Overexpression of OsABCG48 Lowers Cadmium in Rice (Oryza sativa L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Culture and Treatment
2.2. RNA-seq Library Construction, Sequencing and Data Analysis
2.3. qRT-PCR
2.4. Cd Tolerance Assay in S. Pombe
2.5. Expression of OsABCG48 in Plants
3. Results
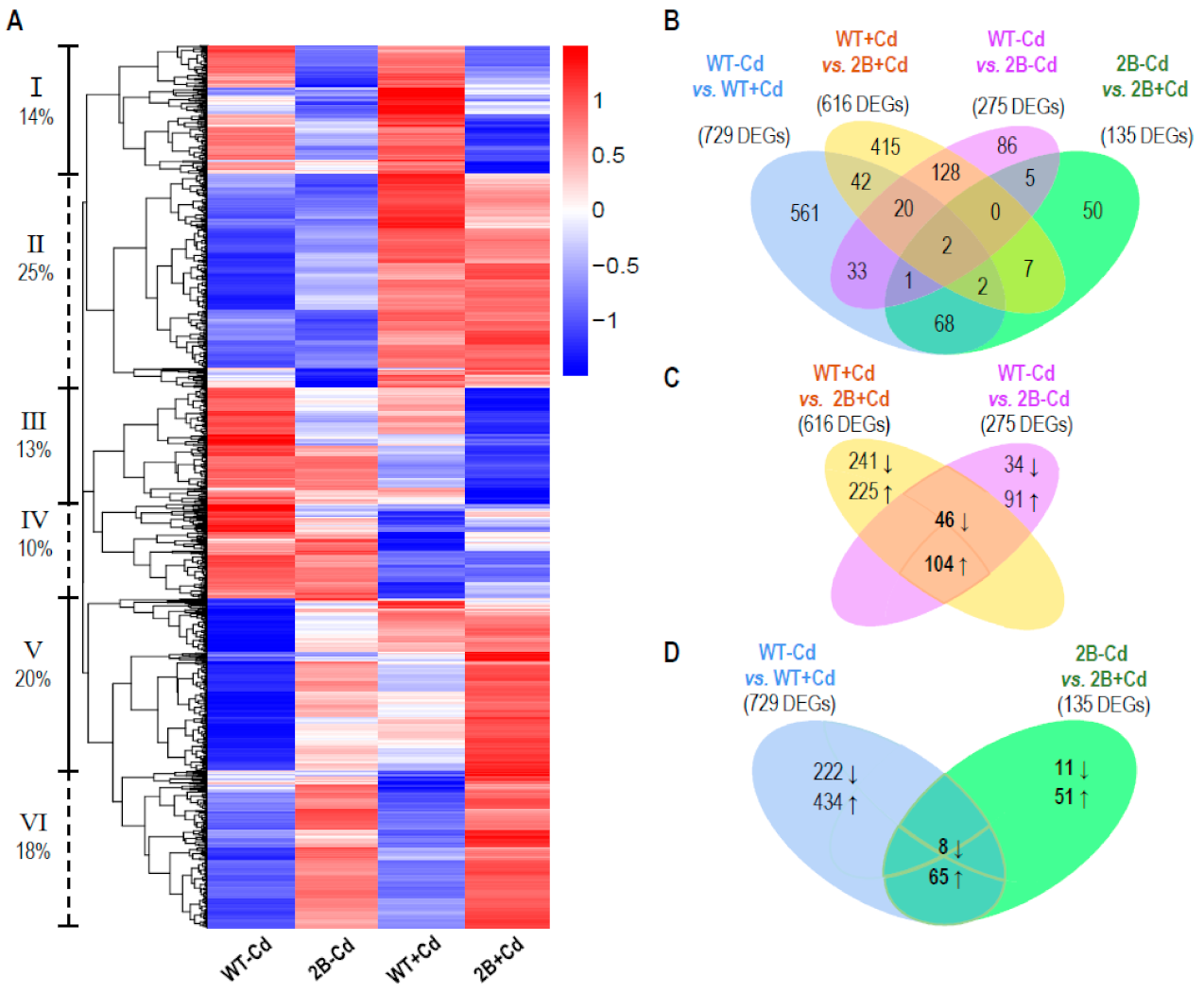
3.1. RNA-Seq Analysis and Identification of Differentially Expressed Genes
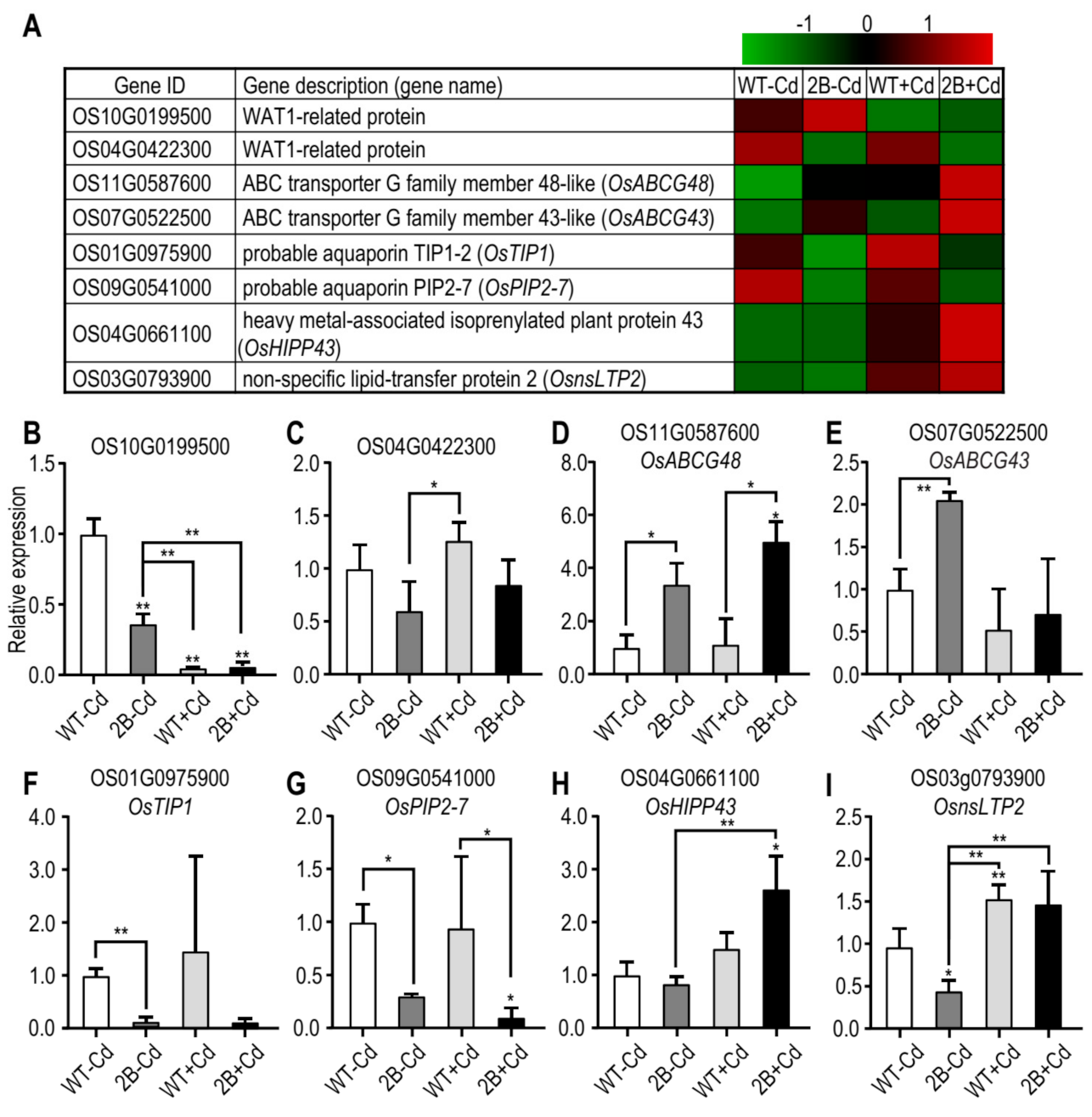
3.2. Verification of DEGs Possibly Related to Ion Transportation
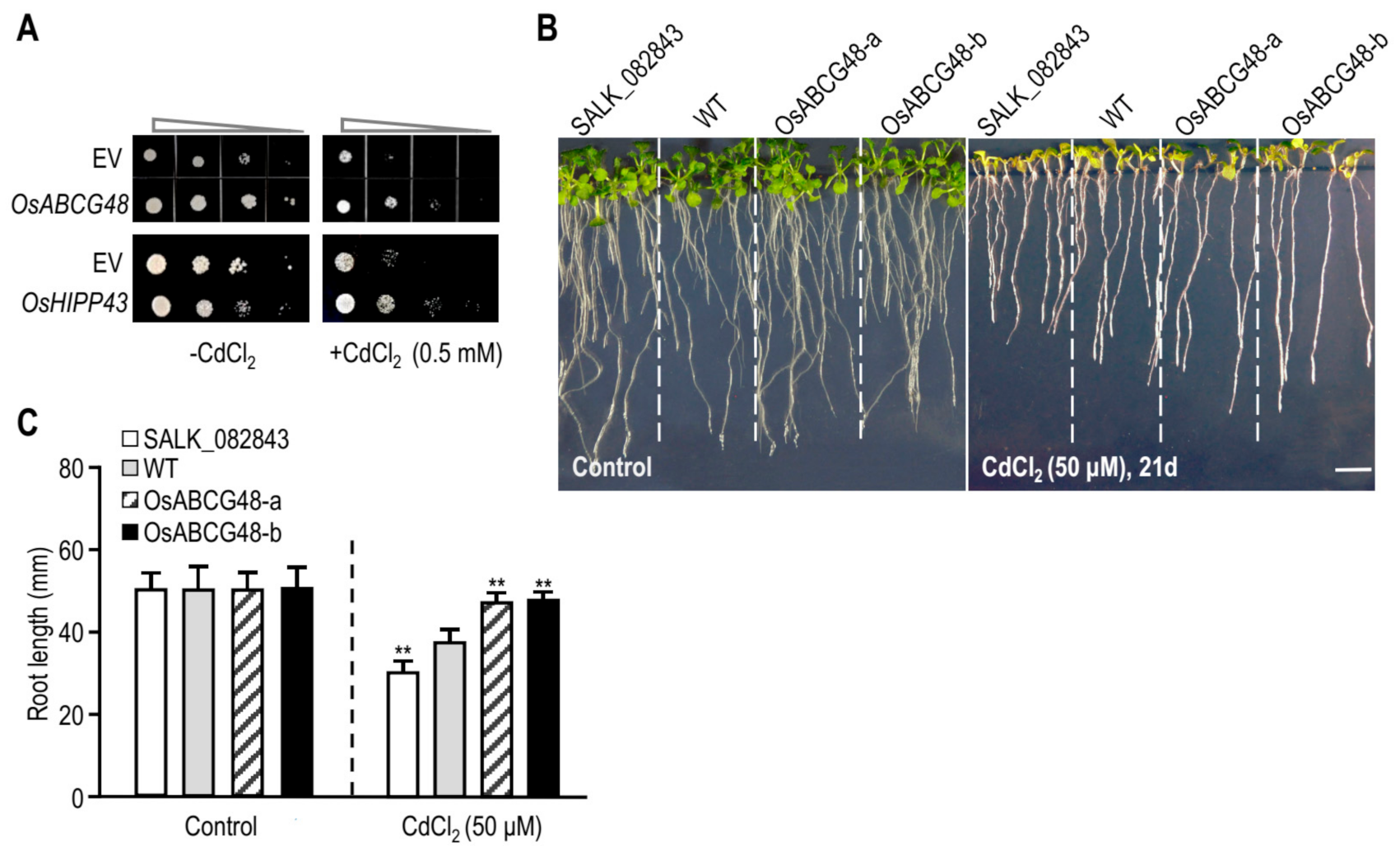
3.3. Heterologous Expression of OsABCG48 Conferred Tolerance to Cd in S. pombe and Arabidopsis
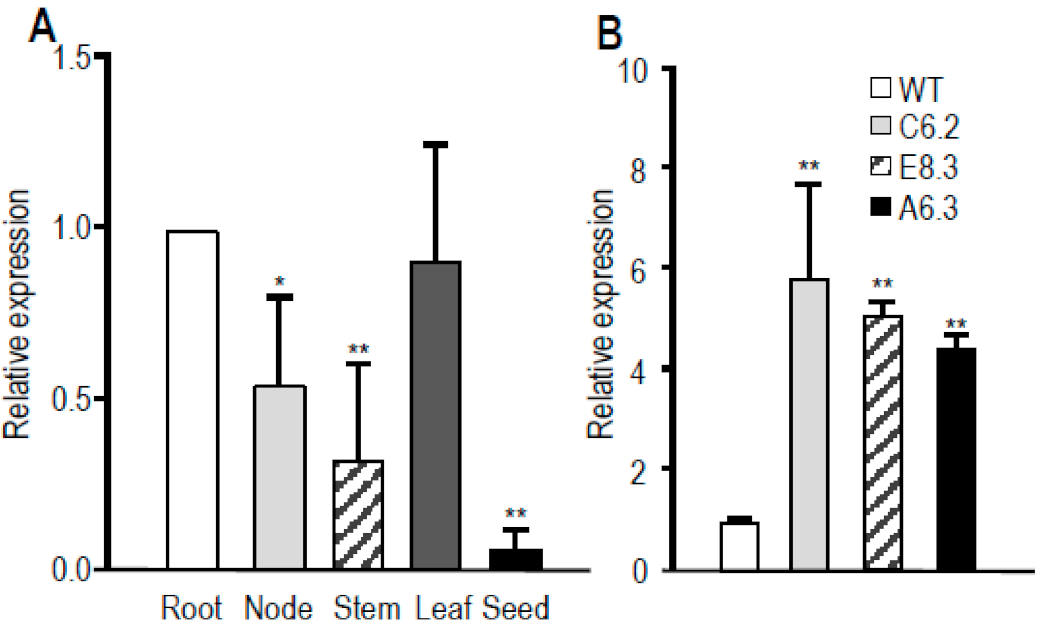
3.4. Overexpressing OsABCG48 Enhances Cd Tolerance and Lowers Cd Accumulation in Rice
