s1, Table S1: Data quality statistics, Figure S1: Inhibition effect of NNPCN against Rhizoctonia solani (A: CK; B: 40 μg/mL).
Author Contributions
Y.Z. designed the study; C.W. performed the experiments; S.L. and X.L. analyzed the data; Y.Z. wrote the manuscript. All authors read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 32072473, the Nature Science Foundation of Hunan Province, grant number 2019JJ40125, the double first-class construction project of Hunan Agricultural University, grant number SYL2019033, and the Scientific Research Fund of Hunan Provincial Education Department, grant number 18B115.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors declare that the data supporting this study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Singh, P.; Mazumdar, P.; Harikrishna, J.A.; Babu, S. Sheath blight of rice: A review and identification of priorities for future research. Planta 2019, 250, 1387–1407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zrenner, R.; Verwaaijen, B.; Genzel, F.; Flemer, B.; Grosch, R. Transcriptional changes in potato sprouts upon interaction with Rhizoctonia solani indicate pathogen-induced interference in the defence pathways of potato. Int. J. Mol. Sci. 2021, 22, 3094. [Google Scholar] [CrossRef]
- Chang, K.F.; Hwang, S.F.; Ahmed, H.U.; Strelkov, S.E.; Harding, M.W.; Conner, R.L.; McLaren, D.L.; Gossen, B.D.; Turnbull, G.D. Disease reaction to Rhizoctonia solani and yield losses in soybean. Can. J. Plant Sci. 2018, 1, 115–124. [Google Scholar] [CrossRef] [Green Version]
- Tomaso-Peterson, M.; Trevathan, L.E. Rhizoctonia solani AG-13 isolated from corn in Mississippi. Plant Dis. 2004, 8, 908. [Google Scholar] [CrossRef] [PubMed]
- Brown, L.; Harrington, S.; Harrington, M.; Murdock, M.R.; Pizolotto, C.A.; Woodhall, J.W. Rhizoctonia solani AG 2-2 IIIB causing root rot of onion in Idaho. Plant Dis. 2021, 2, 498. [Google Scholar] [CrossRef] [PubMed]
- Ogoshi, A. Ecology and pathogenicity of anastomosis and intraspecific groups of Rhizoctonia solani K€uhn. Annu. Rev. Phytopathol. 1987, 25, 125–143. [Google Scholar] [CrossRef]
- Feng, S.; Shu, C.; Wang, C.; Jiang, S.; Zhou, E. Survival of Rhizoctonia solani AG-1 IA, the Causal Agent of Rice Sheath Blight, under Different Environmental Conditions. J. Phytopathol. 2017, 165, 44–52. [Google Scholar] [CrossRef]
- Molla, K.A.; Karmakar, S.; Molla, J.; Bajaj, P.; Rajeev, K. Understanding sheath blight resistance in rice: The road behind and the road ahead. Plant Biotechnol. J. 2020, 18, 895–915. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, I.M.; Jesuraj, A.; Kamboj, R.; Devanna, B.N.; Botella, J.R.; Sharma, T.R. Host Delivered RNAi, an efficient approach to increase rice resistance to sheath blight pathogen (Rhizoctonia solani). Sci. Rep. 2017, 7, 7521. [Google Scholar] [CrossRef]
- Kumar, B. Validation of integrated management modules against sheath blight disease of rice. Indian Phytopathol. 2021, 74, 235–239. [Google Scholar] [CrossRef]
- Choudhary, P.; Rai, P.; Yadav, J.; Verma, S.; Chakdar, H.; Goswami, S.K.; Srivastava, A.K.; Kashyap, P.L.; Saxena, A.K. A rapid colorimetric LAMP assay for detection of Rhizoctonia solani AG-1 IA causing sheath blight of rice. Sci. Rep. 2020, 10, 22022. [Google Scholar] [CrossRef]
- Peters, F.Á.R.; Datnoff, L.E.; Korndörfer, G.H.; Seebold, K.W.; Rush, M.C. Effect of Silicon and Host Resistance on Sheath Blight Development in Rice. Plant Dis. 2001, 85, 827–832. [Google Scholar]
