Interactions between Plants and Plant-Soil in Functionally Complex Mixtures including Grass Pea, Faba Bean and Niger, Intercropped with Oilseed Rape
Abstract
:1. Introduction
2. Materials and Methods
2.1. Species Choice
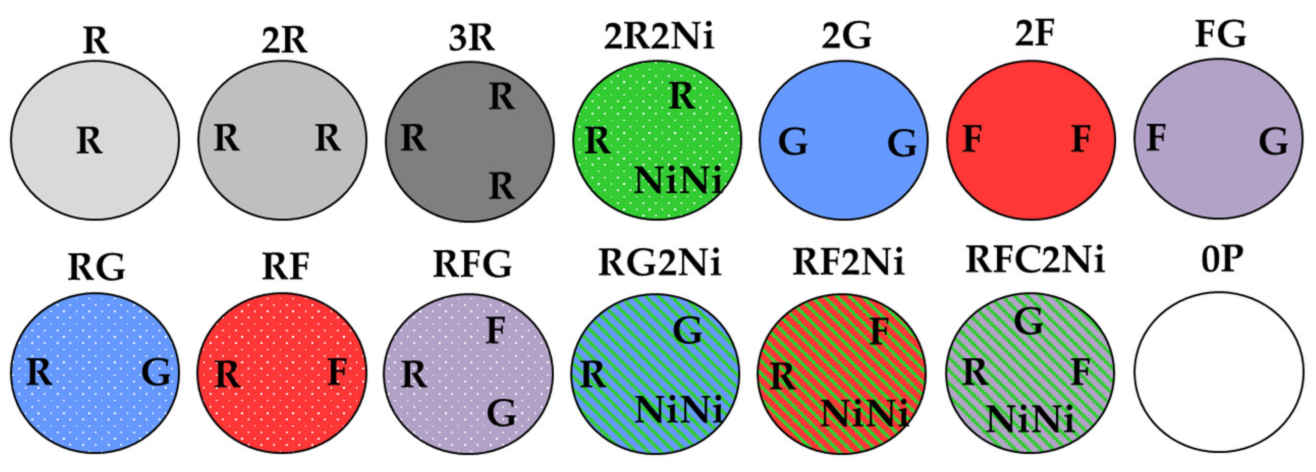
2.2. Experimental Design
2.3. Plant and Soil Sampling and Chemical Analyses
2.4. Soil Microbial Activity
2.5. Calculations
2.5.1. Percentage of Nitrogen Derived from Fixation (%Ndfa)
2.5.2. Competition Index
2.6. Statistical Analysis
3. Results
3.1. Dry Weight and N Accumulated per Mesocosm
3.2. Dry Weight, Total N and N Derived from Fixation in Service Plant Species
3.2.1. Shoot Dry Weights and Shoot N per Species
3.2.2. Nitrogen Derived from Biological Fixation in the Legumes
3.2.3. Oilseed Rape Dry Weight and N, P Status
3.2.4. Interactions between Plants
3.3. Soil Properties
3.3.1. Chemical Analyses
3.3.2. Soil Microbial Activity
4. Discussion
4.1. Drivers of DW and N Accumulation in the Service Plant Mixtures
4.2. Grass Pea and Faba Bean: Two Contrasting Legumes
4.3. Consequences of Service Plants on Winter Oilseed Rape Growth
4.4. Microbial Activity
4.5. Which Species to Mix for Which Services?
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. FAOSTAT. Available online: http://www.fao.org/faostat/en/#home (accessed on 2 May 2020).
