Plant-Based Protein Hydrolysate Improves Salinity Tolerance in Hemp: Agronomical and Physiological Aspects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Setting and Design
2.2. Crop Management, Saline Irrigation and Biostimulant Application
2.3. Soil Electrical Conductivity Measurements
2.4. Yield Measurements
2.5. Chlorophyll Fluorescence Measurements
2.6. Statistics
3. Results
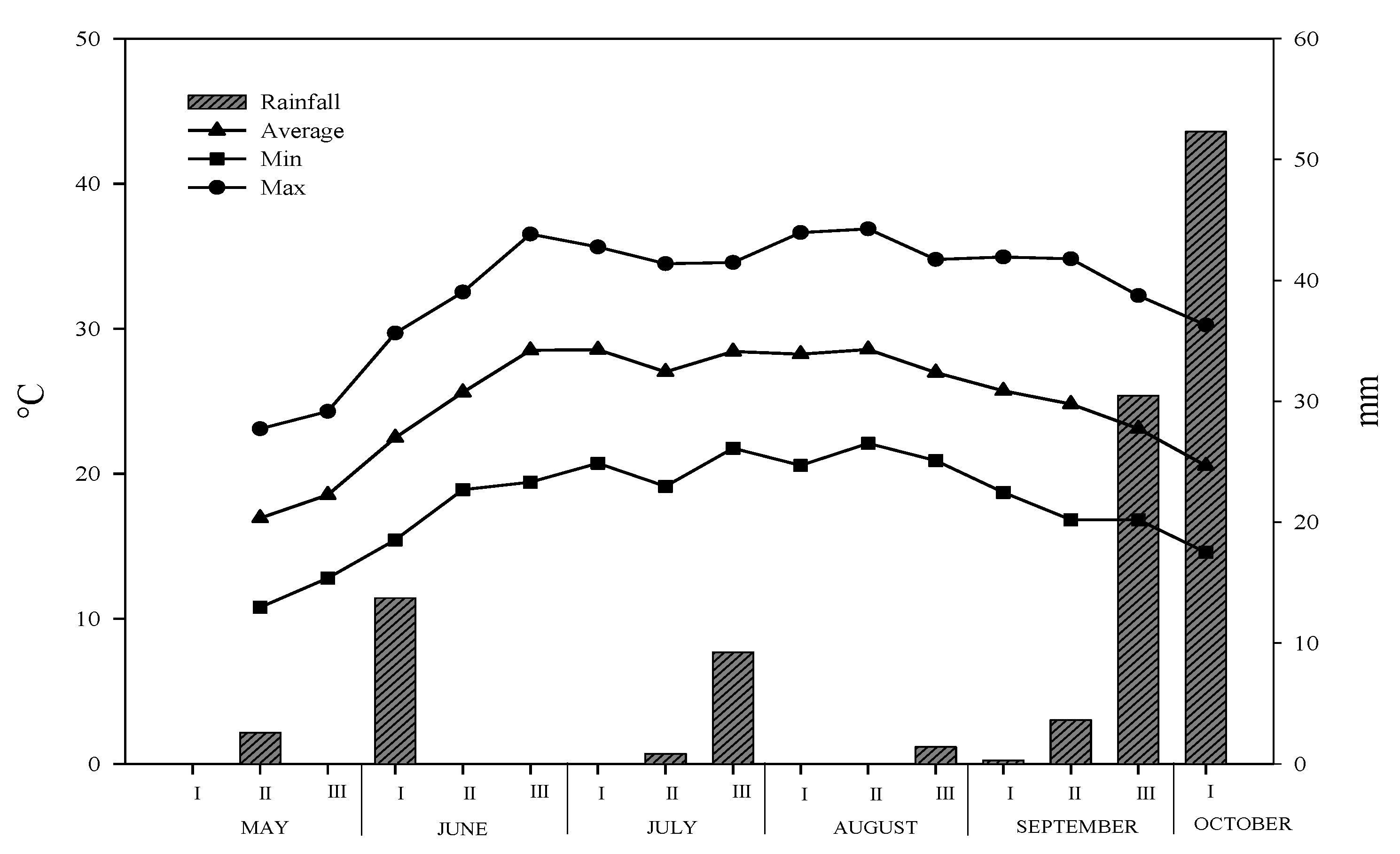
3.1. Climate Characteristics of Experimental Site
3.2. Electrical Conductivity of Soil
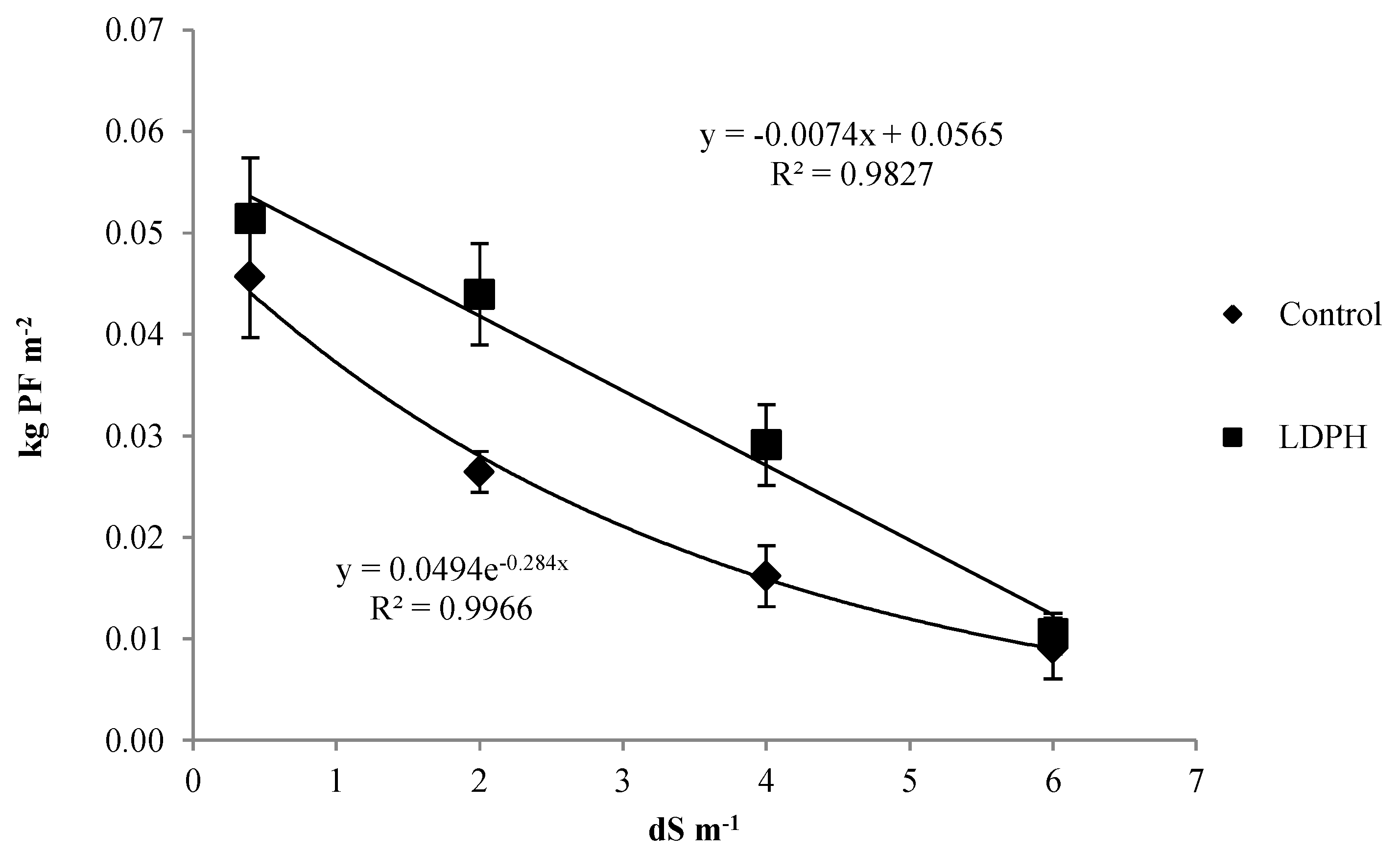
3.3. Seeds Production and Its Parameters
3.4. Residual Biomass and Its Parameters
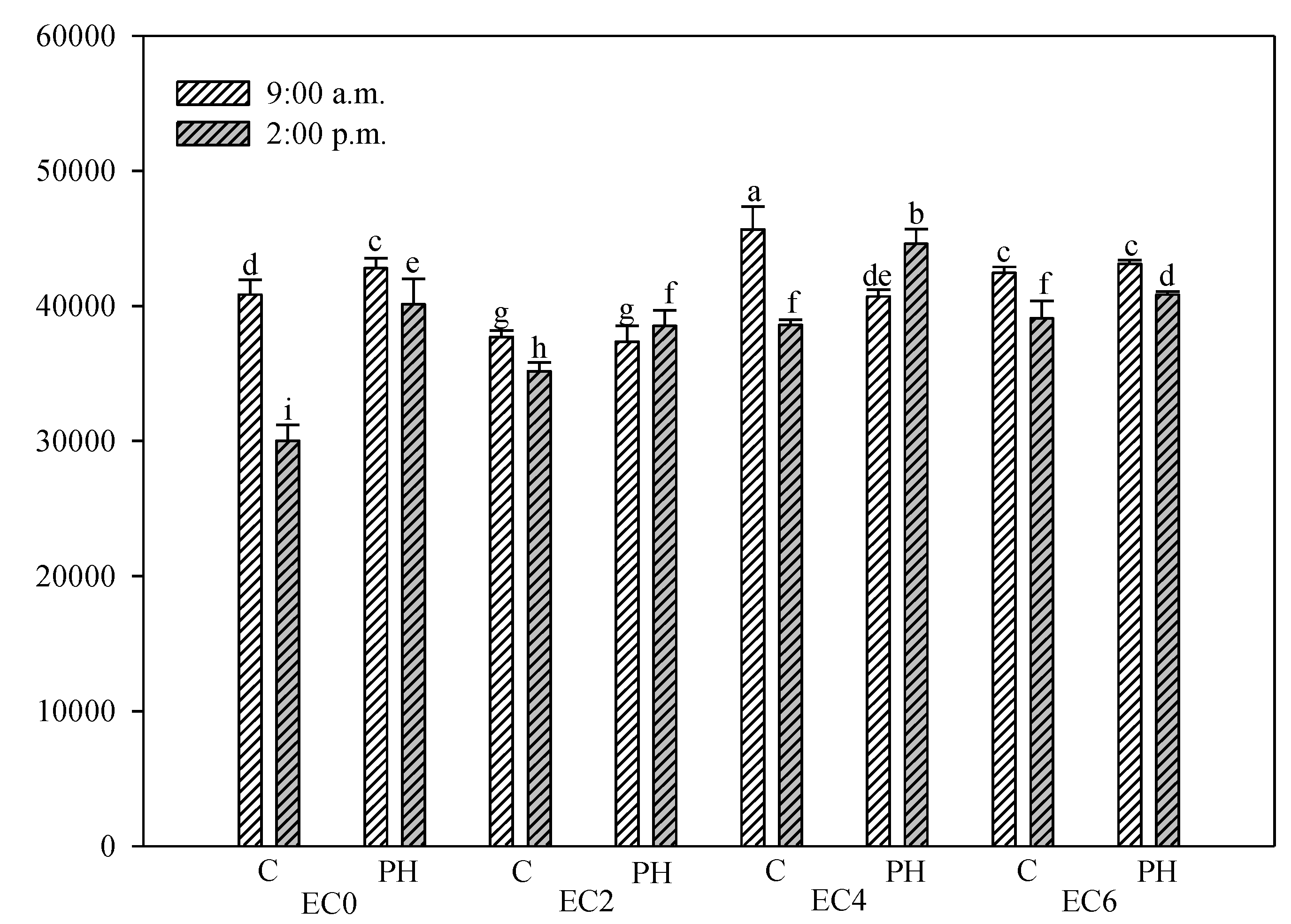
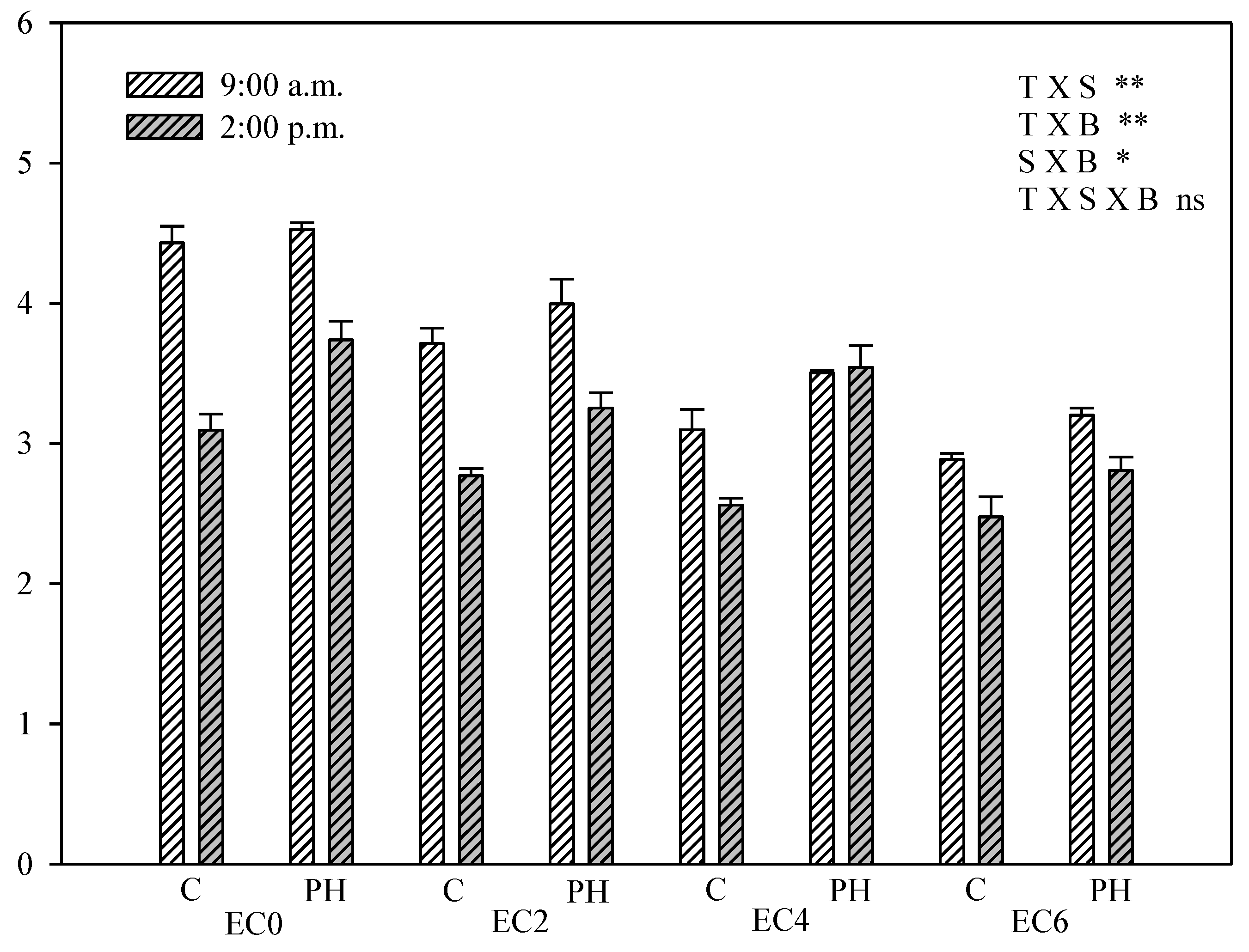
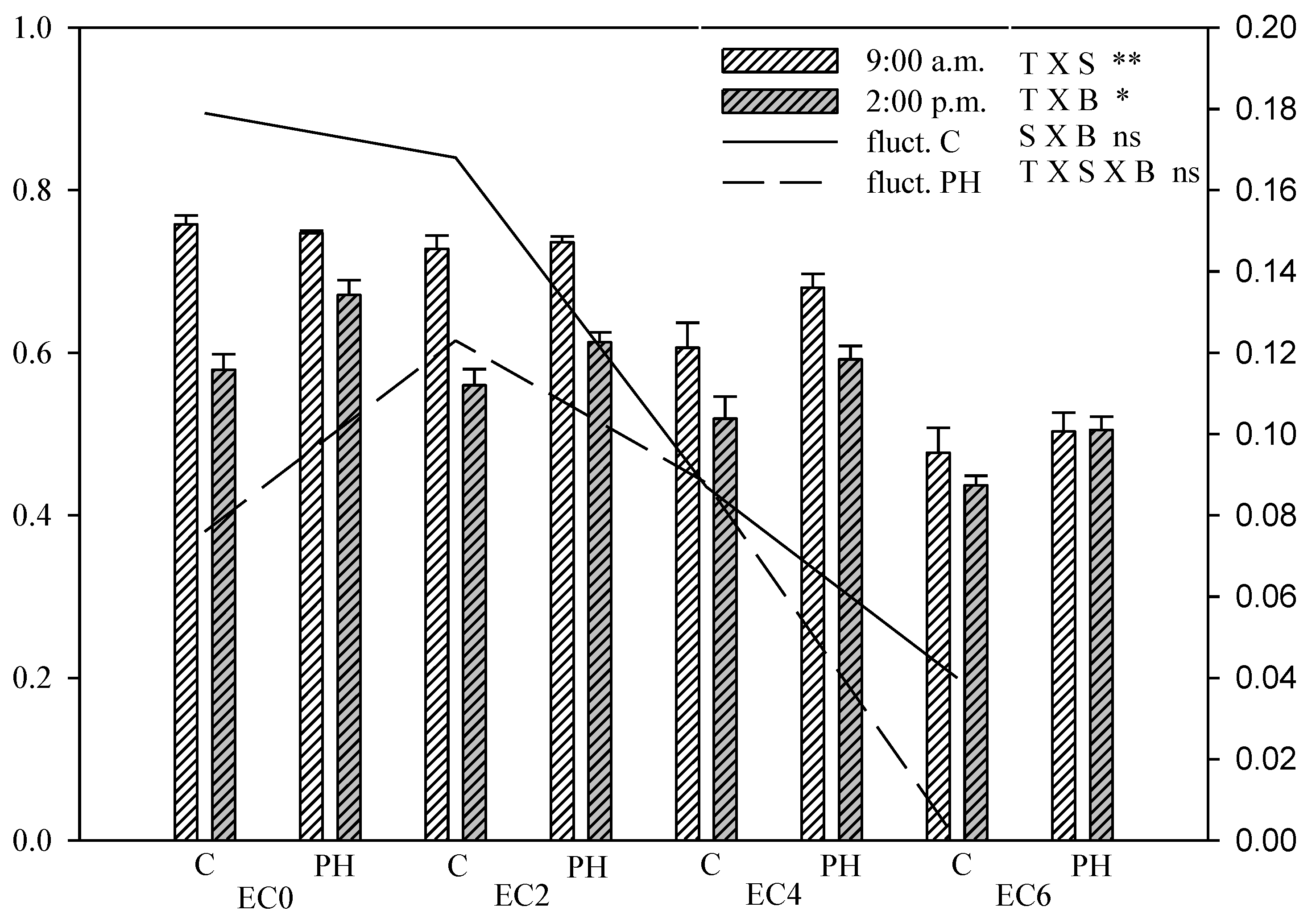
3.5. Chlorophyll Fluorescence Measurements
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EC | electrical conductivity |
| PH | protein hydrolysate |
| LDPH | legume-derived protein hydrolysate |
| Chl a | chlorophyll a |
| DAS | dark-adapted state |
| LAS | light adapted state |
References
- Karus, M.; Vogt, D. European hemp industry: Cultivation, processing and product lines. Euphytica 2004, 140, 7–12. [Google Scholar] [CrossRef]
- Jarabo, R.; Fuente, E.; Monte, M.C.; Savastano, H., Jr.; Mutjé, P.; Negro, C. Use of cellulose fibres from hemp core in fibre-cement production. Effect on flocculation, retention, drainage and product properties. Ind. Crops Prod. 2012, 39, 89–96. