Genetic Diversity and Population Structure of Japanese Plum-Type (Hybrids of P. salicina) Accessions Assessed by SSR Markers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. DNA Extraction and SSR Analysis
2.3. Genetic Diversity Analysis and Genetic Relationships among Accessions
3. Results
3.1. SSR Genotyping
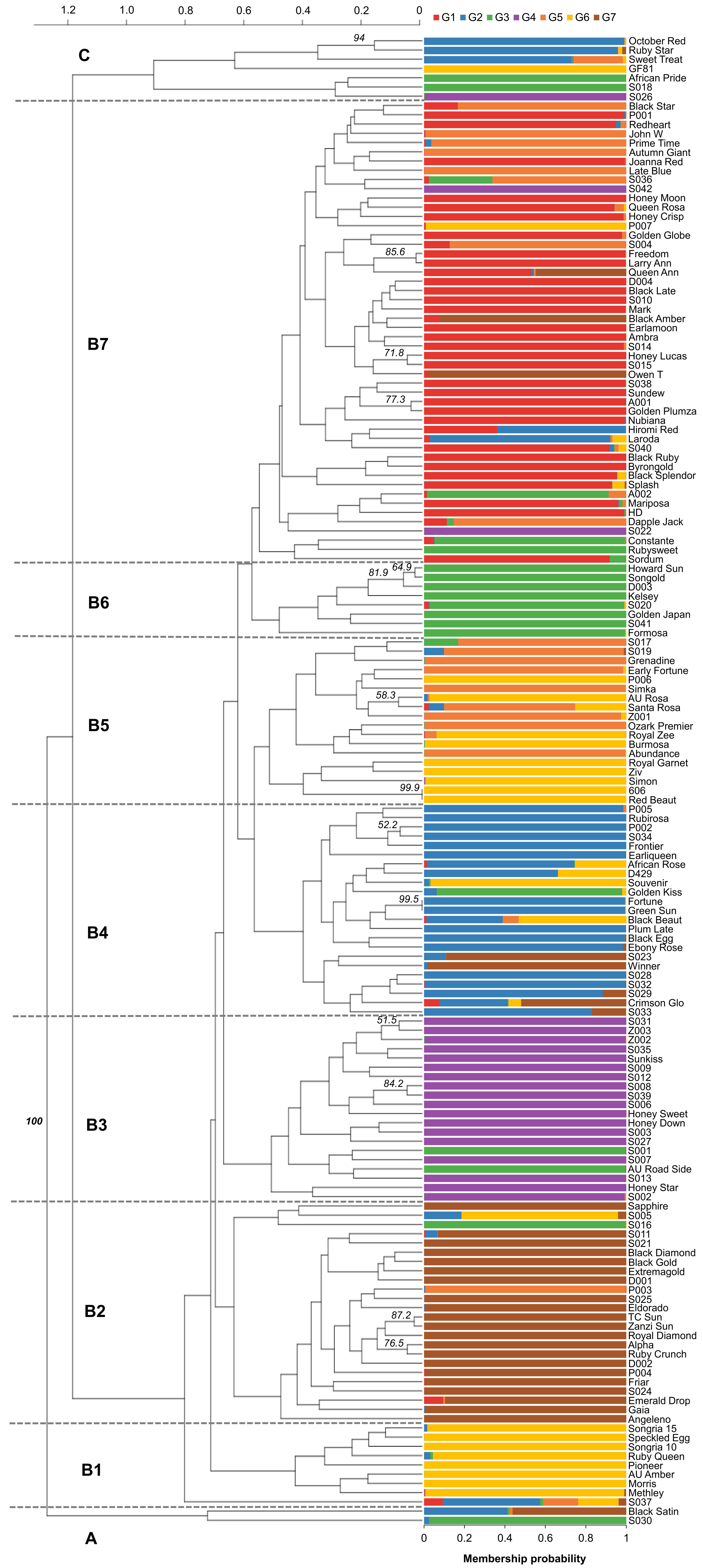
3.2. Genetic Relationships among Accessions
3.3. Analysis Genetic Structure
3.4. Genetic Diversity among Groups
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rehder, A. Manual of Cultivated Trees and Shrubs Hardy in North America: Exclusive of the Subtropical and Warmer Temperate Regions, 2nd ed.; MacMillan: New York, NY, USA, 1940. [Google Scholar]
- Faust, M.; Surányi, D. Origin and dissemination of plums. In Origin and Dissemination of Prunus Crops: Peach, Cherry, Apricot, Plum and Almond; Janick, J., Ed.; Horticultural Reviews: Gent-Oostakker, Belgium, 2011; pp. 139–186. ISBN 978-90-6605-436-3. [Google Scholar]
- Yoshida, M. The origin of fruits. 2: Plums. Fruit Japan 1987, 42, 49–53. (In Japanese) [Google Scholar]
- Okie, W.R.; Weinberger, J.H. Plums. In Fruit Breeding. Volume 1: Tree and Tropical Fruits; Janick, J., Moore, J.N., Eds.; John Wiley and Sons, Inc.: New York, NY, USA, 1996; pp. 559–608. [Google Scholar]
- Milošević, T.; Milošević, N. Plum (Prunus spp.) breeding. In Advances in Plant Breeding Strategies: Fruits; Al-Khayri, J., Jain, S., Eds.; Springer: Cham, Switzerland, 2018; Volume 3, pp. 165–215. ISBN 9783319919447. [Google Scholar]
- Topp, B.L.; Russell, D.M.; Neumüller, M.; Dalbó, M.A.; Liu, W. Plum. In Fruit Breeding; Badenes, M.L., Byrne, D.H., Eds.; Springer: Boston, MA, USA, 2012; pp. 571–621. ISBN 978-1-4419-0763-9. [Google Scholar]
- Burbank, L. Luther Burbank: His Methods and Discoveries and Their Practical Application; Luther Burbank Press: New York, NY, USA, 1914; ISBN 3192400897. [Google Scholar]
- Okie, W.R. Introgression of Prunus species in plum. N. Y. Fruit Q. 2006, 14, 29–37. [Google Scholar]
- Okie, W.R.; Ramming, D.W. Plum breeding worldwide. Horttechnology 1999, 9, 162–176. [Google Scholar] [CrossRef] [Green Version]
- Batlle, I.; Iglesias, I.; Cantin, C.M.; Badenes, M.L.; Ríos, G.; Ruiz, D.; Dicenta, F.; Egea, J.; López-Corrales, M.; Guerra, M.E.; et al. Frutales de hueso y pepita. In Influencia del Cambio Climático en la Mejora Genética de Plantas; García-Brunton, J., Tornero, O., Cos-Terrer, J., Ruíz-García, L., Sanchez, E., Eds.; Sociedad Española de Ciencias Hortícolas: Murcia, Spain, 2018; pp. 79–132. ISBN 978-84-948233-8-1. [Google Scholar]
- Community Plant Variety Office. CPVO Annual Statistics. Available online: https://cpvo.europa.eu/ (accessed on 28 May 2021).
- Guerra, M.E.; Rodrigo, J. Japanese plum pollination: A review. Sci. Hortic. 2015, 197, 674–686. [Google Scholar] [CrossRef]
- Guerra, M.E.; Guerrero, B.I.; Casadomet, C.; Rodrigo, J. Self-(in)compatibility, S-RNase allele identification, and selection of pollinizers in new Japanese plum-type cultivars. Sci. Hortic. 2020, 261, 109022. [Google Scholar] [CrossRef]
- Guerra, M.E.; Wunsch, A.; López-Corrales, M.; Rodrigo, J. Flower emasculation as the cause for lack of fruit set in Japanese plum crosses. J. Amer. Soc. Hortic. Sci. 2010, 135, 556–562. [Google Scholar] [CrossRef]