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, S.; Hao, S.; Qiu, Z.; Wang, Y.; Zhao, Y.; Li, Y.; Gao, W.; Wu, Y.; Liu, C.; Xu, X.; et al. Cadmium disrupts the DNA damage response by destabilizing RNF168. Food Chem. Toxicol. 2019, 133, 110745. [Google Scholar] [CrossRef] [PubMed]
- Reynders, H.; Van Campenhout, K.; Bervoets, L.; De Coen, W.M.; Blust, R. Dynamics of cadmium accumulation and effects in common carp (Cyprinus carpio) during simultaneous exposure to water and food (Tubifex tubifex). Environ. Toxicol. Chem. 2006, 25, 1558–1567. [Google Scholar] [CrossRef] [PubMed]
- Assche, F.V.; Clijsters, H. Effects of metals on enzyme activity in plants. Plant Cell and Environ. 1990, 13, 195–206. [Google Scholar] [CrossRef]
- Dietz, K.J.; Baier, M.; Krämer, U. Free radicals and reactive oxygen species as mediators of heavy metal toxicity in plants. In Heavy Metal Stress in Plants; Springer: Berlin/Heidelberg, Germany, 1999; pp. 73–97. [Google Scholar]
- Hernández, L.E.; Cooke, D.T. Modification of the root plasma membrane lipid composition of cadmium-treated Pisum sativum. J. Exp. Bot. 1997, 48, 1375–1381. [Google Scholar] [CrossRef]
- Järup, L.; Akesson, A. Current status of cadmium as an environmental health problem. Toxicol. Appl. Pharmacol. 2009, 238, 201–208. [Google Scholar] [CrossRef]
- Järup, L.; Berglund, M.; Elinder, C.G.; Nordberg, G.; Vahter, M. Health effects of cadmium exposure-a review of the literature and a risk estimate. Scand. J. Work. Environ. Health 1998, 24 (Suppl. 1), 1–51. [Google Scholar]
- Egan, S.K.; Bolger, P.M.; Carrington, C.D. Update of US FDA’s Total Diet Study food list and diets. J. Expo. Sci. Environ. Epidemiol. 2007, 17, 573–582. [Google Scholar] [CrossRef]