- Valantin-Morison, M.; Meynard, J. Diagnosis of limiting factors of organic oilseed rape yield. A survey of farmers’ fields. Agron. Sustain. Dev. 2008, 28, 527–539. [Google Scholar] [CrossRef] [Green Version]
- Rathke, G.-W.; Behrens, T.; Diepenbrock, W. Integrated nitrogen management strategies to improve seed yield, oil content and nitrogen efficiency of winter oilseed rape (Brassica napus L.): A review. Agric. Ecosyst. Environ. 2006, 117, 80–108. [Google Scholar] [CrossRef]
- Bélanger, G.; Ziadi, N.; Pageau, D.; Grant, C.; Lafond, J.; Nyiraneza, J. Shoot growth, phosphorus–nitrogen relationships, and yield of canola in response to mineral phosphorus fertilization. Agron. J. 2015, 107, 1458–1464. [Google Scholar] [CrossRef]
- Tilman, D.; Fargione, J.; Wolff, B.; D’antonio, C.; Dobson, A.; Howarth, R.; Schindler, D.; Schlesinger, W.H.; Simberloff, D.; Swackhamer, D. Forecasting agriculturally driven global environmental change. Science 2001, 292, 281–284. [Google Scholar] [CrossRef] [Green Version]
- Crews, T.; Peoples, M. Legume versus fertilizer sources of nitrogen: Ecological tradeoffs and human needs. Agric. Ecosyst. Environ. 2004, 102, 279–297. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2014: Mitigation of Climate Change; Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2014; p. 1435. [Google Scholar]
- Kim, K.-H.; Kabir, E.; Jahan, S.A. Exposure to pesticides and the associated human health effects. Sci. Total Environ. 2017, 575, 525–535. [Google Scholar] [CrossRef] [PubMed]
- Altieri, M.A. The ecological role of biodiversity in agroecosystems. Agric. Ecosyst. Environ. 1999, 74, 19–31. [Google Scholar] [CrossRef] [Green Version]
- Duru, M.; Therond, O.; Martin, G.; Martin-Clouaire, R.; Magne, M.-A.; Justes, E.; Journet, E.-P.; Aubertot, J.-N.; Savary, S.; Bergez, J.-E. How to implement biodiversity-based agriculture to enhance ecosystem services: A review. Agron. Sustain. Dev. 2015, 35, 1259–1281. [Google Scholar] [CrossRef]
- Gaba, S.; Lescourret, F.; Boudsocq, S.; Enjalbert, J.; Hinsinger, P.; Journet, E.-P.; Navas, M.-L.; Wery, J.; Louarn, G.; Malézieux, E. Multiple cropping systems as drivers for providing multiple ecosystem services: From concepts to design. Agron. Sustain. Dev. 2015, 35, 607–623. [Google Scholar] [CrossRef] [Green Version]
- Verret, V.; Gardarin, A.; Makowski, D.; Lorin, M.; Cadoux, S.; Butier, A.; Valantin-Morison, M. Assessment of the benefits of frost-sensitive companion plants in winter rapeseed. Eur. J. Agron. 2017, 91, 93–103. [Google Scholar] [CrossRef]
- Willey, R. Intercropping: Its importance and research needs. Part 1 Competition and crop yield advantages. Field Crop Abstr. 1979, 32, 1–10. [Google Scholar]
- Bedoussac, L.; Journet, E.-P.; Hauggaard-Nielsen, H.; Naudin, C.; Corre-Hellou, G.; Jensen, E.S.; Prieur, L.; Justes, E. Ecological principles underlying the increase of productivity achieved by cereal-grain legume intercrops in organic farming: A review. Agron. Sustain. Dev. 2015, 35, 911–935. [Google Scholar] [CrossRef]
- Brooker, R.W.; Bennett, A.E.; Cong, W.F.; Daniell, T.J.; George, T.S.; Hallett, P.D.; Hawes, C.; Iannetta, P.P.; Jones, H.G.; Karley, A.J. Improving intercropping: A synthesis of research in agronomy, plant physiology and ecology. New Phytol. 2015, 206, 107–117. [Google Scholar] [CrossRef]