- Guerra, M.E.; Rodrigo, J.; López-Corrales, M.; Wünsch, A. S-RNase genotyping and incompatibility group assignment by PCR and pollination experiments in Japanese plum. Plant Breed. 2009, 128, 304–311. [Google Scholar] [CrossRef]
- Guerra, M.E.; Wünsch, A.; López-Corrales, M.; Rodrigo, J. Lack of fruit set caused by ovule degeneration in Japanese plum. J. Am. Soc. Hortic. Sci. 2011, 136, 375–381. [Google Scholar] [CrossRef] [Green Version]
- Pereira-Lorenzo, S.; Ramos-Cabrer, A.M.; Fischer, M. Breeding apple (Malus × domestica Borkh). In Breeding Plantation Tree Crops: Temperate Species; Mohan, S., Priyadarshan, P.M., Eds.; Springer: New York, NY, USA, 2009; pp. 33–82. ISBN 978-0-387-71202-4. [Google Scholar]
- Campoy, J.A.; Lerigoleur-Balsemin, E.; Christmann, H.; Beauvieux, R.; Girollet, N.; Quero-García, J.; Dirlewanger, E.; Barreneche, T. Genetic diversity, linkage disequilibrium, population structure and construction of a core collection of Prunus avium L. landraces and bred cultivars. BMC Plant Biol. 2016, 16, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Mas-Gómez, J.; Cantín, C.M.; Moreno, M.; Prudencio, Á.S.; Gómez-Abajo, M.; Bianco, L.; Troggio, M.; Martínez-Gómez, P.; Rubio, M.; Martínez-García, P.J. Exploring genome-wide diversity in the national peach (Prunus persica) germplasm collection at CITA (Zaragoza, Spain). Agronomy 2021, 11, 481. [Google Scholar] [CrossRef]
- Mason, A.S. SSR genotyping. Methods Mol. Biol. 2015, 1245, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Lucia, M.; Vieira, C.; Santini, L.; Diniz, A.L.; Munhoz, C.D.F. Microsatellite markers: What they mean and why they are so useful. Genet. Mol. Biol. 2016, 39, 312–328. [Google Scholar] [CrossRef]
- Guilford, P.; Prakash, S.; Zhu, J.M.; Rikkerink, E.; Gardiner, S.; Bassett, H.; Forster, R. Microsatellites in Malus × domestica (apple): Abundance, polymorphism and cultivar identification. Theor. Appl. Genet. 1997, 94, 249–254. [Google Scholar] [CrossRef]
- Hokanson, S.C.; Szewc-McFadden, A.K.; Lamboy, W.F.; McFerson, J.R. Microsatellite (SSR) markers reveal genetic identities, genetic diversity and relationships in a Malus × domestica Borkh. core subset collection. Theor. Appl. Genet. 1998, 97, 671–683. [Google Scholar] [CrossRef]
- Pereira-Lorenzo, S.; Ramos-Cabrer, A.M.; Díaz-Hernández, M.B. Evaluation of genetic identity and variation of local apple cultivars (Malus × domestica Borkh.) from Spain using microsatellite markers. Genet. Resour. Crop Evol. 2007, 54, 405–420. [Google Scholar] [CrossRef]
- Cabe, P.R.; Baumgarten, A.; Onan, K.; Luby, J.J.; Bedford, D.S. Using microsatellite analysis to verify breeding records: A study of “Honeycrisp” and other cold-hardy apple cultivars. HortScience 2005, 40, 15–17. [Google Scholar] [CrossRef] [Green Version]
- Cipriani, G.; Lot, G.; Huang, W.G.; Marrazzo, M.T.; Peterlunger, E.; Testolin, R. AC/GT and AG/CT microsatellite repeats in peach [Prunus persica (L) Batsch]: Isolation, characterisation and cross-species amplification in Prunus. Theor. Appl. Genet. 1999, 99, 65–72. [Google Scholar] [CrossRef]
- Cantini, C.; Iezzoni, A.F.; Lamboy, W.F.; Boritzki, M.; Struss, D. DNA fingerprinting of tetraploid cherry germplasm using simple sequence repeats. J. Am. Soc. Hortic. Sci. 2001, 126, 205–209. [Google Scholar] [CrossRef]
- Downey, S.L.; Iezzoni, A.F. Polymorphic DNA markers in black cherry (Prunus serotina) are identified using sequences from sweet cherry, peach, and sour cherry. J. Am. Soc. Hortic. Sci. 2000, 125, 76–80. [Google Scholar] [CrossRef] [Green Version]