- Wu, W.; Shah, F.; Shah, F. Rice sheath blight evaluation as affected by fertilization rate and planting density. Australas. Plant Pathol. 2015, 44, 183–189. [Google Scholar] [CrossRef]
- Wan, G.K.; Chang, K.K. Density of overwintered sclerotia in paddy fields in Korea, viability of the sclerotia and pathogenicity of the sclerotial fungi. Plant Biotechnol. J. 1988, 4, 207–217. [Google Scholar]
- Shu, C.; Zhao, M.; Anderson, J.P.; Garg, G.; Zhou, E. Transcriptome analysis reveals molecular mechanisms of sclerotial development in the rice sheath blight pathogen Rhizoctonia solani AG1-IA. Funct. Integr. Genom. 2019, 19, 743–758. [Google Scholar] [CrossRef]
- Slaton, N.A.; Cartwright, R.D.; Meng, J.; Edward, E.G.; Richard, J. Sheath Blight Severity and Rice Yield as Affected by Nitrogen Fertilizer Rate, Application Method, and Fungicide. Agron. J. 2003, 95, 1489–1496. [Google Scholar] [CrossRef]
- Zhang, C.Q.; Liu, Y.H.; Ma, X.Y.; Feng, Z.; Ma, Z.H. Characterization of sensitivity of Rhizoctonia solani, causing rice sheath blight, to mepronil and boscalid. Crop Prot. 2009, 28, 381–386. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, A.F.; Wang, W.X.; Zhang, Y.; Gao, T.C. Baseline sensitivity and efficacy of thifluzamide in Rhizoctonia solani. Ann. Appl. Biol. 2012, 161, 247–254. [Google Scholar] [CrossRef]
- Yang, R.; Jiang, S.; Wen, X.; Song, X.; Chen, Z. The Antifungal Activity and Mode of Action of a Streptomyces-Derived Anti-Microbial, Ningnanmycin, on the Tea Gray Blight Disease Pathogen Pseudopestalotiopsis camelliae-sinensis. Phytopathology 2021. online ahead of print. [Google Scholar] [CrossRef]
- Kasuga, K.; Sasaki, A.; Matsuo, T.; Yamamoto, C.; Minato, Y.; Kuwahara, N.; Fujii, C.; Kobayashi, M.; Agematu, H.; Tamura, T.; et al. Heterologous production of kasugamycin, an aminoglycoside antibiotic from Streptomyces kasugaensis, in Streptomyces lividans and Rhodococcus erythropolis L-88 by constitutive expression of the biosynthetic gene cluster. Appl. Microbiol. Biotechnol. 2017, 101, 4259–4268. [Google Scholar] [CrossRef]
- Osada, H. Special issue: Nucleoside antibiotics, polyoxin and beyond. J. Antibiot. 2019, 72, 853–854. [Google Scholar] [CrossRef]
- Smith, D.L.; Dushoff, J.; Morris, G., Jr. Agricultural antibiotics and human health. Public Libr. Sci. Med. 2005, 2, e232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Liu, S.; Wang, C.; Liao, X.L. Fungicidal activity and biological characteristics of a novel natural product fungicide: Phenazine-1-carboxamide-derived 18-1. Int. J. Agric. Biol. 2018, 20, 2378–2386. [Google Scholar]
- Xiong, Z.; Niu, J.; Liu, H.; Xu, Z.; Li, J.; Wu, Q. Synthesis and bioactivities of phenazine-1-carboxylic acid derivatives based on the modification of PCA carboxyl group. Bioorg. Med. Chem. Lett. 2017, 27, 2010–2013. [Google Scholar] [CrossRef]
- Zhang, J.F.; Zhao, W.J.; Fu, R.; Fu, C.L.; Wang, L.X.; Liu, H.N.; Li, S.C.; Deng, Q.M.; Wang, S.Q.; Zhu, J.; et al. Comparison of gene co-networks reveals the molecular mechanisms of the rice (Oryza sativa L.) response to Rhizoctonia solani AG1 IA infection. Funct. Integr. Genom. 2018, 5, 545–557. [Google Scholar] [CrossRef]
- Yan, X.; Qin, W.; Sun, L.; Qi, S.H.; Yuan, H.Z. Study of inhibitory effects and action mechanism of the novel fungicide pyrimorph against Phytophthora capsici. J. Agric. Food. Chem. 2010, 58, 2720–2725. [Google Scholar] [CrossRef] [PubMed]