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Wang, X.; Wang, L. Properties of hemp fibre reinforced concrete composites. Compos. Part A Appl. Sci. Manuf. 2006, 37, 497–505. [Google Scholar] [CrossRef] [Green Version]
- Elfordy, S.; Lucas, F.; Tancret, F.; Scudeller, Y.; Goudet, L. Mechanical and thermal properties of lime and hemp concrete (‘hempcrete’) manufactured by a projection process. Constr. Build. Mater. 2008, 22, 2116–2123. [Google Scholar] [CrossRef]
- Bénézet, J.C.; Stanojlovic-Davidovic, A.; Bergeret, A.; Ferry, L.; Crespy, A. Mechanical and physical properties of expanded starch, reinforced by naturalfibres. Ind. Crops Prod. 2012, 37, 435–440. [Google Scholar]
- Holbery, J.; Houston, D. Natural-fibre-reinforced polymer composites in auto-motive applications. J. Miner. Met. Mater. Soc. 2006, 58, 80–86. [Google Scholar] [CrossRef]
- Kreuger, E.; Prade, T.; Escobar, F.; Svensson, S.E.; Englund, J.E.; Björnsson, L. Anaerobic digestion of industrial hemp—Effect of harvest time on methane energy yield per hectare. Biomass Bioenergy 2011, 35, 893–900. [Google Scholar] [CrossRef]
- Prade, T.; Svensson, S.E.; Andersson, A.; Mattsson, J.A. Biomass and energy yield of industrial hemp grown for biogas and solid fuel. Biomass Bioenergy 2011, 35, 3040–3049. [Google Scholar] [CrossRef]