- Shimbo, S.; Zhang, Z.W.; Watanabe, T.; Nakatsuka, H.; Matsuda-Inoguchi, N.; Higashikawa, K.; Ikeda, M. Cadmium and lead contents in rice and other cereal products in Japan in 1998-2000. Sci. Total Environ. 2001, 281, 165–175. [Google Scholar] [CrossRef]
- Jin, T.; Nordberg, M.; Frech, W.; Dumont, X.; Bernard, A.; Ye, T.T.; Kong, Q.; Wang, Z.; Li, P.; Lundström, N.G.; et al. Cadmium biomonitoring and renal dysfunction among a population environmentally exposed to cadmium from smelting in China (ChinaCad). Biometals 2002, 15, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Rivai, I.F.; Koyama, H.; Suzuki, S. Cadmium content in rice and its daily intake in various countries. Bull. Environ. Contam. Toxicol. 1990, 44, 910–916. [Google Scholar] [CrossRef]
- Honda, R.; Swaddiwudhipong, W.; Nishijo, M.; Mahasakpan, P.; Teeyakasem, W.; Ruangyuttikarn, W.; Satarug, S.; Padungtod, C.; Nakagawa, H. Cadmium induced renal dysfunction among residents of rice farming area downstream from a zinc-mineralized belt in Thailand. Toxicol. Lett. 2010, 198, 26–32. [Google Scholar] [CrossRef]
- Rizwan, M.; Ali, S.; Abbas, T.; Zia-Ur-Rehman, M.; Hannan, F.; Keller, C.; Al-Wabel, M.I.; Ok, Y.S. Cadmium minimization in wheat: A critical review. Ecotoxicol. Environ. Saf. 2016, 130, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Horiguchi, H.; Teranishi, H.; Niiya, K.; Aoshima, K.; Katoh, T.; Sakuragawa, N.; Kasuya, M. Hypoproduction of erythropoietin contributes to anemia in chronic cadmium intoxication: Clinical study on Itai-itai disease in Japan. Arch. Toxicol. 1994, 68, 632–636. [Google Scholar] [CrossRef]
- Japan for Sustainability Home Page. Available online: https://www.japanfs.org/en/news/archives/-news_id032147.html (accessed on 14 July 2020).