- Struss, D.; Ahmad, R.; Southwick, S.M.; Boritzki, M. Analysis of sweet cherry (Prunus avium L.) cultivars using SSR and AFLP markers. J. Am. Soc. Hortic. Sci. 2003, 128, 904–909. [Google Scholar] [CrossRef] [Green Version]
- Sosinski, B.; Gannavarapu, M.; Hager, L.D.; Beck, L.E.; KIng, J.J.; Ryder, C.D.; Rajapakse, S.; Baird, W.V.; Ballard, R.E.; Abbot, A.G. Characterization of microsatellite markers in peach [Prunus persica (L.) Batsch]. Theor. Appl. Genet. 2000, 101, 421–428. [Google Scholar] [CrossRef]
- Dirlewanger, E.; Cosson, P.; Tavaud, M.; Aranzana, M.; Poizat, C.; Zanetto, A.; Arús, P.; Laigret, F. Development of microsatellite markers in peach [Prunus persica (L.) Batsch] and their use in genetic diversity analysis in peach and sweet cherry (Prunus avium L.). Theor. Appl. Genet. 2002, 105, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Wang, K.; Wang, W.; Chen, M.; Wu, D.; Xu, C.; Chen, K. Development of high quality EST-SSR markers without stutter bands in peach and their application in cultivar discrimination and hybrid authentication. HortScience 2017, 52, 24–30. [Google Scholar] [CrossRef] [Green Version]
- Testolin, R.; Marrazzo, T.; Cipriani, G.; Quarta, R.; Verde, I.; Dettori, M.T.; Pancaldi, M.; Sansavini, S. Microsatellite DNA in peach (Prunus persica L. Batsch) and its use in fingerprinting and testing the genetic origin of cultivars. Genome 2000, 43, 512–520. [Google Scholar] [CrossRef]
- Messina, R.; Lain, O.; Marrazzo, M.T.; Cipriano, G.; Testolin, R. New set of microsatellite loci isolated in apricot. Mol. Ecol. Notes 2004, 4, 432–434. [Google Scholar] [CrossRef]
- Mnejja, M.; Garcia-Mas, J.; Howad, W.; Badenes, M.L.; Arús, P. Simple-sequence repeat (SSR) markers of Japanese plum (Prunus salicina Lindl.) are highly polymorphic and transferable to peach and almond. Mol. Ecol. Notes 2004, 4, 163–166. [Google Scholar] [CrossRef]
- Sánchez-Pérez, R.; Ballester, J.; Dicenta, F.; Arús, P.; Martínez-Gómez, P. Comparison of SSR polymorphisms using automated capillary sequencers, and polyacrylamide and agarose gel electrophoresis: Implications for the assessment of genetic diversity and relatedness in almond. Sci. Hortic. 2006, 108, 310–316. [Google Scholar] [CrossRef]
- Halász, J.; Kodad, O.; Galiba, G.M.; Skola, I.; Ercisli, S.; Ledbetter, C.A.; Hegedűs, A. Genetic variability is preserved among strongly differentiated and geographically diverse almond germplasm: An assessment by simple sequence repeat markers. Tree Genet. Genomes 2019, 15, 12. [Google Scholar] [CrossRef] [Green Version]
- Aranzana, M.; Pineda, A.; Cosson, P.; Dirlewanger, E.; Ascasibar, J.; Cipriani, G.; Ryder, C.D.; Testolin, R.; Abbot, A.; King, G.J.; et al. A set of simple-sequence repeat (SSR) markers covering the Prunus genome. Theor. Appl. Genet. 2003, 106, 819–825. [Google Scholar] [CrossRef]
- Mnejja, M.; Garcia-Mas, J.; Audergon, J.M.; Arús, P. Prunus microsatellite marker transferability across Rosaceous crops. Tree Genet. Genomes 2010, 6, 689–700. [Google Scholar] [CrossRef]
- Hormaza, J.I. Molecular characterization and similarity relationships among apricot (Prunus armeniaca L.) genotypes using simple sequence repeats. Theor. Appl. Genet. 2002, 104, 321–328. [Google Scholar] [CrossRef]