- Jin, P.F.; Wang, H.N.; Tan, Z.; Xuan, Z.; Dahar, G.Y.; Li, Q.X.; Miao, W.; Liu, W. Antifungal mechanism of bacillomycin D from Bacillus velezensis HN-2 against Colletotrichum gloeosporioides Penz. Pestic. Biochem. Physiol. 2020, 163, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.W.; Bao, Y.; Cao, J.J.; Zhang, H.L.; Chen, L.M. Transcriptome analysis on symbiotic molecular mechanism of Armillaria mellea and Gastrodia elata. Chin. Tradit. Herb. Drugs 2018, 49, 4125–4130. [Google Scholar]
- Chen, S.; Zhou, Y.Q.; Chen, Y.R.; Gu, J. Fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 17, i1884–i1890. [Google Scholar] [CrossRef] [PubMed]
- Sirén, J.; Välimäki, N.; Mäkinen, V. Indexing graphs for path queries with applications in genome research. IEEE/ACM Trans. Comput. Biol. Bioinf. 2014, 11, 375–388. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Langmead, B.; Salzberg, S.L. HISAT: A fast spliced aligner with low memory requirements. Nat. Methods 2015, 12, 357–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pertea, M.; Pertea, G.M.; Antonescu, C.M.; Chang, T.C.; Mendell, J.T.; Salzberg, S.L. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads Nature Biotechnology. Nat. Biotechnol. 2015, 3, 290–295. [Google Scholar] [CrossRef] [Green Version]
- Pertea, M.; Kim, D.; Pertea, G.M.; Leek, J.T.; Salzberg, S.L. Transcript-level expression analysis of RNA-seq experiments with HISAT, StringTie and Ballgown. Nat. Protoc. 2016, 11, 1650–1667. [Google Scholar] [CrossRef]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [Green Version]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 10, R106. [Google Scholar] [CrossRef] [Green Version]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. ClusterProfiler: An R package for comparing biological themes among gene clusters. OMICS 2012, 5, 284–287. [Google Scholar] [CrossRef]
- Jiang, Y.M. Incidence of anthracnose in relation to chitinase, β-1,3-glucanase and dopamine of banana fruits after harvest. Acta Phytophysiol. Sin. 1997, 2, 158–162. [Google Scholar]
- Latoud, C.; Peypoux, F.; Michel, G. Action of iturin A, an antifungal antibiotic from Bacillus subtilis, on the yeast Saccharomyces cerevisiae: Modifications of membrane permeability and lipid composition. J. Antibiot. 1987, 11, 1588–1895. [Google Scholar] [CrossRef] [Green Version]
- Wei, S.J. Research on the Disease Prevention Potential and Mechanism of Streptomyces 702 on Rice; Jiangxi Agricultural University: Nanchang, China, 2013. [Google Scholar]
- Liu, L.R.; Miao, J.J.; Zhao, A.R.; Pan, L.Q. Understanding Homology Modeling and Molecular Docking of Bivalve Estrogen Receptors. Period. Ocean. Univ. China 2021, 51, 17–25. [Google Scholar]
- Liu, X.; Li, W.; Hu, B.C.; Wang, M.X.; Wang, J.; Guan, L.J. Identification of isobavachalcone as a potential drug for rice blast disease caused by the fungus Magnaporthe grisea. J. Biomol. Struct. Dyn. 2019, 37, 3399–3409. [Google Scholar] [CrossRef]
- Yang, C.; Hamel, C.; Vujanovic, V.; Gan, Y. Fungicide: Modes of action and possible impact on nontarget microorganisms. ISRN Ecol. 2011, 130289, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Deising, H.B.; Reimann, S.; Pascholati, S.F. Mechanisms and significance of fungicide resistance. Braz. J. Microbiol. 2008, 2, 286–295. [Google Scholar] [CrossRef] [Green Version]