- Sipos, B.; Kreuger, E.; Svensson, S.E.; Réczey, K.; Björnsson, L.; Guido, Z. Steam pre-treatment of dry and ensiled industrial hemp for ethanol production. Biomass Bioenergy 2010, 34, 1721–1731. [Google Scholar] [CrossRef] [Green Version]
- Aluru, M.; Kukk, L.; Astover, A.; Shanskiy, M.; Loit, E. An agro-economic analysis of briquette production from fibre hemp and energy sunflower. Ind. Crops Prod. 2013, 51, 186–193. [Google Scholar] [CrossRef]
- Callaway, J.C. Hempseed as a nutritional resource. Euphytica 2004, 140, 65–72. [Google Scholar] [CrossRef]
- Matthäus, B.; Brühl, L. Virgin hemp seed oil: An interesting niche product. Eur. J. Lipid Sci. Technol. 2008, 110, 655–661. [Google Scholar] [CrossRef]
- Hessle, A.; Eriksson, M.; Nadeau, E.; Turner, T.; Johansson, B. Cold-pressed hempseed cake as a protein feed for growing cattle. Acta Agric. Scand. A Anim. Sci. 2008, 58, 136–145. [Google Scholar] [CrossRef]
- Goldberg, E.M.; Gakhar, N.; Ryland, D.; Aliani, M.; Gibson, R.A.; House, J.D. Fatty acid profile and sensory characteristics of table eggs from laying hens fed hempseed and hempseed oil. J. Food Sci. 2012, 77, 153–160. [Google Scholar] [CrossRef]
- Sapino, S.; Carlotti, M.E.; Peira, E.; Gallarate, M. Hemp-seed and olive oils: Their stability against oxidation and use in O/W emulsions. J. Cosmet. Sci. 2005, 56, 227–251. [Google Scholar] [CrossRef] [PubMed]