- Bourguiba, H.; Scotti, I.; Sauvage, C.; Zhebentyayeva, T.; Ledbetter, C.; Krška, B.; Remay, A.; D’Onofrio, C.; Iketani, H.; Christen, D.; et al. Genetic structure of a worldwide germplasm collection of Prunus armeniaca L. reveals three major diffusion routes for varieties coming from the species’ center of origin. Front. Plant Sci. 2020, 11, 638. [Google Scholar] [CrossRef]
- Urrestarazu, J.; Errea, P.; Miranda, C.; Santesteban, L.G.; Pina, A. Genetic diversity of Spanish Prunus domestica L. germplasm reveals a complex genetic structure underlying. PLoS ONE 2018, 13, e0195591. [Google Scholar]
- Gharbi, O.; Wünsch, A.; Rodrigo, J. Characterization of accessions of “Reine Claude Verte” plum using Prunus SRR and phenotypic traits. Sci. Hortic. 2014, 169, 57–65. [Google Scholar] [CrossRef] [Green Version]
- Sitther, V.; Zhang, D.; Dhekney, S.A.; Harris, D.L.; Yadav, A.K.; Okie, W.R. Cultivar identification, pedigree verification, diversity analysis among peach cultivars simple sequence repeat markers. J. Am. Soc. Hortic. Sci. 2012, 137, 114–121. [Google Scholar] [CrossRef] [Green Version]
- Wünsch, A. Cross-transferable polymorphic SSR loci in Prunus species. Sci. Hortic. 2009, 120, 348–352. [Google Scholar] [CrossRef]
- Öz, M.H.; Vurgun, H.; Bakir, M.; Büyük, I.; Yüksel, C.; Ünlü, H.M.; Çukadar, K.; Karadoǧan, B.; Köse, Ö.; Ergül, A. Molecular analysis of East Anatolian traditional plum and cherry accessions using SSR markers. Genet. Mol. Res. 2013, 12, 5310–5320. [Google Scholar] [CrossRef]
- Wünsch, A.; Hormaza, J.I. Molecular characterisation of sweet cherry (Prunus avium L.) genotypes using peach [Prunus persica (L.) Batsch] SSR sequences. Heredity 2002, 89, 56–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Byrne, D.H.; Littleton, T.G. Electrophoretic characterization of diploid plums of the southeastern United States. J. Am. Soc. Hortic. Sci. 1988, 113, 918–924. [Google Scholar]
- Boonprakob, U.; Byrne, D.H.; Graham, C.J.; Smith, B.R. Genetic relationships among cultivated diploid plums and their progenitors as determined by RAPD markers. J. Am. Soc. Hortic. Sci. 2001, 126, 451–461. [Google Scholar] [CrossRef] [Green Version]
- Qiao, Y.S.; Fang, J.G.; Cong, Y.; Zhou, J.; Zhang, Z. Analysis of genetic diversity of Japanese plum cultivars based on RAPD, ISSR and SSR markers. Acta Hortic. 2007, 763, 177–183. [Google Scholar] [CrossRef]
- Klabunde, G.H.F.; Dalbó, M.A.; Nodari, R.O. DNA fingerprinting of Japanese plum (Prunus salicina) cultivars based on microsatellite markers. Crop Breed. Appl. Biotechnol. 2014, 14, 139–145. [Google Scholar] [CrossRef] [Green Version]
- Nyawo, T.A. Fingerprinting and Molecular Characterisation of ARC’s Apricot and Plum Collection. Master’s Thesis, Stellenbosch University, Stellenbosch, South Africa, 2017. [Google Scholar]
- Carrasco, B.; Díaz, C.; Moya, M.; Gebauer, M.; García-González, R. Genetic characterization of Japanese plum cultivars (Prunus salicina) using SSR and ISSR molecular markers. Cienc. Investig. Agrar. 2012, 39, 533–543. [Google Scholar] [CrossRef]
- Abdallah, D.; Baraket, G.; Perez, V.; Ben Mustapha, S.; Salhi-Hannachi, A.; Hormaza, J.I. Analysis of self-incompatibility and genetic diversity in diploid and hexaploid plum genotypes. Front. Plant Sci. 2019, 10, 896. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, R.; Potter, D.; Southwick, S.M. Identification and characterization of plum and pluot cultivars by microsatellite markers. J. Hortic. Sci. Biotechnol. 2016, 79, 164–169. [Google Scholar] [CrossRef]