- Malézieux, E.; Crozat, Y.; Dupraz, C.; Laurans, M.; Makowski, D.; Ozier-Lafontaine, H.; Rapidel, B.; De Tourdonnet, S.; Valantin-Morison, M. Mixing plant species in cropping systems: Concepts, tools and models: A review. Agron. Sustain. Dev. 2009, 29, 43–62. [Google Scholar] [CrossRef] [Green Version]
- Pelzer, E.; Bazot, M.; Makowski, D.; Corre-Hellou, G.; Naudin, C.; Al Rifaï, M.; Baranger, E.; Bedoussac, L.; Biarnès, V.; Boucheny, P. Pea–wheat intercrops in low-input conditions combine high economic performances and low environmental impacts. Eur. J. Agron. 2012, 40, 39–53. [Google Scholar] [CrossRef]
- Dowling, A.; Sadras, V.O.; Roberts, P.; Doolette, A.; Zhou, Y.; Denton, M.D. Legume-oilseed intercropping in mechanised broadacre agriculture—A review. Field Crops Res. 2021, 260, 107980. [Google Scholar] [CrossRef]
- Duchene, O.; Vian, J.-F.; Celette, F. Intercropping with legume for agroecological cropping systems: Complementarity and facilitation processes and the importance of soil microorganisms: A review. Agric. Ecosyst. Environ. 2017, 240, 148–161. [Google Scholar] [CrossRef]
- Hinsinger, P.; Betencourt, E.; Bernard, L.; Brauman, A.; Plassard, C.; Shen, J.; Tang, X.; Zhang, F. P for two, sharing a scarce resource: Soil phosphorus acquisition in the rhizosphere of intercropped species. Plant Physiol. 2011, 156, 1078–1086. [Google Scholar] [CrossRef] [Green Version]
- Drut, B.; Cassagne, N.; Cannavacciuolo, M.; Le Floch, G.; Cobo, J.; Fustec, J. Mobilization of interactions between functional diversity of plant and soil organisms on nitrogen availability and use. Asp. Appl. Biol. 2018, 138, 31–42. [Google Scholar]
- Génard, T.; Etienne, P.; Diquélou, S.; Yvin, J.-C.; Revellin, C.; Laîné, P. Rapeseed-legume intercrops: Plant growth and nitrogen balance in early stages of growth and development. Heliyon 2017, 3, e00261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jamont, M.; Piva, G.; Fustec, J. Sharing N resources in the early growth of rapeseed intercropped with faba bean: Does N transfer matter? Plant Soil 2013, 371, 641–653. [Google Scholar] [CrossRef]
- Carlsson, G.; Huss-Danell, K. Nitrogen fixation in perennial forage legumes in the field. Plant Soil 2003, 253, 353–372. [Google Scholar] [CrossRef]
- Thilakarathna, M.S.; McElroy, M.S.; Chapagain, T.; Papadopoulos, Y.A.; Raizada, M.N. Belowground nitrogen transfer from legumes to non-legumes under managed herbaceous cropping systems. A review. Agron. Sustain. Dev. 2016, 36, 58. [Google Scholar] [CrossRef] [Green Version]
- Louarn, G.; Pereira-Lopès, E.; Fustec, J.; Mary, B.; Voisin, A.-S.; de Faccio Carvalho, P.C.; Gastal, F. The amounts and dynamics of nitrogen transfer to grasses differ in alfalfa and white clover-based grass-legume mixtures as a result of rooting strategies and rhizodeposit quality. Plant Soil 2015, 389, 289–305. [Google Scholar] [CrossRef]
- Andersen, M.K.; Hauggaard-Nielsen, H.; Ambus, P.; Jensen, E.S. Biomass production, symbiotic nitrogen fixation and inorganic N use in dual and tri-component annual intercrops. Plant Soil 2005, 266, 273–287. [Google Scholar] [CrossRef] [Green Version]
- Corre-Hellou, G.; Fustec, J.; Crozat, Y. Interspecific competition for soil N and its interaction with N2 fixation, leaf expansion and crop growth in pea–barley intercrops. Plant Soil 2006, 282, 195–208. [Google Scholar] [CrossRef]
- Jensen, E.S. Grain yield, symbiotic N2 fixation and interspecific competition for inorganic N in pea-barley intercrops. Plant Soil 1996, 182, 25–38. [Google Scholar] [CrossRef]