- Vogl, C.R.; Mölleken, H.; Lissek-Wolf, G.; Surböck, A.; Kobert, J. Hemp (Cannabis sativa L.) as a resource for green cosmetics: Yield of seed and fatty acid compositions of 20 varieties under the growing conditions of organic farming in Austria. J. Ind. Hemp 2004, 9, 51–68. [Google Scholar] [CrossRef]
- Appendino, G.; Gibbons, S.; Giana, A.; Pagani, A.; Grassi, G.; Stavri, M.; Smith, E.; Rahman, M.M. Antibacterial cannabinoids from Cannabis sativa: Astructure–activity study. J. Nat. Prod. 2008, 71, 1427–1430. [Google Scholar] [CrossRef] [PubMed]
- Mukhtar, T.; Kayani, M.Z.; Hussain, M.A. Nematicidal activities of Cannabissativa L. and Zanthoxylum alatum Roxb. against Meloidogyne incognita. Ind. Crops Prod. 2013, 42, 447–453. [Google Scholar] [CrossRef]
- Amaducci, S.; Zatta, A.; Raffanini, M.; Venturi, G. Characterisation of hemp (Cannabis sativa L.) roots under different growing conditions. Plant Soil 2008, 313, 227–235. [Google Scholar] [CrossRef]
- Cheng, X.; Deng, G.; Su, Y.; Liu, J.J.; Yang, Y.; Du, G.H.; Chen, Z.Y.; Liu, F.H. Protein mechanisms in response to NaCl-stress of salt-tolerant and salt-sensitive industrial hemp based on iTRAQ technology. Ind. Crops Prod. 2016, 83, 444–452. [Google Scholar] [CrossRef]
- Food and Agriculture Organization of the United Nations. World Soil Resources Reports No.106. Rome: International Soil Classification System for Naming Soils and Creating Legends for Soil Maps. Available online: http://www.opengrey.eu/item/display/10068/310015 (accessed on 25 November 2020).