- Pérez, V.; Larrañaga, N.; Abdallah, D.; Wünsch, A.; Hormaza, J.I. Genetic diversity of local peach (Prunus persica) accessions from La Palma Island (Canary Islands, Spain). Agronomy 2020, 10, 457. [Google Scholar] [CrossRef] [Green Version]
- Guerra, M.E.; López-Corrales, M.; Wünsch, A. Improved S-genotyping and new incompatibility groups in Japanese plum. Euphytica 2012, 186, 445–452. [Google Scholar] [CrossRef]
- Guerrero, B.I.; Guerra, M.E.; Rodrigo, J. Establishing pollination requirements in Japanese plum by phenological monitoring, hand pollinations, fluorescence microscopy and molecular genotyping. J. Vis. Exp. 2020, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Covarrubias-Pazaran, G.; Diaz-Garcia, L.; Schlautman, B.; Salazar, W.; Zalapa, J. Fragman: An R package for fragment analysis. BMC Genet. 2016, 17, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Team, R.C. R: A Language and Environment for Statistical Computing. 2020. Available online: https://www.r-project.org/ (accessed on 20 July 2021).
- Aranzana, M.J.; García-Mas, J.; Carbó, J.; Arús, P. Development and variability analysis of microsatellite markers in peach. Plant Breed. 2002, 121, 87–92. [Google Scholar] [CrossRef]
- Jombart, T. Adegenet: A R package for the multivariate analysis of genetic markers. Bioinformatics 2008, 24, 1403–1405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goudet, J. Hierfstat, a package for R to compute and test hierarchical F-statistics. Mol. Ecol. Notes 2005, 5, 184–186. [Google Scholar] [CrossRef] [Green Version]
- Paradis, E. Pegas: An R package for population genetics with an integrated-modular approach. Bioinformatics 2010, 26, 419–420. [Google Scholar] [CrossRef] [Green Version]
- Adamack, A.; Gruber, B. PopGenReport: Simplifying basic population genetic analyses in R. Methods Ecol. Evol. 2014, 5, 384–387. [Google Scholar] [CrossRef]
- Wei, T.; Simko, V. R Package “Corrplot”: Visualization of a Correlation Matrix, Version 0.90. 2021. Available online: https://github.com/taiyun/corrplot (accessed on 25 July 2021).
- Nei, M.; Li, W.H. Mathematical model for studying genetic variation in terms of restriction endonucleases. Proc. Natl. Acad. Sci. USA 1979, 76, 5269–5273. [Google Scholar] [CrossRef] [Green Version]
- Kamvar, Z.N.; Tabima, J.F.; Grünwald, N.J. Poppr: An R package for genetic analysis of populations with clonal, partially clonal, and/or sexual reproduction. PeerJ 2014, 2, e281. [Google Scholar] [CrossRef] [Green Version]
- Wünsch, A.; Hormaza, J.I. Cultivar identification and genetic fingerprinting of temperate fruit tree species using DNA markers. Euphytica 2002, 125, 59–67. [Google Scholar] [CrossRef]
- Botstein, D.; White, R.; Skolnick, M.; Davis, R. Construction of a linkage map using restriction fragment length polymorphisms. Am. J. Hum. Genet. 1980, 32, 314–331. [Google Scholar] [PubMed]
- Howard, W.L. Luther Burbank’s Plant Contributions; University of California: Berkeley, CA, USA, 1945. [Google Scholar]
- Hedrick, U.P. The Plums of New York; J.B. Lyon Company, State Printers: Albany, NY, USA, 1911. [Google Scholar]
- Agricultural Research Council. Product Catalogue. Available online: https://www.arc.agric.za/arc-infruitec-nietvoorbij/Pages/Product-Catalogue.aspx (accessed on 26 June 2021).