- Rodriguez, C.; Carlsson, G.; Englund, J.-E.; Flöhr, A.; Pelzer, E.; Jeuffroy, M.-H.; Makowski, D.; Jensen, E.S. Grain legume-cereal intercropping enhances the use of soil-derived and biologically fixed nitrogen in temperate agroecosystems: A meta-analysis. Eur. J. Agron. 2020, 118, 126077. [Google Scholar] [CrossRef]
- Lorin, M.; Jeuffroy, M.-H.; Butier, A.; Valantin-Morison, M. Undersowing winter oilseed rape with frost-sensitive legume living mulch: Consequences for cash crop nitrogen nutrition. Field Crops Res. 2016, 193, 24–33. [Google Scholar] [CrossRef]
- Lorin, M.; Jeuffroy, M.-H.; Butier, A.; Valantin-Morison, M. Undersowing winter oilseed rape with frost-sensitive legume living mulches to improve weed control. Eur. J. Agron. 2015, 71, 96–105. [Google Scholar] [CrossRef]
- Cadoux, S.; Sauzet, G.; Valantin-Morison, M.; Pontet, C.; Champolivier, L.; Robert, C.; Lieven, J.; Flénet, F.; Mangenot, O.; Fauvin, P. Intercropping frost-sensitive legume crops with winter oilseed rape reduces weed competition, insect damage, and improves nitrogen use efficiency. OCL 2015, 22, D302. [Google Scholar] [CrossRef] [Green Version]
- Altieri, M.A.; Gliessman, S.R. Effects of plant diversity on the density and herbivory of the flea beetle, Phyllotreta cruciferae Goeze, in California collard (Brassica oleracea) cropping systems. Crop Prot. 1983, 2, 497–501. [Google Scholar] [CrossRef]
- Altieri, M.A.; Wilson, R.C.; Schmidt, L.L. The effects of living mulches and weed cover on the dynamics of foliage-and soil-arthropod communities in three crop systems. Crop Prot. 1985, 4, 201–213. [Google Scholar] [CrossRef]
- Jamont, M.; Crépellière, S.; Jaloux, B. Effect of extrafloral nectar provisioning on the performance of the adult parasitoid Diaeretiella rapae. Biol. Control 2013, 65, 271–277. [Google Scholar] [CrossRef]
- Wendling, M.; Charles, R.; Herrera, J.; Amossé, C.; Jeangros, B.; Walter, A.; Büchi, L. Effect of species identity and diversity on biomass production and its stability in cover crop mixtures. Agric. Ecosyst. Environ. 2019, 281, 81–91. [Google Scholar] [CrossRef] [Green Version]
- Finney, D.M.; Murrell, E.G.; White, C.M.; Baraibar, B.; Barbercheck, M.E.; Bradley, B.A.; Cornelisse, S.; Hunter, M.C.; Kaye, J.P.; Mortensen, D.A.; et al. Ecosystem services and disservices are bundled in simple and diverse cover cropping systems. Agric. Environ. Lett. 2017, 2, 170033. [Google Scholar] [CrossRef]
- Finney, D.M.; White, C.M.; Kaye, J.P. Biomass production and carbon/nitrogen ratio influence ecosystem services from cover crop mixtures. Agron. J. 2016, 108, 39–52. [Google Scholar] [CrossRef] [Green Version]
- Baux, A.; Schumacher, P. Dévelopement du colza associé: Avis des producteurs suisses. Rech. Agron. Suisse 2019, 10, 128–133. [Google Scholar]
- Magrini, M.-B.; Cabanac, G.; Lascialfari, M.; Plumecocq, G.; Amiot, M.-J.; Anton, M.; Arvisenet, G.; Baranger, A.; Bedoussac, L.; Chardigny, J.-M. Peer-reviewed literature on grain legume species in the WoS (1980–2018): A comparative analysis of soybean and pulses. Sustainability 2019, 11, 6833. [Google Scholar] [CrossRef] [Green Version]
- Büchi, L.; Gebhard, C.-A.; Liebisch, F.; Sinaj, S.; Ramseier, H.; Charles, R. Accumulation of biologically fixed nitrogen by legumes cultivated as cover crops in Switzerland. Plant Soil 2015, 393, 163–175. [Google Scholar] [CrossRef]