- Sasaki, A.; Yamaji, N.; Yokosho, K.; Ma, J.F. Nramp5 is a major transporter responsible for manganese and cadmium uptake in rice. Plant Cell 2012, 24, 2155–2167. [Google Scholar] [CrossRef]
- Ishikawa, S.; Ishimaru, Y.; Igura, M.; Kuramata, M.; Abe, T.; Senoura, T.; Hase, Y.; Arao, T.; Nishizawa, N.K.; Nakanishi, H. Ion-beam irradiation, gene identification, and marker-assisted breeding in the development of low-cadmium rice. Proc. Natl. Acad. Sci. USA 2012, 109, 19166–19171. [Google Scholar] [CrossRef]
- Ueno, D.; Yamaji, N.; Kono, I.; Huang, C.F.; Ando, T.; Yano, M.; Ma, J.F. Gene limiting cadmium accumulation in rice. Proc. Natl. Acad. Sci. USA 2010, 107, 16500–16505. [Google Scholar] [CrossRef]
- Uraguchi, S.; Kamiya, T.; Sakamoto, T.; Kasai, K.; Sato, Y.; Nagamura, Y.; Yoshida, A.; Kyozuka, J.; Ishikawa, S.; Fujiwara, T. Low-affinity cation transporter (OsLCT1) regulates cadmium transport into rice grains. Proc. Natl. Acad. Sci. USA 2011, 108, 20959–20964. [Google Scholar] [CrossRef] [PubMed]
- Shimo, H.; Ishimaru, Y.; An, G.; Yamakawa, T.; Nakanishi, H.; Nishizawa, N.K. Low cadmium (LCD), a novel gene related to cadmium tolerance and accumulation in rice. J. Exp. Bot. 2011, 62, 5727–5734. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Guo, W.; Ye, S.; Wei, P.; Ow, D.W. Reduction of Cd in rice through expression of OXS3-like gene fragments. Mol. Plant 2016, 9, 301–304. [Google Scholar] [CrossRef]
- Wang, C.; Guo, W.; Cai, X.; Li, R.; Ow, D.W. Engineering low-cadmium rice through stress-inducible expression of OXS3-family member genes. N. Biotechnol. 2019, 48, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Blanvillain, R.; Kim, J.H.; Wu, S.; Lima, A.; Ow, D.W. OXIDATIVE STRESS 3 is a chromatin-associated factor involved in tolerance to heavy metals and oxidative stress. Plant J. 2009, 57, 654–665. [Google Scholar] [CrossRef]
- Liang, M.; Xiao, S.; Cai, J.; Ow, D.W. OXIDATIVE STRESS 3 regulates drought-induced flowering through APETALA 1. Biochem. Biophys. Res. Commun. 2019, 519, 585–590. [Google Scholar] [CrossRef]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Benjamini, Y.; Hochberg, Y. Controlling the False discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. B. 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Weghe, J.G.V.; Ow, D.W. A fission yeast gene for mitochondrial sulfide oxidation. J. Biol. Chem. 1999, 274, 13250–13257. [Google Scholar] [CrossRef] [PubMed]
- Gietz, R.D.; Woods, R.A. Yeast transformation by the LiAc/SS Carrier DNA/PEG method. Methods Mol. Biol. 2006, 313, 107–120. [Google Scholar] [PubMed]
- Zhang, X.R.; Henriques, R.; Lin, S.S.; Niu, Q.W.; Chua, N.H. Agrobacterium-mediated transformation of Arabidopsis thaliana using the floral dip method. Nat.Protoc. 2006, 1, 641–646. [Google Scholar] [CrossRef]
- Hiei, Y.; Ohta, S.; Komari, T.; Kumashiro, T. Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J. 1994, 6, 271–282. [Google Scholar] [CrossRef]
- Luo, J.S.; Huang, J.; Zeng, D.L.; Peng, J.S.; Zhang, G.B.; Ma, H.L.; Guan, Y.; Yi, H.Y.; Fu, Y.L.; Han, B.; et al. A defensin-like protein drives cadmium efflux and allocation in rice. Nat. Commun. 2018, 9, 645. [Google Scholar] [CrossRef]