- Okie, W.R. Register of new fruit and nut varieties. HortScience 2019, 39, 1509–1523. [Google Scholar] [CrossRef] [Green Version]
- Jain, S.M.; Priyadarshan, P.M. Breeding Plantation Tree Crops: Temperate Species, 1st ed.; Springer: New York, NY, USA, 2009; ISBN 9780387712031. [Google Scholar]
- García-Gómez, B.; Razi, M.; Salazar, J.A.; Prudencio, A.S.; Ruiz, D.; Dondini, L.; Martínez-Gómez, P. Comparative analysis of SSR markers developed in exon, intron, and intergenic regions and distributed in regions controlling fruit quality traits in Prunus species: Genetic diversity and association studies. Plant Mol. Biol. Rep. 2018, 36, 23–35. [Google Scholar] [CrossRef]
- Kalinowski, S.T. Counting alleles with rarefaction: Private alleles and hierarchical sampling designs. Conserv. Genet. 2004, 5, 539–543. [Google Scholar] [CrossRef]
- Lee, K.J.; Lee, J.R.; Sebastin, R.; Shin, M.J.; Kim, S.H.; Cho, G.T.; Hyun, D.Y. Genetic diversity assessed by genotyping by sequencing (GBS) in watermelon germplasm. Genes 2019, 10, 822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, K.J.; Lee, J.R.; Sebastin, R.; Cho, G.T.; Hyun, D.Y. Molecular genetic diversity and population structure of ginseng germplasm in RDA-geneBank: Implications for breeding and conservation. Agronomy 2020, 10, 68. [Google Scholar] [CrossRef] [Green Version]
- Wright, S. Evolution and the genetics of populations. In Variability within and among Natural Populations; University of Chicago Press: Chicago, IL, USA, 1978. [Google Scholar]
- Urrestarazu, J.; Royo, J.B.; Santesteban, L.G.; Miranda, C. Evaluating the influence of the microsatellite marker set on the genetic structure inferred in Pyrus communis L. PLoS ONE 2015, 10, e0138417. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Accessions | Origin | Accessions | Origin |
|---|---|---|---|
| 606 | Reedley Nursery, USA | P006 | Provedo, Spain |
| A001 | Unknown | P007 | Provedo, Spain |
| A002 | Unknown | Pioneer | ARC Infruitec, South Africa |
| Abundance | Imported from Japan | Plum Late | Unknown |
| African Pride | ARC Infruitec, South Africa | Prime Time | Wuhl, USA |
| African Rose | ARC Infruitec, South Africa | Queen Ann | USDA, USA |
| Alpha | Selected in New Jersey | Queen Rosa | USDA, USA |
| Ambra | Unknown | Red Beaut | Reedley Nursery, USA |
| Angeleno | Garabedian, USA | Redheart | Reedley Nursery, USA |
| AU Amber | Auburn University, USA | Royal Diamond | Kitahara Farms, USA |
| AU Road Side | Auburn University, USA | Royal Garnet | Reedley Nursery, USA |
| AU Rosa | Auburn University, USA | Royal Zee | Zaiger, USA |
| Autumn Giant | Zaiger, USA | Rubirosa | Zaiger, USA |
| Black Amber | USDA, USA | Ruby Crunch | ARC Infruitec, South Africa |
| Black Beaut | Reedley Nursery, USA | Ruby Queen | USDA, USA |
| Black Diamond | Superior Farming Co, USA | Ruby Star | ARC Infruitec, South Africa |
| Black Egg | Ben Dor, Israel | Ruby Sweet | USDA, USA |
| Black Gold | Superior Farming Co, USA | S001 | Stargrow, South Africa |
| Black Late | Unknown | S002 | Stargrow, South Africa |
| Black Ruby | USDA, USA | S003 | Stargrow, South Africa |
| Black Satin | Zaiger, USA | S004 | Stargrow, South Africa |
| Black Splendor | USDA, USA | S005 | Stargrow, South Africa |
| Black Star | Unknown | S006 | Stargrow, South Africa |
| Burmosa | USDA, USA | S007 | Stargrow, South Africa |
| Byrongold | USDA, USA | S008 | Stargrow, South Africa |
| Constante | Unknown | S009 | Stargrow, South Africa |
| Crimson Glo | Zaiger, USA | S010 | Stargrow, South Africa |
| D001 | Unknown | S011 | Stargrow, South Africa |
| D002 | Unknown | S012 | Stargrow, South Africa |
| D003 | Unknown | S013 | Stargrow, South Africa |
| D004 | Unknown | S014 | Stargrow, South Africa |
| D42 | Ben Dor, Israel | S015 | Stargrow, South Africa |