- Rozema, J.; Flowers, T. Crops for a salinized world. Science 2008, 322, 1478–1480. [Google Scholar] [CrossRef]
- Brady, N.C.; Weil, R.R. The Nature and Properties of Soils, 14th ed.; Prentice-Hall: Upper Saddle River, NJ, USA, 2008. [Google Scholar]
- Machado, R.; Serralheiro, R. Soil salinity: Effect on vegetable crop growth. Management practices to prevent and mitigate soil salinization. Horticulturae 2017, 3, 30. [Google Scholar] [CrossRef]
- Di Mola, I.; Guida, G.; Mistretta, C.; Giorio, P.; Albrizio, R.; Visconti, D.; Fagnano, M.; Mori, M. Agronomic and physiological response of giant reed (Arundo donax L.) to soil salinity. Ital. J. Agron. 2018, 13, 31–39. [Google Scholar] [CrossRef] [Green Version]
- Munns, R. Genes and salt tolerance: Bringing them together. New Phytol. 2017, 167, 645–663. [Google Scholar] [CrossRef] [PubMed]
- Ntatsi, G.K.A.; Aliferis, Y.; Rouphael, F.; Napolitano, K.; Makris, G.; Kalala, G.; Katopodis, G.; Savvas, D. Salinity source alters mineral composition and metabolism of Cichorium spinosum. Environ. Exp. Bot. 2017, 141, 113–123. [Google Scholar] [CrossRef]
- Akram, N.A.; Shafiq, F.; Ashraf, M.; Iqbal, M.; Ahmad, P. Advances in Salt Tolerance of Some Major Fiber Crops Through Classical and Advanced Biotechnological Tools: A Review. J. Plant Growth Regul. 2020. [Google Scholar] [CrossRef]
- Rouphael, Y.; Cardarelli, M.; Bassal, A.; Leonardi, C.; Giuffrida, F.; Colla, G. Vegetable quality as affected by genetic, agronomic and environmental factors. J. Food Agric. Environ. 2012, 10, 680–688. [Google Scholar]
- Rouphael, Y.; Cardarelli, M.; Bonini, P.; Colla, G. Synergistic Action of a Microbial-based Biostimulant and a Plant Derived-Protein Hydrolysate Enhances Lettuce Tolerance to Alkalinity and Salinity. Front. Plant Sci. 2017, 8, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stirbet, A.; Lazar, D.; Kromdijk, J. Chlorophyll a fluorescence induction: Can just a one-second measurement be used to quantify abiotic stress responses? Photosynthetica 2018, 56, 86–104. [Google Scholar] [CrossRef]
- Rastogi, A.; Kovar, M.; He, X.; Zivcak, M.; Kataria, S.; Kalaji, H.M.; Skalicky, M.; Ibrahimova, U.F.; Hussain, S.; Mbarki, S.; et al. JIP-test as a tool to identify salinity tolerance in sweet sorghum genotypes. Photosynthetica 2019, 58, 518–528. [Google Scholar] [CrossRef] [Green Version]
- Di Mola, I.; Rouphael, Y.; Colla, G.; Fagnano, M.; Paradiso, R.; Mori, M. Morpho physiological traits and nitrate content of greenhouse lettuce as affected by irrigation with saline water. HortScience 2017, 52, 1716–1721. [Google Scholar] [CrossRef] [Green Version]
- Mori, M.; Amato, M.; Di Mola, I.; Caputo, R.; Quaglietta Chiaranda, F.; Di Tommaso, T. Productive behaviour of “cherry”-type tomato irrigated with saline water in relation to nitrogen fertilization. Eur. J. Agron. 2008, 29, 135–143. [Google Scholar] [CrossRef]
- Mori, M.; Di Mola, I.; Quaglietta Chiarandà, F. Salt stress and transplant time in snap bean: Growth and productive behaviour. Int. J. Plant Prod. 2011, 5, 49–64. [Google Scholar]
- Parida, A.K.; Das, A.B. Salt tolerance and salinity effects on plants: A review. Ecotoxicol. Environ. Safe 2005, 60, 324–349. [Google Scholar] [CrossRef] [PubMed]
- Acosta-Motos, J.R.; Ortuño, M.F.; Bernal-Vicente, A.; Diaz-Vivancos, P.; Sanchez-Blanco, M.J.; Hernandez, J.A. Responses to salt stress: Adaptive mechanisms. Agronomy 2017, 7, 18. [Google Scholar] [CrossRef] [Green Version]
- Colla, G.; Rouphael, Y.; Cardarelli, M.; Rea, E. Effect of salinity on yield, fruit quality, leaf gas exchange, and mineral composition of grafted watermelon plants. HortScience 2006, 41, 622–627. [Google Scholar] [CrossRef] [Green Version]
- Rouphael, Y.; Cardarelli, M.; Rea, E.; Battistelli, A.; Colla, G. Comparison of the subirrigation and drip-irrigation systems for greenhouse zucchini squash production using saline and non-saline nutrient solution. Agric. Water Manag. 2006, 82, 99–117. [CrossRef]
- Wungrampha, S.; Joshi, R.; Singla-Pareek, S.L.; Pareek, A. Photosynthesis and salinity: Are these mutually exclusive? Photosynthetica 2018, 56, 366–381. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef]
- Roháček, K.; Soukupová, J.; Barták, M. Chlorophyll fluorescence: A wonderful tool to study plant physiology and plant stress. In Plant Cell Compartments—Selected Topics; Schoefs, B., Ed.; Research Signpost: Kerala, India, 2008; pp. 41–104. ISBN 978-81-308-0104-9. [Google Scholar]
- Kalaji, H.M.; Goltsev, V.N.; Zuk-Golaszewska, K.; Zivcak, M.; Brestic, M. Chlorophyll Fluorescence: Understanding Crop Performance—Basics and Applications; CRC Press: Boca Raton, FL, USA, 2017; p. 222. [Google Scholar]
- Govindjee, G. Chlorophyll a Fluorescence: A Bit of Basics and History. In Chlorophyll a Fluorescence: A Signature of Photosynthesis. Advances in Photosynthesis and Respiration; Papageorgiou, G.C., Govindjee, G., Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 1–42. [Google Scholar]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the chlorophyll a fluorescence transient. In Chlorophyll a Fluorescence: A Signature of Photosynthesis. Advances in Photosynthesis and Respiration; Papageorgiou, G.C., Govindjee, G., Eds.; Springer: Dordrecht, The Netherlands, 2004; pp. 321–362. [Google Scholar]