- Dayoub, E.; Naudin, C.; Piva, G.; Shirtliffe, S.J.; Fustec, J.; Corre-Hellou, G. Traits affecting early season nitrogen uptake in nine legume species. Heliyon 2017, 3, e00244. [Google Scholar] [CrossRef] [Green Version]
- Guinet, M.; Nicolardot, B.; Revellin, C.; Durey, V.; Carlsson, G.; Voisin, A.-S. Comparative effect of inorganic N on plant growth and N2 fixation of ten legume crops: Towards a better understanding of the differential response among species. Plant Soil 2018, 432, 207–227. [Google Scholar] [CrossRef]
- Taylor, B.N.; Simms, E.L.; Komatsu, K.J. More than a functional group: Diversity within the legume–rhizobia mutualism and Its relationship with ecosystem function. Diversity 2020, 12, 50. [Google Scholar] [CrossRef] [Green Version]
- Wendling, M.; Büchi, L.; Amossé, C.; Sinaj, S.; Walter, A.; Charles, R. Influence of root and leaf traits on the uptake of nutrients in cover crops. Plant Soil 2016, 409, 419–434. [Google Scholar] [CrossRef] [Green Version]
- Tribouillois, H.; Fort, F.; Cruz, P.; Charles, R.; Flores, O.; Garnier, E.; Justes, E. A functional characterisation of a wide range of cover crop species: Growth and nitrogen acquisition rates, leaf traits and ecological strategies. PLoS ONE 2015, 10, e0122156. [Google Scholar] [CrossRef]
- Lorin, M.; Butier, A.; Jeuffroy, M.; Valantin-Morison, M. Choisir et gérer des légumineuses gélives associées au colza d’hiver pour le contrôle des adventices et la fourniture d’azote. Innov. Agron. 2017, 60, 77–89. [Google Scholar]
- Paterson, E.; Gebbing, T.; Abel, C.; Sim, A.; Telfer, G. Rhizodeposition shapes rhizosphere microbial community structure in organic soil. New Phytol. 2007, 173, 600–610. [Google Scholar] [CrossRef]
- Taschen, E.; Amenc, L.; Tournier, E.; Deleporte, P.; Malagoli, P.; Fustec, J.; Bru, D.; Philippot, L.; Bernard, L. Cereal-legume intercropping modifies the dynamics of the active rhizospheric bacterial community. Rhizosphere 2017, 3, 191–195. [Google Scholar] [CrossRef]
- Bobille, H.; Limami, A.M.; Robins, R.J.; Cukier, C.; Le Floch, G.; Fustec, J. Evolution of the amino acid fingerprint in the unsterilized rhizosphere of a legume in relation to plant maturity. Soil. Biol. Biochem. 2016, 101, 226–236. [Google Scholar] [CrossRef]
- Fustec, J.; Lesuffleur, F.; Mahieu, S.; Cliquet, J.-B. Nitrogen rhizodeposition of legumes. A review. Agron. Sustain. Dev. 2010, 30, 57–66. [Google Scholar] [CrossRef] [Green Version]
- Dennis, P.G.; Miller, A.J.; Hirsch, P.R. Are root exudates more important than other sources of rhizodeposits in structuring rhizosphere bacterial communities? FEMS Microbiol. Ecol. 2010, 72, 313–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richardson, A.E.; Barea, J.-M.; McNeill, A.M.; Prigent-Combaret, C. Acquisition of phosphorus and nitrogen in the rhizosphere and plant growth promotion by microorganisms. Plant Soil 2009, 321, 305–339. [Google Scholar] [CrossRef]
- Wahbi, S.; Prin, Y.; Thioulouse, J.; Sanguin, H.; Baudoin, E.; Maghraoui, T.; Oufdou, K.; Le Roux, C.; Galiana, A.; Hafidi, M. Impact of wheat/faba bean mixed cropping or rotation systems on soil microbial functionalities. Front. Plant Sci. 2016, 7, 1364. [Google Scholar] [CrossRef] [Green Version]
- Drut, B.; Cassagne, N.; Mario, C.; Le Floch, G.; Cobo-Díaz, J.F.; Fustec, J. Improving complementarity effect of legume intercrop by earthworm facilitation for wheat performance. J. Agric. Sci. 2018, 10, 1–14. [Google Scholar] [CrossRef]