| Dapple Jack | Zaiger, USA | S016 | Stargrow, South Africa |
| Earlamoon | Ben Dor, Israel | S017 | Stargrow, South Africa |
| Earliqueen | Zaiger, USA | S018 | Stargrow, South Africa |
| Early Fortune | Azienda Agricola Martelli, Italy | S019 | Stargrow, South Africa |
| Ebony Rose | Zaiger, USA | S020 | Stargrow, South Africa |
| Eldorado | Terry, USA | S021 | Stargrow, South Africa |
| Emerald Drop | Zaiger, USA | S022 | Stargrow, South Africa |
| Extremagold | Unknown | S023 | Stargrow, South Africa |
| Formosa | Fancher Creek Nursery, USA | S024 | Stargrow, South Africa |
| Fortune | USDA, USA | S025 | Stargrow, South Africa |
| Freedom | USDA, USA | S026 | Stargrow, South Africa |
| Friar | USDA, USA | S027 | Stargrow, South Africa |
| Frontier | USDA, USA | S028 | Stargrow, South Africa |
| Gaia | Azienda Agricola Martelli, Italy | S029 | Stargrow, South Africa |
| GF81 | INRA, Francia | S030 | Stargrow, South Africa |
| Golden Globe | Zaiger, USA | S031 | Stargrow, South Africa |
| Golden Japan | Imported from Japan | S032 | Stargrow, South Africa |
| Golden Kiss | ARC Infruitec, South Africa | S033 | Stargrow, South Africa |
| Golden Plumza | Vivai F.lli Zanzi, Italy | S034 | Stargrow, South Africa |
| Green Sun | Chamberlin, USA | S035 | Stargrow, South Africa |
| Grenadine | Zaiger, USA | S036 | Stargrow, South Africa |
| HD | Ben Dor, Israel | S037 | Stargrow, South Africa |
| Hiromi Red | Zaiger, USA | S038 | Stargrow, South Africa |
| Honey Crisp | Unknown | S039 | Stargrow, South Africa |
| Honey Down | Stargrow, South Africa | S040 | Stargrow, South Africa |
| Honey Lucas | Unknown | S041 | Stargrow, South Africa |
| Honey Moon | Stargrow, South Africa | S042 | Stargrow, South Africa |
| Honey Star | Stargrow, South Africa | Santa Rosa | Burbank, USA |
| Honey Sweet | INRA, Francia | Sapphire | ARC Infruitec, South Africa |
| Howard Sun | Agri Sun Nursery, USA | Simka | Coche D Simonian, USA |
| Joanna Red | Zaiger, USA | Simon | Simon Brothers, USA |
| John W | USDA, USA | Songold | ARC Infruitec, South Africa |
| Kelsey | Imported from Japan | Songria 10 | Planasa, Spain |
| Laroda | USDA, USA | Songria 15 | Planasa, Spain |
| Larry Ann | Topfruit, South Africa | Sordum | Imported from Japan |
| Late blue | Zaiger, USA | Souvenir | ARC Infruitec, South Africa |
| Mariposa | Armstrong Nursery, USA | Splash | Zaiger, USA |
| Mark | Ben Dor, Israel | Speckled Egg | Ben Dor, Israel |
| Methley | Burbank, USA | Sundew | ARC Infruitec, South Africa |
| Morris | Texas AM, USA | Sunkiss | ARC Infruitec, South Africa |
| Nubiana | USDA, USA | Sweet Treat | Zaiger, USA |
| October Red | Unknown | Tc Sun | Chamberlin, USA |
| Owen T | USDA, USA | Winner | Ben Dor, Israel |
| Ozark Premier | Missouri State Univ., USA | Z001 | Zaiger, USA |
| P001 | Provedo, Spain | Z002 | Zaiger, USA |
| P002 | Provedo, Spain | Z003 | Zaiger, USA |
| P003 | Provedo, Spain | Zanzi Sun | Unknown |
| P004 | Provedo, Spain | Ziv | Ben Dor, Israel |
| P005 | Provedo, Spain |
| Mp | Locus | LG | Dye | PC (µM) | Primer Sequence | SSR Motif | Size Range (bp) | Species |
|---|---|---|---|---|---|---|---|---|
| M01 | CPPCT029 * [61] | G1 | VIC | 0.2 | F: CCAAATTCCAAATCTCCTAACA | (CT)24 | 170–194 | Peach |
| R: TGATCAACTTTGAGATTTGTTGAA | ||||||||
| pchgms2 [30] | G4 | 6-FAM | 0.2 | F: GTCAATGAGTTCAGTGTTACACTC | (CT)24 | 130–200 | Peach | |
| R: AATCATAACATCATTCAGCCACTGC | ||||||||
| CPPCT033 [61] | G7 | NED | 0.2 | F: TCAGCAAACTAGAAACAAACC | (CT)16 | 151 | Peach | |
| R: TTGCAATCTGGTTGATGTT | ||||||||
| M02 | UDP96-008 * [26] | G3 | PET | 0.3 | F: TTGTACACACCCTCAGCCTG | (CA)23 | 140–160 | Sweet cherry |
| R: TGCTGAGGTTCAGGTGAGTG | ||||||||
| UDP98-412 * [26] | G6 | NED | 0.15 | F: AGGGAAAGTTTCTGCTGCAC | (AG)28 | 100–140 | Peach | |