- Stirbet, A.; Riznichenko, G.Y.; Rubin, A.B. Modeling Chlorophyll a Fluorescence Transient: Relation to Photosynthesis. Biochemistry (Moscow) 2014, 79, 291–323. [Google Scholar] [CrossRef]
- Strasser, R.J. On the O-J-I-P transient in leaves and D1 mutants of Chlamydomonas reinhardtii. In Research in Photosynthesis; Murata, N., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1992; Volume 2, pp. 29–32. [Google Scholar]
- Tsimilli-Michael, M. Revisiting JIP-test: An educative review on concepts, assumptions, approximations, definitions and terminology. Photosynthetica 2019, 1, 90–107. [Google Scholar] [CrossRef] [Green Version]
- Kalaji, H.M.; Bosa, K.; Koscielniak, J.; Zuk-Golaszewska, K. Effects of salt stress on photosystem II efficiency and CO2 assimilation of two Syrian barley landraces. Environ. Exp. Bot. 2011, 73, 64–72. [Google Scholar] [CrossRef]
- Genty, B.; Briantais, J.M.; Baker, N.R. The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 1989, 990, 87–92. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J.; Pinheiro, C. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef] [Green Version]
- Allakhverdiev, S.I.; Murata, N. Salt stress inhibits photosystem II and I in cynobacteria. Photosynth. Res. 2008, 98, 529–539. [Google Scholar] [CrossRef] [PubMed]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef] [Green Version]
- Consentino, B.B.; Virga, G.; La Placa, G.G.; Sabatino, L.; Rouphael, Y.; Ntatsi, G.; Iapichino, G.; La Bella, S.; Mauro, R.P.; D’Anna, F.; et al. Celery (Apium graveolens L.) Performances as Subjected to Different Sources of Protein Hydrolysates. Plants 2020, 9, 1633. [Google Scholar] [CrossRef]
- Dell’Aversana, E.; D’Amelia, L.; De Pascale, S.; Carillo, P. Use of Biostimulants to Improve Salinity Tolerance in Agronomic Crops. In Agronomic Crops; Hasanuzzaman, M., Ed.; Springer: Singapore, 2020. [Google Scholar] [CrossRef]
- Colla, G.; Hoagland, L.; Ruzzi, M.; Cardarelli, M.; Bonini, P.; Canaguier, R.; Rouphael, Y. Biostimulant Action of Protein Hydrolysates: Unraveling Their Effects on Plant Physiology and Microbiome. Front. Plant Sci. 2017, 8, 2202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Di Mola, I.; Cozzolino, E.; Ottaiano, L.; Nocerino, S.; Rouphael, Y.; Colla, G.; El-Nakhel, C.; Mori, M. Nitrogen Use and Uptake Efficiency and Crop Performance of Baby Spinach (Spinacia oleracea L.) and Lamb’s Lettuce (Valerianella locusta L.) Grown under Variable Sub-Optimal N Regimes Combined with Plant-Based Biostimulant Application. Agronomy 2020, 10, 278. [Google Scholar] [CrossRef] [Green Version]
- Di Mola, I.; Cozzolino, E.; Ottaiano, L.; Giordano, M.; Rouphael, Y.; Colla, G.; Mori, M. Effect of vegetal- and seaweed extract-based biostimulants on agronomical and leaf quality traits of plastic tunnel-grown baby lettuce under four regimes of nitrogen fertilization. Agronomy 2019, 9, 571. [Google Scholar] [CrossRef] [Green Version]
- Di Mola, I.; Ottaiano, L.; Cozzolino, E.; Senatore, M.; Giordano, M.; El-Nakhel, C.; Sacco, A.; Rouphael, Y.; Colla, G.; Mori, M. Plant-Based Biostimulants Influence the Agronomical, Physiological, and Qualitative Responses of Baby Rocket Leaves under Diverse Nitrogen Conditions. Plants 2019, 8, 522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rouphael, Y.; Giordano, M.; Cardarelli, M.; Cozzolino, E.; Mori, M.; Kyriacou, M.; Bonini, P.; Colla, G. Plant and seaweed-based extracts increase yield but differentially modulate nutritional quality of greenhouse spinach through biostimulant action. Agronomy 2018, 8, 126. [Google Scholar] [CrossRef] [Green Version]
- Björkman, O.; Demmig, B. Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77 K among vascular plants of diverse origins. Planta 1987, 170, 489–504. [Google Scholar] [CrossRef] [PubMed]
- Pimentel, D.; Berger, B.; Filiberto, D.; Newton, M.; Wolfe, B.; Karabinakis, E.; Clark, S.; Poon, E.; Abbett, E.; Nandagopal, S. Water resources: Agricultural and environmental issues. Bioscience 2004, 54, 909–918. [Google Scholar] [CrossRef] [Green Version]
- Okur, B.; Örçen, N. Soil Salinization and Climate Change. In Climate Change and Soil Interactions; Elsevier: Amsterdam, The Netherlands, 2020; pp. 331–350. [Google Scholar]
- Benazzouk, S.; Dobrev, P.I.; Djazouli, Z.E.; Motyka, V.; Lutts, S. Positive impact of vermicompost leachate on salt stress resistance in tomato (Solanum lycopersicum L.) at the seedling stage: Aphytohormonal approach. Plant Soil 2020, 446, 145–162. [Google Scholar] [CrossRef]
- Hu, H.; Liu, H.; Liu, F. Seed germination of hemp (Cannabis sativa L.) cultivars responds differently to the stress of salt type and concentration. Ind. Crops Prod. 2018, 123, 254–261. [Google Scholar] [CrossRef]
- Lucini, L.; Rouphael, Y.; Cardarelli, M.; Canaguier, R.; Kumar, P.; Colla, G. The effect of a plant-derived biostimulant on metabolic profiling and crop performance of lettuce grown under saline conditions. Sci. Hortic. 2015, 182, 124–133. [Google Scholar] [CrossRef]
- Ertani, A.; Schiavon, M.; Muscolo, A.; Nardi, S. Alfalfa plant-derived biostimulant stimulate short-term growth of salt stressed Zea mays L. plants. Plant Soil 2013, 364, 145–158. [Google Scholar] [CrossRef]