- Laguerre, G.; Mazurier, S.I.; Amarger, N. Plasmid profiles and restriction fragment length polymorphism of Rhizobium leguminosarum bv. viciae in field populations. FEMS Microbiol. Lett. 1992, 101, 17–26. [Google Scholar] [CrossRef]
- Campbell, C.D.; Chapman, S.J.; Cameron, C.M.; Davidson, M.S.; Potts, J.M. A rapid microtiter plate method to measure carbon dioxide evolved from carbon substrate amendments so as to determine the physiological profiles of soil microbial communities by using whole soil. Appl. Environ. Microbiol. 2003, 69, 3593–3599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brechenmacher, L.; Lei, Z.; Libault, M.; Findley, S.; Sugawara, M.; Sadowsky, M.J.; Sumner, L.W.; Stacey, G. Soybean metabolites regulated in root hairs in response to the symbiotic bacterium Bradyrhizobium japonicum. Plant Physiol. 2010, 153, 1808–1822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vančura, V. Root exudates of plants. Plant Soil 1964, 21, 231–248. [Google Scholar] [CrossRef]
- Turner, B.L.; Papházy, M.J.; Haygarth, P.M.; McKelvie, I.D. Inositol phosphates in the environment. Philos. Trans. R. Soc. Lond. B Ser. B Biol. Sci. 2002, 357, 449–469. [Google Scholar] [CrossRef] [Green Version]
- Dakora, F.D.; Phillips, D.A. Root exudates as mediators of mineral acquisition in low-nutrient environments. Plant Soil 2002, 245, 35–47. [Google Scholar] [CrossRef]
- Anderson, J.P.; Domsch, K.H. A physiological method for the quantitative measurement of microbial biomass in soils. Soil Biol. Biochem. 1978, 10, 215–221. [Google Scholar] [CrossRef]
- Anderson, T.H.; Domsch, K.H. Determination of ecophysiological maintenance carbon requirements of soil microorganisms in a dormant state. Biol. Fertil. Soils 1985, 1, 81–89. [Google Scholar] [CrossRef]
- Sassi, M.B.; Dollinger, J.; Renault, P.; Tlili, A.; Bérard, A. The FungiResp method: An application of the MicroResp™ method to assess fungi in microbial communities as soil biological indicators. Ecol. Indic. 2012, 23, 482–490. [Google Scholar] [CrossRef]
- Hart, S.C.; Stark, J.M.; Davidson, E.A.; Firestone, M.K. Nitrogen mineralization, immobilization, and nitrification. In Methods of Soil Analysis: Part 2 Microbiological and Biochemical Properties; Weaver, R.W., Angle, S., Bottomley, P., Bezdicek, D., Smith, S., Tabatabai, A., Wollum, A., Eds.; Soil Science Society of America, Inc.: Madison, WI, USA, 1994; Volume 5, pp. 985–1018. [Google Scholar]
- McAuliffe, C.; Chamblee, D.S.; Uribe-Arango, H.; Woodhouse, W.W., Jr. Influence of inorganic nitrogen on nitrogen fixation by legumes as revealed by N15 1. Agron. J. 1958, 50, 334–337. [Google Scholar] [CrossRef]
- Armas, C.; Ordiales, R.; Pugnaire, F.I. Measuring plant interactions: A new comparative index. Ecology 2004, 85, 2682–2686. [Google Scholar] [CrossRef] [Green Version]
- Coulis, M.; Bernard, L.; Gerard, F.; Hinsinger, P.; Plassard, C.; Villeneuve, M.; Blanchart, E. Endogeic earthworms modify soil phosphorus, plant growth and interactions in a legume-cereal intercrop. Plant Soil 2014, 379, 149–160. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2020; Available online: https://www.R-project.org/ (accessed on 1 December 2020).
- Pohlert, T. The Pairwise Multiple Comparison of Mean Ranks Package (PMCMR). Available online: https://CRAN.R-project.org/package=PMCMR (accessed on 1 December 2020).