- Kataoka, S.; Takagaki, M.; Kaku, K.; Shimizu, T. Mechanism of action and selectivity of a novel fungicide, pyribencarb. J. Pestic. Sci. 2010, 2, 99–106. [Google Scholar] [CrossRef] [Green Version]
- Young, D.H.; Wang, N.X.; Meyer, S.T.; Avlia-Adame, C. Characterization of the mechanism of action of the fungicide fenpicoxamid and its metabolite UK-2A. Pest Manag. Sci. 2018, 2, 489–498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, H.; Sun, L.; Bi, K.; Zhong, G.; Hu, M. The effect of phenazine-1-carboxylic acid on the morphological, physiological, and molecular characteristics of Phellinus noxius. Molecules 2016, 5, 613. [Google Scholar] [CrossRef] [Green Version]
- Shao, X.; Cheng, S.; Wang, H.; Yu, D.; Mungal, C. The possible mechanism of antifungal action of tea tree oil on Botrytis cinerea. J. Appl. Microbiol. 2013, 6, 1642–1649. [Google Scholar] [CrossRef]
- He, L.; Yuan, H.; Tang, J.; Yang, D.; Yan, X. Preliminary studies on the action mechanism of the novel fungicide LH-2010A against Phytophthora capsici. Chin. J. Pestic. Sci. 2016, 18, 185–193. [Google Scholar]
- Xiang, Y.; Zhang, Y.; Wang, C.; Liu, S.Q.; Liao, X.L. Effects and inhibition mechanism of phenazine-1-carboxamide on the mycelial morphology and ultrastructure of Rhizoctonia solani. Pestic. Biochem. Physiol. 2018, 147, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Pham, T.A.T.; Kyriacou, B.A.; Schwerdt, J.G.; Shirley, N.J.; Xing, X.H.; Bulone, V. Alan Little Composition and biosynthetic machinery of the Blumeria graminis f. sp. hordei conidia cell wall. Cell Surf. 2019, 5, 100029. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez-Armijos, L.R.; Sussmann, R.A.C.; Silber, A.M.; Cortez, M.; Hernandez, A. Abnormal sterol-induced cell wall glucan deficiency in yeast is due to impaired glucan synthase transport to the plasma membrane. Biochem. J. 2020, 24, 4729–4744. [Google Scholar] [CrossRef] [PubMed]
- Burden, R.S.; Cooke, D.T.; Hargreaves, J.A. Mechanism of action of herbicidal and fungicidal compounds on cell membranes. Pestic. Sci. 1990, 30, 125–140. [Google Scholar] [CrossRef]
- Han, Z.Y.; Tian, P.F. Applications of KEGG database in research of biosynthesis. Biotechnol. Bull. 2011, 1, 76–82. [Google Scholar]
- Li, X.Z.; Li, Z.P.; Li, J.; Fang, H.S. Recent progress and application of KEGG database in the research of bioinformatics. Pharm. Biotechnol. 2012, 6, 535–539. [Google Scholar]
- Morales, D.K.; Jacobs, N.J.; Rajamani, S.; Krishnamurthy, M.; Cubillos-Ruiz, J.R.; Hogan, D.A. Antifungal mechanisms by which a novel Pseudomonas aeruginosa phenazine toxin kills Candida albicans in biofilms. Mol. Microbiol. 2010, 6, 1379–1392. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Zeng, Y.; Zhao, X.; Zou, S.S.; He, Y.W.; Liang, Y.H. A genetic screen in combination with biochemical analysis in Saccharomyces cerevisiae indicates that phenazine-1-carboxylic acid is harmful to vesicular trafficking and autophagy. Sci. Rep. 2017, 1, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pan, J.L. Antifungal Mechanism of Matrine to Botryosphaeria Dothidea; Northeast Forestry University: Halbin, China, 2018. [Google Scholar]
- Qiu, M.; Li, Y.; Ye, W.; Zheng, X.B.; Wang, Y.C. A CRISPR/Cas9-mediated in situ complementation method for Phytophthora sojae mutants. Mol. Plant Pathol. 2021, 3, 373–381. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).