- Hu, H.; Liu, H.; Du, G.; Fei, Y.; Deng, G.; Yang, Y.; Feihu, L. Fiber and seed type of hemp (Cannabis sativa L.) responded differently to salt-alkali stress in seedling growth and physiological indices. Ind. Crops Prod. 2019, 129, 624–630. [Google Scholar] [CrossRef]
- Maggio, A.; De Pascale, S.; Fagnano, M.; Barbieri, G. Saline agriculture in Mediterranean environments. Ital. J. Agron. 2011, 6, 36–43. [Google Scholar] [CrossRef]
- Colla, G.; Rouphael, Y.; Leonardi, C.; Bie, Z. Role of grafting in vegetable crops grown under saline conditions. Sci. Hortic. 2010, 127, 147–155. [Google Scholar] [CrossRef]
- Munns, R.; Gilliham, M. Salinity tolerance of crops—What is the cost? New Phytol. 2015, 208, 668–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in plant science: A global perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, C.; Mou, B. Drench application of fish-derived protein hydrolysates affects lettuce growth, chlorophyll content, and gas exchange. HortTechnology 2017, 27, 539–543. [Google Scholar] [CrossRef]
- Paul, K.; Sorrentino, M.; Lucini, L.; Rouphael, Y.; Cardarelli, M.; Bonini, P.; Reynaud, H.; Canaguier, R.; Trtiílek, M.; Panzarovaá, K.; et al. Understanding the Biostimulant Action of Vegetal-Derived Protein Hydrolysates by High-Throughput Plant Phenotyping and Metabolomics: A Case Study on Tomato. Front. Plant Sci. 2019, 10, 47. [Google Scholar] [CrossRef]
- Botta, A. Enhancing plant tolerance to temperature stress with amino acids: An approach to their mode of action. Acta Hortic. 2013, 1009, 29–35. [Google Scholar] [CrossRef]
- Kauffman, G.L., III; Kneival, D.P.; Watschke, T.L. Effects of biostimulant on the heat tolerance associated with photosynthetic capacity, membrane thermostability, and polyphenol production of perennial ryegrass. Crop Sci. 2007, 47, 261–267. [Google Scholar] [CrossRef]
- Haldimann, P.; Fracheboud, Y.; Stamp, P. Photosynthetic performance and resistance to photoinhibition of Zea mays L. leaves grown at sub-optimal temperature. Plant Cell Environ. 1996, 19, 85–92. [Google Scholar] [CrossRef]
- González, A.; Tezara, W.; Rengifo, E.; Herrera, A. Ecophysiological responses to drought and salinity in the cosmopolitan invader Nicotiana glauca. Braz. J. Plant Physiol. 2012, 24, 213–222. [Google Scholar]
- Tattini, M.; Sebastiani, F.; Brunetti, C.; Fini, A.; Torre, S.; Gori, A.; Centritto, M.; Ferrini, F.; Landi, M.; Guidi, L. Dissecting molecular and physiological response mechanisms to high solar radiation in cyanic and acyanic leaves: A case study on red and green basil. J. Exp. Bot. 2017, 68, 2425–2437. [Google Scholar] [CrossRef]
- Zribi, L.; Gharbi, F.; Rezgui, F.; Rejeb, S.; Nahdi, H.; Rejeb, M.N. Application of chlorophyll fluorescence for the diagnosis of salt stress in tomato “Solanum lycopersicum (variety Rio Grande)”. Sci. Hortic. 2009, 120, 367–372. [Google Scholar] [CrossRef]
- Lu, K.X.; Cao, B.H.; Feng, X.P.; He, Y.; Jiang, D.A. Photosynthetic response of salt tolerant and sensitive soybean varieties. Photosynthetica 2009, 47, 381–387. [Google Scholar] [CrossRef]
- Hanachi, S.; Van Labeke, M.C.; Mehouachi, T. Application of chlorophyll fluorescence to screen eggplant (Solanum melongena L.) cultivars for salt tolerance. Photosynthetica 2014, 52, 57–62. [Google Scholar] [CrossRef]
- He, Y.; Zhu, Z.; Yang, J.; Ni, X.; Zhu, B. Grafting increases the salt tolerance of tomato by improvement of photosynthesis and enhancement of antioxidant enzymes activity. Environ. Exp. Bot. 2009, 66, 270–278. [Google Scholar] [CrossRef]
- Lutts, S.; Kinet, J.M.; Bouharmont, J. NaCl-induced senescence in leaves of rice (Oryza sativa L.) cultivars differing in salinity resistance. Ann. Bot. 1996, 78, 389–398. [Google Scholar] [CrossRef]
- Aro, E.M.; Virgin, I.; Andersson, B. Photoinhibition of photosystem II. Inactivation, protein damage and turnover. Biochim. Biophys. Acta 1993, 1143, 113–134. [Google Scholar] [CrossRef]
- Banks, J.M. Continuous excitation chlorophyll fluorescence parameters: A review for practitioners. Tree Physiol. 2017, 37, 1128–1136. [Google Scholar] [CrossRef]
- Gao, Y.; Lu, Y.; Wu, M.; Liang, E.; Li, Y.; Zhang, D.; Yin, Z.; Ren, X.; Dai, Y.; Deng, D.; et al. Ability to remove Na+ and retain K+ correlates with salt tolerance in two maize inbred lines seedlings. Front. Plant Sci. 2016, 7, 1716. [Google Scholar] [CrossRef] [Green Version]
- Zaghdoudi, M.; Msilini, N.; Govindachary, S.; Lachaâl, M.; Ouerghi, Z.; Carpentier, R. Inhibition of photosystems I and II activities in salt stress-exposed Fenugreek (Trigonella foenum graecum). J. Photochem. Photobiol. 2011, 105, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Demmig-Adams, B.; Adams, W.W., III. Photoprotection and other responses of plants to high light stress. Annu. Rev. Plant Physiol. 1992, 43, 599–626. [Google Scholar] [CrossRef]
- Babani, F.; Lichtenthaler, H.K. Light-induced and age-dependent development of chloroplasts in etiolated barley leaves as visualized by determination of photosynthetic pigments, CO2 assimilation rates and different kinds of chlorophyll fluorescence ratios. J. Plant Physiol. 1996, 148, 555–566. [Google Scholar] [CrossRef]