- Freund, L.; Mariotte, P.; Santonja, M.; Buttler, A.; Jeangros, B. Species identity, rather than species mixtures, drives cover crop effects on nutrient partitioning in unfertilized agricultural soil. Plant Soil 2020, 460, 149–162. [Google Scholar] [CrossRef]
- Schappert, A.; Schumacher, M.; Gerhards, R. Weed control ability of single sown cover crops compared to species mixtures. Agronomy 2019, 9, 294. [Google Scholar] [CrossRef] [Green Version]
- Tilman, D.; Knops, J.; Wedin, D.; Reich, P.; Ritchie, M.; Siemann, E. The influence of functional diversity and composition on ecosystem processes. Science 1997, 277, 1300–1302. [Google Scholar] [CrossRef] [Green Version]
- Wendling, M.; Büchi, L.; Amossé, C.; Jeangros, B.; Walter, A.; Charles, R. Specific interactions leading to transgressive overyielding in cover crop mixtures. Agric. Ecosyst. Environ. 2017, 241, 88–99. [Google Scholar] [CrossRef]
- Fan, F.; Zhang, F.; Song, Y.; Sun, J.; Bao, X.; Guo, T.; Li, L. Nitrogen fixation of faba bean (Vicia faba L.) interacting with a non-legume in two contrasting intercropping systems. Plant Soil 2006, 283, 275–286. [Google Scholar] [CrossRef]
- Naudin, C.; Corre-Hellou, G.; Pineau, S.; Crozat, Y.; Jeuffroy, M.-H. The effect of various dynamics of N availability on winter pea–wheat intercrops: Crop growth, N partitioning and symbiotic N2 fixation. Field Crops Res. 2010, 119, 2–11. [Google Scholar] [CrossRef]
- Streeter, J.; Wong, P.P. Inhibition of legume nodule formation and N2 fixation by nitrate. Crit. Rev. Plant Sci. 1988, 7, 1–23. [Google Scholar] [CrossRef]
- Turpin, J.; Herridge, D.; Robertson, M. Nitrogen fixation and soil nitrate interactions in field-grown chickpea (Cicer arietinum) and fababean (Vicia faba). Aust. J. Agric. Res. 2002, 53, 599–608. [Google Scholar] [CrossRef]
- Martínez-Hidalgo, P.; Hirsch, A.M. The nodule microbiome: N2-fixing rhizobia do not live alone. Phytobiomes 2017, 1, 70–82. [Google Scholar] [CrossRef] [Green Version]
- Corre-Hellou, G.; Crozat, Y. Assessment of Root System Dynamics of Species Grown in Mixtures under Field Conditions using Herbicide Injection and 15N Natural Abundance Methods: A Case Study with Pea, Barley and Mustard. Plant Soil 2005, 276, 177–192. [Google Scholar] [CrossRef]
- Hauggaard-Nielsen, H.; Ambus, P.; Jensen, E.S. Temporal and spatial distribution of roots and competition for nitrogen in pea-barley intercrops—A field study employing 32P technique. Plant Soil 2001, 236, 63–74. [Google Scholar] [CrossRef] [Green Version]
- Amossé, C.; Jeuffroy, M.-H.; Celette, F.; David, C. Relay-intercropped forage legumes help to control weeds in organic grain production. Eur. J. Agron. 2013, 49, 158–167. [Google Scholar] [CrossRef]
- Bowsher, A.W.; Evans, S.; Tiemann, L.K.; Friesen, M.L. Effects of soil nitrogen availability on rhizodeposition in plants: A review. Plant Soil 2018, 423, 59–85. [Google Scholar] [CrossRef]
- Hauggaard-Nielsen, H.; Ambus, P.; Jensen, E.S. Interspecific competition, N use and interference with weeds in pea–barley intercropping. Field Crops Res. 2001, 70, 101–109. [Google Scholar] [CrossRef]
- Xu, Q.; Wang, X.; Tang, C. The effects of elevated CO2 and nitrogen availability on rhizosphere priming of soil organic matter under wheat and white lupin. Plant Soil 2018, 425, 375–387. [Google Scholar] [CrossRef]
- Brolsma, K.M.; Vonk, J.A.; Mommer, L.; Van Ruijven, J.; Hoffland, E.; De Goede, R.G. Microbial catabolic diversity in and beyond the rhizosphere of plant species and plant genotypes. Pedobiologia 2017, 61, 43–49. [Google Scholar] [CrossRef]