| R: GCTGAAGACGACGATGATGA | ||||||||
| UDP98-409 * [26] | G8 | 6-FAM | 0.3 | F: GCTGATGGGTTTTATGGTTTTC | (AG)19 | 125–165 | Peach | |
| R: CGGACTCTTATCCTCTATCAACA | ||||||||
| UDP98-406 * [26] | G2 | VIC | 0.2 | F: TCGGAAACTGGTAGTATGAACAGA | (AG)15 | 30–100 | Peach | |
| R: ATGGGTCGTATGCACAGTCA | ||||||||
| M03 | BPPCT-007 [31] | G3 | 6-FAM | 0.2 | F: TCATTGCTCGTCATCAGC | (AG)22(CG)2(AG)4 | 143–151 | Peach |
| R: CAGATTTCTGAAGTTAGCGGTA | ||||||||
| UDP96-005 [26] | G1 | VIC | 0.3 | F: GTAACGCTCGCTACCACAAA | (AC)16TG(CT)2CA(CT)11 | 100–250 | Peach | |
| R: CCTGCATATCACCACCCAG | ||||||||
| M04 | BPPCT-039 [31] | G3 | PET | 0.3 | F: ATTACGTACCCTAAAGCTTCTGC | (GA)20 | 148–158 | Peach |
| R: GATGTCATGAAGATTGGAGAGG | ||||||||
| BPPCT-025 [31] | G6 | VIC | 0.3 | F: TCCTGCGTAGAAGAAGGTAGC | (GA)29 | 178–202 | Peach | |
| R: CGACATAAAGTCCAAATGGC | ||||||||
| M05 | CPSCT026 [35] | G7 | 6-FAM | 0.3 | F: TCTCACACGCTTTCGTCAAC | (CT)16 | 177–213 | Japanese plum |
| R: AAAAAGCCAAAAGGGGTTGT | ||||||||
| M06 | CPSCT005 [35] | G4 | NED | 0.3 | F: CTGCAAGCACTGCGGATCTC | (CT)15 | 171–191 | Japanese plum |
| R: CCCATATTCCCAACCCATTA |
| Locus | NA | Allele Size (bp) | PIC | Ho | He | FIS | FST |
|---|---|---|---|---|---|---|---|
| pchgms2 | 11 | 130–170 | 0.77 | 0.85 | 0.75 | −0.13 | 0.07 |
| CPPCT033 | 9 | 129–147 | 0.56 | 0.47 | 0.51 | 0.07 | 0.15 |
| BPPCT007 | 16 | 117–155 | 0.83 | 0.82 | 0.74 | −0.11 | 0.13 |
| BPPCT039 | 13 | 121–167 | 0.73 | 0.45 | 0.55 | 0.18 | 0.30 |
| BPPCT025 | 13 | 140–194 | 0.75 | 0.52 | 0.67 | 0.22 | 0.13 |
| CPSCT026 | 15 | 156–208 | 0.83 | 0.66 | 0.77 | 0.14 | 0.11 |
| CPSCT005 | 13 | 165–193 | 0.84 | 0.76 | 0.80 | 0.05 | 0.06 |
| UDP96005 | 14 | 93–153 | 0.67 | 0.68 | 0.68 | 0.00 | 0.06 |
| Mean | 13 | - | 0.75 | 0.65 | 0.68 | 0.05 | 0.12 |
| Source of Variation | df | Sum of Square | Mean Sum of Square | % of the Variance | Phi |
|---|---|---|---|---|---|
| Among groups | 6 | 281 | 46.9 | 14.2 | 0.182 |
| Among accessions within groups | 154 | 876 | 5.7 | 4.0 | 0.046 |
| Within accessions | 161 | 834 | 5.2 | 81.8 * | 0.142 |
| Total | 321 | 1991 | 6.2 | 100.0 |
| Group | n | NA PER LOCUS | NA TOTAL | AR | PA | Ho | He | FIS |
|---|---|---|---|---|---|---|---|---|
| G1 | 32 | 7.38 | 59 | 6.06 | 3 | 0.59 | 0.63 | 0.04 |
| G2 | 23 | 7.63 | 61 | 7.04 | 10 | 0.64 | 0.67 | 0.06 |
| G3 | 18 | 7.25 | 58 | 7.25 | 2 | 0.69 | 0.73 | 0.05 |
| G4 | 21 | 6.25 | 50 | 6.01 | 3 | 0.67 | 0.68 | 0.00 |
| G5 | 18 | 7.13 | 57 | 7.13 | 1 | 0.66 | 0.66 | 0.00 |
| G6 | 22 | 6.50 | 52 | 6.23 | 3 | 0.61 | 0.71 | 0.15 |
| G7 | 27 | 6.75 | 54 | 6.17 | 4 | 0.69 | 0.69 | −0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerrero, B.I.; Guerra, M.E.; Herrera, S.; Irisarri, P.; Pina, A.; Rodrigo, J. Genetic Diversity and Population Structure of Japanese Plum-Type (Hybrids of P. salicina) Accessions Assessed by SSR Markers. Agronomy 2021, 11, 1748. https://doi.org/10.3390/agronomy11091748
Guerrero BI, Guerra ME, Herrera S, Irisarri P, Pina A, Rodrigo J. Genetic Diversity and Population Structure of Japanese Plum-Type (Hybrids of P. salicina) Accessions Assessed by SSR Markers. Agronomy. 2021; 11(9):1748. https://doi.org/10.3390/agronomy11091748
Chicago/Turabian StyleGuerrero, Brenda I., M. Engracia Guerra, Sara Herrera, Patricia Irisarri, Ana Pina, and Javier Rodrigo. 2021. "Genetic Diversity and Population Structure of Japanese Plum-Type (Hybrids of P. salicina) Accessions Assessed by SSR Markers" Agronomy 11, no. 9: 1748. https://doi.org/10.3390/agronomy11091748
APA StyleGuerrero, B. I., Guerra, M. E., Herrera, S., Irisarri, P., Pina, A., & Rodrigo, J. (2021). Genetic Diversity and Population Structure of Japanese Plum-Type (Hybrids of P. salicina) Accessions Assessed by SSR Markers. Agronomy, 11(9), 1748. https://doi.org/10.3390/agronomy11091748