- Govindachary, S.; Bukhov, N.G.; Joly, D.; Carpentier, R. Photosystem II inhibition by moderate light under low temperature in intact leaves of chilling-sensitive and -tolerant plants. Physiol. Plant 2004, 121, 322–333. [Google Scholar] [CrossRef]
- Kana, R.; Lazar, D.; Prasil, O.; Naus, J. Experimental and theoretical studies on the excess capacity of photosystem II. Photosynth. Res. 2002, 72, 271–284. [Google Scholar] [CrossRef]
- Isayenkov, S.V.; Maathuis, F.J.M. Plant Salinity Stress: Many unanswered questions remain. Front. Plant Sci. 2019, 10, 80. [Google Scholar] [CrossRef] [Green Version]
- Sitohy, M.Z.; Desoky, E.S.M.; Osman, A.; Rady, M.M. Pumpkin seed protein hydrolysate treatment alleviates salt stress effects on Phaseolus vulgaris by elevating antioxidant capacity and recovering ion homeostasis. Sci. Hortic. 2020, 271, 109495. [Google Scholar] [CrossRef]
- Sudhir, P.R.; Pogoryelov, D.; Kovacs, L.; Garab, G.; Murthy, D.S. The effects of salt stress on photosynthetic electron transport and thylakoid membrane proteins in the cyanobacterium Spirulina platensis. J. Biochem. Mol. Biol. 2005, 38, 481–485. [Google Scholar] [CrossRef] [Green Version]
- Suo, J.; Zhao, Q.; David, L.; Chen, S.; Dai, S. Salinity response in chloroplasts: Insights from gene characterization. Int. J. Mol. Sci. 2017, 18, 1011. [Google Scholar] [CrossRef] [PubMed]
- Blokhina, O.; Virolainen, E.; Fagerstedt, K.V. Antioxidants, oxidative damage and oxygen deprivation stress: A review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colla, G.; Rouphael, Y.; Canaguier, R.; Svecova, E.; Cardarelli, M. Biostimulant action of a plant-derived protein hydrolysate produced through enzymatic hydrolysis. Front. Plant Sci. 2014, 5, 448. [Google Scholar] [CrossRef] [Green Version]
- Carillo, P.; Colla, G.; Fusco, G.M.; Dell’Aversana, E.; El-Nakhel, C.; Giordano, M.; Pannico, A.; Cozzolino, E.; Mori, M.; Reynaud, H.; et al. Morphological and physiological responses induced by protein hydrolysate-based biostimulant and nitrogen rates in greenhouse spinach. Agronomy 2019, 9, 450. [Google Scholar] [CrossRef] [Green Version]
- Mir, A.R.; Siddiqui, H.; Alam, P.; Hayat, S. Foliar spray of Auxin/IAA modulates photosynthesis, elemental composition, ROS localization and antioxidant machinery to promote growth of Brassica juncea. Physiol. Mol. Biol. Plants 2020, 26, 2503–2520. [Google Scholar] [CrossRef] [PubMed]


| Treatments | Electrical Conductivity (dS m−1) | ||||
|---|---|---|---|---|---|
| June | July | August | September | October | |
| EC0 | 0.166 ± 0.007 | 0.195 ± 0.013 | 0.251 ± 0.013 | 0.152 ± 0.007 | 0.161 ± 0.009 |
| EC2 | 0.178 ± 0.015 | 0.428 ± 0.087 | 1.019 ± 0.094 | 0.392 ± 0.057 | 0.252 ± 0.027 |
| EC4 | 0.158 ± 0.012 | 0.786 ± 0.093 | 1.769 ± 0.147 | 0.552 ± 0.078 | 0.247 ± 0.014 |
| EC6 | 0.162 ± 0.008 | 0.998 ± 0.106 | 2.212 ± 0.118 | 0.705 ± 0.072 | 0.228 ± 0.011 |
| Treatments | Seeds | ||
|---|---|---|---|
| % on Inflorescence | n° plant−1 | g 1000−1 | |
| Water Salinity (S) | |||
| EC0 | 20.6 b | 478.7 a | 21.7 a |
| EC2 | 18.5 b | 425.3 b | 18.1 b |
| EC4 | 21.5 b | 288.7 c | 17.0 b |
| EC6 | 24.1 a | 152.0 d | 13.1 c |
| Biostimulant (B) | |||
| Control | 22.1 | 315.7 b | 16.2 b |
| LPDH | 20.2 | 395.8 a | 18.7 a |
| Significance | |||
| Salinity | * | ** | ** |
| Biostimulant | NS | * | ** |
| S × B | NS | NS | NS |
| Treatments | Residual Biomass | Average Weight | Height | HI |
|---|---|---|---|---|
| kg m−2 | g Female Plant−1 | Cm | ||
| Water Salinity | ||||
| EC0 | 0.10 a | 20.7 a | 89.1 a | 0.26 a |
| EC2 | 0.08 b | 15.1 b | 74.4 b | 0.25 a |
| EC4 | 0.07 b | 13.2 b | 77.0 b | 0.21 b |
| EC6 | 0.04 c | 7.3 c | 69.3 b | 0.18 c |
| Biostimulant | ||||
| Control | 0.06 b | 12.1 b | 72.5 b | 0.22 |
| LPDH | 0.08 a | 16.0 a | 82.4 a | 0.23 |
| Significance | ||||
| Salinity | ** | ** | ** | * |
| Biostimulant | * | * | ** | NS |
| S × B | NS | NS | NS | NS |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di Mola, I.; Conti, S.; Cozzolino, E.; Melchionna, G.; Ottaiano, L.; Testa, A.; Sabatino, L.; Rouphael, Y.; Mori, M. Plant-Based Protein Hydrolysate Improves Salinity Tolerance in Hemp: Agronomical and Physiological Aspects. Agronomy 2021, 11, 342. https://doi.org/10.3390/agronomy11020342
Di Mola I, Conti S, Cozzolino E, Melchionna G, Ottaiano L, Testa A, Sabatino L, Rouphael Y, Mori M. Plant-Based Protein Hydrolysate Improves Salinity Tolerance in Hemp: Agronomical and Physiological Aspects. Agronomy. 2021; 11(2):342. https://doi.org/10.3390/agronomy11020342
Chicago/Turabian StyleDi Mola, Ida, Stefano Conti, Eugenio Cozzolino, Giuseppe Melchionna, Lucia Ottaiano, Antonino Testa, Leo Sabatino, Youssef Rouphael, and Mauro Mori. 2021. "Plant-Based Protein Hydrolysate Improves Salinity Tolerance in Hemp: Agronomical and Physiological Aspects" Agronomy 11, no. 2: 342. https://doi.org/10.3390/agronomy11020342
APA StyleDi Mola, I., Conti, S., Cozzolino, E., Melchionna, G., Ottaiano, L., Testa, A., Sabatino, L., Rouphael, Y., & Mori, M. (2021). Plant-Based Protein Hydrolysate Improves Salinity Tolerance in Hemp: Agronomical and Physiological Aspects. Agronomy, 11(2), 342. https://doi.org/10.3390/agronomy11020342