- Mwafulirwa, L.; Baggs, E.M.; Russell, J.; George, T.; Morley, N.; Sim, A.; de la Fuente Cantó, C.; Paterson, E. Barley genotype influences stabilization of rhizodeposition-derived C and soil organic matter mineralization. Soil Biol. Biochem. 2016, 95, 60–69. [Google Scholar] [CrossRef]
- Kaye, J.P.; Hart, S.C. Competition for nitrogen between plants and soil microorganisms. Trends Ecol. Evol. 1997, 12, 139–143. [Google Scholar] [CrossRef]
- Moreau, D.; Pivato, B.; Bru, D.; Busset, H.; Deau, F.; Faivre, C.; Matejicek, A.; Strbik, F.; Philippot, L.; Mougel, C. Plant traits related to nitrogen uptake influence plant-microbe competition. Ecology 2015, 96, 2300–2310. [Google Scholar] [CrossRef]
- Song, Y.N.; Marschner, P.; Li, L.; Bao, X.G.; Sun, J.H.; Zhang, F.S. Community composition of ammonia-oxidizing bacteria in the rhizosphere of intercropped wheat (Triticum aestivum L.), maize (Zea mays L.), and faba bean (Vicia faba L.). Biol. Fertil. Soils 2007, 44, 307–314. [Google Scholar] [CrossRef]
- Corre-Hellou, G.; Dibet, A.; Hauggaard-Nielsen, H.; Crozat, Y.; Gooding, M.; Ambus, P.; Dahlmann, C.; von Fragstein, P.; Pristeri, A.; Monti, M. The competitive ability of pea–barley intercrops against weeds and the interactions with crop productivity and soil N availability. Field Crops Res. 2011, 122, 264–272. [Google Scholar] [CrossRef] [Green Version]
- Cheriere, T.; Lorin, M.; Corre-Hellou, G. Species choice and spatial arrangement in soybean-based intercropping: Levers that drive yield and weed control. Field Crops Res. 2020, 256, 107923. [Google Scholar] [CrossRef]




| Source of Variance | Legume Biomass | Legume N | Amount of N Derived from Biological Fixation | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Df | F-Value | p-Value | Df | F-Value | p-Value | Df | F-Value | p-Value | |
| Leg | 1 | 41.77 | <0.001 *** | 1 | 69.49 | <0.001 *** | 1 | 74.946 | <0.001 *** |
| Pl-pl inter | 2 | 8.088 | <0.01 ** | 2 | 7.063 | <0.01 ** | 2 | 2.073 | 0.155 |
| Leg × Pl-pl inter | 2 | 1.153 | 0.338 | 2 | 0.372 | 0.694 | 2 | 0.424 | 0.661 |
| Residuals | 18 | 18 | 18 | ||||||
| 2F/2G | FG | RF/RG | RFG | RF2Ni/RG2Ni | RFG2Ni | p-Value | |
|---|---|---|---|---|---|---|---|
| Grass pea | 14 ± 13.9 b | 16 ± 5.9 ab | 63 ± 20.8 ab | 52 ± 31.9 ab | 51 ± 35.3 ab | 65 ± 12.5a | <0.05 * |
| Faba bean | 54 ± 8.7 c | 71 ± 8.3 bc | 90 ± 0.9 a | 90 ± 2.2 a | 89 ± 1.6 ab | 88 ± 0.9 ab | <0.001 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bousselin, X.; Cassagne, N.; Baux, A.; Valantin-Morison, M.; Herrera, J.M.; Lorin, M.; Hédan, M.; Fustec, J. Interactions between Plants and Plant-Soil in Functionally Complex Mixtures including Grass Pea, Faba Bean and Niger, Intercropped with Oilseed Rape. Agronomy 2021, 11, 1493. https://doi.org/10.3390/agronomy11081493
Bousselin X, Cassagne N, Baux A, Valantin-Morison M, Herrera JM, Lorin M, Hédan M, Fustec J. Interactions between Plants and Plant-Soil in Functionally Complex Mixtures including Grass Pea, Faba Bean and Niger, Intercropped with Oilseed Rape. Agronomy. 2021; 11(8):1493. https://doi.org/10.3390/agronomy11081493
Chicago/Turabian StyleBousselin, Xavier, Nathalie Cassagne, Alice Baux, Muriel Valantin-Morison, Juan Manuel Herrera, Mathieu Lorin, Marie Hédan, and Joëlle Fustec. 2021. "Interactions between Plants and Plant-Soil in Functionally Complex Mixtures including Grass Pea, Faba Bean and Niger, Intercropped with Oilseed Rape" Agronomy 11, no. 8: 1493. https://doi.org/10.3390/agronomy11081493
APA StyleBousselin, X., Cassagne, N., Baux, A., Valantin-Morison, M., Herrera, J. M., Lorin, M., Hédan, M., & Fustec, J. (2021). Interactions between Plants and Plant-Soil in Functionally Complex Mixtures including Grass Pea, Faba Bean and Niger, Intercropped with Oilseed Rape. Agronomy, 11(8), 1493. https://doi.org/10.3390/agronomy11081493