- Yan, H.; Xu, W.; Xie, J.; Gao, Y.; Wu, L.; Sun, L.; Feng, L.; Chen, X.; Zhang, T.; Dai, C.; et al. Variation of a major facilitator superfamily gene contributes to differential cadmium accumulation between rice subspecies. Nat. Commun. 2019, 10, 2562. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, X.; Liu, J.; Niu, Y.; Chen, Y.; Hao, Y.; Zhao, J.; Sun, L.; Wang, H.; Xiao, J.; et al. Characterization of the Heavy-Metal-Associated Isoprenylated Plant Protein (HIPP) Gene Family from Triticeae Species. Int. J. Mol.Sci. 2020, 21, 6191. [Google Scholar] [CrossRef]
- Robinson, N.J.; Winge, D.R. Copper Metallochaperones. Annu. Rev. Biochem. 2010, 79, 537–562. [Google Scholar] [CrossRef] [PubMed]
- Oda, K.; Otani, M.; Uraguchi, S.; Akihiro, T.; Fujiwara, T. Rice ABCG43 is cd inducible and confers Cd tolerance on Yeast. Biosci. Biotechnol. Biochem. 2011, 75, 1211–1213. [Google Scholar] [CrossRef]
- Kang, J.; Park, J.; Choi, H.; Burla, B.; Kretzschmar, T.; Lee, Y.; Martinoia, E. Plant ABC Transporters. Arab. Book 2011, 9, e0153. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.Y.; Bovet, L.; Maeshima, M.; Martinoia, E.; Lee, Y. The ABC transporter AtPDR8 is a cadmium extrusion pump conferring heavy metal resistance. Plant J. 2007, 50, 207–218. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Song, W.Y.; Ko, D.; Eom, Y.; Hansen, T.H.; Schiller, M.; Lee, T.G.; Martinoia, E.; Lee, Y. The phytochelatin transporters AtABCC1 and AtABCC2 mediate tolerance to cadmium and mercury. Plant J. 2012, 69, 278–288. [Google Scholar] [CrossRef]
- Brunetti, P.; Zanella, L.; De Paolis, A.; Di Litta, D.; Cecchetti, V.; Falasca, G.; Barbieri, M.; Altamura, M.M.; Costantino, P.; Cardarelli, M. Cadmium-inducible expression of the ABC-type transporter AtABCC3 increases phytochelatin-mediated cadmium tolerance in Arabidopsis. J. Exp. Bot. 2015, 66, 3815–3829. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Lu, Y.; Zhang, X.; Yang, G.; Chao, D.; Wang, Z.; Shi, M.; Chen, J.; Chao, D.Y.; Li, R.; et al. The ABC transporter ABCG36 is required for cadmium tolerance in rice. J. Exp. Bot. 2019, 70, 5909–5918. [Google Scholar] [CrossRef]
- Pegler, J.L.; Oultram, J.M.J.; Nguyen, D.Q.; Grof, C.P.L.; Eamens, A.L. MicroRNA-mediated responses to cadmium stress in Arabidopsis thaliana. Plants 2021, 10, 130. [Google Scholar] [CrossRef]
- Navarro-León, E.; Ruiz, J.M.; Albacete, A.; Blasco, B. Tolerance to cadmium toxicity and phytoremediation potential of three Brassica rapa cax1a tilling mutants. Ecotoxicol. Environ. Saf. 2020, 189, 109961. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cai, X.; Wang, M.; Jiang, Y.; Wang, C.; Ow, D.W. Overexpression of OsABCG48 Lowers Cadmium in Rice (Oryza sativa L.). Agronomy 2021, 11, 918. https://doi.org/10.3390/agronomy11050918
Cai X, Wang M, Jiang Y, Wang C, Ow DW. Overexpression of OsABCG48 Lowers Cadmium in Rice (Oryza sativa L.). Agronomy. 2021; 11(5):918. https://doi.org/10.3390/agronomy11050918
Chicago/Turabian StyleCai, Xingzhe, Meng Wang, Yucong Jiang, Changhu Wang, and David W. Ow. 2021. "Overexpression of OsABCG48 Lowers Cadmium in Rice (Oryza sativa L.)" Agronomy 11, no. 5: 918. https://doi.org/10.3390/agronomy11050918
APA StyleCai, X., Wang, M., Jiang, Y., Wang, C., & Ow, D. W. (2021). Overexpression of OsABCG48 Lowers Cadmium in Rice (Oryza sativa L.). Agronomy, 11(5), 918. https://doi.org/10.3390/agronomy11050918





